A Comparative Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Different Extracts of Caulerpa prolifera
Abstract
1. Introduction
2. Results
2.1. Yields, Phenols, and Flavonoids Contents
2.2. Fatty Acid Analysis
2.3. HPLC Analysis of C. prolifera Extracts
2.4. Antioxidant Activity
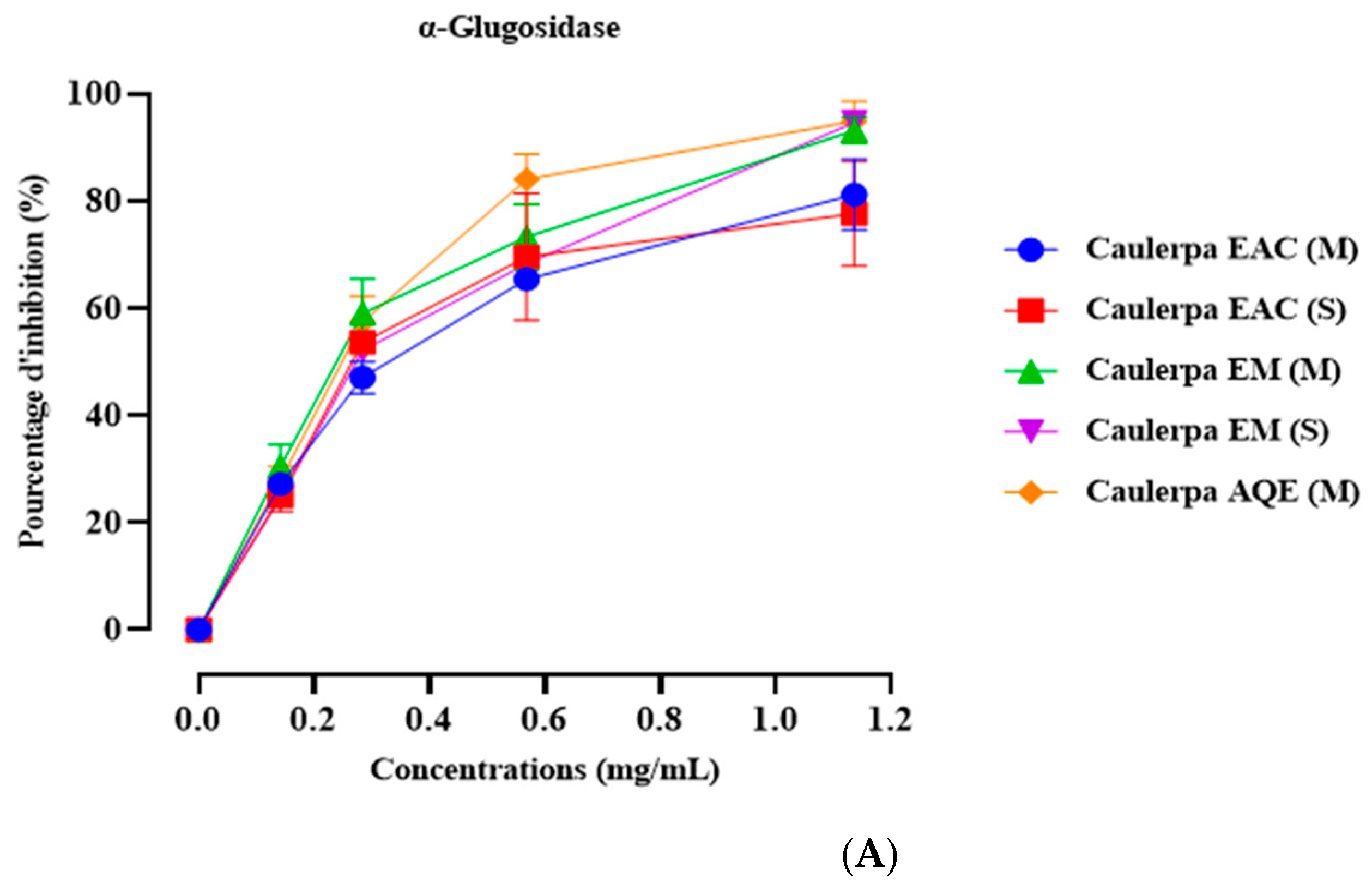
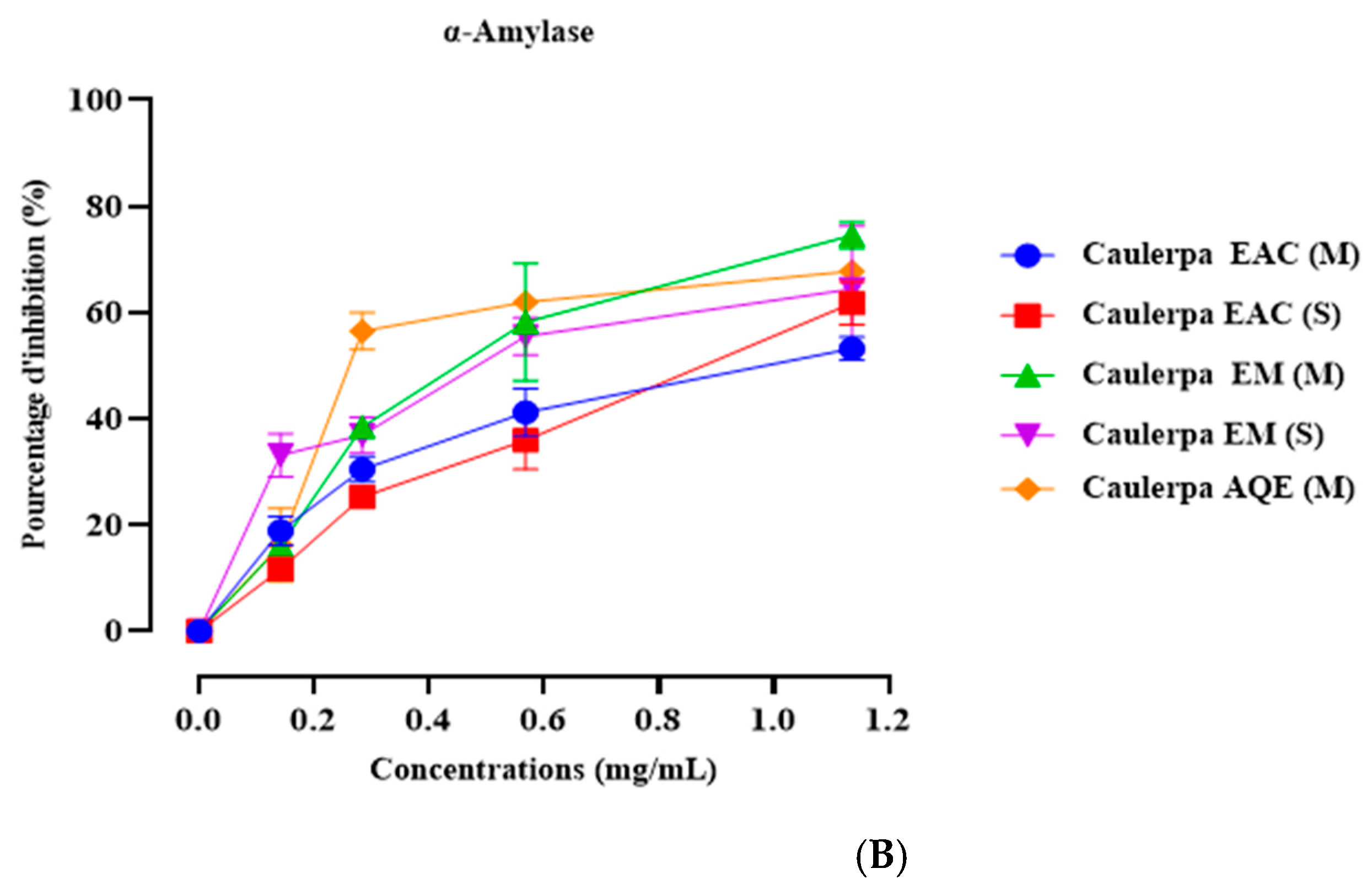
2.5. In Vitro α-Amylase Inhibition
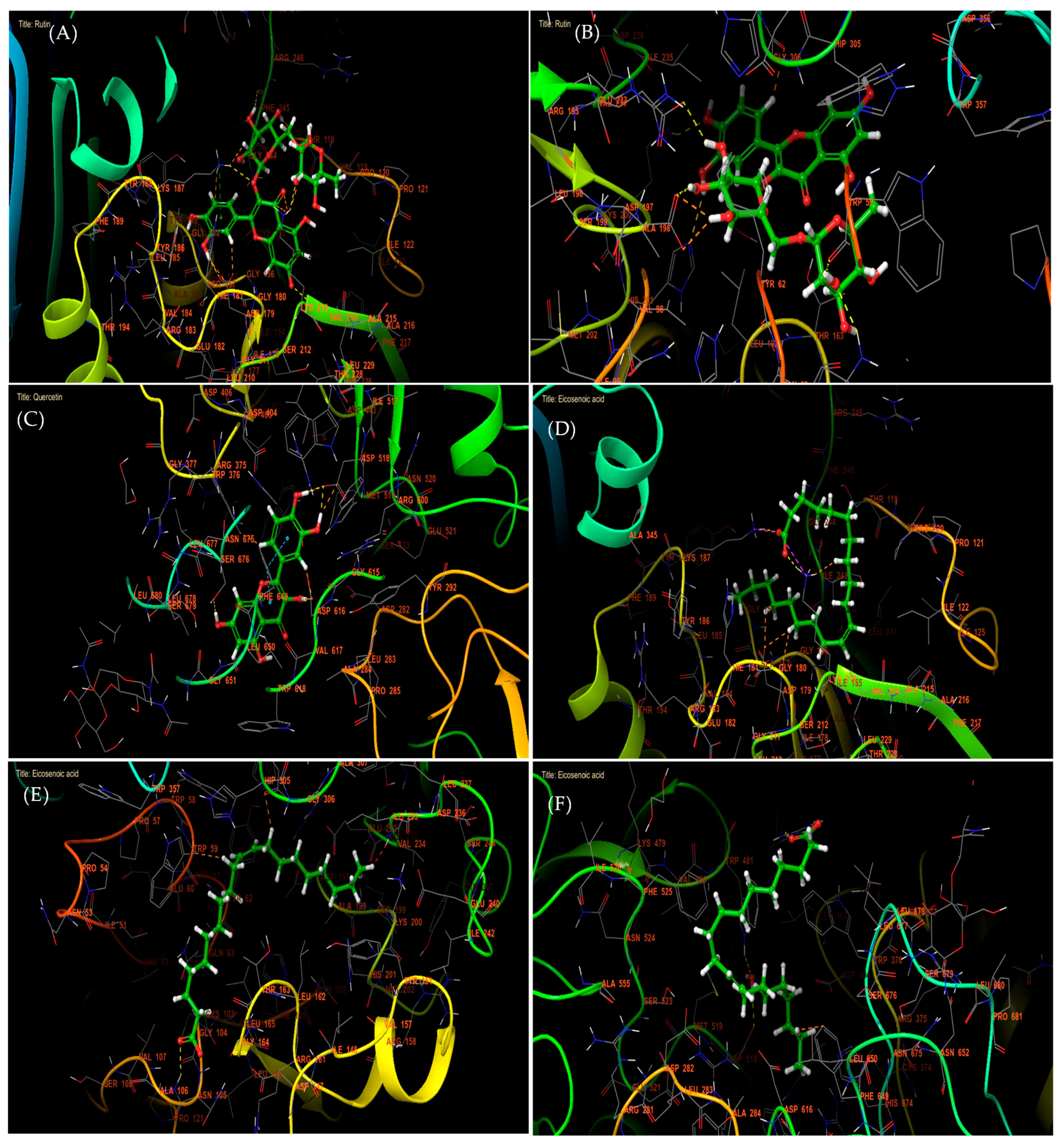
2.6. Molecular Modeling Studies
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extraction
4.2.1. Maceration Extraction
4.2.2. Soxhlet Extraction
4.3. Phytochemical Compounds
4.3.1. Quantification of Total Phenolic Constituents
4.3.2. Measurement of Total Flavonoid Content
4.4. Fatty Acid GC-MS Analysis of C. prolifera Extracts
4.5. HPLC Analyses of C. prolifera Extracts
4.6. Antioxidant Activity
4.6.1. Scavenging 2,2-Diphenyl-1-picrylhydrazyl Radical Test
4.6.2. β-Carotene Bleaching Assay
4.7. In Vitro α-Amylase Inhibition
4.8. In Vitro α-Glucosidase Inhibition Assay
4.9. Theoretical Study
4.9.1. Ligands Preparation
4.9.2. Receptor Preparation
4.9.3. Grid Generation and Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snoussi, M.; Ouchani, T.; Khouakhi, A.; Niang-Diop, I. Impacts of Sea-Level Rise on the Moroccan Coastal Zone: Quantifying Coastal Erosion and Flooding in the Tangier Bay. Geomorphology 2009, 107, 32–40. [Google Scholar] [CrossRef]
- Benhissoune, S.; Boudouresque, C.; Verlaque, M. A CheckList of Marine Seaweeds of the Mediterranean and Atlantic Coasts of Morocco. I. Chlorophyceae Wille s. l. Bot. Mar. 2001, 44, 171–182. [Google Scholar] [CrossRef]
- Benhissoune, S.; Boudouresque, C.; Perret-Boudouresque, M.; Verlaque, M. A Checklist of the Seaweeds of the Mediterranean and Atlantic Coasts of Morocco. III. Rhodophyceae (Excluding Ceramiales). Bot. Mar. 2002, 45, 391–412. [Google Scholar] [CrossRef]
- Elasri, O.; Ramdani, M.; Latrach, L.; Benyounes, H.; Mohamed elamin, A.; Mohammed, R. Comparison of Energy Recovery after Anaerobic Digestion of Three Marchica Lagoon Algae (Caulerpa prolifera, Colpomenia sinuosa, Gracilaria bursa-pastoris). Sustain. Mater. Technol. 2017, 11, 47–52. [Google Scholar]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Crockett, P.F.; Keough, M.J. Ecological Niches of Three Abundant Caulerpa Species in Port Phillip Bay, Southeast Australia. Aquat. Bot. 2014, 119, 120–131. [Google Scholar] [CrossRef]
- de Senerpont Domis, L.; Famà, P.; Bartlett, A.; Prud’homme van Reine, W.; Espinosa, C.; Trono, J. Gavino Defining Taxon Boundaries in Members of the Morphologically and Genetically Plastic Genus Caulerpa (Caulerpales, Chlorophyta). J. Phycol. 2003, 39, 1019–1037. [Google Scholar] [CrossRef]
- Fernández-García, C.; Cortés, J.; Alvarado, J.J.; Nivia-Ruiz, J. Physical Factors Contributing to the Benthic Dominance of the Alga Caulerpa sertularioides (Caulerpaceae, Chlorophyta) in the Upwelling Bahía Culebra, North Pacific of Costa Rica. Rev. Biol. Trop. 2015, 60, 93. [Google Scholar] [CrossRef]
- Gacia, E.; Rodríguez-Prieto, C.; Delgado, O.; Ballesteros, E. Seasonal Light and Temperature Responses of Caulerpa Taxifolia from the Northwestern Mediterranean. Aquat. Bot. 1996, 53, 215–225. [Google Scholar] [CrossRef]
- Ohba, H.; Nashima, H.; Enomoto, S. Culture Studies on Caulerpa (Caulerpales, Chlorophyceae) III. Reproduction, Development and Morphological Variation of Laboratory-Cultured, C. racemosa Var. peltata. Bot. Mag. 1992, 105, 589–600. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical Marine Macroalgae as Potential Sources of Nutritionally Important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Nagappan, T.; Vairappan, C.S. Nutritional and Bioactive Properties of Three Edible Species of Green Algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- de Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa Consumption, Nutritional Value and Farming in the Indo-Pacific Region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Paul, N.A.; Neveux, N.; Magnusson, M.; de Nys, R. Comparative Production and Nutritional Value of “Sea Grapes”—The Tropical Green Seaweeds Caulerpa lentillifera and C. racemosa. J. Appl. Phycol. 2014, 26, 1833–1844. [Google Scholar] [CrossRef]
- Ghosh, P.; Adhikari, U.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. In Vitro Anti-Herpetic Activity of Sulfated Polysaccharide Fractions from Caulerpa Racemosa. Phytochemistry 2004, 65, 3151–3157. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Hayashi, T.; Lee, J.-B.; Srisomporn, P.; Maeda, M.; Ozawa, T.; Sakuragawa, N. Inhibition of Thrombin by Sulfated Polysaccharides Isolated from Green Algae. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2000, 1543, 86–94. [Google Scholar] [CrossRef]
- Ji, H.; Shao, H.; Zhang, C.; Hong, P.; Xiong, H. Separation of the Polysaccharides in Caulerpa racemosa and Their Chemical Composition and Antitumor Activity. J. Appl. Polym. Sci. 2008, 110, 1435–1440. [Google Scholar] [CrossRef]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Immunostimulatory Activity of Polysaccharides Isolated from Caulerpa lentillifera on Macrophage Cells. Biosci. Biotechnol. Biochem. 2012, 76, 501–505. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Neto, É.M.; Teixeira, L.A.C.; de Paula, R.C.M.; Mourão, P.A.d.S.; Benevides, N.M.B. Structural Features and Inactivation of Coagulation Proteases of a Sulfated Polysaccharidic Fraction from Caulerpa cupressoides Var. Lycopodium (Caulerpaceae, Chlorophyta). Acta Sci. Technol. 2013, 35, 611–619. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Doty, M.S.; Aguilar-Santos, G. Caulerpicin, a Toxic Constituent of Caulerpa. Nature 1966, 211, 990. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.V.; Naik, S.R.; Zine, S.; Ved, H.; Doshi, G. Antidiabetic Activity of Caulerpa Racemosa: Role of Proinflammatory Mediators, Oxidative Stress, and Other Biomarkers. Planta Medica Int. Open 2022, 9, e60–e71. [Google Scholar] [CrossRef]
- Fajriah, S.; Rizki, I.F.; Sinurat, E. Characterization and Analysis of the Antidiabetic Activities of Sulphated Polysaccharide Extract from Caulerpa lentillifera. Pharmacia 2021, 68, 869–875. [Google Scholar] [CrossRef]
- Bradshaw, P.C. Cytoplasmic and Mitochondrial NADPH-Coupled Redox Systems in the Regulation of Aging. Nutrients 2019, 11, 504. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of Extraction Methods on Yield, Phytochemical Constituents and Antioxidant Activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effects of Salinity and Nutrients on the Growth and Chlorophyll Fluorescence of Caulerpa lentillifera. Chin. J. Ocean. Limnol. 2015, 33, 410–418. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of Macroalgae as Feedstocks for Biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva Lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Qudus, B.; Aroyehun, A.; Abdul Razak, S.; Palaniveloo, K.; Nagappan, T.; Suraiza Nabila Rahmah, N.; Wee Jin, G.; Chellappan, D.K.; Chellian, J.; Kunnath, A.P. Bioprospecting Cultivated Tropical Green Algae, Caulerpa Racemosa (Forsskal) J. Agardh: A Perspective on Nutritional Properties, Antioxidative Capacity and Anti-Diabetic Potential. Foods 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Perveen, S.; Ahmad, V.U.; Bano, N.; Shameel, M. Chemical Constituents of Endarachne binghamiae (Scytosiphonales, Phaeophyta) from the Karachi Coast. Bot. Mar. 1987, 30, 371–372. [Google Scholar] [CrossRef]
- Bano, S.; Hayee, A.; Ahmad, V.U.; Shaikh, W.; Usmanghani, K.; Shameel, M. Marine Natural Products. Part VII. Steroids from a Red Alga Asparagopsis sandfordiana. Pol. J. Chem. 1988, 62, 905–906. [Google Scholar]
- Gunstone, F.D. Fatty Acids and Glycerides. Nat. Prod. Rep. 1987, 4, 95. [Google Scholar] [CrossRef]
- Wood, B.J.B. Fatty Acids and Lipids in Algae. In Microbial Lipids; Academic Press: New York, NY, USA, 1988; pp. 807–867. [Google Scholar]
- Hertog, M.G.L.; Feskens, E.J.M.; Kromhout, D.; Hertog, M.G.L.; Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Dietary Antioxidant Flavonoids and Risk of Coronary Heart Disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and Prevention of Cardiovascular Diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B. Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review. JAMA 2005, 294, 97. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Duthie, G.; Morrice, P. Antioxidant Capacity of Flavonoids in Hepatic Microsomes Is Not Reflected by Antioxidant Effects In Vivo. Oxidative Med. Cell. Longev. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of Solvent on the Antioxidant and Antimicrobial Properties of Walnut (Juglans regia L.) Green Husk Extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 Diabetes Mellitus: From a Metabolic Disorder to an Inflammatory Condition. World J. Diabetes 2015, 6, 598. [Google Scholar] [CrossRef]
- Arayne, M.; Sultana, N.; Mirza, A.; MH, Z.; Siddiqui, F. In Vitro Hypoglycemic Activity of Methanolic Extracts of Some Indigenous Plants. Pak. J. Pharm. Sci. 2007, 20, 268–273. [Google Scholar]
- Daoudi, N.E.; Bouziane, O.; Bouhrim, M.; Bnouham, M. Natural Aldose Reductase Inhibitors for Treatment and Prevention of Diabetic Cataract: A Review. Herba Pol. 2022, 68, 35–58. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of Oxidative Stress in Diabetic Complications: A New Perspective on an Old Paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Dalli, M.; Daoudi, N.E.; Abrigach, F.; Azizi, S.; Bnouham, M.; Kim, B.; Gseyra, N. In Vitro α-Amylase and Hemoglobin Glycation Inhibitory Potential of Nigella Sativa Essential Oil, and Molecular Docking Studies of Its Principal Components. Front. Pharmacol. 2022, 13, 1036129. [Google Scholar] [CrossRef]
- Nainu, F.; Frediansyah, A.; Mamada, S.S.; Permana, A.D.; Salampe, M.; Chandran, D.; Emran, T.B.; Simal-Gandara, J. Natural Products Targeting Inflammation-Related Metabolic Disorders: A Comprehensive Review. Heliyon 2023, 9, e16919. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of Gallic Acid on α-Amylase and α-Glucosidase Inhibitory Properties of Acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.-L.; Hammouti, B.; Rhazi, L.; et al. Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Gracilaria Bursa-Pastoris Extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical Screening, Polyphenols, Flavonoids and Tannin Content, Antioxidant Activities and FTIR Characterization of Marrubium vulgare L. from 2 Different Localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, K.; George, E.; Sathyanarayanan, S.; George, B.P.; Abrahamse, H.; Thamburaj, S.; Thangaraj, P. Phenolics, Tannins, Flavonoids and Anthocyanins Contents Influenced Antioxidant and Anticancer Activities of Rubus Fruits from Western Ghats, India. Food Sci. Hum. Wellness 2019, 8, 73–81. [Google Scholar] [CrossRef]
- Belkacemi, L.; Belalia, M.; Djendara, A.; Bouhadda, Y. Antioxidant and Antibacterial Activities and Identification of Bioactive Compounds of Various Extracts of Caulerpa Racemosa from Algerian Coast. Asian Pac. J. Trop. Biomed. 2020, 10, 87. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Legssyer, A.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory Effect of Roasted/ Unroasted Argania Spinosa Seeds Oil on α- Glucosidase, α-Amylase and Intestinal Glucose Absorption Activities. S. Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- Lakshmanasenthil, S.; Vinoth Kumar, T.; Geetharamani, D.; Shanthi Priya, S. α-Amylase and α-Glucosidase InhibitoryActivity of Tetradecanoic Acid (TDA) from Sargassum wightii with Relevance to Type 2 Diabetes Mellitus. J. Biol. Act. Prod. Nat. 2018, 8, 180–191. [Google Scholar] [CrossRef]
- Salazar, M.O.; Osella, M.I.; Arcusin, D.E.J.; Lescano, L.E.; Furlan, R.L.E. New α-Glucosidase Inhibitors from a Chemically Engineered Essential Oil of Origanum vulgare L. Ind. Crops Prod. 2020, 156, 112855. [Google Scholar] [CrossRef]
- Wuttke, A.; Idevall-Hagren, O.; Tengholm, A. P2Y 1 Receptor-dependent Diacylglycerol Signaling Microdomains in β Cells Promote Insulin Secretion. FASEB J. 2013, 27, 1610–1620. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, Separation, and Detection Methods for Phenolic Acids and Flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Taibi, M.; Loukili, E.H.; Elbouzidi, A.; Baraich, A.; Haddou, M.; Bellaouchi, R.; Saalaoui, E.; Asehraou, A.; Addi, M.; Bourhia, M.; et al. Exploring the Pharmacological Potential of the Chemically Characterized Essential Oil from Clinopodium nepeta Subsp. Ascendens: A Combined In Vitro and In Silico Analysis. Moroc. J. Chem. 2024, 12, 997–1021. [Google Scholar] [CrossRef]
- Loukili, E.H.; Abrigach, F.; Bouhrim, M.; Bnouham, M.; Fauconnier, M.; Ramdani, M. Chemical Composition and Physicochemical Analysis of Opuntia dillenii Extracts Grown in Morocco. J. Chem. 2021, 2021, 8858929. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bekkouch, O.; Harnafi, M.; Touiss, I.; Khatib, S.; Harnafi, H.; Alem, C.; Amrani, S. In Vitro Antioxidant and In Vivo Lipid-Lowering Properties of Zingiber officinale Crude Aqueous Extract and Methanolic Fraction: A Follow-Up Study. Evid.-Based Complement. Altern. Med. 2019, 2019, 9734390. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M.; et al. Artemisia Absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Lafraxo, S.; El Moussaoui, A.; A Bin Jardan, Y.; El Barnossi, A.; Chebaibi, M.; Baammi, S.; Ait Akka, A.; Chebbac, K.; Akhazzane, M.; Chelouati, T.; et al. GC-MS Profiling, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Essential Oil of Juniperus thurifera Bark. Evid.-Based Complement. Altern. Med. 2022, 2022, 6305672. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutiérrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.-S. Chemical Constituents, In Vitro Antioxidant Activity and In Silico Study on NADPH Oxidase of Allium sativum L. (Garlic) Essential Oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]
- El Abdali, Y.; Mahraz, A.M.; Beniaich, G.; Mssillou, I.; Chebaibi, M.; Bin Jardan, Y.A.; Lahkimi, A.; Nafidi, H.-A.; Aboul-Soud, M.A.M.; Bourhia, M.; et al. Essential Oils of Origanum compactum Benth: Chemical Characterization, in Vitro, in Silico, Antioxidant, and Antibacterial Activities. Open Chem. 2023, 21, 20220282. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Elmadbouh, O.H.M.; Chebaibi, M.; Soufi, B.; Conte, R.; Slighoua, M.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Zair, T.; et al. Evaluation of the Toxicity of Caralluma europaea (C.E) Extracts and Their Effects on Apoptosis and Chemoresistance in Pancreatic Cancer Cells. J. Biomol. Struct. Dyn. 2023, 41, 8517–8534. [Google Scholar] [CrossRef]


| Solvent | Extraction Methods | Yield (%) | Polyphenols (mg GAE/g) | Flavonoids (mg QE/g) |
|---|---|---|---|---|
| Hexane | M | 1.30 ± 0.01 | - | - |
| S | 2.23 ± 0.02 | - | - | |
| Ethyl acetate | M | 4.46 ± 0.03 | 179.88 ± 0.03 | 154.64 ± 0.02 |
| S | 6.81 ± 0.05 | 112.87 ± 0.07 | 40.35 ± 0.04 | |
| Methanol | M | 7.12 ± 0.02 | 99.47 ± 0.06 | 22.93 ± 0.01 |
| S | 8.41 ± 0.04 | 53.61 ± 0.02 | 16.70 ± 0.03 | |
| Water | M | 8.51 ± 0.02 | 402.34 ± 0.08 | 196.65 ± 0.09 |
| Fatty Acids | RT (min) | HE (%) | EAcE (%) | ||
|---|---|---|---|---|---|
| M | S | M | S | ||
| Lauric acid (C1:0) | 17.83 | 2.61 ± 0.03 | 2.31 ± 0.02 | 12.13 ± 0.04 | 12.45 ± 0.05 |
| Eicosenoic acid (C20:1) | 20.08 | 3.58 ± 0.02 | 3.20 ± 0.02 | 5.52 ± 0.03 | nd |
| Myristic acid (C14:0) | 20.39 | 7.15 ± 0.04 | 8.19 ± 0.04 | 2.29 ± 0.01 | 5.28 ± 0.02 |
| 7,10-Hexadecadienoic acid (C16:2) | 21.17 | 7.09 ± 0.02 | 6.52 ± 0.03 | 9.32 ± 0.03 | 9.50 ± 0.04 |
| Palmitoleic acid (C16:1) | 23.11 | 1.19 ± 0.01 | 2.15 ± 0.02 | 6.35 ± 0.02 | 6.81 ± 0.03 |
| Palmitic acid (C16:0) | 23.31 | 59.54 ± 0.06 | 60.21 ± 0.05 | 39.31 ± 0.12 | 40.76 ± 0.08 |
| Linoleic acid (C18:2) | 25.03 | 12.25 ± 0.03 | 10.14 ± 0.03 | 15.62 ± 0.05 | 15.66 ± 0.04 |
| Linolenic acid (C18:3) | 25.09 | 6.59 ± 0.02 | 7.28 ± 0.03 | 9.46 ± 0.03 | 9.49 ± 0.03 |
| SFA a | 69.30 | 70.71 | 53.73 | 58.49 | |
| UFA b | 30.70 | 29.29 | 46.27 | 41.46 | |
| UFA/SFA c | 0.44 | 0.41 | 0.86 | 0.71 | |
| N° | Compounds | RT (min) | EAcE (%) | ME (%) | ||
|---|---|---|---|---|---|---|
| M | S | M | S | |||
| 1 | Gallic acid | 15.47 | nd | 0.99 | nd | 0.36 |
| 2 | Catechin | 18.68 | 1.23 | 0.87 | 1.94 | 0.90 |
| 3 | 4-hydroxy-benzoic acid | 18.91 | 0.39 | 1.75 | nd | 0.87 |
| 4 | Chlorogenic acid | 19.15 | 0.63 | 1.50 | 2.55 | 0.77 |
| 5 | Caffeic acid | 19.45 | 0.47 | 2.22 | 0.91 | 0.74 |
| 6 | Syringic acid | 19.74 | 1.36 | 1.72 | 1.99 | 1.01 |
| 7 | Vanillin | 23.10 | 13.16 | 2.52 | 10.48 | 8.94 |
| 8 | p-Coumaric acid | 23.63 | 3.72 | nd | 16.24 | 12.84 |
| 9 | Sinapic acid | 24.09 | 18.54 | 21.28 | 7.27 | 6.82 |
| 10 | 7,3′,4′-flavon-3-ol | 24.92 | 12.26 | 7.77 | 17.29 | 14.43 |
| 11 | Rutin | 25.16 | 3.27 | 6.03 | nd | 7.07 |
| 12 | Salicylic acid | 25.32 | 2.27 | 4.99 | 3.21 | 4.38 |
| 13 | Quercetin | 25.46 | 3.98 | 3.13 | 2.72 | 3.30 |
| 14 | Cinnamic acid | 25.48 | 5.98 | 6.05 | 6.85 | 11.13 |
| 15 | Luteolin | 25.64 | 2.86 | 3.52 | 1.67 | nd |
| 16 | Apigenin | 25.87 | 4.59 | 4.90 | 4.59 | 9.54 |
| 17 | Kaempferol | 26.10 | 6.03 | 27.00 | 16.30 | 2.99 |
| 18 | Flavone | 26.92 | 3.71 | nd | nd | 3.07 |
| 19 | Flavonone | 27.41 | 15.57 | 3.75 | 6.00 | 10.85 |
| Extracts | IC50 (mg/mL) | ||
|---|---|---|---|
| DPPH | β-Carotene | ||
| EAcE | M | 0.702 ± 0.311 | 0.01 ± 0.18 |
| S | 0.767 ± 0.063 | 0.02 ± 0.25 | |
| ME | M | 0.691 ± 0.041 | 0.29 ± 0.09 |
| S | 0.723 ± 0.020 | 0.31 ± 0.31 | |
| AQE | M | 0.091 ± 0.091 | 0.381 ± 0.11 |
| Ascorbic Acid | 0.06 | - | |
| BHA | - | 0.02 | |
| Inhibitors | IC50 (mg/mL) | ||
|---|---|---|---|
| α-Amylase | α-Glucosidase | ||
| Acarbose | 0.35 ± 0.08 | 0.39 ± 0.04 | |
| EAcE | M | 0.88 ± 0.08 | 0.48 ± 0.02 |
| S | 0.83 ± 0.01 | 0.43 ± 0.07 | |
| ME | M | 0.62 ± 0.11 | 0.35 ± 0.08 |
| S | 0.63 ± 0.14 | 0.29 ± 0.05 | |
| Compound Name | Glide Gscore (Kcal/mol) | |||
|---|---|---|---|---|
| NADPH Oxidase (PDB: 2CDU) | Alpha Amylase (PDB: 1B2Y) | Alpha Glucosidase (PDB: 5NN8) | ||
| Chemical compounds by HPLC analysis | 4-hydroxybenzoic acid | −5.355 | −5.016 | −4.366 |
| 7,3,4-flavon-3-ol | −6.803 | −6.961 | −5.558 | |
| Apigenin | −6.405 | −7.130 | −5.202 | |
| Caffeic acid | −5.484 | −5.953 | −4.237 | |
| Catechin | −5.550 | −6.283 | −4.908 | |
| Chlorogenic acid | −4.812 | −6.254 | −3.738 | |
| Cinnamic acid | −4.637 | −3.713 | −3.353 | |
| Flavone | −5.040 | −5.175 | −4.326 | |
| Flavonone | −5.192 | −5.389 | −4.624 | |
| Gallic acid | −5.878 | −5.333 | −4.32 | |
| Kaempferol | −5.543 | −6.617 | −5.698 | |
| Luteolin | −6.574 | −6.807 | −5.425 | |
| p-Coumaric acid | −5.017 | −5.742 | −3.558 | |
| Quercetin | −6.587 | −6.817 | −7.035 | |
| Rutin | −6.889 | −7.615 | −5.237 | |
| Salicylic acid | −5.469 | −4.565 | −3.911 | |
| Sinapic acid | −5.299 | −4.35 | −3.543 | |
| Syringic acid | −6.132 | −5.973 | −3.472 | |
| Vanillin | −6.603 | −6.012 | −4.651 | |
| Fatty Acid by GC-MS | 7,10-Hexadecadienoic acid | −1.001 | - | - |
| Eicosenoic acid | −3.048 | −2.289 | −2.008 | |
| Lauric acid | - | - | - | |
| Linoleic acid | −0.817 | - | - | |
| Linolenic acid | −0.546 | - | −0.873 | |
| Margaric acid | - | - | - | |
| Myristic acid | - | - | - | |
| Oleic acid | −0.665 | - | - | |
| Palmitic acid | −0.006 | - | - | |
| Palmitoleic acid | - | - | - | |
| Stearic acid | −0.552 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouahabi, S.; Daoudi, N.E.; Chebaibi, M.; Mssillou, I.; Rahhou, I.; Bnouham, M.; Hammouti, B.; Fauconnier, M.-L.; Ayerdi Gotor, A.; Rhazi, L.; et al. A Comparative Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Different Extracts of Caulerpa prolifera. Mar. Drugs 2025, 23, 259. https://doi.org/10.3390/md23070259
Ouahabi S, Daoudi NE, Chebaibi M, Mssillou I, Rahhou I, Bnouham M, Hammouti B, Fauconnier M-L, Ayerdi Gotor A, Rhazi L, et al. A Comparative Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Different Extracts of Caulerpa prolifera. Marine Drugs. 2025; 23(7):259. https://doi.org/10.3390/md23070259
Chicago/Turabian StyleOuahabi, Safae, Nour Elhouda Daoudi, Mohamed Chebaibi, Ibrahim Mssillou, Ilyesse Rahhou, Mohamed Bnouham, Belkheir Hammouti, Marie-Laure Fauconnier, Alicia Ayerdi Gotor, Larbi Rhazi, and et al. 2025. "A Comparative Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Different Extracts of Caulerpa prolifera" Marine Drugs 23, no. 7: 259. https://doi.org/10.3390/md23070259
APA StyleOuahabi, S., Daoudi, N. E., Chebaibi, M., Mssillou, I., Rahhou, I., Bnouham, M., Hammouti, B., Fauconnier, M.-L., Ayerdi Gotor, A., Rhazi, L., & Ramdani, M. (2025). A Comparative Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Different Extracts of Caulerpa prolifera. Marine Drugs, 23(7), 259. https://doi.org/10.3390/md23070259









