Macroalgae-Inspired Brominated Chalcones as Cosmetic Ingredients with the Potential to Target Skin Inflammaging
Abstract
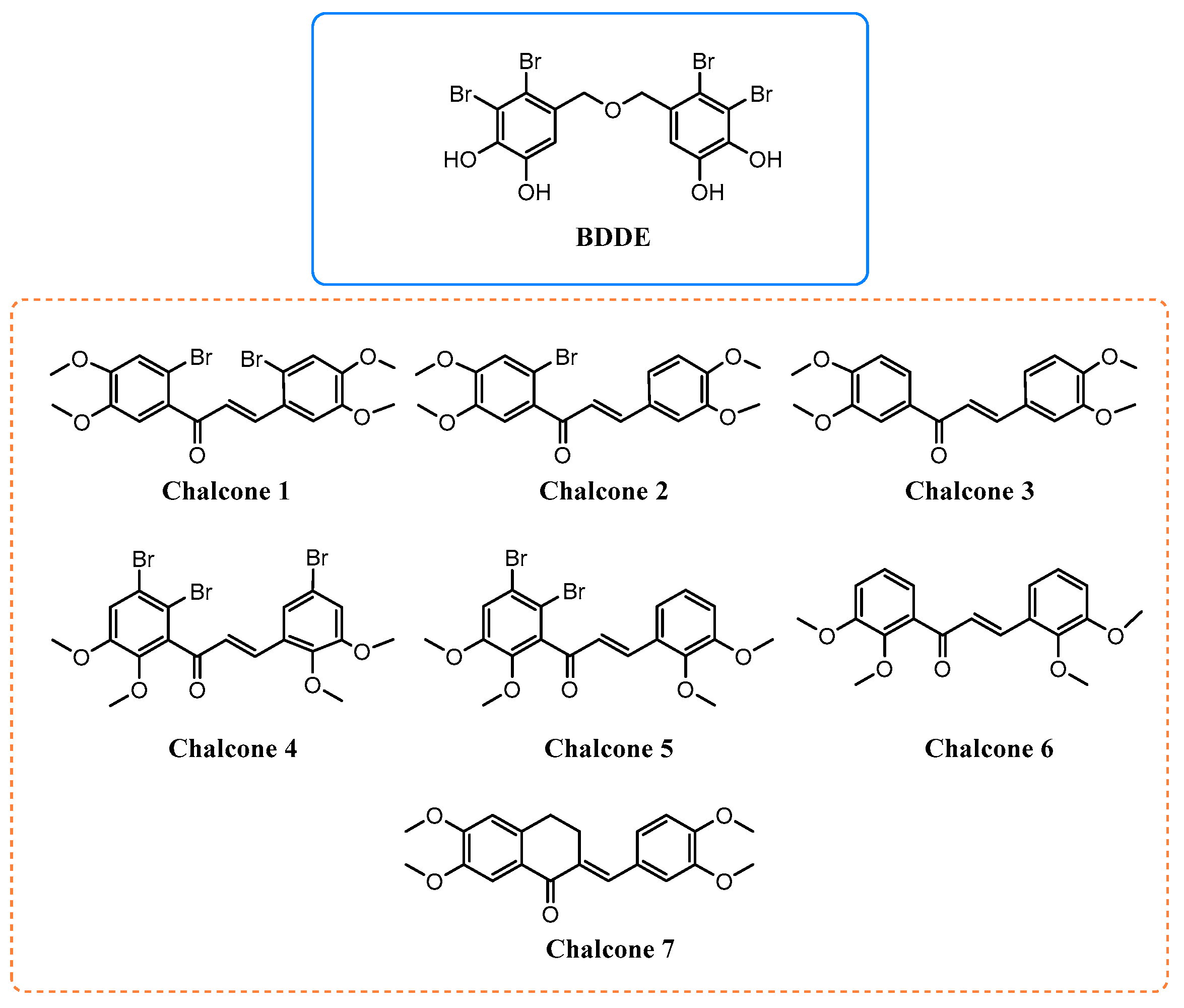
1. Introduction
2. Results and Discussion
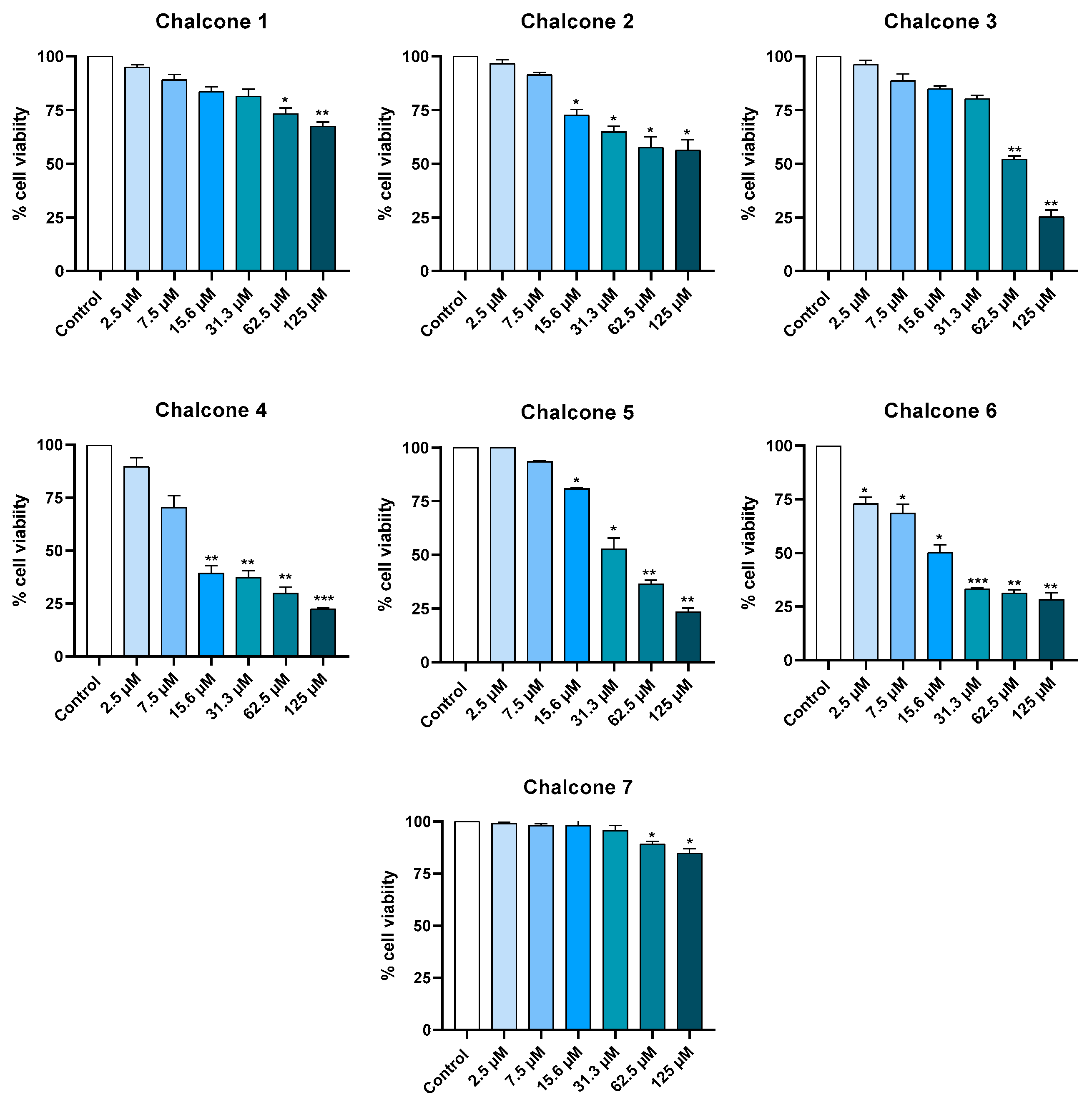
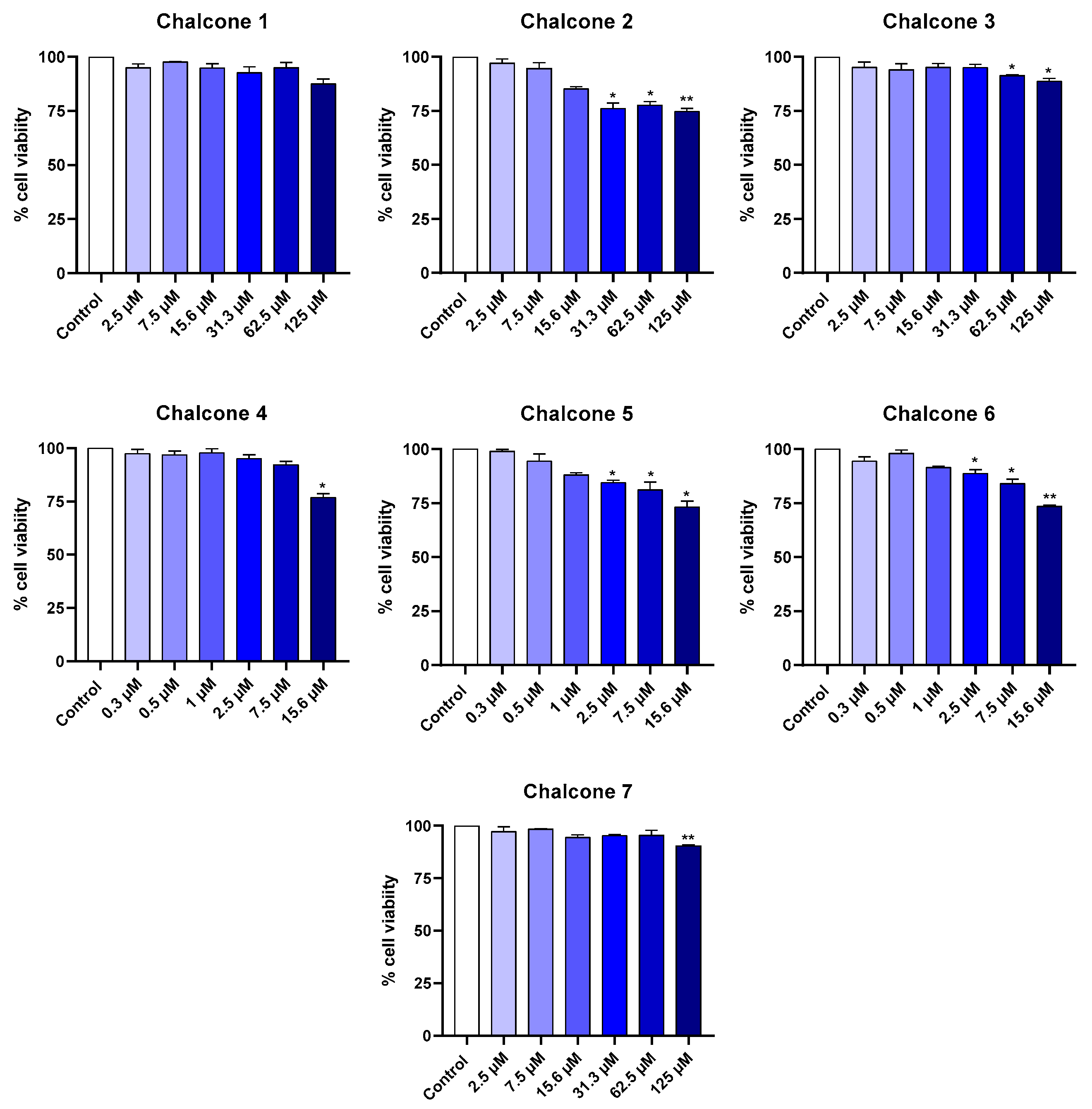
2.1. Cytotoxicity in Cell Lines
2.1.1. Keratinocyte Cell Line
2.1.2. Macrophage Cell Line
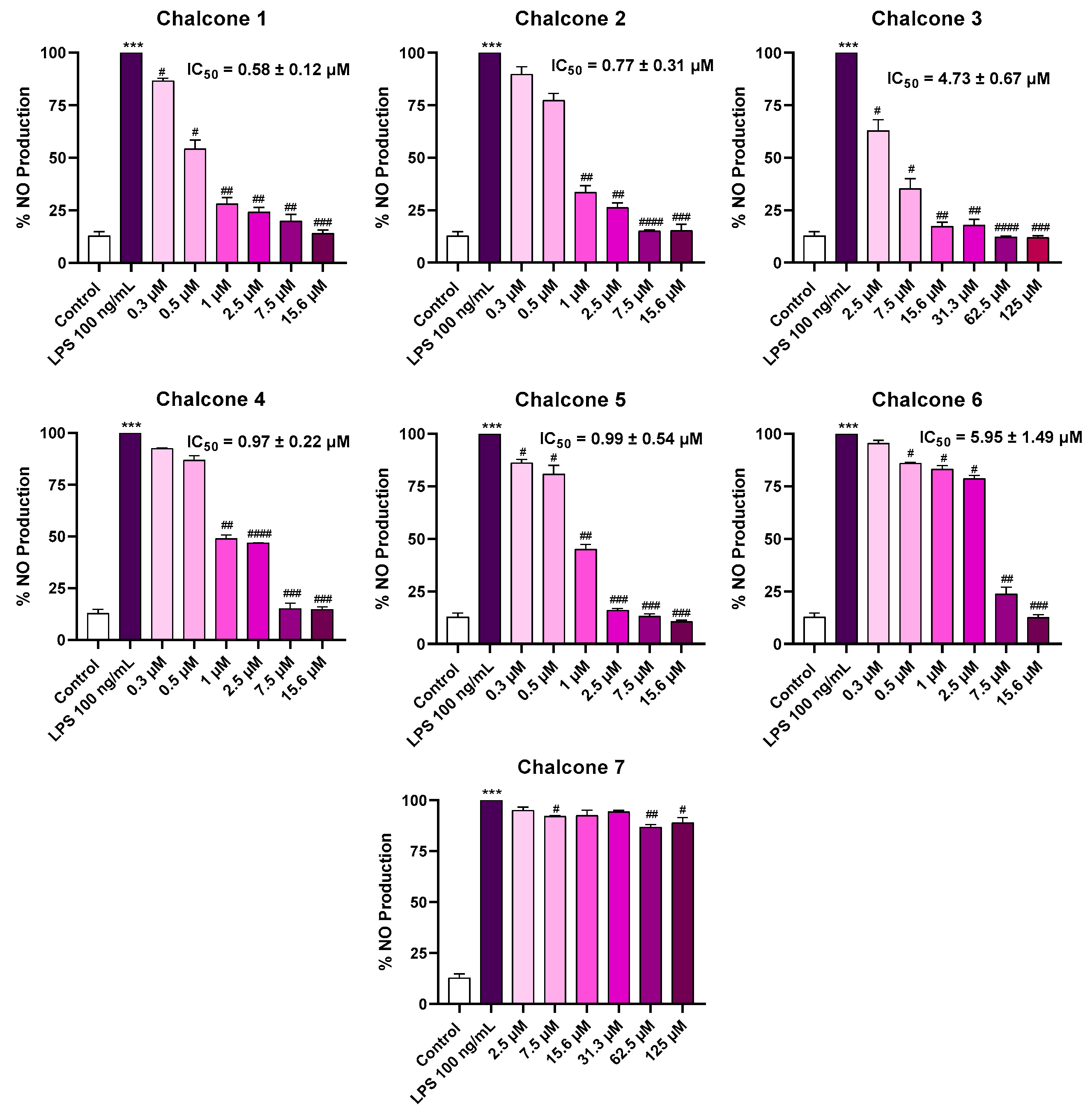
2.2. Anti-Inflammatory Activity
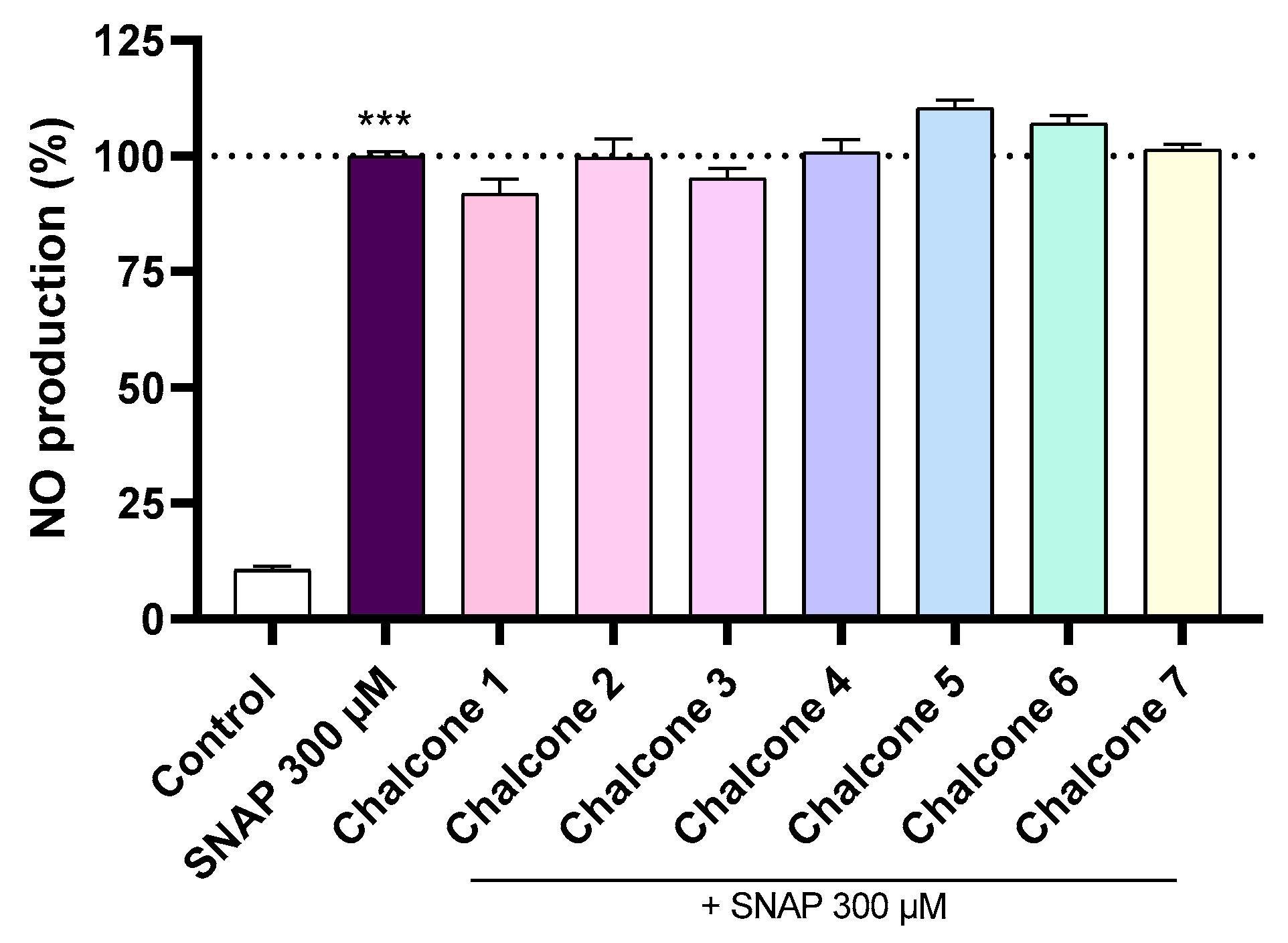
2.3. NO Scavenging Assay (SNAP)
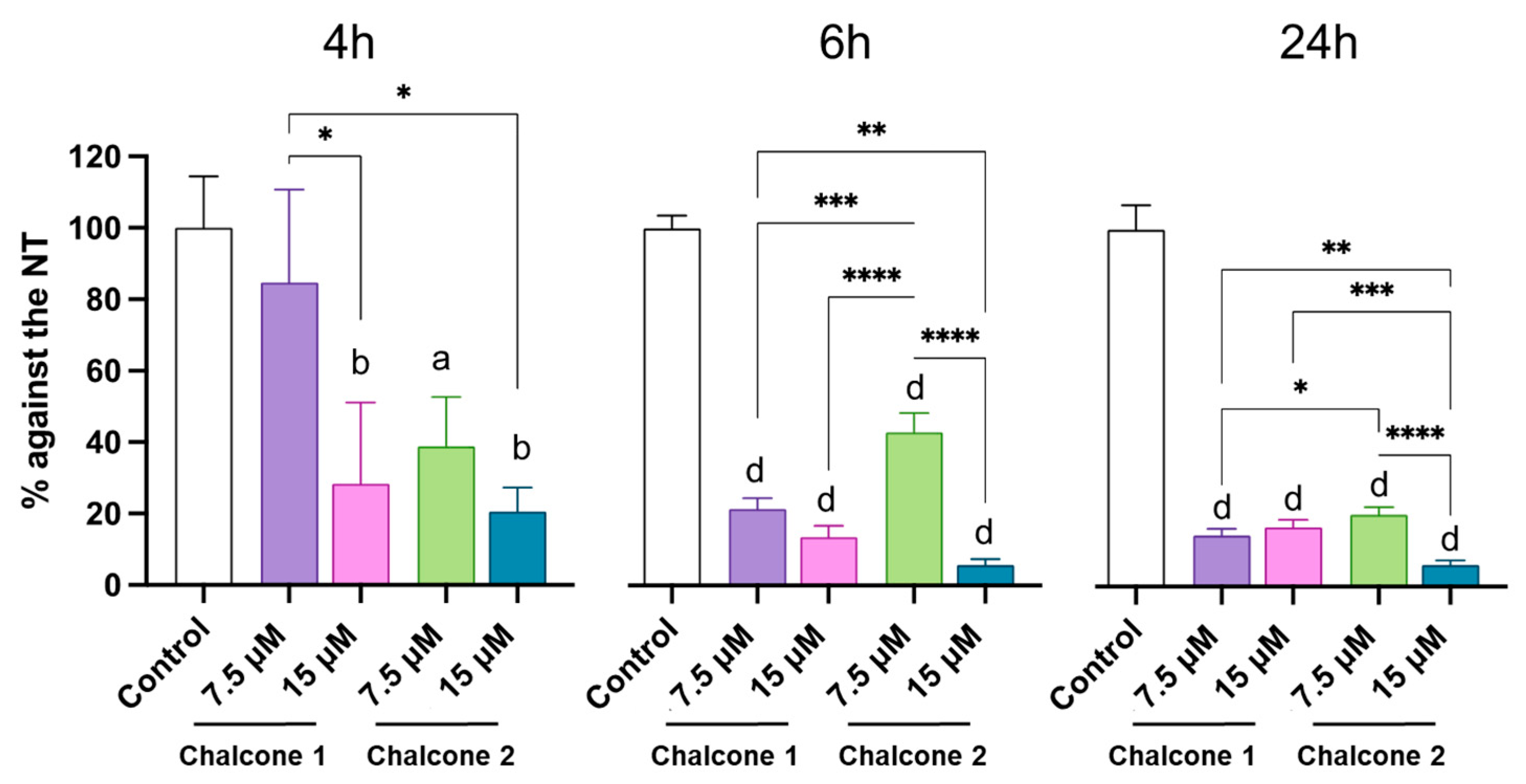
2.4. iNOS Expression
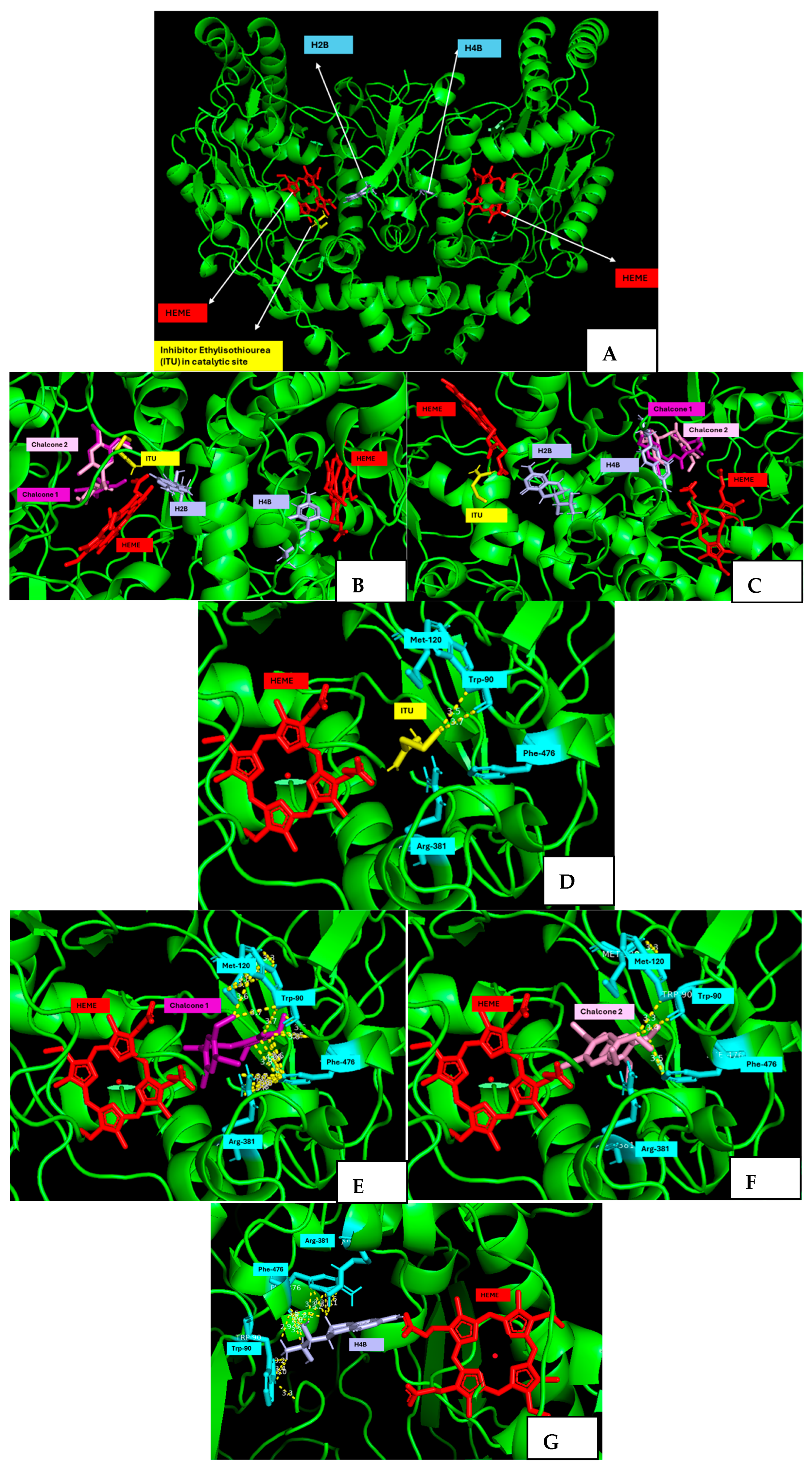
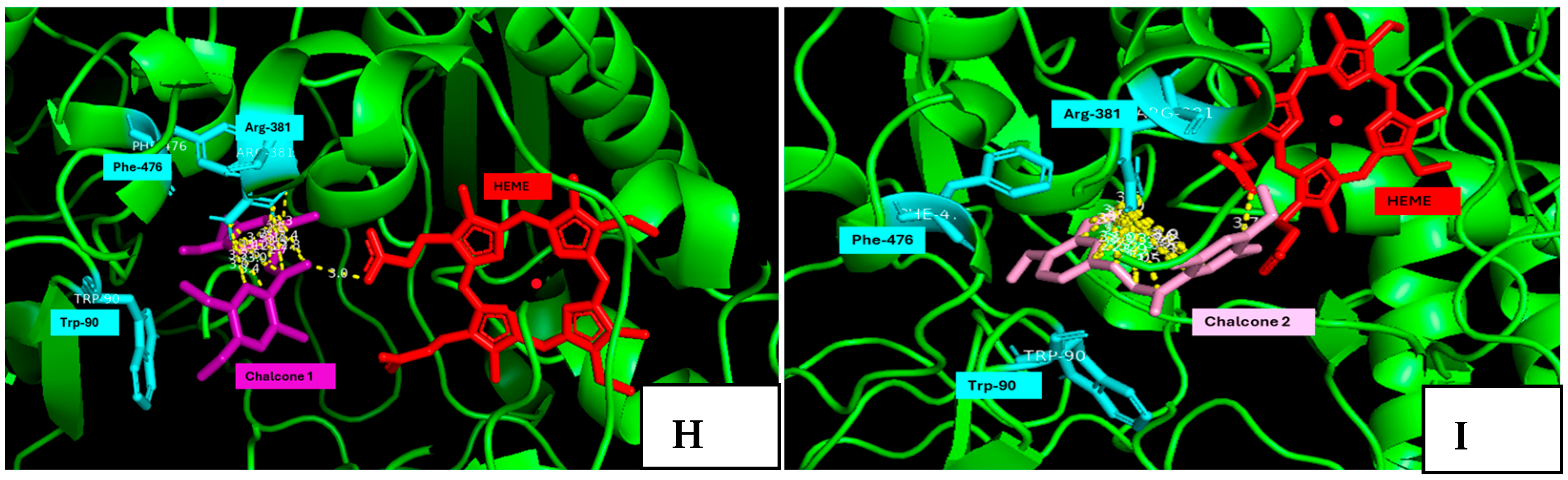
2.5. Molecular Docking Studies
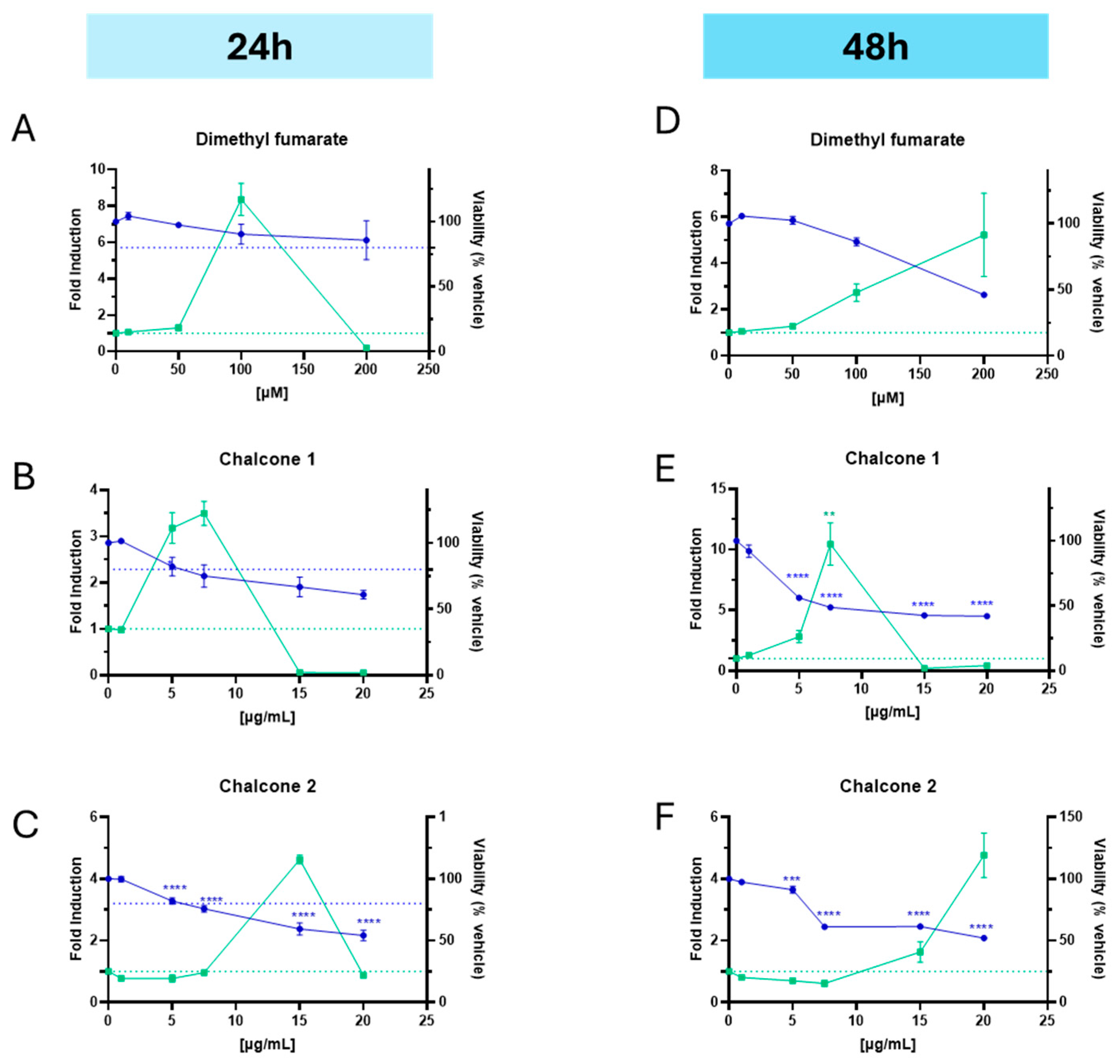
2.6. Nrf2 Activation
3. Materials and Methods
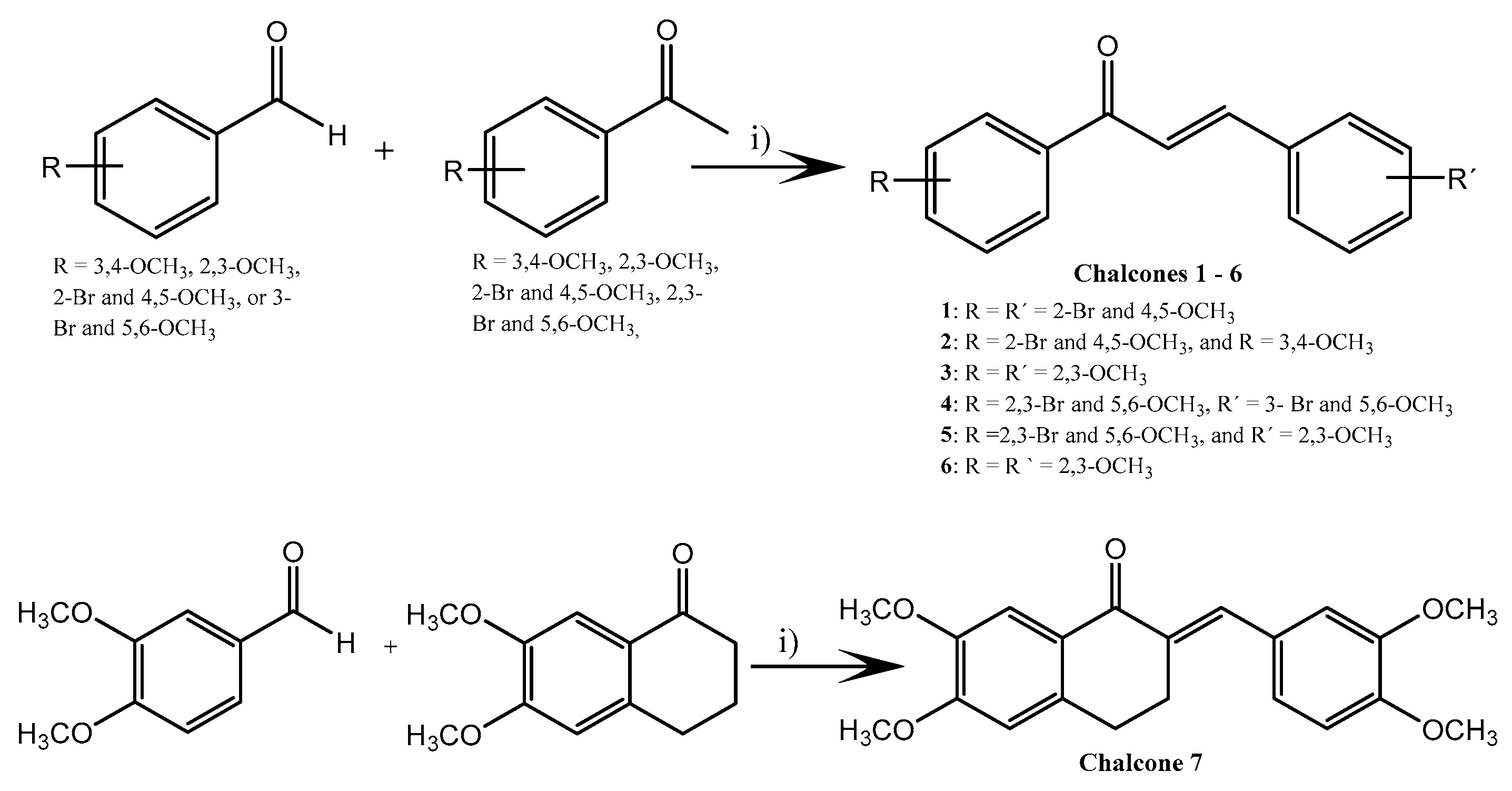
3.1. Synthesis of Chalcones 1–7
3.2. Materials
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Anti-Inflammatory Activity (NO Production)
3.6. NO Scavenging Potential (SNAP Assay)
3.7. Western Blot Analysis of iNOS Expression
3.8. Docking Studies
3.9. Evaluation of the Nrf2 Activation
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Gegotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Hurtova, M.; Kanova, K.; Dobiasova, S.; Holasova, K.; Cakova, D.; Hoang, L.; Biedermann, D.; Kuzma, M.; Cvacka, J.; Kren, V.; et al. Selectively Halogenated Flavonolignans-Preparation and Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 15121. [Google Scholar] [CrossRef]
- Dao, T.T.; Chi, Y.S.; Kim, J.; Kim, H.P.; Kim, S.; Park, H. Synthesis and inhibitory activity against COX-2 catalyzed prostaglandin production of chrysin derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1165–1167. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Perz, M.; Szymanowska, D.; Janeczko, T.; Kostrzewa-Suslow, E. Antimicrobial Properties of Flavonoid Derivatives with Bromine, Chlorine, and Nitro Group Obtained by Chemical Synthesis and Biotransformation Studies. Int. J. Mol. Sci. 2024, 25, 5540. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Rodrigues, M.; Florêncio, M.H.; Mira, L. Structure and antioxidant activity of brominated flavonols and flavanones. J. Mass Spectrom. 2009, 44, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Pinto, M.; Correia-da-Silva, M.; Cidade, H. Recent Advances in Bioactive Flavonoid Hybrids Linked by 1,2,3-Triazole Ring Obtained by Click Chemistry. Molecules 2021, 27, 230. [Google Scholar] [CrossRef]
- Herencia, F.; Ferrándiz, M.L.; Ubeda, A.; Domínguez, J.; Charris, J.E.; Lobo, G.M.; Alcaraz, M.J. Synthesis and anti-inflammatory activity of chalcone derivatives. Bioorg. Med. Chem. Lett. 1998, 8, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Iqtadar, R.; Naz, A.; Shah, S.A.A.; Ali, S.; Abdullah, S. New halogenated chalcones as potential anti-inflammatory agents: A comprehensive In-Silico, In-Vitro, and In-Vivo study with ADME profiling. J. Mol. Struct. 2025, 1326, 141055. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.; Park, H.; Kim, H.P. Anti-inflammatory activity of the synthetic chalcone derivatives: Inhibition of inducible nitric oxide synthase-catalyzed nitric oxide production from lipopolysaccharide-treated RAW 264.7 cells. Biol. Pharm. Bull. 2007, 30, 1450–1455. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two New Bromophenols from the Red Alga Odonthalia corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Ye, B.R.; Kim, E.A.; Kim, J.; Kim, M.S.; Lee, W.W.; Ahn, G.N.; Kang, N.; Jung, W.K.; Heo, S.J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Domínguez, J.N.; Charris, J.E.; Lobo, G.; Payá, M.; Ferrándiz, M.L. Synthesis and inhibitory activity of dimethylamino-chalcone derivatives on the induction of nitric oxide synthase. Eur. J. Med. Chem. 2002, 37, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.M.; Beshr, E.A.M.; Salem, I.M.; Ahmed, O.A.A.; Ibrahim, T.S.; Elsayed Abouzed, D.E.; Mahmoud, A.M.; Mohamed, M.F.A. Novel chalcone candidates as potential in vitro and in vivo anti-inflammatory agents: Synthesis, in silico docking, multitarget bioevaluation and molecular dynamic simulation. Bioorg. Chem. 2025, 161, 108540. [Google Scholar] [CrossRef]
- Jesus, A.; Duraes, F.; Szemeredi, N.; Freitas-Silva, J.; da Costa, P.M.; Pinto, E.; Pinto, M.; Spengler, G.; Sousa, E.; Cidade, H. BDDE-Inspired Chalcone Derivatives to Fight Bacterial and Fungal Infections. Mar. Drugs 2022, 20, 315. [Google Scholar] [CrossRef]
- Deng, S.; Yu, K.; Zhang, B.; Yao, Y.; Wang, Z.; Zhang, J.; Zhang, X.; Liu, G.; Li, N.; Liu, Y.; et al. Toll-Like Receptor 4 Promotes NO Synthesis by Upregulating GCHI Expression under Oxidative Stress Conditions in Sheep Monocytes/Macrophages. Oxid. Med. Cell. Longev. 2015, 2015, 359315. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Abdelall, E.K.A.; Labib, M.B.; Fadaly, W.A.A.; Zidan, T.H. Synthesis of novel halogenated triarylpyrazoles as selective COX-2 inhibitors: Anti-inflammatory activity, histopatholgical profile and in-silico studies. Bioorg. Chem. 2020, 105, 104418. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.-E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Ko, H.-H.; Tsao, L.-T.; Yu, K.-L.; Liu, C.-T.; Wang, J.-P.; Lin, C.-N. Structure–activity relationship studies on chalcone derivatives: The potent inhibition of chemical mediators release. Bioorg. Med. Chem. 2003, 11, 105–111. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.-W.; Park, W.-K.; Kong, J.-Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.-K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Structural and Chemical Biology of the Interaction of Cyclooxygenase with Substrates and Non-Steroidal Anti-Inflammatory Drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef] [PubMed]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Oliveira, A.S.; Vaz, C.V.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.M.F.; Palmeira-de-Oliveira, A.; et al. Anti-inflammatory potential of Portuguese thermal waters. Sci. Rep. 2020, 10, 22313. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Augustine, R.; Chaudhry, M.; Akhtar, U.A.; Zahid, A.A.; Tariq, M.; Falahati, M.; Ahmad, I.S.; Hasan, A. Nitric oxide-releasing biomaterials for promoting wound healing in impaired diabetic wounds: State of the art and recent trends. Biomed. Pharmacother. 2022, 149, 112707. [Google Scholar] [CrossRef]
- Uffort, D.G.; Grimm, E.A.; Ellerhorst, J.A. NF-kappaB mediates mitogen-activated protein kinase pathway-dependent iNOS expression in human melanoma. J. Investig. Dermatol. 2009, 129, 148–154. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.-K.; Shin, J.-H.; Lee, J.-K. Up-down Regulation of HO-1 and iNOS Gene Expressions by Ethyl Pyruvate via Recruiting p300 to Nrf2 and Depriving It from p65. Free Rad. Bio. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Nelin, L.D. Extracellular Signal-regulated Kinase Mediates Expression of Arginase II but Not Inducible Nitric-oxide Synthase in Lipopolysaccharide-stimulated Macrophages*. J. Biol. Chem. 2015, 290, 2099–2111. [Google Scholar] [CrossRef]
- Jacobs, A.T.; Ignarro, L.J. Lipopolysaccharide-induced Expression of Interferon-β Mediates the Timing of Inducible Nitric-oxide Synthase Induction in RAW 264.7 Macrophages*. J. Biol. Chem. 2001, 276, 47950–47957. [Google Scholar] [CrossRef]
- Ganster, R.W.; Geller, D.A. Chapter 8—Molecular Regulation of Inducible Nitric Oxide Synthase. In Nitric Oxide; Ignarro, L.J., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 129–156. [Google Scholar]
- Fischmann, T.O.; Hruza, A.; Niu, X.D.; Fossetta, J.D.; Lunn, C.A.; Dolphin, E.; Prongay, A.J.; Reichert, P.; Lundell, D.J.; Narula, S.K.; et al. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat. Struct. Biol. 1999, 6, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.R.; Arvai, A.S.; Ghosh, D.K.; Wu, C.; Getzoff, E.D.; Stuehr, D.J.; Tainer, J.A. Structure of Nitric Oxide Synthase Oxygenase Dimer with Pterin and Substrate. Science 1998, 279, 2121–2126. [Google Scholar] [CrossRef]
- Crane, B.R.; Arvai, A.S.; Gachhui, R.; Wu, C.; Ghosh, D.K.; Getzoff, E.D.; Stuehr, D.J.; Tainer, J.A. The Structure of Nitric Oxide Synthase Oxygenase Domain and Inhibitor Complexes. Science 1997, 278, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Levita, J.; Patala, R.; Kolina, J.; Milanda, T.; Mutakin, M.; Puspitasari, I.M.; Saptarii, N.M.; Sumiwi, S.A. Pharmacophore modeling and molecular docking of phytoconstituents in Morus sp. and Arcangelisia flava against nitric oxide synthase for antiinflammatory discovery. J. Appl. Pharm. Sci. 2018, 8, 53–59. [Google Scholar] [CrossRef]
- Andres, E.; Sá-Rocha, V.M.; Barrichello, C.; Haupt, T.; Ellis, G.; Natsch, A. The sensitivity of the KeratinoSens™ assay to evaluate plant extracts: A pilot study. Toxicol. Vitr. 2013, 27, 1220–1225. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for the Testing of Chemicals. 2024. Test Guideline No. 442D In Vitro Skin Sensitisation. Assay Addressing the Adverse Outcome Pathway Key Event on Keratinocyte Activation; OECD: Paris, France, 2024. [Google Scholar]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, A.; Gimondi, S.; Pinho, S.A.; Ferreira, H.; Neves, N.M.; Palmeira, A.; Sousa, E.; Almeida, I.F.; Cruz, M.T.; Cidade, H. Macroalgae-Inspired Brominated Chalcones as Cosmetic Ingredients with the Potential to Target Skin Inflammaging. Mar. Drugs 2025, 23, 278. https://doi.org/10.3390/md23070278
Jesus A, Gimondi S, Pinho SA, Ferreira H, Neves NM, Palmeira A, Sousa E, Almeida IF, Cruz MT, Cidade H. Macroalgae-Inspired Brominated Chalcones as Cosmetic Ingredients with the Potential to Target Skin Inflammaging. Marine Drugs. 2025; 23(7):278. https://doi.org/10.3390/md23070278
Chicago/Turabian StyleJesus, Ana, Sara Gimondi, Sónia A. Pinho, Helena Ferreira, Nuno M. Neves, Andreia Palmeira, Emília Sousa, Isabel F. Almeida, Maria T. Cruz, and Honorina Cidade. 2025. "Macroalgae-Inspired Brominated Chalcones as Cosmetic Ingredients with the Potential to Target Skin Inflammaging" Marine Drugs 23, no. 7: 278. https://doi.org/10.3390/md23070278
APA StyleJesus, A., Gimondi, S., Pinho, S. A., Ferreira, H., Neves, N. M., Palmeira, A., Sousa, E., Almeida, I. F., Cruz, M. T., & Cidade, H. (2025). Macroalgae-Inspired Brominated Chalcones as Cosmetic Ingredients with the Potential to Target Skin Inflammaging. Marine Drugs, 23(7), 278. https://doi.org/10.3390/md23070278












