Effects of Salinity on the Growth Performance and Docosahexaenoic Acid Positional Distribution in Triacylglycerols of the Newly Isolated Schizochytrium sp. FJ-1
Abstract
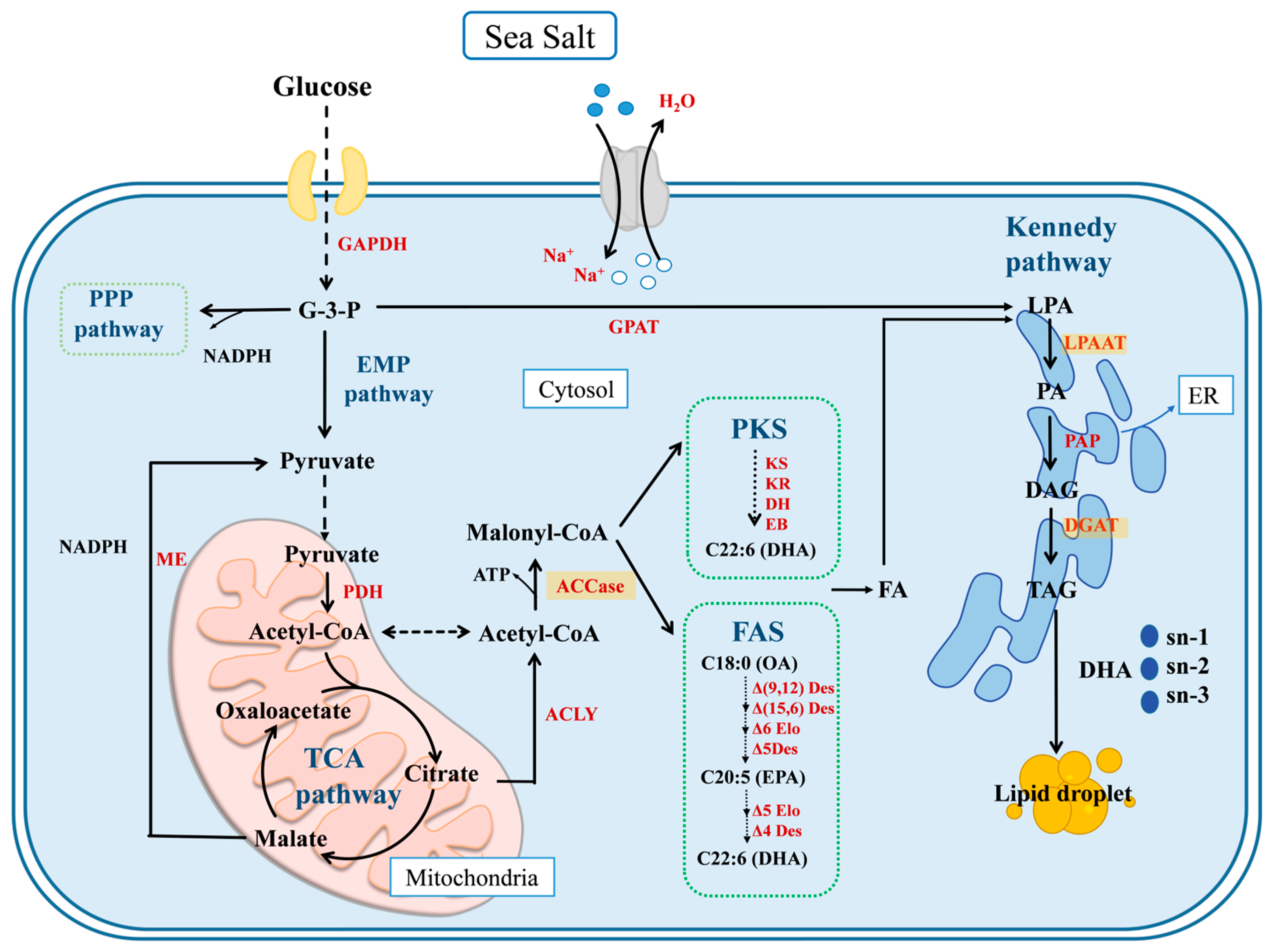
1. Introduction
2. Results and Discussion
2.1. Molecular Identification of Schizochytrium sp. FJ-1
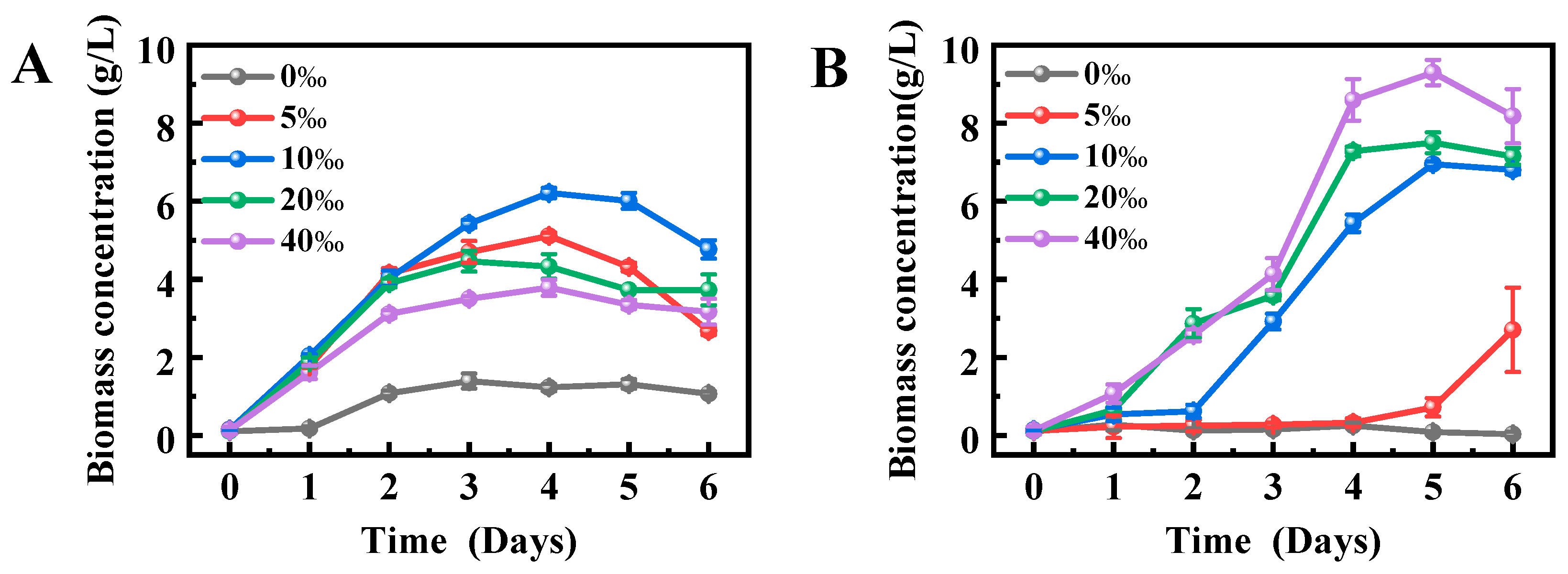
2.2. Effects of Salinity on the Growth Performance of Schizochytrium sp. FJ-1 and 20888
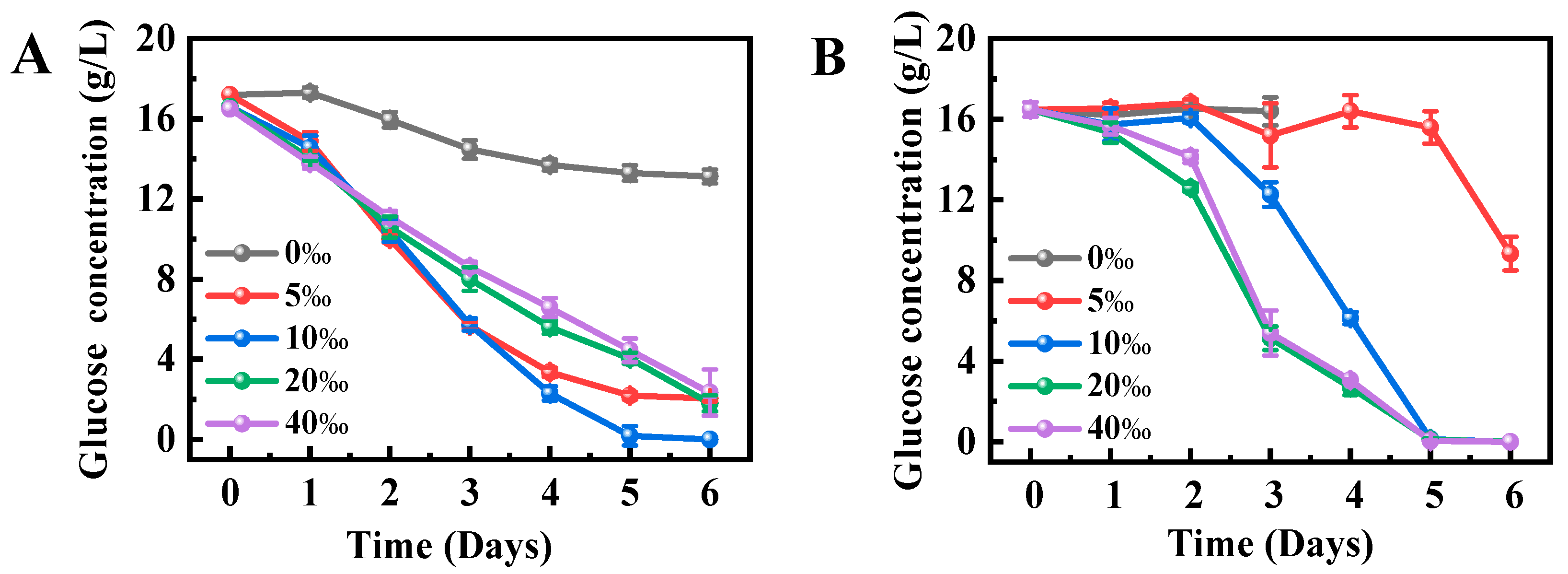
2.3. Effects of Salinity on Glucose Utilization by Schizochytrium sp. FJ-1 and 20888
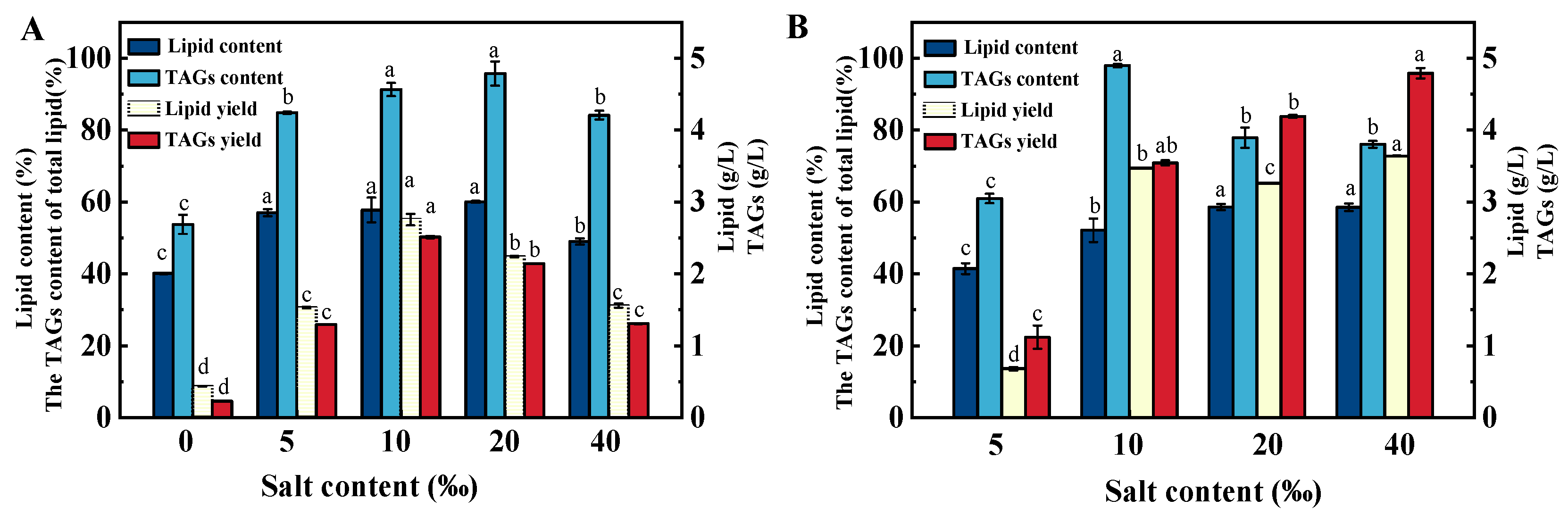
2.4. Effects of Salinity on the Total Lipid Content and TAG Fraction Percentage of Schizochytrium sp. FJ-1 and 20888
2.5. Effects of Salinity on the Fatty Acid Composition of Total Lipids from Schizochytrium sp. FJ-1 and 20888
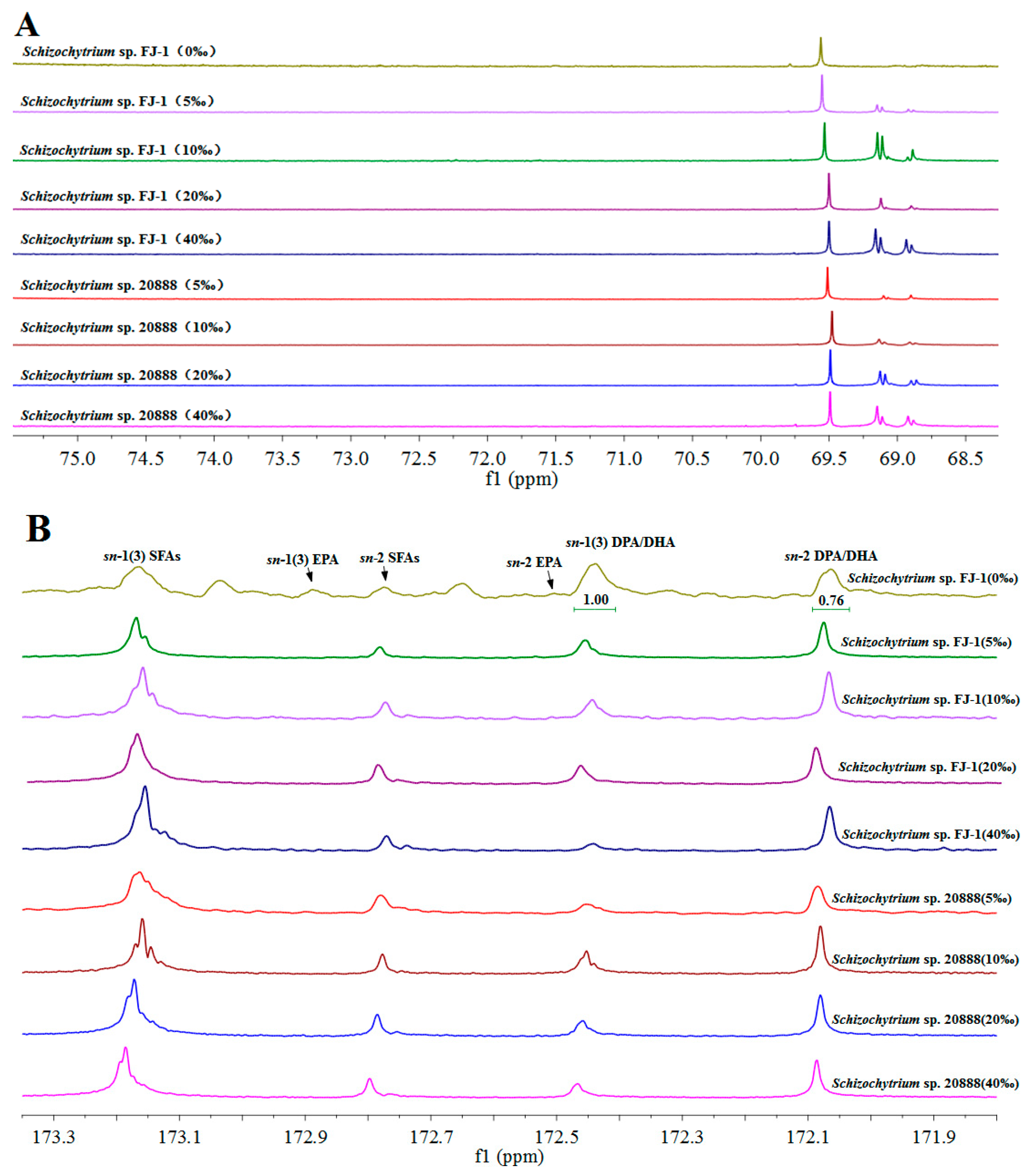
2.6. Effects of Salinity on the Fatty Acid Composition of the TAG Fraction from Schizochytrium sp. FJ-1 and 20888
3. Materials and Methods
3.1. Strains of Schizochytrium
3.2. Culture Media
3.3. Effects of Different Salinity Levels on the Fermentation Performance of Schizochytrium sp. FJ-1 and 20888
3.4. Analytical Methods
3.4.1. Determination of Schizochytrium sp. Biomass and Glucose Concentration of the Culture Medium
3.4.2. Determination of Total Lipid Content of Schizochytrium Cells
3.4.3. Determination of the TAG Fraction Percentage of Total Lipids in Schizochytrium sp.
3.4.4. Determination of Fatty Acid Composition
3.4.5. C-13 Nuclear Magnetic Resonance (13C-NMR) Analysis of Schizochytrium-Derived TAGs
3.5. Data Processing and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Omachi, D.O.; Aryee, A.N.A.; Onuh, J.O. Functional Lipids and Cardiovascular Disease Reduction: A Concise Review. Nutrients 2024, 16, 2453. [Google Scholar] [CrossRef] [PubMed]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Fleith, M.; Clandinin, M.T. Dietary PUFA for Preterm and Term Infants: Review of Clinical Studies. Crit. Rev. Food Sci. Nutr. 2005, 45, 205–229. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Birch, C.S.; Bonwick, G.A.; English, A.; Metcalfe, P.; Li, W. Optimal omegas—Barriers and novel methods to narrow omega-3 gaps. A narrative review. Front. Nutr. 2024, 11, 1325099. [Google Scholar] [CrossRef]
- Sehl, A.; Caderby, E.; Bouhouda, S.; Rébeillé, F.; Griffiths, H.; Da Rocha Gomes, S. How do algae oils change the omega-3 polyunsaturated fatty acids market? OCL 2022, 29, 20. [Google Scholar] [CrossRef]
- Chi, G.; Xu, Y.; Cao, X.; Li, Z.; Cao, M.; Chisti, Y.; He, N. Production of polyunsaturated fatty acids by Schizochytrium (Aurantiochytrium) spp. Biotechnol. Adv. 2022, 55, 107897. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of oil from Schizochytrium sp. (strain CABIO-A-2) for use in infant and follow-on formula as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2023, 21, e8415. [Google Scholar]
- Puri, M.; Gupta, A.; Sahni, S. Schizochytrium sp. Trends. Microbiol. 2023, 31, 872–873. [Google Scholar]
- Zhang, T.; Lou, F.; Tao, G.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Composition and Structure of Single Cell Oil Produced by Schizochytrium limacinum SR31. J. Am. Oil Chem. Soc. 2016, 93, 1337–1346. [Google Scholar] [CrossRef]
- Li, F.; Yibing, N.; Yiren, Z.; Huidong, H.; Qingbin, Y.; Xingguo, W.; Wei, W. Positional distribution of DHA in triacylglycerols: Natural sources, synthetic routes, and nutritional properties. Crit. Rev. Food Sci. Nutr. 2025, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. High Sn-2 Docosahexaenoic Acid Lipids for Brain Benefits, and Their Enzymatic Syntheses: A Review. Engineering 2020, 6, 424–431. [Google Scholar] [CrossRef]
- Christensen, M.S.; Høy, C.E.; Becker, C.C.; Redgrave, T.G. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: Dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 1995, 61, 56–61. [Google Scholar] [CrossRef]
- Wang, Q.; Han, W.; Jin, W.; Gao, S.; Zhou, X. Docosahexaenoic acid production by Schizochytrium sp.: Review and prospect. Food Biotechnol. 2021, 35, 111–135. [Google Scholar] [CrossRef]
- Sahin, D.; Tas, E.; Altindag, U.H. Enhancement of docosahexaenoic acid (DHA) production from Schizochytrium sp. S31 using different growth medium conditions. AMB Express. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Półbrat, T.; Konkol, D.; Korczyński, M. Optimization of docosahexaenoic acid production by Schizochytrium sp.—A review. Biocatal. Agric. Biotechnol. 2021, 35, 102076. [Google Scholar] [CrossRef]
- Sun, L.; Ren, L.; Zhuang, X.; Ji, X.; Yan, J.; Huang, H. Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresour. Technol. 2014, 159, 199–206. [Google Scholar]
- Gao, M.; Song, X.; Feng, Y.; Li, W.; Cui, Q. Isolation and characterization of Aurantiochytrium species: High docosahexaenoic acid (DHA) production by the newly isolated microalga, Aurantiochytrium sp. SD116. J. Oleo Sci. 2013, 62, 143–151. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, P.; Zhu, Y.; Xie, C.; Ma, L.; Wang, X.; Bao, Z.; Yu, L. Improvement in the docosahexaenoic acid production of Schizochytrium sp. S056 by replacement of sea salt. Bioprocess Biosyst. Eng. 2016, 39, 315–321. [Google Scholar] [CrossRef]
- Ludevese-Pascual, G.; Dela Peña, M.; Tornalejo, J. Biomass production, proximate composition and fatty acid profile of the local marine thraustochytrid isolate, Schizochytrium sp. LEY 7 using low-cost substrates at optimum culture conditions. Aquac. Res. 2016, 47, 318–328. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Zhu, S.; Zhang, Y.; Sun, X.; Hu, X.; Huang, H.; Ren, L.J. Integration of lipidomic and transcriptomic profiles reveals novel genes and regulatory mechanisms of Schizochytrium sp. in response to salt stress. Bioresour. Technol. 2019, 294, 122231. [Google Scholar] [CrossRef]
- Wu, S.-T.; Yu, S.-T.; Lin, L.-P. Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem. 2005, 40, 3103–3108. [Google Scholar] [CrossRef]
- Hu, X.-C.; Ren, L.-J.; Chen, S.-L.; Zhang, L.; Ji, X.-J.; Huang, H. The roles of different salts and a novel osmotic pressure control strategy for improvement of DHA production by Schizochytrium sp. Bioprocess Biosyst. Eng. 2015, 38, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, F.; Chen, L.; Zhang, W. Metabolomic analysis reveals the responses of docosahexaenoic-acid-producing Schizochytrium under hyposalinity conditions. Algal Res. 2023, 70, 102987. [Google Scholar] [CrossRef]
- Wang, W.; Xue, Y.; Li, B.; Sheng, X.; Shi, Y.; Zou, Q.; Li, J.; Li, T.; Wang, X.; Xue, J. Effect of peroxisome proliferation and salt stress on enhancing the potential of microalgae as biodiesel feedstock. Renew. Sustain. Energy Rev. 2025, 212, 115398. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.-F.; Li, R.-Y.; Yang, W.-D.; Liu, J.-S.; Lin, C.S.K.; Balamurugan, S.; Li, H.-Y. TAG pathway engineering via GPAT2 concurrently potentiates abiotic stress tolerance and oleaginicity in Phaeodactylum tricornutum. Biotechnol. Biofuels 2020, 13, 160. [Google Scholar] [CrossRef]
- An, M.; Mou, S.; Zhang, X.; Zheng, Z.; Ye, N.; Wang, D.; Zhang, W.; Miao, J. Expression of fatty acid desaturase genes and fatty acid accumulation in Chlamydomonas sp. ICE-L under salt stress. Bioresour. Technol. 2013, 149, 77–83. [Google Scholar] [CrossRef]
- Atikij, T.; Syaputri, Y.; Iwahashi, H.; Praneenararat, T.; Sirisattha, S.; Kageyama, H.; Waditee-Sirisattha, R. Enhanced lipid production and molecular dynamics under salinity stress in green microalga Chlamydomonas reinhardtii (137C). Mar. Drugs 2019, 17, 484. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Ji, L.; Song, X.; Kuang, C. Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochem. 2007, 42, 210–214. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Li, C.; Li, H.; Chen, W.; Li, D. Transcriptome analyses reveal the DHA enhancement mechanism in Schizochytrium limacinum LD11 mutant. Algal Res. 2022, 67, 102868. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Wu, H.-X.; Lin, Y.-C.; Xu, Y.-S.; Ma, W.; Sun, X.-M.; Huang, H. Polyketide synthase acyltransferase domain swapping for enhanced epa recognition and efficient coproduction of EPA and DHA in Schizochytrium sp. J. Agric. Food Chem. 2024, 73, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Qin, Y.; Feng, S.; Yang, H. Dual stress factors adaptive evolution for high EPA production in Schizochytrium sp. and metabolomics mechanism analysis. Bioprocess Biosyst. Eng. 2024, 47, 863–875. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, T.; Li, L.; Lou, F.; Ma, M.; Liu, R.; Jin, Q.; Wang, X. Choreography of multiple omics reveals the mechanism of lipid turnover in Schizochytrium sp. S31. Algal Res. 2021, 54, 102182. [Google Scholar] [CrossRef]
- Sutton, G.C.; Quinn, P.J.; Russell, N.J. The effect of salinity on the composition of fatty acid double-bond isomers and sn-1/sn-2 positional distribution in membrane phospholipids of a moderately halophilic Eubacterium. Curr. Microbiol. 1990, 20, 43–46. [Google Scholar] [CrossRef]
- Wayne, L.L.; Gachotte, D.J.; Graupner, P.R.; Adelfinskaya, Y.; McCaskill, D.G.; Metz, J.G.; Zirkle, R.; Walsh, T.A. Plant and algal lysophosphatidic acid acyltransferases increase docosahexaenoic acid accumulation at the sn-2 position of triacylglycerol in transgenic Arabidopsis seed oil. PLoS ONE 2021, 16, e0256625. [Google Scholar] [CrossRef]
- Shen, L.; Li, F.; Jiang, C.; Cao, X.; Jin, J.; Wang, X.; Wei, W. Comparative analysis of DHA positional distribution and triacylglycerol molecular species in algal oil (Schizochytrium sp.) from different oil processing. Food Biosci. 2024, 58, 103634. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, L.; Yang, B.; Zhao, J.; Chen, W.; Chen, H. Dynamic Changes in Lipid Composition Reveal the Mechanism of Arachidonic Acid Accumulation in Mortierella alpina. Food Biosci. 2025, 2025, 106019. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Luo, M.; Chen, F. Utilization of enzymatic cell disruption hydrolysate of Chlorella pyrenoidosa as potential carbon source in algae mixotrophic cultivation. Algal Res. 2020, 45, 101730. [Google Scholar] [CrossRef]
- He, Y.; Huang, Z.; Zhong, C.; Guo, Z.; Chen, B. Pressurized liquid extraction with ethanol as a green and efficient technology to lipid extraction of Isochrysis biomass. Bioresour. Technol. 2019, 293, 122049. [Google Scholar] [CrossRef]

| Fatty Acid | Salinity Concentration (‰) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Schizochytrium sp. FJ-1 | Schizochytrium sp. 20888 | ||||||||
| 0 | 5 | 10 | 20 | 40 | 5 | 10 | 20 | 40 | |
| C12:0 | 6.35 ± 2.32 B | 4.83 ± 1.17 B | 5.11 ± 0.59 B | 6.63 ± 0.23 B | 16.68 ± 1.83 A | - | - | - | - |
| C14:0 | 1.02 ± 0.06 | 0.73 ± 0.17 | 0.59 ± 0.15 | 0.85 ± 0.12 | 1.47 ± 0.34 | 2.69 ± 0.85 B | 2.65 ± 0.25 B | 3.28 ± 0.22 B | 6.66 ± 0.08 A |
| C15:0 | 1.78 ± 0.47 C | 10.59 ± 1.90 B | 8.76 ± 1.66 B | 16.57 ± 1.26 A | 11.82 ± 1.64 B | 9.58 ± 1.40 A | 5.50 ± 0.22 B | 3.80 ± 0.11 C | 1.52 ± 0.04 C |
| C16:0 | 15.69 ± 1.81 A | 16.49 ± 2.24 A | 8.43 ± 0.07 B | 9.84 ± 0.68 B | 7.82 ± 0.51 B | 28.49 ± 5.42 A | 15.48 ± 1.01 C | 15.30 ± 0.74 C | 24.30 ± 0.59 B |
| C16:1 | 0.64 ± 0.16 B | 1.88 ± 0.25 B | 3.05 ± 0.68 B | 3.44 ± 0.63 B | 10.65 ± 1.77 A | 0.90 ± 0.28 A | 0.43 ± 0.05 B | 0.29 ± 0.07 B | 0.61 ± 0.03 AB |
| C17:0 | 0.79 ± 0.20 C | 8.14 ± 1.30 A | 4.63 ± 0.27 B | 6.24 ± 0.62 AB | 4.47 ± 0.75 B | 4.10 ± 0.70 A | 2.26 ± 0.06 A | 1.55 ± 0.06 A | 0.69 ± 0.10 B |
| C18:0 | 9.64 ± 0.52 A | 1.85 ± 0.21 B | 1.56 ± 0.44 B | 1.98 ± 0.26 B | 1.90 ± 0.46 B | 1.34 ± 0.16 | 0.90 ± 0.03 | 0.90 ± 0.11 | 0.92 ± 0.16 |
| C18:1 | 6.73 ± 2.70 A | 1.06 ± 0.64 B | 1.54 ± 1.09 B | 0.81 ± 0.65 B | 0.72 ± 0.07 B | 1.80 ± 0.71 A | 0.16 ± 0.01 B | 0.10 ± 0.01 B | 0.16 ± 0.01 B |
| EPA | 4.36 ± 2.14 B | 2.75 ± 0.16 B | 7.05 ± 0.76 A | 6.44 ± 0.56 A | 7.17 ± 0.40 A | 0.55 ± 0.06 B | 1.64 ± 0.10 A | 1.83 ± 0.20 AB | 0.91 ± 0.02 B |
| DPA | 21.12 ± 1.69 A | 17.04 ± 1.28 A | 17.92 ± 0.52 A | 13.83 ± 1.22 B | 11.16 ± 0.13 C | 12.89 ± 1.59 C | 20.10 ± 0.15 A | 19.64 ± 0.23 AB | 16.41 ± 0.20 B |
| DHA | 31.90 ± 3.56 C | 34.64 ± 5.57 B | 41.38 ± 0.85 A | 33.37 ± 1.55 B | 26.15 ± 1.15 C | 37.63 ± 3.77 C | 50.89 ± 0.81 A | 53.31 ± 1.03 A | 47.79 ± 0.63 B |
| ΣSAFs | 35.27 ± 0.69 A | 42.63 ± 0.43A | 29.07 ± 0.54 C | 43.28 ± 0.89 A | 44.15 ± 0.91 A | 43.69 ± 0.19 A | 26.78 ± 1.02 C | 24.84 ± 1.46 C | 34.08 ± 0.56 B |
| ΣMUFAs | 7.37 ± 2.83 B | 2.94 ± 0.98 C | 4.59 ± 0.77 B | 4.52 ± 1.51 B | 11.37 ± 1.80 A | 2.13 ± 0.14 A | 0.59 ± 0.04 B | 0.38 ± 0.14 B | 0.76 ± 0.03 B |
| ΣPUFAs | 57.37 ± 0.41 B | 54.43 ± 0.56 C | 66.35 ± 2.12 A | 52.21 ± 0.62 B | 44.48 ± 0.90 C | 54.13 ± 0.39 C | 72.63 ± 1.07 A | 74.78 ± 1.56 A | 65.20 ± 0.56 B |
| Strain | Total Lipid (%) | EPA (%) | DPA (%) | DHA (%) | Ref. |
|---|---|---|---|---|---|
| S. limacinum SR21 | 41.33 | 0.71 | 7.63 | 36.65 | [29] |
| S. limacinum B4D1 | 55.98 | 0.42 | 7.71 | 38.70 | [30] |
| Schizochytrium sp. HX-308 | 50.82 | 0.80 | 18.10 | 48.19 | [31] |
| Schizochytrium sp. S056 | 47.27 | 0.67 | 7.25 | 40.23 | [19] |
| Schizochytrium sp. M20231041 | 60.70 | 7.20 | 21.18 | 33.41 | [32] |
| Schizochytrium sp. S31 (ATCC 20888) | 51.70 | 2.82 | 19.95 | 50.43 | [33] |
| Schizochytrium sp. FJ-1 | 57.81 | 7.05 | 17.92 | 41.38 | This work |
| Fatty Acid | Salinity Concentration (‰) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Schizochytrium sp. FJ-1 | Schizochytrium sp. 20888 | ||||||||
| 0 | 5 | 10 | 20 | 40 | 5 | 10 | 20 | 40 | |
| C12:0 | 1.59 ± 0.25 | 0.78 ± 0.29 | 0.64 ± 0.19 | 0.81 ± 0.26 | 1.36 ± 1.18 | - | - | - | - |
| C14:0 | 1.46 ± 0.01 | 0.69 ± 0.16 | 0.9 ± 0.16 | 1.56 ± 0.01 | 1.37 ± 0.55 | 2.55 ± 0.73 B | 3.01 ± 0.40 A | 3.88 ± 0.34 A | 6.81 ± 0.83 A |
| C15:0 | 1.84 ± 0.63 B | 7.57 ± 1.13 C | 12.53 ± 2.29 B | 24.43 ± 1.22 A | 15.54 ± 0.26 B | 8.99 ± 1.61 A | 5.51 ± 0.75 AB | 4.38 ± 0.19 B | 1.48 ± 0.2 C |
| C16:0 | 25.41 ± 1.68 A | 20.51 ± 3.14 AB | 11.53 ± 0.61 C | 14.39 ± 3.32 B | 16.89 ± 0.84 B | 27.61 ± 5.01 A | 16.23 ± 2.41 B | 18.27 ± 0.84 B | 23.9 ± 2.49 A |
| C16:1 | 3.67 ± 2.36 B | 8.35 ± 6.67 AB | 3.17 ± 0.75 B | 2.66 ± 2.02 C | 13.20 ± 2.32 A | 0.98 ± 0.17 | 0.57 ± 0.21 | 0.50 ± 0.07 | 0.73 ± 0.15 |
| C17:0 | 0.91 ± 0.22 B | 6.76 ± 1.02 A | 6.18 ± 0.75 A | 9.08 ± 0.6 A | 5.90 ± 1.19 A | 3.69 ± 0.78 A | 2.15 ± 0.34 AB | 1.72 ± 0.11 B | 0.74 ± 0.10 B |
| C18:0 | 20.58 ± 1.77 A | 6.22 ± 1.9 B | 2.6 ± 0.23 C | 3.41 ± 2.07 B | 7.51 ± 0.15 B | 1.79 ± 0.60 | 0.90 ± 0.16 | 1.17 ± 0.17 | 0.87 ± 0.16 |
| C18:1 | 2.86 ± 0.45 A | 0.46 ± 0.19 B | 0.34 ± 0.11 B | 0.25 ± 0.16 B | 0.29 ± 0.13 B | 1.83 ± 0.65 A | 0.12 ± 0.01 B | 0.14 ± 0.05 B | 0.17 ± 0.01 B |
| EPA | 2.99 ± 0.04 B | 1.99 ± 0.42 B | 4.88 ± 0.8 A | 3.23 ± 0.23 B | 3.50 ± 0.25 B | 0.58 ± 0.05 | 1.59 ± 0.26 | 1.75 ± 0.21 | 0.90 ± 0.09 |
| DPA | 15.23 ± 0.11 A | 13.88 ± 2.86 A | 17.06 ± 0.67 A | 12.31 ± 2.51 AB | 10.18 ± 2.01 B | 12.80 ± 1.30 B | 19.32 ± 0.53 A | 18.72 ± 0.11 A | 16.17 ± 0.65 A |
| DHA | 23.62 ± 0.19 C | 32.79 ± 7.74 B | 40.18 ± 1.6 A | 27.94 ± 6.59 BC | 24.27 ± 2.73 C | 39.20 ± 3.50 B | 50.61 ± 3.50 A | 49.46 ± 1.03 A | 48.23 ± 3.17 A |
| ΣSAFs | 51.63 ± 0.45 AB | 42.53 ± 0.42 C | 37.37 ± 0.8 C | 53.61 ± 0.23 A | 48.56 ± 1.31 B | 44.61 ± 0.13 A | 27.79 ± 0.34 B | 29.43 ± 0.21 B | 33.8 ± 0.07 B |
| ΣMUFAs | 6.53 ± 0.67 B | 8.81 ± 0.75 AB | 0.51 ± 1.02 C | 2.91 ± 0.60 C | 13.49 ± 0.70 A | 2.81 ± 1.03 | 0.69 ± 0.16 | 0.64 ± 0.53 | 0.9 ± 0.11 |
| ΣPUFAs | 41.84 ± 0.22 B | 48.66 ± 1.9 B | 62.12 ± 0.75 A | 43.48 ± 2.14 B | 37.95 ± 0.67 C | 52.58 ± 0.13 C | 71.52 ± 2.48 A | 69.93 ± 0.16 A | 65.3 ± 1.23 B |
| Schizochytrium Strain | EPA and DPA/DHA at the sn-1,3 Positions | EPA and DPA/DHA at the sn-2 Position | ||
|---|---|---|---|---|
| EPA | DPA/DHA | EPA | DPA/DHA | |
| Schizochytrium sp. FJ-1 | ||||
| 0‰ salinity | 46.67 ± 3.53 | 56.80 ± 3.08 | 53.33 ± 2.86 | 43.20 ± 2.78 |
| 5‰ salinity | 30.76 ± 2.67 | 35.34 ± 2.77 | 69.23 ± 4.32 | 64.66 ± 2.58 |
| 10‰ salinity | 32.33 ± 2.82 | 36.76 ± 2.31 | 67.67 ± 2.55 | 63.24 ± 4.02 |
| 20‰ salinity | 31.58 ± 2.64 | 37.04 ± 2.69 | 68.42 ± 3.58 | 62.96 ± 2.96 |
| 40‰ salinity | 35.45 ± 3.05 | 18.76 ± 1.58 | 64.55 ± 3.06 | 81.24 ± 4.05 |
| Schizochytrium sp. 20888 | ||||
| 5‰ salinity | 57.69 ± 2.56 | 38.46 ± 2.47 | 42.31 ± 1.96 | 61.54 ± 2.66 |
| 10‰ salinity | 32.08 ± 2.53 | 35.46 ± 1.98 | 67.92 ± 2.55 | 64.54 ± 2.07 |
| 20‰ salinity | 51.10 ± 2.66 | 32.05 ± 2.38 | 48.90 ± 2.75 | 67.95 ± 3.81 |
| 40‰ salinity | 48.25 ± 2.96 | 31.15 ± 1.63 | 51.75 ± 2.82 | 68.84 ± 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Wang, X.; Zhou, Y.; Xiao, X.; Liu, P.; Chi, C.; Sun, P.; Zheng, M.; Chen, B.; Mao, R.; et al. Effects of Salinity on the Growth Performance and Docosahexaenoic Acid Positional Distribution in Triacylglycerols of the Newly Isolated Schizochytrium sp. FJ-1. Mar. Drugs 2025, 23, 260. https://doi.org/10.3390/md23070260
Ye S, Wang X, Zhou Y, Xiao X, Liu P, Chi C, Sun P, Zheng M, Chen B, Mao R, et al. Effects of Salinity on the Growth Performance and Docosahexaenoic Acid Positional Distribution in Triacylglycerols of the Newly Isolated Schizochytrium sp. FJ-1. Marine Drugs. 2025; 23(7):260. https://doi.org/10.3390/md23070260
Chicago/Turabian StyleYe, Sitong, Xiaonan Wang, Youcai Zhou, Xuehua Xiao, Pingying Liu, Chengdeng Chi, Peipei Sun, Mingmin Zheng, Bilian Chen, Ruoyu Mao, and et al. 2025. "Effects of Salinity on the Growth Performance and Docosahexaenoic Acid Positional Distribution in Triacylglycerols of the Newly Isolated Schizochytrium sp. FJ-1" Marine Drugs 23, no. 7: 260. https://doi.org/10.3390/md23070260
APA StyleYe, S., Wang, X., Zhou, Y., Xiao, X., Liu, P., Chi, C., Sun, P., Zheng, M., Chen, B., Mao, R., & He, Y. (2025). Effects of Salinity on the Growth Performance and Docosahexaenoic Acid Positional Distribution in Triacylglycerols of the Newly Isolated Schizochytrium sp. FJ-1. Marine Drugs, 23(7), 260. https://doi.org/10.3390/md23070260








