Overcoming Extraction Hurdles and Assessing Biological Activity in a Major Invasive Seaweed Species in Europe, Rugulopteryx okamurae
Abstract
1. Introduction
2. Results
2.1. Dry Matter and Mineral Matter Yields
2.2. Total Polyphenol Content
2.3. Biological Activity
3. Discussion
3.1. Dry Matter and Mineral Matter Yields
3.2. Total Polyphenol Content
3.3. Biological Activity
4. Materials and Methods
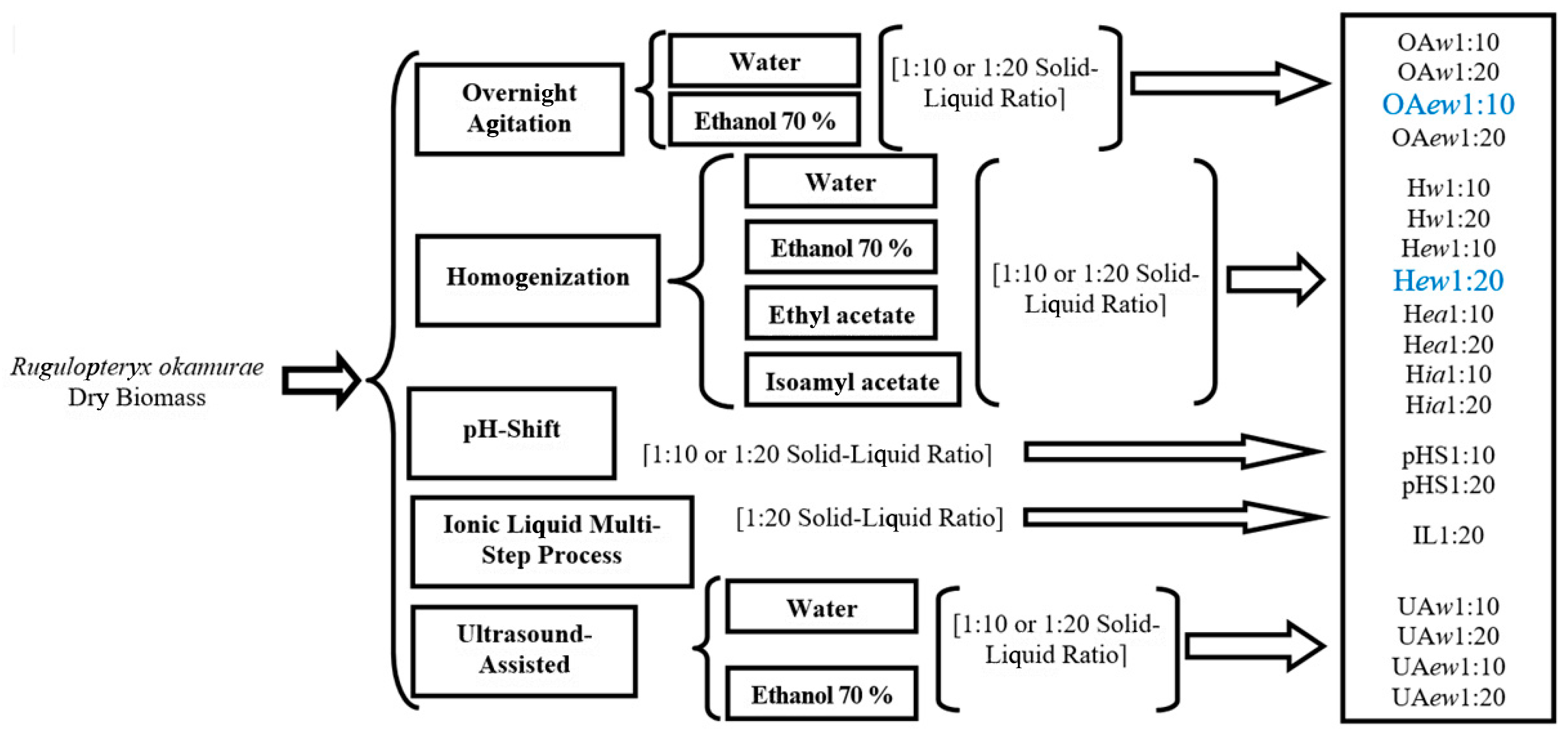
4.1. Experimental Design
4.2. Seaweed Source, Collection, and Preparation
4.3. Seaweed Extraction Methodologies
4.3.1. Overnight Agitation (OA)
4.3.2. Homogenization (H)
4.3.3. pH-Shift (pHS)
4.3.4. Ionic Liquid Multi-Step Extraction (IL)
4.3.5. Ultrasound-Assisted Extraction (UA)
4.4. Determination of Moisture and Ash and Calculation of Yields
4.5. Total Polyphenol Content
4.6. Antioxidant Activity as Measured by DPPH Method
4.7. Antioxidant Activity as Measured by FRAP Method
4.8. Antioxidant Activity as Measured by ABTS Method
4.9. Anti-Inflammatory Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA Eq. | Ascorbic Acid Equivalent |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| COX | Cyclooxygenase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| dw | Dry Weight |
| ea | Ethyl Acetate |
| ew | Ethanol–Water 7:3 |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalent |
| H | Homogenization |
| HSD | Honestly Significant Difference |
| ia | Isoamyl Acetate |
| IL | Ionic Liquid Multi-Step Extraction |
| μmol Fe2+ Eq. | Micromole Iron (II) Equivalent |
| OA | Overnight Agitation |
| pHS | pH-Shift Extraction |
| Trolox Eq. | Trolox Equivalent |
| UA | Ultrasound-Assisted Extraction |
| w | Water |
| w/v | Weight/Volume |
References
- Algaebase. Global Algal Database of Taxonomic, Nomenclatural and Distributional Information. 2018. Available online: http://www.algaebase.org/ (accessed on 19 September 2018).
- Faria, J.; Prestes, A.C.L.; Moreu, I.; Martins, G.M.; Neto, A.I.; Cacabelos, E. Arrival and proliferation of the invasive seaweed Rugulopteryx okamurae in NE Atlantic islands. Bot. Mar. 2022, 65, 45–50. [Google Scholar]
- Barcellos, L.; Pham, C.K.; Menezes, G.; Bettencourt, R.; Rocha, N.; Carvalho, M.; Felgueiras, H.P. A concise review on the potential applications of Rugulopteryx okamurae macroalgae. Mar. Drugs 2023, 21, 40. [Google Scholar] [CrossRef]
- Patón, D.; García-Gomez, J.C.; Loring, J.; Torres, A. Composting the invasive toxic seaweed Rugulopteryx okamurae using five invertebrate species, and a mini-review on composting macroalgae. Waste Biomass Valorization 2023, 14, 167–184. [Google Scholar]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W. Quantification of polyphenolic antioxidants and free radical scavengers in marine algae. J. Appl. Phycol. 2018, 30, 113–120. [Google Scholar]
- Figueroa, F.L.; Vega, J.; Flórez-Fernández, N.; Mazón, J.; Torres, M.D.; Domínguez, H.; Pereira, L. Challenges and opportunities of the exotic invasive macroalga Rugulopteryx okamurae (Phaeophyceae, Heterokontophyta). J. Appl. Phycol. 2025, 37, 579–595. [Google Scholar]
- Ferreira-Anta, T.; Flórez-Fernández, N.; Torres, M.D.; Mazon, J.; Domínguez, H. Microwave-assisted hydrothermal processing of Rugulopteryx okamurae. Mar. Drugs 2023, 21, 319. [Google Scholar] [CrossRef]
- Vega, J.; Catala, T.S.; García-Márquez, J.; Speidel, L.G.; Arijo, S.; Kunz, N.C.; Geisler, C.; Figueroa, F.L. Molecular diversity and biochemical content in two invasive alien species: Looking for chemical similarities and bioactivities. Mar. Drugs 2023, 21, 5. [Google Scholar]
- Cordoba-Granados, J.J.; Jimenez-Hierro, M.J.; Zuasti, E.; Ochoa-Hueso, R.; Puertas, B.; Zarraonaindia, I.; Hachero-Cruzado, I.; Cantos-Villar, E. Biochemical characterization and potential valorization of the invasive seaweed Rugulopteryx okamurae. J. Appl. Phycol. 2025, 37, 567–577. [Google Scholar]
- Cuevas, B.; Arroba, A.I.; de los Reyes, C.; Gómez-Jaramillo, L.; González-Montelongo, M.C.; Zubía, E. Diterpenoids from the brown alga Rugulopteryx okamurae and their anti-inflammatory activity. Mar. Drugs 2021, 19, 677. [Google Scholar] [CrossRef]
- Reboleira, J.; Ganhão, R.; Mendes, S.; Adão, P.; Andrade, M.; Vilarinho, F.; Sanches-Silva, A.; Sousa, D.; Mateus, A.; Bernardino, S. Optimization of extraction conditions for Gracilaria gracilis extracts and their antioxidative stability as part of microfiber food coating additives. Molecules 2020, 25, 4060. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.P.; Robinson, L.F.; Gomez, L.D. Valorisation strategies for brown seaweed biomass production in a European context. Algal Res. 2023, 75, 103248. [Google Scholar]
- Fernández-Medina, P.; Álvarez-Gallego, C.J.; Caro, I. Yield evaluation of enzyme hydrolysis and dark fermentation of the brown seaweed Rugulopteryx okamurae hydrothermally pretreated by microwave irradiation. J. Environ. Chem. Eng. 2022, 10, 108817. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Mazón, J.; Domínguez, H.; Torres, M.D. Relevance of drying treatment on the extraction of high valuable compounds from invasive brown seaweed Rugulopteryx okamurae. Algal Res. 2023, 69, 102917. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Cartan-Moya, S.; Martínez-Sanz, M.; Gómez-Cortés, P.; Calvo, M.V.; López-Rubio, A.; Martínez-Abad, A. Characterization of the invasive macroalgae Rugulopteryx okamurae for potential biomass valorisation. Food Chem. 2024, 440, 138241. [Google Scholar]
- Agabo-García, C.; Romero-García, L.I.; Álvarez-Gallego, C.J.; Blandino, A. Valorisation of the invasive alga Rugulopteryx okamurae through the production of monomeric sugars. Appl. Microbiol. Biotechnol. 2023, 107, 1971–1982. [Google Scholar]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Matos, G.S.; Pereira, S.G.; Genisheva, Z.A.; Gomes, A.M.; Teixeira, J.A.; Rocha, C.M.R. Advances in extraction methods to recover added-value compounds from seaweeds: Sustainability and functionality. Foods 2021, 10, 516. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized liquid extraction for the recovery of bioactive compounds from seaweeds for food industry application: A review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2023, 22, 1509–1540. [Google Scholar]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Ann. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, M.; Barcala, M.; Rosell, C.M.; Sineiro, J.; Moreira, R. Aqueous extracts characteristics obtained by ultrasound-assisted extraction from Ascophyllum nodosum seaweeds: Effect of operation conditions. J. Appl. Phycol. 2021, 33, 3297–3308. [Google Scholar] [CrossRef]
- Panjaitan, R.S.; Yuliana, W. Antioxidant and toxicity activities of Gracilaria gracilis methanol extract based on different extraction methods. J. Kim. Ris. 2022, 7, 141–151. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- De La Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Garrido-Fernández, A.; Borja, R. Process optimization of the extraction of reducing sugars and total phenolic compounds from the invasive alga Rugulopteryx okamurae by response surface methodology (RSM). Algal Res. 2024, 80, 103500. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of Ultrasound-Assisted Extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application. Bioresour. Technol. 2016, 211, 190–199. [Google Scholar] [CrossRef]
- Le Guillard, C.; Bergé, J.-P.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Baron, R.; Fleurence, J.; Dumay, J. Soft liquefaction of the red seaweed Grateloupia turuturu Yamada by ultrasound-assisted enzymatic hydrolysis process. J. Appl. Phycol. 2016, 28, 2575–2585. [Google Scholar] [CrossRef]
- León-Marcos, L.; Fuente-Zapico, E.; Romero-Vargas, A.; Blandino, A.; Romero-García, L.I. Ultrasound pretreatment of third-generation biomass (invasive macroalga Rugulopteryx okamurae) to obtain platform biocommodities. J. Appl. Phycol. 2024, 36, 2807–2821. [Google Scholar]
- Kashyap, M.; Ghosh, S.; Levkov, K.; Livney, Y.D.; Israel, A.; Goldberg, A. High-voltage pulsed electric fields and pH shift process for protein and solute release from Gracilaria sp., red edible seaweed. Food Bioprocess Technol. 2024, 17, 5273–5284. [Google Scholar]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar]
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) bioprospection: A study of fatty acid profiles and bioactivity. Int. J. Food Sci. Technol. 2019, 54, 880–890. [Google Scholar] [CrossRef]
- Fonseca, I.; Guarda, I.; Mourato, M.; Martins, L.L.; Gomes, R.; Matos, J.; Gomes-Bispo, A.; Bandarra, N.M.; Cardoso, C.; Afonso, C. Undervalued Atlantic brown seaweed species (Cystoseira abies-marina and Zonaria tournefortii): Influence of treatment on their nutritional and bioactive potential and bioaccessibility. Eur. Food Res. Technol. 2021, 247, 221–232. [Google Scholar]
- Belhadj, R.N.A.; Mellinas, C.; Jiménez, A.; Bordehore, C.; Garrigós, M.C. Invasive seaweed Rugulopteryx okamurae: A potential source of bioactive compounds with antioxidant activity. Antioxidants 2024, 13, 1298. [Google Scholar] [CrossRef]
- Julião, D.R.; Afonso, C.; Gomes-Bispo, A.; Bandarra, N.M.; Cardoso, C. The effect of drying on undervalued brown and red seaweed species: Bioactivity alterations. Phycol. Res. 2021, 69, 246–257. [Google Scholar]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar]
- Boopathy, N.S.; Kathiresan, K. Anticancer drugs from marine Flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ragonese, C.; Tedone, L.; Beccaria, M.; Torre, G.; Cichello, F.; Cacciola, F.; Dugo, P.; Mondello, L. Characterisation of lipid fraction of marine macroalgae by means of chromatography techniques coupled to mass spectrometry. Food Chem. 2014, 145, 932–940. [Google Scholar] [CrossRef]
- Jin, D.Q.; Lim, C.S.; Sung, J.Y.; Choi, H.G.; Ha, I.; Han, J.S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 2006, 402, 154–158. [Google Scholar] [CrossRef]
- Montalvão, S.; Demirel, Z.; Devi, P.; Lombardi, V.; Hongisto, V.; Perälä, M.; Hattara, J.; Imamoglu, E.; Tilvi, S.S.; Turan, G.; et al. Large-scale bioprospecting of cyanobacteria, micro- and macroalgae from the Aegean Sea. N. Biotechnol. 2018, 33, 399–406. [Google Scholar] [CrossRef]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Anti-inflammatory effect of Petalonia binghamiae in LPS-induced macrophages is mediated by suppression of iNOS and COX-2. Int. J. Agric. Biol. Eng. 2010, 12, 754–758. [Google Scholar]
- Cuevas, B.; Arroba, A.I.; de los Reyes, C.; Zubía, E. Rugulopteryx-derived spatane, secospatane, prenylcubebane and prenylkelsoane diterpenoids as inhibitors of nitric oxide production. Mar. Drugs 2023, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Díaz de Rada, J.R.; Donázar-Aramendia, I.; Chacón, M.; Quintero, J.J.; Magariño, S.; Megina, C. Monitoring extreme impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (biosphere reserve). Showing radical changes in the underwater seascape. Front. Ecol. Evol. 2021, 9, 639161. [Google Scholar] [CrossRef]
- Bellissimo, G.; Altamirano, M.; Muñoz, A.R.; De la Rosa, J.; Hung, T.H.; Rizzuto, G.; Vizzini, S.; Tomasello, A. The invasive brown seaweed Rugulopteryx okamurae (Dictyotales, Ochrophyta) continues to expand: First record in Italy. BioInvasions Rec. 2024, 13, 385–401. [Google Scholar] [CrossRef]
- Yang, J.-I.; Yeh, C.-C.; Lee, J.-C.; Yi, S.-C.; Huang, H.-W.; Tseng, C.-N.; Chang, H.-W. Aqueous extracts of the edible Gracilaria tenuistipitata are protective against H2O2-induced DNA damage, growth inhibition, and cell cycle arrest. Molecules 2012, 17, 7241–7254. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O’Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021, 110, 90–106. [Google Scholar]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.-G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar]
- Martins, M.; Ventura, S.P.M. Emerging seaweed extraction techniques using ionic liquids—Chapter 12. In Sustainable Seaweed Technologies—Cultivation, Biorefinery, and Applications—Advances in Green and Sustainable Chemistry; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–311. [Google Scholar]
- Maia, M.L.; Vieira, E.F.; Grosso, C.; Lopes, G.; Vasconcelos, V.; Hilliou, L.; Delerue-Matos, C. Integrated approach applying ultrasound-assisted extraction to recover bioactive material from Chondrus crispus. LWT Food Sci. Technol. 2023, 188, 115344. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
| Extraction Method | Operational Parameter | Code | Dry Matter Yield (%) | Mineral Matter Yield (%) |
|---|---|---|---|---|
| Overnight Agitation (OA) | Biomass–Water 1:10 (w/v) | OAw1:10 | 20.7 ± 0.1 d | 64.7 ± 0.4 d |
| Biomass–Water 1:20 (w/v) | OAw1:20 | 21.1 ± 0.1 de | 56.2 ± 3.3 c | |
| Biomass–Ethanol 70% 1:10 (w/v) | OAew1:10 | 28.3 ± 0.0 fg | 54.0± 4.2 c | |
| Biomass–Ethanol 70% 1:20 (w/v) | OAew1:20 | 24.5 ± 0.0 e | 36.4 ± 0.3 b | |
| Mechanical Homogenization (H) | Biomass–Water 1:10 (w/v) | Hw1:10 | 24.4 ± 0.2 e | 71.0 ± 0.4 e |
| Biomass–Water 1:20 (w/v) | Hw1:20 | 26.3 ± 0.1 ef | 82.3 ± 0.1 f | |
| Biomass–Ethanol 70% 1:10 (w/v) | Hew1:10 | 27.8 ± 0.0 f | 62.5 ± 1.3 cd | |
| Biomass–Ethanol 70% 1:20 (w/v) | Hew1:20 | 29.8 ± 0.2 g | 59.6 ± 1.1 cd | |
| Biomass–Ethyl Acetate 1:10 (w/v) | Hea1:10 | 5.7 ± 0.0 b | 0.0 ± 0.0 a | |
| Biomass–Ethyl Acetate 1:20 (w/v) | Hea1:20 | 5.6 ± 0.0 b | 0.0 ± 0.0 a | |
| Biomass–Isoamyl Acetate 1:10 (w/v) | Hia1:10 | 5.7 ± 0.0 b | 0.0 ± 0.0 a | |
| Biomass–Isoamyl Acetate 1:20 (w/v) | Hia1:20 | 5.3 ± 0.0 b | 0.0 ± 0.0 a | |
| pH-Shift (pHS) | Biomass–1 M HCl 1:10 (w/v) | pHS1:10 | 14.5 ± 0.2 c | 64.8 ± 0.5 d |
| Biomass–1 M HCl 1:20 (w/v) | pHS1:20 | 13.3± 0.0 c | 64.4 ± 3.5 d | |
| Ionic Liquid (IL) | Biomass–Ionic Liquid 1:20 (w/v) | IL1:20 | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| Ultrasound Agitation (UA) | Biomass–Water 1:10 (w/v) | UAw1:10 | 19.7 ± 0.0 cd | 60.3 ± 5.3 cd |
| Biomass–Water 1:20 (w/v) | UAw1:20 | 20.9 ± 0.3 de | 53.4 ± 2.0 c | |
| Biomass–Ethanol 70% 1:10 (w/v) | UAew1:10 | 19.6 ± 0.0 cd | 33.9 ± 1.1 b | |
| Biomass–Ethanol 70% 1:20 (w/v) | UAew1:20 | 21.6 ± 0.0 de | 26.9 ± 2.4 b |
| Extraction Method | Operational Parameter | Code | Total Polyphenol Content (mg GAE/100 g dw of Seaweed) |
|---|---|---|---|
| Overnight Agitation (OA) | Biomass–Water 1:10 (w/v) | OAw1:10 | 118.5 ± 19.1 d |
| Biomass–Water 1:20 (w/v) | OAw1:20 | 170.8 ± 0.2 f | |
| Biomass–Ethanol 70% 1:10 (w/v) | OAew1:10 | 260.7 ± 7.5 h | |
| Biomass–Ethanol 70% 1:20 (w/v) | OAew1:20 | 240.0 ± 12.6 g | |
| Mechanical Homogenization (H) | Biomass–Water 1:10 (w/v) | Hw1:10 | 127.2 ± 1.5 de |
| Biomass–Water 1:20 (w/v) | Hw1:20 | 157.0 ± 8.6 ef | |
| Biomass–Ethanol 70% 1:10 (w/v) | Hew1:10 | 255.6 ± 12.8 gh | |
| Biomass–Ethanol 70% 1:20 (w/v) | Hew1:20 | 310.7 ± 22.1 i | |
| Biomass–Ethyl Acetate 1:10 (w/v) | Hea1:10 | 83.4 ± 9.4 bc | |
| Biomass–Ethyl Acetate 1:20 (w/v) | Hea1:20 | 148.3 ± 22.0 e | |
| Biomass–Isoamyl Acetate 1:10 (w/v) | Hia1:10 | 114.1 ± 19.5 cd | |
| Biomass–Isoamyl Acetate 1:20 (w/v) | Hia1:20 | 167.8 ± 7.3 f | |
| pH-Shift (pHS) | Biomass–1 M HCl 1:10 (w/v) | pHS1:10 | 71.6 ± 6.2 b |
| Biomass–1 M HCl 1:20 (w/v) | pHS1:20 | 96.0 ± 6.4 c | |
| Ionic Liquid (IL) | Biomass–Ionic Liquid 1:20 (w/v) | IL1:20 | 31.7 ± 1.7 a |
| Ultrasound Agitation (UA) | Biomass–Water 1:10 (w/v) | UAw1:10 | 116.0 ± 0.5 d |
| Biomass–Water 1:20 (w/v) | UAw1:20 | 129.0 ± 6.3 de | |
| Biomass–Ethanol 70% 1:10 (w/v) | UAew1:10 | 192.1 ± 8.9 fg | |
| Biomass–Ethanol 70% 1:20 (w/v) | UAew1:20 | 236.5 ± 20.7 g |
| Extraction Method | Operational Parameter | Code | DPPH (mg AA Eq./100 g dw of Seaweed) |
|---|---|---|---|
| Overnight Agitation (OA) | Biomass–Ethanol 70% 1:10 (w/v) | OAew1:10 | 18.8 ± 0.1 a |
| Mechanical Homogenization (H) | Biomass–Ethanol 70% 1:20 (w/v) | Hew1:20 | 40.8 ± 0.5 b |
| Extraction Method | Operational Parameter | Code | FRAP (μmol Fe2+ Eq./g dw of Seaweed) |
|---|---|---|---|
| Overnight Agitation (OA) | Biomass–Ethanol 70% 1:10 (w/v) | OAew1:10 | 9.4 ± 0.4 a |
| Mechanical Homogenization (H) | Biomass–Ethanol 70% 1:20 (w/v) | Hew1:20 | 15.7 ± 0.7 b |
| Extraction Method | Operational Parameter | Code | ABTS (μmol Trolox Eq./100 g dw of Seaweed) |
|---|---|---|---|
| Overnight Agitation (OA) | Biomass–Ethanol 70% 1:10 (w/v) | OAew1:10 | 1034.5 ± 71.7 a |
| Mechanical Homogenization (H) | Biomass–Ethanol 70% 1:20 (w/v) | Hew1:20 | 1489.2 ± 107.6 b |
| Activity or Phenolic Content | Phenolic Content | DPPH | FRAP | ABTS |
|---|---|---|---|---|
| Phenolic content | 1.00 | 0.87 | 0.93 | 0.91 |
| DPPH | 0.87 | 1.00 | 0.99 | 0.95 |
| FRAP | 0.93 | 0.99 | 1.00 | 0.97 |
| ABTS | 0.91 | 0.95 | 0.97 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo, C.; Matos, J.; Afonso, C.; Cardoso, C. Overcoming Extraction Hurdles and Assessing Biological Activity in a Major Invasive Seaweed Species in Europe, Rugulopteryx okamurae. Mar. Drugs 2025, 23, 141. https://doi.org/10.3390/md23040141
Paulo C, Matos J, Afonso C, Cardoso C. Overcoming Extraction Hurdles and Assessing Biological Activity in a Major Invasive Seaweed Species in Europe, Rugulopteryx okamurae. Marine Drugs. 2025; 23(4):141. https://doi.org/10.3390/md23040141
Chicago/Turabian StylePaulo, Carolina, Joana Matos, Cláudia Afonso, and Carlos Cardoso. 2025. "Overcoming Extraction Hurdles and Assessing Biological Activity in a Major Invasive Seaweed Species in Europe, Rugulopteryx okamurae" Marine Drugs 23, no. 4: 141. https://doi.org/10.3390/md23040141
APA StylePaulo, C., Matos, J., Afonso, C., & Cardoso, C. (2025). Overcoming Extraction Hurdles and Assessing Biological Activity in a Major Invasive Seaweed Species in Europe, Rugulopteryx okamurae. Marine Drugs, 23(4), 141. https://doi.org/10.3390/md23040141






