Abstract
This study investigates the sequential extraction of lipids and saponins from C. frondosa viscera. Lipids were extracted using supercritical carbon dioxide (scCO2) in the presence of ethanol (EtOH) as a co-solvent. Subsequently, the lipid-extracted viscera underwent three saponin extraction approaches, scCO2-scCO2, scCO2-EtOH, and scCO2-hot water, resulting in saponin-rich extracts. Process parameter investigation for saponin extraction from scCO2-defatted viscera revealed minimal effects of temperature, pressure, extraction time, static extraction, and EtOH concentration on saponin yields, allowing for milder operational conditions (35 °C, 20 MPa, 30 min dynamic extraction, 75% EtOH at 0.5 mL/min) to achieve energy-efficient recovery. Continuous EtOH feeding predominates the scCO2 extraction of saponins. The sequential scCO2 extraction of lipid and saponins yielded saponins at 9.13 mg OAE/g, while scCO2 extraction of lipid followed by a 24 h 70% EtOH extraction of saponins achieved 16.26 mg OAE/g, closely matching the optimized ultrasonic-assisted extraction of saponins (17.31 mg OAE/g) from hexane-defatted samples. Antioxidant activities of saponin-rich extracts obtained in the sequential scCO2-EtOH extraction (17.12 ± 4.20% DPPH scavenging) and the sequential scCO2-scCO2 extraction (16.14 ± 1.98%) were comparable to BHT (20.39 ± 0.68%), surpassing that of hexane-defatted ultrasonic extracts (8.11 ± 1.16%). The optimized scCO2-EtOH method offers a sustainable alternative, eliminating toxic solvents while maintaining high saponin yields and bioactivity.
1. Introduction
Saponins are high-molecular-weight glycosides composed of diverse sugar moieties attached to triterpene or steroid aglycones [1]. While they are predominantly found in higher plants as defence compounds against pathogens and herbivores [2], increasing evidence suggests their presence in lower marine animals, particularly in the marine phylum Echinodermata, including the classes Holothuroidea (sea cucumbers) and Asteroidea (starfishes) [3]. As research into their pharmacological and biological properties develops, saponins have been identified as bioactive ingredients of traditional Oriental medicines and pharmaceutical drugs, exhibiting diverse biological activities such as immunostimulatory properties, as well as anticancer, antimicrobial, antifungal, anti-oxidative, anti-inflammatory, and antiviral activities [1,4]. Additionally, they have been shown to combat obesity, improve cardiovascular health, lower hypertension, and reduce diabetes risk [5,6,7,8,9,10].
Recent studies have identified sea cucumbers as a particularly rich source of triterpene glycosides, which function both as chemical defences against predators and as bioactive compounds with remarkable pharmaceutical potential [4,6,7,10,11,12]. Among their diverse biological activities, sea cucumber-derived saponins have been reported to exhibit antioxidant potential. Studies have shown that saponins isolated from various sea cucumber species possess radical-scavenging and reducing activities, contributing to their therapeutic relevance [13]. To date, approximately 300 triterpene glycosides have been identified across various sea cucumber species. The saponins extracted from C. frondosa include a diverse range of triterpene glycosides, with frondoside A being the most well-documented [14]. Other identified saponins include frondosides B, C, D, E, F, isofrondoside C, frondoside A derivatives, and the dimeric pentasaccharide frondecaside [11,14,15,16]. A recent study identified six previously undetected saponins and two unknown compounds in C. frondosa extracts [17].
While the body wall is commonly studied as the main source of sea cucumber saponins [18,19], the viscera, often discarded during processing, have emerged as a promising and underutilized alternative. Rich in saponins, as well as lipids and polysaccharides, the viscera offer potential for integrated biorefinery applications [20,21]. Their use not only adds value to processing by-products but also supports sustainability goals by reducing waste. The high saponin content and ready availability of viscera make them an economically and environmentally attractive raw material for extraction.
Developing an efficient method for extracting and quantifying saponins from marine by-products is crucial for maximizing their utilization. Extraction efficiency is influenced by factors such as biomass feedstock properties (e.g., moisture content, particle size), solvent type, and extraction conditions (e.g., temperature, solvent-to-feedstock ratio). Biomass pretreatment, including drying, particle size reduction, and defatting with lipophilic solvents like ethyl acetate or hexane, is often required to improve solvent penetration and enhance mass transfer during extraction [22,23]. Conventional saponin extraction on marine biomass typically involves solvents such as methanol (MeOH), ethanol (EtOH), water, or aqueous alcohol under mild heating [24]. The extracts are partitioned with n-butanol, and the pooled fractions are concentrated to obtain crude saponins for analysis [23,25]. However, traditional extraction methods have drawbacks, including prolonged extraction times, high solvent consumption, and limited efficiency. Consequently, researchers are exploring greener and more efficient alternatives, such as ultrasonic-assisted extraction [17], with supercritical carbon dioxide (scCO2) extraction emerging as a promising approach.
Supercritical fluid extraction is an efficient method for valorizing food by-products, utilizing supercritical fluids that combine the permeation ability of gases with the solvency of liquids. ScCO2 is commonly used due to its non-toxic, non-flammable, cost-effective, and eco-friendly properties [21]. Although the scCO2 extraction technique has been extensively investigated for plant-derived saponins, including those from tea camellia seed cake [26], quinoa bran [27], spina Gleditsiae [28], Brazilian ginseng [2], and sisal hemp [29], with optimal conditions falling in ~45–60 °C and ~30–50 MPa using EtOH as co-solvent, its application to marine-derived saponins remains underexplored. The limited research available has primarily focused on the scCO2 extraction of saponins from C. frondosa body wall, reporting a yield of 1.1 g triterpene glycosides per 60 g of freeze-dried samples [30]. Since marine-derived saponins often coexist with lipids and other non-polar compounds [11], the scCO2 extraction technique is regarded as a promising alternative, offering tunable selectivity, mild extraction conditions, and reduced reliance on organic solvents. It also enables efficient sequential extraction of both lipophilic and hydrophilic components.
Sequential extraction techniques have emerged as a practical approach for the sustainable valorization of food by-products through multiple extraction steps. ScCO2 extraction is commonly used as the first step, helping to extract lipids [31]. Once the lipids are removed, the remaining defatted biomass can be processed further with techniques like pressurized liquid extraction [32,33], ultrasound-assisted extraction [33], enzyme-assisted extraction [34], or subcritical water hydrolysis [35] to recover other valuable bioactive compounds, such as phenolics and bio-peptides. This approach not only increases the efficiency of extraction but also supports the sustainable use of food waste by maximizing the recovery of different compounds from the same raw material. However, the sequential valorization of sea cucumber processing by-products has yet to be thoroughly explored in the literature.
Liquid chromatography–mass spectrometry and similar chromatographic techniques are commonly used to analyze sea cucumber saponin fractions, such as frondoside A and cladoloside A, with reported concentrations ranging from 0.01 to 0.15 mg/g in dried samples (0.05 to 0.73 mg/g in dried extracts) [17,36]. While these methods offer accurate analysis, multiple saponin fractions with varying structures and chromatographic behaviours, along with some saponins being present at low concentrations below detection limits, complicate quantification. This requires specialized knowledge, complex sample preparation, and optimized chromatographic conditions. Additionally, the high cost and time-intensive nature of the process limit their application. Spectrophotometric quantification of total saponins is becoming popular for its simplicity and cost-effectiveness. It relies on colorimetric reactions, where saponins turn lavender to purple upon reacting with vanillin–acetic acid–perchloric acid reagents. The resulting chromophore is measured with an ultraviolet–visible (UV-Vis) spectrophotometer, and saponin concentrations are determined through standard calibration [37,38]. However, spectrophotometric saponin quantification faces challenges such as matrix interferences from lipids [39]. Thus, there is a clear gap in understanding the importance of sample pretreatment and matrix correction to improve the accuracy of saponin quantification.
This study explored the subsequent extraction of saponins from seafood processing wastes, C. frondosa viscera, using scCO2, EtOH, and hot water following scCO2 lipid extraction. The process variables influencing the sequential scCO2 extraction of sea cucumber saponins, including temperature, pressure, co-solvent EtOH concentration, and extraction time, were examined using a factorial and screening design for the first time. Additionally, the study assessed whether saponins were co-extracted during scCO2 lipid extraction and evaluated the feasibility of subsequent scCO2 extraction for saponin recovery from lipid-extracted viscera. The impact of interference compounds (e.g., lipids) in the viscera matrix on saponin extraction was further examined. Yields and bioactivities of saponins obtained via various extraction techniques were compared to determine the most efficient and viable extraction method. Through this systematic investigation, the study seeks to advance scalable and sustainable sequential extraction methodologies for marine by-product-derived bioactive compounds.
2. Results and Discussion
2.1. Impacts of Interference Compounds on Saponin Quantification and Extraction
The conventional EtOH extraction of native and defatted C. frondosa viscera samples without further purification resulted in significantly different analytical saponin yields, which is associated with the interference of compounds such as lipids (Table 1). Shang (2016) reported that glucose, glycine, and palmitic acid can react to form pigments exhibiting maximum UV absorbance at 540 nm [39]. Furthermore, a colorimetric sulfo-phospho-vanillin method was developed for rapid quantitative analysis of lipids, examining that similar reagents could cause colour reactions with lipids [40]. Since sugars, amino acids, and fatty acids are commonly co-present with or within saponins, these components may contribute to overestimating the total saponin contents. Shang (2016) observed that the contribution of these constituents varied between 1.82% and 8.57% in ginseng [39]. In the current study, the interference caused by nontarget compounds in saponin yield determination was up to 28.03% (i.e., compared yields resulting from native and hexane-defatted viscera using 100% or 70% EtOH-(38.22–10.19) mg oleanolic acid equivalent (OAE)/g = 28.03 mg OAE/g = 28.03%, (36.34–16.44) mg OAE/g = 19.9 mg OAE/g = 19.9%; Table 1). Recently, Le Bot et al. (2022) developed a colorimetric method for quantifying total triterpenoid and steroidal saponins in plant materials and also incorporated a dichloromethane pretreatment prior to saponin extraction [37].

Table 1.
Comparison of saponin yields (mg OAE/g of samples on dry weight basis, n = 3, mean ± SD) resulting from reflux and EtOH extraction on native and defatted C. frondosa viscera.
Moreover, reflux extraction of native and hexane-defatted C. frondosa viscera, followed by purification, excluded the interference from undesired compounds but showed significant differences in the saponin yields (4.18 vs. 6.71 mg OAE/g; Table 1). The phenomenon suggests lipid extraction improves saponin yields by removing hydrophobic barriers, enhancing solvent penetration, and disrupting cellular structures that trap saponins.
Therefore, defatting and purification are key steps in improving saponin extraction from sea cucumber viscera by reducing lipid interference, enhancing solvent penetration, and boosting yields. Defatting removes non-polar lipids that block the extraction of saponins by polar solvents, while purification helps eliminate the overestimation caused by interfering compounds, ensuring more accurate quantification. For instance, while scCO2 extraction removes most lipids in the first step, some residual lipids may remain in the system. These residual lipids can then be carried over into the second collection vial during subsequent saponin extraction, leading to an inflated estimate of the saponin yields if not properly purified.
2.2. Sequential ScCO2 Extraction of Saponins
To systematically explore the conditions for subsequent saponin extraction using scCO2, a stepwise experimental approach was implemented. This included preliminary single-factor experiments (Table S1) followed by a definitive screening design (Table S2(1–13)) and ultimately replicated single-factor validations (Table S2(A1–A3)), allowing for a comprehensive assessment of critical process variables.
Besides those variables that have been proven to impact the saponin extraction in the conventional procedure (temperature, pressure, extraction time, and EtOH concentration), static extraction time and co-solvent loading parameters were explored under a fixed condition: 55 °C, 35 MPa, 45 min of dynamic extraction, and 75% EtOH used as co-solvent. The one-single-factor experimental results recommended 10 min of static extraction time with an initial addition of 3.75 mL EtOH, followed by continuous feeding of EtOH at 0.5 mL/min.
Subsequently, a definitive screening design (resolution IV design) was implemented to examine the impacts and the ranges of proposed parameters, temperature (Temp, 35–75 °C), pressure (Pres, 20–50 MPa), dynamic extraction time (DET, 30–90 min), static extraction time (SET, 0–30 min), initial EtOH volume (Vol, 0–7.5 mL), and EtOH concentration (Conc, 50–100 vol%) (Table S2(1–13)). Based on the Pareto chart of standardized effects (Figure S1), none of the main effects, interaction terms, or quadratic terms had a statistically meaningful impact on total saponin yields (i.e., all bars on the chart were grey, further confirming that no terms were retained in the model during stepwise regression). The results suggest that within the studied parameter ranges, saponin extraction remains relatively unaffected by changes in temperature, pressure, extraction times, initial EtOH volume, or EtOH concentration.
To further validate the insignificance of the two main scCO2 system criteria and reaffirm the definitive screening design findings, single-factor experiments with varying temperatures (35 vs. 75 °C) and pressures (20 vs. 50 MPa) were conducted (Table S2(A1–A3)), which reinforced that these variables do not critically influence saponin recovery, with p = 0.0849 and 0.6945 > 0.05.
Moreover, saponins were co-extracted during the scCO2 lipid extraction, as these amphiphilic natural products can easily attach to lipids [41], facilitating their simultaneous recovery. The noticeable differences in saponin contents in the lipid-rich extracts were attributed to the uneven extracts that remained within the supercritical fluid system after the initial extraction. During subsequent extraction cycles, these residual compounds were gradually eluted, thereby influencing the overall saponin measurements in the second stage. The current study found (2.90 ± 1.33) and (2.59 ± 1.24) mg of OAE/g of samples on a dry weight basis in the lipid-rich extracts and the rinse (washing solutions from the empty system after lipid extraction), showing a dynamic distribution of saponins during the extraction process.
Noticeably, 70% EtOH extraction significantly enhances the saponin yields compared with 100% EtOH extraction (16.44 vs. 10.19 mg OAE/g; Table 1). The improvement can be attributed to the synergistic effects of water in the extraction process. As amphiphilic molecules with hydrophilic sugar moieties and hydrophobic aglycone structures [42], saponins exhibit higher solubility in aqueous EtOH [43]. Water in 70% EtOH improves the balance between polar and non-polar properties, promoting matrix penetration and facilitating saponin release and diffusion. In contrast, absolute EtOH lacks the polar water component, limiting the ability to dissolve the hydrophilic sugar chains in saponins and disrupt hydrogen bonding, resulting in lower extraction efficiency.
Although EtOH was used as a co-solvent in the scCO2 extraction system, varying its concentrations did not significantly affect saponin yield within the tested range. This outcome suggests that once a certain polarity threshold is reached, further changes in ethanol concentration offer limited benefit to saponin diffusion under supercritical conditions.
In practical terms, 75% EtOH proved more favourable than 50% EtOH as a co-solvent because of improved process performance during depressurization. This is attributed to the physicochemical properties of EtOH–water mixtures. The viscosity of the water/EtOH reaches a maximum at about 50% ethanol [44], and a higher EtOH concentration lowers the surface tension [45]. Additionally, 75% EtOH has a lower viscosity and a reduced surface tension, allowing CO2 to escape more readily during depressurization. As such, 75% EtOH was selected as the recommended co-solvent concentration.
The continuous addition of the EtOH co-solvent is the principal factor modulating scCO2 polarity and governing saponin elution from the marine biological matrix. Bitencourt et al. (2014) also reported higher yields and better surfactant activity of Brazilian ginseng saponins with the aid of continuous co-solvent draining (EtOH or a 70% hydroalcoholic solution) in scCO2 extraction, among which 70% EtOH yielded the majority of saponins, revealing that the synergistic use of EtOH with scCO2 is crucial for both yields and functionality of saponins [2]. Other process parameters appear secondary, offering little yield enhancement within the tested range, as indicated by the definitive screening design results. These findings suggest that the extraction of saponins using scCO2 is highly dependent on surpassing a certain polarity threshold, which is enabled by co-solvents, after which the marginal gains from increasing pressure, temperature, or extraction duration diminish significantly. A study supported this observation by finding that raising pressure from 20.7 to 48.3 MPa, with other conditions fixed at 110 °C and a modifier to solid ratio of 4:1, had no significant effect on the total ginsenoside yield from Panax quinquefolius [46]. However, the minimal effects of temperature changes may be attributed to a lack of a single supercritical operating phase. A higher concentration of co-solvent can significantly influence the critical temperature of the CO2-co-solvent system. For instance, when the EtOH mole fraction reaches 30%, the critical temperature of the CO2-EtOH mixture rises to around 104 °C, compared to 31 °C for pure CO2 [47]. As a result, the proposed conditions may fall outside a true single supercritical phase, reducing the sensitivity of the saponin extraction to temperature changes. Although higher temperatures (>100 °C) raise safety and sustainability concerns, future studies should investigate the phase behaviour of CO2-EtOH-water in supercritical systems and better clarify the roles of temperature and co-solvent interactions in saponin extraction.
The lack of significant differences across the tested ranges indicates that the process is highly tolerant to operational fluctuations, which is a desirable trait for large-scale industrial applications where the consistent performance of the scCO2 extraction technique is critical due to its inherent variations and the challenge of precise control over these factors. From a process engineering standpoint, these findings support the use of simplified, milder extraction conditions, such as 35 °C, 20 MPa, 30 min dynamic extraction, 10 min of static extraction with an initial addition of 3.75 mL EtOH, followed by continuous feeding of 75 vol% EtOH at 0.5 mL/min. While these conditions do not necessarily maximize recovery, they ensure comparable yields while enhancing energy efficiency by operating at lower thermal and pressure settings for a shorter duration. This streamlined approach enhances process scalability and demonstrates strong potential for sustainable industrial implementation.
2.3. Sequential Extraction: ScCO2 Extraction Followed by Conventional Extraction
To explore the alternative to sequential scCO2 extraction with higher saponin yields, scCO2 extraction followed by hot water or 70% EtOH extraction and the effect of extraction time were investigated (Table 2). The extraction time of 24 h was determined as the optimal extraction in both cases since the yields at 24 h extraction achieved peak yields with minimal additional benefit afterward (Figure 1). As such, the existing methods in the literature, such as two hours of hot water extraction and 72 h of ethanol extraction [17], are either insufficient for efficient saponin recovery or unnecessarily time-consuming.

Table 2.
Comparison of saponin extraction yields (mg of OAE/g of samples on dry weight basis, n = 3, mean ± SD) from hot water and EtOH extraction of scCO2-pretreated, lipid-removed C. frondosa viscera over time. Means in the same row with different letters are significantly different at α = 0.05 level by Tukey comparison.
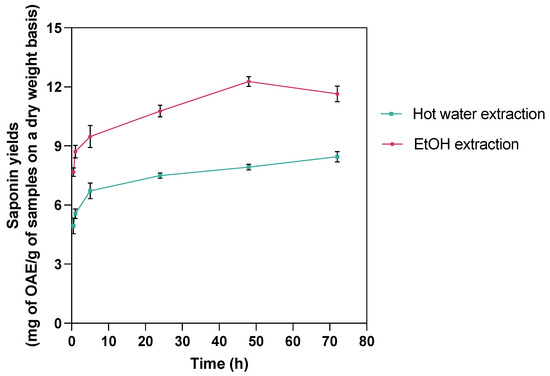
Figure 1.
Time-dependent changes in saponin yields from hot water and 70% EtOH extractions following scCO2 lipid extraction at various time intervals (0.5, 1, 5, 24, 48, 72 h). Results demonstrate variation in extraction efficiency over time, highlighting optimal time for maximum saponin recovery for each extraction method.
Compared with the subsequent scCO2 extraction (Table S2(A1–A3)) that yielded saponins at (6.48 ± 0.36) mg OAE/g, subsequent 24 h hot water extraction (7.50 ± 0.25) mg OAE/g) and EtOH extraction (10.77 ± 0.51) mg OAE/g) produced significantly more saponins (p ≤ 0.05), among which, 70% EtOH recovered the highest total saponin amount (~16.26 mg OAE/g in scCO2-EtOH extraction, comparable to 16.44 mg OAE/g in hexane defatting–70% EtOH extraction and 17.31 mg OAE/g in hexane defatting–ultrasonic-assisted extraction). Therefore, while the scCO2 technique is not the most effective green tool for saponin extraction from C. frondosa viscera, a sequential approach integrating scCO2 with EtOH extraction emerges as a practicable alternative.
2.4. Comparison of Saponin Yields from Different Methods
The comparison of the performance of sequential scCO2 extraction and other sequential extraction methods on saponins derived from C. frondosa viscera is presented in Table 3 and Figure 2. Several saponin extraction methods, especially hexane extraction followed by hot water extraction (22.76% recovery efficiency), exhibited lower recovery efficiencies than the benchmark resulting from hexane defatting–ultrasonic-assisted extraction. In contrast, 70% EtOH extraction on hexane-defatted samples (16.44 ± 0.21 mg OAE/g) and scCO2 extraction followed by 70% EtOH extraction (16.26 ± 2.47 mg OAE/g) exhibited comparable saponin yields compared to hexane defatting–ultrasonic-assisted extraction (17.31 ± 0.60 mg OAE/g, p = 0.992 and 0.976 > 0.05). Additionally, 100% EtOH extraction on hexane-defatted viscera (10.19 ± 0.20 mg OAE/g), hexane extraction followed by reflux extraction (7.17 ± 0.78 mg OAE/g), and sequential scCO2 extraction (9.13 ± 1.30 mg OAE/g) yielded proportionate saponins (p > 0.05). Since the temperature, pressure, EtOH concentration, and extraction time of scCO2 extraction did not affect the sea cucumber saponin extraction yields, the relatively mild conditions (35 °C and 20 MPa or even the critical point of scCO2) can be used for better elution of saponins in a short period (30 min). Therefore, sequential scCO2 extraction outperforms certain conventional extraction methods (i.e., hexane extraction followed by 100% EtOH or reflux extraction) regarding efficiency and sustainability, offering a timesaving, environmentally friendly alternative for saponin isolation. While scCO2 alone yielded modest results (9.13 mg OAE/g), its integration with subsequent solvent extraction steps significantly enhanced performance. ScCO2 extraction followed by 24 h 70% EtOH extraction achieved 16.26 mg OAE/g, nearly matching the hexane defatting–ultrasonic-assisted method (17.31 mg OAE/g). Also, scCO2-coupled 24 h hot water extraction led to 12.99 mg OAE/g, with no statistical significance compared to 70% EtOH-participated ones.

Table 3.
Comparison of C. frondosa viscera saponin yields (mg OAE/g of samples on dry weight basis, mean ± SD, n = 3) resulting from different sequential extraction methods. Means in the same column with different letters are significantly different at α = 0.05 level by Tukey comparison.
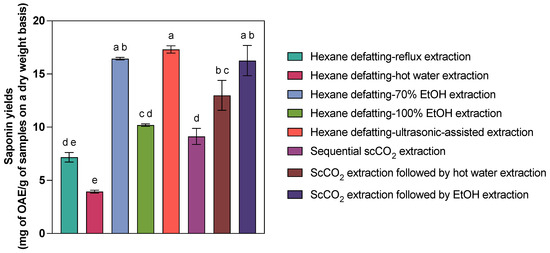
Figure 2.
Comparison of saponin yields (mg OAE/g of samples on a dry weight basis) from C. frondosa viscera via different sequential extraction methods. Bars labelled with different letters indicate statistically significant differences among extraction methods based on Tukey’s HSD test at α = 0.05 level.
Effective saponin extraction and analysis require the elimination of lipid interference, making pretreatment a crucial step in the process. The initial scCO2 extraction step served as a pretreatment by disrupting the structural integrity of the viscera matrix, thereby enhancing mass transfer efficiency during subsequent extraction and promoting saponin release. This mechanistic hypothesis aligns with prior research on scCO2 extraction of lipids from C. frondosa viscera, which documented significant microstructural alterations in the tissue matrix following lipid removal (i.e., pore formation and increased surface area) [21]. These structural modifications likely facilitated solvent penetration and saponin diffusion during downstream extraction. Comparison between conventional hexane defatting–hot water extraction and scCO2 extraction followed by hot water extraction indicates that the latter compensates for the relatively weak solvating power of water, leading to higher saponin yields even after one-hour extraction (5.57 ± 0.41 mg OAE/g, p = 0.0081 ≤ 0.05). While a three-day extraction with 70% ethanol on hexane-defatted samples yielded comparable levels (16.44 mg OAE/g), the scCO2-EtOH sequential extraction approach eliminates the use of toxic hexane, significantly reduces extraction time, and has been previously validated for superior fatty acid recovery [21].
2.5. Antioxidant Activities of Extracts
Given the reported antioxidant properties of sea cucumber saponins, such as their ability to scavenge DPPH radicals [18,19], the DPPH assay was conducted to assess whether different extraction methods influenced not only the yield but also the functional bioactivity of the resulting fractions from C. frondosa viscera (Table 4 and Figure 3). Previous studies have demonstrated that processing methods can significantly impact the antioxidant and physicochemical properties of sea cucumber products. For example, microwave-treated Holothuria scabra powder exhibited higher DPPH radical-scavenging activity compared to smoking and steaming treatments [48]. In this context, assessing antioxidant activity helps provide insight into the preservation or enhancement of bioactive quality through different extraction strategies.
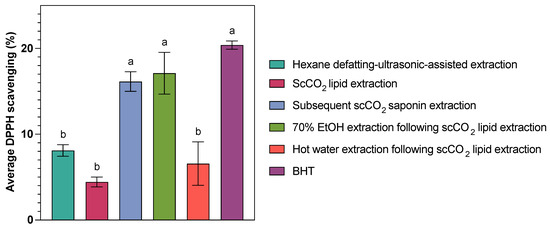
Figure 3.
Comparison of average DPPH scavenging (%) of saponin extracts from C. frondosa viscera obtained from different methods. Bars labelled with different letters indicate statistically significant differences among extraction methods based on Tukey’s HSD test at α = 0.05 level.
The DPPH radical is a commonly used model system for evaluating the free radical-scavenging activity of samples based on their ability to transfer electrons or donate hydrogen. The results showed that the crude extracts have a limited ability to decrease the DPPH concentration, with a 2.41–19.47% radical-scavenging ability at 10 mg/mL under the test conditions, among which the lipids-rich scCO2 extracts (from original samples) and the hot water extracts (from samples defatted by scCO2 technique) showed the lowest antioxidant activity at (4.43 ± 1.00) and (6.57 ± 4.39)%, respectively. In contrast, the subsequent scCO2 extracts (from samples defatted by scCO2 technique) and the EtOH extracts (from samples defatted by scCO2 technique) exhibited comparable high antioxidant activity at (16.14 ± 1.98) and (17.12 ± 4.20)%. The scCO2 extraction and subsequent ethanol extraction following scCO2 lipid removal yielded more than twice the antioxidant activity compared to both hexane defatting–ultrasonic-assisted extraction and hot water extraction after scCO2 lipid removal. The strong antioxidant activity observed in the subsequent EtOH and scCO2 extracts can be attributed to the relatively mild extraction conditions (relatively low temperatures and an inert (oxygen-free) atmosphere of scCO2) and the high efficiency of these solvent systems in extracting saponins and other antioxidant compounds (e.g., carotenoids).
Although ultrasound generates cavitation bubbles that collapse violently, producing localized high temperatures (~5000 K) and pressures (up to 1800 atm) [49], then disrupting cells to release saponins, excessive energy input can damage their structure, such as glycosidic bond cleavage [50], reducing solubility and altering radical-scavenging capacity. A similar phenomenon in Ganoderma lucidum polysaccharides extracted by ultrasound and hot water was reported by Kang et al. (2019) [51].

Table 4.
The antioxidant activity of saponin-containing extracts from C. frondosa viscera obtained through different treatments (n = 3, mean ± SD).
Table 4.
The antioxidant activity of saponin-containing extracts from C. frondosa viscera obtained through different treatments (n = 3, mean ± SD).
| Extract 1 | Hexane Defatting–Ultrasonic-Assisted Extraction | ScCO2 Lipid Extraction | Subsequent scCO2 Saponin Extraction | 70% EtOH Extraction Following scCO2 Lipid Extraction | Hot water Extraction Following scCO2 Lipid Extraction | Butylated Hydroxytoluene (BHT) |
|---|---|---|---|---|---|---|
| Average DPPH scavenging (%) 2 | 8.11 ± 1.16 b | 4.43 ± 1.00 b | 16.14 ± 1.98 a | 17.12 ± 4.20 a | 6.57 ± 4.39 b | 20.39 ± 0.68 a (tested)/23.13 (theoretical 3) |
| Antioxidant power (%) 4 | 39.75 | 21.74 | 79.14 | 83.92 | 32.23 | \ |
1 The concentration of the extracts is 10 mg/mL (final conc.: 1.11 mg/mL), and the concentration of BHT is 100 μM (final conc.: 11.11 μM). The examined extracts were obtained through ultrasonic-assisted extraction from samples that were defatted using hexane, scCO2 extraction of native samples, subsequent scCO2, 24 h 70% EtOH, and hot water extraction, respectively, of samples that have undergone defatting via scCO2 extraction. 2 Average DPPH scavenging (%) values that do not share a letter are significantly different at α = 0.05 level by Tukey comparison. 3 The theoretical calculation follows the finding that BHT can scavenge 1.85 DPPH radicals per molecule [52]. 4 The antioxidant power is expressed as the fraction of the average DPPH scavenging % between extracts and BHT.
Furthermore, the results suggest that the initial stage of scCO2 extraction primarily removes a higher proportion of lipids enriched in polyunsaturated fatty acids (PUFAs), resulting in a lower relative concentration of potent antioxidants and consequently reducing the overall scavenging capacity. PUFAs are highly susceptible to lipid peroxidation, a chain reaction triggered by free radicals. In the presence of DPPH, PUFAs may undergo oxidation, consuming free radicals while generating secondary oxidation products (e.g., lipid hydroperoxides) that do not effectively quench DPPH [53]. The DPPH scavenging activity of the extracts from defatted samples further corroborated this finding, suggesting that lipid removal enhanced the antioxidant capacity of saponin-containing extracts.
Compared with the reference standard, BHT, the extracts from subsequent scCO2 extraction and EtOH extraction following scCO2 lipid extraction showed comparable antioxidant ability (p ≤ 0.05). This suggests that these natural extracts, when prepared at a concentration of 10 mg/mL, could serve as effective alternatives to BHT at a concentration of 100 μM.
2.6. Green and Efficient Valorization via Sequential Extraction
Beyond extraction efficiency and antioxidant activity, it is also important to consider the broader environmental implications, particularly in the context of green chemistry and future industrial scalability. Among all evaluated methods, scCO2 extraction followed by 24 h 70% EtOH extraction represents a favourable process with respect to extraction efficiency, environmental performance, and adherence to green chemistry principles. This sequential approach achieved high saponin yields and superior fatty acid recovery while relying only on low-toxicity solvents. In contrast to conventional solvent systems employing hazardous volatile organics like hexane, which contribute to photochemical smog and require strict hazardous waste management [54], this method significantly reduces worker exposure risks and minimizes the potential for environmental contamination.
When operated in a closed-loop system, scCO2 extraction virtually eliminates solvent emissions during operation. Furthermore, CO2 used in this method is recyclable and can be sourced from industrial by-products, contributing to sustainability. The process is also compatible with renewable energy for heating and pressurization, supporting broader environmental goals. As such, although the scCO2 technique involves higher energy inputs and therefore higher global warming potential, this trade-off is offset by the elimination of solvent hazards and improved recovery of high-value bioactive compounds. A life cycle assessment indicated that energy use for heating and solvent production is the primary contributor to environmental impacts in lab-scale extractions. However, these impacts can be substantially mitigated through process scale-up, the use of energy-efficient equipment, and the integration of renewable energy sources. For example, transitioning from small-scale extraction to a 30-litre system powered by a biomass boiler substantially lowered global warming potential and overall environmental burden [55].
Additionally, first-stage scCO2 extraction significantly contributes to the effectiveness of the sequential protocol by enabling the selective recovery of lipid fractions while enhancing the accessibility of saponin-rich components. Under optimized conditions, scCO2 extraction yielded high levels of fatty acids, particularly omega-3 fatty acids (yielding 16.30 ± 0.66 g of total fatty acids and 3.38 ± 0.20 g of EPA and DHA per 100 g of dried samples), and surpassed conventional organic solvent methods in both efficiency and selectivity [21]. As both fatty acids and saponins are key targets, removing lipids in the first step reduces interference and allows for more efficient and selective saponin extraction in the subsequent ethanol stage.
Considering its superior extraction performance, favourable antioxidant activity of the recovered fractions, and alignment with green chemistry principles, including safer solvent use, recyclability, and waste minimization, the sequential scCO2-EtOH extraction method represents a sustainable and scalable strategy for the valorization of sea cucumber processing waste.
3. Materials and Methods
3.1. Chemicals and Materials
Hot air-dried C. frondosa viscera (moisture content is around 6.45%) were provided by AKSO Marine Biotech Inc. (Hacketts Cove, NS, Canada). The viscera samples were ground into powder with sizes smaller than 18-mesh. The ground samples were packed in zipper bags and stored in the freezer for future use. All the chemicals and reagents with at least ACS reagent grade were purchased from Sigma-Aldrich (Oakville, ON, Canada), Thermo Fisher Scientific (Ottawa, ON, Canada), and VWR (Mississauga, ON, Canada).
3.2. Sequential ScCO2 Extraction of Lipids and Saponins
Hot air-dried, ground C. frondosa viscera (~1.5 g, Mettler Toledo AT261 Analytical Balance, Columbus, OH, USA) were subject to scCO2 extraction (SFE-110, Supercritical Fluid Technologies Inc., Newark, DE, USA) under the optimal conditions (75 °C and 45 MPa, with a 20 min static and a 30 min dynamic extraction, and an initial loaded co-solvent at 2:1 of 95% EtOH to feedstock mass ratio) for lipid extraction as previously described [21], obtaining the lipid-rich extracts (the 1st extracts). Defatted residual samples that remained in the scCO2 vessel were subjected to further extraction for saponin recovery. Without removing the residues, a certain amount of EtOH (concentration at 50–100% v/v, 0–7.5 mL) was pumped into the extraction vessel. Once the temperature (35–75 °C) and pressure (20–50 MPa) reached the set point, the static stage (0–30 min) was recorded. After the pre-determined static soaking, the static/dynamic valve was opened, and the restrictor valve was slightly adjusted to ensure scCO2 was continuously drained through the vessel at approximately 10 mL/min. The co-solvent pump introduced the EtOH into the system at 0.5 mL/min during the entire dynamic extraction time (30–90 min). The continuous feeding of EtOH co-solvent was determined in the preliminary single-factor experiments. After completing the two-step extraction, the system was vented until the pressure inside the vessel dropped to atmospheric pressure and the temperature reached room temperature. The vessel was opened to remove the residues and then reconnected to the system for washing. Then, 95% EtOH was purged using the co-solvent pump to rinse the line and the vessel to collect the remaining extract. The extract obtained from system washing was combined with the extracts obtained in the subsequent extraction, regarding the 2nd extracts (Figure 4). All the extracts were dried under reduced pressure using a rotary evaporator and stored in the fridge for further purification. The single-factor experiments were performed in duplicate, while the definitive screening experiments were generated by Minitab 21.1.0 (Minitab Inc., State College, PA, USA).
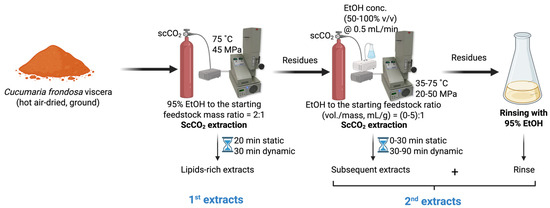
Figure 4.
The scheme of sequential scCO2 extraction of C. frondosa viscera.
3.3. Conventional Extraction of Saponins
To evaluate the scCO2-assisted sequential extraction, documented conventional extraction methods (Figure 5) were performed. Pre-weighed ground, hot air-dried C. frondosa viscera was defatted by soaking in hexane three times (~30 min), with the final soak left overnight. The conventionally defatted samples after vacuum oven drying (105 °C overnight, Across International, Livingston, NJ, USA) were subjected to the following traditional methods for saponin extraction.
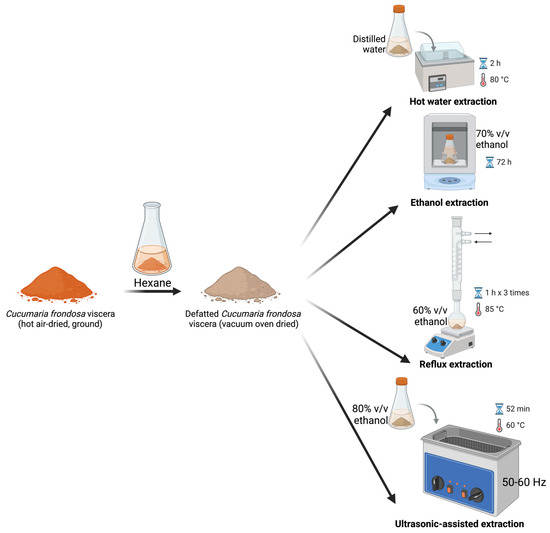
Figure 5.
Conventional extraction of saponins from hexane-defatted C. frondosa viscera.
3.3.1. EtOH Extraction
The extraction method was adapted from Fagbohun et al. (2024) [17]. Native and defatted C. frondosa viscera (~5 g) were combined with 100 mL of absolute or 70% EtOH, respectively, and placed in a shaker (Benchtop Shaking Incubator, Corning, NY, USA) at 200 rpm for 72 h. The different extraction solvent concentrations were set to examine their affinity to saponins. Each extraction was performed in three replicate samples. To explore the effects of lipid interference, the resulting extracts were not subject to purification.
3.3.2. Reflux Extraction
A reflux extraction of saponins using the 60% EtOH method developed by Gao et al. (2014) [25] was adopted. Native and defatted C. frondosa viscera (~1.5 g) were subjected to 20 mL of 60% EtOH in a reflux setup at 85 °C for one hour. The extraction was repeated three times. The combined extracts were concentrated using a rotary evaporator under reduced pressure. Each extraction was performed in three replicate samples. The dried extracts were further purified before analysis.
3.3.3. Hot Water Extraction
Defatted C. frondosa viscera (~5 g) were soaked in 100 mL of distilled water and placed in a water bath (Precision 280 series water bath, Thermo Fisher Scientific, Waltham, MA, USA) at 80 °C for two hours [17]. The extraction was performed in three replicate samples.
3.3.4. Ultrasonic-Assisted Extraction
Defatted C. frondosa viscera (~5 g) were soaked in 100 mL of 80% EtOH and placed in an ultrasonic water bath (50–60 Hz, VWR Aquasonic 750D Ultrasonic Cleaner, VWR, Radnor, PA, USA), with a temperature set at 60 °C for 52 min [17]. The extraction was performed in three replicate samples.
3.4. ScCO2 Extraction Followed by Conventional Extraction
Hot air-dried, ground C. frondosa viscera (~1.5 g) were subject to scCO2 extraction under the optimal lipid extraction conditions: 75 °C and 45 MPa, with a 20 min static and a 30 min dynamic extraction, and co-solvent (95% EtOH) to feedstock mass ratio of 2:1 [21]. The residues were removed from the scCO2 system and placed in centrifuge tubes filled with 50 mL of distilled water for hot water extraction or 70% EtOH for EtOH extraction. The operating procedure was the same as described above. To determine the extraction yield plateau and shorter durations with the maximum yields, the saponin contents in the extracts were measured in half an hour, one hour, five hours, 24 h, 48 h, and 72 h (Figure 6). Each extraction was performed in three replicate samples.
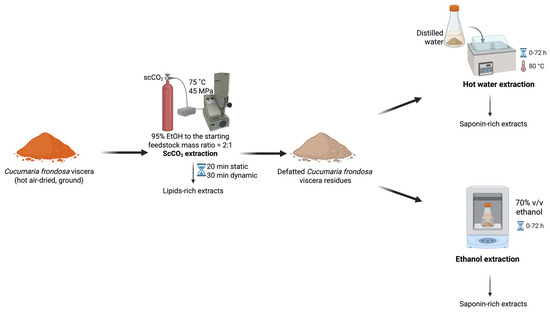
Figure 6.
ScCO2 extraction followed by hot water extraction or EtOH extraction on C. frondosa viscera.
3.5. Purification
The purification procedure was adapted from Gao et al. (2014) [25]. The dried extracts were suspended in 25 mL of distilled water and partitioned against 20 mL of n-hexane thrice. The biphasic mixtures were centrifuged (Eppendorf Centrifuge 5804, Eppendorf, Hamburg, Germany) at 10,000 rpm for 10 min, followed by removing the upper n-hexane layer using Pasteur pipettes. The aqueous layer was partitioned using 15 mL of water pre-saturated n-butanol three times. Then, the combined n-butanol fraction was partitioned against 30 mL of n-butanol pre-saturated water. The collected n-butanol fraction was dried under reduced pressure for colorimetric analysis in a UV-Vis spectrophotometer (DR6000 Benchtop Spectrophotometer, Hach, Loveland, CO, USA).
3.6. Saponin Yield Determination
3.6.1. Standard Solution and Stock Solution Preparation
To prepare the standard oleanolic acid solution, 9.96 mg oleanolic acid was weighed and dissolved in MeOH, followed by transferring to a 50 mL volumetric flask. The 5% vanillin–acetic acid solution was prepared by dissolving 0.50 g vanillin in glacial acetic acid and then adjusting the volume in a 10 mL volumetric flask.
3.6.2. Standard Curve Preparation
The standard oleanolic solution was drawn at 0 (blank), 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 mL in 12 mL test tubes with lids. The solutions were concentrated under the nitrogen stream and then reconstituted using 0.2 mL of 5% vanillin–acetic acid solution and 0.8 mL of perchloric acid. The well-mixed solutions were placed on a reactor block at 60 °C for 15 min and then cooled in the ice water bath. Before colorimetric analysis, 5 mL of acetic acid was added, and the mixtures were set at room temperature for 10 min. The solutions were subjected to a UV-Vis spectrophotometer at 400–650 nm to find the wavelength demonstrating maximum absorbance for oleanolic acid. The wavelength with the maximum absorbance was found at 546 nm. Each tube was measured at 546 nm six times to obtain the average absorbance. A standard curve (Figure S2) was plotted using the amount of OAE as the X-axis and the absorbance as the Y-axis.
3.6.3. Total Saponin Content Determination
The dried fractions (from extraction or purification) were dissolved in 25 mL of 60% MeOH. Then 0.25 mL of the extract-rich solution was pipetted into the 12 mL test tubes with lids. The solutions were concentrated under the nitrogen stream and then reconstituted using 0.2 mL of 5% vanillin–acetic acid solution and 0.8 mL of perchloric acid. The well-mixed solutions were placed on a reactor block at 60 °C for 15 min and then cooled in the ice water bath. Prior to colorimetric analysis at 546 nm, 5 mL of acetic acid was added, and the mixtures were set at room temperature for 10 min. Each tube was measured six times, and the OAE in the samples (OAE mg/g of samples on a dry weight basis) was calculated based on the standard curve (Y = 10.874X − 0.0426).
3.7. Antioxidant Activity Test
The DPPH free radical-scavenging activity of sea cucumber saponin extracts was assayed using the DPPH method according to the modified procedure from the previous work [56]. The 0.10 mM DPPH solution was prepared by dissolving 1.97 mg of DPPH in 70% MeOH in a 50 mL amber volumetric flask. To determine the DPPH radicals scavenged, 0.25 mL of the extracts in 70% MeOH (10 mg/mL) was mixed with 2 mL of 0.10 mM DPPH solution in a 12 mL test tube. A control was prepared by mixing 0.25 mL 70% MeOH with 2 mL of DPPH solution. A blank with 2.25 mL of 70% MeOH was set to zero the absorbance. Samples were vortexed for 15 s and held at room temperature in the dark (wrapped with the foil) for 30 min. After incubation, the samples and the blank were measured for their absorbance six times at 517 nm using a UV-Vis spectrophotometer. Then 100 µM of BHT was used as reference for comparison. The percentage of DPPH radical-scavenging (% inhibition) was calculated:
3.8. Statistical Analysis
The results are presented as mean ± standard deviation (mean ± SD). Statistical analyses were performed using T-tests or one-way ANOVA followed by Tukey comparison, with differences considered significant at p ≤ 0.05.
To evaluate the impacts of the proposed process variables on the scCO2 extraction of sea cucumber saponins, preliminary single-factor experiments were conducted to establish a fundamental understanding. A definitive screening design, a three-level experiment design, was then implemented to investigate six potential variables simultaneously: temperature, pressure, static extraction time, dynamic extraction time, co-solvent amount in the static stage, and co-solvent concentration. Stepwise analysis (α = 0.05 or 0.15 for both entry and removal) was applied to identify significant linear, interaction, or quadratic terms. If any terms met the criteria for model entry, a response surface design would be conducted around those variables to determine optimized extraction conditions. However, as no terms were retained in the model, follow-up single-factor experiments were performed to confirm the observed trends and assess reproducibility under selected conditions.
4. Conclusions
This study successfully demonstrated that the sequential use of scCO2 extraction followed by hot water extraction or EtOH extraction offers an effective and green method for extracting saponins from C. frondosa viscera residues. The sequential scCO2-EtOH extraction method yielded 16.26 mg OAE/g, comparable to the benchmark conventional hexane defatted–ultrasonic-assisted extraction (17.31 mg OAE/g) but without intensive energy inputs and toxic organic solvent use. This streamlined process enhances operational efficiency and sustainability, making it suitable for industrial applications.
Regarding sequential scCO2 extraction, the suggested scCO2 conditions in the second step (35 °C, 20 MPa, 30 min dynamic extraction with continuous feeding of 75% EtOH at 0.5 mL/min) achieved a relatively average recovery (9.13 mg OAE/g) while maintaining higher energy efficiency, mild operating conditions, and less solvent intensity. The results indicated that varying process variables of the scCO2 technique did not help the recovery of saponins from C. frondosa viscera residues, and a mild supercritical condition with continued co-solvent feeding can flush out a moderate amount of saponins. The absence of significant differences across the tested ranges demonstrates the robustness of the process to minor fluctuations. Given the non-negligible variations inherent to supercritical systems, it is a highly desirable attribute for large-scale industrial applications where maintaining consistent scCO2 extraction performance is essential.
Defatting and purification steps were found to be crucial in improving saponin recovery, effectively reducing lipid interference and enhancing solvent penetration. The first scCO2 extraction for lipid removal, acting like a pretreatment for subsequent saponin extraction, improved the performance of the subsequent extraction by disrupting the structural integrity of the biological matrix, promoting better mass transfer and saponin elution. The antioxidant activities of scCO2-EtOH extracts (17.12% DPPH scavenging) and sequential scCO2 extracts (16.14%) were comparable to the synthetic antioxidant BHT, further supporting the bioactivity potential of these extracts.
Overall, this study, along with the previous study [21], highlights the potential of combining optimized scCO2 and EtOH extraction as a viable method for the sequential recovery of fatty acids and saponins from sea cucumber by-products. This sequential extraction approach offers a scalable, solvent-conscious, and resource-efficient solution for the valorization of marine processing residues.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md23070272/s1. Table S1: The preliminary one-single-factor experiments for subsequent scCO2 extraction of saponins from C. frondosa viscera; Table S2: The definitive screening design and additional single-factor experiments for subsequent scCO2 extraction of saponins from C. frondosa viscera; Figure S1: Pareto chart of the standardized effects for total saponin yields; Figure S2: The standard curve of the amount of OAE as the X-axis and the absorbance as the Y-axis.
Author Contributions
Conceptualization, A.K.-p., J.L. and G.J.; methodology, J.L.; software, J.L.; validation, J.L.; formal analysis, J.L.; investigation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, A.K.-p., G.J. and J.L.; visualization, J.L.; supervision, A.K.-p.; funding acquisition, A.K.-p. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mitacs Accelerate Canada, grant number IT16732.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Acknowledgments
J.L. is thankful for the Nova Scotia Graduate Scholarship.
Conflicts of Interest
Author Guangling Jiao was employed by the company AKSO Marine Biotech Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, Y.; Hao, R.; Chen, J.; Li, S.; Huang, K.; Cao, H.; Farag, M.A.; Battino, M.; Daglia, M.; Capanoglu, E.; et al. Health Benefits of Saponins and Its Mechanisms: Perspectives from Absorption, Metabolism, and Interaction with Gut. Crit. Rev. Food Sci. Nutr. 2024, 64, 9311–9332. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Queiroga, C.L.; Junior, Í.M.; Cabral, F.A. Fractionated Extraction of Saponins from Brazilian Ginseng by Sequential Process Using Supercritical CO2, Ethanol and Water. J. Supercrit. Fluids 2014, 92, 272–281. [Google Scholar] [CrossRef]
- Thimmappa, R.; Wang, S.; Zheng, M.; Misra, R.C.; Huang, A.C.; Saalbach, G.; Chang, Y.; Zhou, Z.; Hinman, V.; Bao, Z.; et al. Biosynthesis of Saponin Defensive Compounds in Sea Cucumbers. Nat. Chem. Biol. 2022, 18, 774–781. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Joseph, J.S.; Oriyomi, O.V.; Rupasinghe, H.P.V. Saponins of North Atlantic Sea Cucumber: Chemistry, Health Benefits, and Future Prospectives. Mar. Drugs 2023, 21, 262. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Dong, P.; Li, Z.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea Cucumber Saponin Liposomes Ameliorate Obesity-Induced Inflammation and Insulin Resistance in High-Fat-Diet-Fed Mice. Food Funct. 2018, 9, 861–870. [Google Scholar] [CrossRef]
- Guo, Y.; Han, X.; Che, H.; Li, Z.; Dong, P.; Xue, C.; Zhang, T.; Wang, Y. Synergistic Effect of Eicosapentaenoic Acid-Enriched Phospholipids and Sea Cucumber Saponin on Orotic Acid-Induced Non-Alcoholic Fatty Liver Disease in Rats. R. Soc. Open Sci. 2018, 5, 172182. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, L.; Ding, L.; Shi, H.; Xue, C.; Zhang, T.; Wang, Y. Synergistic Effect of Sea Cucumber Saponins and EPA-Enriched Phospholipids on Insulin Resistance in High-Fat Diet-Induced Obese Mice. Food Funct. 2019, 10, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Qiu, L.; Yu, Y.; Wang, C. Saponins of Panax Notoginseng: Chemistry, Cellular Targets and Therapeutic Opportunities in Cardiovascular Diseases. Expert Opin. Investig. Drugs 2014, 23, 523–539. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, C.; Zhang, T.; Wang, Y. Saponins from Sea Cucumber and Their Biological Activities. J. Agric. Food Chem. 2018, 66, 7222–7237. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.A.; Elbandy, M.; Lawrence, A.; Paget, T.; Rae-Rho, J.; Binnaser, Y.S.; Ali, I. Extraction, Identification and Biological Activities of Saponins in Sea Cucumber Pearsonothuria Graeffei. Comb. Chem. High Throughput Screen. 2018, 21, 222–231. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef]
- Findlay, J.A.; Yayli, N.; Radics, L. Novel Sulfated Oligosaccharides from the Sea Cucumber Cucumaria frondosa. J. Nat. Prod. 1992, 55, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea Cucumbers Triterpene Glycosides, the Recent Progress in Structural Elucidation and Chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Yayli, N. Minor Saponins from the Sea Cucumber Cucumaria frondosa. Indian J. Chem. Sect. B 2001, 40B, 399–404. [Google Scholar]
- Fagbohun, O.F.; Hui, J.P.M.; Zhang, J.; Jiao, G.; Rupasinghe, H.P.V. Application of Response Surface Methodology and Artificial Neural Network to Optimize the Extraction of Saponins and Polyphenols from North Atlantic Sea Cucumber. Food Chem. Adv. 2024, 5, 100748. [Google Scholar] [CrossRef]
- Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M.; Ghandourah, M.; Al-Farawati, R. Sulfated Triterpene Glycosides from the Saudi Red Sea Cucumber Holothuria Atra with Antioxidant and Cytotoxic Activities. Thalass. Int. J. Mar. Sci. 2021, 37, 817–824. [Google Scholar] [CrossRef]
- Nugroho, A.; Harahap, I.A.; Ardiansyah, A.; Bayu, A.; Rasyid, A.; Murniasih, T.; Setyastuti, A.; Putra, M.Y. Antioxidant and Antibacterial Activities in 21 Species of Indonesian Sea Cucumbers. J. Food Sci. Technol. 2022, 59, 239–248. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; MM Franco, C. Distribution of Saponins in the Sea Cucumber Holothuria Lessoni; the Body Wall versus the Viscera, and Their Biological Activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Brooks, M.S.; Budge, S.M.; Kermanshahi-Pour, A. Extraction of Omega-3 Fatty Acids from Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Using Supercritical Carbon Dioxide. Mar. Drugs 2024, 22, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ebrahim, Z.M.; Tao, L.; Shi, W.; Li, W.; Lu, W. Optimized Extraction of Saponins from Camelia Oleifera Using Ultrasonic-Assisted Enzymes and Their Surface Performance Evaluation. Processes 2025, 13, 1063. [Google Scholar] [CrossRef]
- Majinda, R. Extraction and Isolation of Saponins. In Natural Products Isolation; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 864, pp. 415–426. ISBN 978-1-61779-624-1. [Google Scholar]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and Quantification of Saponins: A Review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, R.; Guo, F.; Li, Y. Determination Total Saponin Content in Sea Cucumbers. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 89–92. [Google Scholar] [CrossRef]
- Wang, L.; Fang, X.; Du, M.; Long, Q. Effects of Supercritical CO2 Extraction on the Quality of Oil and Physicochemical Properties of Tea Saponin in Oil-Tea Camellia Seed Cake. China Oils Fats 2020, 45, 109–114. [Google Scholar] [CrossRef]
- Yang, D. Optimization of Supercritical CO2 Extraction Process of Quinoa Bran Saponin. Food Res. Dev. 2019, 40, 149–154. [Google Scholar] [CrossRef]
- Liu, X. Optimization of Supercritical CO2 Fluid Extraction Conditions of Saponins from Spina Gleditsiae. IOP Conf. Ser. Mater. Sci. Eng. 2018, 439, 042027. [Google Scholar] [CrossRef]
- Deng, C.; Nie, F. Extraction of Steroidal Saponins from Sisal Hemp by Supercritical CO2. Food Res. Dev. 2008, 2, 41–44. [Google Scholar]
- Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Hang, C.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica semper, 1868. Molecules 2020, 25, 4088. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H. Valorization of Bioactive Compounds from Food By-Products Using Supercritical Fluid Extraction: A Technological and Industrial Perspective. Food Chem. 2025, 484, 144277. [Google Scholar] [CrossRef]
- Carvalho, L.M.S.; Oliveira, A.M.B.; Grimaldi, R.; de Souza, P.T.; Batista, E.A.C.; Martínez, J. Supercritical Fluid and Pressurized Liquid Extraction of Spent Tucumã-Do-Amazonas (Astrocaryum aculeatum) Almonds. J. Supercrit. Fluids 2024, 209, 106238. [Google Scholar] [CrossRef]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Domínguez, M.C. Valorization of Granadilla Waste (Passiflora Ligularis, Juss.) by Sequential Green Extraction Processes Based on Pressurized Fluids to Obtain Bioactive Compounds. J. Supercrit. Fluids 2023, 194, 105833. [Google Scholar] [CrossRef]
- Marcuzzo, N.; Draszewski, C.P.; Wagner, R.; Cordeiro, M.W.S.; Castilhos, F.; Mayer, F.D.; Flores, D.C.B.; Nora, F.M.D.; Abaide, E.R.; Rosa, C.S. Obtaining Oil and Fermentable Sugars from Olive Pomace Using Sequential Supercritical Fluid Extraction and Enzymatic Hydrolysis. J. Supercrit. Fluids 2024, 211, 106288. [Google Scholar] [CrossRef]
- Park, J.; Roy, V.C.; Kim, S.; Lee, S.; Chun, B. Extraction of Edible Oils and Amino Acids from Eel By-Products Using Clean Compressed Solvents: An Approach of Complete Valorization. Food Chem. 2022, 388, 132949. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, X.; Ge, K.; Wu, J.; Wang, Z.; Du, J.; Song, L.; Zhou, Z. Isolation, Identification, and Quantitative Determination of Saponin in Apostichopus Japonicus by HPLC-DAD. J. Ocean Univ. China 2022, 21, 473–478. [Google Scholar] [CrossRef]
- Le Bot, M.; Thibault, J.; Pottier, Q.; Boisard, S.; Guilet, D. An Accurate, Cost-Effective and Simple Colorimetric Method for the Quantification of Total Triterpenoid and Steroidal Saponins from Plant Materials. Food Chem. 2022, 383, 132597. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Huo, Y.; Yang, W.; Chen, J.; Gao, Z.; Yang, Z. Ultrasonic Extraction and Antioxidant Evaluation of Oat Saponins. Ultrason. Sonochem. 2024, 109, 106989. [Google Scholar] [CrossRef]
- Shang, J. Analytical Method Development for Quantification of Total Saponins. Master’s Thesis, National University of Singapore, Singapore, 2016. [Google Scholar]
- Cheng, Y.; Zheng, Y.; VanderGheynst, J.S. Rapid Quantitative Analysis of Lipids Using a Colorimetric Method in a Microplate Format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef]
- Ondevilla, J.C.; Hanashima, S.; Mukogawa, A.; Miyazato, D.G.; Umegawa, Y.; Murata, M. Effect of the Number of Sugar Units on the Interaction between Diosgenyl Saponin and Membrane Lipids. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184145. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin Surfactants Used in Drug Delivery Systems: A New Application for Natural Medicine Components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, H.; Song, L.; Tao, F.; Ji, X.; Yu, Y.; Cao, Y.; Tang, S.; Xue, P. Optimization of Green Deep Eutectic Solvent (DES) Extraction of Chenopodium Quinoa Willd. Husks Saponins by Response Surface Methodology and Their Antioxidant Activities. RSC Adv. 2023, 13, 29408–29418. [Google Scholar] [CrossRef] [PubMed]
- Ostellari, P.; Tajoli, F.; Fortunati, I.; Carofiglio, T.; Badocco, D.; Pastore, P.; Gross, S. Eu(Iii)-Doped Calcium Molybdate Nano- and Microstructures: Microfluidic Synthesis and Morphology Tuning via Solvent Dielectric Constant and Viscosity Control. CrystEngComm 2024, 26, 6052–6064. [Google Scholar] [CrossRef]
- Dufour, R.; Perry, G.; Harnois, M.; Coffinier, Y.; Thomy, V.; Senez, V.; Boukherroub, R. From Micro to Nano Reentrant Structures: Hysteresis on Superomniphobic Surfaces. Colloid Polym. Sci. 2013, 291, 409–415. [Google Scholar] [CrossRef]
- Wood, J.A.; Bernards, M.A.; Wan, W.; Charpentier, P.A. Extraction of Ginsenosides from North American Ginseng Using Modified Supercritical Carbon Dioxide. J. Supercrit. Fluids 2006, 39, 40–47. [Google Scholar] [CrossRef]
- Yeo, S.; Park, S.; Kim, J.; Kim, J. Critical Properties of Carbon Dioxide + Methanol, + Ethanol, + 1-Propanol, and + 1-Butanol. J. Chem. Eng. Data 2000, 45, 932–935. [Google Scholar] [CrossRef]
- Ansharullah; Tamrin; Patadjai, A.B.; Asranuddin. Powder Production of Sea Cucumber (Holothuria Scabra): Effect of Processing Methods on the Antioxidant Activities and Physico-Chemical Characteristics. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012024. [Google Scholar] [CrossRef]
- Shanei, A.; Sazgarnia, A. An Overview of Therapeutic Applications of Ultrasound Based on Synergetic Effects with Gold Nanoparticles and Laser Excitation. Iran. J. Basic Med. Sci. 2019, 22, 848–855. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, H.; Zheng, T.; Jiang, J.; Xu, Y.; Jia, F.; He, K.; Yang, Y. Quantitative Changes and Transformation Mechanisms of Saponin Components in Chinese Herbal Medicines during Storage and Processing: A Review. Molecules 2024, 29, 4486. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on Characterization and Antioxidant Activity of Polysaccharides from Ganoderma Lucidum by Ultrasound and Conventional Extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef]
- Higgins, C.L.; Filip, S.V.; Afsar, A.; Colquhoun, H.M.; Hayes, W. From Food to Mobility: Investigating a Screening Assay for New Automotive Antioxidants Using the Stable Radical DPPH. ChemistrySelect 2021, 6, 9179–9184. [Google Scholar] [CrossRef]
- Kodali, S.T.; Kauffman, P.; Kotha, S.R.; Yenigalla, A.; Veeraraghavan, R.; Pannu, S.R.; Hund, T.J.; Satoskar, A.R.; McDaniel, J.C.; Maddipati, R.K.; et al. Oxidative Lipidomics: Analysis of Oxidized Lipids and Lipid Peroxidation in Biological Systems with Relevance to Health and Disease. In Measuring Oxidants and Oxidative Stress in Biological Systems; Berliner, L.J., Parinandi, N.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–92. ISBN 978-3-030-47318-1. [Google Scholar]
- Khan, F.I.; Ghoshal, A.K. Removal of Volatile Organic Compounds from Polluted Air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life Cycle Assessment of Polyphenols Extraction Processes from Waste Biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Kermanshahi Pour, A. Extraction of Anthocyanins from Haskap Berry Pulp Using Supercritical Carbon Dioxide: Influence of Co-Solvent Composition and Pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).