Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics
Abstract
1. Introduction
2. Results
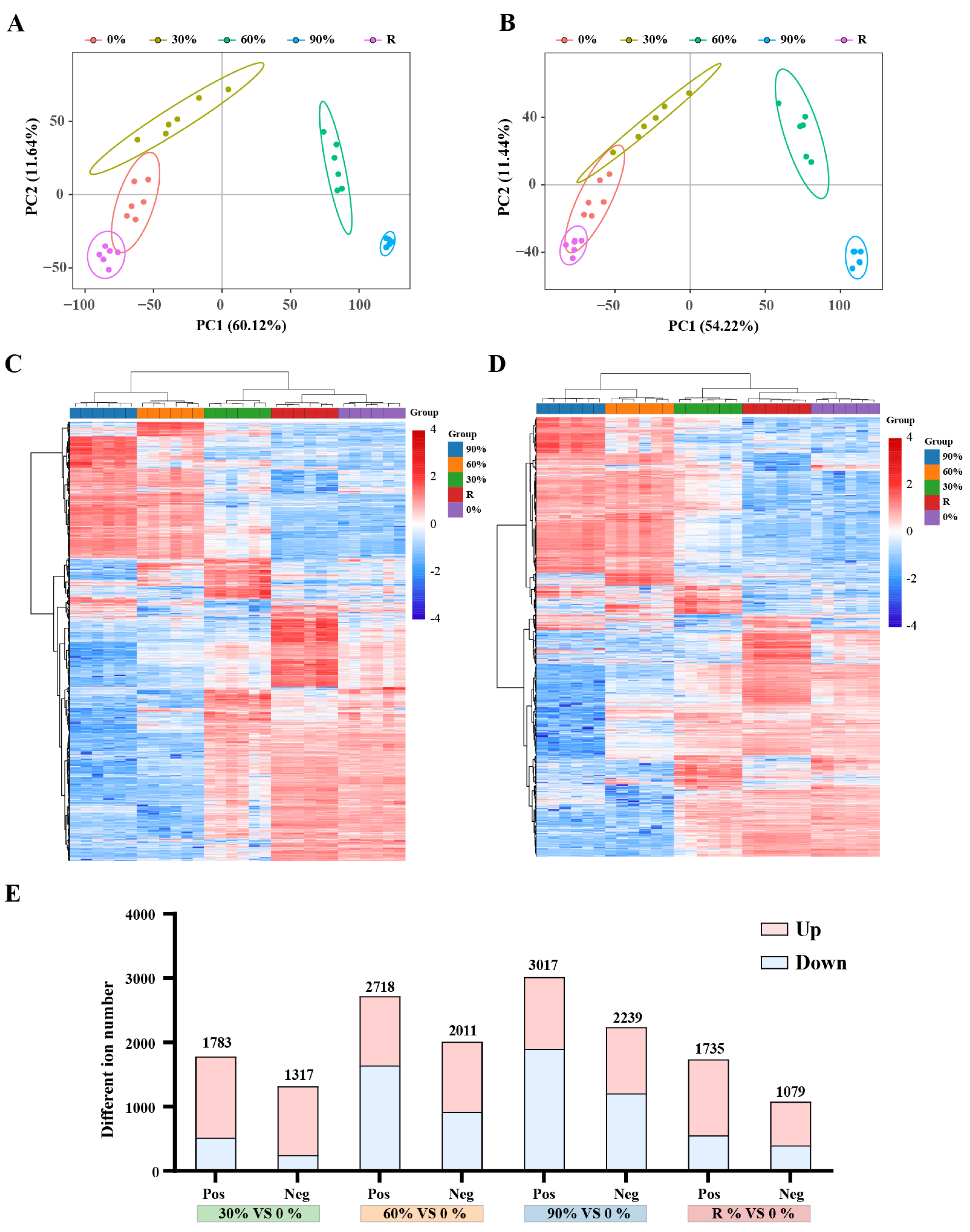
2.1. Evaluation of Metabolomics Reproducibility
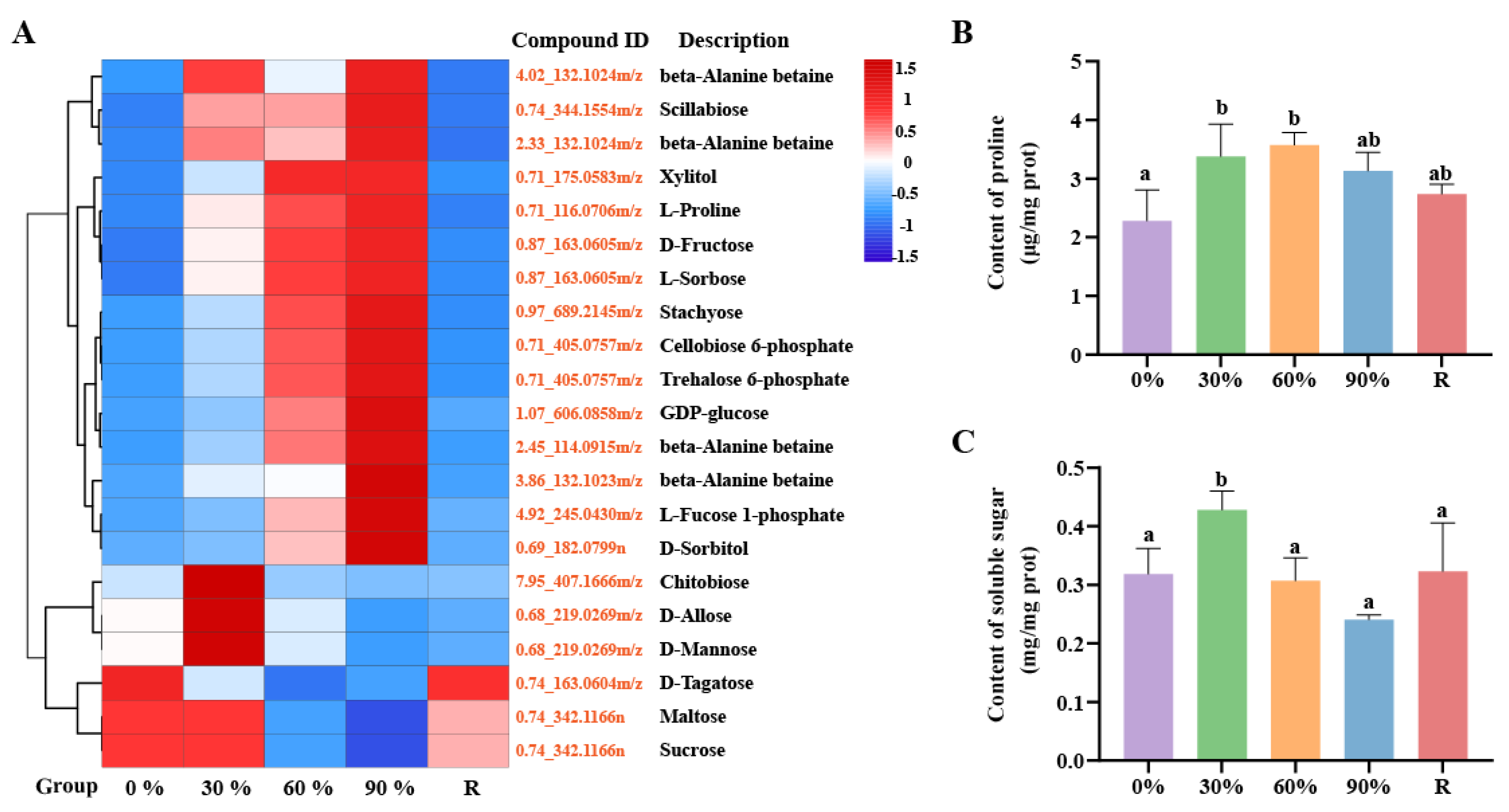
2.2. The Adjustments of Osmotic System
2.3. The Activation of Antioxidant Systems
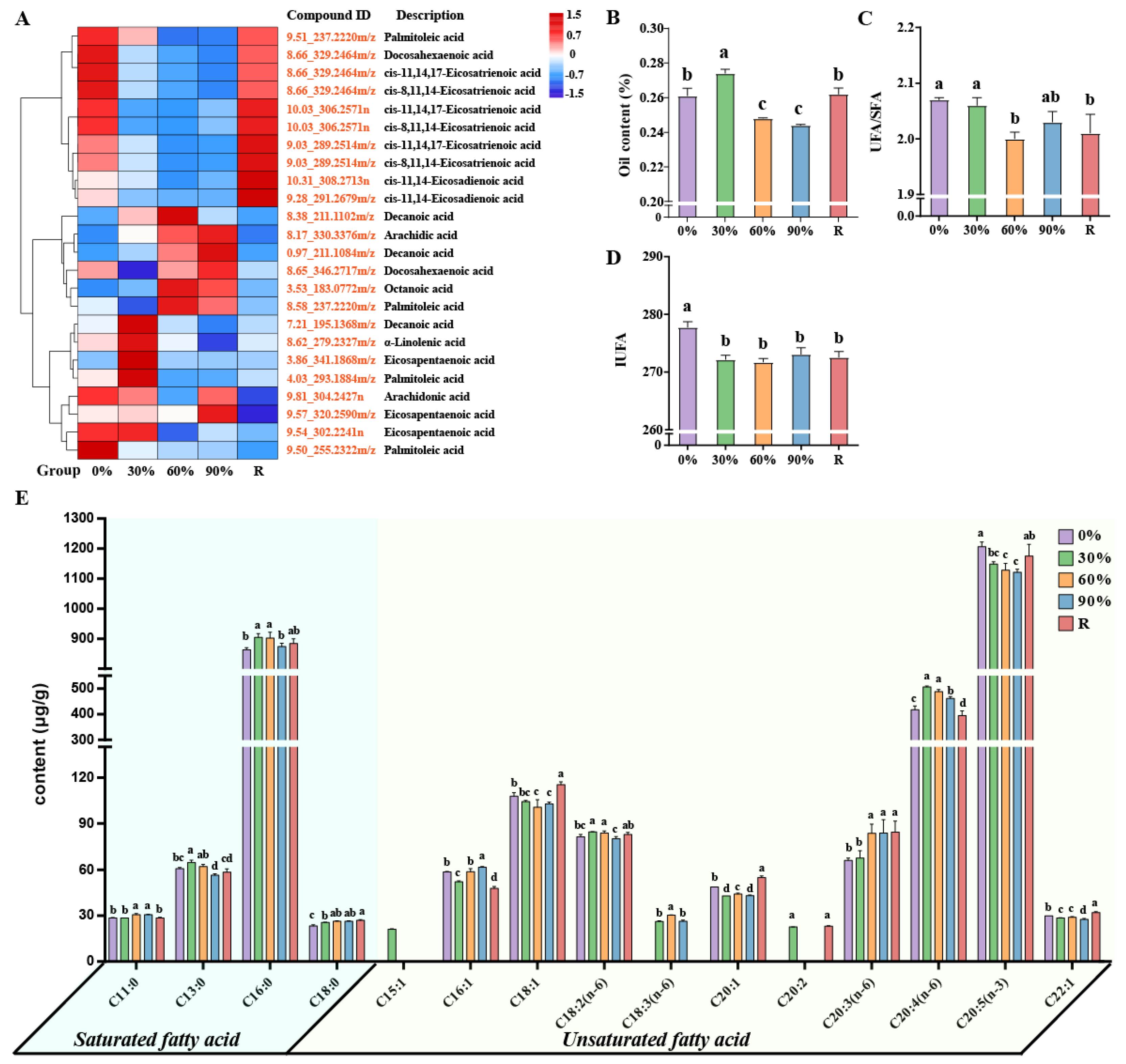
2.4. The Adjustments of Fatty Acids Metabolism
2.5. The Adjustments of Plant Hormones Metabolism
3. Discussion
3.1. Osmotic Stress Response Mechanism
3.2. Oxidative Stress Response Mechanism
3.3. Fatty Acid Metabolism Response Mechanism
3.4. Plant Hormones Metabolism Response Mechanism
4. Materials and Methods
4.1. Materials and Desiccation Treatment
4.2. Physiological Measurements
4.2.1. Soluble Protein Content Determination
4.2.2. Determination of Reactive Oxygen Species and Malondialdehyde Content
4.2.3. Determination of Antioxidant Enzyme Activities
4.2.4. Determination of Osmotic Regulation Substances
4.3. Metabolite Extraction and Measurement
4.4. Liquid Phase Parameters
4.5. Mass Spectrometry Parameters
4.6. Peak Extraction and Identification
4.7. Screening of Differential Ions
4.8. Determination of Fatty Acids Contents
4.9. Determination of Plant Hormone Content
4.10. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| APX | ascorbate peroxidase |
| C10:0 | decanoic acid |
| C16:1 | Palmitoleic acid |
| C18:3 | α-linolenic acid |
| C20:0 | arachidic acid |
| C20:2 | cis-11,14-Eicosadienoic acid |
| C20:3 N3 | cis-11,14,17-Eicosatrienoic acid |
| C20:3 N6 | cis-8,11,14-Eicosatrienoic acid |
| C20:4 | arachidonic acid |
| C20:5 | eicosapentaenoic acid |
| C22:6 | docosahexaenoic acid |
| C8:0 | octanoic acid |
| CAT | catalase |
| CKs | cytokinins |
| FA | fatty acid |
| GAs | gibberellins |
| GSH | glutathione |
| IAA | Indole-3-acetic acid |
| IUFA | index of unsaturated fatty acid |
| JA | jasmonic acid |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SFA | saturated fatty acids |
| SOD | superoxide dismutase |
| UFA | unsaturated fatty acids |
References
- Dittami, S.M.; Scornet, D.; Petit, J.-L.; Ségurens, B.; Da Silva, C.; Corre, E.; Dondrup, M.; Glatting, K.-H.; König, R.; Sterck, L. Global expression analysis of the brown alga Ectocarpus siliculosus (Phaeophyceae) reveals large-scale reprogramming of the transcriptome in response to abiotic stress. Genome Biol. 2009, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Brawley, S.H.; Blouin, N.A.; Ficko-Blean, E.; Wheeler, G.L.; Lohr, M.; Goodson, H.V.; Jenkins, J.W.; Blaby-Haas, C.E.; Helliwell, K.E.; Chan, C.X. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc. Natl. Acad. Sci. USA 2017, 114, E6361–E6370. [Google Scholar] [CrossRef] [PubMed]
- Huan, L.; Xie, X.; Zheng, Z.; Sun, F.; Wu, S.; Li, M.; Gao, S.; Gu, W.; Wang, G. Positive correlation between PSI response and oxidative pentose phosphate pathway activity during salt stress in an intertidal macroalga. Plant Cell Physiol. 2014, 55, 1395–1403. [Google Scholar] [CrossRef]
- Chen, H.; Chu, J.S.-C.; Chen, J.; Luo, Q.; Wang, H.; Lu, R.; Zhu, Z.; Yuan, G.; Yi, X.; Mao, Y. Insights into the ancient adaptation to intertidal environments by red algae based on a genomic and multiomics investigation of Neoporphyra haitanensis. Mol. Biol. Evol. 2022, 39, msab315. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hackett, J.D.; Ciniglia, C.; Pinto, G.; Bhattacharya, D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 2004, 21, 809–818. [Google Scholar] [CrossRef]
- Wen, J.; Wang, W.; Xu, K.; Ji, D.; Xu, Y.; Chen, C.; Xie, C. Comparative Analysis of Proteins Involved in Energy Metabolism and Protein Processing in Pyropia haitanensis at Different Salinity Levels. Front. Mar. Sci. 2020, 7, 415. [Google Scholar] [CrossRef]
- Wang, W.; Ge, Q.; Wen, J.; Zhang, H.; Guo, Y.; Li, Z.; Xu, Y.; Ji, D.; Chen, C.; Guo, L.; et al. Horizontal gene transfer and symbiotic microorganisms regulate the adaptive evolution of intertidal algae, Porphyra sense lato. Commun. Biol. 2024, 7, 976. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, Y.; Xu, K.; Ji, D.; Chen, C.; Xie, C. Investigating the mechanisms underlying the hyposaline tolerance of intertidal seaweed, Pyropia haitanensis. Algal Res. 2020, 47, 101886. [Google Scholar] [CrossRef]
- Park, J.-S.; Jeong, Y.-R.; Chun, B.-S. Physiological activities and bioactive compound from laver (Pyropia yezoensis) hydrolysates by using subcritical water hydrolysis. J. Supercrit. Fluids 2019, 148, 130–136. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics—Yearbook; FAO: Rome, Italy, 2025. [Google Scholar]
- Zhang, Z.; Wang, X.; Pan, Y.; Wang, G.; Mao, G. The degraded polysaccharide from Pyropia haitanensis represses amyloid beta peptide-induced neurotoxicity and memory in vivo. Int. J. Biol. Macromol. 2020, 146, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, H.; Fu, X. Fermentation of Pyropia spp. seaweed: A comprehensive review on processing conditions, biological activities and potential applications in the food industry. Crit. Rev. Food Sci. Nutr. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Luo, Q.; Zhu, S.; Chen, J.; Yang, R.; Niu, T.; Wang, T.; Zhang, P.; Chen, H. The extensive cultivation of Pyropia haitanensis along the coastal areas influences the ecological dynamics of nearby intertidal mudflat pond water. Front. Mar. Sci. 2025, 12, 1442210. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G. The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J. Exp. Bot. 2012, 63, 4349–4358. [Google Scholar] [CrossRef]
- Behera, D.P.; Ingle, K.N.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Sahu, S.K.; Krishnan, M.G.; Shinde, P.B.; Ganesan, M.; Mantri, V.A. Epiphytism, diseases and grazing in seaweed aquaculture: A comprehensive review. Rev. Aquac. 2022, 14, 1345–1370. [Google Scholar] [CrossRef]
- Li, X.-l.; Wang, W.-j.; Liu, F.-l.; Liang, Z.-r.; Sun, X.-t.; Yao, H.-q.; Wang, F.-j. Periodical drying or no drying during aquaculture affects the desiccation tolerance of a sublittoral Pyropia yezoensis strain. J. Appl. Phycol. 2018, 30, 697–705. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Y.; Kong, F.; Cao, M.; Sun, P. Genome-wide expression profiles of Pyropia haitanensis in response to osmotic stress by using deep sequencing technology. BMC Genom. 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Im, S.; Lee, H.-N.; Jung, H.S.; Yang, S.; Park, E.-J.; Hwang, M.S.; Jeong, W.-J.; Choi, D.-W. Transcriptome-based identification of the desiccation response genes in marine red algae Pyropia tenera (Rhodophyta) and enhancement of abiotic stress tolerance by PtDRG2 in Chlamydomonas. Mar. Biotechnol. 2017, 19, 232–245. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; López-Cristoffanini, C.; Meynard, A.; Kumar, M. Tolerance pathways to desiccation stress in seaweeds. Syst. Biol. Mar. Ecosyst. 2017, 13–33. [Google Scholar]
- Yin, J.; Sun, Y.; Miao, X.; Qu, J.; Zhang, K.; Qing Han, X.; Li, Y.; Sun, J.; Kong, F. Dynamic changes and transcriptome analyses reveal the microfilament skeleton response to water stress in thalli of Neopyropia yezoensis inhabiting the intertidal zone. Plant Stress 2025, 15, 100762. [Google Scholar] [CrossRef]
- Xu, K.; Xu, Y.; Ji, D.; Xie, J.; Chen, C.; Xie, C. Proteomic analysis of the economic seaweed Pyropia haitanensis in response to desiccation. Algal Res. 2016, 19, 198–206. [Google Scholar] [CrossRef]
- Shi, J.; Wang, W.; Lin, Y.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Insight into transketolase of Pyropia haitanensis under desiccation stress based on integrative analysis of omics and transformation. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Saito, K.; Matsuda, F. Metabolomics for functional genomics, systems biology, and biotechnology. Annu. Rev. Plant Biol. 2010, 61, 463–489. [Google Scholar] [CrossRef]
- Hama, J.R.; Hooshmand, K.; Laursen, B.B.; Vestergård, M.; Fomsgaard, I.S. Clover root uptake of cereal benzoxazinoids (BXs) caused accumulation of BXs and BX transformation products concurrently with substantial increments in clover flavonoids and abscisic acid. J. Agric. Food Chem. 2022, 70, 14633–14640. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Thakur, R.S.; Baghel, R.S.; Reddy, C.; Jha, B. Seaweed metabolomics: A new facet of functional genomics. Adv. Bot. Res. 2014, 71, 31–52. [Google Scholar]
- Ye, Y.; Yang, R.; Lou, Y.; Chen, J.; Yan, X.; Tang, H. Effects of food processing on the nutrient composition of Pyropia yezoensis products revealed by NMR-based metabolomic analysis. J. Food Nutr. Res. 2014, 2, 749–756. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, H.; Zhu, S.; Niu, T.; Luo, Q.; Chen, J.; Yang, R.; Zhang, P.; Wang, T.; Chen, H. Metabolome analysis reveals the involvement of oxylipins in regulating the maturation of conchosporangia in Pyropia haitanensis. Algal Res. 2025, 86, 103933. [Google Scholar] [CrossRef]
- Jian, Q.; Zhu, X.; Chen, J.; Zhu, Z.; Yang, R.; Luo, Q.; Chen, H.; Yan, X. Analysis of global metabolome by gas chromatography-mass spectrometry of Pyropia haitanensis stimulated with 1-octen-3-ol. J. Appl. Phycol. 2017, 29, 2049–2059. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zou, P.; Tian, X.; Dong, B.; Zhang, C. Size effects of chitooligomers with certain degrees of polymerization on the chilling tolerance of wheat seedlings. Carbohydr. Polym. 2017, 160, 194–202. [Google Scholar] [CrossRef]
- Zhao, S.; Zeng, W.; Li, Z.; Peng, Y. Mannose regulates water balance, leaf senescence, and genes related to stress tolerance in white clover under osmotic stress. Biol. Plant. 2020, 64, 406–416. [Google Scholar] [CrossRef]
- Yobi, A.; Wone, B.W.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol. Plant 2013, 6, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation tolerance: Avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef]
- Bray, E.A. Plant responses to water deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Xu, Y.; Yi, Y. How the xerophytic moss Pogonatum inflexum tolerates desiccation. Plant Cell Rep. 2024, 43, 39. [Google Scholar] [CrossRef]
- Asami, P.; Rupasinghe, T.; Moghaddam, L.; Njaci, I.; Roessner, U.; Mundree, S.; Williams, B. Roots of the resurrection plant Tripogon loliiformis survive desiccation without the activation of autophagy pathways by maintaining energy reserves. Front. Plant Sci. 2019, 10, 459. [Google Scholar] [CrossRef]
- Dai, T.; Ban, S.; Han, L.; Li, L.; Zhang, Y.; Zhang, Y.; Zhu, W. Effects of exogenous glycine betaine on growth and development of tomato seedlings under cold stress. Front. Plant Sci. 2024, 15, 1332583. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Zhang, H.; Shi, L.; Cao, F.; Guo, L.; Xie, Y.; Wang, T.; Yan, X.; Dai, S. Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J. Proteome Res. 2010, 9, 6561–6577. [Google Scholar] [CrossRef]
- Pandey, V.; Ranjan, S.; Deeba, F.; Pandey, A.K.; Singh, R.; Shirke, P.A.; Pathre, U.V. Desiccation-induced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. J. Plant Physiol. 2010, 167, 1351–1359. [Google Scholar] [CrossRef]
- Shivaraj, Y.N.; Plancot, B.; Ramdani, Y.; Gügi, B.; Kambalagere, Y.; Jogaiah, S.; Driouich, A.; Govind, S.R. Physiological and biochemical responses involved in vegetative desiccation tolerance of resurrection plant Selaginella brachystachya. 3 Biotech 2021, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, D.; Yu, B.; Yu, X.; Li, W. Maintenance or collapse: Responses of extraplastidic membrane lipid composition to desiccation in the resurrection plant Paraisometrum mileense. PLoS ONE 2014, 9, e103430. [Google Scholar] [CrossRef] [PubMed]
- Flores-Molina, M.R.; Thomas, D.; Lovazzano, C.; Núnez, A.; Zapata, J.; Kumar, M.; Correa, J.A.; Contreras-Porcia, L. Desiccation stress in intertidal seaweeds: Effects on morphology, antioxidant responses and photosynthetic performance. Aquat. Bot. 2014, 113, 90–99. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, V.; Trivedi, N.; Kumari, P.; Bijo, A.; Reddy, C.; Jha, B. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ. Exp. Bot. 2011, 72, 194–201. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2011, 62, 1815–1829. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Mi, C.; Wang, Q.; Zhao, Y.; Zhang, C.; Sun, C.; Liu, Z.; Lin, L. Changes in the differentially expressed proteins and total fatty acid contents in winter rapeseed (Brassica rapa L.) leaves under drought stress. Russ. J. Plant Physiol. 2022, 69, 31. [Google Scholar] [CrossRef]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Fujii, S.; Uenaka, M.; Nakayama, S.; Yamamoto, R.; Mantani, S. Effects of sodium chloride on the fatty acids composition in Boekelovia hooglandii (Ochromonadales, Chrysophyceae). Phycol. Res. 2001, 49, 73–77. [Google Scholar] [CrossRef]
- Xu, L.; Han, L.; Huang, B. Membrane fatty acid composition and saturation levels associated with leaf dehydration tolerance and post-drought rehydration in Kentucky bluegrass. Crop Sci. 2011, 51, 273–281. [Google Scholar] [CrossRef]
- Miao, X.; Zhang, L.; Chen, X.; Wu, S.; Niu, D.; FU, H. The relationship of fatty acid composition and resistance of Artemisia sphaerocephala seedlings under water stress. Acta Prataculturae Sin. 2015, 24, 55. [Google Scholar]
- Liu, X.; Huang, B. Changes in fatty acid composition and saturation in leaves and roots of creeping bentgrass exposed to high soil temperature. J. Am. Soc. Hortic. Sci. 2004, 129, 795–801. [Google Scholar] [CrossRef]
- Pham Thi, A.T.; Vieira Da Silva, J.; Mazliak, P. The role of membrane lipids in drought resistance of plants. Bull. Société Bot. France. Actual. Bot. 1990, 137, 99–114. [Google Scholar] [CrossRef]
- Yin, L.; Xu, J.; Zhang, L.; Liu, D.; Zhang, C.; Liu, T.; Wang, S.; Deng, X. Altered fatty acid composition confers improved drought acclimation in maize. Plant Physiol. Biochem. 2024, 206, 108274. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Qi, L.; Yin, L.; Deng, X. Galactolipid remodeling is involved in drought-induced leaf senescence in maize. Environ. Exp. Bot. 2018, 150, 57–68. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Zorin, B.; Pal-Nath, D.; Lukyanov, A.; Smolskaya, S.; Kolusheva, S.; Didi-Cohen, S.; Boussiba, S.; Cohen, Z.; Khozin-Goldberg, I.; Solovchenko, A. Arachidonic acid is important for efficient use of light by the microalga Lobosphaera incisa under chilling stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 853–868. [Google Scholar] [CrossRef]
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarraj, S.; Albasher, G.; Schnitzler, J.-P.; Ache, P.; Hedrich, R.; Rennenberg, H. Metabolic responses of date palm (Phoenix dactylifera L.) leaves to drought differ in summer and winter climate. Tree Physiol. 2021, 41, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Plant responses to exogenous salicylic and jasmonic acids under drought stress. Jasmonates Salicylates Signal. Plants 2021, 65–85. [Google Scholar]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Tak, Y.; Asthir, B. Salicylic acid: A key signal molecule ameliorating plant stresses. Cereal Res. Commun. 2022, 50, 617–626. [Google Scholar] [CrossRef]
- Liu, A.; Wang, M.; Dong, J.; Yan, Z.; Wang, X.; Li, J.; Song, H. Foliar application of exogenous salicylic acid mitigates the detrimental effects caused by salt stress in sunflower seedlings. Ind. Crops Prod. 2024, 222, 119854. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Chen, H.-I.; Li, P.-F.; Yang, C.-H. NAC-like gene GIBBERELLIN SUPPRESSING FACTOR regulates the gibberellin metabolic pathway in response to cold and drought stresses in Arabidopsis. Sci. Rep. 2019, 9, 19226. [Google Scholar] [CrossRef]
- Shohat, H.; Cheriker, H.; Kilambi, H.V.; Illouz Eliaz, N.; Blum, S.; Amsellem, Z.; Tarkowská, D.; Aharoni, A.; Eshed, Y.; Weiss, D. Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytol. 2021, 232, 1985–1998. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.; Pandey, M. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Yang, R.; Luo, Q.; Wang, T.; Zhang, P.; Chen, H. Abscisic acid activates desiccation tolerance responses in intertidal seaweed Neoporphyra haitanensis. Front. Mar. Sci. 2022, 9, 1007193. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the basal role of ABA–roles outside of stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Avramova, Z. Defence-related priming and responses to recurring drought: Two manifestations of plant transcriptional memory mediated by the ABA and JA signalling pathways. Plant Cell Environ. 2019, 42, 983–997. [Google Scholar] [CrossRef]
- Chen, C.; Ji, D.; Xie, C.; Xu, Y.; Liang, Y.; Zheng, Y.; Shi, X.; Wang, F.; Zhao, L. Preliminary study on selecting the high temperature resistance strains and economic traits of Porphyra haitanensis. Acta Oceanol. Sin. 2008, 30, 100–106. [Google Scholar]
- Tukozkan, N.; Erdamar, H.; Seven, I. Measurement of total malondialdehyde in plasma and tissues by high-performance liquid chromatography and thiobarbituric acid assay. Firat Tip Derg. 2006, 11, 88–92. [Google Scholar]
- Zhang, C.; Yi, X.; Gao, X.; Wang, M.; Shao, C.; Lv, Z.; Chen, J.; Liu, Z.; Shen, C. Physiological and biochemical responses of tea seedlings (Camellia sinensis) to simulated acid rain conditions. Ecotoxicol. Environ. Saf. 2020, 192, 110315. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Chen, Z.-Y.; Jiang, Y.; Duan, B.-B.; Xi, Z.-M. Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 256, 108596. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Beisson, F.; Pollard, M.; Ohlrogge, J. Oil content of Arabidopsis seeds: The influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 2006, 67, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Floková, K.; Tarkowská, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novák, O. UHPLC–MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Shi, J.; Meng, M.; Xu, K.; Xu, Y.; Ji, D.; Wang, W.; Xie, C. Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics. Mar. Drugs 2025, 23, 203. https://doi.org/10.3390/md23050203
Wen J, Shi J, Meng M, Xu K, Xu Y, Ji D, Wang W, Xie C. Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics. Marine Drugs. 2025; 23(5):203. https://doi.org/10.3390/md23050203
Chicago/Turabian StyleWen, Jian, Jianzhi Shi, Muhan Meng, Kai Xu, Yan Xu, Dehua Ji, Wenlei Wang, and Chaotian Xie. 2025. "Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics" Marine Drugs 23, no. 5: 203. https://doi.org/10.3390/md23050203
APA StyleWen, J., Shi, J., Meng, M., Xu, K., Xu, Y., Ji, D., Wang, W., & Xie, C. (2025). Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics. Marine Drugs, 23(5), 203. https://doi.org/10.3390/md23050203





