Marine-Derived Compounds: A New Horizon in Cancer, Renal, and Metabolic Disease Therapeutics
Abstract
1. Introduction
2. Methodology of Literature Review
3. Marine-Derived Compounds in Cancer Therapy
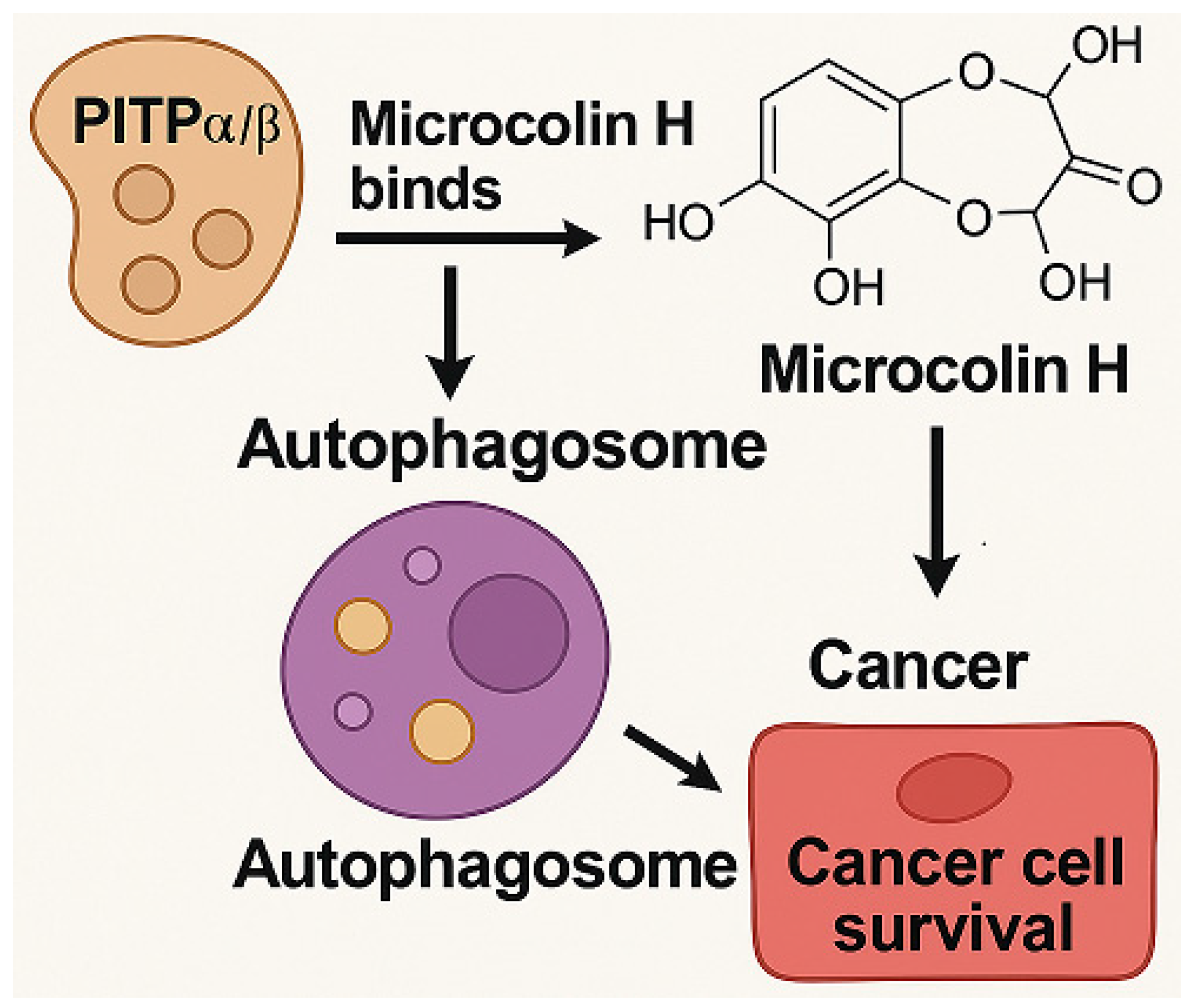
3.1. Microcolin H
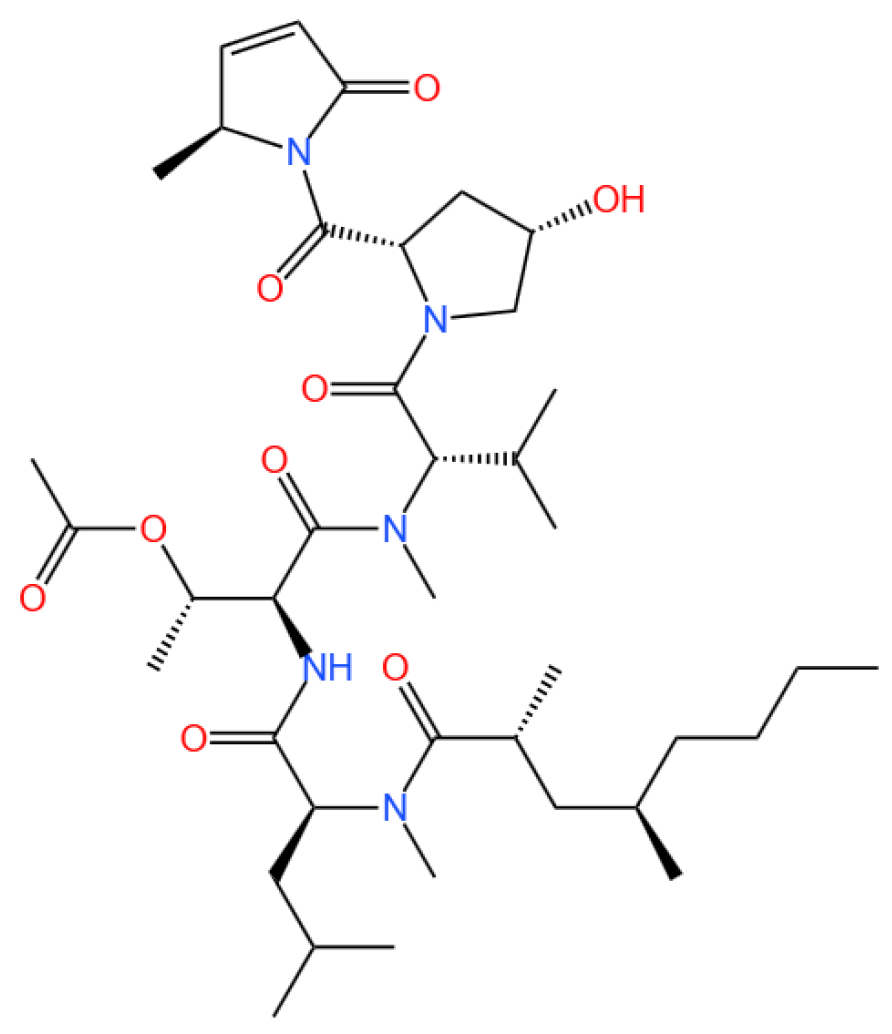
3.1.1. Background and Discovery
3.1.2. Mechanism of Action
3.1.3. Experimental Evidence
3.1.4. Future Perspectives
3.2. Marine Alkaloids: Nortopsentin and Topsentin
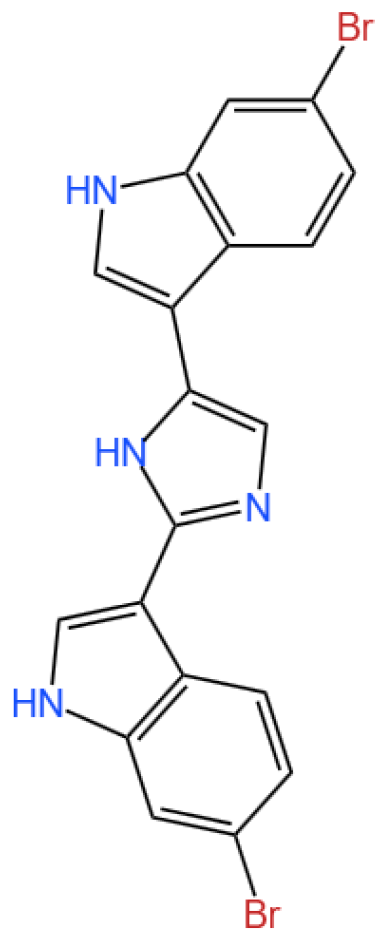
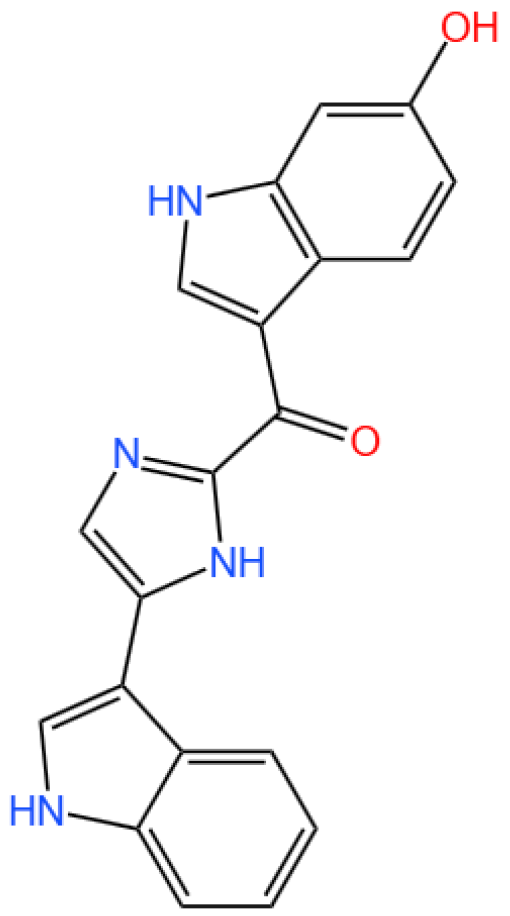
3.2.1. Background and Discovery
3.2.2. Mechanism of Action
3.2.3. Experimental Evidence
3.2.4. Future Perspectives
3.3. Bryostatin

3.3.1. Background and Discovery
3.3.2. Mechanism of Action
3.3.3. Experimental Evidence
3.3.4. Future Perspectives
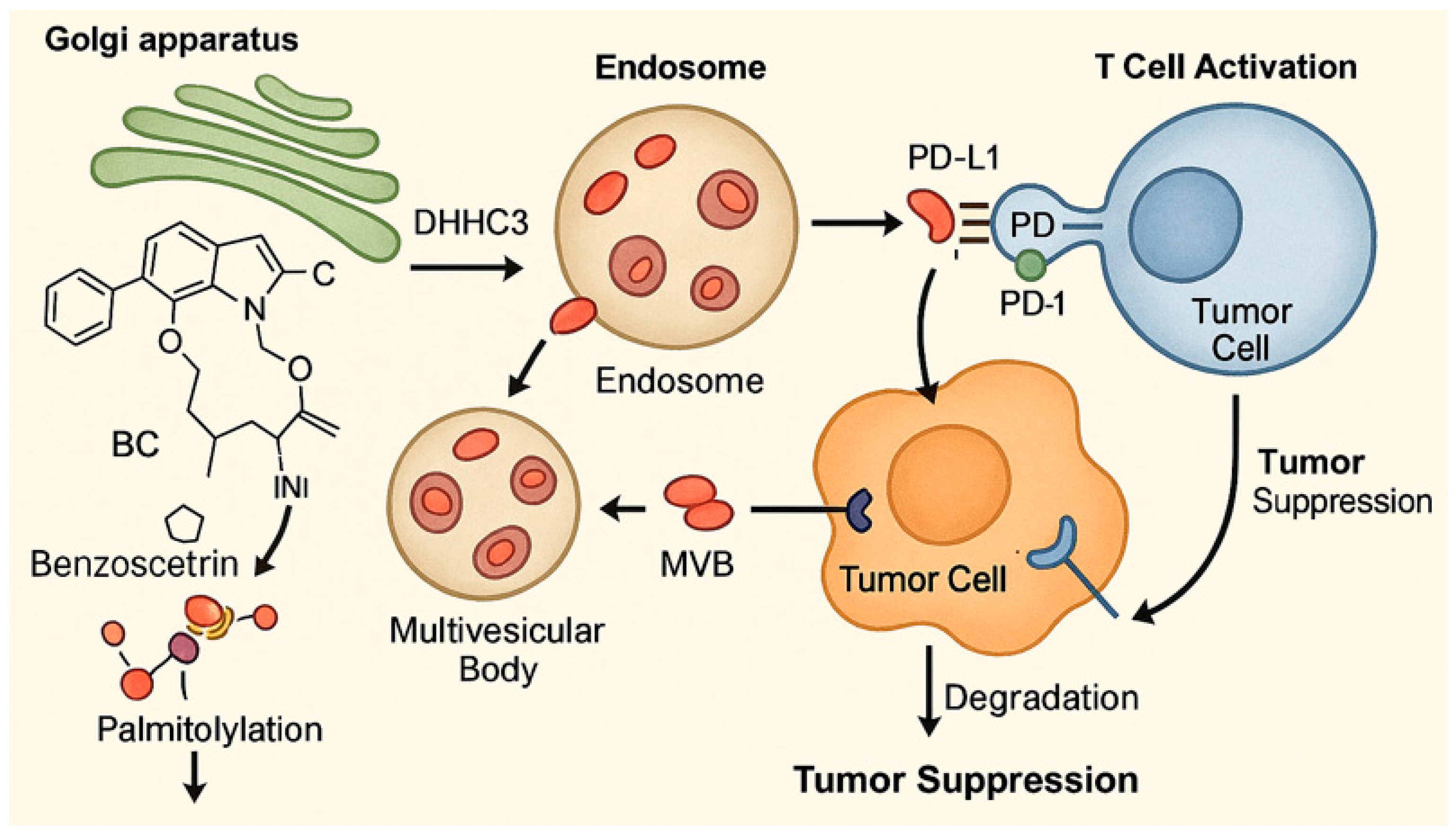
3.4. Benzosceptrin C
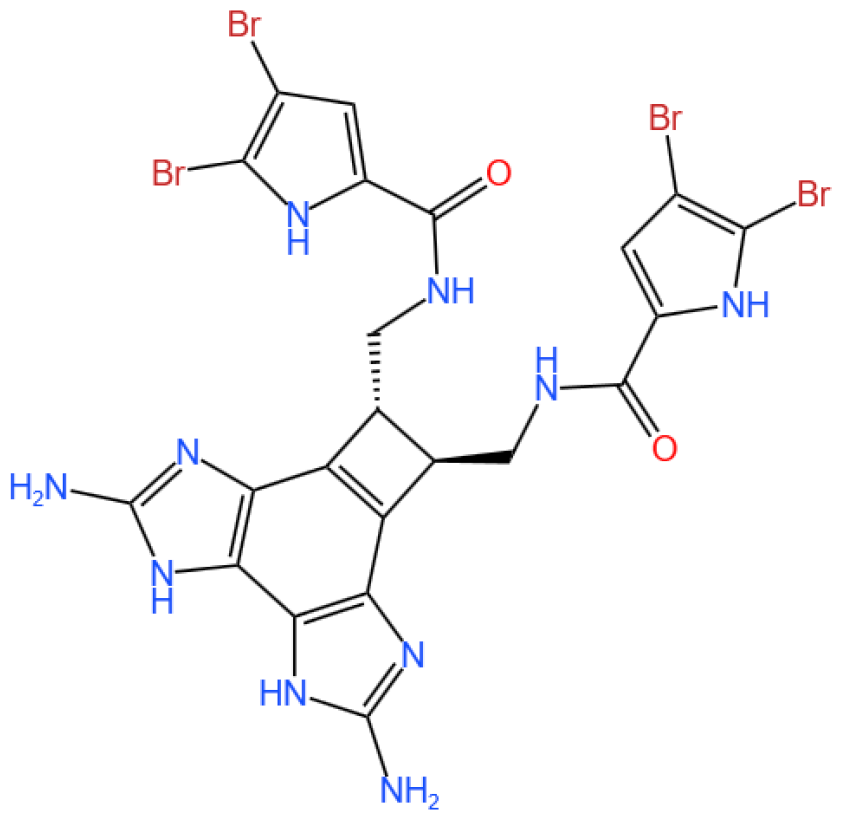
3.4.1. Background and Discovery
3.4.2. Immunotherapy Context
3.4.3. In Vivo and In Vitro Results, and Mechanism of Benzosceptrin C
3.4.4. Future Research Directions
3.5. Glycosides
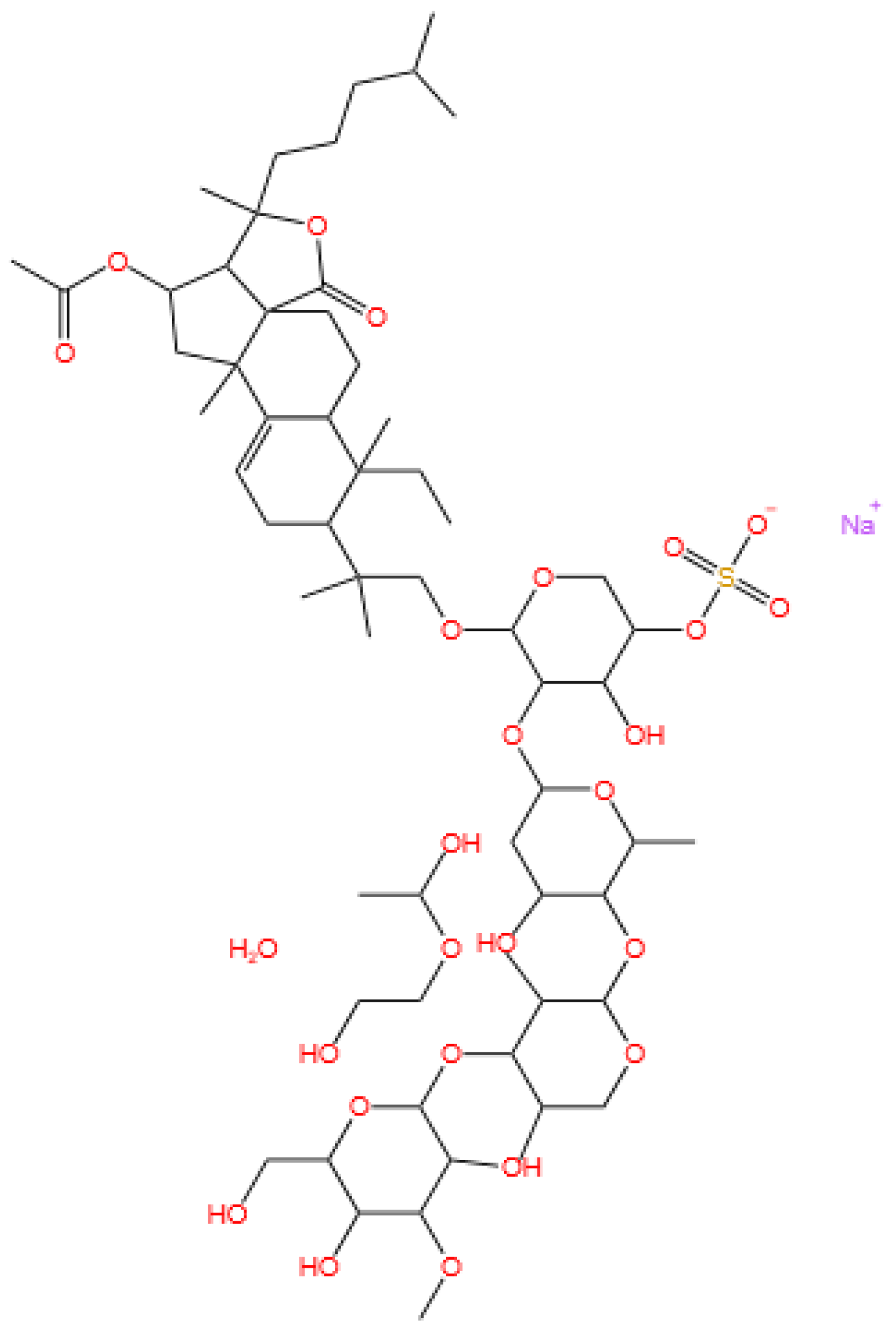
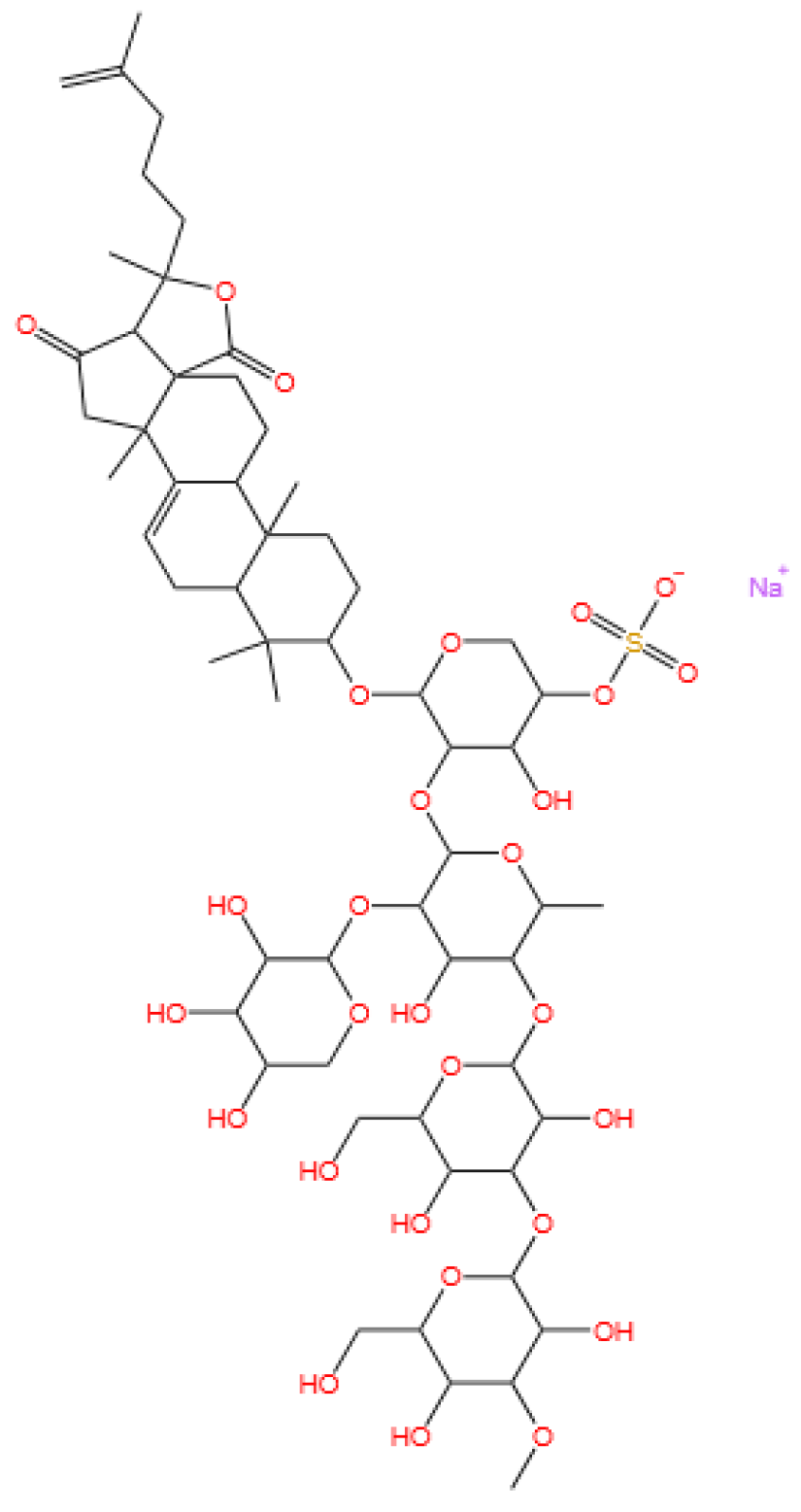
3.5.1. Background and Discovery
3.5.2. Mechanism of Action
3.5.3. Experimental Evidence
3.5.4. Future Perspectives
3.6. Ilimaquinone
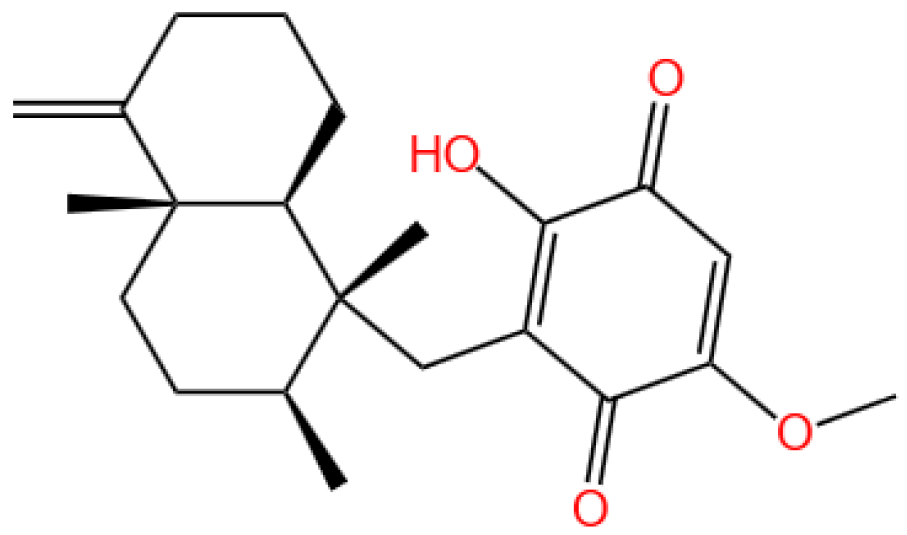
3.6.1. Background and Discovery
3.6.2. Mechanism of Action
3.6.3. Experimental Evidence
3.6.4. Future Perspectives
3.7. Aplidin (Plitidepsin or Dehydrodidemnin B)

3.7.1. Background and Discovery
3.7.2. Mechanism of Action
3.7.3. Experimental Evidence
3.7.4. Future Perspectives
3.8. Summary of the Section
4. Marine-Derived Compounds in Renal Disease Treatment
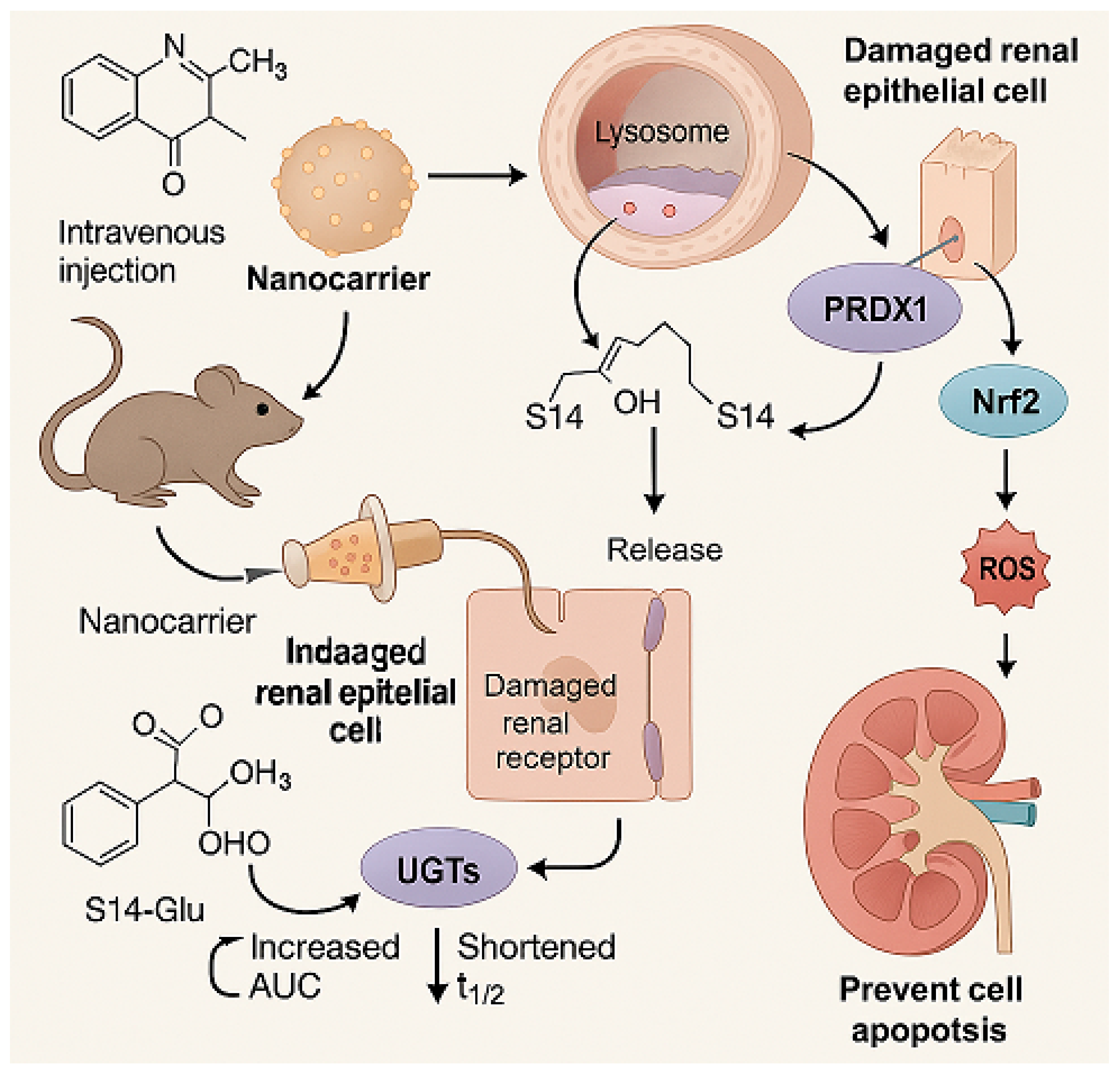
4.1. S14
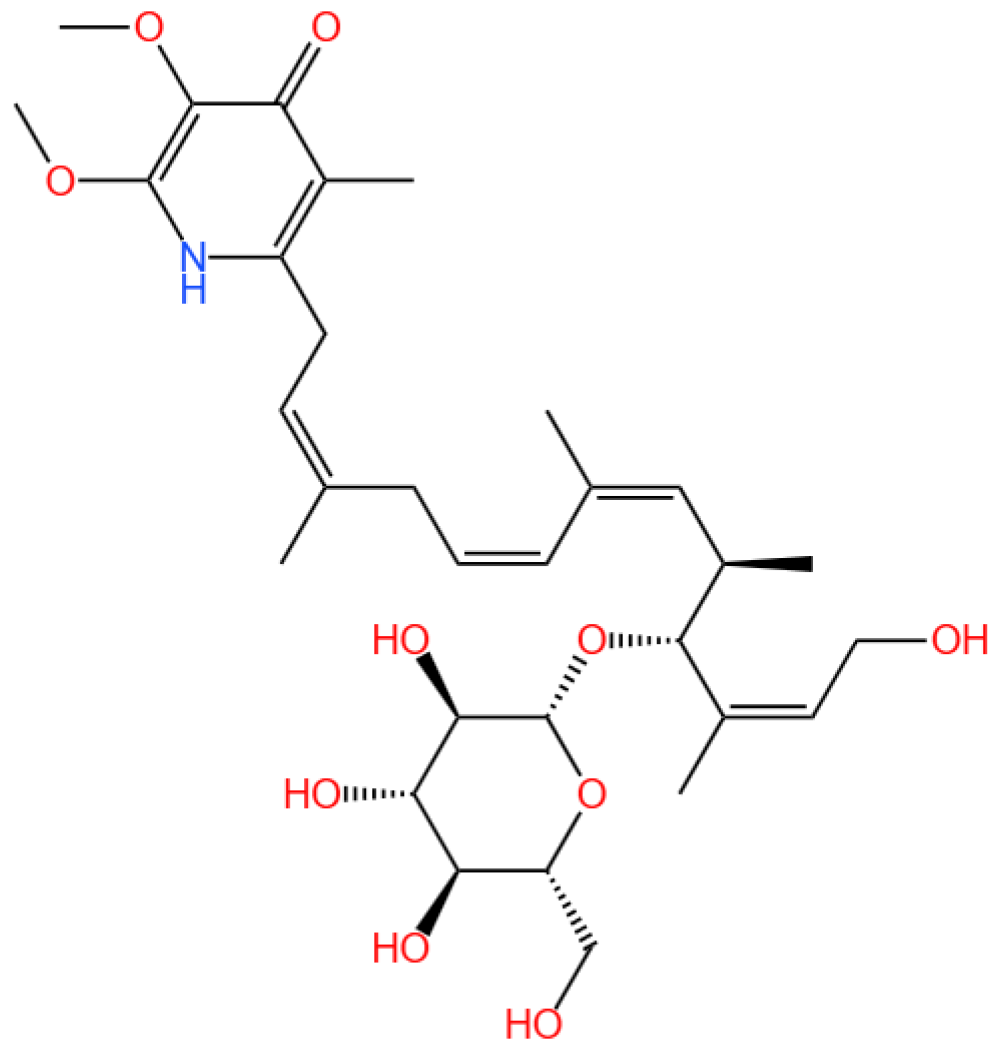
4.1.1. Renal Disease Landscape
4.1.2. The Role of S14
4.1.3. Delivery System and Efficacy
4.1.4. Future Research
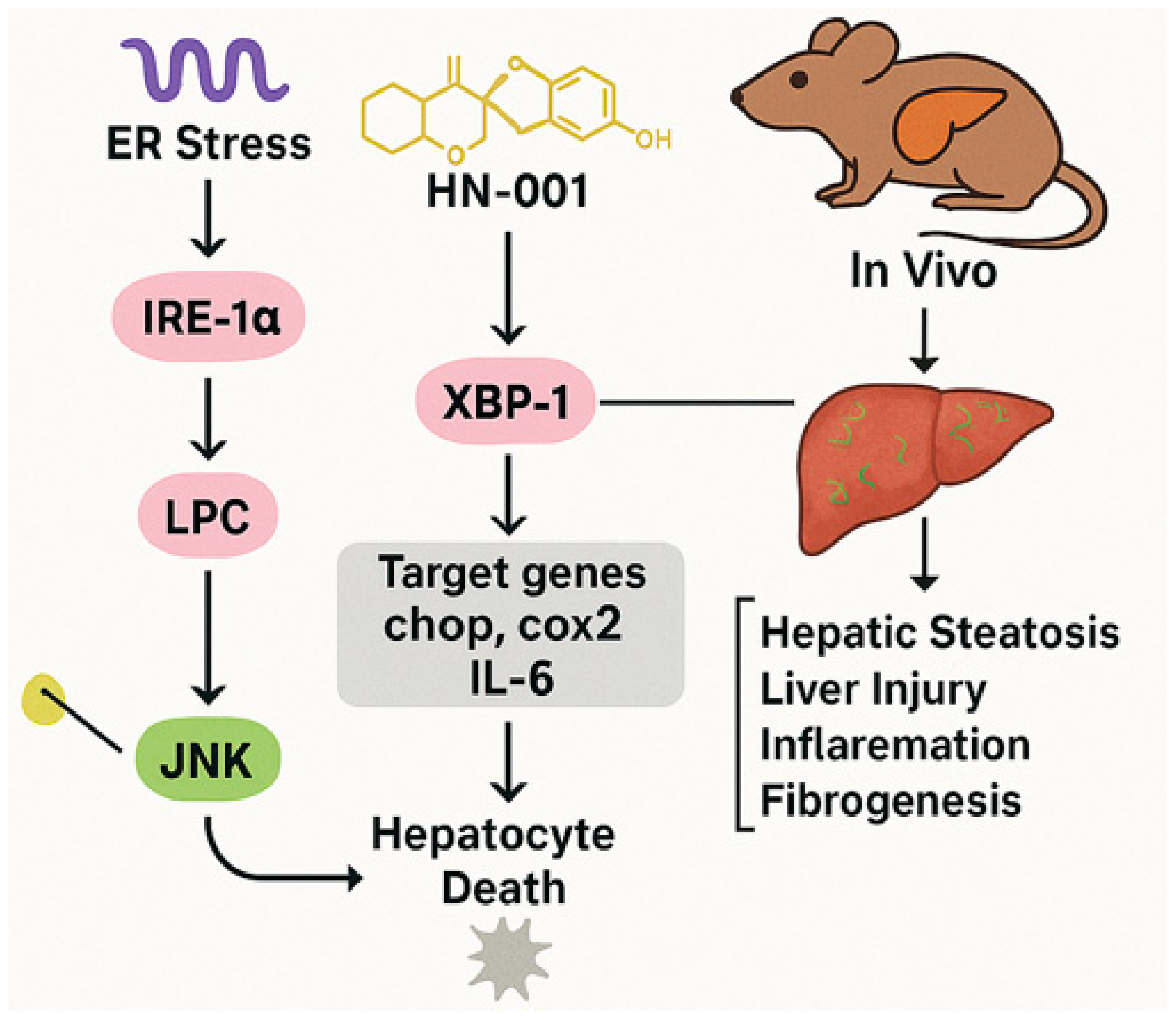
4.2. HN-001
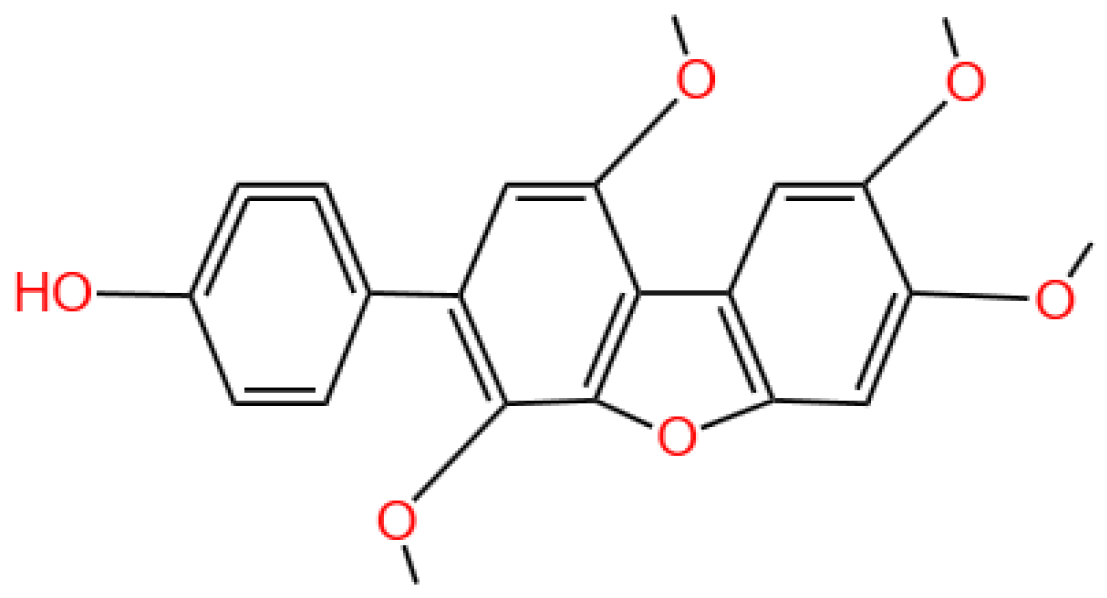
4.2.1. MAFLD Prevalence and Mechanisms
4.2.2. HN-001’s Mechanism
4.2.3. In Vivo and In Vitro Findings
4.2.4. Future Research Needs
4.3. Summary of the Section
5. Marine-Derived Compounds in Atherosclerosis Treatment
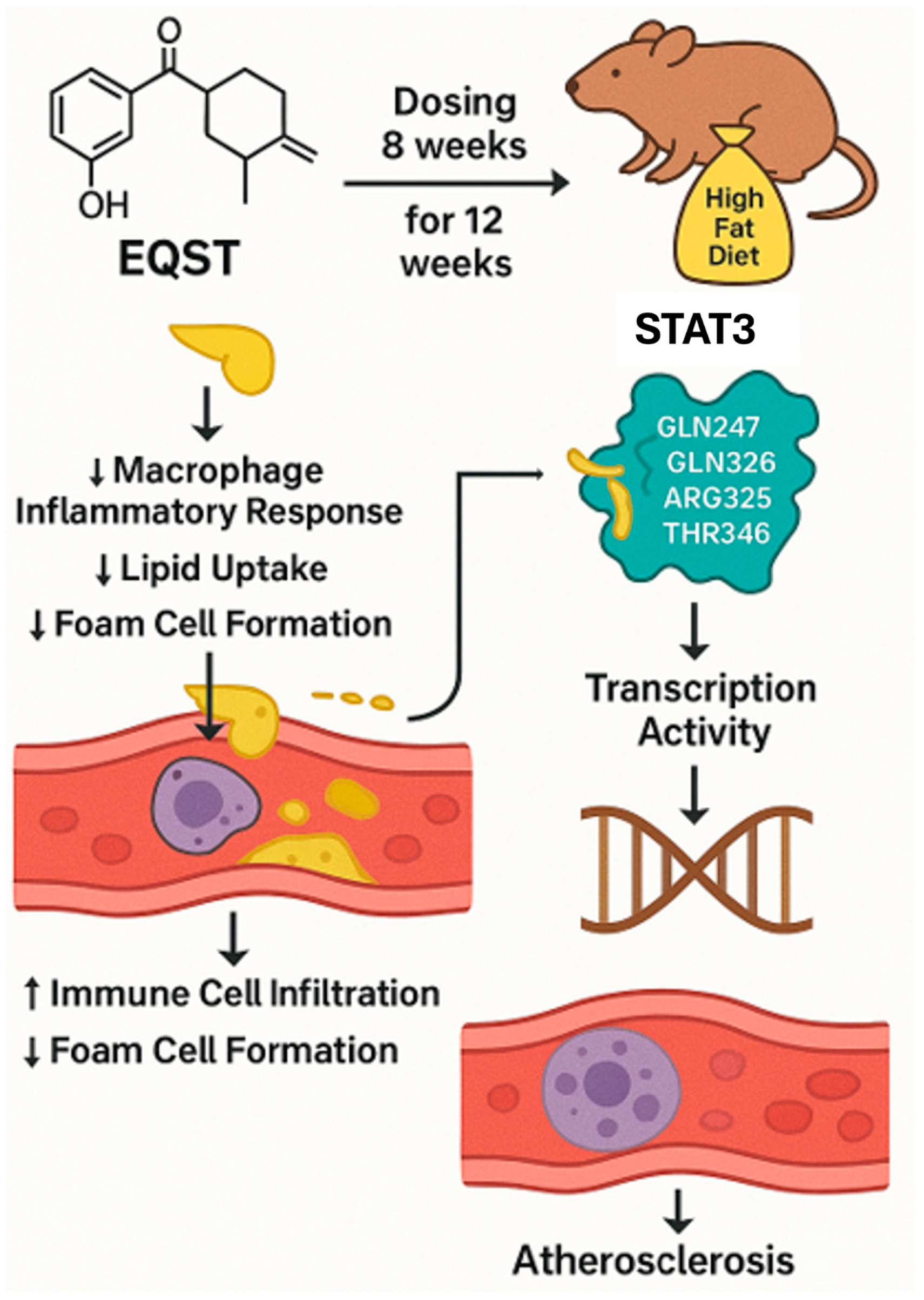
5.1. Equisetin
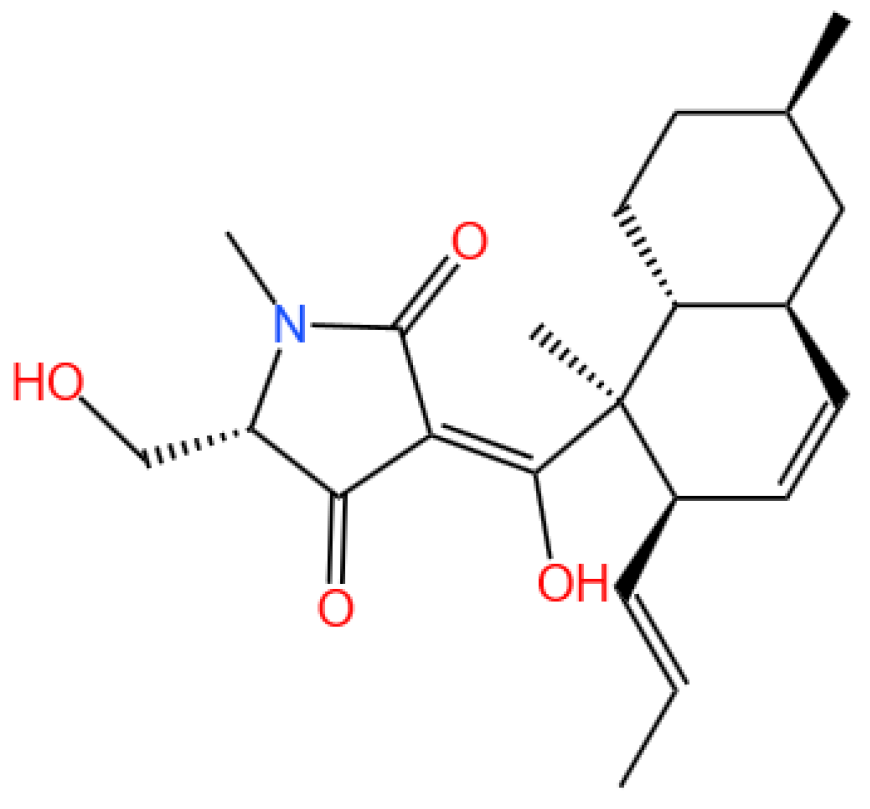
5.1.1. Background and Discovery
5.1.2. Mechanism of Action
5.1.3. Future Perspectives
5.2. Summary of the Section
6. Other Marine-Derived Compounds
6.1. Fucoidan
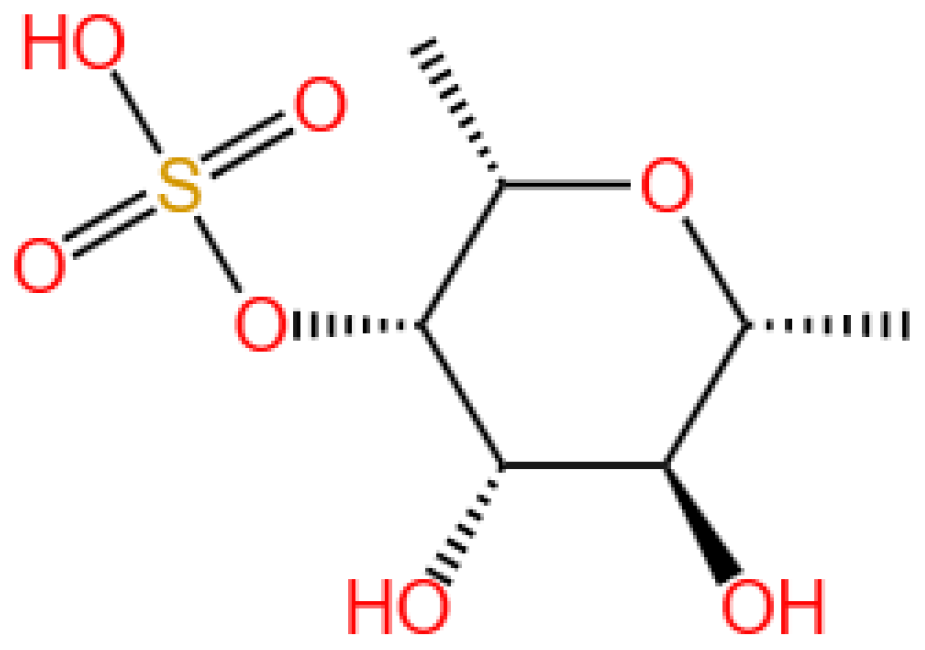
6.1.1. Background and Discovery
6.1.2. Mechanism of Action
6.1.3. Experimental Evidence
6.1.4. Future Perspectives
7. Pharmacokinetics of Marine-Derived Compounds
7.1. Background and Characteristics
7.2. Key Pharmacokinetic Parameters and Mechanisms
7.2.1. Absorption and Distribution
7.2.2. Metabolism and Excretion
7.2.3. Structure–Activity Relationships
7.2.4. Clinical Implications and Challenges
7.3. Future Perspectives
8. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| MAFLD | Metabolic-associated fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| DDR | DNA damage response |
| PRDX1 | Peroxiredoxin 1 |
| Kim-1 | Kidney injury molecule-1 |
| PD-1 | Programmed cell death-1 |
| PD-L1 | Programmed cell death ligand-1 |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CAR T cells | Chimeric antigen receptor T cells |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-derived suppressor cells |
| TNBC | Triple-negative breast cancer |
| PITPα/β | Phosphatidylinositol transfer protein alpha/beta isoform |
| LKB1 | Liver kinase B1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| ROS | Reactive oxygen species |
| IRE-1α | Inositol-requiring enzyme 1 alpha |
| XBP-1s | Spliced X-box binding protein 1 |
| JNK | c-Jun N-terminal kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| PLA2 | Phospholipase A2 |
| CK1 | Casein kinase 1 |
| GSK-3β | Glycogen synthase kinase-3 beta |
| CDK1/2/4/6 | Cyclin-dependent kinase 1/2/4/6 |
| PKC | Protein kinase C |
| SPR | Surface plasmon resonance |
| CETSA | Cellular thermal shift assay |
| UIRI | Unilateral ischemia-reperfusion injury |
| AUC | Area under the curve |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| HFD | High-fat diet |
| oxLDL | Oxidized low-density lipoprotein |
| BMDMs | Bone marrow-derived macrophages |
| EQST | Equisetin |
| S14 | 13-hydroxyglucopiericidin A |
| HN-001 | A compound derived from Aspergillus sp. C1 |
| BC | Benzosceptrin C |
| HCQ | Hydroxychloroquine |
| PTX | Paclitaxel |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Bonavida, B. Review Article: Probiotics-Mediated Enhancement of Checkpoint Inhibitor Blockade for HER2+ Breast Cancer. Crit. Rev. Oncog. 2025, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Dalle Grave, R.; Suppini, A.; Marchesini, G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology 2008, 47, 746–754. [Google Scholar] [CrossRef]
- Tamargo, C.; Hanouneh, M.; Cervantes, C.E. Treatment of Acute Kidney Injury: A Review of Current Approaches and Emerging Innovations. J. Clin. Med. 2024, 13, 2455. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Wang, C.; Zhang, H.; Yi, J.; Wang, K.; Hou, Y.; Ji, P.; Jin, X.; Li, C.; et al. Microcolin H, a novel autophagy inducer, exerts potent antitumour activity by targeting PITPα/β. Signal Transduct. Target. Ther. 2023, 8, 428. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Yu, D.; Zhang, Q.; Hu, H.; Xu, M.; Zhang, H.; Tian, S.; Zheng, G.; Lu, D.; et al. Benzosceptrin C induces lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting DHHC3. Cell Rep. Med. 2024, 5, 101357. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hui, Z.; Huang, S.; Cai, M.; Shi, W.; Lin, Y.; Shen, J.; Sui, M.; Lai, Q.; et al. A Semisynthesis Platform for the Efficient Production and Exploration of Didemnin-Based Drugs. Angew. Chem. Int. Ed. 2024, 63, e202318784. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Wang, X.; Zhang, Y.; Min, W.; Yu, P.; Miao, J.; Shen, W.; Chen, S.; Zhou, S.; et al. A new LKB1 activator, piericidin analogue S14, retards renal fibrosis through promoting autophagy and mitochondrial homeostasis in renal tubular epithelial cells. Theranostics 2022, 12, 7158–7179. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Su, R.; Wu, C.; Chai, X.; Li, J.; Yang, G.; Wu, J.; Fu, T.; Jiang, Z.; Guo, Z.; et al. Identification of a natural PLA2 inhibitor from the marine fungus Aspergillus sp. c1 for MAFLD treatment that suppressed lipotoxicity by inhibiting the IRE-1α/XBP-1s axis and JNK signaling. Acta Pharm. Sin. B 2024, 14, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Gu, T.; Rao, Y.; Liang, W.; Zhang, X.; Jiang, H.; Lu, J.; She, J.; Guo, J.; Yang, W.; et al. A novel marine-derived anti-acute kidney injury agent targeting peroxiredoxin 1 and its nanodelivery strategy based on ADME optimization. Acta Pharm. Sin. B 2024, 14, 3232–3250. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Anees, M.; Zofair, S.F.F.; Rasool, F.; Khan, M.A.; Moin, S.; Younus, H. Fucoidan based polymeric nanoparticles encapsulating epirubicin: A novel and effective chemotherapeutic formulation against colorectal cancer. Int. J. Pharm. 2024, 664, 124622. [Google Scholar] [CrossRef]
- Pecoraro, C.; Terrana, F.; Panzeca, G.; Parrino, B.; Cascioferro, S.; Diana, P.; Giovannetti, E.; Carbone, D. Nortopsentins as Leads from Marine Organisms for Anticancer and Anti-Inflammatory Agent Development. Molecules 2023, 28, 6450. [Google Scholar] [CrossRef]
- Carbone, D.; Pecoraro, C.; Panzeca, G.; Xu, G.; Roeten, M.S.F.; Cascioferro, S.; Giovannetti, E.; Diana, P.; Parrino, B. 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Nortopsentin Derivatives against Pancreatic Ductal Adenocarcinoma: Synthesis, Cytotoxic Activity, and Inhibition of CDK1. Mar. Drugs 2023, 21, 412. [Google Scholar] [CrossRef]
- Abdelmegeed, H.; Abo-Salem, H.M.; Zayed, E.M.; El-Sawy, E.R. Anti colorectal cancer activity and in silico studies of novel pyridine nortopsentin analog as cyclin dependent kinase 6 inhibitor. Sci. Rep. 2024, 14, 26327. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, D.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Deng, D.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cusimano, M.G.; et al. 1,2,4-Oxadiazole topsentin analogs as staphylococcal biofilm inhibitors targeting the bacterial transpeptidase sortase A. Eur. J. Med. Chem. 2021, 209, 112892. [Google Scholar] [CrossRef]
- Sun, L.; Lamb, J.G.; Niu, C.; Serna, S.N.; Romero, E.G.; Deering-Rice, C.E.; Schmidt, E.W.; Golkowski, M.; Reilly, C.A. Bryostatins 1 and 3 inhibit TRPM8 and modify TRPM8- and TRPV1-mediated lung epithelial cell responses to a proinflammatory stimulus via protein kinase C. Mol. Pharmacol. 2025, 107, 100042. [Google Scholar] [CrossRef]
- Li, L.; Zhao, M.; van Meurs, M.; Brouwers-Haspels, I.; den Dekker, R.J.H.; Wilmsen, M.E.P.; Grashof, D.G.B.; van de Werken, H.J.G.; Rao, S.; Rokx, C.; et al. Bryostatin-1 enhances the proliferation and functionality of exhausted CD8+ T cells by upregulating MAP Kinase 11. Front. Immunol. 2025, 15, 1509874. [Google Scholar] [CrossRef]
- Gharibani, P.; Abramson, E.; Shanmukha, S.; Smith, M.D.; Godfrey, W.H.; Lee, J.J.; Hu, J.; Baydyuk, M.; Dorion, M.-F.; Deng, X.; et al. The protein kinase C modulator bryostatin-1 therapeutically targets microglia to attenuate neuroinflammation and promote remyelination. Sci. Transl. Med. 2025, 17, eadk3434. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Park, S.-K.; Mookherjee, S.; Peters, E.C.; Pires, P.W.; Symons, J.D. Bryostatin-1 improves function in arteries with suppressed endothelial cell autophagy. GeroScience 2025. [Google Scholar] [CrossRef]
- Kisaka, J.K.; Rauch, D.; Griffith, M.; Kyei, G.B. A macrophage-cell model of HIV latency reveals the unusual importance of the bromodomain axis. Virol. J. 2024, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Lan, J.; Li, C.; Shi, H.; Brosseau, J.-P.; Wang, H.; Lu, H.; Fang, C.; Zhang, Y.; Liang, L.; et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 2019, 3, 306–317. [Google Scholar] [CrossRef]
- Chuang, W.-H.; Pislyagin, E.; Lin, L.-Y.; Menchinskaya, E.; Chernikov, O.; Kozhemyako, V.; Gorpenchenko, T.; Manzhulo, I.; Chaikina, E.; Agafonova, I.; et al. Holothurian triterpene glycoside cucumarioside A2-2 induces macrophages activation and polarization in cancer immunotherapy. Cancer Cell Int. 2023, 23, 292. [Google Scholar] [CrossRef]
- Menchinskaya, E.S.; Dyshlovoy, S.A.; Venz, S.; Jacobsen, C.; Hauschild, J.; Rohlfing, T.; Silchenko, A.S.; Avilov, S.A.; Balabanov, S.; Bokemeyer, C.; et al. Anticancer Activity of the Marine Triterpene Glycoside Cucumarioside A2-2 in Human Prostate Cancer Cells. Mar. Drugs 2023, 22, 20. [Google Scholar] [CrossRef]
- Pislyagin, E.; Manzhulo, I.; Gorpenchenko, T.; Dmitrenok, P.; Avilov, S.; Silchenko, A.; Wang, Y.-M.; Aminin, D. Cucumarioside A2-2 Causes Macrophage Activation in Mouse Spleen. Mar. Drugs 2017, 15, 341. [Google Scholar] [CrossRef]
- Carpi, S.; Scoditti, E.; Polini, B.; Brogi, S.; Calderone, V.; Proksch, P.; Ebada, S.S.; Nieri, P. Pro-Apoptotic Activity of the Marine Sponge Dactylospongia elegans Metabolites Pelorol and 5-epi-Ilimaquinone on Human 501Mel Melanoma Cells. Mar. Drugs 2022, 20, 427. [Google Scholar] [CrossRef]
- Jiso, A.; Demuth, P.; Bachowsky, M.; Haas, M.; Seiwert, N.; Heylmann, D.; Rasenberger, B.; Christmann, M.; Dietrich, L.; Brunner, T.; et al. Natural Merosesquiterpenes Activate the DNA Damage Response via DNA Strand Break Formation and Trigger Apoptotic Cell Death in p53-Wild-Type and Mutant Colorectal Cancer. Cancers 2021, 13, 3282. [Google Scholar] [CrossRef]
- Kwak, C.-H.; Jin, L.; Han, J.H.; Han, C.W.; Kim, E.; Cho, M.; Chung, T.-W.; Bae, S.-J.; Jang, S.B.; Ha, K.-T. Ilimaquinone Induces the Apoptotic Cell Death of Cancer Cells by Reducing Pyruvate Dehydrogenase Kinase 1 Activity. Int. J. Mol. Sci. 2020, 21, 6021. [Google Scholar] [CrossRef]
- Losada, A.; Berlanga, J.J.; Molina-Guijarro, J.M.; Jiménez-Ruiz, A.; Gago, F.; Avilés, P.; de Haro, C.; Martínez-Leal, J.F. Generation of endoplasmic reticulum stress and inhibition of autophagy by plitidepsin induces proteotoxic apoptosis in cancer cells. Biochem. Pharmacol. 2020, 172, 113744. [Google Scholar] [CrossRef]
- Losada, A.; Muñoz-Alonso, M.J.; García, C.; Sánchez-Murcia, P.A.; Martínez-Leal, J.F.; Domínguez, J.M.; Lillo, M.P.; Gago, F.; Galmarini, C.M. Translation Elongation Factor eEF1A2 is a Novel Anticancer Target for the Marine Natural Product Plitidepsin. Sci. Rep. 2016, 6, 35100. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Kurihara, N.; Atkinson, E.G.; Nelson, J.; Miyagawa, K.; Galmarini, C.M.; Roodman, G.D.; Bellido, T. Aplidin (plitidepsin) is a novel anti-myeloma agent with potent anti-resorptive activity mediated by direct effects on osteoclasts. Oncotarget 2019, 10, 2709–2721. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Ocio, E.M.; Pandiella, A.; Maiso, P.; Gajate, C.; Garayoa, M.; Vilanova, D.; Montero, J.C.; Mitsiades, N.; McMullan, C.J.; et al. Aplidin, a Marine Organism–Derived Compound with Potent Antimyeloma Activity In vitro and In vivo. Cancer Res. 2008, 68, 5216–5225. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Cibeira, M.T.; Richardson, P.G.; Prosper, F.; Oriol, A.; de la Rubia, J.; Lahuerta, J.J.; García-Sanz, R.; Extremera, S.; Szyldergemajn, S.; et al. Phase II Clinical and Pharmacokinetic Study of Plitidepsin 3-Hour Infusion Every Two Weeks Alone or with Dexamethasone in Relapsed and Refractory Multiple Myeloma. Clin. Cancer Res. 2010, 16, 3260–3269. [Google Scholar] [CrossRef]
- Landete, P.; Caliman-Sturdza, O.-A.; Lopez-Martin, J.A.; Preotescu, L.; Luca, M.-C.; Kotanidou, A.; Villares, P.; Iglesias, S.-P.; Guisado-Vasco, P.; Saiz-Lou, E.-M.; et al. A Phase III Randomized Controlled Trial of Plitidepsin, a Marine-Derived Compound, in Hospitalized Adults With Moderate COVID-19. Clin. Infect. Dis. 2024, 79, 910–919. [Google Scholar] [CrossRef]
- Vesonder, R.F.; Tjarks, L.W.; Rohwedder, W.K.; Burmeister, H.R.; Laugal, J.A. Equisetin, an antibiotic from Fusarium equiseti NRRL 5537, identified as a derivative of N-methyl-2,4-pyrollidone. J. Antibiot. 1979, 32, 759–761. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Tian, Y.; Li, M.; Xu, S.; Zhang, L.; Luo, X.; Tan, Y.; Liang, H.; Chen, M. Equisetin protects from atherosclerosis in vivo by binding to STAT3 and inhibiting its activity. Pharmacol. Res. 2024, 206, 107289. [Google Scholar] [CrossRef]
- Yunianta, Y.; Laeliocattleya, R.A.; Wulan, S.N.; Risjani, Y. Fucoidan extract from brown seaweed (Sargassum echinocarpum): Molecular weight, elemental composition, selectivity and anticancer activity against breast cancer cells. Nat. Prod. Res. 2024, 39, 3093–3101. [Google Scholar] [CrossRef]
- Chan, C.-H.; Deng, Y.-H.; Peng, B.-Y.; Chiang, P.-C.; Wu, L.-A.; Lee, Y.-Y.; Tsao, W.; Mao, H.-H.; Wu, C.-Y.; Deng, W.-P. Anti-Colorectal Cancer Effects of Fucoidan Complex-Based Functional Beverage Through Retarding Proliferation, Cell Cycle and Epithelial–Mesenchymal Transition Signaling Pathways. Integr. Cancer Ther. 2023, 22, 15347354231213613. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Peng, X.; Li, J.; Zhu, C. The Natural Product Fucoidan Inhibits Proliferation and Induces Apoptosis of Human Ovarian Cancer Cells: Focus on the PI3K/Akt Signaling Pathway. Cancer Manag. Res. 2020, 12, 6195–6207. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Ohba, K.; Mitsunari, K.; Mukae, Y.; Otsubo, A.; Harada, J.; Matsuda, T.; Kondo, T.; Sakai, H. Present Status, Limitations and Future Directions of Treatment Strategies Using Fucoidan-Based Therapies in Bladder Cancer. Cancers 2020, 12, 3776. [Google Scholar] [CrossRef]
- Ramanjooloo, A.; Chummun Phul, I.; Goonoo, N.; Bhaw-Luximon, A. Electrospun polydioxanone/fucoidan blend nanofibers loaded with anti-cancer precipitate from Jaspis diastra and paclitaxel: Physico-chemical characterization and in-vitro screening. Int. J. Biol. Macromol. 2024, 259, 129218. [Google Scholar] [CrossRef]
- Panwong, S.; Phinyo, K.; Duangjan, K.; Sattayawat, P.; Pekkoh, J.; Tragoolpua, Y.; Yenchitsomanus, P.-t.; Panya, A. Inhibition of dengue virus infection in vitro by fucoidan and polysaccharide extract from marine alga Sargassum spp. Int. J. Biol. Macromol. 2024, 276, 133496. [Google Scholar] [CrossRef]
- Moore, R.E.; Entzeroth, M. Majusculamide D and deoxymajusculamide D, two cytotoxins from Lyngbya majuscula. Phytochemistry 1988, 27, 3101–3103. [Google Scholar] [CrossRef]
- Caro-Diaz, E.J.E.; Valeriote, F.A.; Gerwick, W.H. Highly Convergent Total Synthesis and Assignment of Absolute Configuration of Majusculamide D, a Potent and Selective Cytotoxic Metabolite from Moorea sp. Org. Lett. 2019, 21, 793–796. [Google Scholar] [CrossRef]
- Koehn, F.E.; Longley, R.E.; Reed, J.K. Microcolins A and B, New Immunosuppressive Peptides from the Blue-Green Alga Lyngbya majuscula. J. Nat. Prod. 2004, 55, 613–619. [Google Scholar] [CrossRef]
- Takamatsu, S.; Nagle, D.; Gerwick, W. Secondary Metabolites from Marine Cyanobacteria and Algae Inhibit LFA-1/ICAM-1 Mediated Cell Adhesion. Planta Medica 2004, 70, 127–131. [Google Scholar] [CrossRef]
- Meickle, T.; Matthew, S.; Ross, C.; Luesch, H.; Paul, V. Bioassay-Guided Isolation and Identification of Desacetylmicrocolin B fromLyngbyacf.polychroa. Planta Medica 2009, 75, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.A.; Harmody, D.; Pitts, T.P.; Vera-Diaz, B.; Winder, P.L.; Yu, Y.; Wright, A.E. Inhibition of IL-8 secretion on BxPC-3 and MIA PaCa-2 cells and induction of cytotoxicity in pancreatic cancer cells with marine natural products. Anti-Cancer Drugs 2017, 28, 153–160. [Google Scholar] [CrossRef]
- Yu, H.-B.; Glukhov, E.; Li, Y.; Iwasaki, A.; Gerwick, L.; Dorrestein, P.C.; Jiao, B.-H.; Gerwick, W.H. Cytotoxic Microcolin Lipopeptides from the Marine Cyanobacterium Moorea producens. J. Nat. Prod. 2019, 82, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Fu, V.; Liu, G.; Tang, T.; Konradi, A.W.; Peng, X.; Kemper, E.; Cravatt, B.F.; Franklin, J.M.; Wu, Z.; et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat Chem Biol 2022, 18, 1076–1086. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Nile, A.H.; Bankaitis, V.A.; Grabon, A. Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clin. Lipidol. 2017, 5, 867–897. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Shi, Y.; Lin, Q.; Yang, W.; Luo, Q.; Cen, Y.; Li, J.; Fang, X.; Jiang, W.G.; Gong, C. Attenuation of PITPNM1 Signaling Cascade Can Inhibit Breast Cancer Progression. Biomolecules 2021, 11, 1265. [Google Scholar] [CrossRef]
- Shintani, T.; Klionsky, D.J. Autophagy in Health and Disease: A Double-Edged Sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef]
- Huang, Y.; You, X.; Wang, L.; Zhang, G.; Gui, S.; Jin, Y.; Zhao, R.; Zhang, D. Pyridinium-Substituted Tetraphenylethylenes Functionalized with Alkyl Chains as Autophagy Modulators for Cancer Therapy. Angew. Chem. Int. Ed. 2020, 59, 10042–10051. [Google Scholar] [CrossRef]
- Pardo, R.; Lo Ré, A.; Archange, C.; Ropolo, A.; Papademetrio, D.L.; Gonzalez, C.D.; Alvarez, E.M.; Iovanna, J.L.; Vaccaro, M.I. Gemcitabine Induces the VMP1 -Mediated Autophagy Pathway to Promote Apoptotic Death in Human Pancreatic Cancer Cells. Pancreatology 2010, 10, 19–26. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Thorburn, A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol. Ther. 2011, 131, 130–141. [Google Scholar] [CrossRef]
- Alvarado, S.; Roberts, B.F.; Wright, A.E.; Chakrabarti, D. The Bis(Indolyl)Imidazole Alkaloid Nortopsentin A Exhibits Antiplasmodial Activity. Antimicrob. Agents Chemother. 2013, 57, 2362–2364. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cho, E.; Hwang, J.-Y.; Park, S.C.; Chung, B.; Kwon, O.-S.; Sim, C.J.; Oh, D.-C.; Oh, K.-B.; Shin, J. Bioactive Bis(indole) Alkaloids from a Spongosorites sp. Sponge. Mar. Drugs 2020, 19, 3. [Google Scholar] [CrossRef]
- Pecoraro, C.; Parrino, B.; Cascioferro, S.; Puerta, A.; Avan, A.; Peters, G.J.; Diana, P.; Giovannetti, E.; Carbone, D. A New Oxadiazole-Based Topsentin Derivative Modulates Cyclin-Dependent Kinase 1 Expression and Exerts Cytotoxic Effects on Pancreatic Cancer Cells. Molecules 2021, 27, 19. [Google Scholar] [CrossRef]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef]
- Carbone, D.; Parrino, B.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cirrincione, G.; Diana, P. 1,2,4-Oxadiazole Topsentin Analogs with Antiproliferative Activity against Pancreatic Cancer Cells, Targeting GSK3β Kinase. ChemMedChem 2020, 16, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Gu, X.H. Syntheses and cytotoxicity evaluation of bis(indolyl)thiazole, bis(indolyl)pyrazinone and bis(indolyl)pyrazine: Analogues of cytotoxic marine bis(indole) alkaloid. Bioorg. Med. Chem. 2000, 8, 363–371. [Google Scholar] [CrossRef]
- Naaz, F.; Ahmad, F.; Lone, B.A.; Pokharel, Y.R.; Fuloria, N.K.; Fuloria, S.; Ravichandran, M.; Pattabhiraman, L.; Shafi, S.; Shahar Yar, M. Design and synthesis of newer 1,3,4-oxadiazole and 1,2,4-triazole based Topsentin analogues as anti-proliferative agent targeting tubulin. Bioorg. Chem. 2020, 95, 103519. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Hassan, G.S.; Al-Rashood, S.T.; Alkahtani, H.M.; Almehizia, A.A.; Al-Ansary, G.H. Marine-Inspired Bis-indoles Possessing Antiproliferative Activity against Breast Cancer; Design, Synthesis, and Biological Evaluation. Mar. Drugs 2020, 18, 190. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, D.; Park, J.S.; Park, H.J.; Shin, J.; Lee, S.K. Photoprotective Activity of Topsentin, A Bis(Indole) Alkaloid from the Marine Sponge Spongosorites genitrix, by Regulation of COX-2 and Mir-4485 Expression in UVB-Irradiated Human Keratinocyte Cells. Mar. Drugs 2020, 18, 87. [Google Scholar] [CrossRef]
- Kamel, M.M.; Abdel-hameid, M.K.; El-Nassan, H.B.; El-Khouly, E.A. Synthesis and Cytotoxic Activity of Novel Mono- and Bis-Indole Derivatives: Analogues of Marine Alkaloid Nortopsentin. Med. Chem. 2021, 17, 779–789. [Google Scholar] [CrossRef]
- Di Franco, S.; Parrino, B.; Gaggianesi, M.; Pantina, V.D.; Bianca, P.; Nicotra, A.; Mangiapane, L.R.; Lo Iacono, M.; Ganduscio, G.; Veschi, V.; et al. CHK1 inhibitor sensitizes resistant colorectal cancer stem cells to nortopsentin. iScience 2021, 24, 102664. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Rayko, M.; Komissarov, A.; Kwan, J.C.; Lim-Fong, G.; Rhodes, A.C.; Kliver, S.; Kuchur, P.; O’Brien, S.J.; Lopez, J.V. Draft genome of Bugula neritina, a colonial animal packing powerful symbionts and potential medicines. Sci. Data 2020, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, R.; Bharate, S.B. Preclinical and Clinical Studies on Bryostatins, A Class of Marine-Derived Protein Kinase C Modulators: A Mini-Review. Curr. Top. Med. Chem. 2020, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, Y.; Zhang, H.; Wang, D.; Li, B.; Jiang, D.; Tu, X.; Gao, X.; Zhang, C.; Qin, Y.; et al. Total Syntheses of Bryostatins 1, 7, 9 and 9-N3. Angew. Chem. Int. Ed. 2025, 64, e202423465. [Google Scholar] [CrossRef]
- Trost, B.M.; Wang, Y.; Buckl, A.K.; Huang, Z.; Nguyen, M.H.; Kuzmina, O. Total synthesis of bryostatin 3. Science 2020, 368, 1007–1011. [Google Scholar] [CrossRef]
- Izgutdina, A.; Rashid, T.; Temple, W.C.; Patiño-Escobar, B.; Walunj, S.; Geng, H.; Takamatsu, H.; Gil-Alós, D.; Kang, A.S.; Ramos, E.; et al. Affinity-matured CD72-targeting Nanobody CAR T-cells Enhance Elimination of Antigen-Low B-cell Malignancies. bioRxiv 2025. [Google Scholar] [CrossRef]
- Kou, Y.-Y.; Liu, J.; Chang, Y.-T.; Liu, L.-Y.; Sun, F.; Li, Y.-L.; Leng, J.-R.; Lin, H.-W.; Yang, F. Marine derived macrolide bryostatin 4 inhibits the TGF-β signaling pathway against acute erythroleukemia. Cell. Oncol. 2024, 47, 1863–1878. [Google Scholar] [CrossRef]
- Alkon, D.L.; Sun, M.-K.; Tuchman, A.J.; Thompson, R.E.; Avila, J. Advanced Alzheimer’s Disease Patients Show Safe, Significant, and Persistent Benefit in 6-Month Bryostatin Trial. J. Alzheimer’s Dis. 2023, 96, 759–766. [Google Scholar] [CrossRef]
- Thompson, R.E.; Tuchman, A.J.; Alkon, D.L.; Moreira, P. Bryostatin Placebo-Controlled Trials Indicate Cognitive Restoration Above Baseline for Advanced Alzheimer’s Disease in the Absence of Memantine1. J. Alzheimer’s Dis. 2022, 86, 1221–1229. [Google Scholar] [CrossRef]
- Douek, D.C.; French, A.J.; Natesampillai, S.; Krogman, A.; Correia, C.; Peterson, K.L.; Alto, A.; Chandrasekar, A.P.; Misra, A.; Li, Y.; et al. Reactivating latent HIV with PKC agonists induces resistance to apoptosis and is associated with phosphorylation and activation of BCL2. PLoS Pathog. 2020, 16, e1008906. [Google Scholar] [CrossRef]
- Sloane, J.L.; Benner, N.L.; Keenan, K.N.; Zang, X.; Soliman, M.S.A.; Wu, X.; Dimapasoc, M.; Chun, T.-W.; Marsden, M.D.; Zack, J.A.; et al. Prodrugs of PKC modulators show enhanced HIV latency reversal and an expanded therapeutic window. Proc. Natl. Acad. Sci. USA 2020, 117, 10688–10698. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wen, H.; Zuo, L.; Song, X.; Geng, Z.; Ge, S.; Ge, Y.; Wu, R.; Chen, S.; Yu, C.; et al. Bryostatin-1 attenuates intestinal ischemia/reperfusion-induced intestinal barrier dysfunction, inflammation, and oxidative stress via activation of Nrf2/HO-1 signaling. FASEB J. 2023, 37, e22948. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Luu-Nguyen, Q.H.; Sloane, J.L.; Ranjan, A. Trimethylene Methane Dianion Equivalent for the Asymmetric Consecutive Allylation of Aldehydes: Applications to Prins-Driven Macrocyclizations for the Synthesis of Bryostatin 1 and Analogues. J. Org. Chem. 2022, 87, 15925–15937. [Google Scholar] [CrossRef]
- Wu, W.-C.; Tian, J.; Xiao, D.; Guo, Y.-X.; Xiao, Y.; Wu, X.-Y.; Casella, G.; Rasouli, J.; Yan, Y.-P.; Rostami, A.; et al. Engineered extracellular vesicles encapsulated Bryostatin-1 as therapy for neuroinflammation. Nanoscale 2022, 14, 2393–2410. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Liao, B.-Y.; Xiao, D.; Wu, W.-C.; Xiao, Y.; Alexander, T.; Song, S.-J.; Zhao, Z.-H.; Zhang, Y.; Wang, Z.-H.; et al. Encapsulation of bryostatin-1 by targeted exosomes enhances remyelination and neuroprotection effects in the cuprizone-induced demyelinating animal model of multiple sclerosis. Biomater. Sci. 2022, 10, 714–727. [Google Scholar] [CrossRef]
- Maki, J.; Oshimura, A.; Shiotani, Y.; Yamanaka, M.; Okuda, S.; Yanagita, R.C.; Kitani, S.; Igarashi, Y.; Saito, Y.; Sakakibara, Y.; et al. Validation of machine learning-assisted screening of PKC ligands: PKC binding affinity and activation. Biosci. Biotechnol. Biochem. 2025, 89, 668–679. [Google Scholar] [CrossRef]
- Hardman, C.; Ho, S.; Shimizu, A.; Luu-Nguyen, Q.; Sloane, J.L.; Soliman, M.S.A.; Marsden, M.D.; Zack, J.A.; Wender, P.A. Synthesis and evaluation of designed PKC modulators for enhanced cancer immunotherapy. Nat. Commun. 2020, 11, 1879. [Google Scholar] [CrossRef]
- Jaimalai, T.; Meeroekyai, S.; Suree, N.; Prangkio, P. Drug Delivery System Targeting CD4+ T Cells for HIV-1 Latency Reactivation Towards the Viral Eradication. J. Pharm. Sci. 2020, 109, 3013–3020. [Google Scholar] [CrossRef]
- La Cognata, V.; D’Amico, A.G.; Maugeri, G.; Morello, G.; Guarnaccia, M.; Magrì, B.; Aronica, E.; Alkon, D.L.; D’Agata, V.; Cavallaro, S. The ε-Isozyme of Protein Kinase C (PKCε) Is Impaired in ALS Motor Cortex and Its Pulse Activation by Bryostatin-1 Produces Long Term Survival in Degenerating SOD1-G93A Motor Neuron-like Cells. Int. J. Mol. Sci. 2023, 24, 12825. [Google Scholar] [CrossRef]
- Jiang, R.; Huang, W.; Qiu, X.; Chen, J.; Luo, R.; Zeng, R.; Tong, S.; Lyu, Y.; Sun, P.; Lian, Q.; et al. Unveiling promising drug targets for autism spectrum disorder: Insights from genetics, transcriptomics, and proteomics. Brief. Bioinform. 2024, 25, bbae353. [Google Scholar] [CrossRef]
- Tilvi, S.; Moriou, C.; Martin, M.-T.; Gallard, J.-F.; Sorres, J.; Patel, K.; Petek, S.; Debitus, C.; Ermolenko, L.; Al-Mourabit, A. Agelastatin E, Agelastatin F, and Benzosceptrin C from the Marine Sponge Agelas dendromorpha. J. Nat. Prod. 2010, 73, 720–723. [Google Scholar] [CrossRef]
- Kovalerchik, D.; Singh, R.P.; Schlesinger, P.; Mahajni, A.; Shefer, S.; Fridman, M.; Ilan, M.; Carmeli, S. Bromopyrrole Alkaloids of the Sponge Agelas oroides Collected Near the Israeli Mediterranean Coastline. J. Nat. Prod. 2020, 83, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Stout, E.P.; Morinaka, B.I.; Wang, Y.-G.; Romo, D.; Molinski, T.F. De Novo Synthesis of Benzosceptrin C and Nagelamide H from 7-15N-Oroidin: Implications for Pyrrole–Aminoimidazole Alkaloid Biosynthesis. J. Nat. Prod. 2012, 75, 527–530. [Google Scholar] [CrossRef]
- Tran, M.Q.; Ermolenko, L.; Retailleau, P.; Nguyen, T.B.; Al-Mourabit, A. Reaction of Quinones and Guanidine Derivatives: Simple Access to Bis-2-aminobenzimidazole Moiety of Benzosceptrin and Other Benzazole Motifs. Org. Lett. 2014, 16, 920–923. [Google Scholar] [CrossRef]
- Benchekroun, M.; Ermolenko, L.; Tran, M.Q.; Vagneux, A.; Nedev, H.; Delehouzé, C.; Souab, M.; Baratte, B.; Josselin, B.; Iorga, B.I.; et al. Discovery of simplified benzazole fragments derived from the marine benzosceptrin B as necroptosis inhibitors involving the receptor interacting protein Kinase-1. Eur. J. Med. Chem. 2020, 201, 112337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020, 9, 8086–8121. [Google Scholar] [CrossRef]
- Li, K.; Tian, H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J. Drug Target. 2018, 27, 244–256. [Google Scholar] [CrossRef]
- von Knethen, A.; Brüne, B. PD-L1 in the palm of your hand: Palmitoylation as a target for immuno-oncology. Signal Transduct. Target. Ther. 2019, 4, 18. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Chingizova, E.A.; Menchinskaya, E.S.; Zelepuga, E.A.; Kalinovsky, A.I.; Avilov, S.A.; Tabakmakher, K.M.; Popov, R.S.; Dmitrenok, P.S.; Dautov, S.S.; et al. Composition of Triterpene Glycosides of the Far Eastern Sea Cucumber Cucumaria conicospermium Levin et Stepanov; Structure Elucidation of Five Minor Conicospermiumosides A3-1, A3-2, A3-3, A7-1, and A7-2; Cytotoxicity of the Glycosides Against Human Breast Cancer Cell Lines; Structure–Activity Relationships. Mar. Drugs 2024, 22, 560. [Google Scholar] [CrossRef]
- Yang, W.-S.; Qi, X.-R.; Xu, Q.-Z.; Yuan, C.-H.; Yi, Y.-H.; Tang, H.-F.; Shen, L.; Han, H. A new sulfated triterpene glycoside from the sea cucumber Colochirus quadrangularis, and evaluation of its antifungal, antitumor and immunomodulatory activities. Bioorg. Med. Chem. 2021, 41, 116188. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Z.; Wang, D.; Yan, Z.; Sun, X.; Li, X.; Yin, X.; Wang, S.; Li, K. Pectiniferosides A–J: Diversified Glycosides of Polyhydroxy Steroids Isolated from the Sea Star Patiria (=Asterina) pectinifera. Mar. Drugs 2024, 22, 545. [Google Scholar] [CrossRef]
- Okamura, R.; Kikuchi, K.; Taniguchi, A.; Nagai, K.; Seki, R.; Ohte, S.; Ohshiro, T.; Ando, M.; Tanaka, T.; Fukuda, T. The new seriniquinone glycoside by biological transformation using the deep sea-derived bacterium Bacillus licheniformis KDM612. J. Antibiot. 2024, 77, 515–521. [Google Scholar] [CrossRef]
- Han, X.; Wang, H.; Li, B.; Chen, X.; Li, T.; Yan, X.; Ouyang, H.; Lin, W.; He, S. New Diterpenes and Diterpene Glycosides with Antibacterial Activity from Soft Coral Lemnalia bournei. Mar. Drugs 2024, 22, 157. [Google Scholar] [CrossRef]
- Ru, R.; Chen, G.; Liang, X.; Cao, X.; Yuan, L.; Meng, M. Sea Cucumber Derived Triterpenoid Glycoside Frondoside A: A Potential Anti-Bladder Cancer Drug. Nutrients 2023, 15, 378. [Google Scholar] [CrossRef]
- Yun, S.-H.; Sim, E.-H.; Han, S.-H.; Han, J.-Y.; Kim, S.-H.; Silchenko, A.; Stonik, V.; Park, J.-I. Holotoxin A1 Induces Apoptosis by Activating Acid Sphingomyelinase and Neutral Sphingomyelinase in K562 and Human Primary Leukemia Cells. Mar. Drugs 2018, 16, 123. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Dmitrenok, P.S.; Chingizova, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dautov, S.S.; et al. Structures and Bioactivities of Quadrangularisosides A, A1, B, B1, B2, C, C1, D, D1–D4, and E from the Sea Cucumber Colochirus quadrangularis: The First Discovery of the Glycosides, Sulfated by C-4 of the Terminal 3-O-Methylglucose Residue. Synergetic Effect on Colony Formation of Tumor HT-29 Cells of these Glycosides with Radioactive Irradiation. Mar. Drugs 2020, 18, 394. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides O, P, P1-P3 and R, triterpene glycosides with two novel types of carbohydrate chains from the sea cucumberCladolabes schmeltzii. Inhibition of cancer cells colony formation and its synergy with radioactive irradiation. Carbohydr. Res. 2018, 468, 73–79. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides C4, D1, D2, M, M1, M2, N and Q, new triterpene glycosides with diverse carbohydrate chains from sea cucumber Cladolabes schmeltzii. An uncommon 20,21,22,23,24,25,26,27-okta-nor-lanostane aglycone. The synergism of inhibitory action of non-toxic dose of the glycosides and radioactive irradiation on colony formation of HT-29 cancer cells. Carbohydr. Res. 2018, 468, 36–44. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Popov, R.S.; Chingizova, E.A. Structures and Bioactivities of Psolusosides B1, B2, J, K, L, M, N, O, P, and Q from the Sea Cucumber Psolus fabricii. The First Finding of Tetrasulfated Marine Low Molecular Weight Metabolites. Mar. Drugs 2019, 17, 631. [Google Scholar] [CrossRef]
- Kumar, R.; Subramani, R.; Aalbersberg, W. Three bioactive sesquiterpene quinones from the Fijian marine sponge of the genusHippospongia. Nat. Prod. Res. 2013, 27, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- McConnell, O.J.; Longley, R.; Gunasekera, M. Isometachromin, a new cytotoxic sesquiterpenoid from a deep water sponge of the family Spongiidae. Experientia 1992, 48, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-H.; Chueh, S.-C.; Kung, F.-L.; Pan, S.-L.; Shen, Y.-C.; Guh, J.-H. Ilimaquinone, a marine sponge metabolite, displays anticancer activity via GADD153-mediated pathway. Eur. J. Pharmacol. 2007, 556, 45–54. [Google Scholar] [CrossRef]
- Takizawa, P.A.; Yucel, J.K.; Veit, B.; Faulkner, D.J.; Deerinck, T.; Soto, G.; Ellisman, M.; Malhotra, V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 1993, 73, 1079–1090. [Google Scholar] [CrossRef]
- Son, H.; Noh, K.; Park, I.; Na, M.; Oh, S.; Shin, B.S.; Kang, W. Stereo-Selective Pharmacokinetics of Ilimaquinone Epimers Extracted from a Marine Sponge in Rats. Mar. Drugs 2019, 17, 171. [Google Scholar] [CrossRef]
- Surti, M.; Patel, M.; Redhwan, A.; Al-Keridis, L.A.; Adnan, M.; Alshammari, N.; Reddy, M.N. Ilimaquinone (Marine Sponge Metabolite) Induces Apoptosis in HCT-116 Human Colorectal Carcinoma Cells via Mitochondrial-Mediated Apoptosis Pathway. Mar. Drugs 2022, 20, 582. [Google Scholar] [CrossRef]
- Son, Y.; Lim, D.; Park, S.; Song, I.-S.; Kim, J.-H.; Shin, S.; Jang, H.; Liu, K.-H.; Song, G.-Y.; Kang, W.; et al. Ilimaquinone inhibits neovascular age-related macular degeneration through modulation of Wnt/β-catenin and p53 pathways. Pharmacol. Res. 2020, 161, 105146. [Google Scholar] [CrossRef]
- Lin, C.-W.; Bai, L.-Y.; Su, J.-H.; Chiu, C.-F.; Lin, W.-Y.; Huang, W.-T.; Shih, M.-C.; Huang, Y.-T.; Hu, J.-L.; Weng, J.-R. Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines 2020, 8, 296. [Google Scholar] [CrossRef]
- Bai, L.-Y.; Su, J.-H.; Chiu, C.-F.; Lin, W.-Y.; Hu, J.-L.; Feng, C.-H.; Shu, C.-W.; Weng, J.-R. Antitumor Effects of a Sesquiterpene Derivative from Marine Sponge in Human Breast Cancer Cells. Mar. Drugs 2021, 19, 244. [Google Scholar] [CrossRef]
- Surti, M.; Patel, M.; Adnan, M.; Moin, A.; Ashraf, S.A.; Siddiqui, A.J.; Snoussi, M.; Deshpande, S.; Reddy, M.N. Ilimaquinone (marine sponge metabolite) as a novel inhibitor of SARS-CoV-2 key target proteins in comparison with suggested COVID-19 drugs: Designing, docking and molecular dynamics simulation study. RSC Adv. 2020, 10, 37707–37720. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martin, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Dev. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef]
- Galmarini, C.; D’Incalci, M.; Allavena, P. Trabectedin and Plitidepsin: Drugs from the Sea that Strike the Tumor Microenvironment. Mar. Drugs 2014, 12, 719–733. [Google Scholar] [CrossRef]
- Sachse, M.; Tenorio, R.; Fernández de Castro, I.; Muñoz-Basagoiti, J.; Perez-Zsolt, D.; Raïch-Regué, D.; Rodon, J.; Losada, A.; Avilés, P.; Cuevas, C.; et al. Unraveling the antiviral activity of plitidepsin against SARS-CoV-2 by subcellular and morphological analysis. Antivir. Res. 2022, 200, 105270. [Google Scholar] [CrossRef]
- Muñoz-Alonso, M.J.; González-Santiago, L.; Zarich, N.; Martínez, T.; Alvarez, E.; Rojas, J.M.; Muñoz, A. Plitidepsin Has a Dual Effect Inhibiting Cell Cycle and Inducing Apoptosis via Rac1/c-Jun NH2-Terminal Kinase Activation in Human Melanoma Cells. J. Pharmacol. Exp. Ther. 2008, 324, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Suárez, Y.; González-Santiago, L.; Zarich, N.; Dávalos, A.; Aranda, J.F.; Alonso, M.A.; Lasunción, M.A.; Rojas, J.M.; Muñoz, A. Plitidepsin Cellular Binding and Rac1/JNK Pathway Activation Depend on Membrane Cholesterol Content. Mol. Pharmacol. 2006, 70, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Varona, J.F.; Landete, P.; Lopez-Martin, J.A.; Estrada, V.; Paredes, R.; Guisado-Vasco, P.; Fernandez de Orueta, L.; Torralba, M.; Fortun, J.; Vates, R.; et al. Preclinical and randomized phase I studies of plitidepsin in adults hospitalized with COVID-19. Life Sci. Alliance 2022, 5, e202101200. [Google Scholar] [CrossRef]
- van Andel, L.; Fudio, S.; Rosing, H.; Munt, S.; Miguel-Lillo, B.; González, I.; Tibben, M.M.; de Vries, N.; de Vries Schultink, A.H.M.; Schellens, J.H.M.; et al. Pharmacokinetics and excretion of 14C–Plitidepsin in patients with advanced cancer. Investig. New Drugs 2017, 35, 589–598. [Google Scholar] [CrossRef]
- Barboza, N.M.; Medina, D.J.; Budak-Alpdogan, T.; Aracil, M.; Jimeno, J.M.; Bertino, J.R.; Banerjee, D. Plitidepsin (Aplidin) is a potent inhibitor of diffuse large cell and Burkitt lymphoma and is synergistic with rituximab. Cancer Biol. Ther. 2014, 13, 114–122. [Google Scholar] [CrossRef]
- Schöffski, P.; Guillem, V.; Garcia, M.; Rivera, F.; Tabernero, J.; Cullell, M.; Lopez-Martin, J.A.; Pollard, P.; Dumez, H.; Garcia del Muro, X.; et al. Phase II Randomized Study of Plitidepsin (Aplidin), Alone or in Association with L-carnitine, in Patients with Unresectable Advanced Renal Cell Carcinoma. Mar. Drugs 2009, 7, 57–70. [Google Scholar] [CrossRef]
- Gerhardt, L.M.S.; Liu, J.; Koppitch, K.; Cippa, P.E.; McMahon, A.P. Single-nuclear transcriptomics reveals diversity of proximal tubule cell states in a dynamic response to acute kidney injury. Proc. Natl. Acad. Sci. USA 2021, 118, e2026684118. [Google Scholar] [CrossRef] [PubMed]
- Gonsalez, S.R.; Cortes, A.L.; Silva, R.C.D.; Lowe, J.; Prieto, M.C.; Silva Lara, L.D. Acute kidney injury overview: From basic findings to new prevention and therapy strategies. Pharmacol. Ther. 2019, 200, 1–12. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, Z.; Li, K.; Fang, W.; Tian, Y.; Luo, X.; Chen, Y.; Zhan, Z.; Zhang, T.; Liao, S.; et al. Exploring the Natural Piericidins as Anti-Renal Cell Carcinoma Agents Targeting Peroxiredoxin 1. J. Med. Chem. 2019, 62, 7058–7069. [Google Scholar] [CrossRef]
- Li, K.; Liang, Z.; Chen, W.; Luo, X.; Fang, W.; Liao, S.; Lin, X.; Yang, B.; Wang, J.; Tang, L.; et al. Iakyricidins A–D, Antiproliferative Piericidin Analogues Bearing a Carbonyl Group or Cyclic Skeleton from Streptomyces iakyrus SCSIO NS104. J. Org. Chem. 2019, 84, 12626–12631. [Google Scholar] [CrossRef]
- Li, K.; Su, Z.; Gao, Y.; Lin, X.; Pang, X.; Yang, B.; Tao, H.; Luo, X.; Liu, Y.; Zhou, X. Cytotoxic Minor Piericidin Derivatives from the Actinomycete Strain Streptomyces psammoticus SCSIO NS126. Mar. Drugs 2021, 19, 428. [Google Scholar] [CrossRef]
- Gao, L.; Zhong, X.; Jin, J.; Li, J.; Meng, X.-m. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct. Target. Ther. 2020, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sanyal, A.J. Management of NAFLD: A stage-based approach. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Sasako, T.; Ohsugi, M.; Kubota, N.; Itoh, S.; Okazaki, Y.; Terai, A.; Kubota, T.; Yamashita, S.; Nakatsukasa, K.; Kamura, T.; et al. Hepatic Sdf2l1 controls feeding-induced ER stress and regulates metabolism. Nat. Commun. 2019, 10, 947. [Google Scholar] [CrossRef]
- Yan, H.; Gao, Y.; Zhang, Y. Inhibition of JNK suppresses autophagy and attenuates insulin resistance in a rat model of nonalcoholic fatty liver disease. Mol. Med. Rep. 2017, 15, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, M.M.; Ashwin, J.K.M.; Soundari, A.; Sathiskumar, S.; Priyadharshini, U.; Paramasivam, D.; Liu, W.C.; Balasubramanian, B. Mycofabrication of AgONPs derived from Aspergillus terreus FC36AY1 and its potent antimicrobial, antioxidant, and anti-angiogenesis activities. Mol. Biol. Rep. 2021, 48, 7933–7946. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, X.; Lin, J.; Zhang, Y.; He, H.; Zhao, G. Lipoxin A4 activates ALX/FPR2 to attenuate inflammation in Aspergillus fumigatus keratitis. Int. Immunopharmacol. 2021, 96, 107785. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naime, W.A.; Kimishima, A.; Setiawan, A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S.; Arai, M. Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp. Mar. Drugs 2020, 18, 555. [Google Scholar] [CrossRef]
- Lio, Y.-C.; Reynolds, L.J.; Balsinde, J.; Dennis, E.A. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1996, 1302, 55–60. [Google Scholar] [CrossRef]
- Sacco, R.L.; Roth, G.A.; Reddy, K.S.; Arnett, D.K.; Bonita, R.; Gaziano, T.A.; Heidenreich, P.A.; Huffman, M.D.; Mayosi, B.M.; Mendis, S.; et al. The Heart of 25 by 25: Achieving the Goal of Reducing Global and Regional Premature Deaths From Cardiovascular Diseases and Stroke: A Modeling Study From the American Heart Association and World Heart Federation. Circulation 2016, 133, e674–e690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Pentikäinen, M.O.; Öörni, K.; Ala-Korpela, M.; Kovanen, P.T. Modified LDL—Trigger of atherosclerosis and inflammation in the arterial intima. J. Intern. Med. 2001, 247, 359–370. [Google Scholar] [CrossRef]
- Bevilacqua, M.P.; Stengelin, S.; Gimbrone, M.A.; Seed, B. Endothelial Leukocyte Adhesion Molecule 1: An Inducible Receptor for Neutrophils Related to Complement Regulatory Proteins and Lectins. Science 1989, 243, 1160–1165. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Liu, X.; Lin, W.; Zhu, K. Equisetin Restores Colistin Sensitivity against Multi-Drug Resistant Gram-Negative Bacteria. Antibiotics 2021, 10, 1263. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Zhu, X.; Yu, W.; Gong, Q. Equisetin as potential quorum sensing inhibitor of Pseudomonas aeruginosa. Biotechnol. Lett. 2018, 40, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ratnaweera, P.B.; de Silva, E.D.; Williams, D.E.; Andersen, R.J. Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Complement. Altern. Med. 2015, 15, 220. [Google Scholar] [CrossRef]
- König, T.; Kapus, A.; Sarkadi, B. Effects of equisetin on rat liver mitochondria: Evidence for inhibition of substrate anion carriers of the inner membrane. J. Bioenerg. Biomembr. 1993, 25, 537–545. [Google Scholar] [CrossRef]
- Ma, J.; Yue, S.; Liu, Y.; Gong, L.; He, P.; Yang, Y.; Fu, Z.; Han, D.; Hu, Q.; Liao, F.; et al. Fucoxanthin ameliorates ulcerative colitis by maintaining the epithelial barrier via blocking JAK2/STAT3 signaling pathway. Toxicol. Appl. Pharmacol. 2025, 495, 117213. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, H.; Ma, Z.; Dong, D.; Yang, Z.; Tian, J. Astaxanthin ameliorates lipopolysaccharide-induced acute lung injuryviainhibition of inflammatory reactions and modulation of the SOCS3/JAK2/STAT3 signaling pathways in mice. Food Funct. 2022, 13, 11638–11651. [Google Scholar] [CrossRef] [PubMed]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-Derived Polysaccharides and Their Potential Health Benefits in Nutraceutical Applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef]
- Bodur, M.; Yilmaz, B.; Ağagündüz, D.; Ozogul, Y. Immunomodulatory Effects of Omega-3 Fatty Acids: Mechanistic Insights and Health Implications. Mol. Nutr. Food Res. 2025, 69, e202400752. [Google Scholar] [CrossRef]
- Shah, F.I.; Imran, H.; Akram, F.; Khalid, T.; Shehzadi, S. Marine Carotenoids: Unlocking Advanced Antioxidant Mechanisms and Therapeutic Applications for Oxidative Stress. Mol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Farooqi, A.A.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Opportunities and challenges of fucoidan for tumors therapy. Carbohydr. Polym. 2024, 324, 121555. [Google Scholar] [CrossRef]
- Turrini, E.; Maffei, F.; Fimognari, C. Ten Years of Research on Fucoidan and Cancer: Focus on Its Antiangiogenic and Antimetastatic Effects. Mar. Drugs 2023, 21, 307. [Google Scholar] [CrossRef]
- Jin, J.-O.; Yadav, D.; Madhwani, K.; Puranik, N.; Chavda, V.; Song, M. Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review. Molecules 2022, 27, 6032. [Google Scholar] [CrossRef]
- Cao, J.; Qin, L.; Liu, M.; Yao, M.; Wang, K.; Lin, H.; Qu, C.; He, Y.; Xue, C.; Miao, J. Fucoidan from sea cucumber cooking liquid: Structural analysis, physicochemical properties, and anti-Helicobacter pylori potential. Int. J. Biol. Macromol. 2025, 306, 141593. [Google Scholar] [CrossRef]
- Ren, P.; Liu, M.; Wei, B.; Tang, Q.; Wang, Y.; Xue, C. Fucoidan exerts antitumor effects by regulating gut microbiota and tryptophan metabolism. Int. J. Biol. Macromol. 2025, 300, 140334. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Lapina, I.M.; Kulminskaya, A.A.; Zhurishkina, E.V.; Shikov, A.N. Comparative Evaluation of Dynamic Maceration and Ultrasonic Assisted Extraction of Fucoidan from Four Arctic Brown Algae on Its Antioxidant and Anticancer Properties. Mar. Drugs 2025, 23, 230. [Google Scholar] [CrossRef]
- Deng, Z.; Qishan, S.; Zhang, Q.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Low molecular weight fucoidan LF2 improves the immunosuppressive tumor microenvironment and enhances the anti-pancreatic cancer activity of oxaliplatin. Biomed. Pharmacother. 2024, 173, 116360. [Google Scholar] [CrossRef]
- Zueva, A.O.; Silchenko, A.S.; Rasin, A.B.; Malyarenko, O.S.; Kusaykin, M.I.; Kalinovsky, A.I.; Ermakova, S.P. Production of high- and low-molecular weight fucoidan fragments with defined sulfation patterns and heightened in vitro anticancer activity against TNBC cells using novel endo-fucanases of the GH107 family. Carbohydr. Polym. 2023, 318, 121128. [Google Scholar] [CrossRef]
- Zhang, W.; Park, H.-B.; An, E.-K.; Kim, S.-J.; Ryu, D.; Kim, D.; Lim, D.; Hwang, J.; Kwak, M.; You, S.; et al. Fucoidan from Durvillaea Antarctica enhances the anti-cancer effect of anti-PD-L1 antibody by activating dendritic cells and T cells. Int. J. Biol. Macromol. 2024, 280, 135922. [Google Scholar] [CrossRef]
- Tao, J.; Wang, B.; Dong, Y.; Chen, X.; Li, S.; Jiang, T.; Zhao, X. Photothermal and Acid-Responsive Fucoidan-CuS Bubble Pump Microneedles for Combined CDT/PTT/CT Treatment of Melanoma. ACS Appl. Mater. Interfaces 2023, 15, 40267–40279. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Chen, B.; Zhu, F.; Wang, Z.; Cheong, K.-L.; Ye, S.; Zhong, S.; Chen, J. Selenium nanoparticles decorated by fucoidan induce ferroptosis in HepG2 cells. Int. J. Biol. Macromol. 2025, 289, 138841. [Google Scholar] [CrossRef]
- Laeliocattleya, R.A.; Yunianta, Y.; Risjani, Y.; Wulan, S.N. In silico molecular docking, molecular dynamics, ADMET analysis of fucoidan against receptor frizzled-8 and coreceptor LRP6 in Wnt/β-Catenin pathway and in vitro analysis of fucoidan extract from Sargassum echinocarpum as β-catenin inhibitor in breast cancer cell line (MCF-7). J. Biomol. Struct. Dyn. 2023, 42, 11828–11843. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, S.; Mi, L.; Niu, D.; Hu, F.; Han, W.; Li, B. Fucoidan MF4 from Fucus vesiculosus inhibits Lewis lung cancer via STING–TBK1–IRF3 pathway. Int. J. Biol. Macromol. 2024, 267, 131336. [Google Scholar] [CrossRef]
- Al-Duais, M.A.; El Rabey, H.A.; Mohammed, G.M.; Al-Awthan, Y.S.; Althiyabi, A.S.; Attia, E.S.; Rezk, S.M.; Tayel, A.A. The anticancer activity of fucoidan coated selenium nanoparticles and curcumin nanoparticles against colorectal cancer lines. Sci. Rep. 2025, 15, 287. [Google Scholar] [CrossRef]
- Qiongyan, F.; Yin, C.; Yan, C.; Huaiyu, Z. Preparation and property study of self-assembled nanoparticles from thiolated fucoidan and doxorubicin. Int. J. Biol. Macromol. 2025, 305, 140830. [Google Scholar] [CrossRef]
- Li, H.; Dong, T.; Tao, M.; Zhao, H.; Lan, T.; Yan, S.; Gong, X.; Hou, Q.; Ma, X.; Song, Y. Fucoidan enhances the anti-tumor effect of anti-PD-1 immunotherapy by regulating gut microbiota. Food Funct. 2024, 15, 3463–3478. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Wang, B.; Su, Y.; Jiang, T.; Gan, T.; Zhao, X. Fucoidan based Ce6-chloroquine self-assembled hydrogel as in situ vaccines to enhance tumor immunotherapy by autophagy inhibition and macrophage polarization. Carbohydr. Polym. 2024, 346, 122637. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Y.; Zhang, N.; Yang, H.; Huang, Y.; Yang, Y.; Zhang, X.; Ye, J.; Xiao, M. Fucoidan treats chemotherapy-induced alopecia and helps cyclophosphamide treat tumors. Int. J. Biol. Macromol. 2025, 287, 138321. [Google Scholar] [CrossRef]
- Ghahtan, N.; Dehghan, N.; Ullah, M.; Khoradmehr, A.; Habibi, H.; Nabipour, I.; Baghban, N. From seaweed to healing: The potential of fucoidan in wound therapy. Nat. Prod. Res. 2024, 39, 1345–1358. [Google Scholar] [CrossRef]
- Moreno, I.; Hernández, T.; Calvo, E.; Fudio, S.; Kahatt, C.; Martínez, S.; Iglesias, J.L.; Calafati, R.O.; Pérez-Ramos, L.; Montilla, L.; et al. Pharmacokinetics and Safety of Lurbinectedin Administrated with Itraconazole in Cancer Patients: A Drug–Drug Interaction Study. Mar. Drugs 2024, 22, 178. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Sebak, M.; Molham, F.; Greco, C.; Tammam, M.A.; Sobeh, M.; El-Demerdash, A. Chemical diversity, medicinal potentialities, biosynthesis, and pharmacokinetics of anthraquinones and their congeners derived from marine fungi: A comprehensive update. RSC Adv. 2022, 12, 24887–24921. [Google Scholar] [CrossRef] [PubMed]
- Ouassaf, M.; Bourougaa, L.; Al-Mijalli, S.H.; Abdallah, E.M.; Bhat, A.R.; Kawsar, S.M.A. Marine-Derived Compounds as Potential Inhibitors of Hsp90 for Anticancer and Antimicrobial Drug Development: A Comprehensive In Silico Study. Molecules 2023, 28, 8074. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Aviles, P.; Ly, C.; Lee, W.; Guillen, M.; Munt, S.; Cuevas, C.; Faircloth, G. Quantitative analysis of Variolin analog (PM01218) in mouse and rat plasma by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2006, 832, 268–273. [Google Scholar] [CrossRef]
- Soares, D.G.; Machado, M.S.; Rocca, C.J.; Poindessous, V.; Ouaret, D.; Sarasin, A.; Galmarini, C.M.; Henriques, J.A.P.; Escargueil, A.E.; Larsen, A.K. Trabectedin and Its C Subunit Modified Analogue PM01183 Attenuate Nucleotide Excision Repair and Show Activity toward Platinum-Resistant Cells. Mol. Cancer Ther. 2011, 10, 1481–1489. [Google Scholar] [CrossRef]
- Mia, M.M.; Hasan, M.; Miah, M.M.; Hossain, M.A.S.; Islam, S.; Shanta, V. Inhibitory Potentiality of Secondary Metabolites Extracted from Marine Fungus Target on Avian Influenza Virus-A Subtype H5N8 (Neuraminidase) and H5N1 (Nucleoprotein): A Rational Virtual Screening. Vet. Anim. Sci. 2022, 15, 100231. [Google Scholar] [CrossRef]
| No. | Disease | Drug Name | Species | Dose, Route, Time | MOA | Model Inducers | Outcomes/Biological Effects | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Cancer | Microcolin H | Moorea producens | 0.1–0.5 nM (in vitro); 1–10 mg/kg i.p. | Targets PITPα/β, inhibits proliferation, induces autophagy | N/A | Suppressed tumor growth, low toxicity | [7] |
| 2 | Cancer | Nortopsentin | Topsentia sponge | Submicromolar (in vitro) | Inhibits CK1/GSK-3β, disrupts Wnt/β-catenin pathway | N/A | Cytotoxic to cancer cells, induces cell cycle arrest | [15,16] |
| 3 | Cancer | Topsentin | Spongosorites sponge | Targets CDK1/tubulin, disrupts mitosis | Active against breast cancer, inhibits biofilm | [17,18] | ||
| 4 | Cancer | Bryostatin | Bugula neritina | Clinically tested (various doses) | Activates PKC, enhances immune cell function, modulates autophagy | N/A | Reduced tumor growth, improved immune response | [19,20,21,22,23] |
| 5 | Cancer | Benzosceptrin C | Agelas dendromorpha | 10–20 μM (in vitro); 5–10 mg/kg i.p. | Induces PD-L1 degradation via DHHC3, enhances T cell activity | N/A | Suppressed tumor growth, increased CD8+ T cells | [8,24] |
| 6 | Cancer | Cucumarioside A2-2 | Cucumaria japonica | 1.2–2.8 μM (in vitro); 5 mg/kg i.p. | Induces apoptosis, activates macrophages via TLR4/NF-κB | N/A | Reduced tumor volume, enhanced M1 macrophage infiltration | [25,26,27] |
| 7 | Cancer | Ilimaquinone | Hippospongia spp. | 4.2 μM (in vitro); 10 mg/kg i.p. | Induces mitochondrial apoptosis, activates DDR, inhibits PDK1 | N/A | Suppressed tumor growth, induced DNA damage | [28,29,30] |
| 8 | Cancer | Aplidin | Didemnum molle | 0.1–1 nM (in vitro); 1–5 mg/kg i.p. | Binds eEF1A2, disrupts protein synthesis, induces ER stress | N/A | Inhibited myeloma cell growth, reduced tumor size | [31,32,33,34,35] |
| 9 | COVID-19 | Aplidin | Didemnum molle | 0.3 mg/kg i.v. (clinical) | Disrupts viral replication, modulates immune response | SARS-CoV-2 | Shortened clinical improvement time in patients | [36] |
| 10 | Renal | S14 | Streptomyces spp. | 5 mg/kg (free); 5 mg/kg (nanoparticles) i.v. | Activates PRDX1/Nrf2, reduces oxidative stress, enhances autophagy | UIRI mouse model | Improved renal function, reduced injury markers | [13] |
| 11 | MAFLD | HN-001 | Aspergillus sp. C1 | Not specified (in vivo/model) | Inhibits PLA2, reduces LPC, suppresses JNK/ER stress pathway | High-fat diet | Alleviated hepatic steatosis, reduced inflammation | [12] |
| 12 | Atherosclerosis | Equisetin | Fusarium equiseti | Not specified (in vivo model) | Binds STAT3, inhibits its activation, reduces lipid uptake | ApoE−/− HFD mouse | Reduced plaque size, decreased inflammatory markers | [37,38] |
| 13 | Cancer | Fucoidan | Brown algae/sea cucumbers | 50–200 μg/mL (in vitro); 800 μg/kg i.p. | Activates immune cells, induces apoptosis, inhibits angiogenesis | N/A | Suppressed tumor growth, enhanced immune response | [39,40,41,42] |
| 14 | COVID-19 | Fucoidan | Brown algae/sea cucumbers | Not specified (clinical) | Interferes with viral entry, reduces inflammation | SARS-CoV-2 | Reduced viral load in patients | [43,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J. Marine-Derived Compounds: A New Horizon in Cancer, Renal, and Metabolic Disease Therapeutics. Mar. Drugs 2025, 23, 283. https://doi.org/10.3390/md23070283
Zhang J. Marine-Derived Compounds: A New Horizon in Cancer, Renal, and Metabolic Disease Therapeutics. Marine Drugs. 2025; 23(7):283. https://doi.org/10.3390/md23070283
Chicago/Turabian StyleZhang, Jinwei. 2025. "Marine-Derived Compounds: A New Horizon in Cancer, Renal, and Metabolic Disease Therapeutics" Marine Drugs 23, no. 7: 283. https://doi.org/10.3390/md23070283
APA StyleZhang, J. (2025). Marine-Derived Compounds: A New Horizon in Cancer, Renal, and Metabolic Disease Therapeutics. Marine Drugs, 23(7), 283. https://doi.org/10.3390/md23070283






