Sesquiterpenes from Brown Algae
Abstract
1. Introduction
2. Sesquiterpenes and Sesquiterpenoids in Brown Algae
2.1. Sesquiterpenes and Sesquiterpenoids with Monocyclic Skeleton
- Germacranes
- Bisabolol
2.2. Sesquiterpenes and Sesquiterpenoids with Bicyclic Skeleton
- Selinanes
- Cadinanes
- Spiroaxanes
- Oplopanes
2.3. Sesquiterpenoids with Tricyclic Skeleton
2.4. Other Sesquiterpenoids
- Sesquiterpene + Isoprene Unit
- Norsesquiterpenoids (C14 structure)
- Dinorsesquiterpenoids
- Merosesquiterpenoids with aromatic rings
2.5. Volatile Organic Compounds (VOCs) in Brown Algae
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, R.E.; Pettus, J.A., Jr.; Mistysyn, J. Odoriferous C11 hydrocarbons from Hawaiian dictyopteris. J. Org. Chem. 1974, 39, 2201–2207. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Roussis, V. Natural Products from Seaweeds. In Plant-Derived Natural Products: Synthesis, Function, and Application; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 51–81. [Google Scholar]
- Vlietinck, A.J.; De Bruyne, T.; Vanden Berghe, D.A. Plant Substances As Antiviral Agents. Curr. Org. Chem. 1997, 1, 307–344. [Google Scholar] [CrossRef]
- Alassali, A.; Cybulska, I.; Brudecki, G.; Husain Farzanah, R.; Thomsen, M. Methods for Upstream Extraction and Chemical Characterization of Secondary Metabolites from Algae Biomass. Adv. Tech. Biol. Med. 2016, 4, 1000163. [Google Scholar] [CrossRef]
- Manoylov, K.M. Taxonomic identification of algae (morphological and molecular): Species concepts, methodologies, and their implications for ecological bioassessment. J. Phycol. 2014, 50, 409–424. [Google Scholar] [CrossRef]
- Garson, M.J. Biosynthetic studies on marine natural products. Nat. Prod. Rep. 1989, 6, 143. [Google Scholar] [CrossRef]
- Andersen, R.A. Diversity of eukaryotic algae. Biodivers. Conserv. 1992, 1, 267–292. [Google Scholar] [CrossRef]
- De Reviers, B.; Rousseau, F. Towards a new classification of the brown algae. Prog. Phycol. Res. 1999, 13, 107–201. [Google Scholar]
- Remya, R.R.; Samrot, A.V.; Kumar, S.S.; Mohanavel, V.; Karthick, A.; Chinnaiyan, V.K.; Umapathy, D.; Muhibbullah, M. Bioactive potential of brown algae. Adsorpt. Sci. Technol. 2022, 2022, 9104835. [Google Scholar] [CrossRef]
- Bringloe, T.T.; Starko, S.; Wade, R.M.; Vieira, C.; Kawai, H.; De Clerck, O.; Cock, J.M.; Coelho, S.M.; Destombe, C.; Valero, M.; et al. Phylogeny and Evolution of the Brown Algae. Crit. Rev. Plant Sci. 2020, 39, 281–321. [Google Scholar] [CrossRef]
- Amico, V. Marine brown algae of family cystoseiraceae: Chemistry and chemotaxonomy. Phytochemistry 1995, 39, 1257. [Google Scholar] [CrossRef]
- Faulkner, D.J. Interesting aspects of marine natural products chemistry. Tetrahedron 1977, 33, 1421. [Google Scholar] [CrossRef]
- Capon, R.J. Marine sesquiterpene/quinones. Stud. Nat. Prod. Chem. 1995, 15, 289–326. [Google Scholar]
- Segawa, M.; Yamano, K.; Shirahama, H. A germacrane-type sesquiterpene from the brown alga Dictyopteris divaricata. Phytochemistry 1990, 29, 973–974. [Google Scholar] [CrossRef]
- De Rosa, S.; De Giulio, A.; Iodice, C.; Zavodink, N. Sesquiterpenes from the brown alga Taonia atomaria. Phytochemistry 1994, 37, 1327–1330. [Google Scholar] [CrossRef]
- Fattorusso, E.; Magno, S.; Mayol, L.; Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C. Isolation of (2R,8R)-germacra-1(11),5(12),E6-trien-2-ol acetate from the brown alga Dilophus fasciola. Tetrahedron Lett. 1978, 19, 4149–4152. [Google Scholar] [CrossRef]
- Othmani, A.; Bunet, R.; Bonnefont, J.-L.; Briand, J.-F.; Culioli, G. Settlement inhibition of marine biofilm bacteria and barnacle larvae by compounds isolated from the Mediterranean brown alga Taonia atomaria. J. Appl. Phycol. 2016, 28, 1975–1986. [Google Scholar] [CrossRef]
- Sethupathy, S.; Shanmuganathan, B.; Kasi, P.D.; Karutha Pandian, S. Alpha-bisabolol from brown macroalga Padina gymnospora mitigates biofilm formation and quorum sensing controlled virulence factor production in Serratia marcescens. J. Appl. Phycol. 2015, 28, 1987–1996. [Google Scholar] [CrossRef]
- Shanmuganathan, B.; Sathya, S.; Balasubramaniam, B.; Balamurugan, K.; Devi, K.P. Amyloid-beta induced neuropathological actions are suppressed by Padina gymnospora (Phaeophyceae) and its active constituent α-bisabolol in Neuro2a cells and transgenic Caenorhabditis elegans Alzheimer’s model. Nitric Oxide 2019, 91, 52–66. [Google Scholar] [CrossRef]
- Ji, N.Y.; Wen, W.; Li, X.M.; Xue, Q.Z.; Xiao, H.L.; Wang, B.G. Brominated selinane sesquiterpenes from the marine brown alga Dictyopteris divaricata. Mar. Drugs 2009, 7, 355–360. [Google Scholar] [CrossRef]
- Kurosawa, E.; Izawa, M.; Yamamoto, K.; Masamune, T.; Irie, T. Sesquiterpenes from Dictyopteris Divaricata. II. Dictyopterol and Dictyopterone. Bull. Chem. Soc. Jpn. 1966, 39, 2509–2512. [Google Scholar] [CrossRef] [PubMed]
- Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C.; Fattorusso, E.; Magno, S.; Mayol, L. Sesquiterpenes based on the cadalane skeleton from the brown alga Dilophus fasciola. Experientia 1979, 35, 450–451. [Google Scholar] [CrossRef]
- Tringali, C.; Piattelli, M.; Spatafora, C. Sesquiterpenes and geranylgeranylglycerol from the brown algae Taonia lacheana and Taonia atomaria f. ciliata: Their chemotaxonomic significance. Phytochemistry 1995, 40, 827–831. [Google Scholar] [CrossRef]
- Fenical, W.; Sims, J.J.; Wing, R.M.; Radlick, P.C. Zonarene, a sesquiterpene from the brown seaweed Dictyopteris zonarioides. Phytochemistry 1972, 11, 1161–1163. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D. Concerted application of a shift reagent and 2D NOESY to the structure determination of new natural products from the tropical brown alga Dictyopteris delicatula. Magn. Reson. Chem. 1995, 33, 178–183. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Ji, N.Y.; Wen, W.; Yin, X.L.; Xue, Q.Z. A new epoxy-cadinane sesquiterpene from the marine brown alga Dictyopteris divaricata. Mar. Drugs 2009, 7, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Song, Y.P.; Miao, F.P.; Liang, X.R. Three cadinane derivatives from the marine brown alga Dictyopteris divaricata. Magn. Reson. Chem. 2016, 54, 88–90. [Google Scholar] [CrossRef]
- Song, F.; Fan, X.; Xu, X.; Zhao, J.; Yang, Y.; Shi, J. Cadinane sesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2004, 67, 1644–1649. [Google Scholar] [CrossRef]
- Wen, W.; Li, F.; Ji, N.Y.; Li, X.M.; Cui, C.M.; Li, X.D.; Zhang, L.N.; Xue, Q.Z.; Wang, B.G. A new cadinane sesquiterpene from the marine brown alga Dictyopteris divaricata. Molecules 2009, 14, 2273–2277. [Google Scholar] [CrossRef]
- Song, F.; Xu, X.; Li, S.; Wang, S.; Zhao, J.; Yang, Y.; Fan, X.; Shi, J.; He, L. Minor sesquiterpenes with new carbon skeletons from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2006, 69, 1261–1266. [Google Scholar] [CrossRef]
- Hirschfeld, D.R.; Fenical, W.; Lin, G.H.Y.; Wing, R.M.; Radlick, P.; Sims, J.J. Marine natural products. VIII. Pachydictyol A, an exceptional diterpene alcohol from the brown alga, Pachydictyon coriaceum. J. Am. Chem. Soc. 1973, 95, 4049–4050. [Google Scholar] [CrossRef]
- Song, F.; Xu, X.; Li, S.; Wang, S.; Zhao, J.; Cao, P.; Yang, Y.; Fan, X.; Shi, J.; He, L.; et al. Norsesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2005, 68, 1309–1313. [Google Scholar] [CrossRef]
- Song, F.H.; Fan, X.; Xu, X.L.; Zhao, J.L.; Han, L.J.; Shi, J.G. Chemical constituents of the brown alga Dictyopteris divaricata. J. Asian Nat. Prod. Res. 2005, 7, 777–781. [Google Scholar] [CrossRef]
- Yu, M.; Gong, Y.-B.; Chen, Y.; Zhang, Y.-J.; He, X.-L.; Huang, G.-L.; Zheng, C.-J. A New Sesquiterpene from the Brown Algae Sargassum polycystum. Chem. Nat. Compd. 2023, 59, 505–507. [Google Scholar] [CrossRef]
- Ishibashi, F.; Sato, S.; Sakai, K.; Hirao, S.; Kuwano, K. Algicidal sesquiterpene hydroquinones from the brown alga Dictyopteris undulata. Biosci. Biotechnol. Biochem. 2013, 77, 1120–1122. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Capon, R.J. A New Sesquiterpene/Phenol from the Australian Marine Brown Alga Perithalia caudata. J. Nat. Prod. 1994, 57, 849–851. [Google Scholar] [CrossRef]
- Cimino, G.; de Stefano, S.; Fenical, W.; Minale, L.; Sims, J.J. Zonaroic acid from the brown seaweed Dictyopteris undulata (=zonarioides). Experientia 1975, 31, 1250–1251. [Google Scholar] [CrossRef]
- Fenical, W.; Sims, J.J.; Squatrito, D.; Wing, R.M.; Radlick, P. Marine natural products. VII. Zonarol and isozonarol, fungitoxic hydroquinones from the brown seaweed Dictyopteris zonarioides. J. Org. Chem. 1973, 38, 2383–2386. [Google Scholar] [CrossRef]
- Joshi Bipin, C.; Kazaoka, M.; Trischman Jacqueline, A. New sesquiterpene hydroquinones from marine brown alga Dictyopteris undulata. Res. J. Chem. Sci. 2012, 2, 9–13. [Google Scholar]
- Kumagai, M.; Nishikawa, K.; Matsuura, H.; Umezawa, T.; Matsuda, F.; Okino, T. Antioxidants from the Brown Alga Dictyopteris undulata. Molecules 2018, 23, 1214. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Suzuki, M. Cyclozonarone, a sesquiterpene-substituted benzoquinone derivative from the brown alga Dictyopteris undulata. Phytochemistry 1996, 41, 749–752. [Google Scholar] [CrossRef]
- Ochi, M.; Kotsuki, H.; Muraoka, K.; Tokoroyama, T. The Structure of Yahazunol, a New Sesquiterpene-substituted Hydroquinone from the Brown Seaweed Dictyopteris undulata Okamura. Bull. Chem. Soc. Jpn. 1979, 52, 629–630. [Google Scholar] [CrossRef]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef]
- Fenical, W.; McConnell, O. Chromazonarol and isochromazonarol, new chromanols from the brown seaweed Dictyopteris undulata (zonarioides). Experientia 1975, 31, 1004. [Google Scholar] [CrossRef]
- Schröder, J.; Magg, C.; Seifert, K. Total synthesis of the marine sesquiterpene hydroquinones zonarol and isozonarol and the sesquiterpene quinones zonarone and isozonarone. Tetrahedron Lett. 2000, 41, 5469–5473. [Google Scholar] [CrossRef]
- Laube, T.; Schröder, J.; Stehle, R.; Seifert, K. Total synthesis of yahazunol, zonarone and isozonarone. Tetrahedron 2002, 58, 4299–4309. [Google Scholar] [CrossRef]
- Ishibashi, H.; Ishihara, K.; Yamamoto, H. A New Artificial Cyclase for Polyprenoids: Enantioselective Total Synthesis of (−)-Chromazonarol, (+)-8-epi-Puupehedione, and (−)-11′-Deoxytaondiol Methyl Ether. J. Am. Chem. Soc. 2004, 126, 11122–11123. [Google Scholar] [CrossRef]
- El Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of various extraction methods for identification and determination of volatile metabolites from the brown alga Dictyopteris membranacea. J. Chromatogr. A 2007, 1143, 1–7. [Google Scholar] [CrossRef]
- Radman, S.; Cagalj, M.; Simat, V.; Jerkovic, I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef]
- Radman, S.; Cagalj, M.; Simat, V.; Jerkovic, I. Seasonal Monitoring of Volatiles and Antioxidant Activity of Brown Alga Cladostephus spongiosus. Mar. Drugs 2023, 21, 415. [Google Scholar] [CrossRef]
- Bouzidi, N.; Seridi, H.; Daghbouche, Y.; Piovetti, L.; El Hattab, M. Comparison of the chemical composition of “Cystoseira sedoides (Desfontaines) C. Agardh” volatile compounds obtained by different extraction techniques. Rec. Nat. Prod. 2016, 10, 58–67. [Google Scholar]
- Jerkovic, I.; Kranjac, M.; Marijanovic, Z.; Roje, M.; Jokic, S. Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- Cagalj, M.; Radman, S.; Simat, V.; Jerkovic, I. Detailed Chemical Prospecting of Volatile Organic Compounds Variations from Adriatic Macroalga Halopteris scoparia. Molecules 2022, 27, 4997. [Google Scholar] [CrossRef] [PubMed]
- De Grazia, G.; Cucinotta, L.; Sciarrone, D.; Donato, P.; Trovato, E.; Riad, N.; Hattab, M.E.; Mondello, L.; Rotondo, A. Preparative three-dimensional GC and nuclear magnetic resonance for the isolation and identification of two sesquiterpene ethers from Dictyota Dichotoma. J. Sep. Sci. 2023, 46, e2300261. [Google Scholar] [CrossRef]
- Jerkovic, I.; Marijanovic, Z.; Roje, M.; Kus, P.M.; Jokic, S.; Coz-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, J.; Chen, L.; Shi, L.; Liu, H. Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China. Mar. Drugs 2021, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, T.; Hatanaka, A.; Tanaka, Y.; Kawai, T.; Ishihara, M.; Tsuneya, T.; Fujimura, T. Volatile constituents from marine brown algae of japanese Dictyopteris. Phytochemistry 1989, 28, 636–639. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Kodama, K.; Ochi, S.; Fujimura, T. Dictyopterenes from three Japanese brown algae. Phytochemistry 1991, 30, 1805–1807. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kodama, K.; Hatanaka, A. Male-attracting substance in marine brown algae the genus Dictyopteris. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 771. [Google Scholar] [CrossRef][Green Version]
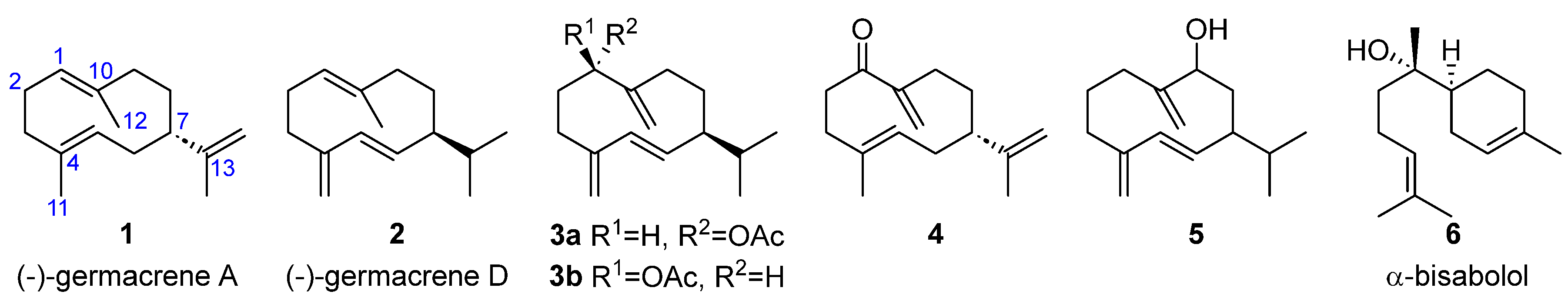

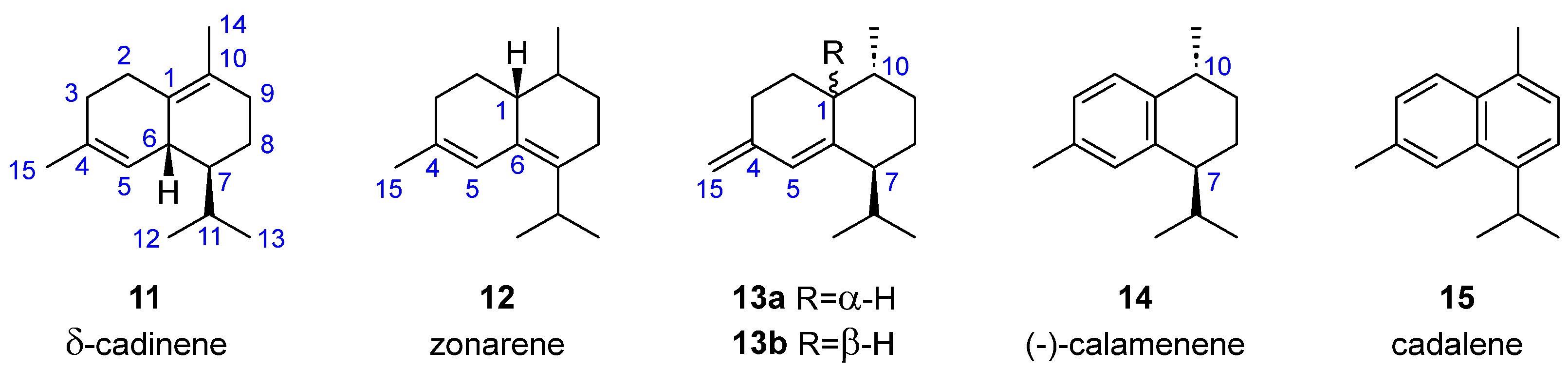
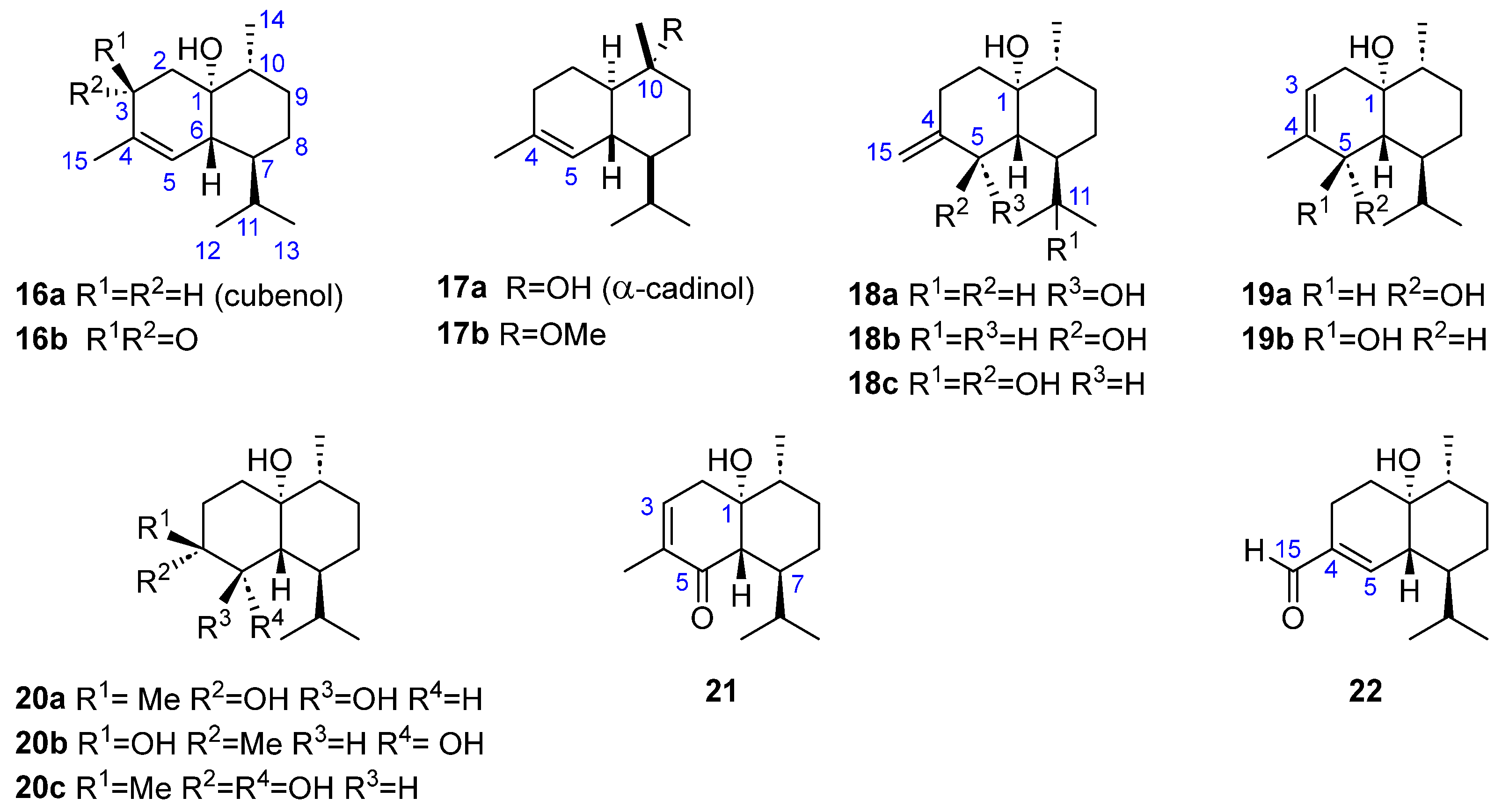
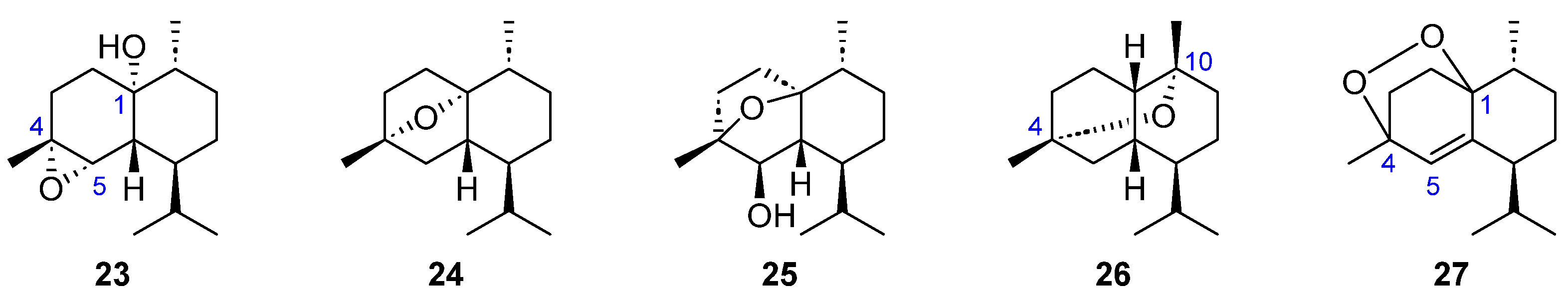






| Compound 1 | Dictyopteris divaricata | Dilophus fasciola | Padina gymnospora | Taonia atomaria |
|---|---|---|---|---|
| 1 | [15] | |||
| 2 | [16] | |||
| 3a | [17] | |||
| 3b | [18] | |||
| 4 | [15] | |||
| 5 | [18] | |||
| 6 | [19] |
| Compound 1 | Dictyopteris delicatula | Dictyopteris divaricata | Dictyopteris zonaroides | Dilophus fasciola | Taonia atomaria |
|---|---|---|---|---|---|
| 7a,7b | [21] | ||||
| 8a,8b | [22] | ||||
| 9 | [21] | ||||
| 10a, 10b | [21] | ||||
| 11 | [23] | [24] | |||
| 12 | [25] | ||||
| 13a | [23] | ||||
| 13b | [16] | ||||
| 14 | [23] | [18] | |||
| 15 | [21] | ||||
| 16a | [26] | [27] | [23] | [24] | |
| 16b | [27] | ||||
| 17a | [26] | [27] | |||
| 17b | [18] | ||||
| 18a, 18b | [28,29] | ||||
| 18c | [29] | ||||
| 19a, 19b | [29] | ||||
| 20a | [26] | ||||
| 20b | [27] | ||||
| 20c | [29] | ||||
| 21 | [29] | ||||
| 22–23 | [27] | ||||
| 24 | [30] | ||||
| 25 | [30] | ||||
| 26 | [24] | ||||
| 27 | [18] | ||||
| 28 | [17,18] | ||||
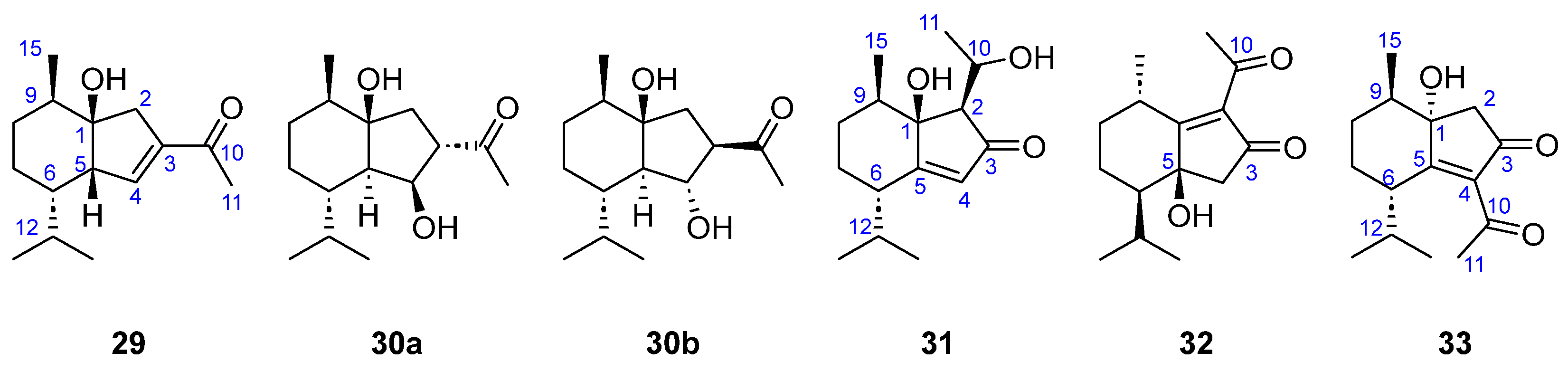
| 29–33 | [31] |
| Compound 1 | Taonia atomaria | Taonia lacheana |
|---|---|---|
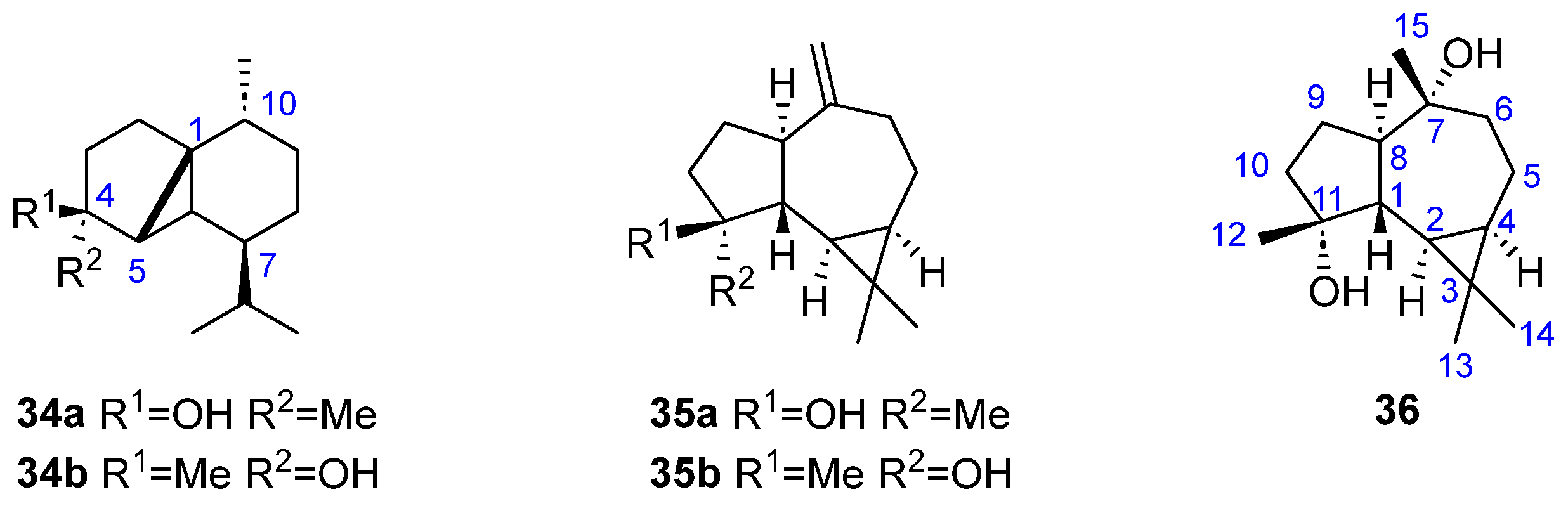
| 34a, 34b | [16] | |
| 35a, 35b | [24] | |
| 36 | [24] |
| Compound 1 | Dictyopteris divaricata | Dictyopteris zonaroides | Perithalia caudata | Dictyopteris undulata | Pachydictyon coriaceum | Sargassum polycystum | Padina gymnospora |
|---|---|---|---|---|---|---|---|
| 37 | [32] | ||||||
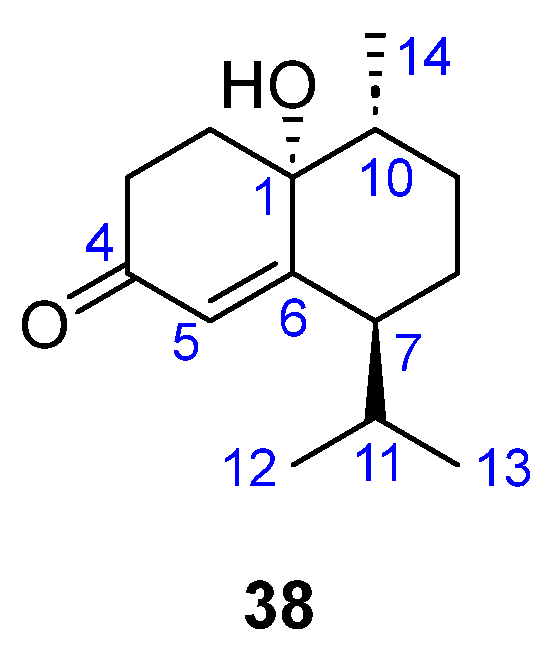
| 38–42 | [34] | ||||||
| 43 | [35] | ||||||
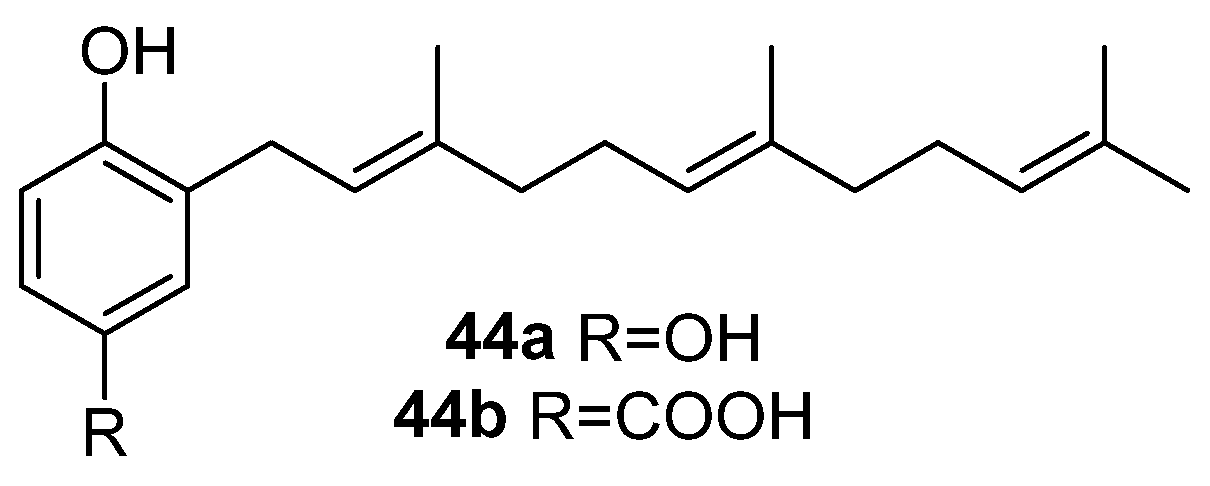
| 44a | [15,33] | [36] | [37] | ||||
| 44b | [36] | [37] | |||||
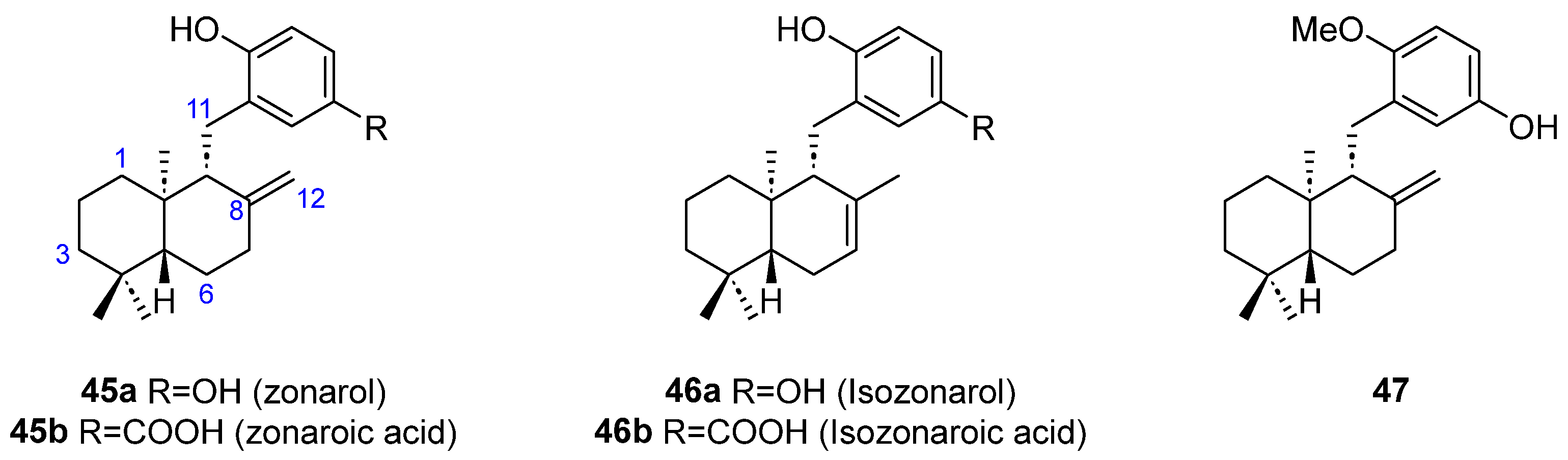
| 45a | [36,38,39,40,41,42,43,44] | ||||||
| 45b | [36,38,41,42] | ||||||
| 46a | [36,39,40,41,42] | ||||||
| 46b | [41] | ||||||
| 47 | [41] | ||||||
| 48 | [40,42] | ||||||
| 49 | [42] | ||||||
| 50 | [36,40,43] | ||||||
| 51 | [34] | ||||||
| 52 | [36] | ||||||
| 53 | [34] | [36,38,41,42,45] | |||||
| 54 | [36,38,45] |
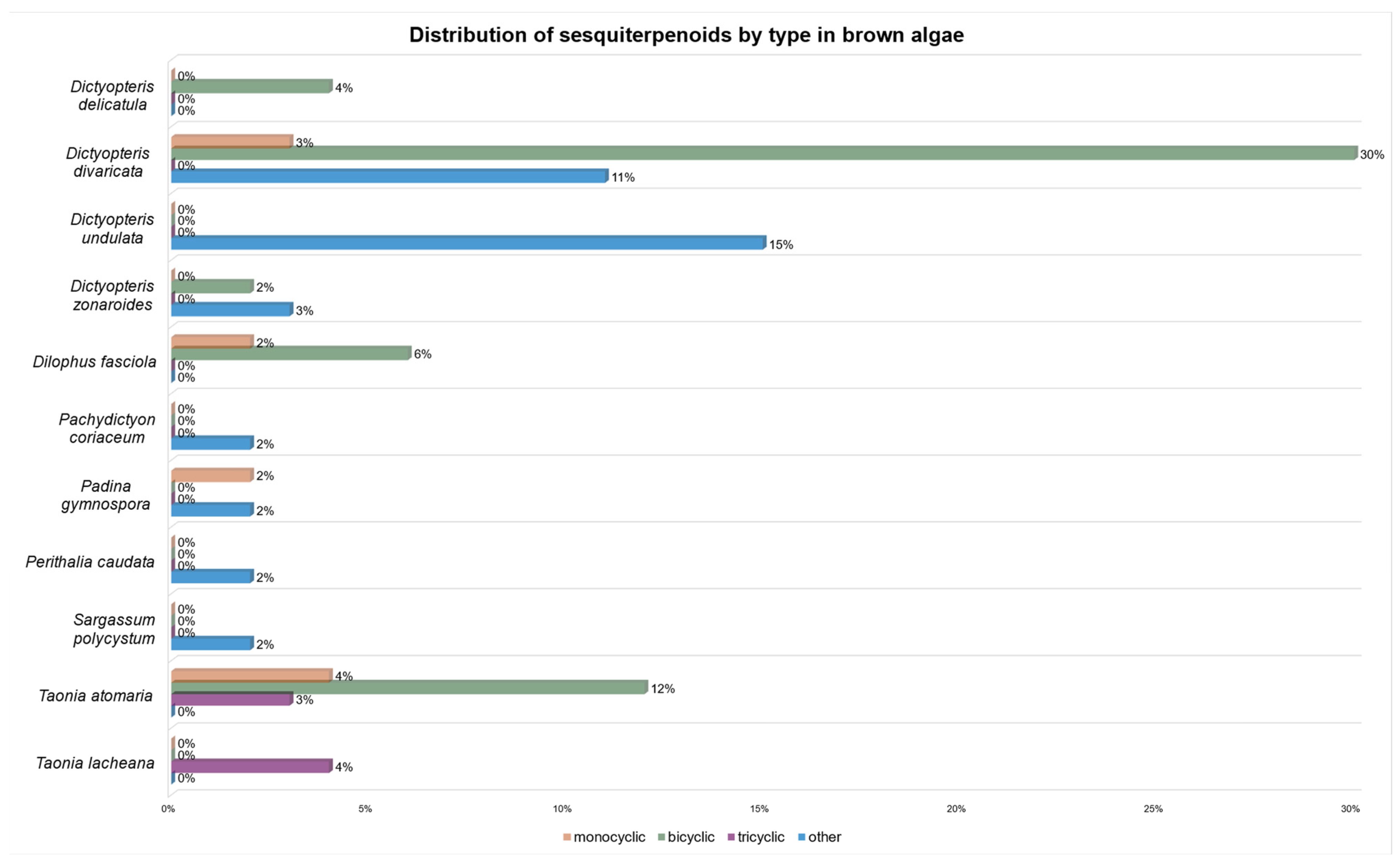
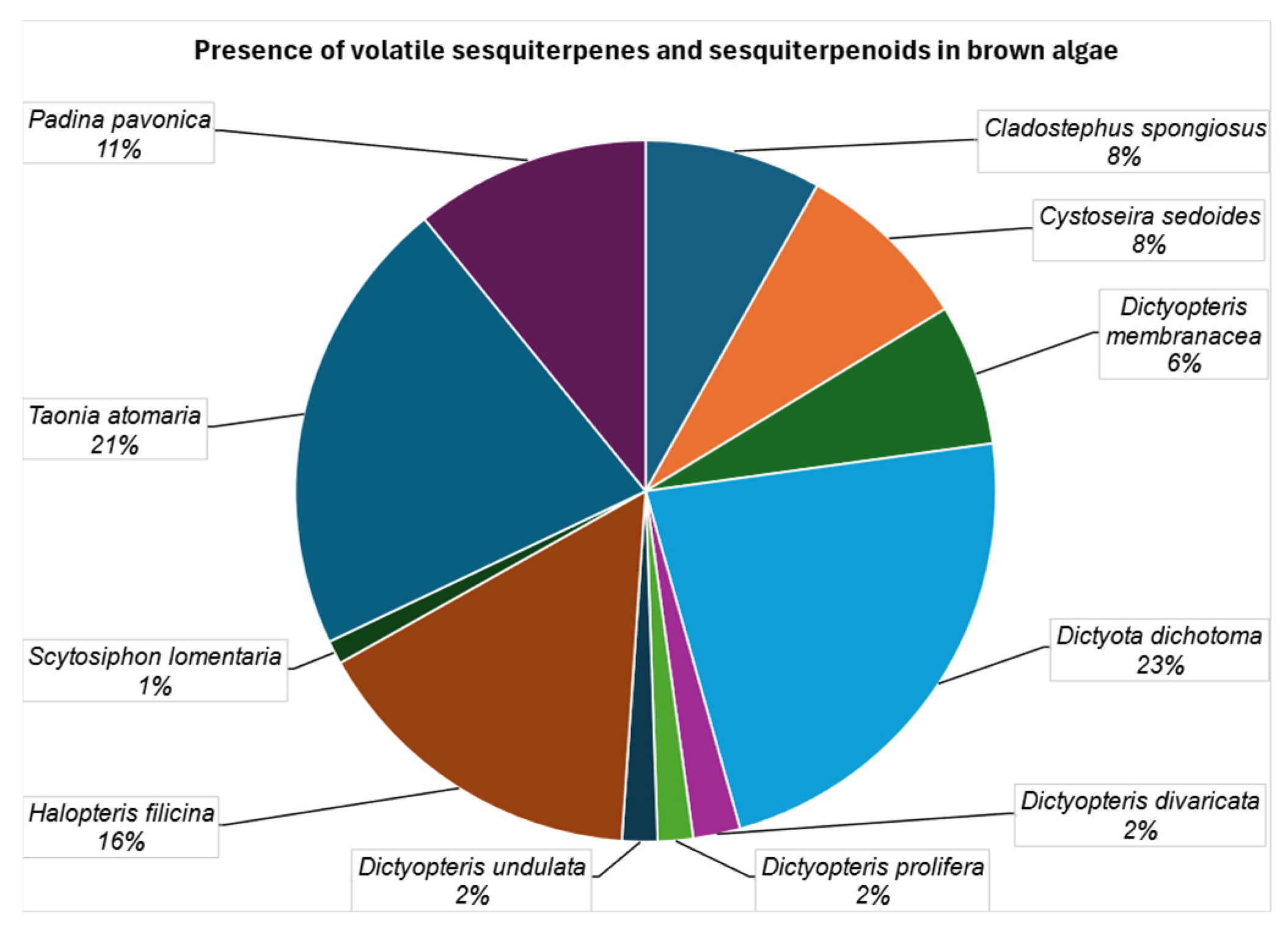
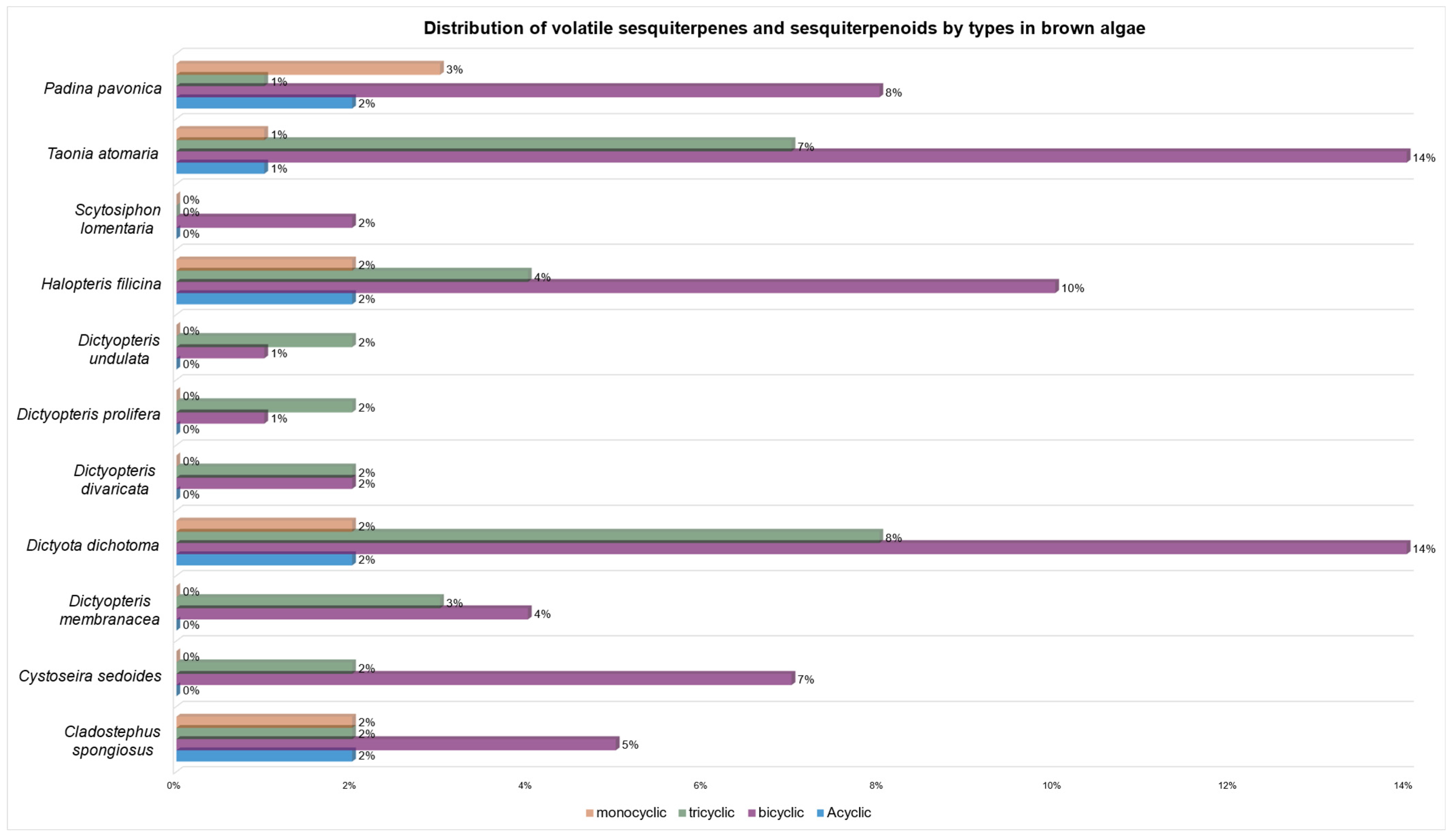
| Compound | I1 | II2 | III3 | IV4 | V5 | VI6 | VII7 | VIII8 | IX9 | X10 | XI11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acyclic | |||||||||||
| α-farnesene (55) | [53] | ||||||||||
| (E)-β-farnesene (56) | [55] | [56] | [53] | [53] | |||||||
| (E,E)-farnesyl acetone (57) | [51] | ||||||||||
| (E)-geranylacetone (58) | [57] | ||||||||||
| hexahydrofarnesyl acetone (59) | [51] | [54] | |||||||||
| Monocyclic | |||||||||||
| (E)-α-bisabolene (60) | [53] | ||||||||||
| β-bisabolene (61) | [53] | ||||||||||
| trans-γ-bisabolene (62) | [56] | ||||||||||
| Ar-curcumene (63) | [56] | ||||||||||
| γ-curcumene (64) | [50] | ||||||||||
| β-elemene (65) | [58] | [59] | |||||||||
| γ-elemene (66) | [53] | ||||||||||
| germacrene-4-ol (67) | [51] | ||||||||||
| germacra-4(15),5,10(14)-trien-1α-ol (68) | [52] | ||||||||||
| germacrene A (1) | [55] | ||||||||||
| germacrene B (69) | [56] | ||||||||||
| germacrene C (70) | [50] | ||||||||||
| germacrene D (2) | [51] | [52] | [49] | [50,56,57] | [58] | [54,56] | [53] | ||||
| α-humulene (71) | [50] | [53] | |||||||||
| Bicylic | |||||||||||
| bicyclogermacrene (72) | [56] | [56] | [53] | ||||||||
| 9-epi-(E)-caryphyllene (73) | [55] | ||||||||||
| gleenol (28) | [51] | [52] | [49] | [50,55] | [54] | [53] | |||||
| (E)-β-guaiene (74) | [53] | ||||||||||
| γ-gurjunene (75) | [52] | [55] | [53] | ||||||||
| pachydictyol A (37) | [53] | ||||||||||
| isopachydictyol A (76) | [54] | [53] | |||||||||
| β-santalene (77) | [53] | ||||||||||
| epi-β-santalene (78) | [53] | ||||||||||
| trans-α-bergamotene (79) | [53] | [53] | |||||||||
| junenol (80) | [53] | [53] | |||||||||
| α-selinene (81) | [57] | ||||||||||
| δ-selinene (82) | [56] | ||||||||||
| α-amorphene (83) | [49] | [50,55] | [56] | [53] | |||||||
| γ-amorphene (84) | [55] | [53] | |||||||||
| cadalene (15) | [52] | ||||||||||
| epi-bicyclosesquiphellandrene (85) | [51] | [49] | [50] | [53] | [53] | ||||||
| cadina-3,5-diene (86) | [53] | [53] | |||||||||
| cadina-4,9-diene (87) | [51] | ||||||||||
| cadina-1,4-diene (88) | [49] | [50] | |||||||||
| trans-cadina-1,4-diene (89) | [52] | [50,55] | [56] | [53] | |||||||
| 4,10(14)-cadinadien-8β-ol (90) | [53] | [53] | |||||||||
| α-cadinene (91) | [56] | [53] | |||||||||
| β-cadinene (92) | [50,55,56,57] | [56] | [53] | ||||||||
| γ-cadinene (93) | [51] | [50,55,57] | [56] | [53] | |||||||
| δ-cadinene (11) | [51] | [49] | [50,55,56,57] | [58] | [58] | [58] | [54,56] | [59] | [53] | ||
| α-cadinol (94) | [51] | [52] | [56] | [53] | |||||||
| δ-cadinol (95) | [51] | [53] | |||||||||
| τ-cadinol (96) | [51] | [50] | [53] | ||||||||
| α-calacorene (97) | [52] | [49] | [50] | [56] | [53] | ||||||
| cis-calamenene (98) | [52] | [53] | |||||||||
| trans-calamenene (99) | [53] | ||||||||||
| cubenol (16a) | [52] | [50,55] | [58] | [54] | |||||||
| epi-cubenol (100) | [50,55] | [59] | |||||||||
| cis-muurola-3,5-diene (101) | [57] | ||||||||||
| trans-muurola-3,5-diene (102) | [55] | ||||||||||
| cis-muurola-4(15),5-diene (103) | [55] | [53] | [53] | ||||||||
| muurola-4,9-diene (104) | [57] | ||||||||||
| α-muurolene (105) | [55,56,57] | [54,56] | [53] | ||||||||
| γ-muurolene (106) | [50,55,57] | [56] | [53] | ||||||||
| α-muurolol (107) | [55] | [53] | |||||||||
| τ-muurolol (108) | [56] | ||||||||||
| zonarene (12) | [51] | [49] | [55] | [54] | [53] | ||||||
| epi-zonarene (109) | [52] | [56] | [53] | ||||||||
| 14-nor-cadin-5-en-4-one (110) | [52] | ||||||||||
| α-eudesmol (111) | [52] | ||||||||||
| β-oplopenone (112) | [52] | ||||||||||
| Tricyclic | |||||||||||
| alloaromadendrene (113) | [53] | [53] | |||||||||
| aromandrene (114) | [56] | [53] | |||||||||
| β-gurjunene (115) | [50,57] | ||||||||||
| α-cedrene (116) | [57] | ||||||||||
| β-cedrene (117) | [57] | ||||||||||
| β-patchoulene (118) | [53] | ||||||||||
| β-bourbonene (119) | [51] | [52] | [49] | [50,55,56,57] | [54,56] | [53] | |||||
| cubebol (34) | [55] | [53] | |||||||||
| 1,10-di-epi-cubebol (120) | [49] | [53] | |||||||||
| α-copaene (121) | [49] | [50,55,56,57] | [58] | [58] | [58] | [56] | [53] | ||||
| β-copaene (122) | [55] | [53] | |||||||||
| α-cubebene (123) | [52] | [49] | [50,55,57] | [58] | [58] | [58] | [54,56] | [53] | |||
| β-cubebene (124) | [51] | [52] | [49] | [50,55] | [54,56] | [53] | |||||
| cyclosativene (125) | [55] | [53] | |||||||||
| cycloisosativene (126) | [56] | ||||||||||
| cis-4,10-epoxy-amorphane (127) | [55] | ||||||||||
| sativene (128) | [55] | ||||||||||
| α-ylangene (129) | [56] | [53] | |||||||||
| β-ylangene (130) | [55] | [53] | [53] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Gutiérrez, I.; Berenguel-Gómez, S.; Muñoz-Dorado, M.; Álvarez-Corral, M.; Rodríguez-García, I. Sesquiterpenes from Brown Algae. Mar. Drugs 2025, 23, 210. https://doi.org/10.3390/md23050210
Moreno-Gutiérrez I, Berenguel-Gómez S, Muñoz-Dorado M, Álvarez-Corral M, Rodríguez-García I. Sesquiterpenes from Brown Algae. Marine Drugs. 2025; 23(5):210. https://doi.org/10.3390/md23050210
Chicago/Turabian StyleMoreno-Gutiérrez, Irene, Sonia Berenguel-Gómez, Manuel Muñoz-Dorado, Míriam Álvarez-Corral, and Ignacio Rodríguez-García. 2025. "Sesquiterpenes from Brown Algae" Marine Drugs 23, no. 5: 210. https://doi.org/10.3390/md23050210
APA StyleMoreno-Gutiérrez, I., Berenguel-Gómez, S., Muñoz-Dorado, M., Álvarez-Corral, M., & Rodríguez-García, I. (2025). Sesquiterpenes from Brown Algae. Marine Drugs, 23(5), 210. https://doi.org/10.3390/md23050210







