Paralytic Shellfish Toxins in Coastal Waters of Changdao Island (China): Toxin Profiles, Potential Producers, and Environmental Conditions
Abstract
1. Introduction
2. Results
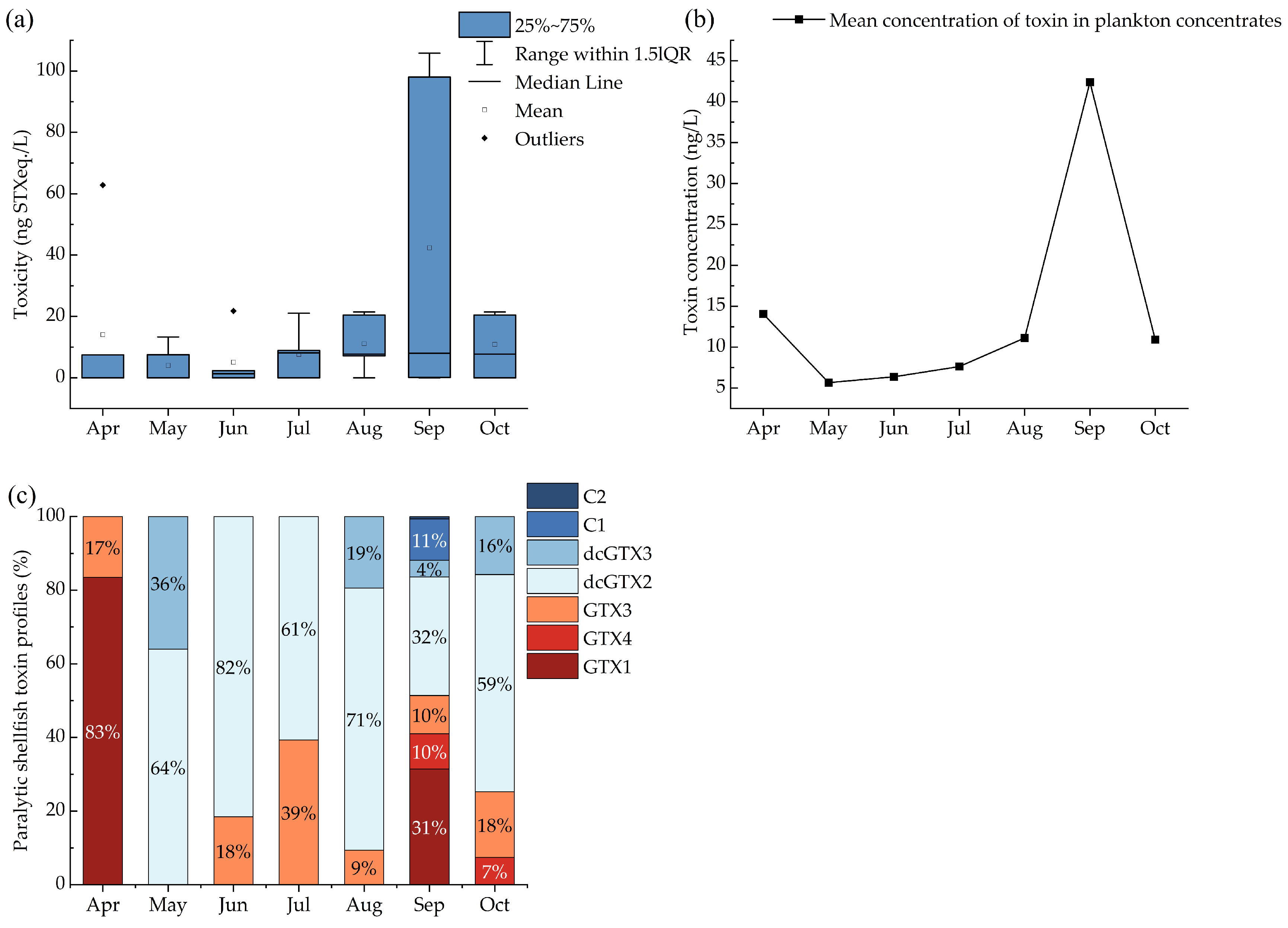
2.1. Toxin Profiles and Concentrations of PSTs in Plankton Filters
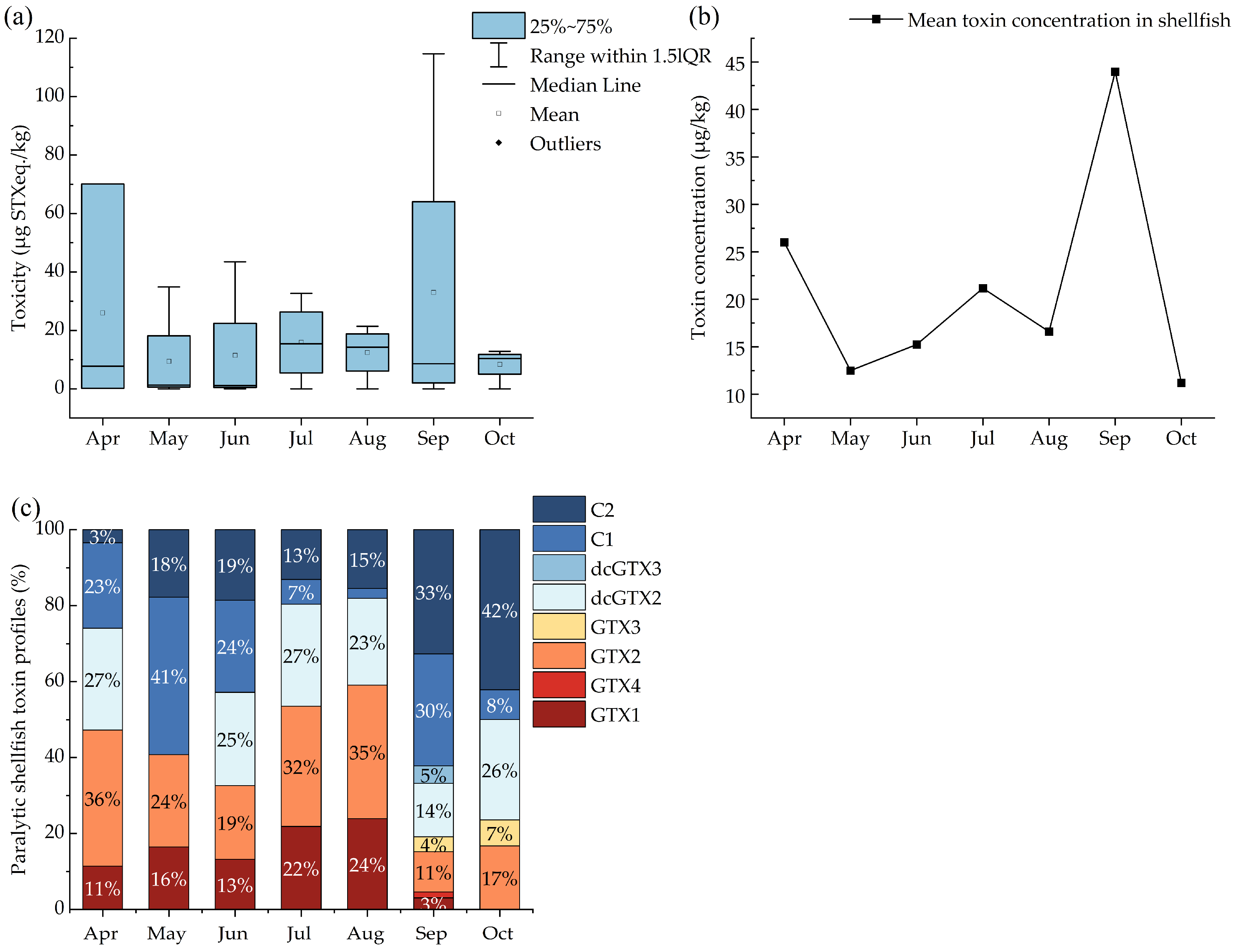
2.2. Seasonal Distribution of PSTs in Shellfish Samples
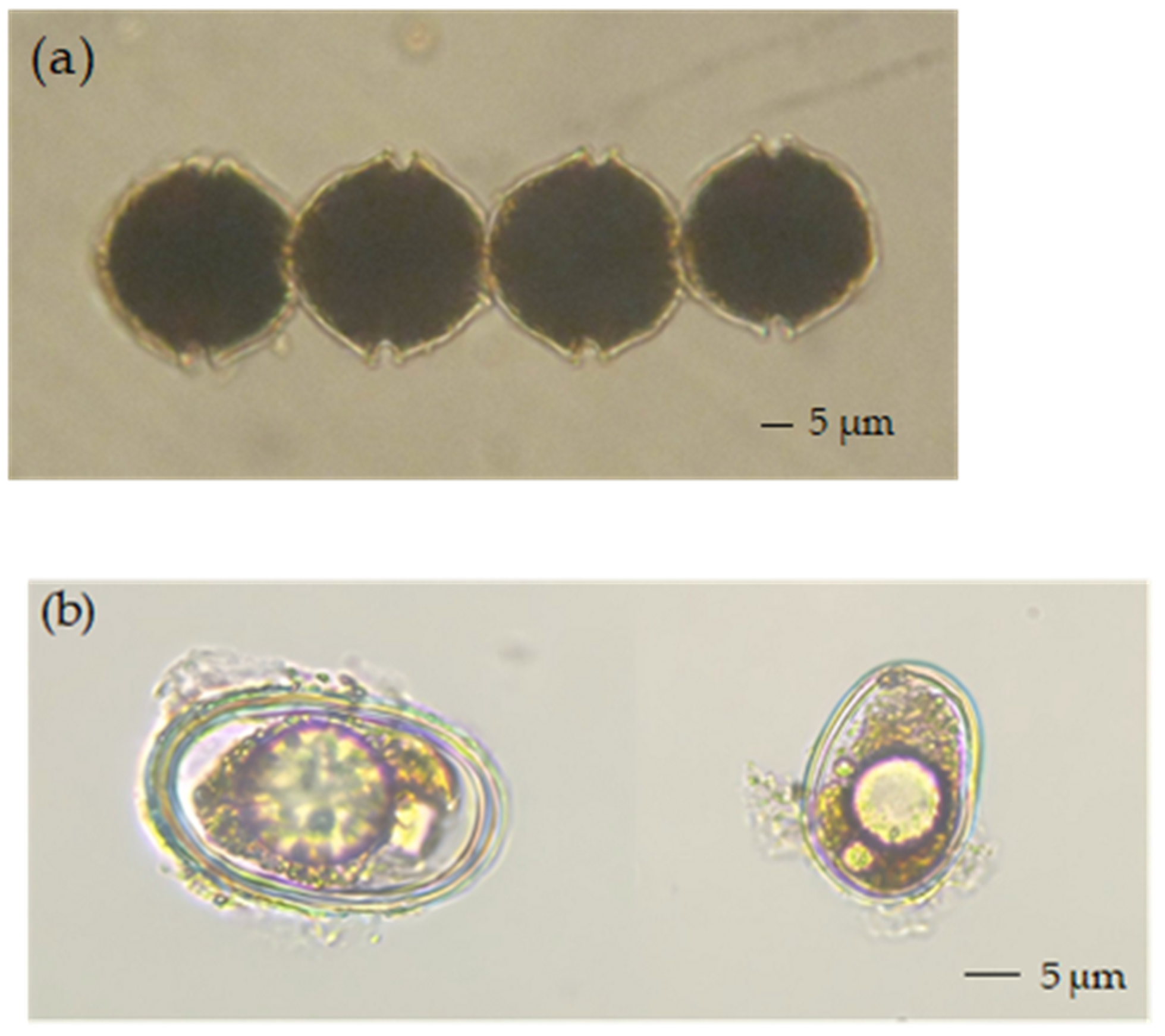
2.3. Preliminary Identification of the PST-Producing Algal Genera
2.4. Monthly Variations in Environmental Parameters
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Field Sample Collection, Storage, and Analysis
4.3. Sample Processing for Toxin Extraction and Analyses
4.4. LC-MS/MS Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, W.J.; Su, S.K.; Mohamed, H.F.; Xiao, J.M.; Kang, J.H.; Krock, B.; Xie, B.; Luo, Z.H.; Chen, B. Assessing the global distribution and risk of harmful microalgae: A focus on three toxic Alexandrium dinoflagellates. Sci. Total Environ. 2024, 948, 174767. [Google Scholar] [CrossRef]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Soliño, L.; Carnicer, O.; Diogène, J.; Campàs, M. Alternative Methods for the Detection of Emerging Marine Toxins: Biosensors, Biochemical Assays and Cell-Based Assays. Mar. Drugs 2014, 12, 5719–5763. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Ann. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Li, S.W.; He, J.; Sun, J.F.; Pan, F.; Zhou, P.P.; Zhang, L.; Yang, X.; Cao, C.B.; Yu, X.J.; Cao, P. Risk assessment and spatial analysis of paralytic shellfish toxin (PST) concentrations and acute dietary exposure of commercially available shellfish in coastal areas in China. Food Control 2024, 160, 110331. [Google Scholar] [CrossRef]
- Cao, Y.D.; Qiu, J.B.; Li, A.F.; Zhang, L.; Yan, G.W.; Ji, Y.; Zhang, J.R.; Zhao, P.; Wu, X.Z. Occurrence and spatial distribution of paralytic shellfish toxins in seawater and marine organisms in the coastal waters of Qinhuangdao, China. Chemosphere 2023, 315, 137746. [Google Scholar] [CrossRef]
- Samantha, C.F. Saxitoxin and the induction of paralytic shellfish poisoning. J. Young Investig. 2012, 23, 1–7. Available online: https://api.semanticscholar.org/CorpusID:9424533 (accessed on 20 November 2024).
- Van Dolah, F.M.; Ramsdell, J.S. Review and assessment of in vitro detection methods for algal toxins. J. AOAC Int. 2001, 84, 1617–1625. [Google Scholar] [CrossRef]
- Yu, R.C.; Zhang, Q.C.; Liu, Y.; Chen, Z.F.; Geng, H.X.; Dai, L.; Lin, Z.R.; Tang, W.J.; Kong, F.Z.; Yan, T.; et al. The dinoflagellate Alexandrium catenella producing only carbamate toxins may account for the seafood poisonings in Qinhuangdao, China. Harmful Algae 2021, 103, 101980. [Google Scholar] [CrossRef]
- Morse, E.V. Paralytic shellfish poisoning: A review. JAVMA 1977, 171, 1178–1180. [Google Scholar] [CrossRef]
- Lin, Z.R.; Geng, H.X.; Yu, R.C. Potential roles of hydroxybenzoate paralytic shellfish toxins in modulating toxin biokinetics in scallops. J. Hazard. Mater. 2024, 469, 133896. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.P.; Bolch, C.J.S.; Geier, S.; Green, D.H.; Park, T.G.; Blackburn, S.I. Widespread presence of hydrophobic paralytic shellfish toxins in Gymnodinium catenatum. Harmful Algae 2007, 6, 774–780. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Shin, C.; Jo, H.; Kim, S.H.; Kang, G.J. Exposure assessment to paralytic shellfish toxins through the shellfish consumption in Korea. Food Res. Int. 2018, 108, 274–279. [Google Scholar] [CrossRef]
- Alonso, E.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Evaluation of toxicity equivalent factors of paralytic shellfish poisoning toxins in seven human sodium channels types by an automated high throughput electrophysiology system. Arch. Toxicol. 2016, 90, 479–488. [Google Scholar] [CrossRef]
- Leal, J.F.; Cristiano, M.L.S. Marine paralytic shellfish toxins: Chemical properties, mode of action, newer analogues, and structure-toxicity relationship. Nat. Prod. Rep. 2022, 39, 33–57. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Chain, E. Marine biotoxins in shellfish-Saxitoxin group. EFSA J. 2009, 7, 1019. [Google Scholar]
- Humpage, A.R.; Magalhaes, V.F.; Froscio, S.M. Comparison of analytical tools and biological assays for detection of paralytic shellfish poisoning toxins. Anal. Bioanal. Chem. 2010, 397, 1655–1671. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Lara-Martin, P.A. Analysis of paralytic shellfish toxins in seafood by liquid chromatography: A critical review of the methodologies developed. Trends. Environ. Anal. 2023, 37, e00190. [Google Scholar] [CrossRef]
- Turner, A.D.; Hatfield, R.G.; Rapkova, M.; Higman, W.; Algoet, M.; Suarez-Isla, B.A.; Cordova, M.; Caceres, C.; van de Riet, J.; Gibbs, R.; et al. Comparison of AOAC 2005.06 LC official method with other methodologies for the quantitation of paralytic shellfish poisoning toxins in UK shellfish species. Anal. Bioanal. Chem. 2011, 399, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Wu, Y.R.; Lü, S.H.; Lu, D.D.; Tang, Y.Z.; Qi, Y.Z. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae 2022, 111, 102059. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Zeng, N.; Liu, T.T.; Yang, W.D.; Müller, A.; Krock, B. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae 2013, 27, 68–81. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.Y.; Chai, Z.Y.; Hu, Z.X.; Tang, Y.Z. A combined approach detected novel species diversity and distribution of dinoflagellate cysts in the Yellow Sea, China. Mar. Pollut. Bull. 2023, 187, 114567. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, R.C.; Chen, J.H.; Zhang, Q.C.; Kong, F.Z.; Zhou, M.J. Distribution of Alexandrium fundyense and A. pacificum (Dinophyceae) in the Yellow Sea and Bohai Sea. Mar. Pollut. Bull. 2015, 96, 210–219. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, R.C.; Kong, F.Z.; Chen, Z.F.; Dai, L.; Gao, Y.; Zhang, Q.C.; Wang, Y.F.; Yan, T.; Zhou, M.J. Paralytic shellfish toxins in phytoplankton and shellfish samples collected from the Bohai Sea, China. Mar. Pollut. Bull. 2017, 115, 324–331. [Google Scholar] [CrossRef]
- Wu, H.Y.; Dong, C.F.; Zheng, G.C.; Zhang, Z.H.; Zhang, Y.Y.; Tan, Z.J.; Gu, H.F. Formation mechanism and environmental drivers of Alexandrium catenella bloom events in the coastal waters of Qinhuangdao, China. Environ. Pollut. 2022, 313, 120241. [Google Scholar] [CrossRef]
- Reis Costa, P. Impact and effects of paralytic shellfish poisoning toxins derived from harmful algal blooms to marine fish. Fish Fish. 2014, 17, 226–248. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, L.; Huang, Y.L.; Li, S.W.; Wang, X.D.; Pan, F.; Yu, X.J.; Sun, J.F.; Liang, J.; Zhou, P.P.; et al. Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China. Foods 2024, 13, 361. [Google Scholar] [CrossRef]
- Yu, R.C.; Lü, S.H.; Qi, Y.Z.; Zhou, M.J. Progress and perspectives of harmful algal bloom studies in China. Oceanol. Limnol. Sin. 2020, 51, 769–788. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.; Wu, Z.H.; Wang, Q.X.; Wang, Y.; Gao, X. The basic characteristics and prevention countermeasures of red tide in Shandong coast waters. Mar. Environ. 2020, 39, 538–543. [Google Scholar] [CrossRef]
- Fishery Bureau of the Ministry of Agriculture and Rural Affairs of China. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024; pp. 27–28. Available online: https://pdc.capub.cn/search.html#/detail?id=ofp2n3qfea (accessed on 20 November 2024).
- Chen, J.H.; Yang, J.B.; He, X.P.; Wang, J.M.; Pan, L.; Xin, M.; Chen, F.R.; Liang, S.K.; Wang, B.D. Prevalence of the neurotoxin domoic acid in the aquatic environments of the Bohai and Northern Yellow seas in China. Sci. Total. Environ. 2023, 876, 162732. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.B.; Li, A.F.; Chen, J.H.; Tan, Z.J.; Tong, M.M.; Liu, Z.R.; Qiu, J.B.; Yu, R.C. Progress on the investigation and monitoring of marine phycotoxins in China. Harmful Algae 2022, 111, 102152. [Google Scholar] [CrossRef]
- Chen, J.H.; Yu, R.C.; Gao, Y.; Kong, F.Z.; Wang, Y.F.; Zhang, Q.C.; Kang, Z.; Yan, T.; Zhou, M.J. Tracing the origin of paralytic shellfish toxins in scallop Patinopecten yessoensis in the northern Yellow Sea. Food. Addit. Contam. Part A 2013, 30, 1933–1945. [Google Scholar] [CrossRef]
- Zheng, G.C.; Xu, X.Z.; Wu, H.Y.; Fan, L.Q.; Wang, Q.R.; Peng, J.X.; Guo, M.M.; Yang, D.J.; Tan, Z.J. Contamination Status and Risk Assessment of Paralytic Shellfish Toxins in Shellfish along the Coastal Areas of China. Mar. Drugs. 2024, 22, 64. [Google Scholar] [CrossRef]
- Li, P.Y.; Qiu, J.B.; Han, L.L.; Li, A.F.; Ji, Y. Dynamic variations in profile of intra- and extracellular paralytic shellfish toxins during the growth cycle of Alexandrium species and their accumulation in mussels. Aquaculture 2024, 592, 741266. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Q.; Gibson, K.; Chen, Y.; Chen, N.S. Molecular characterization of harmful algal blooms in the Bohai Sea using metabarcoding analysis. Harmful Algae 2021, 106, 102066. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Du, J.; Li, N.; Song, G.J.; Wang, K.; Sun, M.; Wang, P. The Characteristics and Distribution of Eukaryotic Phytoplankton Community in Liaodong Bay, China. Ocean Sci. J. 2019, 54, 183–203. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, R.C.; Murray, S.A.; Chen, J.H.; Kang, Z.J.; Zhang, Q.C.; Kong, F.Z.; Zhou, M.J. High Specificity of a Quantitative PCR Assay Targeting a Saxitoxin Gene for Monitoring Toxic Algae Associated with Paralytic Shellfish Toxins in the Yellow Sea. Appl. Environ. Microbiol. 2015, 81, 6973–6981. [Google Scholar] [CrossRef]
- Ding, X.X.; Liu, S.Y.; Cui, Z.M.; Zhao, Y.F.; Chen, N.S. Differential spatial–temporal dynamics of Alexandrium species revealed using metabarcoding analysis by the 18S rDNA V4 region in Jiaozhou Bay, China. J. Appl. Phycol. 2023, 35, 1727–1742. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, L. An autecological study of the potentially toxic dinoflagellate Alexandrium affine isolated from Vietnamese waters. Harmful Algae 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Kwong, R.W.; Wang, W.X.; Lam, P.K.; Yu, P.K. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquat. Toxicol. 2006, 80, 82–91. [Google Scholar] [CrossRef]
- Tan, K.S.; Ransangan, J. Factors influencing the toxicity, detoxification and biotransformation of paralytic shellfish toxins. Rev. Environ. Contam. Toxicol. 2015, 235, 1–25. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic Shellfish Toxins in Bivalve Molluscs: Occurrence, Transfer Kinetics, and Biotransformation. Rev. Fish. Sci. 2010, 6, 315–383. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, Y.C.; Zhang, Q.R.; Zheng, G.C.; Geng, Q.Q.; Tan, Z.J. Immune and physiological responses of Mytilus unguiculatus to Alexandrium spp. with varying paralytic shellfish toxin profiles. Sci. Total. Environ. 2024, 935, 173483. [Google Scholar] [CrossRef]
- Wang, H.; Kim, H.; Park, H.; Ki, J.S. Temperature influences the content and biosynthesis gene expression of saxitoxins (STXs) in the toxigenic dinoflagellate Alexandrium pacificum. Sci. Total. Environ. 2022, 802, 149801. [Google Scholar] [CrossRef]
- Alonso-Rodriguez, R.; Pichardo-Velarde, J.G. Effects of temperature and nutrients on growth and toxicity of Alexandrium affine from southeastern Gulf of California. Mar. Pollut. Bull. 2024, 203, 116464. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yin, T.C.; Pang, M.; Wang, J. Toxicity variability in Alexandrium pacificum isolated from East China Sea: Impact of temperature and light intensity. J. Oceanol. Limnol. 2024, 1, 147–158. [Google Scholar] [CrossRef]
- Wang, Q.X.; Yan, Q.; Zhu, L.; Cui, Z.G.; Qu, K.M.; Wei, Y.Q. Environmental controls on the seasonal variations of diatoms and dinoflagellates in the Qingdao coastal region, the Yellow Sea. Mar. Environ. Res. 2024, 198, 106524. [Google Scholar] [CrossRef]
- Hinder, S.L.; Hays, G.C.; Edwards, M.; Roberts, E.C.; Walne, A.W.; Gravenor, M.B. Changes in marine dinoflagellate and diatom abundance under climate change. Nat. Clim. Chang. 2012, 2, 271–275. [Google Scholar] [CrossRef]
- Wang, Q.N.; Li, X.D.; Yan, T.; Song, J.J.; Yu, R.C.; Zhou, M.J. Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom. J. Oceanol. Limnol. 2020, 39, 500–507. [Google Scholar] [CrossRef]
- Ishikawa, A.; Hattori, M.; Ishii, K.I.; Kulis, D.M.; Anderson, D.M.; Imai, I. In situ dynamics of cyst and vegetative cell populations of the toxic dinoflagellate Alexandrium catenella in Ago Bay, central Japan. J. Plankton. Res. 2014, 36, 1333–1343. [Google Scholar] [CrossRef]
- Natsuike, M.; Yokoyama, K.; Nishitani, G.; Yamada, Y.; Yoshinaga, I.; Ishikawa, A. Germination fluctuation of toxic Alexandrium fundyense and A. pacificum cysts and the relationship with bloom occurrences in Kesennuma Bay, Japan. Harmful Algae 2017, 62, 52–59. [Google Scholar] [CrossRef]
- Yang, I.; Beszteri, S.; Tillmann, U.; Cembella, A.; John, U. Growth- and nutrient-dependent gene expression in the toxigenic marine dinoflagellate Alexandrium minutum. Harmful Algae 2011, 12, 55–69. [Google Scholar] [CrossRef]
- Lee, T.C.; Kwok, O.T.; Ho, K.C.; Lee, F.W. Effects of different nitrate and phosphate concentrations on the growth and toxin production of an Alexandrium tamarense strain collected from Drake Passage. Mar. Environ. Res. 2012, 81, 62–69. [Google Scholar] [CrossRef]
- Maso, M.; Garces, E. Harmful microalgae blooms (HAB) problematic and conditions that induce them. Mar. Pollut. Bull. 2006, 53, 620–630. [Google Scholar] [CrossRef]
- Jiang, H.C.; He, J.L.; Cheng, L.; Liu, N.; Fu, P.; Wang, N.; Jiang, X.Y.; Sun, S.; Zhang, J. Long-term response of plankton assemblage to differentiated nutrient reductions in Laizhou Bay, China. J. Sea Res. 2024, 198. [Google Scholar] [CrossRef]
- Lin, Z.R.; Geng, H.X.; Zhang, Q.C.; Chen, Z.F.; Dai, L.; Yu, R.C. Toxin production of dinoflagellate Gymnodinium catenatum isolated from the East China Sea. Harmful Algae 2022, 113, 102188. [Google Scholar] [CrossRef]
- Liu, M.; Gu, H.; Krock, B.; Luo, Z.; Zhang, Y. Toxic dinoflagellate blooms of Gymnodinium catenatum and their cysts in Taiwan Strait and their relationship to global populations. Harmful Algae 2020, 97, 101868. [Google Scholar] [CrossRef]
- Chen, Z.F.; Zhang, Q.C.; Kong, F.Z.; Liu, Y.; Zhao, Y.; Zhou, Z.X.; Geng, H.X.; Dai, L.; Zhou, M.J.; Yu, R.C. Resolving phytoplankton taxa based on high-throughput sequencing during brown tides in the Bohai Sea, China. Harmful Algae 2019, 84, 127–138. [Google Scholar] [CrossRef]
- Cheung, M.K.; Au, C.H.; Chu, K.H.; Kwan, H.S.; Wong, C.K. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Gibson, K.; Song, H.Y.; Chen, N.S. Metabarcoding analysis of microbiome dynamics during a Phaeocystis globosa bloom in the Beibu Gulf, China. Harmful Algae 2022, 114, 102217. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Qiu, J.B.; Zhang, L.; Cao, Y.D.; Zhao, P.; Zhang, J.R.; Li, A.F. Bioaccessibility of paralytic shellfish toxins in different cooked shellfish using the simulated digestive model. Food. Chem. 2022, 390, 133094. [Google Scholar] [CrossRef]
- Zheng, G.C.; Che, H.Y.; Wu, H.Y.; Cheng, L.; Deng, Y.X.; Guo, M.M.; Peng, J.X.; Liu, L.J.; Tan, Z.J. Risk characteristics of shellfish toxins in Mytilus unguiculatus around the Zhoushan Islands, East China Sea. Mar. Pollut. Bull. 2024, 199, 115955. [Google Scholar] [CrossRef]
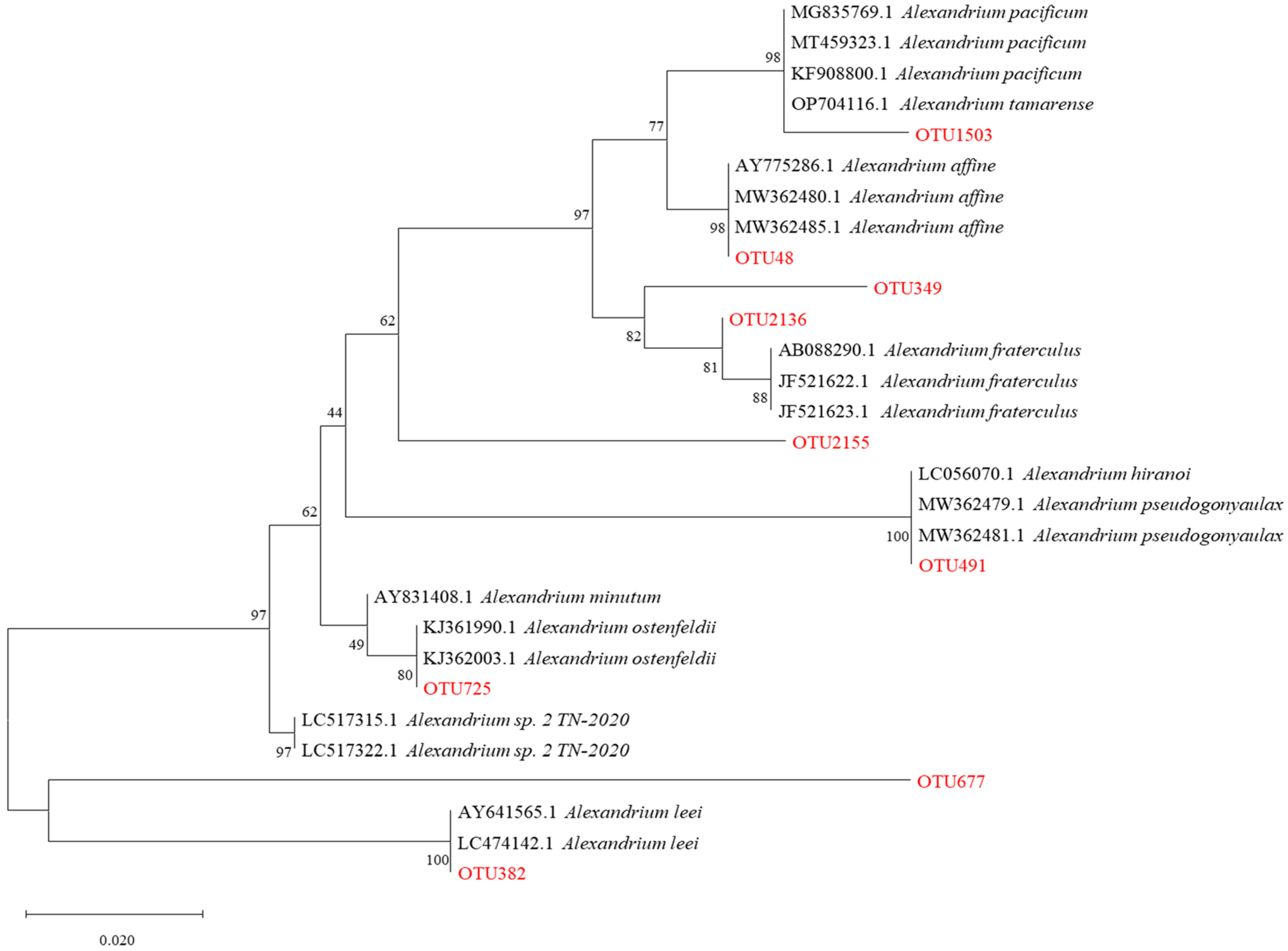



| OTUs | Species Assigned | Query Cover | Percent Identity |
|---|---|---|---|
| OTU1503 | A. pacificum/tamarense | 100% | 98% |
| OTU48 | A. affine | 100% | 98% |
| OTU349 | A. fraterculus | 100% | 95% |
| OTU2155 | A. fraterculus | 100% | 93% |
| OTU491 | A. hiranoi/pseudogonyaulax | 100% | 100% |
| OTU725 | A. ostenfeldii | 100% | 92% |
| OTU382 | A. leei | 100% | 100% |
| OTU677 | A. leei | 100% | 99% |
| OTU2136 | A. fraterculus | 100% | 93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, G.; Deng, Y.; Wu, H.; Li, X.; Cheng, L.; Yuan, C.; Liu, M.; Tan, Z. Paralytic Shellfish Toxins in Coastal Waters of Changdao Island (China): Toxin Profiles, Potential Producers, and Environmental Conditions. Mar. Drugs 2025, 23, 217. https://doi.org/10.3390/md23050217
Zheng G, Deng Y, Wu H, Li X, Cheng L, Yuan C, Liu M, Tan Z. Paralytic Shellfish Toxins in Coastal Waters of Changdao Island (China): Toxin Profiles, Potential Producers, and Environmental Conditions. Marine Drugs. 2025; 23(5):217. https://doi.org/10.3390/md23050217
Chicago/Turabian StyleZheng, Guanchao, Yuxiang Deng, Haiyan Wu, Xiaokang Li, Ling Cheng, Chengxu Yuan, Minlu Liu, and Zhijun Tan. 2025. "Paralytic Shellfish Toxins in Coastal Waters of Changdao Island (China): Toxin Profiles, Potential Producers, and Environmental Conditions" Marine Drugs 23, no. 5: 217. https://doi.org/10.3390/md23050217
APA StyleZheng, G., Deng, Y., Wu, H., Li, X., Cheng, L., Yuan, C., Liu, M., & Tan, Z. (2025). Paralytic Shellfish Toxins in Coastal Waters of Changdao Island (China): Toxin Profiles, Potential Producers, and Environmental Conditions. Marine Drugs, 23(5), 217. https://doi.org/10.3390/md23050217






