A Fresh Perspective on Cyanobacterial Paralytic Shellfish Poisoning Toxins: History, Methodology, and Toxicology
Abstract
1. Paralytic Shellfish Poisoning Toxins
Understanding Freshwater Paralytic Shellfish Poisoning Toxins: How Do Marine PSPTs Compare to Their Freshwater Counterparts?
2. Structures of Paralytic Shellfish Poisoning Toxins
2.1. PSPT Structures
| Name | Elemental Formula | M.W. | Exact Mass | Original Elucidation | |
|---|---|---|---|---|---|
| 1. | Neosaxitoxin (NEO) | C10H19N7O52+ | 317.30 | 317.14367 | [37,38] |
| 2. | Saxitoxin (STX) * | C10H19N7O42+ | 301.30 | 301.14875 | [39,40] |
| 3. | 12,12-dido-dcSTX | C9H18N6O2+ | 226.28 | 226.15311 | [26,33] |
| 4. | 12α-do-doSTX | C9H18N6O2+ | 226.28 | 226.15311 | [26] |
| 5. | LWTX4 (12β-do-dcSTX) | C9H18N6O22+ | 242.28 | 242.14803 | [15,27] |
| 6. | 12α-do-dcSTX | C9H18N6O22+ | 242.28 | 242.14803 | [26] |
| 7. | 12β-do-doSTX | C9H18N6O2+ | 226.28 | 226.15311 | [26] |
| 8. | 12β-do-STX | C10H19N7O32+ | 285.30 | 285.15384 | [31] |
| 9. | 12,12-dido-doSTX | C9H18N62+ | 210.28 | 210.15820 | [33] |
| 10. | C1 | C10H17N7O11S2 | 475.41 | 475.04275 | [41,42] |
| 11. | C2 | C10H17N7O11S2 | 475.41 | 475.04275 | [42] |
| 12. | C3 | C10H17N7O12S2 | 491.41 | 491.03766 | [37] |
| 14. | C4 | C10H17N7O12S2 | 491.41 | 491.03766 | [37] |
| 15. | GTX1 | C10H18N7O9S+ | 412.36 | 412.08812 | [21,43,44] |
| 16. | GTX2 * | C10H18N7O8S+ | 396.36 | 396.09321 | [20,22] |
| 17. | GTX3 * | C10H18N7O8S+ | 396.36 | 396.09321 | [20,22] |
| 18. | GTX4 | C10H18N7O9S+ | 412.35 | 412.08812 | [43,45] |
| 19. | GTX5 (B1) | C10H18N7O7S+ | 380.36 | 380.09829 | [46] |
| 20. | GTX6 (B2) | C10H18N7O8S+ | 396.35 | 396.09321 | [46] |
| 21. | dcGTX1 | C9H17N6O8S+ | 369.33 | 369.08231 | [47] |
| 22. | dcGTX2 | C9H17N6O7S+ | 353.33 | 353.08739 | [15,48] |
| 23. | dcGTX3 | C9H17N6O7S+ | 353.33 | 353.08739 | [15,48] |
| 24. | dcGTX4 | C9H17N6O8S+ | 369.33 | 369.08231 | [47] |
| 25. | dcNEO | C9H18N6O42+ | 274.28 | 274.13786 | [49,50] |
| 26. | dcSTX | C9H18N6O32+ | 258.28 | 258.14294 | [51,52,53] ** |
| 27. | doSTX | C9H18N6O22+ | 242.28 | 242.14803 | [49,50] |
| 28. | LWTX1 | C11H19N6O7S+ | 379.37 | 379.10304 | [15] |
| 29. | LWTX2 | C11H19N6O8S+ | 395.37 | 395.09796 | [15] |
| 30. | LWTX3 | C11H19N6O8S+ | 395.37 | 395.09796 | [15] |
| 31. | LWTX5 | C11H20N6O42+ | 300.31 | 300.15351 | [15] |
| 32. | LWTX6 | C11H20N6O32+ | 284.31 | 284.15859 | [15] |
| 33. | 11α-OH GTX5 (M1α) † | C10H18N7O8S+ | 396.36 | 396.09321 | [23] |
| 34. | 11α-OH STX (M2α) † | C10H19N7O52+ | 317.30 | 317.14367 | [20,24] |
| 35. | 11β-OH GTX5 (M1β) | C10H18N7O8S+ | 396.36 | 396.09321 | [24] |
| 36. | 11β-OH STX (M2β) | C10H19N7O52+ | 317.30 | 317.14367 | [20,24] |
| 37. | 11β-OH dcSTX (dcM2β) | C9H18N6O42+ | 274.28 | 274.13786 | [23,43] |
| 38. | 11α-OH dcSTX (dcM2α) † | C9H18N6O42+ | 274.28 | 274.13786 | [23,43] |
| 39. | 11,11-OH GTX5 (M3) | C10H18N7O9S+ | 412.09 | 412.08812 | [24] |
| 40. | 11,11-OH STX (M4) | C10H19N7O72+ | 349.30 | 349.13350 | [24] |
| 41. | 11,11-OH dcSTX (dcM4) | C9H18N6O62+ | 306.28 | 306.12769 | [23] |
| 42. | M5 | C10H20N6O10S+ | 430.37 | 430.09869 | [23,24] |
| 43. | M6 | C10H21N7O72+ | 351.31 | 351.14915 | [23] |
| 44. | dcM6 | C9H20N6O62+ | 308.29 | 308.14334 | [23] |
| 45. | 11α-OH GTX6 (M7α) † | C10H18N7O9S+ | 412.36 | 412.08812 | [23] |
| 46. | 11β-OH GTX6 (M7β) | C10H18N7O9S+ | 412.36 | 412.08812 | [23] |
| 47. | 11α-OH NEO (M8α) † | C10H19N7O62+ | 333.30 | 333.13858 | [23] |
| 48. | 11β-OH NEO (M8β) | C10H19N7O62+ | 333.30 | 333.13858 | [23] |
| 49. | 11α-OH dcNEO (dcM8α) † | C9H18N6O52+ | 290.28 | 290.13277 | [23] |
| 50. | 11β-OH NEO (dcM8β) | C9H18N6O52+ | 290.28 | 290.13277 | [23] |
| 51. | 11,11-OH GTX6 (M9) | C10H18N7O10S+ | 428.36 | 428.08304 | [23] |
| 52. | 11,11-OH NEO (M10) | C10H19N7O72+ | 349.30 | 349.13350 | [23] |
| 53. | 11,11-OH dcNEO (dcM10) | C9H18N6O62+ | 306.27 | 306.12769 | [23] |
| 54. | M11 | C10H20N7O10S+ | 430.37 | 430.09869 | [23] |
| 55. | M12 | C10H21N7O72+ | 351.32 | 351.14915 | [23] |
| 56. | dcM12 | C9H20N6O62+ | 308.29 | 308.14000 | [23] |
| 57. | 12β-do-GTX3 | C10H18N7O7S+ | 380.36 | 380.09829 | [20,32] |
| 58. | 12β-do-GTX2 | C10H18N7O7S+ | 380.36 | 380.09829 | [20] |
| 59. | 12β-do-GTX5 | C10H18N7O6S+ | 364.36 | 364.10338 | [31] |
| 60. | M5-HA | C10H19N7O52+ | 317.30 | 317.14367 | [25] |
| 61. | M6-HA | C10H18N7O9S+ | 412.35 | 412.08812 | [25] |
| 62. | 12β-do-doSTX (doLWTX4) | C9H18N6O2+ | 226.28 | 226.15311 | [26] |
| 63. | 12α/β-do-GTX4 | C11H20N7O8S+ | 410.38 | 410.10886 | [54] |
| 64. | GC1 | C16H21N6O9S+ | 473.44 | 473.10852 | [55] |
| 65. | GC2 | C16H21N6O9S+ | 473.44 | 473.10852 | [55] |
| 66. | GC3 | C16H22N6O52+ | 378.38 | 378.16407 | [55] |
| 67. | GC4 | C16H21N6O10S+ | 489.44 | 489.10344 | [56] |
| 68. | GC5 | C16H21N6O10S+ | 489.44 | 489.10344 | [56] |
| 69. | GC6 | C16H22N6O62+ | 394.38 | 394.15899 | [56] |
| 70. | GC1a | C16H21N6O10S+ | 489.44 | 489.10344 | [56] |
| 71. | GC2a | C16H21N6O10S+ | 489.44 | 489.10344 | [56] |
| 72. | GC3a | C16H22N6O62+ | 394.39 | 394.15899 | [56] |
| 73. | GC4a | C16H21N6O11S+ | 505.43 | 505.09835 | [56] |
| 74. | GC5a | C16H21N6O11S+ | 505.43 | 505.09835 | [56] |
| 75. | GC6a | C16H22N6O72+ | 410.38 | 410.15390 | [56] |
| 76. | GC1b | C16H20N6O12S2+ | 552.49 | 552.05806 | [56] |
| 77. | GC2b | C16H20N6O12S2 | 552.49 | 552.05806 | [56] |
| 78. | GC3b | C16H21N6O8S+ | 457.44 | 457.11361 | [56] |
| 79. | GC4b | C16H20N6O13S2 | 568.49 | 568.05298 | [56] |
| 80. | GC5b | C16H20N6O13S2 | 568.49 | 568.05298 | [56] |
| 81. | GC6b | C16H21N6O9S+ | 473.44 | 473.10852 | [56] |
2.2. Important Chemical Features of PSPTs
2.3. Abiotic and Biological Conversions of PSPTs and Relevance to Freshwaters
3. Paralytic Shellfish Poisoning Toxins in Freshwater Environments
3.1. Production of PSPTs by Planktonic and Benthic Cyanobacteria
3.2. History of Detection of PSPTs in Freshwater Environments
| Date Collected | Location (Country) | Freshwater Source | Benthic/Open | Cyanobacterium | Detections Method | PSPT Congeners | Notes | Authors |
|---|---|---|---|---|---|---|---|---|
| 1967 | New Hampshire, United States | Kezar Lake | O | Aphanizomenon flos-aquae | MBA, Patch-clamp | “the toxin” | Toxins later elucidated as PSPTs | Sawyer et al., 1968 [70] |
| 1968 | - | - | O | Aphanizomenon flos-aquae | Infrared Spectroscopy, Chemical Assay, TLC | STX | Lab culture | Jackim & Gentile, 1968 [2] |
| 1970 | New Hampshire, United States | Kezar Lake | O | Aphanizomenon flos-aquae | MBA, HPLC, TLC | STX, * | Alam et al., 1978 [67] | |
| ~1970s | New Hampshire, United States | Kezar Lake, North Sutton | O | Aphanizomenon flos-aquae | MBA, HPLC | STX(?), 3 unknowns, * | Lab culture | Sasner et al., 1981 [65] |
| 1980 | New Hampshire, United States | Pond near Durham, NH | O | Aphanizomenon flos-aquae | MBA, PCOX, TLC | STX, NEO | Lab Culture | Ikawa et al., 1982 [69] |
| 1980 | New Hampshire, United States | Pond near Durham, NH | O | Aphanizomenon flos-aquae | MBA, HPLC | STX, NEO | Lab culture | Mahmood & Carmichael, 1986 [64] |
| 1990–1993 | Australia | Murray-Darling Basin (Millbrook Reservoir) | O | Anabaena circinalis | MBA, Electrophysiology, HPLC, FAB-MS | STX, GTX1,2,3,4,6, C1, C2, dcGTX2(?), 3(?), NEO(?) | Natural and Cultured | Humpage et al., 1994 [112] |
| 1991–1994 | Alabama, United States | Guntersville Reservoir, Tennessee River | B | Microseira (Lyngbya) wollei | MBA, RBA, PCOX | GTX?, dcSTX?, dcGTX2,3 | Natural and Cultured | Carmichael et al., 1997 [121] |
| 1993 | Alabama, United States | Guntersville Reservoir, Tennessee River | B | Microseira (Lyngbya) wollei | MBA, PCOX, MS, NMR | dcSTX, dcGTX2,3, LWTX1-6 | Toxins in situ and culture | Onodera et al., 1997 [15] |
| 1993–1994 | Multiple | Multiple | O/B | Dolichospermum (Anabaena) circinalis, Raphidiopsis (Cylindrospermopsis) raciborskii, Lyngbya (Microseira) wollei | PCOX, MS | Multiple | Lab culture, see Table S1 | Onodera et al., 1996, 2000 [105,106] |
| 1994 | Australia | Dam near Forbes, Central New South Wales | O | Dolichospermum (Anabaena) circinalus | MBA, PCOX | STX, C1,2, dcGTX2,3, GTX2,3,5, dcSTX | Sheep mortality | Negri et al., 1995 [90] |
| 1994 & 1996 | Brazil | Amparo City and Billings Reservoirs, São Paulo | O | Raphidiopsis (Cylindrospermopsis) raciborskii | MBA, PCOX, LC-MS/MS | STX, NEO, GTX2,3, * | Toxin only detected in culture | Lagos et al., 1999 [62] |
| 1996 | Portugal | Crestuma-Lever Reservoir, Douro River | O | Aphanizomenon flos-aquae | MBA, PCOX | STX, NEO, dcSTX, GTX1,2,3,4 | Natural and Cultured | Ferreire et al., 2001 [122] |
| 1996 | Portugal | Montargil Reservoir | O | Aphanizomenon flos-aquae | MBA, PCOX, LC-MS/MS | STX, NEO, dcSTX, GTX5,6 | Natural and Cultured | Pereira et al., 2000 [123] |
| 1996 † | Australia | Burrinjuck Dam, New South Wales | O | Dolichospermum (Anabaena) circinalis | PCOX | C1,2, dcGTX2,3, GTX2,3 | Stability over 90 days | Jones & Negri, 1997 [81] |
| 1997 | Italy | Lake Varese | O | Planktothrix agardhii | preCOX and PCOX, LC-MS/MS, Patch-Clamp | STX | Natural and Cultured | Pomati et al., 2000 [124] |
| 1997 | Italy | Lake Varese | O | Oscillatoria, Aphanizomenon, Anabaena * | preCOX, Patch-Clamp | STX | Natural and Cultured | Giovannardi et al., 1999 [125] |
| 1998 | Brazil | Tabocas Reservoir | O | Raphidiopsis (Cylindrospermopsis) raciborskii | MBA, PCOX, LC-MS/MS | STX, GTX6, dcSTX, NEO, dcNEO | Lab culture | Molica et al., 2002 [61] |
| 1998–2000 | New Zealand | Waikanae and Mataura Rivers | B/O | “Oscillatoria-like” | MBA, Neuroblastoma assay | STX, NEO | Hamill, 2001 [126] | |
| 2000 | Brazil | Armando Ribeiro Gonçalves Reservoir and Pataxó channel | O | - | preCOX | C1,2, GTX (?), GTX5 (B1), STX | Costa et al., 2006 [127] | |
| 2000 | Portugal | Lake Crato | O | Aphanizomenon gracile | PCOX | STX, NEO, GTX1 | Natural and Cultured | Pereira et al., 2004 [128] |
| 2000 | Brazil | Billings Reservoir, Sao Paulo | O | Microcystis aeruginosa | MBA, PCOX, LC-MS/MS | GTX1,2,3,4 | Lab culture | Sant’Anna et al., 2011 [107] |
| 2002 | Brazil | Tapacurá Reservoir | O | Dolichospermum (Anabaena) spiroides | MBA, PCOX | STX, NEO, dcSTX | Natural and Cultured | Molica et al., 2005 [129] |
| 2002–2003 | New Zealand | Waikato River | O | - | ELISA (manufacturer unspecified) | - | Kouzminov et al., 2007 [130] | |
| 2003 | China | Lake Dianchi | O | Aphanizomenon sp. | MBA, PCOX | STX, NEO, dcSTX, dcGTX2,3, GTX4 | Natural and Cultured | Liu et al., 2006 [131] |
| 2004 | Brazil | Billings Reservoir, Sao Paulo | O | - | PCOX | STX, NEO, GTX2,3 | dos Anjos et al., 2006 [132] | |
| 2005–2008 | France | Champs-sur-Marne, Paris | O | Aphanizomenon gracile | Neuro-2a cell-based assay, LC-MS/MS | STX, NEO | Natural and Cultured | Ledreux et al., 2010 [133] |
| 2006 | Brazil | Lake Lagoa do Peri | O | - | PCOX | NEO, GTX4 | Adsorption testing | Romero et al., 2014 [134] |
| 2006 | Bulgaria | Borovitsa Reservoir | O | - | HPLC, Ridascreen™ ELISA | STX | Teneva et al., 2010[135] | |
| 2006–2013 | Canada | St. Lawrence River | B | Microseira (Lyngbya) wollei | LC-MS/MS | LWTX1 | Only looked for LWTX1 | Hudon et al., 2016 [13] |
| 2008 | Germany | 5 lakes in NE Germany | O | Aphanizomenon gracile | Abraxis ELISA, LC-MS/MS | STX, NEO, GTX5, dcSTX | Lab culture | Ballot et al., 2010 [136] |
| 2008 † | Mexico | Lake Catemaco | O | - | Abraxis ELISA | - | Seston, water, and snails | Berry & Lind, 2010 [120] |
| 2008–2009 | Greece | Lake Pamvotis | O | - | Abraxis ELISA | - | Gkelis et al., 2014 [137] | |
| 2009 | Artic | Northern Baffin Island near Cape Hatt | ? | - | Abraxis ELISA, preCOX | - | Kleinteich et al., 2013 [138] | |
| 2009 | Guatemala | Lake Atitlan | O | - | Abraxis ELISA | - | Rejmánková et al., 2011 [139] | |
| 2009–2010 | Florida, United States | Silver Glen and Blue Hole Springs | B | Microseira (Lyngbya) wollei | LC-MS/MS | dcSTX, dcGTX2,3 LWTX1,2,3,4,5,6 | Foss et al., 2012 [14] | |
| 2009–2011 | Brazil | 4 Reservoirs of Rio Grande do Norte | O | - | Beacon ELISA | - | Fonseca et al., 2015 [140] | |
| 2010 | Russia | Lake Baikal | O | - | Abraxis ELISA | - | Belykh et al., 2015 [141] | |
| 2010 | Russia | Lake Baikal and Reservoirs of the Angara River | O | - | Abraxis ELISA, MALDI-TOF | STX, NEO, dcGTX2/3, dcGTX1/4 | Paired with above study | Belykh et al., 2015 [59] |
| 2010 | Australia | Murray and Edward River systems | O | - | preCOX, Abraxis ELISA, Jellet rapid test strips | - | <LOD except for 3 samples by ELISA | Bowling et al., 2013 [142] |
| 2010 | Canada | St. Lawrence River (Lake St. Louis) | B | Microseira (Lyngbya) wollei | LC-MS/MS, LC-QToF | LWTX1,6 | Lajeunesse et al., 2012 [96] | |
| 2011 | New Zealand | Drinking-water Reservoir and Groynes Lakes in South Island | B/O | Heteroscytonema cf. crispum | preCOX, Jellett PSP Rapid Test Kit | STX | Natural and Cultured | Smith et al., 2011 [143] |
| 2011 | Brazil | Mundau River basin and Araripe, Ceara | B | Geitlerinema amphibium, Geitlerinema lemmermannii, Cylindrospermum stagnale and Phormidium uncinatum | PCOX | NEO, STX, dcSTX, GTX1,4 | Lab culture, suspect LC | Borges et al., 2015 [144] |
| 2011 | Brazil | Itupararanga Reservoir, São Paulo | O | Raphidiopsis (Cylindrospermopsis) raciborskii | Beacon ELISA | - | Reported STX quota per trichome and per L | Casali et al., 2017 [145] |
| 2013–2015 | Russia | Central European Russia and West Siberia | O | - | LC-MS/MS | STX | Chernova et al., 2017 [146] | |
| 2013–2015 | Greece | Lake Karla and Kalamaki Reservoir | O | - | Abraxis ELISA, LC-MS/MS | STX, NEO | Gkelis et al., 2017 [147] | |
| 2014 | Greece | Lake Vistonis | O | - | LC-MS/MS | STX, NEO | Moustaka-Gouni et al., 2017 [148] | |
| 2014–2017 | New York, United States | Chautauqua Lake | O | - | PCOX, RBA, Abraxis ELISA | * | Unknown PSPTs | Smith, Z.J. et al., 2020 [149] |
| 2015 | Russia | Lake Baikal | B | - | Abraxis ELISA, MALDI-ToF | STX, NEO, GTX5, dcSTX, dcNEO, dcGTX2/3, dcGTX1/4, doGTX 2/3/4(?) | Constitutional isomers of doGTX2/3 not determined | Belykh et al., 2016 [59] |
| 2016 | Greece | Karla Reservoir | O | - | Abraxis ELISA | - | Pelican death | Papadimitriou et al., 2018 [150] |
| 2016–2017 | Brazil | Paraíba River Basin | O | - | Abraxis ELISA | - | dos Santos Silva et al., 2019 [151] | |
| 2017 | New York, United States | Butterfield Lake | B | Microseira (Lyngbya) wollei | PCOX, Abraxis ELISA, LC-MS/MS | STX, GTX3,5(?), dcGTX2,3, dcSTX, LWTX2/3, LWTX5, * | Smith, Z.J. et al., 2019 [12] | |
| 2017 | Canada | St. Lawrence River | B | Microseira (Lyngbya) wollei | LC-MS/MS | LWTX1 | Only looked for LWTX-1 | Poirier-Larabie et al., 2020 [152] |
| 2017 | Russia | Irkutsk Reservoir near Lake Baikal | O | - | LC-MS (TOF), Abraxis ELISA | STX | Precolumn modification of STX | Grachev et al., 2018 [153] |
| 2017–2018 | New York, United States | Cayuga Lake | O | - | PCOX, ELISA, RBA, LCMS | STX, * | Unknown PSPTs | Smith and Boyer, 2024 [60] |
| 2018 | Denmark | Northern Zealand Lakes | O | - | PCOX | STX, NEO, dcNEO, dcSTX, GTX2,3 | Podduturi et al., 2021 [154] | |
| 2018–2019 | Ohio, United States | Western Lake Erie | B/O | - | Abraxis ELISA | - | Nauman et al., 2024 [155] | |
| 2018–2019 | South Carolina, United States | Lake Wateree | B | Microseira wollei | LC-MS/MS | LWTX1,4,5,6 | Putnam et al., 2022 [156] | |
| 2019 | South Carolina, United States | Lake Wateree | B/O | Microseira wollei | LC-MS/MS | LWTX1,4,5,6, dcSTX | Export, stability, and degradation study | Metz et al., 2022 [97] |
| 2019 | China | West Lake in Hangzhou | B | Microseira wollei | LC-MS/MS | STX, NEO, dcSTX, GTX2,3,5, dcGTX2,3, C1, LWTX1 | Lab culture | Chen et al., 2024 [99] |
| 2019 | Multiple | Multiple | O | LC-MS/MS | 35 analogs | Re-evaluate PSPT producers (in culture), PSPT structures incorrect | D’Agostino et al., 2019 [26] | |
| 2021 | Kansas, United States | Blue River, Mill Creek, and Indian Creek | B | - | Abraxis ELISA | - | ELISA does not detect all toxins | Rider et al., 2024 [157] |
3.3. Historical Large-Scale Measurement of PSPTs
| State/Country | Lakes with PSPTs/Lakes Surveyed (%) | PSPT Range (Median) [µg PSPTs/L] * | Analytical Method | Notes |
|---|---|---|---|---|
| Australia [112] | 12/12 (100%) | 14.7–568.6 | MBA, PCOX | |
| Brazil [161] | 37/37 (100%) | 0.1–0.63 | Abraxis ELISA | Toxicology assessment by ELISA only |
| Bulgaria [162] | 4/120 (3%) | 0.01–2.5 | HPLC-DAD, LCMS, ELISA, in vitro cytotests | Compiled data from 35 studies |
| Czech Republic [163] | 2/19 (10.5%) | 0.03–0.04 (0.03) | Abraxis ELISA | |
| Denmark [164] | 8–11/96 | 6–224 (34.1) µg STX eq./g DW−1 | MBA, PCOX | Net tows and seston study. Mostly STX and GTX4, others present |
| Finland [165] | 50 | 13–1070 | Jellett rapid PSP test strips, RBA (Na-Ch and SXPN), PCOX, LCMS | |
| Finland [166] | 21/32 (66%) (13–59% of samples) | <0.01–1.47 (0.031) | PCOX | Mostly STX, some dcSTX |
| France [167] | 10 (14% of samples) | <0.05 | LC-MS/MS | Lakes with toxin not reported |
| Germany [168] | 10/29 (34%) | NA | ELISA, LC-MS/MS | |
| Greece [137] | 3/6 (50%) | 0.4–1.2 | Abraxis ELISA | |
| Greece [169] | 5/14 (36%) | 0.1–6.65 (0.73) | LC-MS/MS (various extraction/separation methods) | Median is sum of STX + NEO in entire sample set |
| New Zealand [170] | 38/42 (90%) | 0.001–0.99 (0.00113) | ELISA, Neuroblastoma Assay | Only analyzed toxigenic cyanobacteria |
| Ohio, United States [171] | 25/105 (24%) | 0.022–0.880 | ELISA, LC-MS/MS | Includes water treatment finished water |
| Poland [172] | 12/34 (35%) | <0.01–0.57 | PCOX | Dead fish and shellfish also analyzed. Detected STX and dcSTX |
| Sweden [173] | 98 sites/blooms (47% of samples) | 72 ± 190.4 † | LC-MS/MS | Unclear description of abundance and sample percentages. Only measured STX, NEO, dcNEO |
| United States [174] | 6/1161 (<1%) | >0.2 (0.03) | Abraxis ELISA | |
| United States [175] | 4/23 (17%) | 0.02–0.2 (0.03) | ELISA | |
| New York, United States [4] | 29–36/245 | 0.38–923 (6.1–14) | PCOX, ELISA, RBA, LC-MS/MS | Many unknown PSPT-like compounds |
| United States [176] | 6/11 (55%) | ND-0.913 | ELISA (and qPCR) | |
| Texas, United States [177] | 8/20 (40%) | ND-0.016 (0.003) | LC-MS/MS | Only measured STX |
| Uruguay [178] | 18 | ND-14.62 (1.74) | Abraxis ELISA |
4. Overview of Detection Methods
4.1. Receptor Binding Assay
4.2. Saxitoxin and Neosaxitoxin Enzyme Linked Immunosorbent Assay (ELISA)
4.3. Oxidation and Fluorescence Detection with High Performance Liquid Chromatography
4.4. Mass Spectrometry
5. Selecting an Appropriate Method for Freshwater Paralytic Shellfish Poisoning Toxin Analyses
5.1. Requirements for Selecting an Analytical Method
5.2. PSPT Method Limitations and Practical Application—Confirming Toxin Presence and Interpretating Results
5.2.1. Receptor Binding Assay
5.2.2. STX ELISA
5.2.3. Chemical Oxidation (PCOX and PreCOX)
5.2.4. Mass Spectrometry
5.3. Monitoring Recommendations for PSPTs
6. Toxicology and Human Health Concerns from Freshwater PSPTs
6.1. Acute and Chronic/Sub-Chronic Exposure to PSPTs
6.2. Medical Treatment of PSP Syndrome
6.3. Toxicological Gaps and Concerns
7. Protecting Human Health from Freshwater Paralytic Shellfish Poisoning Toxins
7.1. Quantification of Toxic Potential
7.2. Toxicity Equivalency Factors
7.3. Proposed Toxicity Equivalency Factors for M2α and M2β (“11-Hydroxysaxitoxin”) and dcM2α and dcM2β (“11-Hydroxydecarbamoylsaxitoxin”)
7.4. PSPT Regulatory Guidelines
| Source | Drinking Water Exposure | Recreational (Short Term) Exposure | Recreational (Subchronic) Exposure |
|---|---|---|---|
| Australia [293] | 3 | - | - |
| Brazil [294] | 3 | - | - |
| New Zealand [295] | 3 | - | - |
| Oregon, United States [296,297] * | 1 or 1.6 or 3 | - | 8 or 10 |
| Ohio, United States [298,299] | 0.3 | 3 (2016 only) | 0.8 |
| Utah, United States [300] | - | 75 | 8 |
| Washington, United States [301,302] | 3 | 75 | - |
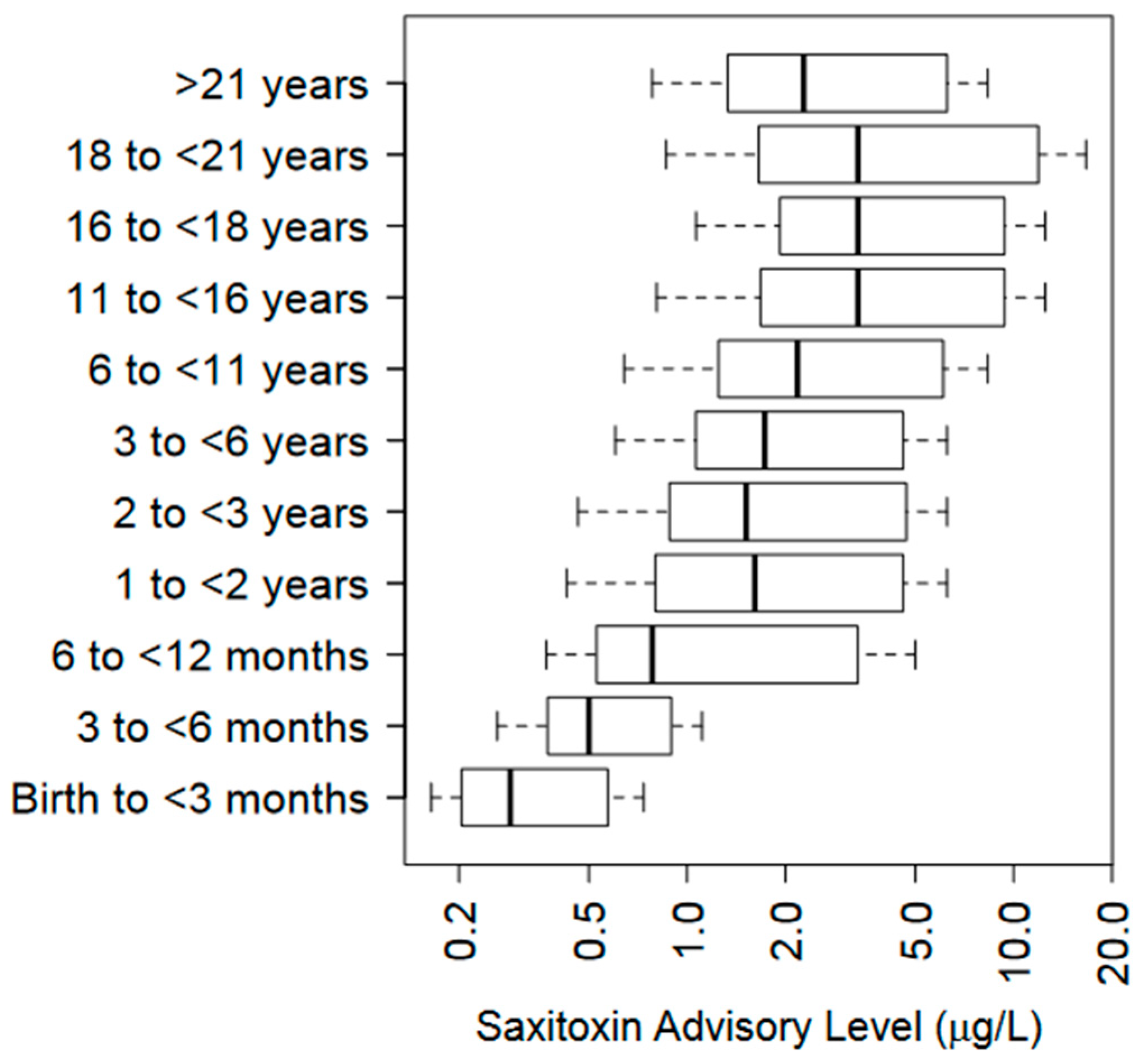
7.5. Reporting Units
7.6. Exposure Risk Assessment from Consuming PSPTs in Freshwater Systems
8. Important Research Gaps for Freshwater Paralytic Shellfish Poisoning Toxins
8.1. Limited Availability and Type of Standards
8.2. Freshwater PSPT Congener Profiles and Environmental Drivers
8.3. Acute and Chronic Toxicity of PSPTs
9. Final Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, K.; Sommer, H.; Schoenholz, P. Mussel Poisoning. J. Prev. Med. 1928, 2, 365–394. [Google Scholar]
- Jackim, E.; Gentile, J. Toxins of a Blue-Green Alga: Similarity to Saxitoxin. Science 1968, 162, 915–916. [Google Scholar] [CrossRef]
- Terlau, H.; Heinemann, S.H.; Stühmer, W.; Pusch, M.; Conti, F.; Imoto, K.; Numa, S. Mapping the Site of Block by Tetrodotoxin and Saxitoxin of Sodium Channel II. FEBS Lett. 1991, 293, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.J. Freshwater Paralytic Shellfish Poisoning Toxins and Other Cyanobacterial Neurotoxins in New York Lakes. Ph.D. Thesis, State University of New York College of Environmental Science and Forestry, Syracuse, NY, USA, 2019; pp. 1–385. [Google Scholar]
- Carmichael, W.W.; Boyer, G.L. Health Impacts from Cyanobacteria Harmful Algae Blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria Secondary Metabolites—The Cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M. A Review of Harmful Algal Blooms and Their Apparent Global Increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- RaLonde, R. Paralytic Shellfish Poisoning: The Alaska Problem. Mar. Resour. 1996, 8, 1–20. [Google Scholar]
- Yin, Q.; Carmichael, W.W.; Evans, W.R. Factors Influencing Growth and Toxin Production by Cultures of the Freshwater Cyanobacterium Lyngbya wollei Farlow Ex Gomont. J. Appl. Phycol. 1997, 9, 55–63. [Google Scholar] [CrossRef]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental Conditions That Influence Toxin Biosynthesis in Cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Dias, E.; Pereira, P.; Franca, S. Production of Paralytic Shellfish Toxins by Aphanizomenon Sp. Lmecya 31 (Cyanobacteria). J. Phycol. 2002, 38, 705–712. [Google Scholar] [CrossRef]
- Smith, Z.J.; Martin, R.M.; Wei, B.; Wilhelm, S.W.; Boyer, G.L. Spatial and Temporal Variation in Paralytic Shellfish Toxin Production by Benthic Microseira (Lyngbya) wollei in a Freshwater New York Lake. Toxins 2019, 11, 44. [Google Scholar] [CrossRef]
- Hudon, C.; Gagnon, P.; Poirier Larabie, S.; Gagnon, C.; Lajeunesse, A.; Lachapelle, M.; Quilliam, M.A. Spatial and Temporal Variations of a Saxitoxin Analogue (LWTX-1) in Lyngbya wollei (Cyanobacteria) Mats in the St. Lawrence River (Québec, Canada). Harmful Algae 2016, 57, 69–77. [Google Scholar] [CrossRef]
- Foss, A.J.; Phlips, E.J.; Yilmaz, M.; Chapman, A. Characterization of Paralytic Shellfish Toxins from Lyngbya wollei Dominated Mats Collected from Two Florida Springs. Harmful Algae 2012, 16, 98–107. [Google Scholar] [CrossRef]
- Onodera, H.; Satake, M.; Oshima, Y.; Yasumoto, T.; Carmichael, W.W. New Saxitoxin Analogues from the Freshwater Filamentous Cyanobacterium Lyngbya wollei. Nat. Toxins 1997, 5, 146–151. [Google Scholar] [CrossRef]
- Kenins, A. Notulae Algarum No.43. Available online: https://www.notulaealgarum.com/documents/Notulae%20algarum%20No.%2043.pdf (accessed on 2 May 2025).
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P.; et al. Acute Toxicity of Dihydroanatoxin-a from Microcoleus autumnalis in Comparison to Anatoxin-a. Chemosphere 2021, 263, 127937. [Google Scholar] [CrossRef]
- Munday, R.; Reeve, J. Risk Assessment of Shellfish Toxins. Toxins 2013, 5, 2109–2137. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.L.; Schantz, E.J.; Schnoes, H.K. Characterization of 11-Hydroxysaxitoxin Sulphate, a Major Toxin in Scallops Exposed to Blooms of the Poisonous Dinoflagellate Gonyaulax tamarensis. J. Chem. Soc. Chem. Commun. 1978, 889–890. [Google Scholar] [CrossRef]
- Shimizu, Y.; Alam, M.; Oshima, Y.; Fallon, W.E. Presence of Four Toxins in Red Tide Infested Clams and Cultured Gonyaulax tamarensis Cells. Biochem. Biophys. Res. Commun. 1975, 66, 731–737. [Google Scholar] [CrossRef]
- Shimizu, Y.; Buckley, L.J.; Alam, M.; Oshima, Y.; Fallon, W.E.; Kasai, H.; Miura, I.; Gullo, V.P.; Nakanishi, K. Structures of Gonyautoxin II and III from the East Coast Toxic Dinoflagellate Gonyaulax tamarensis. J. Am. Chem. Soc. 1976, 98, 5414–5416. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Li, A.; Lewis, N.; McCarron, P.; Thomas, K.M.; Walter, J.A. Biotransformation and Chemical Degradation of Paralytic Shellfish Toxins in Mussels. In Proceedings of the 17th International Conference on Harmful Algae, Florianópolis, Brazil, 9–14 October 2016. [Google Scholar]
- Dell’Aversano, C.; Walter, J.A.; Burton, I.W.; Stirling, D.J.; Fattorusso, E.; Quilliam, M.A. Isolation and Structure Elucidation of New and Unusual Saxitoxin Analogues from Mussels. J. Nat. Prod. 2008, 71, 1518–1523. [Google Scholar] [CrossRef]
- Numano, S.; Kudo, Y.; Cho, Y.; Konoki, K.; Kaga, Y.; Nagasawa, K.; Yotsu-Yamashita, M. Two New Skeletal Analogues of Saxitoxin Found in the Scallop, Patinopecten yessoensis, as Possible Metabolites of Paralytic Shellfish Toxins. Chemosphere 2021, 278, 130224. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Boundy, M.J.; Harwood, T.D.; Carmichael, W.W.; Neilan, B.A.; Wood, S.A. Re-Evaluation of Paralytic Shellfish Toxin Profiles in Cyanobacteria Using Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. Toxicon 2019, 158, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Tsuchiya, S.; Yoshioka, R.; Omura, T.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. The Presence of 12β-Deoxydecarbamoylsaxitoxin in the Japanese Toxic Dinoflagellate Alexandrium Determined by Simultaneous Analysis for Paralytic Shellfish Toxins Using HILIC-LC–MS/MS. Harmful Algae 2015, 49, 58–67. [Google Scholar] [CrossRef]
- McGregor, G.B. Freshwater Cyanobacteria of North-Eastern Australia: 3. Nostocales. AlgaeBase 2018, 359, 9. [Google Scholar] [CrossRef]
- Sendall, B.C.; McGregor, G.B. Cryptic Diversity within the Scytonema Complex: Characterization of the Paralytic Shellfish Toxin Producer Heterosyctonema crispum, and the Establishment of the Family Heteroscytonemataceae (Cyanobacteria/Nostocales). Harmful Algae 2018, 80, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Berrendero Gómez, E.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The Polyphasic Analysis of Two Native Raphidiopsis Isolates Supports the Unification of the Genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). AlgaeBase 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Akamatsu, M.; Hirozumi, R.; Cho, Y.; Kudo, Y.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. First Identification of 12β-Deoxygonyautoxin 5 (12α-Gonyautoxinol 5) in the Cyanobacterium Dolichospermum circinale (TA04) and 12β-Deoxysaxitoxin (12α-Saxitoxinol) in D. circinale (TA04) and the Dinoflagellate Alexandrium pacificum (Group IV) (120518KureAC). Mar. Drugs 2022, 20, 166. [Google Scholar] [CrossRef]
- Minowa, T.; Cho, Y.; Oshima, Y.; Konoki, K.; Yotsu-Yamashita, M. Identification of a Novel Saxitoxin Analogue, 12β-Deoxygonyautoxin 3, in the Cyanobacterium, Anabaena circinalis (TA04). Toxins 2019, 11, 539. [Google Scholar] [CrossRef]
- Hakamada, M.; Tokairin, C.; Ishizuka, H.; Adachi, K.; Osawa, T.; Aonuma, S.; Hirozumi, R.; Tsuchiya, S.; Cho, Y.; Kudo, Y.; et al. Synthesis and Identification of decarbamoyloxySaxitoxins in Toxic Microalgae and Their Reactions with the Oxygenase, SxtT, Reveal Saxitoxin Biosynthesis. Chem.–A Eur. J. 2024, 30, e202304238. [Google Scholar] [CrossRef]
- Arakawa, O.; Nishio, S.; Noguchi, T.; Shida, Y.; Onoue, Y. A New Saxitoxin Analogue from a Xanthid Crab Atergatis floridus. Toxicon 1995, 33, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Zaman, L.; Arakawa, O.; Shimosu, A.; Shida, Y.; Onoue, Y. Occurrence of a Methyl Derivative of Saxitoxin in Bangladeshi Freshwater Puffers. Toxicon 1998, 36, 627–630. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kim, Y.H.; Dudley, S.C.; Choudhary, G.; Pfahnl, A.; Oshima, Y.; Daly, J.W. The Structure of Zetekitoxin AB, a Saxitoxin Analog from the Panamanian Golden Frog Atelopus zeteki: A Potent Sodium-Channel Blocker. Proc. Natl. Acad. Sci. USA 2004, 101, 4346–4351. [Google Scholar] [CrossRef]
- Hall, S.; Darling, S.D.; Boyer, G.L.; Reichardt, P.B.; Liu, H.-W. Dinoflagellate Neurotoxins Related to Saxitoxin: Structures of Toxins C3 and C4, and Confirmation of the Structure of Neosaxitoxin. Tetrahedron Lett. 1984, 25, 3537–3538. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hsu, C.-P.; Fallon, W.E.; Oshima, Y.; Miura, I.; Nakanishi, K. Structure of Neosaxitoxin. J. Am. Chem. Soc. 1978, 100, 6791–6793. [Google Scholar] [CrossRef]
- Wong, J.L.; Oesterlin, R.; Rapoport, H. Structure of Saxitoxin. J. Am. Chem. Soc. 1971, 93, 7344–7345. [Google Scholar] [CrossRef] [PubMed]
- Schantz, E.J.; Ghazarossian, V.E.; Schnoes, H.K.; Strong, F.M.; Springer, J.P.; Pezzanite, J.O.; Clardy, J. Structure of Saxitoxin. J. Am. Chem. Soc. 1975, 97, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shimizu, Y. Gonyautoxin VIII, a Cryptic Precursor of Paralytic Shellfish Poisons. J. Chem. Soc. Chem. Commun. 1981, 827–828. [Google Scholar] [CrossRef]
- Noguchi, T.; Onoue, Y.; Maruyama, J.; Hashimoto, K.F.A.; Nishio, S.; Ikeda, T. The New Paralytic Shellfish Poisons from Protogonyaulax catenella. Bull. Jpn. Soc. Sci. Fish 1983, 49, 1931. [Google Scholar] [CrossRef]
- Boyer, G. Chemical Investigations of the Toxins Produced by Marine Dinoflagellates. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 1980; pp. 1–226. [Google Scholar]
- Shimizu, Y.; Hsu, C.P. Confirmation of the Structures of Gonyautoxins I–IV by Correlation with Saxitoxin. J. Chem. Soc. Chem. Commun. 1981, 314–315. [Google Scholar] [CrossRef]
- Oshima, Y.; Buckley, L.J.; Alam, M.; Shimizu, Y. Heterogeneity of Paralytic Shellfish Poisons. Three New Toxins from Cultured Gonyaulax tamarensis Cells, Mya arenaria and Saxidomus giganteus. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1977, 57, 31–34. [Google Scholar] [CrossRef]
- Harada, T.; Oshima, Y.; Yasumoto, T. Structures of Two Paralytic Shellfish Toxins, Gonyautoxins V and VI, Isolated from a Tropical Dinoflagellate, Pyrodinium bahamense Var. Compressa. Agric. Biol. Chem. 1982, 46, 1861–1864. [Google Scholar] [CrossRef]
- Lin, H.-P.; Cho, Y.; Yashiro, H.; Yamada, T.; Oshima, Y. Purification and Characterization of Paralytic Shellfish Toxin Transforming Enzyme from Mactra chinensis. Toxicon 2004, 44, 657–668. [Google Scholar] [CrossRef]
- Oshima, Y.; Sugino, K.; Yasumoto, T. Latest Advances in HPLC Analysis of Paralytic Shellfish Toxins. In Mycotoxins and Phycotoxins ’88; Elsevier: Amsterdam, The Netherlands, 1989; pp. 319–326. [Google Scholar]
- Oshima, Y.; Itakura, H.; Lee, K.-C.; Yasumoto, T.; Blackburn, S.; Hallegraeff, G. Toxin Production by the Dinoflagellate Gymnodinium catenatum. In Proceedings of the Fifth International Conference on Toxic Marine Phytoplankton, Newport, RI, USA, 28 October–1 November 1991. [Google Scholar]
- Oshima, Y.; Sugino, K.; Itakura, H.; Hirota, M.; Yasumoto, T. Comparative Studies on Paralytic Shellfish Toxin Profile of Dinoflagellates and Bivalves. In Proceedings of the Fourth International Conference on Toxic Marine Phytoplankton, Lund, Sweden, 26–30 June 1989. [Google Scholar]
- Ghazarossian, V.E.; Schantz, E.J.; Schnoes, H.K.; Strong, F.M. A Biologically Active Acid Hydrolysis Product of Saxitoxin. Biochem. Biophys. Res. Commun. 1976, 68, 776–780. [Google Scholar] [CrossRef]
- Harada, T.; Oshima, Y.; Kamaiya, H.; Yasumoto, T. Confirmation of Paralytic Shellfish Toxins in the Dinoflagellate Pyrodinium bahamense Var. Compressa and Bivalves in Palau. Bull. Jpn. Soc. Sci. Fish 1982, 48, 821–825. [Google Scholar] [CrossRef]
- Harada, T.; Oshima, Y.; Yasumoto, T. Natural Occurrence of Decarbamoylsaxitoxin in Tropical Dinoflagellate and Bivalves. Agric. Biol. Chem. 1983, 47, 191–193. [Google Scholar] [CrossRef]
- Lim, P.-T.; Sato, S.; Van Thuoc, C.; Tu, P.T.; Huyen, N.T.M.; Takata, Y.; Yoshida, M.; Kobiyama, A.; Koike, K.; Ogata, T. Toxic Alexandrium minutum (Dinophyceae) from Vietnam with New Gonyautoxin Analogue. Harmful Algae 2007, 6, 321–331. [Google Scholar] [CrossRef]
- Negri, A.P.; Bolch, C.J.S.; Geier, S.; Green, D.H.; Park, T.-G.; Blackburn, S.I. Widespread Presence of Hydrophobic Paralytic Shellfish Toxins in Gymnodinium catenatum. Harmful Algae 2007, 6, 774–780. [Google Scholar] [CrossRef]
- Vale, P. Complex Profiles of Hydrophobic Paralytic Shellfish Poisoning Compounds in Gymnodinium catenatum Identified by Liquid Chromatography with Fluorescence Detection and Mass Spectrometry. J. Chromatogr. A 2008, 1195, 85–93. [Google Scholar] [CrossRef]
- Vale, P.; Rangel, I.; Silva, B.; Coelho, P.; Vilar, A. Atypical Profiles of Paralytic Shellfish Poisoning Toxins in Shellfish from Luanda and Mussulo Bays, Angola. Toxicon 2009, 53, 176–183. [Google Scholar] [CrossRef]
- Negri, A.; Stirling, D.; Quilliam, M.; Blackburn, S.; Bolch, C.; Burton, I.; Eaglesham, G.; Thomas, K.; Walter, J.; Willis, R. Three Novel Hydroxybenzoate Saxitoxin Analogues Isolated from the Dinoflagellate Gymnodinium catenatum. Chem. Res. Toxicol. 2003, 16, 1029–1033. [Google Scholar] [CrossRef]
- Belykh, O.I.; Tikhonova, I.V.; Kuzmin, A.V.; Sorokovikova, E.G.; Fedorova, G.A.; Khanaev, I.V.; Sherbakova, T.A.; Timoshkin, O.A. First Detection of Benthic Cyanobacteria in Lake Baikal Producing Paralytic Shellfish Toxins. Toxicon 2016, 121, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.J.; Boyer, G.L. Unusual Paralytic Shellfish Poisoning Toxins in Cayuga Lake, New York. Toxicon 2024, 252, 108165. [Google Scholar] [CrossRef]
- Molica, R.; Onodera, H.; García, C.; Rivas, M.; Andrinolo, D.; Nascimento, S.; Meguro, H.; Oshima, Y.; Azevedo, S.; Lagos, N. Toxins in the Freshwater Cyanobacterium Cylindrospermopsis raciborskii (Cyanophyceae) Isolated from Tabocas Reservoir in Caruaru, Brazil, Including Demonstration of a New Saxitoxin Analogue. Phycologia 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Lagos, N.; Onodera, H.; Zagatto, P.A.; Andrinolo, D.; Azevedo, S.M.F.Q.; Oshima, Y. The First Evidence of Paralytic Shellfish Toxins in the Freshwater Cyanobacterium Cylindrospermopsis raciborskii, Isolated from Brazil. Toxicon 1999, 37, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.; Cristiano, M.L.S. Marine Paralytic Shellfish Toxins: Chemical Properties, Mode of Action, Newer Analogues, and Structure–Toxicity Relationship. Nat. Prod. Rep. 2022, 39, 33–57. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Carmichael, W.W. Paralytic Shellfish Poisons Produced by the Freshwater Cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 1986, 24, 175–186. [Google Scholar] [CrossRef]
- Sasner, J.J., Jr.; Ikawa, M.; Foxall, T.L.; Watson, W.H. Studies on Aphantoxin from Aphanizomenon flos-aquae in New Hampshire. In The Water Environment: Algal Toxins and Health; Carmichael, W.W., Ed.; Environmental Science Research; Springer: Boston, MA, USA, 1981; pp. 389–403. ISBN 978-1-4613-3267-1. [Google Scholar]
- Alam, M.; Ikawa, M.; Sasner, J.J.; Sawyer, P.J. Purification of Aphanizomenon flos-aquae Toxin and Its Chemical and Physiological Properties. Toxicon 1973, 11, 65–72. [Google Scholar] [CrossRef]
- Alam, M.; Shimizu, Y.; Ikawa, M.; Sasner, J.J. Reinvestigation of the Toxins from the Blue-green Alga, Aphanizomenon flos-aquae, by a High Performance Chromatographic Method. J. Environ. Sci. Health Part A Environ. Sci. Eng. 1978, 13, 493–499. [Google Scholar] [CrossRef]
- Sasner, J.J., Jr.; Ikawa, M.; Foxall, T.L. Studies on Aphanizomenon and Microcystis Toxins. In Seafood Toxins; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1984; Volume 262, pp. 391–406. ISBN 978-0-8412-0863-6. [Google Scholar]
- Ikawa, M.; Wegener, K.; Foxall, T.L.; Sasner, J.J. Comparison of the Toxins of the Blue-Green Alga Aphanizomenon flos-aquae with the Gonyaulax Toxins. Toxicon 1982, 20, 747–752. [Google Scholar] [CrossRef]
- Sawyer, P.J.; Gentile, J.H.; Sasner, J.J., Jr. Demonstration of a Toxin from Aphanizomenon flos-aquae (L.) Ralfs. Can. J. Microbiol. 1968, 14, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Alam, M. Chemical Studies on Toxins from Gymnodinium breve and Aphanizomenon flos-aquae. Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 1972; pp. 1–95. [Google Scholar]
- Gulavita, N.; Hori, A.; Shimizu, Y.; Laszlo, P.; Clardy, J. Aphanorphine, a Novel Tricyclic Alkaloid from the Blue-Green Alga Aphanizomenon flos-aquae. Tetrahedron Lett. 1988, 29, 4381–4384. [Google Scholar] [CrossRef]
- Li, R.; Carmichael, W.W.; Liu, Y.; Watanabe, M.M. Taxonomic Re-Evaluation of Aphanizomenon flos-aquae NH-5 Based on Morphology and 16S rRNA Gene Sequences. Hydrobiologia 2000, 438, 99–105. [Google Scholar] [CrossRef]
- Rogers, R.S.; Rapoport, H. The pKa’s of Saxitoxin. J. Am. Chem. Soc. 1980, 102, 7335–7339. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Hess, P.; Quilliam, M.A. Hydrophilic Interaction Liquid Chromatography–Mass Spectrometry for the Analysis of Paralytic Shellfish Poisoning (PSP) Toxins. J. Chromatogr. A 2005, 1081, 190–201. [Google Scholar] [CrossRef]
- Laycock, M.; Kralovec, J.; Richards, R. Some In Vitro Chemical Interconversions of Paralytic Shellfish Poisoning (PSP) Toxins Useful in the Preparation of Analytic Standards. J. Mar. Biotechnol. 1994, 3, 121–125. [Google Scholar]
- Wichmann, C.F.; Boyer, G.L.; Divan, C.L.; Schantz, E.J.; Schnoes, H.K. Neurotoxins of Gonyaulax excavata and Bay of Fundy Scallops. Tetrahedron Lett. 1981, 22, 1941–1944. [Google Scholar] [CrossRef]
- Koehn, F.E.; Ghazarossian, V.E.; Schantz, E.J.; Schnoes, H.K.; Strong, F.M. Derivatives of Saxitoxin. Bioorganic Chem. 1981, 10, 412–428. [Google Scholar] [CrossRef]
- Barton, D.H.R.; McCombie, S.W. A New Method for the Deoxygenation of Secondary Alcohols. J. Chem. Soc. Perkin Trans. 1 1975, 1574–1585. [Google Scholar] [CrossRef]
- Norris, K.E.; Kurtz, T.; Wang, S.; Zeng, T.; Leresche, F.; Rosario-Ortiz, F.L. Photochemical Degradation of Saxitoxins in Surface Waters. ACS EST Water 2024, 4, 346–354. [Google Scholar] [CrossRef]
- Jones, G.J.; Negri, A.P. Persistence and Degradation of Cyanobacterial Paralytic Shellfish Poisons (PSPs) in Freshwaters. Water Res. 1997, 31, 525–533. [Google Scholar] [CrossRef]
- Che, Y.; Ding, L.; Qiu, J.; Ji, Y.; Li, A. Conversion and Stability of New Metabolites of Paralytic Shellfish Toxins under Different Temperature and pH Conditions. J. Agric. Food Chem. 2020, 68, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Indrasena, W.M.; Gill, T.A. Storage Stability of Paralytic Shellfish Poisoning Toxins. Food Chem. 2000, 71, 71–77. [Google Scholar] [CrossRef]
- Smith, E.A.; Grant, F.; Ferguson, C.M.J.; Gallacher, S. Biotransformations of Paralytic Shellfish Toxins by Bacteria Isolated from Bivalve Molluscs. Appl. Environ. Microbiol. 2001, 67, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Kayal, N.; Newcombe, G.; Ho, L. Investigating the Fate of Saxitoxins in Biologically Active Water Treatment Plant Filters. Environ. Toxicol. 2008, 23, 751–755. [Google Scholar] [CrossRef]
- Negri, A.P.; Jones, G.J. Bioaccumulation of Paralytic Shellfish Poisoning (PSP) Toxins from the Cyanobacterium Anabaena circinalis by the Freshwater Mussel Alathyria condola. Toxicon 1995, 33, 667–678. [Google Scholar] [CrossRef]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.; Barreto, V.; Ward, C.; Preiser, W.; Poon, G.K.; Neild, G.; Codd, G. Fatal Microcystin Intoxication in Haemodialysis Unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Byth, S. Palm Island Mystery Disease. Med. J. Aust. 1980, 2, 40–42. [Google Scholar] [CrossRef]
- State of Emergency Declared in Toledo Area. Available online: http://www.toledoblade.com/local/2014/08/02/City-of-Toledo-issues-do-no-drink-water-advisery/stories/20140802085 (accessed on 5 October 2019).
- Negri, A.P.; Jones, G.J.; Hindmarsh, M. Sheep Mortality Associated with Paralytic Shellfish Poisons from the Cyanobacterium Anabaena circinalis. Toxicon 1995, 33, 1321–1329. [Google Scholar] [CrossRef]
- Quiblier, C.; Susanna, W.; Isidora, E.-S.; Mark, H.; Aurélie, V.; Jean-François, H. A Review of Current Knowledge on Toxic Benthic Freshwater Cyanobacteria—Ecology, Toxin Production and Risk Management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First Report of Homoanatoxin-a and Associated Dog Neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.-C.; Humbert, J.-F.; Guette, C.; Bernard, C. First Report in a River in France of the Benthic Cyanobacterium Phormidium Favosum Producing Anatoxin-a Associated with Dog Neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef]
- Mez, K.; Beattie, K.; Codd, G.; Hanselmann, K.; Hauser, B.; Naegeli, H.; Preisig, H. Identification of a Microcystin in Benthic Cyanobacteria Linked to Cattle Deaths on Alpine Pastures in Switzerland. Eur. J. Phycol. 1997, 32, 111–117. [Google Scholar] [CrossRef]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of Anatoxin-a Poisoning in Dogs from North America. J. Vet. Diagn. Investig. 2008, 20, 89–92. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Segura, P.A.; Gélinas, M.; Hudon, C.; Thomas, K.; Quilliam, M.A.; Gagnon, C. Detection and Confirmation of Saxitoxin Analogues in Freshwater Benthic Lyngbya wollei Algae Collected in the St. Lawrence River (Canada) by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2012, 1219, 93–103. [Google Scholar] [CrossRef]
- Metz, T.T.; Putnam, S.P.; Scott, G.I.; Ferry, J.L. Shoreline Drying of Microseira (Lyngbya) wollei Biomass Can Lead to the Release and Formation of Toxic Saxitoxin Analogues to the Water Column. Environ. Sci. Technol. 2022, 56, 16866–16872. [Google Scholar] [CrossRef]
- Wazniak, C.; Boyer, G.L.; Hamilton, A.; Hall, S.; McElwee, A.; Ferrier, M.D.; Gaudlip, C.; Hutchinson, B. Detection of Freshwater Saxitoxins in Epipelic Cyanobacteria Mats in Maryland. In Proceedings of the 12th U.S. Symposium On Harmful Algae, Portland, ME, USA, 27 October–1 November 2024. [Google Scholar]
- Chen, Y.; Jiang, Y.; He, Z.; Gao, J.; Li, R.; Yu, G. First Report of PST-Producing Microseira wollei from China Reveals Its Novel Toxin Profile. Harmful Algae 2024, 137, 102655. [Google Scholar] [CrossRef] [PubMed]
- Doucette, G.J.; Kodama, M.; Franca, S.; Gallacher, S. Bacterial Interaction with Harmful Algal Bloom Species: Bloom Ecology, Toxigenesis, and Cytology. In Physiological Ecology of Harmful Algal Blooms; Series G: Ecological Sciences; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; Volume 41, p. 662. [Google Scholar]
- Prol, M.J.; Guisande, C.; Barreiro, A.; Míguez, B.; de la Iglesia, P.; Villar, A.; Gago-Martínez, A.; Combarro, M.P. Evaluation of the Production of Paralytic Shellfish Poisoning Toxins by Extracellular Bacteria Isolated from the Toxic Dinoflagellate Alexandrium minutum. Can. J. Microbiol. 2009, 55, 943–954. [Google Scholar] [CrossRef]
- Kodama, M.; Doucette, G.J.; Green, D.H. Relationships Between Bacteria and Harmful Algae. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg; Germany; New York, NY, USA, 2006; Volume 189. [Google Scholar]
- Michaud, S.; Levasseur, M.; Doucette, G.; Cantin, G. Particle Size Fractionation of Paralytic Shellfish Toxins (PSTs): Seasonal Distribution and Bacterial Production in the St. Lawrence Estuary, Canada. Toxicon 2002, 40, 1451–1462. [Google Scholar] [CrossRef]
- Baker, T.R.; Doucette, G.J.; Powell, C.L.; Boyer, G.L.; Plumley, F.G. GTX4 Imposters: Characterization of Fluorescent Compounds Synthesized by Pseudomonas stutzeri SF/PS and Pseudomonas/Alteromonas PTB-1, Symbionts of Saxitoxin-Producing Alexandrium spp. Toxicon 2003, 41, 339–347. [Google Scholar] [CrossRef]
- Onodera, H.; Watanabe, M.F.; Watanabe, M.M.; Bolch, C.J.S.; Blackburn, S.; Yasumoto, T. Screening of Paralytic Shellfish Toxins in Freshwater Cyanobacteria and Chemical Confirmation of the Toxins in Cultured Anabaena circinalis from Australia. In Proceedings of the Intergovernmental Oceanographic Comission of UNESCO, Paris, France, 24 September 1996; pp. 563–566. [Google Scholar]
- Onodera, H. Chemical Studies on Neurotoxins Produced by Cyanobacteria. Ph.D. Thesis, Tohoku University, Sendai, Japan, 2000; pp. 1–217. [Google Scholar]
- Sant′Anna, C.L.; de Carvalho, L.R.; Fiore, M.F.; Silva-Stenico, M.E.; Lorenzi, A.S.; Rios, F.R.; Konno, K.; Garcia, C.; Lagos, N. Highly Toxic Microcystis aeruginosa Strain, Isolated from São Paulo—Brazil, Produce Hepatotoxins and Paralytic Shellfish Poison Neurotoxins. Neurotox. Res. 2011, 19, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.S.; Fan, T.S. Indirect Enzyme-Linked Immunosorbent Assay for Saxitoxin in Shellfish. J. AOAC Int. 1985, 68, 13–16. [Google Scholar] [CrossRef]
- Cyanotoxins & Marine Toxins ELISA Test Kits Online. Available online: https://www.goldstandarddiagnostics.com/products/environmental-contaminants.html (accessed on 20 November 2015).
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.B. The Structure of Tetrodotoxin. Pure Appl. Chem. 1964, 9, 49–74. [Google Scholar] [CrossRef]
- Humpage, A.R.; Rositano, J.; Bretag, A.H.; Brown, R.; Baker, P.D.; Nicholson, B.C.; Steffensen, D.A. Paralytic Shellfish Poisons from Australian Cyanobacterial Blooms. Aust. J. Mar. Freshw. Res. 1994, 45, 761–771. [Google Scholar] [CrossRef]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-Traditional Vectors for Paralytic Shellfish Poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Zaman, L.; Arakawa, O.; Shimosu, A.; Onoue, Y. Occurrence of Paralytic Shellfish Poison in Bangladeshi Freshwater Puffers. Toxicon 1997, 35, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kungsuwan, A.; Arakawa, O.; Promdet, M.; Onoue, Y. Occurrence of Paralytic Shellfish Poisons in Thai Freshwater Puffers. Toxicon 1997, 35, 1341–1346. [Google Scholar] [CrossRef]
- Ogata, T.; Sato, S.; Kodama, M. Paralytic Shellfish Toxins in Bivalves Which Are Not Associated with Dinoflagellates. Toxicon 1989, 27, 1241–1244. [Google Scholar] [CrossRef]
- Galvão, J.A.; Oetterer, M.; Bittencourt-Oliveira, M.D.C.; Gouvêa-Barros, S.; Hiller, S.; Erler, K.; Luckas, B.; Pinto, E.; Kujbida, P. Saxitoxins Accumulation by Freshwater Tilapia (Oreochromis niloticus) for Human Consumption. Toxicon 2009, 54, 891–894. [Google Scholar] [CrossRef]
- Johnson, A. Blue-Green Algae Toxins in Fish and Sediment from Washington Lakes: Microcystins and Saxitoxin; Washington State Department of Ecology: Olympia, WA, USA, 2010.
- Drobac, D.; Tokodi, N.; Lujić, J.; Marinović, Z.; Subakov-Simić, G.; Dulić, T.; Važić, T.; Nybom, S.; Meriluoto, J.; Codd, G.A. Cyanobacteria and Cyanotoxins in Fishponds and Their Effects on Fish Tissue. Harmful Algae 2016, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.P.; Lind, O. First Evidence of “Paralytic Shellfish Toxins” and Cylindrospermopsin in a Mexican Freshwater System, Lago Catemaco, and Apparent Bioaccumulation of the Toxins in “Tegogolo” Snails (Pomacea patula catemacensis). Toxicon 2010, 55, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Evans, W.R.; Yin, Q.Q.; Bell, P.; Moczydlowski, E. Evidence for Paralytic Shellfish Poisons in the Freshwater Cyanobacterium Lyngbya wollei (Farlow Ex Gomont) Comb. Nov. Appl. Environ. Microbiol. 1997, 63, 3104–3110. [Google Scholar] [CrossRef]
- Ferreira, F.M.B.; Soler, J.M.F.; Fidalgo, M.L.; Fernández-Vila, P. PSP Toxins from Aphanizomenon flos-aquae (Cyanobacteria) Collected in the Crestuma-Lever Reservoir (Douro River, Northern Portugal). Toxicon 2001, 39, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Onodera, H.; Andrinolo, D.; Franca, S.; Araújo, F.; Lagos, N.; Oshima, Y. Paralytic Shellfish Toxins in the Freshwater Cyanobacterium Aphanizomenon flos-aquae, Isolated from Montargil Reservoir, Portugal. Toxicon 2000, 38, 1689–1702. [Google Scholar] [CrossRef]
- Pomati, F.; Sacchi, S.; Rossetti, C.; Giovannardi, S.; Onodera, H.; Oshima, Y.; Neilan, B.A. The Freshwater Cyanobacterium Planktothrix Sp. Fp1: Molecular Identification and Detection of Paralytic Shellfish Poisoning Toxins. J. Phycol. 2000, 36, 553–562. [Google Scholar] [CrossRef]
- Giovannardi, S.; Pollegioni, L.; Pomati, F.; Rossetti, C.; Sacchi, S.; Sessa, L.; Calamari, D. Toxic Cyanobacterial Blooms in Lake Varese (Italy): A Multidisciplinary Approach. Environ. Toxicol. 1999, 14, 127–134. [Google Scholar] [CrossRef]
- Hamill, K.D. Toxicity in Benthic Freshwater Cyanobacteria (Blue-green Algae): First Observations in New Zealand. N. Z. J. Mar. Freshw. Res. 2001, 35, 1057–1059. [Google Scholar] [CrossRef]
- Costa, I.D.; Azevedo, S.; Senna, P.; Bernardo, R.; Costa, S.; Chellappa, N. Occurrence of Toxin-Producing Cyanobacteria Blooms in a Brazilian Semiarid Reservoir. Braz. J. Biol. 2006, 66, 211–219. [Google Scholar] [CrossRef]
- Pereira, P.; Li, R.; Carmichael, W.; Dias, E.; Franca, S. Taxonomy and Production of Paralytic Shellfish Toxins by the Freshwater Cyanobacterium Aphanizomenon gracile LMECYA40. Eur. J. Phycol. 2004, 39, 361–368. [Google Scholar] [CrossRef]
- Molica, R.J.R.; Oliveira, E.J.A.; Carvalho, P.V.V.C.; Costa, A.N.S.F.; Cunha, M.C.C.; Melo, G.L.; Azevedo, S.M.F.O. Occurrence of Saxitoxins and an Anatoxin-a(s)-like Anticholinesterase in a Brazilian Drinking Water Supply. Harmful Algae 2005, 4, 743–753. [Google Scholar] [CrossRef]
- Kouzminov, A.; Ruck, J.; Wood, S.A. New Zealand Risk Management Approach for Toxic Cyanobacteria in Drinking Water. Aust. N. Z. J. Public. Health 2007, 31, 275–281. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Li, D.; Shen, Y.; Liu, Y.; Song, L. Analysis of Paralytic Shellfish Toxins in Aphanizomenon DC-1 from Lake Dianchi, China. Env. Toxicol. 2006, 21, 289–295. [Google Scholar] [CrossRef]
- Dos Anjos, F.M.; do Carmo Bittencourt-Oliveira, M.; Zajac, M.P.; Hiller, S.; Christian, B.; Erler, K.; Luckas, B.; Pinto, E. Detection of Harmful Cyanobacteria and Their Toxins by Both PCR Amplification and LC-MS during a Bloom Event. Toxicon 2006, 48, 239–245. [Google Scholar] [CrossRef]
- Ledreux, A.; Thomazeau, S.; Catherine, A.; Duval, C.; Yéprémian, C.; Marie, A.; Bernard, C. Evidence for Saxitoxins Production by the Cyanobacterium Aphanizomenon gracile in a French Recreational Water Body. Harmful Algae 2010, 10, 88–97. [Google Scholar] [CrossRef]
- Romero, L.; Mondardo, R.; Sens, M.; Grischek, T. Removal of Cyanobacteria and Cyanotoxins during Lake Bank Filtration at Lagoa Do Peri, Brazil. Clean Technol. Environ. Policy 2014, 16, 1133–1143. [Google Scholar] [CrossRef]
- Teneva, I.; Mladenov, R.; Belkinova, D.; Dimitrova-Dyulgerova, I.; Dzhambazov, B. Phytoplankton Community of the Drinking Water Supply Reservoir Borovitsa (South Bulgaria) with an Emphasis on Cyanotoxins and Water Quality. Open Life Sci. 2010, 5, 231–239. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic Shellfish Poisoning Toxin-Producing Cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Zaoutsos, N. Cyanotoxin Occurrence and Potentially Toxin Producing Cyanobacteria in Freshwaters of Greece: A Multi-Disciplinary Approach. Toxicon 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Kleinteich, J.; Wood, S.A.; Puddick, J.; Schleheck, D.; Küpper, F.C.; Dietrich, D. Potent Toxins in Arctic Environments–Presence of Saxitoxins and an Unusual Microcystin Variant in Arctic Freshwater Ecosystems. Chem.-Biol. Interact. 2013, 206, 423–431. [Google Scholar] [CrossRef]
- Rejmánková, E.; Komárek, J.; Dix, M.; Komárková, J.; Girón, N. Cyanobacterial Blooms in Lake Atitlan, Guatemala. Limnologica 2011, 41, 296–302. [Google Scholar] [CrossRef]
- Fonseca, J.R.; Vieira, P.C.S.; Kujbida, P.; da Costa, I.A.S. Cyanobacterial Occurrence and Detection of Microcystins and Saxitoxins in Reservoirs of the Brazilian Semi-Arid Region. Acta Limnol. Bras. 2015, 27, 78–92. [Google Scholar] [CrossRef]
- Belykh, O.I.; Gladkikh, A.S.; Sorokovikova, E.G.; Tikhonova, I.V.; Potapov, S.A.; Butina, T.V. Saxitoxin-Producing Cyanobacteria in Lake Baikal. Contemp. Probl. Ecol. 2015, 8, 186–192. [Google Scholar] [CrossRef]
- Bowling, L.C.; Merrick, C.; Swann, J.; Green, D.; Smith, G.; Neilan, B.A. Effects of Hydrology and River Management on the Distribution, Abundance and Persistence of Cyanobacterial Blooms in the Murray River, Australia. Harmful Algae 2013, 30, 27–36. [Google Scholar] [CrossRef]
- Smith, F.M.J.; Wood, S.A.; van Ginkel, R.; Broady, P.A.; Gaw, S. First Report of Saxitoxin Production by a Species of the Freshwater Benthic Cyanobacterium, Scytonema Agardh. Toxicon 2011, 57, 566–573. [Google Scholar] [CrossRef]
- Borges, H.L.F.; Branco, L.H.Z.; Martins, M.D.; Lima, C.S.; Barbosa, P.T.; Lira, G.A.S.T.; Bittencourt-Oliveira, M.C.; Molica, R.J.R. Cyanotoxin Production and Phylogeny of Benthic Cyanobacterial Strains Isolated from the Northeast of Brazil. Harmful Algae 2015, 43, 46–57. [Google Scholar] [CrossRef]
- Casali, S.P.; Dos Santos, A.C.A.; de Falco, P.B.; Calijuri, M.d.C. Influence of Environmental Variables on Saxitoxin Yields by Cylindrospermopsis raciborskii in a Mesotrophic Subtropical Reservoir. J. Water Health 2017, 15, 509–518. [Google Scholar] [CrossRef]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Voyakina, E.; Babanazarova, O.; Romanov, R.; Kotovshchikov, A.; Mazur-Marzec, H. Dolichospermum and Aphanizomenon as Neurotoxins Producers in Some Russian Freshwaters. Toxicon 2017, 130, 47–55. [Google Scholar] [CrossRef]
- Gkelis, S.; Panou, M.; Chronis, I.; Zervou, S.-K.; Christophoridis, C.; Manolidi, K.; Ntislidou, C.; Triantis, T.M.; Kaloudis, T.; Hiskia, A. Monitoring a Newly Re-Born Patient: Water Quality and Cyanotoxin Occurrence in a Reconstructed Shallow Mediterranean Lake. Adv. Oceanogr. Limnol. 2017, 8, 33–51. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Hiskia, A.; Genitsaris, S.; Katsiapi, M.; Manolidi, K.; Zervou, S.-K.; Christophoridis, C.; Triantis, T.M.; Kaloudis, T.; Orfanidis, S. First Report of Aphanizomenon Favaloroi Occurrence in Europe Associated with Saxitoxins and a Massive Fish Kill in Lake Vistonis, Greece. Mar. Freshw. Res. 2017, 68, 793–800. [Google Scholar] [CrossRef]
- Smith, Z.J.; Conroe, D.E.; Schulz, K.L.; Boyer, G.L. Limnological Differences in a Two-Basin Lake Help to Explain the Occurrence of Anatoxin-a, Paralytic Shellfish Poisoning Toxins, and Microcystins. Toxins 2020, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “Common Suspects” for the Dalmatian Pelican (Pelecanus crispus) Deaths in a Mediterranean Reconstructed Reservoir. Environ. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, R.D.D.S.; Severiano, J.; Oliveira, D.; Mendes, C.; Barbosa, V.; Chia, M.; Barbosa, J. Spatio-Temporal Variation of Cyanobacteria and Cyanotoxins in Public Supply Reservoirs of the Semi-Arid Region of Brazil. J. Limnol. 2019, 79, 13–29. [Google Scholar] [CrossRef]
- Poirier-Larabie, S.; Hudon, C.; Poirier Richard, H.-P.; Gagnon, C. Cyanotoxin Release from the Benthic, Mat-Forming Cyanobacterium Microseira (Lyngbya) wollei in the St. Lawrence River, Canada. Environ. Sci. Pollut. Res. 2020, 27, 30285–30294. [Google Scholar] [CrossRef] [PubMed]
- Grachev, M.; Zubkov, I.; Tikhonova, I.; Ivacheva, M.; Kuzmin, A.; Sukhanova, E.; Sorokovikova, E.; Fedorova, G.; Galkin, A.; Suslova, M.; et al. Extensive Contamination of Water with Saxitoxin Near the Dam of the Irkutsk Hydropower Station Reservoir (East Siberia, Russia). Toxins 2018, 10, 402. [Google Scholar] [CrossRef]
- Podduturi, R.; Schlüter, L.; Liu, T.; Osti, J.A.S.; de Almeida Bispo Moraes, M.; Jørgensen, N.O.G. Monitoring of Saxitoxin Production in Lakes in Denmark by Molecular, Chromatographic and Microscopic Approaches. Harmful Algae 2021, 101, 101966. [Google Scholar] [CrossRef]
- Nauman, C.; Stanislawczyk, K.; Reitz, L.A.; Chaffin, J.D. The Spatiotemporal Distribution of Potential Saxitoxin-Producing Cyanobacteria in Western Lake Erie. J. Great Lakes Res. 2024, 50, 102342. [Google Scholar] [CrossRef]
- Putnam, S.P.; Smith, M.L.; Metz, T.T.; Womer, A.M.; Sellers, E.J.; McClain, S.J.; Crandell, C.A.; Scott, G.I.; Shaw, T.J.; Ferry, J.L. Growth of the Harmful Benthic Cyanobacterium Microseira wollei Is Driven by Legacy Sedimentary Phosphorous. Harmful Algae 2022, 117, 102263. [Google Scholar] [CrossRef]
- Rider, Z.; Percich, A.; Hiripitiyage, Y.; Harris, T.D.; Sturm, B.S.M.; Wilson, A.E.; Pollock, E.D.; Beaver, J.R.; Husic, A. Drivers of Cyanotoxin and Taste-and-Odor Compound Presence within the Benthic Algae of Human-Disturbed Rivers. Water Res. 2024, 253, 121357. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of Climate Variability and Future Climate Change on Harmful Algal Blooms and Human Health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful Algal Blooms: A Climate Change Co-Stressor in Marine and Freshwater Ecosystems. Harmful Algae 2019, 91, 101590. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.R.; Harwood, D.T.; Boundy, M.J.; Holland, P.T.; Hallegraeff, G.; Malhi, N.; Quilliam, M.A. Paralytic Shellfish Toxins–Call for Uniform Reporting Units. Toxicon 2020, 178, 59–60. [Google Scholar] [CrossRef]
- Santos-Silva, R.D.D.; Severiano, J.D.S.; Chia, M.A.; Queiroz, T.M.; Cordeiro-Araújo, M.K.; de Lucena Barbosa, J.E. Unveiling the Link between Raphidiopsis Raciborskii Blooms and Saxitoxin Levels: Evaluating Water Quality in Tropical Reservoirs, Brazil. Environ. Pollut. 2024, 344, 123401. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Descy, J.-P.; Gärtner, G.; Draganova, P.H.; Borisova, C.I.; Pavlova, V.; Mitreva, M. Pilot Application of Drone Observations and Pigment Marker Detection by HPLC in Studies of Cyanobacterial Harmful Algal Blooms in Bulgarian Inland Waters. Mar. Freshw. Res. 2020, 71, 606. [Google Scholar] [CrossRef]
- Jančula, D.; Straková, L.; Sadílek, J.; Maršálek, B.; Babica, P. Survey of Cyanobacterial Toxins in Czech Water Reservoirs—The First Observation of Neurotoxic Saxitoxins. Environ. Sci. Pollut. Res. 2014, 21, 8006–8015. [Google Scholar] [CrossRef] [PubMed]
- Kaas, H.; Henriksen, P. Saxitoxins (PSP Toxins) in Danish Lakes. Water Res. 2000, 34, 2089–2097. [Google Scholar] [CrossRef]
- Rapala, J.; Robertson, A.; Negri, A.P.; Berg, K.A.; Tuomi, P.; Lyra, C.; Erkomaa, K.; Lahti, K.; Hoppu, K.; Lepistö, L. First Report of Saxitoxin in Finnish Lakes and Possible Associated Effects on Human Health. Environ. Toxicol. 2005, 20, 331–340. [Google Scholar] [CrossRef]
- Savela, H.; Spoof, L.; Perälä, N.; Preede, M.; Lamminmäki, U.; Nybom, S.; Häggqvist, K.; Meriluoto, J.; Vehniäinen, M. Detection of Cyanobacterial Sxt Genes and Paralytic Shellfish Toxins in Freshwater Lakes and Brackish Waters on Åland Islands, Finland. Harmful Algae 2015, 46, 1–10. [Google Scholar] [CrossRef]
- Pitois, F.; Fastner, J.; Pagotto, C.; Dechesne, M. Multi-Toxin Occurrences in Ten French Water Resource Reservoirs. Toxins 2018, 10, 283. [Google Scholar] [CrossRef]
- Chorus, I. (Ed.) Cyanotoxins: Occurrence, Causes, Consequences; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–357. ISBN 978-3-642-64004-9. [Google Scholar]
- Christophoridis, C.; Zervou, S.-K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and Diversity of Cyanotoxins in Greek Lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef]
- Wood, S.A.; Holland, P.T.; Stirling, D.J.; Briggs, L.R.; Sprosen, J.; Ruck, J.G.; Wear, R.G. Survey of Cyanotoxins in New Zealand Water Bodies between 2001 and 2004. N. Z. J. Mar. Freshw. Res. 2006, 40, 585–597. [Google Scholar] [CrossRef]
- Ohio Algae Information for Recreational Waters. Available online: https://epa.ohio.gov/habalgae.aspx#147744471-publications-and-helpful-links (accessed on 20 November 2015).
- Savela, H.; Spoof, L.; Höysniemi, N.; Vehniäinen, M.; Mankiewicz-Boczek, J.; Jurczak, T.; Kokociński, M.; Meriluoto, J. First Report of Cyanobacterial Paralytic Shellfish Toxin Biosynthesis Genes and Paralytic Shellfish Toxin Production in Polish Freshwater Lakes. Adv. Ocean. Limnol. 2017, 8. [Google Scholar] [CrossRef]
- Dirks, C.; Cappelli, P.; Blomqvist, M.; Ekroth, S.; Johansson, M.; Persson, M.; Drakare, S.; Pekar, H.; Zuberovic Muratovic, A. Cyanotoxin Occurrence and Diversity in 98 Cyanobacterial Blooms from Swedish Lakes and the Baltic Sea. Mar. Drugs 2024, 22, 199. [Google Scholar] [CrossRef]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in Inland Lakes of the United States: Occurrence and Potential Recreational Health Risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin Mixtures and Taste-and-Odor Compounds in Cyanobacterial Blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Jeon, Y.; Struewing, I.; McIntosh, K.; Tidd, M.; Webb, L.; Ryu, H.; Mash, H.; Lu, J. Spatial and Temporal Variability of Saxitoxin-Producing Cyanobacteria in US Urban Lakes. Toxins 2024, 16, 70. [Google Scholar] [CrossRef]
- Stroski, K.M.; Roelke, D.L.; Kieley, C.M.; Park, R.; Campbell, K.L.; Klobusnik, N.H.; Walker, J.R.; Cagle, S.E.; Labonté, J.M.; Brooks, B.W. What, How, When, and Where: Spatiotemporal Water Quality Hazards of Cyanotoxins in Subtropical Eutrophic Reservoirs. Environ. Sci. Technol. 2024, 58, 1473–1483. [Google Scholar] [CrossRef]
- Bonilla, S.; Haakonsson, S.; Somma, A.; Gravier, A.; Britos, A.; Vidal, L.; De Leon, L.; Brena, B.M.; Pírez, M.; Piccini, C.; et al. Cianobacterias y Cianotoxinas En Ecosistemas Límnicos de Uruguay. Innotec 2015, 10, 9–22. [Google Scholar]
- McFarren, E.F. Report on Collaborative Studies of the Bioassay for Paralytic Shellfish Poison. J. AOAC Int. 1959, 42, 263–271. [Google Scholar] [CrossRef]
- Etheridge, S.M. Paralytic Shellfish Poisoning: Seafood Safety and Human Health Perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef]
- Yakes, B.J.; Kanyuck, K.M.; DeGrasse, S.L. First Report of a Direct Surface Plasmon Resonance Immunosensor for a Small Molecule Seafood Toxin. Anal. Chem. 2014, 86, 9251–9255. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.L.; Doucette, G.J. A Receptor Binding Assay for Paralytic Shellfish Poisoning Toxins: Recent Advances and Applications. Nat. Toxins 1999, 7, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Doucette, G.J.; Logan, M.M.; Ramsdell, J.S.; Van Dolah, F.M. Development and Preliminary Validation of a Microtiter Plate-Based Receptor Binding Assay for Paralytic Shellfish Poisoning Toxins. Toxicon 1997, 35, 625–636. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Fire, S.E.; Leighfield, T.A.; Mikulski, C.M.; Doucette, G.J. Determination of Paralytic Shellfish Toxins in Shellfish by Receptor Binding Assay: Collaborative Study. J. AOAC Int. 2012, 95, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Hartshorne, R.; Catterall, W. The Sodium Channel from Rat Brain. Purification and Subunit Composition. J. Biol. Chem. 1984, 259, 1667–1675. [Google Scholar] [CrossRef]
- Llewellyn, L.E.; Doyle, J.; Negri, A.P. A High-Throughput, Microtiter Plate Assay for Paralytic Shellfish Poisons Using the Saxitoxin-Specific Receptor, Saxiphilin. Anal. Biochem. 1998, 261, 51–56. [Google Scholar] [CrossRef]
- Llewellyn, L.E.; Negri, A.P.; Doyle, J.; Baker, P.D.; Beltran, E.C.; Neilan, B.A. Radioreceptor Assays for Sensitive Detection and Quantitation of Saxitoxin and Its Analogues from Strains of the Freshwater Cyanobacterium, Anabaena circinalis. Environ. Sci. Technol. 2001, 35, 1445–1451. [Google Scholar] [CrossRef]
- Ruberu, S.R.; Liu, Y.-G.; Wong, C.T.; Perera, S.K.; Langlois, G.W.; Doucette, G.J.; Powell, C.L. Receptor Binding Assay for Paralytic Shellfish Poisoning Toxins: Optimization and Interlaboratory Comparison. J. AOAC Int. 2003, 86, 737–745. [Google Scholar] [CrossRef]
- Llewellyn, L.E. The Behavior of Mixtures of Paralytic Shellfish Toxins in Competitive Binding Assays. Chem. Res. Toxicol. 2006, 19, 661–667. [Google Scholar] [CrossRef]
- Mons, M.N.; van Egmond, H.P.; Speijers, G.J.A. Paralytic Shellfish Poisoning: A Review; National Institute of Public Health and Environment (RIVM): Bilthoven, The Netherlands, 1998; pp. 1–47. [Google Scholar]
- Usup, G.; Leaw, C.-P.; Cheah, M.-Y.; Ahmad, A.; Ng, B.-K. Analysis of Paralytic Shellfish Poisoning Toxin Congeners by a Sodium Channel Receptor Binding Assay. Toxicon 2004, 44, 37–43. [Google Scholar] [CrossRef]
- Usleber, E.; Dietrich, R.S.; Buerk, C.; Schneider, E.A.; Märtlbauer, E. Immunoassay Methods for Paralytic Shellfish Poisoning Toxins. J. AOAC Int. 2001, 84, 1649–1656. [Google Scholar] [CrossRef]
- Renz, V.; Terplan, G. Ein Enzymimmunologischer Nachweis von Saxitoxin. Arch. Für Leb. 1988, 3, 30–33. [Google Scholar]
- Hack, R.; Renz, V.; Märtlbauer, E.; Terplan, G. A Monoclonal Antibody to Saxitoxin. Food Agric. Immunol. 1990, 2, 47–48. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Laycock, M.V.; Richards, R.; Usleber, E. Immobilization of Small Molecules on Solid Matrices: A Novel Approach to Enzyme-Linked Immunosorbent Assay Screening for Saxitoxin and Evaluation of Anti-Saxitoxin Antibodies. Toxicon 1996, 34, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Hack, R.; Märtlbauer, E.; Terplan, G. A Monoclonal Antibody to the Trichothecene T-2 Toxin: Screening for the Antibody by a Direct Enzyme Immunoassay. J. Vet. Med. Ser. B 1987, 34, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Di Stefano, S.; Moscone, D.; Palleschi, G.; Marini, S.; Coletta, M.; Draisci, R.; delli Quadri, F. Production of Antibodies and Development of Highly Sensitive Formats of Enzyme Immunoassay for Saxitoxin Analysis. Anal. Bioanal. Chem. 2002, 373, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hsu, K.-H.; Chu, F.S. Direct Competitive Enzyme-Linked Immunosorbent Assay for Saxitoxin and Neosaxitoxin. J. Agric. Food Chem. 1996, 44, 1029–1035. [Google Scholar] [CrossRef]
- Carlson, R.E.; Lever, M.L.; Lee, B.W.; Guire, P.E. Development of Immunoassays for Paralytic Shellfish Poisoning: A Radioimmunoassay for Saxitoxin. In Seafood Toxins; Ragelis, E.P., Ed.; American Chemical Society: Washington, DC, USA, 1984; Volume 262, pp. 181–192. ISBN 978-0-8412-0863-6. [Google Scholar]
- Dietrich, R.; Usleber, E.; Bürk, C.; Märtlbauer, E. Immunochemical Approaches to the Analysis of Paralytic Shellfish Poisoning Toxins. In Immunoassays for Residue Analysis; Beier, R.C., Stanker, L.H., Eds.; American Chemical Society: Washington, DC, USA, 1996; Volume 621, pp. 395–403. ISBN 978-0-8412-3379-9. [Google Scholar]
- Chu, F.S.; Hsu, K.-H.; Huang, X.; Barrett, R.; Allison, C. Screening of Paralytic Shellfish Posioning Toxins in Naturally Occurring Samples with Three Different Direct Competitive Enzyme-Linked Immunosorbent Assays. J. Agric. Food Chem. 1996, 44, 4043–4047. [Google Scholar] [CrossRef]
- Sato, S.; Takata, Y.; Kondo, S.; Kotoda, A.; Hongo, N.; Kodama, M. Quantitative ELISA Kit for Paralytic Shellfish Toxins Coupled with Sample Pretreatment. J. AOAC Int. 2014, 97, 339–344. [Google Scholar] [CrossRef]
- Usleber, E.; Dietrich, R.; Märtlbauer, E.; Terplan, G. Effect of Heterologous Paralytic Shellfish Poisoning Toxin-Enzyme Conjugates on the Cross-Reactivity of a Saxitoxin Enzyme Immunoassay. Lett. Appl. Microbiol. 1994, 18, 337–339. [Google Scholar] [CrossRef]
- Kawatsu, K.; Hamano, Y.; Sugiyama, A.; Hashizume, K.; Noguchi, T. Development and Application of an Enzyme Immunoassay Based on a Monoclonal Antibody against Gonyautoxin Components of Paralytic Shellfish Poisoning Toxins. J. Food Prot. 2002, 65, 1304–1308. [Google Scholar] [CrossRef]
- Oshima, Y. Postcolumn Derivatization Liquid Chromatographic Method for Paralytic Shellfish Toxins. J. AOAC Int. 1995, 78, 528–532. [Google Scholar] [CrossRef]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; van de Riet, J.M.; Burns, B.G.; Thomas, K.M.; Quilliam, M.A. Rapid Postcolumn Methodology for Determination of Paralytic Shellfish Toxins in Shellfish Tissue. J. AOAC Int. 2008, 91, 589–597. [Google Scholar] [CrossRef] [PubMed]
- van de Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; MacNeil, J.D.; Quilliam, M.A. Liquid Chromatography Post-Column Oxidation (PCOX) Method for the Determination of Paralytic Shellfish Toxins in Mussels, Clams, Oysters, and Scallops: Collaborative Study. J. AOAC Int. 2011, 94, 1154–1176. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, M.A.; Janeček, M.; Lawrence, J.F. Characterization of the Oxidation Products of Paralytic Shellfish Poisoning Toxins by Liquid Chromatography/Mass Spectrometry. Rapid. Commun. Mass. Spectrom. 1993, 7, 482–487. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection: Collaborative Study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef]
- Wong, J.L.; Brown, M.S.; Matsumoto, K.; Oesterlin, R.; Rapoport, H. Degradation of Saxitoxin to a Pyrimido [2, 1-b] Purine. J. Am. Chem. Soc. 1971, 93, 4633–4634. [Google Scholar] [CrossRef] [PubMed]
- Bates, H.A.; Rapoport, H. Chemical Assay for Saxitoxin, the Paralytic Shellfish Poison. J. Agric. Food Chem. 1975, 23, 237–239. [Google Scholar] [CrossRef]
- Gago-Martínez, A.; Moscoso, S.A.; Leão Martins, J.M.; Rodriguez Vázquez, J.-A.; Niedzwiadek, B.; Lawrence, J.F. Effect of pH on the Oxidation of Paralytic Shellfish Poisoning Toxins for Analysis by Liquid Chromatography. J. Chromatogr. A 2001, 905, 351–357. [Google Scholar] [CrossRef]
- Sullivan, J.J. High-Performance Liquid Chromatographic Method Applied to Paralytic Shellfish Poisoning Research. In Marine Toxins; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 66–77. ISBN 978-0-8412-1733-1. [Google Scholar]
- House, H.O. Modern Synthetic Reactions, 2nd ed.; Benjamin, W.A., Ed.; Internet Archive: San Francisco, CA, USA, 1972; pp. 1–856. [Google Scholar]
- JOINT FAO/WHO Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs. 2016. Available online: http://www.fao.org/3/a-I5970e.pdf (accessed on 22 September 2019).
- Zakrzewska, S.; Nixon, S.A.; Chen, Z.; Hajare, H.S.; Park, E.R.; Mulcahy, J.V.; Arlinghaus, K.M.; Neu, E.; Konovalov, K.; Provasi, D.; et al. Structural Basis for Saxitoxin Congener Binding and Neutralization by Anuran Saxiphilins. Nat. Commun. 2025, 16, 3885. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)—A Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a Sensitive and Selective Liquid Chromatography–Mass Spectrometry Method for High Throughput Analysis of Paralytic Shellfish Toxins Using Graphitised Carbon Solid Phase Extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Diener, M.; Erler, K.; Christian, B.; Luckas, B. Application of a New Zwitterionic Hydrophilic Interaction Chromatography Column for Determination of Paralytic Shellfish Poisoning Toxins. J. Sep. Sci. 2007, 30, 1821–1826. [Google Scholar] [CrossRef]
- Turrell, E.; Stobo, L.; Lacaze, J.-P.; Piletsky, S.; Piletska, E. Optimization of Hydrophilic Interaction Liquid Chromatography/Mass Spectrometry and Development of Solid-Phase Extraction for the Determination of Paralytic Shellfish Poisoning Toxins. J. AOAC Int. 2008, 91, 1372–1386. [Google Scholar] [CrossRef]
- Cho, Y.; Tsuchiya, S.; Yoshioka, R.; Omura, T.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. Column Switching Combined with Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry for the Analysis of Saxitoxin Analogues, and Their Biosynthetic Intermediates in Dinoflagellates. J. Chromatogr. A 2016, 1474, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Magno, G.S.; Tartaglione, L.; Quilliam, M.A.; Tubaro, A.; Poletti, R. Hydrophilic Interaction Liquid Chromatography/Mass Spectrometry for Determination of Domoic Acid in Adriatic Shellfish. Rapid. Commun. Mass. Spectrom. 2005, 19, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.M.; Beach, D.G.; Reeves, K.L.; Gibbs, R.S.; Kerrin, E.S.; McCarron, P.; Quilliam, M.A. Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry for Quantitation of Paralytic Shellfish Toxins: Validation and Application to Reference Materials. Anal. Bioanal. Chem. 2017, 409, 5675–5687. [Google Scholar] [CrossRef] [PubMed]
- Blay, P.; Hui, J.P.M.; Chang, J.; Melanson, J.E. Screening for Multiple Classes of Marine Biotoxins by Liquid Chromatography–High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 577–585. [Google Scholar] [CrossRef]
- Halme, M.; Rapinoja, M.-L.; Karjalainen, M.; Vanninen, P. Verification and Quantification of Saxitoxin from Algal Samples Using Fast and Validated Hydrophilic Interaction Liquid Chromatography–Tandem Mass Spectrometry Method. J. Chromatogr. B 2012, 880, 50–57. [Google Scholar] [CrossRef]
- Bragg, W.A.; Lemire, S.W.; Coleman, R.M.; Hamelin, E.I.; Johnson, R.C. Detection of Human Exposure to Saxitoxin and Neosaxitoxin in Urine by Online-Solid Phase Extraction-Liquid Chromatography–Tandem Mass Spectrometry. Toxicon 2015, 99, 118–124. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Erdner, D.L.; McClelland, J.W.; Sanderson, M.P.; Anderson, D.M.; Gobler, C.J.; Smith, J.L. Impact of Nitrogen Chemical Form on the Isotope Signature and Toxicity of a Marine Dinoflagellate. Mar. Ecol. Prog. Ser. 2018, 602, 63–76. [Google Scholar] [CrossRef]
- Beach, D.G. Differential Mobility Spectrometry for Improved Selectivity in Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry Analysis of Paralytic Shellfish Toxins. J. Am. Soc. Mass. Spectrom. 2017, 28, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wright, E.J.; Thomas, K.; Li, A.; McCarron, P.; Beach, D.G. Semiquantitation of Paralytic Shellfish Toxins by Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry Using Relative Molar Response Factors. Toxins 2020, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Westerman, D.C.; Putnam, S.P.; Richardson, S.D.; Ferry, J.L. Emerging Lyngbya wollei Toxins: A New High Resolution Mass Spectrometry Method to Elucidate a Potential Environmental Threat. Harmful Algae 2019, 90, 101700. [Google Scholar] [CrossRef]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single-Laboratory Validation of a Multitoxin Ultra-Performance LC-Hydrophilic Interaction LC-MS/MS Method for Quantitation of Paralytic Shellfish Toxins in Bivalve Shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-Performance Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry Method for the Determination of Paralytic Shellfish Toxins and Tetrodotoxin in Mussels, Oysters, Clams, Cockles, and Scallops: Collaborative Study. J. AOAC Int. 2019, 103, 533–562. [Google Scholar] [CrossRef]
- Gallo, P.; Lambiase, S.; Duro, I.; Esposito, M.; Pepe, A. Paralytic Shellfish Poisoning (PSP) Toxins in Bivalve Molluscs from Southern Italy Analysed by Liquid Chromatography Coupled with High-Resolution Mass Spectrometry (UHPLC-HRMS/MS). Toxins 2024, 16, 502. [Google Scholar] [CrossRef]
- Liang, L.; Li, C.; Fang, J.; Li, H.; Wu, S.; Zhao, J.; Li, J.; He, K.; Dong, F. An Integrated Screening Method for Paralytic Shellfish Toxins and Their Analogues Based on Fragmentation Characteristics Using an Orbitrap-Based Ultrahigh-Performance Liquid Chromatography-High-Resolution Mass Spectrometry. Food Chem. 2024, 434, 137502. [Google Scholar] [CrossRef]
- Mattarozzi, M.; Milioli, M.; Bianchi, F.; Cavazza, A.; Pigozzi, S.; Milandri, A.; Careri, M. Optimization of a Rapid QuEChERS Sample Treatment Method for HILIC-MS2 Analysis of Paralytic Shellfish Poisoning (PSP) Toxins in Mussels. Food Control 2016, 60, 138–145. [Google Scholar] [CrossRef]
- Zheng, R.; Huang, L.; Wu, Y.; Lin, S.; Huang, L. Simultaneous Analysis of Paralytic Shellfish Toxins and Tetrodotoxins in Human Serum by Liquid Chromatography Coupled to Q-Exactive High-Resolution Mass Spectrometry. J. Chromatogr. B 2023, 1215, 123565. [Google Scholar] [CrossRef]
- Canada, N.R.C. List of CRM Products | National Research Council Canada. Available online: https://nrc.canada.ca/en/certifications-evaluations-standards/certified-reference-materials/list (accessed on 28 March 2025).
- Cifga Laboratory: Certified Reference Material Producer. Available online: https://cifga.com/ (accessed on 2 June 2025).
- European Food Safety Authority (EFSA). Marine Biotoxins in Shellfish-Saxitoxin Group. EFSA J. 2009, 7, 1–76. [Google Scholar] [CrossRef]
- Turner, A.D.; Lewis, A.M.; Rourke, W.A.; Higman, W.A. Interlaboratory Comparison of Two AOAC Liquid Chromatographic Fluorescence Detection Methods for Paralytic Shellfish Toxin Analysis through Characterization of an Oyster Reference Material. J. AOAC Int. 2014, 97, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Hatfield, R.G.; Rapkova, M.; Higman, W.; Algoet, M.; Suarez-Isla, B.A.; Cordova, M.; Caceres, C.; Riet, J.; Gibbs, R.; et al. Comparison of AOAC 2005.06 LC Official Method with Other Methodologies for the Quantitation of Paralytic Shellfish Poisoning Toxins in UK Shellfish Species. Anal. Bioanal. Chem. 2011, 399, 1257–1270. [Google Scholar] [CrossRef]
- Rodríguez, P.; Alfonso, A.; Botana, A.M.; Vieytes, M.R.; Botana, L.M. Comparative Analysis of Pre- and Post-Column Oxidation Methods for Detection of Paralytic Shellfish Toxins. Toxicon 2010, 56, 448–457. [Google Scholar] [CrossRef]
- CEFAS. Refinement and Validation of the AOAC Method (2005.06) to Improve the Determination of N-Hydroxylated Paralytic Shellfish Poisoning Toxins in King Scallops and Queen Scallops by Liquid Chromatography and Fluorescence Detection; CEFAS: Weymouth, UK, 2011; pp. 1–78. [Google Scholar]
- Gaget, V.; Lau, M.; Sendall, B.; Froscio, S.; Humpage, A.R. Cyanotoxins: Which Detection Technique for an Optimum Risk Assessment? Water Res. 2017, 118, 227–238. [Google Scholar] [CrossRef]
- Schürmann, Q.J.F.; Visser, P.M.; Sollie, S.; Kardinaal, W.E.A.; Faassen, E.J.; Lokmani, R.; van der Oost, R.; Van de Waal, D.B. Risk Assessment of Toxic Cyanobacterial Blooms in Recreational Waters: A Comparative Study of Monitoring Methods. Harmful Algae 2024, 138, 102683. [Google Scholar] [CrossRef]
- Acres, J.; Gray, J. Paralytic Shellfish Poisoning. Can. Med. Assoc. J. 1978, 119, 1195–1197. [Google Scholar] [PubMed]
- Llewellyn, L.E.; Dodd, M.J.; Robertson, A.; Ericson, G.; de Koning, C.; Negri, A.P. Post-Mortem Analysis of Samples from a Human Victim of a Fatal Poisoning Caused by the Xanthid Crab, Zosimus Aeneus. Toxicon 2002, 40, 1463–1469. [Google Scholar] [CrossRef]
- García, C.; del Carmen Bravo, M.; Lagos, M.; Lagos, N. Paralytic Shellfish Poisoning: Post-Mortem Analysis of Tissue and Body Fluid Samples from Human Victims in the Patagonia Fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef]
- Tauxe, R.V.; Kilbourne, E.M.; de Porras, E.; Hall, S.; Velasquez, O.H.; Rodrigue, D.C.; Blake, P.A.; Etzel, R.A. Lethal Paralytic Shellfish Poisoning in Guatemala. Am. J. Trop. Med. Hyg. 1990, 42, 267–271. [Google Scholar] [CrossRef]
- Gessner, B.D.; Middaugh, J.P. Paralytic Shellfish Poisoning in Alaska: A 20-Year Retrospective Analysis. Am. J. Epidemiol. 1995, 141, 766–770. [Google Scholar] [CrossRef]
- Gessner, B.D.; Middaugh, J.P.; Doucette, G.J. Paralytic Shellfish Poisoning in Kodiak, Alaska. West. J. Med. 1997, 167, 351–353. [Google Scholar]
- Long, R.R.; Sargent, J.C.; Hammer, K. Paralytic Shellfish Poisoning: A Case Report and Serial Electrophysiologic Observations. Neurology 1990, 40, 1310–1312. [Google Scholar] [CrossRef]
- de Carvalho, M.; Jacinto, J.; Ramos, N.; de Oliveira, V.; Pinho e Melo, T.; de Sá, J. Paralytic Shellfish Poisoning: Clinical and Electrophysiological Observations. J. Neurol. 1998, 245, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Arnich, N.; Sinno-Tellier, S.; Franchitto, N. Mild Paralytic Shellfish Poisoning (PSP) after Ingestion of Mussels Contaminated below the European Regulatory Limit. Clin. Toxicol. 2021, 59, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Medcof, J.C.; Tennant, A.D. Paralytic Shellfish Poisoning in Eastern Canada; Fisheries Research Board of Canada: Nanaimo, BC, Canada, 1971; pp. 1–87. [Google Scholar]
- Paralytic Shellfish Poisoning—Alaska, 1993–2014; State of Alaska Epidemiology: Anchorage, AK, USA, 2015.
- Paralytic Shellfish Poisoning in Southeast Alaska, May–June 2011; State of Alaska Epidemiology: Anchorage, AK, USA, 2011.
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived Global Increase in Algal Blooms Is Attributable to Intensified Monitoring and Emerging Bloom Impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.; Wolterstorff, C.; MacDonald, R.; Schultz, D. Paralytic Shellfish Poisoning: A Case Series. West. J. Em. Med. 2014, 15, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Sommer, H. The Occurrence of the Paralytic Shell-Fish Poison in the Common Sand Crab. Science 1932, 76, 574–575. [Google Scholar] [CrossRef]
- Sommer, H.; Meyer, K.F. Mussel Poisoning. Cal. West. Med. 1935, 42, 423–426. [Google Scholar]
- Meyer, K.F. Newer Knowledge on Botulism and Mussel Poisoning. Am. J. Public. Health Nations Health 1931, 21, 762–770. [Google Scholar] [CrossRef]
- Gibbard, J.; Naubert, J. Paralytic Shellfish Poisoning on the Canadian Atlantic Coast. Am. J. Public. Health Nations Health 1948, 38, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Sommer, H.; Meyer, K.F. Paralytic Shell-Fish Poisoning. Arch. Pathol. 1937, 24, 560–598. [Google Scholar]
- Schantz, E.J. Chemistry and Biology of Saxitoxin and Related Toxins. Ann. N. Y. Acad. Sci. 1986, 479, 15–23. [Google Scholar] [CrossRef]
- Munday, R.; Thomas, K.; Gibbs, R.; Murphy, C.; Quilliam, M.A. Acute Toxicities of Saxitoxin, Neosaxitoxin, Decarbamoyl Saxitoxin and Gonyautoxins 1&4 and 2&3 to Mice by Various Routes of Administration. Toxicon 2013, 76, 77–83. [Google Scholar] [CrossRef]
- Selwood, A.; Waugh, C.; Harwood, D.; Rhodes, L.; Reeve, J.; Sim, J.; Munday, R. Acute Toxicities of the Saxitoxin Congeners Gonyautoxin 5, Gonyautoxin 6, Decarbamoyl Gonyautoxin 2&3, Decarbamoyl Neosaxitoxin, C-1&2 and C-3&4 to Mice by Various Routes of Administration. Toxins 2017, 9, 73. [Google Scholar] [CrossRef]
- Arnich, N.; Thébault, A. Dose-Response Modelling of Paralytic Shellfish Poisoning (PSP) in Humans. Toxins 2018, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Boente-Juncal, A.; Otero, P.; Rodríguez, I.; Camiña, M.; Rodriguez-Vieytes, M.; Vale, C.; Botana, L.M. Oral Chronic Toxicity of the Safe Tetrodotoxin Dose Proposed by the European Food Safety Authority and Its Additive Effect with Saxitoxin. Toxins 2020, 12, 312. [Google Scholar] [CrossRef]
- Finch, S.C.; Webb, N.G.; Boundy, M.J.; Harwood, D.T.; Munday, J.S.; Sprosen, J.M.; Cave, V.M.; Broadhurst, R.B.; Nicolas, J. Sub-Acute Feeding Study of Saxitoxin to Mice Confirms the Effectiveness of Current Regulatory Limits for Paralytic Shellfish Toxins. Toxins 2021, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Wekell, J.C.; Hurst, J.; Lefebvre, K.A. The Origin of the Regulatory Limits for PSP and ASP Toxins in Shellfish. J. Shellfish. Res. 2004, 23, 927–930. [Google Scholar]
- Food and Agriculture Organization of the United Nations Marine Biotoxins. Available online: https://www.fao.org/4/y5486e/y5486e0r.htm#bm27.1 (accessed on 4 September 2024).
- Kao, C.Y. CHAPTER 4-Paralytic Shellfish Poisoning. In Algal Toxins in Seafood and Drinking Water; Falconer, I.R., Ed.; Academic Press: San Diego, CA, USA, 1993; pp. 75–86. ISBN 978-0-12-247990-8. [Google Scholar]
- Mines, D.; Stahmer, S.; Shepherd, S.M. Poisonings: Food, Fish, Shellfish. Emerg. Med. Clin. N. Am. 1997, 15, 157–177. [Google Scholar] [CrossRef]
- Lan, M.-Y.; Lai, S.-L.; Chen, S.-S.; Hwang, D.-F. Tetrodotoxin Intoxication in a Uraemic Patient. J. Neurol. Neurosurg. Psychiatry 1999, 67, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.T.; Dobmeier, S.G.; Bechtel, L.K.; Holstege, C.P. Food Poisoning. Emerg. Med. Clin. N. Am. 2007, 25, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic Marine Poisoning. Lancet Neurol. 2005, 4, 219–228. [Google Scholar] [CrossRef]
- Finch, S.C.; Boundy, M.J.; Webb, N.G.; Harwood, D.T. The Effect of Experimental Protocol on the Toxicity of Saxitoxin in Mice. Toxins 2023, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, C.P.; Strassburg, A.; Kneip, S.; Barut, A.; Tukey, R.H.; Rodeck, B.; Manns, M.P. Developmental Aspects of Human Hepatic Drug Glucuronidation in Young Children and Adults. Gut 2002, 50, 259–265. [Google Scholar] [CrossRef]
- O’Neill, K.; Musgrave, I.F.; Humpage, A. Low Dose Extended Exposure to Saxitoxin and Its Potential Neurodevelopmental Effects: A Review. Environ. Toxicol. Pharmacol. 2016, 48, 7–16. [Google Scholar] [CrossRef]
- Maucher, J.M.; Ramsdell, J.S. Domoic Acid Transfer to Milk: Evaluation of a Potential Route of Neonatal Exposure. Environ. Health Perspect. 2005, 113, 461–464. [Google Scholar] [CrossRef]
- Vasios, G.; Kosmidi, A.; Kalantzi, O.-I.; Tsantili-Kakoulidou, A.; Kavantzas, N.; Theocharis, S.; Giaginis, C. Simple Physicochemical Properties Related with Lipophilicity, Polarity, Molecular Size and Ionization Status Exert Significant Impact on the Transfer of Drugs and Chemicals into Human Breast Milk. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 1273–1278. [Google Scholar] [CrossRef]
- Ramos, P.B.; Diehl, F.; dos Santos, J.M.; Monserrat, J.M.; Yunes, J.S. Oxidative Stress in Rats Induced by Consumption of Saxitoxin Contaminated Drink Water. Harmful Algae 2014, 37, 68–74. [Google Scholar] [CrossRef]
- Diehl, F.; Ramos, P.B.; dos Santos, J.M.; Barros, D.M.; Yunes, J.S. Behavioral Alterations Induced by Repeated Saxitoxin Exposure in Drinking Water. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 10. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Rude, K.; Weirich, C.A.; Bartlett, S.L.; Seaman, M.; Kozik, C.; Biese, P.; Gosz, T.; Suha, M.; Stempa, C.; et al. Analysis of Cyanobacterial Metabolites in Surface and Raw Drinking Waters Reveals More than Microcystin. Water Res. 2018, 140, 280–290. [Google Scholar] [CrossRef]
- WTOL. “Do Not Drink, Do Not Boil” Water: Crisis Closes out Second Day with Little Information. Available online: http://www.wtol.com/story/26178506/do-not-drink-do-not-boil-water-advisory-issued-for-issued-for-lucas-county-surrounding-area (accessed on 9 October 2016).
- Turner, A.D.; Tarnovius, S.; Hatfield, R.G.; Teixeira-Alves, M.; Broadwater, M.; Dolah, F.V.; Garcia-Mendoza, E.; Medina, D.; Salhi, M.; Goya, A.B.; et al. Application of Six Detection Methods for Analysis of Paralytic Shellfish Toxins in Shellfish from Four Regions within Latin America. Mar. Drugs 2020, 18, 616. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.C.; Webb, N.G.; Boundy, M.J.; Harwood, D.T.; Munday, J.S.; Sprosen, J.M.; Somchit, C.; Broadhurst, R.B. A Sub-Acute Dosing Study of Saxitoxin and Tetrodotoxin Mixtures in Mice Suggests That the Current Paralytic Shellfish Toxin Regulatory Limit Is Fit for Purpose. Toxins 2023, 15, 437. [Google Scholar] [CrossRef] [PubMed]
- Public Health Service, U.S. Food and Drug Administration Office of Food Safety Shellfish and Aquaculture Policy Branch. National Shellfish Sanitation Program (NSSP) Guide for the Control of Molluscan Shellfish: 2023 Revision. Shellfish Laboratory Evaluation Checklist Paralytic Shellfish Poisoning (PSP HPLC-PCOX) 2023. Available online: https://www.issc.org/sites/default/files/uploads/-2023/2023-guide-docs/4-psp-hplc-pcox_final-june-2024.pdf (accessed on 1 April 2025).
- Von Der Leyen, U. COMMISSION IMPLEMENTING REGULATION (EU) 2021/1709 of 23 September 2021 Amending Implementing Regulation (EU) 2019/627 as Regards Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/1709/oj/eng (accessed on 1 April 2025).
- EU Reference Laboratory for Monitoring of Marine Biotoxins Standard Operating Procedures. Available online: https://www.aesan.gob.es/en/CRLMB/web/public_documents/seccion/crlmb_standard_operating_procedures.htm (accessed on 1 April 2025).
- Kao, C.Y.; Kao, P.N.; James-Krace, M.R.; Koehn, F.E.; Wichmann, C.F.; Schnoes, H.K. Actions of Epimers of 12-(OH)-Reduced Saxitoxin and of 11-(OSO3)-Saxitoxin on Squid Axon. Toxicon 1985, 23, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Guidelines for Managing Risks in Recreational Water. Available online: https://guidelines.nhmrc.gov.au/australian-drinking-water-guidelines/part-5/microorganisms/cyanobacteria-and-their-toxins/saxitoxins (accessed on 21 October 2016).
- Brazil Ministry of Health Brazil Saxitoxin Guidelines Ordinance No. 2914. 2011. Available online: https://site.sabesp.com.br/site/uploads/file/asabesp_doctos/portariams291412122011.pdf (accessed on 24 September 2019).
- Drinking-Water Standards for New Zealand 2005 (Revised 2018). Available online: https://www.health.govt.nz/publication/drinking-water-standards-new-zealand-2005-revised-2018 (accessed on 23 September 2019).
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-Based Cyanotoxin Guideline Values Allow for Cyanotoxin-Based Monitoring and Efficient Public Health Response to Cyanobacterial Blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef]
- Oregon Health Authority Public Health Division Center for Health Protection Environmental Public Health Section Advisory Guidelines for Harmful Cyanobacteria Blooms in Recreational Waters. Available online: https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/RECREATION/HARMFULALGAEBLOOMS/Documents/Advisory%20Guidelines%20for%20Harmful%20Cyanobacteria%20Blooms%20in%20Recreational%20Waters.2024_Final.pdf (accessed on 1 May 2025).
- Ohio Microcystin, Anatoxin-a, and Saxitoxins Sampling and Water Quality Guidelines and Public Water Supply Response Strategy. Available online: https://epa.ohio.gov/Portals/28/documents/habs/2019-PWS-HAB-Response-Strategy.pdf (accessed on 17 September 2019).
- DeWine, M.; Husted, J.; Stevenson, L.A.; Mertz, M.; Himes, L. State of Ohio Harmful Algal Bloom Response Strategy For Recreational Waters. 2020. Available online: https://www.scph.org/sites/default/files/editor/EH/5%202020%20HABResponseStrategy.pdf (accessed on 1 May 2025).
- Utah Department of Environmental Quality Recreational Health Advisory Guidance for Harmful Algal Blooms. Available online: https://deq.utah.gov/water-quality/recreational-health-advisory-guidance-for-harmful-algal-blooms (accessed on 19 August 2024).
- Washington State Anatoxin-a and Microcystin Guidelines. Available online: https://www.doh.wa.gov/Portals/1/Documents/4400/334-177-recguide.pdf (accessed on 17 September 2019).
- Washington State Cylindrospermopsin and Saxitoxin Guidelines. Available online: https://www.doh.wa.gov/Portals/1/Documents/4400/332-118-CylindroSax%20Report.pdf (accessed on 6 September 2016).
- Miller, T.; Beversdorf, L.; Weirich, C.; Bartlett, S. Cyanobacterial Toxins of the Laurentian Great Lakes, Their Toxicological Effects, and Numerical Limits in Drinking Water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef]
- US EPA Final Draft for the Drinking Water Criteria Document on Nitrate/Nitrite 1987; United States Environmental Protection Agency: Washington, DC, USA, 1987.
- U.S. National Park Service Toxic Cyanobacteria Bloom in the Virgin River and the Streams of Zion National Park-Zion National Park (U.S. National Park Service). Available online: https://www.nps.gov/zion/planyourvisit/toxic-cyanobacteria-bloom-in-the-virgin-river-and-the-streams-of-zion-national-park.htm (accessed on 4 September 2024).
- Chambers, C.; Grimes, S.; Fire, S.; Reza, M.T. Influence of Biochar on the Removal of Microcystin-LR and Saxitoxin from Aqueous Solutions. Sci. Rep. 2024, 14, 11058. [Google Scholar] [CrossRef]
- Rorar, J.; Garcia, L.D.; Cutright, T. Removal of Saxitoxin and Anatoxin-a by PAC in the Presence and Absence of Microcystin-LR and/or Cyanobacterial Cells. J. Environ. Sci. 2023, 128, 161–170. [Google Scholar] [CrossRef]
- Gawankar, S.; Masten, S.J.; Lahr, R.H. Review of the Occurrence, Treatment Technologies, and Detection Methods for Saxitoxins in Freshwaters. J. Water Health 2024, 22, 1472–1490. [Google Scholar] [CrossRef]
- Miller, T.R.; Xiong, A.; Deeds, J.R.; Stutts, W.L.; Samdal, I.A.; Løvberg, K.E.; Miles, C.O. Microcystin Toxins at Potentially Hazardous Levels in Algal Dietary Supplements Revealed by a Combination of Bioassay, Immunoassay, and Mass Spectrometric Methods. J. Agric. Food Chem. 2020, 68, 8016–8025. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Mazija, L.; Fastner, J.; Dietrich, D.R. Toxin Content and Cytotoxicity of Algal Dietary Supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271. [Google Scholar] [CrossRef]
- Fleming, J.J.; Du Bois, J. A Synthesis of (+)-Saxitoxin. J. Am. Chem. Soc. 2006, 128, 3926–3927. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, J.V.; Walker, J.R.; Merit, J.E.; Whitehead, A.; Du Bois, J. Synthesis of the Paralytic Shellfish Poisons (+)-Gonyautoxin 2, (+)-Gonyautoxin 3, and (+)-11,11-Dihydroxysaxitoxin. J. Am. Chem. Soc. 2016, 138, 5994–6001. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Parsons, W.H.; Du Bois, J. Saxitoxin. Angew. Chem. Int. Ed. 2014, 53, 5760–5784. [Google Scholar] [CrossRef] [PubMed]
- Ghazarossian, V.E. Chemical and Biological Studies of Saxitoxin and Its Derivatives. Ph.D. Thesis, University of Wisconsin Madison, Madison, WI, USA, 1977. [Google Scholar]
- Soeriyadi, A.; Mazmouz, R.; Pickford, R.; Al-Sinawi, B.; Kellmann, R.; Pearson, L.A.; Neilan, B. Heterologous Expression of an Unusual Ketosynthase, SxtA, Leads to Production of Saxitoxin Intermediates in Escherichia Coli. ChemBioChem 2020, 22, 845–849. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Al-Sinawi, B.; Mazmouz, R.; Muenchhoff, J.; Neilan, B.A.; Moffitt, M.C. Identification of Promoter Elements in the Dolichospermum Circinale AWQC131C Saxitoxin Gene Cluster and the Experimental Analysis of Their Use for Heterologous Expression. BMC Microbiol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Kellmann, R.; Neilan, B.A. Process for Making Neosaxitoxin. Patent Number WO 2017/137606 AI; Application Number: PCTEP2017053077, 14 September 2017. [Google Scholar]
- Lukowski, A.L.; Ellinwood, D.C.; Hinze, M.E.; DeLuca, R.J.; Du Bois, J.; Hall, S.; Narayan, A.R.H. C–H Hydroxylation in Paralytic Shellfish Toxin Biosynthesis. J. Am. Chem. Soc. 2018, 140, 11863–11869. [Google Scholar] [CrossRef]
- Kellmann, R.; Neilan, B.A. Biochemical Characterization of Paralytic Shellfish Toxin Biosynthesis in Vitro. J. Phycol. 2007, 43, 497–508. [Google Scholar] [CrossRef]
- Carneiro, R.L.; Dos Santos, M.E.V.; Pacheco, A.B.F.; Azevedo, S.M.F.D.O.E. Effects of Light Intensity and Light Quality on Growth and Circadian Rhythm of Saxitoxins Production in Cylindrospermopsis raciborskii (Cyanobacteria). J. Plankton Res. 2009, 31, 481–488. [Google Scholar] [CrossRef]
- Boundy, M.J.; Harwood, D.T.; Tommasi, E.; Burger, E.; van Ginkel, R.; Waugh, C.; Selwood, A.I.; Finch, S. Acute Toxicity of Decarbamoyl Gonyautoxin 1&4 to Mice by Various Routes of Administration. Toxicon 2021, 204, 56–63. [Google Scholar] [CrossRef] [PubMed]
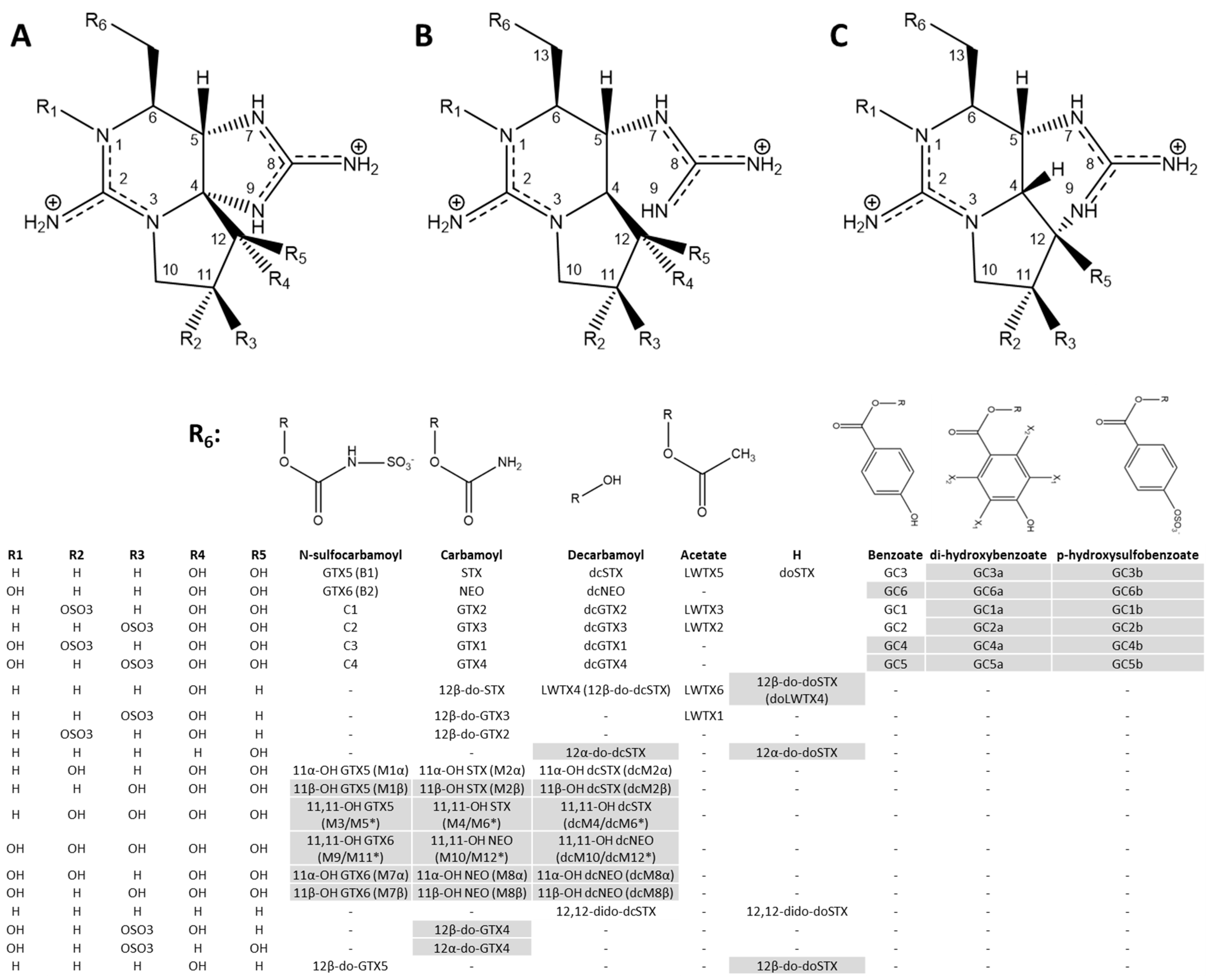
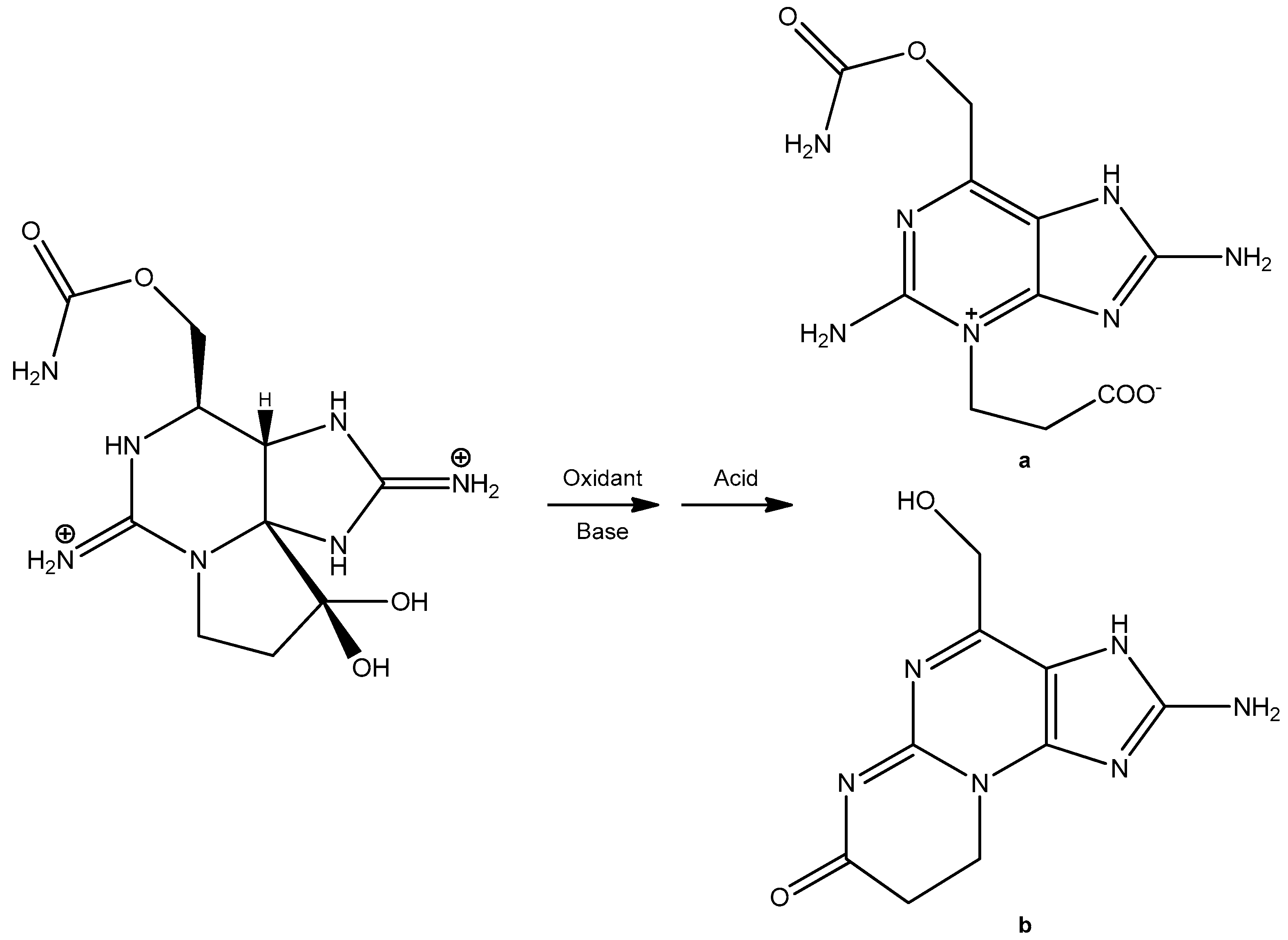
| Toxin | PCOX Relative Response [12] | MBA Relative Toxicity [215] | GSD/A STX-ELISA Cross-Reactivity a [109] | Receptor Binding Assay Relative Binding Affinity [191] † | Receptor Binding Assay Relative Binding Affinity [216] † |
|---|---|---|---|---|---|
| STX | 1 | 1 | 1 | 1 | 1 |
| NEO | 0.41 | 0.5–1.2 | 0.013 | 0.73 | 3 |
| GTX1 | 0.10 | 0.8–1 | <0.02 | 1.04 ** | 0.47 ** |
| GTX2 | 4.66 | 0.4 | 0.23 | 0.34 ** | 0.31 ** |
| GTX3 | 3.52 | 0.6–1.1 | 0.23 | 0.34 ** | 0.31 ** |
| GTX4 | 0.08 | 0.3–0.7 | <0.02 | 1.04 ** | 0.47 ** |
| GTX5 | 0.71 | 0.1–0.2 | 0.23 | 0.033 | 0.005 |
| GTX6 | 0.44 | 0.1 | - | - | 0.017 |
| dcSTX | 1.13 | 0.4–1.02 | 0.29 | 0.10 | 0.53 |
| dcNEO | 0.30 | 0.02–0.4 | 0.06 | - | 0.24 |
| dcGTX1 | - | 0.5 | - | - | - |
| dcGTX2 | 2.71 | 0.2–0.3 | 0.014 | - | 0.075 ** |
| dcGTX3 | 2.46 | 0.2–0.5 | 0.014 | - | 0.075 ** |
| dcGTX4 | - | 0.5 | - | - | - |
| LWTX1 | 0.09 | 0 [15] | 0.13 * | - | - |
| LWTX2 | - | 0.11 [15] | 0.13 * | - | - |
| LWTX3 | - | 0.06 [15] | 0.13 * | - | - |
| LWTX4 | - | 0 [15] | 0.13 * | - | - |
| LWTX5 | - | 0.14 [15] | 0.13 * | - | - |
| LWTX6 | - | 0 [15] | 0.13 * | - | - |
| C1+C2 | 1.20 | 0–0.2 | - | - | 0.007 |
| Toxin | Relative Toxicity by MBA | FAO/WHO Toxicity Equivalency Factor |
|---|---|---|
| STX | 1 | 1 |
| NEO | 0.5–1.2 | 2 |
| GTX1 | 0.8–1 | 1 |
| GTX2 | 0.4 | 0.4 |
| GTX3 | 0.6–1.1 | 0.6 |
| GTX4 | 0.3–0.7 | 0.7 |
| GTX5 | 0.1–0.2 | 0.1 |
| GTX6 | 0.1 | 0.05 |
| dcSTX | 0.4–1.02 | 0.5 |
| dcNEO | 0.02–0.4 | 0.2 |
| dcGTX1 | 0.5 | - |
| dcGTX2 | 0.2–0.3 | 0.2 |
| dcGTX3 | 0.2–0.5 | 0.4 |
| dcGTX4 | 0.5 | - |
| LWTX1 | 0 [15] | - |
| LWTX2 | 0.11 [15] | - |
| LWTX3 | 0.06 [15] | - |
| LWTX4 | 0 [15] | - |
| LWTX5 | 0.14 [15] | - |
| LWTX6 | 0 [15] | - |
| C1+C2 | 0–0.2 | - |
| [STX] | [GTX2,3] | [GTX1,4] | ELISA Result * | True TEF Adjusted Toxin Concentration | ELISA % Error † | |
|---|---|---|---|---|---|---|
| Sample A | 1 | 1 | 0 | 1.23 | 1.50 | 18.00% |
| Sample B | 1.5 | 0.5 | 0 | 1.62 | 1.75 | 7.43% |
| Sample C | 0.5 | 1.5 | 0 | 0.84 | 1.25 | 32.40% |
| Sample D | 10 | 1 | 0 | 10.23 | 10.50 | 2.57% |
| Sample E | 1 | 10 | 0 | 3.30 | 6.00 | 45.00% |
| Sample F | 1 | 1 | 1 | 1.25 | 2.35 | 46.81% |
| Sample G | 1 | 1 | 10 | 1.43 | 10.00 | 85.70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, Z.J.; Arlinghaus, K.M.; Boyer, G.L.; Hapeman, C.J. A Fresh Perspective on Cyanobacterial Paralytic Shellfish Poisoning Toxins: History, Methodology, and Toxicology. Mar. Drugs 2025, 23, 271. https://doi.org/10.3390/md23070271
Smith ZJ, Arlinghaus KM, Boyer GL, Hapeman CJ. A Fresh Perspective on Cyanobacterial Paralytic Shellfish Poisoning Toxins: History, Methodology, and Toxicology. Marine Drugs. 2025; 23(7):271. https://doi.org/10.3390/md23070271
Chicago/Turabian StyleSmith, Zacharias J., Kandis M. Arlinghaus, Gregory L. Boyer, and Cathleen J. Hapeman. 2025. "A Fresh Perspective on Cyanobacterial Paralytic Shellfish Poisoning Toxins: History, Methodology, and Toxicology" Marine Drugs 23, no. 7: 271. https://doi.org/10.3390/md23070271
APA StyleSmith, Z. J., Arlinghaus, K. M., Boyer, G. L., & Hapeman, C. J. (2025). A Fresh Perspective on Cyanobacterial Paralytic Shellfish Poisoning Toxins: History, Methodology, and Toxicology. Marine Drugs, 23(7), 271. https://doi.org/10.3390/md23070271






