The Conopeptide αD-FrXXA, an Inhibitor of Voltage-Gated Potassium Channels
Abstract
1. Introduction
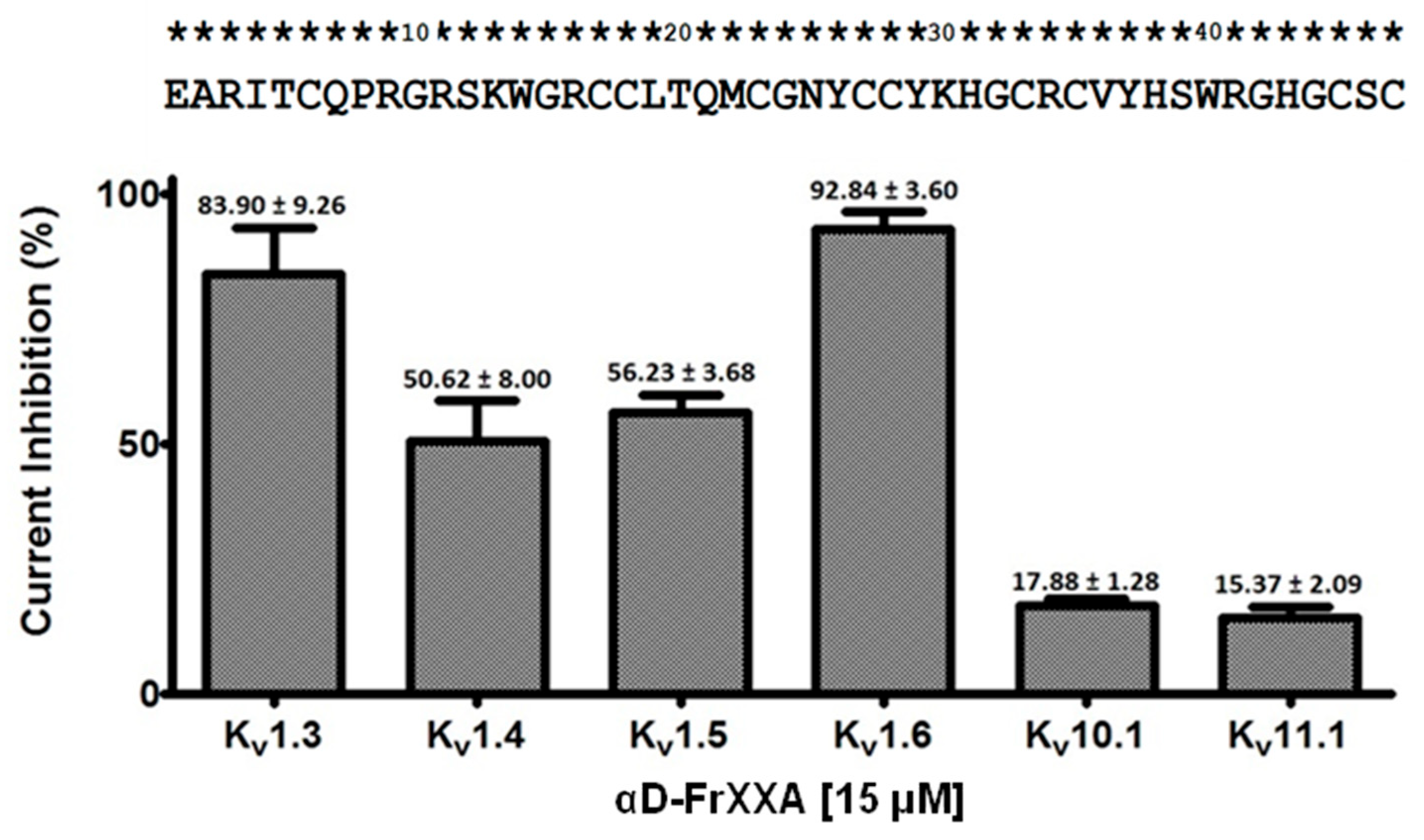
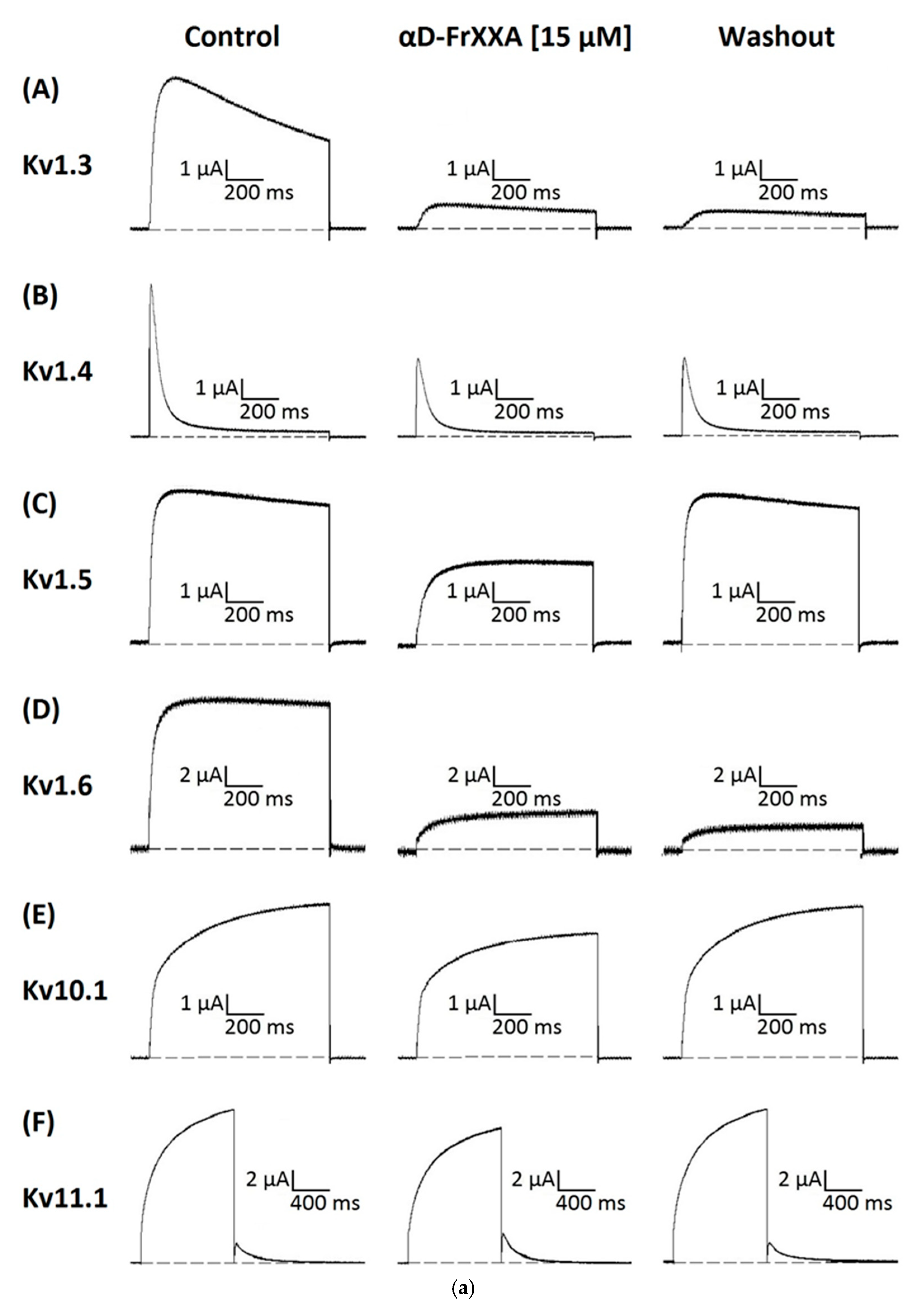
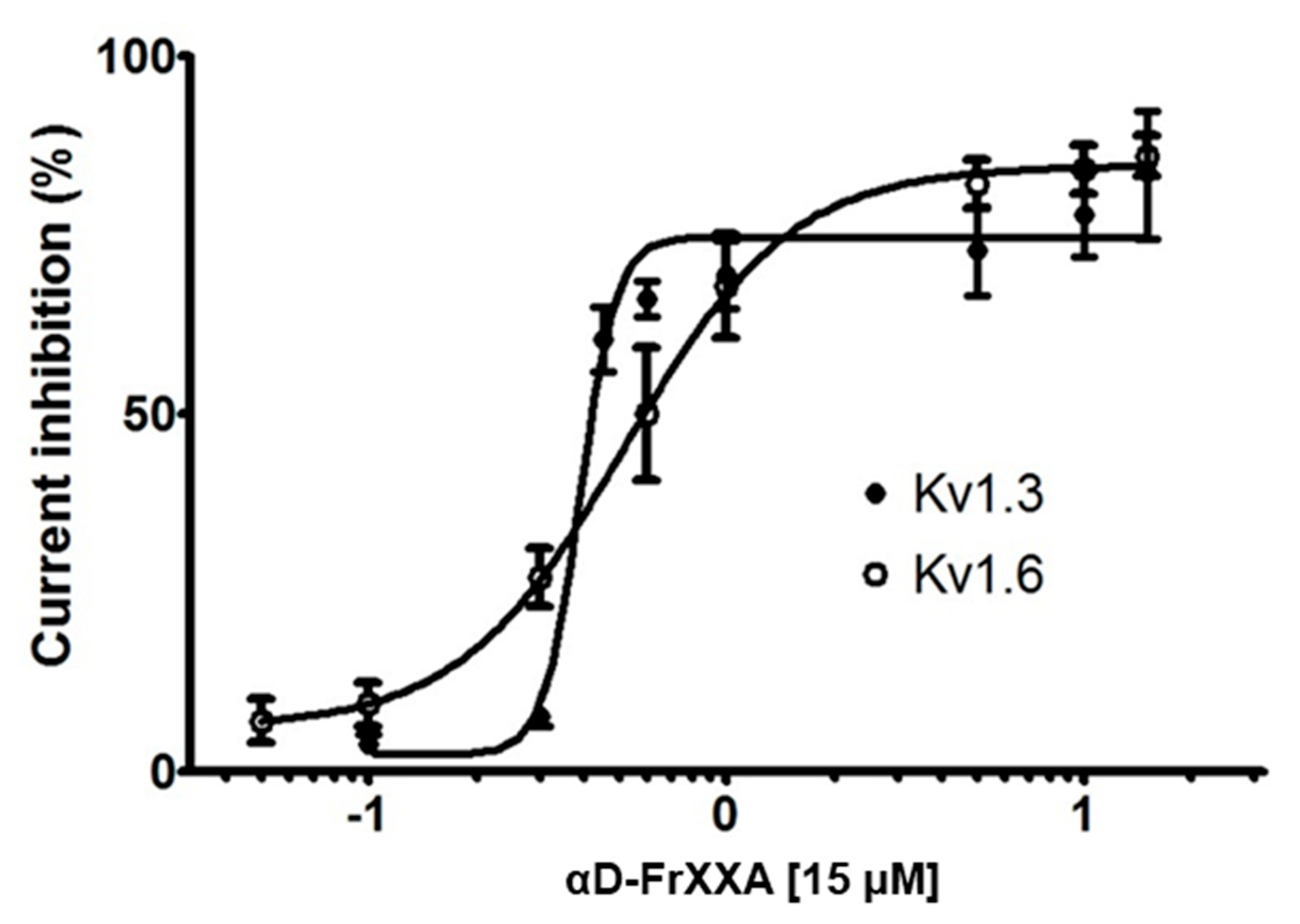
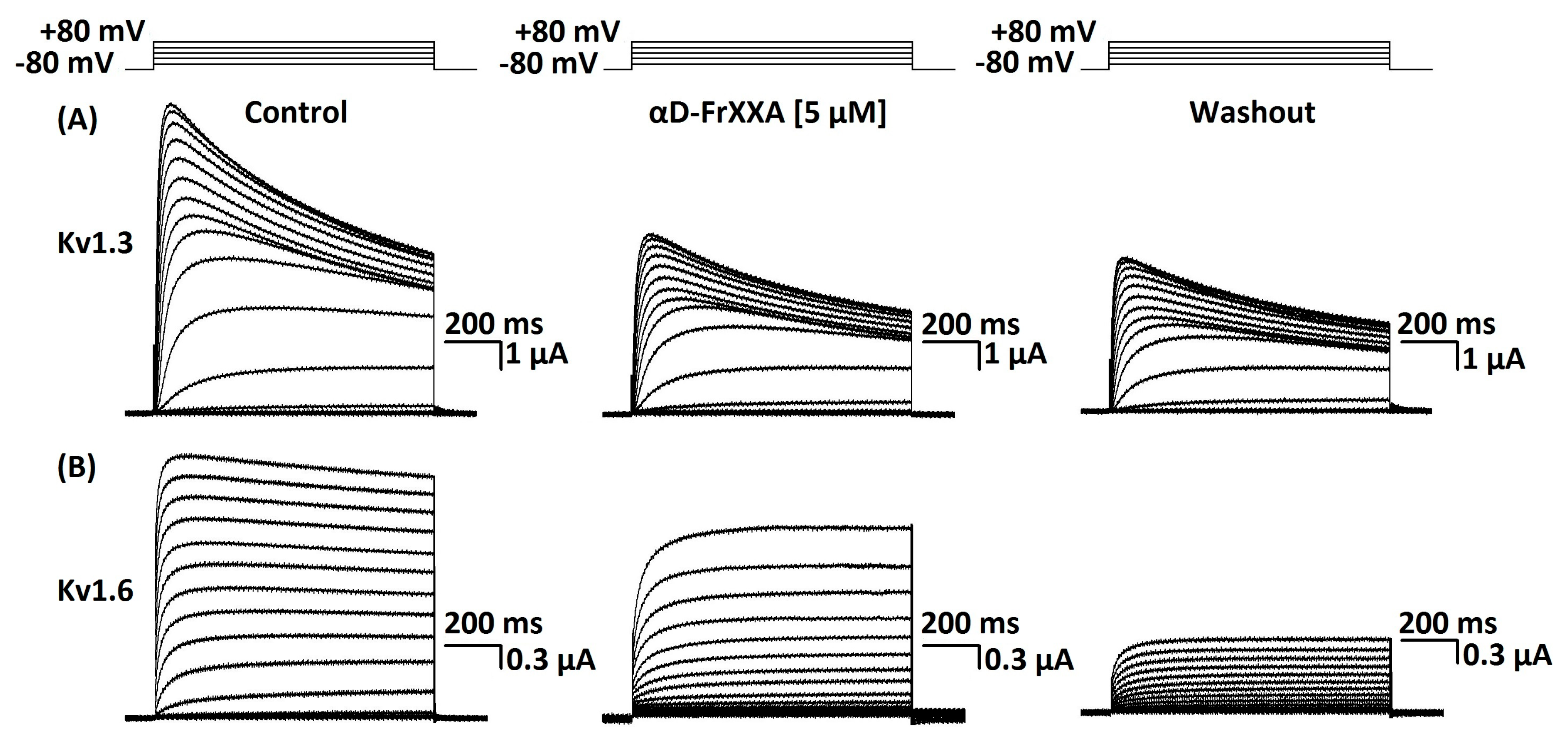
2. Results
Activity of αD-FrXXA on Kv1 and EAG Subfamilies
3. Discussion
4. Materials and Methods
4.1. Isolation of αD-FrXXA from Crude Venom Extract of Conus fergusoni
4.2. Preparation of Vectors Encoding Potassium Channels
4.3. Expression of Voltage-Gated Potassium Channels
4.4. Electrophysiological Recordings
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivera, B.M. A Serendipitous Path to Pharmacology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.T.; Craik, D.J.; Kaas, Q. Bibliometric Review of the Literature on Cone Snail Peptide Toxins from 2000 to 2022. Mar. Drugs 2023, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.A.; Arkhipov, A.; Yum, W.Y.; Lewis, R.J. Comparative Venomics of C. flavidus and C. frigidus and Closely Related Vermivorous Cone Snails. Mar. Drugs 2022, 20, 209. [Google Scholar] [CrossRef]
- Loughnan, M.; Nicke, A.; Jones, A.; Schroeder, C.I.; Nevin, S.T.; Adams, D.J.; Alewood, P.F.; Lewis, R.J. Identification of a novel class of nicotinic receptor antagonists: Dimeric conotoxins VxXIIA, VxXIIB, and VxXIIC from Conus vexillum. JBC 2006, 281, 24745–24755. [Google Scholar] [CrossRef]
- Bjørn-Yoshimoto, W.E.; Ramiro, I.B.L.; Yandell, M.; McIntosh, J.M.; Olivera, B.M.; Ellgaard, L.; Safavi-Hemami, H. Curses or Cures: A Review of the Numerous Benefits Versus the Biosecurity Concerns of Conotoxin Research. Biomedicines 2020, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.H.; Nybakken, J. On the growth stages of Conus fergusoni Sowerby, 1873, and the reinstatement of Conus xanthicus Dall, 1910, and a new species of Conus from the Galapagos Islands. Veliger 1979, 22, 135–144. [Google Scholar]
- Rodriguez-Ruiz, X.C.; Aguilar, M.B.; Ortíz-Arellano, M.A.; Safavi-Hemami, H.; López-Vera, E. A Novel Dimeric Conotoxin, FrXXA, from the Vermivorous Cone Snail Conus fergusoni, of the Eastern Pacific, Inhibits Nicotinic Acetylcholine Receptors. Toxins 2022, 14, 510. [Google Scholar] [CrossRef]
- Martínez-Hernández, L.; López-Vera, E.; Aguilar, M.B. Peptide Toxins from Marine Conus Snails with Activity on Potassium Channels and/or Currents. Toxins 2024, 16, 504. [Google Scholar] [CrossRef]
- Imperial, J.S.; Bansal, P.S.; Alewood, P.F.; Daly, N.L.; Craik, D.J.; Sporning, A.; Terlau, H.; López-Vera, E.; Bandyopadhyay, P.K.; Olivera, B.M. A Novel Conotoxin Inhibitor of Kv1.6 Channel and nAChR Subtypes Defines a New Superfamily of Conotoxins. Biochemistry 2006, 45, 8331–8340. [Google Scholar] [CrossRef]
- Kaas, Q.; Westermann, J.C.; Halai, R.; Wang, C.K.L.; Craik, D.J. ConoServer, a database for conopeptide sequences and structures. Bioinformatics 2008, 24, 445–446. [Google Scholar] [CrossRef]
- Abraham, N.; Lewis, R.J. Neuronal Nicotinic Acetylcholine Receptor Modulators from Cone Snails. Mar. Drugs 2018, 16, 208. [Google Scholar] [CrossRef]
- Kompella, S.N.; Xu, S.; Zhang, T.; Yan, M.; Shao, X.; Chi, C.; Ding, J.; Wang, C.; Adams, D.J. Novel strategy of blocking nAChR revealed by dissecting a dimeric conotoxin αD-GeXXA. Biochem. Pharmacol. 2013, 86, 1229. [Google Scholar] [CrossRef]
- Hernández-Sámano, A.C.; Falcón, A.; Zamudio, F.; Batista, C.V.F.; Michel-Morfín, J.E.; Landa-Jaime, V.; López-Vera, E.; Jeziorski, M.C.; Aguilar, M.B. αD-Conotoxins in Species of the Eastern Pacific: The Case of Conus princeps from Mexico. Toxins 2019, 12, 405. [Google Scholar] [CrossRef]
- Kauferstein, S.; Kendel, Y.; Nicke, A.; Coronas, F.I.V.; Possani, L.D.; Favreau, P.; Krizaj, I.; Wunder, C.; Kauert, G.; Mebs, D. New conopeptides of the D-superfamily selectively inhibiting neuronal nicotinic acetylcholine receptors. Toxicon 2009, 54, 295–301. [Google Scholar] [CrossRef]
- Kryukova, V.E.; Ivanov, A.I.; Lebedev, S.D.; Spirova, N.E.; Egorova, S.N.; Zouridakis, M.; Kasheverov, E.I.; Tzartos, J.S.; Tsetlin, I.V. Orthosteric and/or Allosteric Binding of α-Conotoxins to Nicotinic Acetylcholine Receptors and Their Models. Mar. Drugs 2018, 16, 460. [Google Scholar] [CrossRef]
- Luo, L.; Li, B.; Wang, S.; Wu, F.; Wang, X.; Liang, P.; Ombati, R.; Chen, J.; Lu, X.; Cui, J.; et al. Centipedes subdue giant prey by blocking KCNQ channels. Proc. Natl. Acad. Sci. USA 2018, 115, 1646–1651. [Google Scholar] [CrossRef]
- Jacobsen, R.B.; Koch, D.E.; Lange-Malecki, B.; Stocker, M.; Verhey, J.; Van Wagoner, R.M.; Vyazovkina, A.; Olivera, B.M.; Terlau, H. Single amino acid substitutions in κ-conotoxin PVIIA disrupt interaction with the Shaker K+ channel. J. Biol. Chem. 2000, 275, 24639–24644. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.; Cui, M.; Fu, W.; Yu, K.; Chen, K.; Luo, X.; Shen, J.; Jiang, H. Simulating the Interactions of toxins with K+ channels. Curr. Pharm. Des. 2004, 10, 1057–1067. [Google Scholar] [CrossRef]
- Huang, X.; Dong, F.; Zhou, H.-X. Electrostatic recognition and induced fit in the κ-PVIIA toxin binding to Shaker potassium channel. J. Am. Chem. Soc. 2005, 127, 6836–6849. [Google Scholar] [CrossRef]
- Karbat, I.; Altman-Gueta, H.; Fine, S.; Szanto, T.; Hamer-Rogotner, S.; Dym, O.; Frolow, F.; Gordon, D.; Panyi, G.; Gurevitz, M.; et al. Pore-modulating toxins exploit inherent slow inactivation to block K+ channels. Proc. Natl. Acad. Sci. USA 2019, 116, 18700–18709. [Google Scholar] [CrossRef]
- Saikia, C.; Dym, O.; Altman-Gueta, H.; Gordon, D.; Reuveny, E.; Karbat, I. A Molecular Lid Mechanism of K+ Channel Blocker Action Revealed by a Cone Peptide. J. Mol. Biol. 2021, 433, 166957. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Safo, P.; Rosenbaum, T.; Shcherbatko, A.; Choi, D.Y.; Han, E.; Toledo-Aral, J.J.; Olivera, B.M.; Brehm, P.; Mandel, G. Distinction among Neuronal Subtypes of Voltage-Activated Sodium Channels by μ-Conotoxin PIIIA. J. Neurosci. 2000, 20, 76–80. [Google Scholar] [CrossRef]
- Teichert, R.W.; López-Vera, E.; Gulyas, J.; Watkins, M.; Rivier, J.; Olivera, B.M. Definition and Characterization of the Short αA-Conotoxins: A Single Residue Determines Dissociation Kinetics from the Fetal Muscle Nicotinic Acetylcholine Receptor. Biochemistry 2006, 45, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Dutertre, S.; Vetter, I.; Christie, M. Conus Venom Peptide Pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef]
- Leipold, E.; Ullrich, F.; Thiele, M.; Tietze, A.A.; Terlau, H.; Imhof, D.; Heinemann, S.H. Subtype-specific block of voltage-gated K+ channels by μ-conopeptides. Biochem. Biophys. Res. Commun. 2017, 482, 1135–1140. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, L.; López-Vera, E.; Rodriguez-Ruiz, X.C.; Ortíz-Arellano, M.A. The Conopeptide αD-FrXXA, an Inhibitor of Voltage-Gated Potassium Channels. Mar. Drugs 2025, 23, 237. https://doi.org/10.3390/md23060237
Martínez-Hernández L, López-Vera E, Rodriguez-Ruiz XC, Ortíz-Arellano MA. The Conopeptide αD-FrXXA, an Inhibitor of Voltage-Gated Potassium Channels. Marine Drugs. 2025; 23(6):237. https://doi.org/10.3390/md23060237
Chicago/Turabian StyleMartínez-Hernández, Luis, Estuardo López-Vera, Ximena C. Rodriguez-Ruiz, and Mónica A. Ortíz-Arellano. 2025. "The Conopeptide αD-FrXXA, an Inhibitor of Voltage-Gated Potassium Channels" Marine Drugs 23, no. 6: 237. https://doi.org/10.3390/md23060237
APA StyleMartínez-Hernández, L., López-Vera, E., Rodriguez-Ruiz, X. C., & Ortíz-Arellano, M. A. (2025). The Conopeptide αD-FrXXA, an Inhibitor of Voltage-Gated Potassium Channels. Marine Drugs, 23(6), 237. https://doi.org/10.3390/md23060237







