The Cytotoxic Activity of Secondary Metabolites from Marine-Derived Penicillium spp.: A Review (2018–2024)
Abstract
1. Introduction
2. Secondary Metabolites of Marine-Derived Penicillium spp.
3. Chemical Diversity of Marine-Derived Penicillium Secondary Metabolites
3.1. Polyketides
3.1.1. Azaphilones/Azaphilonoids
3.1.2. Benzopyranoid Derivatives
3.1.3. Chromones
3.1.4. Pyrones
3.1.5. Fatty Acids and Esters
3.1.6. Furans
3.1.7. Xanthone and Benzophenones
3.1.8. Quinones
3.1.9. Phenolics
Phenol
Phenolic Acid
Phenol Ethers
3.1.10. Sorbicillinoids
3.1.11. Pyridinone Derivatives
3.1.12. Others
3.2. Alkaloids
3.2.1. Cytochalasins
3.2.2. Indole Alkaloids
3.2.3. Pyridine Alkaloids
3.2.4. Pyrrole and Pyrrolidine Alkaloids
3.2.5. Quinazolines and Their Analogs
3.2.6. Peptides
3.2.7. Thiodiketopiperazines
3.3. Terpenoids
3.3.1. Sesquiterpenes
3.3.2. Meroterpenoids
3.3.3. Diterpenoids
3.4. Steroids
4. Drugability Assessment
4.1. Dicitrinones G
4.2. Penitrem A
4.3. Penicisulfuranol A
4.4. Secalonic Acid D
4.5. 4,4′-Bond Secalonic Acid D
5. Discussion
5.1. Chemical Diversity and Structural Features of Marine-Derived Penicillium Metabolites
5.2. Current Status of Marine Drug Development
5.3. Challenges and Future Directions
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA-Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA-Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Update. 2021, 59, 100796. [Google Scholar] [CrossRef]
- Visser, K.E.D.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Wei, W.; Khan, B.; Dai, Q.; Lin, J.; Kang, L.; Rajput, N.A.; Yan, W.; Liu, G.Y. Potential of secondary metabolites of diaporthe species associated with terrestrial and marine origins. J. Fungi 2023, 9, 453. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Tamzi, N.N.; Rahman, M.M.; Das, S. Recent advances in marine-derived bioactives towards cancer therapy. Int. J. Transl. Med. 2024, 4, 740–781. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Zhao, K.; Chen, B.; Sun, Z. Natural products modulating MAPK for CRC treatment: A promising strategy. Front. Pharmacol. 2025, 16, 1514486. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2022, 85, 123–154. [Google Scholar] [CrossRef] [PubMed]
- Stramucci, L.; Bossi, G. Approaching the challenges of MKK3/p38delta MAPK targeting for therapeutic purpose in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, W.; Fu, B.; Shi, L.; Wang, X.; Kuca, K. JNK signaling in cancer cell survival. Med. Res. Rev. 2019, 39, 2082–2104. [Google Scholar] [CrossRef]
- Staal, F.J.; Clevers, H.C. WNT signalling and haematopoiesis: A WNT-WNT situation. Nat. Rev. Immunol. 2005, 5, 21–30. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Meesakul, P.; Zhou, J.; Liu, J.; Liu, S.; Wang, C.; Cao, S. Cytotoxic compounds from marine fungi: Sources, structures, and bioactivity. Mar. Drugs 2024, 22, 70. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Refaey, M.S.; Elias, N.; El-Mallah, M.F.; Albaqami, F.M.K.; Dergaa, I.; Du, M.; Salem, M.F.; Tahir, H.E.; Dagliaa, M.; et al. Marine natural products as a source of novel anticancer drugs: An updated review (2019-2023). Nat. Prod. Bioprospect. 2025, 15, 13. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Honecker, F. Marine compounds and cancer: Updates 2022. Mar. Drugs 2022, 20, 759. [Google Scholar] [CrossRef]
- Staats, P.S.; Yearwood, T.; Charapata, S.G.; Presley, R.W.; Wallace, M.S.; Byas-Smith, M.; Fisher, R.; Bryce, D.A.; Mangieri, E.A.; Luther, R.R.; et al. Intrathecal ziconotide in the treatmentof refractory pain in patients with cncer or AIDS. JAMA 2004, 291, 63–70. [Google Scholar] [CrossRef]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentao, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-derived anticancer agents: Clinical benefits, innovative mechanisms, and new targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Coello, L.; Fernandez, R.; Reyes, F.; Rodriguez, A.; Murcia, C.; Garranzo, M.; Mateo, C.; Sanchez-Sancho, F.; Bueno, S.; et al. Isolation and first total synthesis of PM050489 and PM060184, two new marine anticancer compounds. J. Am. Chem. Soc. 2013, 135, 10164–10171. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Sukhikh, S.; Babich, O. Study of marine microorganism metabolites: New resources for bioactive natural products. Front. Microbiol. 2023, 14, 1285902. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine fungi: A source of potential anticancer compounds. Front. Microbiol. 2017, 8, 2536. [Google Scholar] [CrossRef]
- Verissimo, A.C.S.; Pacheco, M.; Silva, A.M.S.; Pinto, D. Secondary metabolites from marine sources with potential use as leads for anticancer applications. Molecules 2021, 26, 4292. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhu, T.H.; Zhu, W.M. New marine natural products of microbial origin from 2010 to 2013. Chinese. J. Org. Chem. 2013, 33, 1195–1234. [Google Scholar] [CrossRef]
- Lee, J.; Currano, J.N.; Carroll, P.J.; Joullie, M.M. Didemnins, tamandarins and related natural products. Nat. Prod. Rep. 2012, 29, 404–424. [Google Scholar] [CrossRef]
- Beasley, V.R.; Bruno, S.J.; Burner, J.S.; Choi, B.W.; Rinehart, K.L.; Koritz, G.D.; Levengood, J.M. Fate of tritiated didemnin B in mice: Excretion and tissue concentrations after an intraperitoneal dose. Biopharm. Drug Dispos. 2005, 26, 341–351. [Google Scholar] [CrossRef]
- Ali, M.A.; Khan, A.U.; Ali, A.; Khaliq, M.; Khan, N.; Mujahid, S.; Calina, D.; Puskulluoglu, M.; Sharifi-Rad, J. Didemnins as marine-derived anticancer agents: Mechanistic insights and clinical potential. Med. Oncol. 2025, 42, 43. [Google Scholar] [CrossRef]
- Aspeslagh, S.; Awada, A.; Matos-Pita, A.S.; Aftimos, P.; Bahleda, R.; Varga, A.; Soria, J.C. Phase I dose-escalation study of plitidepsin in combination with bevacizumab in patients with refractory solid tumors. Anticancer Drugs 2016, 27, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Plummer, R.; Oaknin, A.; Robinson, A.; Pardo, B.; Soto-Matos, A.; Yovine, A.; Szyldergemajn, S.; Calvert, A.H. Phase I study of weekly plitidepsin as 1-hour infusion combined with carboplatin in patients with advanced solid tumors or lymphomas. Investig. New Drugs 2011, 29, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, M.; Song, S.J.; Jung, J. Marine-derived Penicillium Species as producers of cytotoxic metabolites. Mar. Drugs 2017, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.G.; Liu, Q.; Zhu, G.L.; Liu, H.S.; Zhu, W.M. Marine natural products sourced from marine-derived Penicillium fungi. J. Asian Nat. Prod. Res. 2016, 18, 92–115. [Google Scholar] [CrossRef]
- Yang, X.L.; Liu, J.P.; Mei, J.H.; Jiang, R.; Tu, S.Z.; Deng, H.F.; Liu, J.; Yang, S.M.; Li, J. Origins, structures, and bioactivities of secondary metabolites from marine-derived Penicillium fungi. Mini Rev. Med. Chem. 2021, 21, 2000–2019. [Google Scholar] [CrossRef]
- Xu, J.Z.; Yi, M.Q.; Ding, L.J.; He, S. A review of anti-inflammatory compounds from marine fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef]
- Lv, F.; Zeng, Y. Novel bioactive natural products from marine-derived Penicillium fungi: A review (2021–2023). Mar. Drugs 2024, 22, 191. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Q.; Yuan, X.; Xu, K. Newly reported alkaloids produced by marine-derived Penicillium species (covering 2014–2018). Bioorganic Chem. 2020, 99, 103840. [Google Scholar] [CrossRef]
- Koul, M.; Singh, S. Penicillium spp prolific producer for harnessing cytotoxic secondary metabolites. Anti-Cancer Drugs 2017, 28, 11–30. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Cai, L.; Zhao, M.; Kirk, P.M.; Fan, G.; Sun, Q.; Li, B.; Wang, S.; Yu, Z.; et al. Fungal names: A comprehensive nomenclatural repository and knowledge base for fungal taxonomy. Nucleic Acids Res. 2023, 51, D708–D716. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Suetrong, S.; Sakayaroj, J.; Bahkali, A.H.; Abdel-Wahab, M.A.; Boekhout, T.; Pang, K.L. Classification of marine ascomycota, basidiomycota, blastocladiomycota and chytridiomycota. Fungal Divers. 2015, 73, 1–72. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Nonaka, T.; Fujii, I. Fungal type III polyketide synthases. Nat. Prod. Rep. 2014, 31, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Liu, N.; Tang, Y. Coordinated and iterative enzyme catalysis in fungal polyketide biosynthesis. ACS Catalysis 2016, 6, 5935–5945. [Google Scholar] [CrossRef]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Z.; Chang, W.; Zhao, W.; Wang, H.; Chen, H.; Dai, H.; Lv, F. New azaphilones from the marine-derived fungus Penicillium sclerotiorum E23Y-1A with their anti-inflammatory and antitumor activities. Mar. Drugs 2023, 21, 75. [Google Scholar] [CrossRef]
- Wang, H.C.; Ke, T.Y.; Ko, Y.C.; Lin, J.J.; Chang, J.S.; Cheng, Y.B. Anti-inflammatory azaphilones from the edible alga-derived fungus Penicillium sclerotiorum. Mar. Drugs 2021, 19, 529. [Google Scholar] [CrossRef]
- Frank, M.; Hartmann, R.; Plenker, M.; Mandi, A.; Kurtan, T.; Ozkaya, F.C.; Muller, W.E.G.; Kassack, M.U.; Hamacher, A.; Lin, W.; et al. Brominated azaphilones from the sponge-associated fungus Penicillium canescens strain 4.14.6a. J. Nat. Prod. 2019, 82, 2159–2166. [Google Scholar] [CrossRef]
- Xi, Y.D.; Wang, H.N.; Sun, L.X.; Ma, X.Y.; Zhang, S.C.; Zhang, Z. Recent advances in the structures and bioactivities of benzopyrans derived from marine fungi: A review. Front. Pharmacol. 2024, 15, 1482316. [Google Scholar] [CrossRef]
- Fan, H.; Shi, Z.M.; Lei, Y.H.; Si-Tu, M.X.; Zhou, F.G.; Feng, C.; Wei, X.; Shao, X.H.; Chen, Y.; Zhang, C.X. Rare carbon-bridged citrinin dimers from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2. Mar. Drugs 2022, 20, 443. [Google Scholar] [CrossRef]
- Salendra, L.; Lin, X.; Chen, W.; Pang, X.; Luo, X.; Long, J.; Liao, S.; Wang, J.; Zhou, X.; Liu, Y.; et al. Cytotoxicity of polyketides and steroids isolated from the sponge-associated fungus Penicillium citrinum SCSIO 41017. Nat. Prod. Res. 2021, 35, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Chang, C.H.; Liao, H.R.; Cheng, M.J.; Wu, M.D.; Fu, S.L.; Chen, J.J. Rare chromone derivatives from the marine-derived Penicillium citrinum with anti-cancer and anti-inflammatory activities. Mar. Drugs 2021, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Chang, C.H.; Liao, H.R.; Fu, S.L.; Chen, J.J. Anti-cancer and anti-inflammatory activities of three new chromone derivatives from the marine-derived Penicillium citrinum. Mar. Drugs 2021, 19, 408. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Huang, X.F.; Xiao, H.X.; Hao, Y.J.; Xu, L.; Yan, Q.X.; Zou, Z.B.; Xie, C.L.; Xu, Y.Q.; Yang, X.W. Chemical constituents of the marine fungus Penicillium sp. MCCC 3A00228. Chem. Biodivers. 2021, 18, e2100697. [Google Scholar] [CrossRef]
- Yang, P.B.; Hou, P.P.; Liu, F.Y.; Hong, W.B.; Chen, H.Z.; Sun, X.Y.; Li, P.; Zhang, Y.; Ju, C.Y.; Luo, L.J.; et al. Blocking PPARγ interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression. Proc. Natl. Acad. Sci. USA 2020, 117, 27412–27422. [Google Scholar] [CrossRef]
- Alshehri, S.O.; Malatani, R.T.; Bogari, H.A.; Noor, A.O.; Ibrahim, A.K.; Elhady, S.S.; Abdelhameed, R.F.A. Lama-1: A cerebroside isolated from the deep-sea-derived fungus Penicillium chrysogenum. Metabolites 2020, 10, 75. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, S.Q.; Li, X.M.; Li, X.; Wang, B.G.; Li, H.L. Cyclopiumolides A and B, unusual 13-membered macrolides from the deep sea-sourced fungus Penicillium cyclopium SD-413 with antiproliferative activities. Bioorganic Chem. 2022, 128, 106104. [Google Scholar] [CrossRef]
- Hsi, H.Y.; Wang, S.W.; Cheng, C.H.; Pang, K.L.; Leu, J.Y.; Chang, S.H.; Lee, Y.T.; Kuo, Y.H.; Huang, C.Y.; Lee, T.H. Chemical constituents and anti-angiogenic principles from a marine algicolous Penicillium sumatraense SC29. Molecules 2022, 27, 8940. [Google Scholar] [CrossRef]
- Zou, Z.B.; Zhang, G.; Zhou, Y.Q.; Xie, C.L.; Xie, M.M.; Xu, L.; Hao, Y.J.; Luo, L.Z.; Zhang, X.K.; Yang, X.W.; et al. Chemical constituents of the deep-sea-derived Penicillium citreonigrum MCCC 3A00169 and their antiproliferative effects. Mar. Drugs 2022, 20, 736. [Google Scholar] [CrossRef]
- Niu, S.; Xia, M.; Chen, M.; Liu, X.; Li, Z.; Xie, Y.; Shao, Z.; Zhang, G. Cytotoxic polyketides isolated from the deep-sea-derived fungus Penicillium chrysogenum MCCC 3A00292. Mar. Drugs 2019, 17, 686. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Liub, Q.A.; Hua, L.D.; Lia, W.; Zhua, H.J.; Liuc, L.; Caoa, F. New verrucosidin derivatives from the marine-derived fungus Penicillium sp. XL-01. Nat. Prod. Commun. 2018, 13, 1329–1332. [Google Scholar] [CrossRef]
- Tang, X.X.; Liu, S.Z.; Yan, X.; Tang, B.W.; Fang, M.J.; Wang, X.M.; Wu, Z.; Qiu, Y.K. Two new cytotoxic compounds from a deep-sea Penicillum citreonigrum XT20-134. Mar. Drugs 2019, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.Y.; Li, R.D.; Zhang, Y.X.; Pan, X.F.; Jiang, S.C.; Sun, C.C.; Zhang, C.; Lu, X.M. Polyketides isolated from an endophyte Penicillium oxalicum 2021CDF-3 inhibit pancreatic tumor growth. Front. Microbiol. 2022, 13, 1033823. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Kaleem, S.; Wu, B.; Zhan, Z.Z. New antiproliferative compounds against glioma cells from the marine-sourced fungus Penicillium sp. ZZ1750. Mar. Drugs 2021, 19, 483. [Google Scholar] [CrossRef]
- Yu, G.; Sun, P.; Aierken, R.; Sun, C.; Zhang, Z.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, M.; et al. Linear polyketides produced by co-culture of Penicillium crustosum and Penicillium fellutanum. Mar. Life Sci. Technol. 2022, 4, 237–244. [Google Scholar] [CrossRef]
- Huo, R.; Zhang, J.; Niu, S.; Liu, L. New prenylated indole diketopiperazine alkaloids and polyketides from the mangrove-derived fungus Penicillium sp. Front. Mar. Sci. 2022, 9, 1097594. [Google Scholar] [CrossRef]
- Xia, M.W.; Yang, Y.; Xu, R.; Li, C.W.; Cui, C.B. A new polyketide purpurogenic acid: The activated production of polyketides by the diethyl sulphate mutagenesis of marine-derived Penicillium purpurogenum G59. Nat. Prod. Res. 2018, 33, 89–94. [Google Scholar] [CrossRef]
- Zhao, D.L.; Yuan, X.L.; Du, Y.M.; Zhang, Z.F.; Zhang, P. Benzophenone derivatives from an algal-endophytic isolate of Penicillium chrysogenum and their cytotoxicity. Molecules 2018, 23, 3378. [Google Scholar] [CrossRef]
- Zheng, C.J.; Liao, H.X.; Mei, R.Q.; Huang, G.L.; Yang, L.J.; Zhou, X.M.; Shao, T.M.; Chen, G.; Wang, C.Y. Two new benzophenones and one new natural amide alkaloid isolated from a mangrove-derived fungus Penicillium citrinum. Nat. Prod. Res. 2018, 33, 1127–1134. [Google Scholar] [CrossRef]
- Cao, G.P.; Xia, J.L.; Zhao, L.Y.; Tang, Z.Z.; Lin, X.; Liu, Y.H.; Gao, C.H.; Liu, K.; Bai, M. Penicixanthene E, a new xanthene isolated from a mangrove-derived fungus Penicillium sp. J. Antibiot. 2022, 75, 526–529. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.P.; Li, X.X.; Lu, Z.H.; Zheng, Q.H.; Liu, Q.-Y. Isolation of 4,4′-bond secalonic acid D from the marine-derived fungus Penicillium oxalicum with inhibitory property against hepatocellular carcinoma. J. Antibiot. 2018, 72, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Dahlem Junior, M.A.; Nguema Edzang, R.W.; Catto, A.L.; Raimundo, J.M. Quinones as an efficient molecular scaffold in the antibacterial/antifungal or antitumoral arsenal. Int. J. Mol. Sci. 2022, 23, 14108. [Google Scholar] [CrossRef] [PubMed]
- Kaliaperumal, K.; Salendra, L.; Liu, Y.; Ju, Z.; Sahu, S.K.; Elumalai, S.; Subramanian, K.; Alotaibi, N.M.; Alshammari, N.; Saeed, M.; et al. Isolation of anticancer bioactive secondary metabolites from the sponge-derived endophytic fungi Penicillium sp. and in-silico computational docking approach. Front. Microbiol. 2023, 14, 1216928. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.Y.; Zhang, G.J.; Li, D.H.; Zhang, K.J.; Zhang, X.M.; Zhu, T.J.; Che, Q. Overexpression of global regulator pbrlae a leads to the discovery of new polyketide in fungus Penicillium Brocae HDN-12-143. Front. Chem. 2020, 8, 270. [Google Scholar]
- Wang, H.F.; Mei, W.L.; Gai, C.J.; Dai, H.F.; Wang, P.; Tan, Z.Q. Secondary metabolites of marine-derived fungus Penicillium sp. WP-13. Mycosystema 2022, 41, 782–791. [Google Scholar]
- Pang, X.; Cai, G.; Lin, X.; Salendra, L.; Zhou, X.; Yang, B.; Wang, J.; Wang, J.; Xu, S.; Liu, Y. New alkaloids and polyketides from the marine sponge-derived fungus Penicillium sp. SCSIO41015. Mar. Drugs 2019, 17, 398. [Google Scholar] [CrossRef]
- Li, Q.; Xu, W.; Fan, R.; Zhang, J.; Li, Y.; Wang, X.; Han, S.; Liu, W.; Pan, M.; Cheng, Z. Penithoketone and Penithochromones A-L, polyketides from the deep-sea-derived fungus Penicillium thomii YPGA3. J. Nat. Prod. 2020, 83, 2679–2685. [Google Scholar] [CrossRef]
- Zhang, H.M.; Ju, C.X.; Li, G.; Sun, Y.; Peng, Y.; Li, Y.X.; Peng, X.P.; Lou, H.X. Dimeric 1,4-benzoquinone derivatives with cytotoxic activities from the marine-derived fungus Penicillium sp. L129. Mar. Drugs 2019, 17, 383. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Xia, Z.; Chen, C.; Liu, Y.; Jayasinghe, L.; Wang, X.; Zhou, X. New bioactive polyketides from the mangrove-derived fungus Penicillium sp. SCSIO 41411. Mar. Drugs 2024, 22, 384. [Google Scholar] [CrossRef]
- Yong, K.; Kaleem, S.; Ma, M.Z.; Lian, X.Y.; Zhang, Z.Z. Antiglioma natural products from the marine-associated fungus Penicillium sp. ZZ1750. Molecules 2022, 27, 7099. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: A review. Drug Des. Dev. Ther. 2018, 12, 3181–3197. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Seidel, V.; Izabela, M.; Monserrat-Mequida, M.; Sureda, A.; Ormazabal, V.; Zuniga, F.A.; Mangalpady, S.S.; Pezzani, R.; Ydyrys, A.; et al. Phenolic compounds as Nrf2 inhibitors: Potential applications in cancer therapy. Cell Commun. Signal. 2023, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Telkoparan Akillilar, P.; Suzen, S.; Saso, L. Pharmacological applications of Nrf2 Inhibitors as potential antineoplastic Drugs. Int. J. Mol. Sci. 2019, 20, 2025. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Antonov, A.S.; Borkunov, G.V.; Hauschild, J.; Zhuravleva, O.I.; Khudyakova, Y.V.; Menshov, A.S.; Popov, R.S.; Kim, N.Y.; Graefen, M.; et al. New bioactive beta-resorcylic acid derivatives from the alga-derived fungus Penicillium antarcticum KMM 4685. Mar. Drugs 2023, 21, 178. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Kassem, L.T.A.; Ahmed, E.F.; Alghamdi, R.A. Bioactive sulfonyl metabolites from the red sea endophytic fungus Penicillium aculeatum. Nat. Prod. Res. 2022, 36, 2713–2721. [Google Scholar] [CrossRef]
- Xie, C.L.; Zhang, D.; Lin, T.; He, Z.H.; Yan, Q.X.; Cai, Q.; Zhang, X.K.; Yang, X.W.; Chen, H.F. Antiproliferative sorbicillinoids from the deep-sea-derived Penicillium allii-sativi. Front. Microbiol. 2020, 11, 636948. [Google Scholar] [CrossRef]
- Choi, B.K.; Phan, T.H.T.; Hwang, S.; Oh, D.C.; Kang, J.S.; Lee, H.S.; Ngo, T.D.N.; Tran, T.T.V.; Shin, H.J. Resorcinosides A and B, glycosylated alkylresorcinols from a marine-derived strain of the fungus Penicillium janthinellum. J. Nat. Prod. 2019, 82, 3186–3190. [Google Scholar] [CrossRef]
- Anh, N.M.; Huyen, V.T.T.; Quyen, V.T.; Dao, P.T.; Quynh, D.T.; Huong, D.T.M.; Van Cuong, P.; Dat, T.T.H.; Minh, L.T.H. Antimicrobial and cytotoxic secondary metabolites from a marine-derived fungus Penicillium Citrinum VM6. Curr. Microbiol. 2023, 81, 32. [Google Scholar] [CrossRef]
- Dai, L.T.; Yang, L.; Wang, Z.P.; Guo, J.C.; Ma, Q.Y.; Xie, Q.Y.; Dai, H.F.; Yu, Z.F.; Zhao, Y.X. Persteroid, a new steroid from the marine-derived fungus Penicillium sp. ZYX-Z-143. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Kaleem, S.; Qin, L.; Yi, W.; Lian, X.Y.; Zhang, Z. Bioactive metabolites from the mariana trench sediment-derived fungus Penicillium sp. SY2107. Mar. Drugs 2020, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Ji, R.; Li, Y.; Cao, R.; Zhou, S. Network pharmacology, molecular docking, and molecular dynamics simulation analysis reveal the molecular mechanism of halociline against gastric cancer. Mol. Divers. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Tang, J.X.; Bo, Y.H.; Li, Y.Z.; Feng, T.; Zhu, H.W.; Yu, X.; Zhang, X.X.; Zhang, J.L.; Wang, W. Cytotoxic secondary metabolites isolated from the marine alga-associated fungus Penicillium chrysogenum LD-201810. Mar. Drugs 2020, 18, 276. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Zhou, G.L.; Sun, C.X.; Zhang, X.M.; Zhang, G.J.; Zhu, T.J.; Li, J.; Che, Q.; Li, D.H. Penicacids E-G, three new mycophenolic acid derivatives from the marine-derived fungus Penicillium parvum HDN17-478. Chin. J. Nat. Med. 2020, 18, 850–854. [Google Scholar] [CrossRef]
- Anh, N.M.; Minh, L.T.H.; Linh, N.T.; Dao, P.T.; Quynh, D.T.; Huong, D.T.M.; Van Cuong, P.; Huyen, V.T.T.; Dat, T.T.H. Secondary metabolites from marine fungus Penicillium chrysogenum VH17 and their antimicrobial and cytotoxic potential. Biosci. Biotechnol. Bioch. 2024, 88, 1254–1260. [Google Scholar] [CrossRef]
- Wang, Y.R.; Dong, Y.L.; Li, X.M.; Shi, X.S.; Li, H.L.; Meng, L.H.; Xu, R.; Wang, B.G. Curvularin derivatives from the marine mangrove derived fungus Penicillium sumatrense MA-325. Phytochemistry 2024, 220, 114000. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, K.L.; Luo, X.W.; Wu, Z.Y.; Gu, T.W.; Liao, S.R.; Lin, X.P.; Yang, B.; Liu, Y.H.; Zhou, X.F. Sorbicillfurans A and B, two novel sorbicillinoid adducts from the fungus Penicillium citrinum SCSIO41402. Org. Biomol. Chem. 2019, 17, 8721–8725. [Google Scholar] [CrossRef]
- Buachan, P.; Namsa Aid, M.; Sung, H.K.; Peng, C.; Sweeney, G.; Tanechpongtamb, W. Inhibitory effects of terrein on lung cancer cell metastasis and angiogenesis. Oncol. Rep. 2021, 45, 94. [Google Scholar] [CrossRef]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Xu, W.; Liu, W.; Liu, L.; Zhu, D.; Kang, Y.; Luo, Z.; Li, Q. Three new cyclopiane-type diterpenes from a deep-sea derived fungus Penicillium sp. YPGA11 and their effects against human esophageal carcinoma cells. Bioorganic Chem. 2019, 91, 103129. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, N.; Xie, C.L.; Fan, Z.; Luo, Z.; Chen, H.F.; Yang, X.W. Roquefortine J, a novel roquefortine alkaloid, from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Antibiot. 2018, 71, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, J.T.; Ahmadi, P.; Rahmawati, S.I.; Bayu, A.; Putra, M.Y.; Kijjoa, A. Marine-derived indole alkaloids and their biological and pharmacological activities. Mar. Drugs 2021, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Dadashpour, S.; Emami, S. Indole in the target-based design of anticancer agents: A versatile scaffold with diverse mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Zhuravleva, O.I.; Hauschild, J.; Busenbender, T.; Pelageev, D.N.; Yurchenko, A.N.; Khudyakova, Y.V.; Antonov, A.S.; Graefen, M.; Bokemeyer, C.; et al. New marine fungal deoxy-14,15-dehydroisoaustamide resensitizes prostate cancer cells to enzalutamide. Mar. Drugs 2023, 21, 54. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Wang, L.; Lan, D.; Fu, L.; Wu, B. Two new alkaloids from the marine-derived fungus Penicillium sp. LSH-3-1. Chem. Biodivers. 2022, 19, e202200310. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Li, S.; Chen, M.; Cheng, Y.; Yuan, W.; Cheng, Z.; Li, Q. Penicindopene A, a new indole diterpene from the deep-sea fungus Penicillium sp. YPCMAC1. Nat. Prod. Res. 2019, 33, 2988–2994. [Google Scholar] [CrossRef]
- Kong, F.D.; Fan, P.; Zhou, L.M.; Ma, Q.Y.; Xie, Q.Y.; Zheng, H.Z.; Zheng, Z.H.; Zhang, R.S.; Yuan, J.Z.; Dai, H.F.; et al. Penerpenes A–D, four indole terpenoids with potent protein tyrosine phosphatase inhibitory activity from the marine-derived fungus Penicillium sp. KFD28. Org. Lett. 2019, 21, 4864–4867. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, C.Y.; Geng, C.; Zhang, X.W.; Zhu, H.J.; Shao, C.L.; Cao, F. Discovery of bioactive indole-diketopiperazines from the marine-derived fungus Penicillium brasilianum aided by genomic information. Mar. Drugs 2019, 17, 514. [Google Scholar] [CrossRef]
- Song, W.; Ji, L.; Zhang, Y.; Cao, L. New cytotoxic indole derivatives with anti-FADU potential produced by the endophytic fungus Penicillium oxalicum 2021CDF-3 through the OSMAC strategy. Front. Microbiol. 2024, 15, 1400803. [Google Scholar] [CrossRef]
- Wang, N.; Dong, Y.; Yang, Y.; Xu, R.; Li, C.W.; Cui, C.B. Penicimutanin C, a new alkaloidal compound, Isolated from a neomycin-resistant mutant 3-f-31of Penicillium purpurogenum G59. Chem. Biodivers. 2020, 17, e2000241. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Wu, J.; Xu, L.; Hu, M.Y.; Xie, M.M.; Hao, Y.J.; Li, S.J.; Shao, Z.Z.; Yang, X.W. Chemical constituents of the deep-sea-derived Penicillium solitum. Mar. Drugs 2021, 19, 580. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Y.; Xu, S.; Xin, X.; An, F. Perpyrrospirone A, an unprecedented hirsutellone peroxide from the marine-derived Penicillium citrinum. Chinese. Chem. Lett. 2023, 34, 076. [Google Scholar] [CrossRef]
- Song, T.; Tang, M.; Ge, H.; Chen, M.; Lian, X.; Zhang, Z. Novel bioactive Penicipyrroether A and Pyrrospirone J from the marine-derived Penicillium sp. ZZ380. Mar. Drugs 2019, 17, 292. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chen, M.; Chai, W.; Zhang, Z.; Lian, X.Y. New bioactive pyrrospirones C–I from a marine-derived fungus Penicillium sp. ZZ380. Tetrahedron 2018, 74, 884–891. [Google Scholar] [CrossRef]
- Guo, X.C.; Zhang, Y.H.; Gao, W.B.; Pan, L.; Zhu, H.J.; Cao, F. Absolute configurations and chitinase inhibitions of quinazoline-containing diketopiperazines from the marine-derived fungus Penicillium polonicum. Mar. Drugs 2020, 18, 479. [Google Scholar] [CrossRef]
- Gu, Y.; Ding, P.; Liang, Z.; Song, Y.; Liu, Y.; Chen, G.; Li, J.L. Activated production of silent metabolites from marine-derived fungus Penicillium citrinum. Fitoterapia 2018, 127, 207–211. [Google Scholar] [CrossRef]
- Youssef, D.; Alahdal, A. Cytotoxic and antimicrobial compounds from the marine-derived fungus, Penicillium Species. Molecules 2018, 23, 394. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Yang, Z.; Tan, Y.; Peng, B.; Liu, Y.; Zhou, X. Thiodiketopiperazines and alkane derivatives produced by the mangrove sediment-derived fungus Penicillium ludwigii SCSIO 41408. Front. Microbiol. 2022, 13, 857041. [Google Scholar] [CrossRef]
- Sun, L.X.; Wang, H.N.; Yan, M.C.; Sai, C.M.; Zhang, Z. Research advances of bioactive sesquiterpenoids isolated from marine-derived Aspergillus sp. Molecules 2022, 27, 7376. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L.; Nepali, K. Emerging role of terpenoids for the treatment of cancer: A review. Mini Rev. Med. Chem. 2021, 21, 2300–2336. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tian, S.; Xue, J. Isolation and structure determination of a new bisabolane-type sesquiterpenoid with cytotoxicity from Penicillium oxalicum MZY-202312-521. Nat. Prod. Res. 2024, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Ge, H.J.; Yi, W.W.; Wu, B.; Zhang, Z.Z. Bioactive drimane sesquiterpenoids and isocoumarins from the marine-derived fungus Penicillium minioluteum ZZ1657. Tetrahedron Lett. 2020, 61, 151504. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Chingizova, E.A.; Antonov, A.S.; Shlyk, N.P.; Borkunov, G.V.; Berdyshev, D.V.; Chausova, V.E.; Kirichuk, N.N.; Khudyakova, Y.V.; Chingizov, A.R.; et al. New zosteropenillines and pallidopenillines from the seagrass-derived fungus Penicillium yezoense KMM 4679. Mar. Drugs 2024, 22, 317. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Fan, A.; Huang, J.; Lin, W. Eremophilane-type sesquiterpenes from a marine-derived fungus Penicillium Copticola with antitumor and neuroprotective activities. Mar. Drugs 2022, 20, 712. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Antonov, A.S.; Dyshlovoy, S.A.; Berdyshev, D.V.; Hauschild, J.; Zhuravleva, O.I.; Borkunov, G.V.; Menshov, A.S.; Kirichuk, N.N.; Popov, R.S.; et al. Meroantarctines A-C, meroterpenoids with rearranged skeletons from the alga-derived fungus Penicillium antarcticum KMM 4685 with potent p-glycoprotein inhibitory activity. J. Nat. Prod. 2022, 85, 2746–2752. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, X.; Zheng, Z.H.; Zhang, X.X.; Lu, X.H.; Yao, F.H.; Qi, S.H. Penicimeroterpenoids A-C, meroterpenoids with rearrangement skeletons from the marine-derived fungus Penicillium sp. SCSIO 41512. Org. Lett. 2020, 22, 6330–6333. [Google Scholar] [CrossRef]
- Ren, J.; Huo, R.; Liu, G.; Liu, L. New andrastin-type meroterpenoids from the marine-derived fungus Penicillium sp. Mar. Drugs 2021, 19, 189. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Jiang, C.; Chen, Y.; Tang, X.; Li, G.Q. Penisimplinoids A-K, highly oxygenated andrastin-type meroterpenoids with diverse activities from the marine-derived fungus Penicillium simplicissimum. Bioorganic Chem. 2024, 153, 107897. [Google Scholar] [CrossRef]
- Xie, C.L.; Xia, J.M.; Lin, T.; Lin, Y.J.; Lin, Y.K.; Xia, M.L.; Chen, H.F.; Luo, Z.H.; Shao, Z.Z.; Yang, X.W. Andrastone A from the deep-sea-derived fungus Penicillium allii-sativi acts as an inducer of caspase and RXRalpha-dependent apoptosis. Front. Chem. 2019, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, W.; Fan, R.; Han, S.; Li, Y.; Cui, X.; Zhang, J.; Wu, Y.; Lv, X.; Zhang, Y.; et al. Terpenoids from the deep-sea-derived fungus Penicillium thomii YPGA3 and their bioactivities. Mar. Drugs 2020, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Y.; Wang, J.F.; Liao, S.R.; Lin, X.P.; Zhou, X.F.; Li, Y.Q.; Yang, B.; Liu, Y.H. Four new steroids from the marine soft coral-derived fungus Penicillium sp. SCSIO41201. Chin. J. Nat. Med. 2020, 18, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Sun, W.G.; Wang, J.P.; Lin, S.; Li, X.N.; Zhu, H.C.; Luo, Z.W.; Xue, Y.B.; Hu, Z.X.; Zhang, Y.H. A new breviane spiroditerpenoid from the marine-derived fungus Penicillium sp. TJ403-1. Mar. Drugs 2018, 16, 110. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Chen, J.; Cai, X.; Yang, J.; Yang, Y.; Yan, H.; Cheng, X.; Ye, J.; Lu, W.; et al. The natural diterpenoid isoforretin a inhibits thioredoxin-1 and triggers potent ROS-mediated antitumor effects. Cancer Res. 2017, 77, 926–936. [Google Scholar] [CrossRef]
- Tang, P.; Liu, D.; Wu, Z.; Cui, H.; Zhang, R.; Kuang, Z. Inhibitory effects and mechanism of the natural compound diaporthein b extracted from marine-derived fungi on colon cancer cells. Molecules 2022, 27, 2944. [Google Scholar] [CrossRef]
- Hoang, C.K.; Le, C.H.; Nguyen, D.T.; Tran, H.T.N.; Luu, C.V.; Le, H.M.; Tran, H.T.H. Steroid components of marine-derived fungal strain Penicillium levitum N33.2 and their biological activities. Mycobiology 2023, 51, 246–255. [Google Scholar] [CrossRef]
- He, Z.H.; Xie, C.L.; Hao, Y.J.; Xu, L.; Wang, C.F.; Hu, M.Y.; Li, S.J.; Zhong, T.H.; Yang, X.W. Solitumergosterol A, a unique 6/6/6/6/5 steroid from the deep-sea-derived Penicillium solitum MCCC 3A00215. Org. Biomol. Chem. 2021, 19, 9369–9372. [Google Scholar] [CrossRef]
- Xie, C.L.; Zhang, D.; Xia, J.M.; Hu, C.C.; Lin, T.; Lin, Y.K.; Wang, G.H.; Tian, W.J.; Li, Z.P.; Zhang, X.K.; et al. Steroids from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475 induced apoptosis via retinoid X receptor (RXR)-α pathway. Mar. Drugs 2019, 17, 178. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, M.; Fan, H.; Chen, Y.; Dong, S.; Zhou, F.; Wang, B.; Liu, J.; Jin, J.; Luo, Y.; et al. The marine Penicillium sp. GGF16-1-2 metabolite dicitrinone g inhibits pancreatic angiogenesis by regulating the activation of NLRP3 inflammasome. J. Nat. Med. 2023, 78, 78–90. [Google Scholar] [CrossRef]
- Sun, M.H.; Li, X.H.; Xu, Y.; Xu, Y.; Pan, Z.N.; Sun, S.C. Citrinin exposure disrupts organelle distribution and functions in mouse oocytes. Environ. Res. 2020, 185, 109476. [Google Scholar] [CrossRef] [PubMed]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, T.; Uscatu, C.D.; Alexandru, D.O.; MEŞINĂ, C. Correlations between intratumoral microvessel density and histopathological type or neoadjuvant radiotherapy for rectal carcinoma. Curr. Health Sci. J. 2015, 41, 152–157. [Google Scholar]
- Couchie, D.; Vaisman, B.; Abderrazak, A.; Mahmood, D.F.D.; Hamza, M.M.; Canesi, F.; Diderot, V.; Hadri, K.E.; Nègre-Salvayre, A.; Page, A.L.; et al. Human plasma thioredoxin-80 Increases with age and in apoE-/- mice induces inflammation, angiogenesis and atherosclerosis. Circulation 2017, 136, 464–475. [Google Scholar] [CrossRef]
- Su, C.M.; Wang, I.C.; Liu, S.C.; Sun, Y.; Jin, L.; Wang, S.W.; Lee, H.P.; Tseng, W.P.; Tang, C.H. Hypoxia induced mitogenic factor (HIMF) triggers angiogenesis by increasing interleukin-18 production in myoblasts. Sci. Rep. 2017, 7, 7393. [Google Scholar] [CrossRef][Green Version]
- Kobori, T.; Hamasaki, S.; Kitaura, A.; Yamazaki, Y.; Nishinaka, T.; Niwa, A.; Nakao, S.; Wake, H.; Mori, S.; Yoshino, T.; et al. Interleukin-18 amplifies macrophage polarization and morphological alteration, leading to excessive angiogenesis. Front. Immunol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudaha, A.I.; Sayed, K.A.E. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. Med. Chem. Commun. 2013, 4, 1360–1369. [Google Scholar] [CrossRef]
- Goda, A.A.; Siddique, A.B.; Mohyeldin, M.; Ayoub, N.M.; El Sayed, K.A. The maxi-k (BK) channel antagonist penitrem a as a novel breast cancer-targeted therapeutic. Mar. Drugs 2018, 16, 157. [Google Scholar] [CrossRef]
- Schlange, T.; Matsuda, Y.; Lienhard, S.; Huber, A.; Hynes, N.E. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 2007, 9, R63. [Google Scholar] [CrossRef]
- Mei, L.; Lu, W.; Lin, C.; Roberts, M.J.; Waud, W.R.; Piazza, G.A.; Li, Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway. PLoS ONE 2011, 6, e29290. [Google Scholar]
- Sallam, A.A.; Ayoub, N.M.; Foudah, A.I.; Gissendanner, C.R.; Meyer, S.A.; El Sayed, K.A. Indole diterpene alkaloids as novel inhibitors of the Wnt/β-catenin pathway in breast cancer cells. Eur. J. Med. Chem. 2013, 70, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hori, Y.; Inoue, S.; Yamamoto, Y.; Iso, K.; Kamiyama, H.; Yamaguchi, A.; Kimura, T.; Uesugi, M.; Ito, J.; et al. E7386, a selective inhibitor of the interaction between beta-catenin and CBP, exerts antitumor activity in tumor models with activated canonical Wnt signaling. Cancer Res. 2021, 81, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.L.; Zhang, X.M.; Feng, H.M.; Dai, J.J.; Li, J.; Che, Q.; Gu, Q.Q.; Zhu, T.J.; Li, D.H. Penicisulfuranols A-F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 2017, 80, 71–75. [Google Scholar] [CrossRef]
- Hoy, S.M. Pimitespib: First approval. Drugs 2022, 82, 1413–1418. [Google Scholar] [CrossRef]
- Dai, J.; Chen, A.; Zhu, M.; Qi, X.; Tang, W.; Liu, M.; Li, D.; Gu, Q.; Li, J. Penicisulfuranol A, a novel c-terminal inhibitor disrupting molecular chaperone function of Hsp90 independent of ATP binding domain. Biochem. Pharmacol. 2019, 163, 404–415. [Google Scholar] [CrossRef]
- Gayler, K.M.; Lambert, K.M.; Wood, J.L. Synthetic studies towards the penicisulfuranols: Synthesis of an advanced spirocyclic diketopiperazine intermediate. Tetrahedron 2019, 75, 3154–3159. [Google Scholar] [CrossRef]
- Steyn, P.S. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 1970, 26, 51–57. [Google Scholar] [CrossRef]
- Ren, H.; Tian, L.; Gu, Q.; Zhu, W. Secalonic acid D; A cytotoxic constituent from marine lichen-derived fungus gliocladium sp. T31. Arch. Pharm. Res. 2006, 29, 59–63. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Yan, Y.Y.; Dai, C.l.; Xia, X.K.; She, Z.G.; Lin, Y.C.; Fu, L.W. Secalonic acid D induced leukemia cell apoptosis and cell cycle arrest of G1 with involvement of GSK-3β/β-catenin/c-Myc pathway. Cell Cycle 2009, 8, 2444–2450. [Google Scholar] [CrossRef]
- Hong, R. Secalonic acid D as a novel DNA topoisomerase I inhibitor from marine lichen-derived fungus Gliocladium sp. T31. Pharm. Biol. 2011, 49, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, M.M.; Li, Y.P.; Lin, S.F.; Zheng, Q.H.; Liu, Q.Y. 4,4′-bond secalonic acid D targets SP cells and inhibits metastasis in hepatocellular carcinoma. Mol. Med. Rep. 2020, 21, 2624–2632. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, W.; Zhang, Y.; Liang, C.; Tan, J.; Wang, Y. Venetoclax in adult acute myeloid leukemia. Biomed. Pharmacother. 2023, 168, 115820. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Ansari, M.F.; Khan, H.Y.; Tabassum, S.; Arjmand, F. Advances in anticancer alkaloid-derived metallo-chemotherapeutic agents in the last decade: Mechanism of action and future prospects. Pharmacol. Therapeut. 2023, 241, 108335. [Google Scholar] [CrossRef]
- Pennington, L.D.; Moustakas, D.T. The necessary nitrogen atom: A versatile high-impact design element for multiparameter optimization. J. Med. Chem. 2017, 60, 3552–3579. [Google Scholar] [CrossRef]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef]
- Zolottsev, V.A.; Latysheva, A.S.; Pokrovsky, V.S.; Khan, I.I.; Misharin, A.Y. Promising applications of steroid conjugates for cancer research and treatment. Eur. J. Med. Chem. 2021, 210, 113089. [Google Scholar] [CrossRef]
- Chinnappan, R.S.; Kandasamy, K.; Sekar, A. A review on marine based nanoparticles and their potential applications. Afr. J. Biotechnol. 2015, 14, 1525–1532. [Google Scholar] [CrossRef]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug delivery systems of natural products in oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Csermely, P.; Agoston, V.; Pongor, S. The efficiency of multi-target drugs: The network approach might help drug design. Trends Pharmacol. Sci. 2005, 26, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Song, Q.; Zhang, C.; Xu, X.; Li, M.; Yao, D.; Wu, L.; Qu, X.; Guan, H.; Yu, G.; et al. A β-1,3/1,6-glucan from durvillaea antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohyd. Polym. 2019, 222, 114993. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xu, Y.; Zhang, M.; Wu, L.; Liu, S.; Lv, Y.; Hu, T.; Zhao, J.; Zhang, X.; Xu, X.; et al. A β-1,3/1,6-glucan enhances anti-tumor effects of PD1 antibody by reprogramming tumor microenvironment. Int. J. Biol. Macromol. 2024, 279, 134660. [Google Scholar] [CrossRef]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2022, 23, 1044–1054. [Google Scholar] [CrossRef]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Lebrun-Ly, V.; Ray-Coquard, I.; et al. Doxorubicin–trabectedin with trabectedin maintenance in leiomyosarcoma. New Engl. J. Med. 2024, 391, 789–799. [Google Scholar] [CrossRef]
- Atmaca, H.; Oğuz, F.; Ilhan, S. Trabectedin (ET-743) in prostate cancer: Endoplasmic reticulum stress-induced apoptotic effect. Andrologia 2022, 54, e14599. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, F.; Liu, W.X.; Geng, Y.F.; Shi, Y.H.; Tian, Y.; Zhang, B.; Luo, Y.; Sun, X.B. New drug discovery and development from natural products: Advances and strategies. Pharmacol. Therapeut. 2024, 264, 108752. [Google Scholar] [CrossRef]
- Gangwal, A.; Ansari, A.; Ahmad, I.; Azad, A.K.; Wan Sulaiman, W.M.A. Current strategies to address data scarcity in artificial intelligence-based drug discovery: A comprehensive review. Comput. Biol. Med. 2024, 179, 108734. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Cancer drug discovery: Recent innovative approaches to tumor modeling. Expert Opin. Drug Dis. 2016, 11, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, J.E.; Van der Donk, W.A. Genome mining for ribosomally synthesized natural products. Curr. Opin. Chem. Biol. 2011, 15, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Huang, J.; Muhammad, M.; Deng, Z.; Gao, J. Genome mining as a biotechnological tool for the discovery of novel marine natural products. Crit. Rev. Biotechnol. 2020, 40, 571–589. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef]
- Caudal, F.; Tapissier-Bontemps, N.; Edrada-Ebel, R.A. Impact of co-culture on the metabolism of marine microorganisms. Mar. Drugs 2022, 20, 153. [Google Scholar] [CrossRef]
- Pinedo-Rivilla, C.; Aleu, J.; Duran-Patron, R. Cryptic metabolites from marine-derived microorganisms using OSMAC and epigenetic approaches. Mar. Drugs 2022, 20, 84. [Google Scholar] [CrossRef]
- Manouchehri, N.; Bouguila, N. Human activity recognition with an HMM-based generative model. Sensors 2023, 23, 1390. [Google Scholar] [CrossRef]
- Sukmarini, L. Marine bacterial ribosomal peptides: Recent genomics- and synthetic biology-based discoveries and biosynthetic studies. Mar. Drugs 2022, 20, 544. [Google Scholar] [CrossRef]
- Wang, S.W.; Gao, C.; Zheng, Y.M.; Yi, L.; Lu, J.C.; Huang, X.Y.; Cai, J.B.; Zhang, P.F.; Cui, Y.H.; Ke, A.W. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef]
- Tian, J.; Yang, G.; Gu, Y.; Sun, X.; Lu, Y.; Jiang, W. Developing an endogenous quorum-sensing based CRISPRi circuit for autonomous and tunable dynamic regulation of multiple targets in streptomyces. Nucleic Acids Res. 2020, 48, 8188–8202. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yao, L.; Zhou, Y.J. Construction of microbial chassis for terpenoid discovery. Syn. Syst. Biotechno. 2022, 7, 1181–1182. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.L.; Paudel, Y.P.; Ross, A.C. Investigating the biosynthesis of natural products from marine proteobacteria: A survey of molecules and strategies. Mar. Drugs 2017, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Wang, B.; Chen, T.; Chen, J.; Zhang, Y.; Liu, X.; Chen, Q. Activation and enhancement of caerulomycin a biosynthesis in marine-derived Actinoalloteichus sp. AHMU CJ021 by combinatorial genome mining strategies. Microb. Cell Fact. 2020, 19, 159. [Google Scholar] [CrossRef]
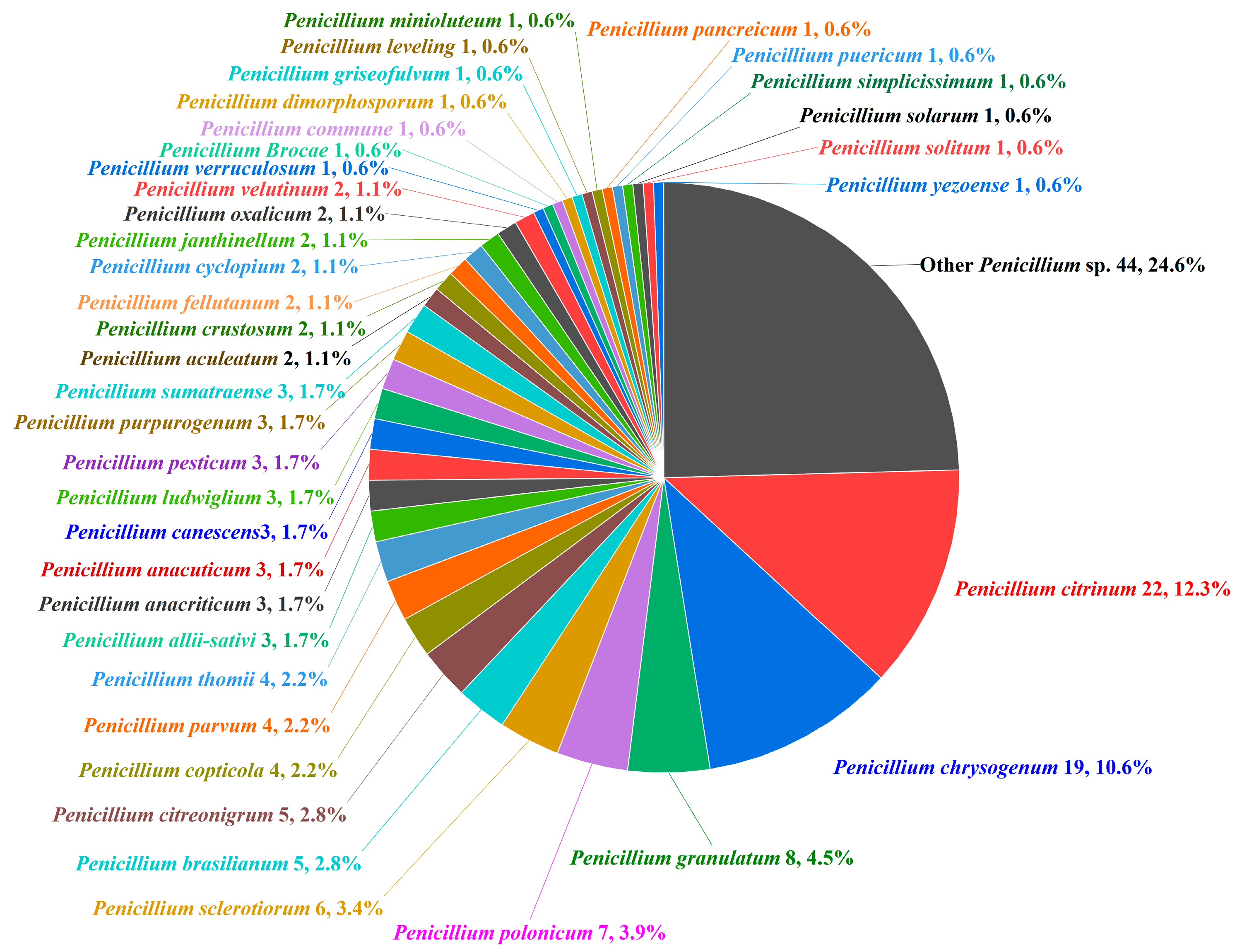
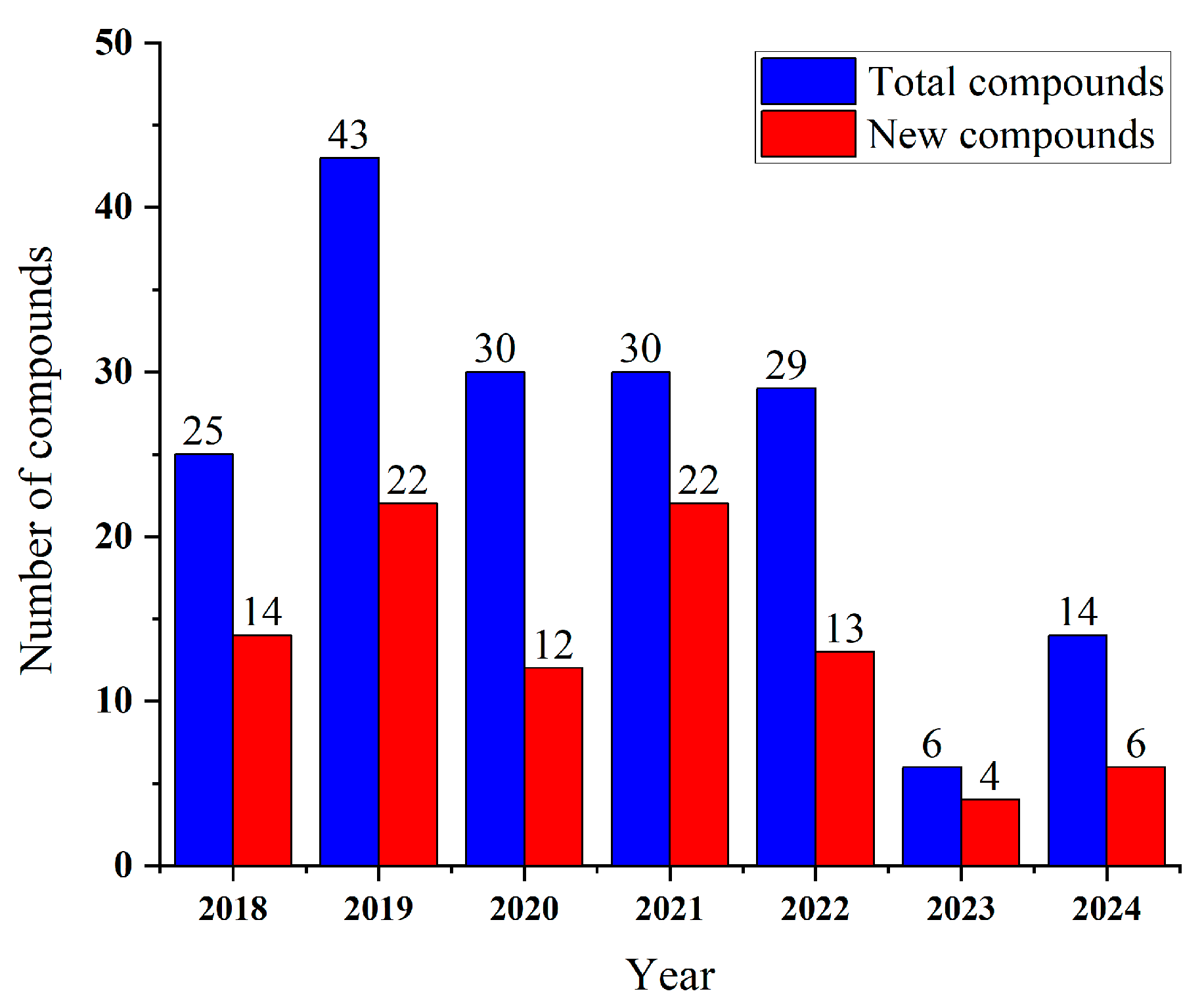
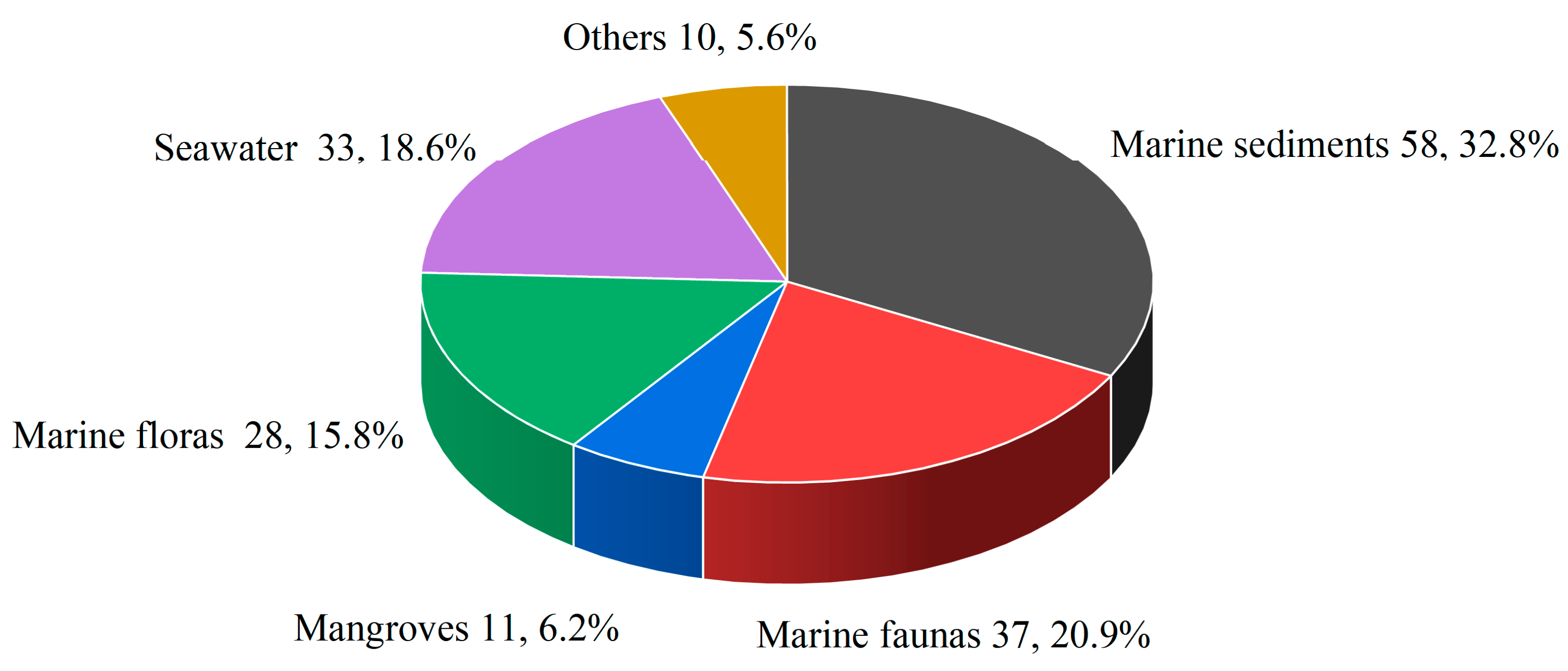
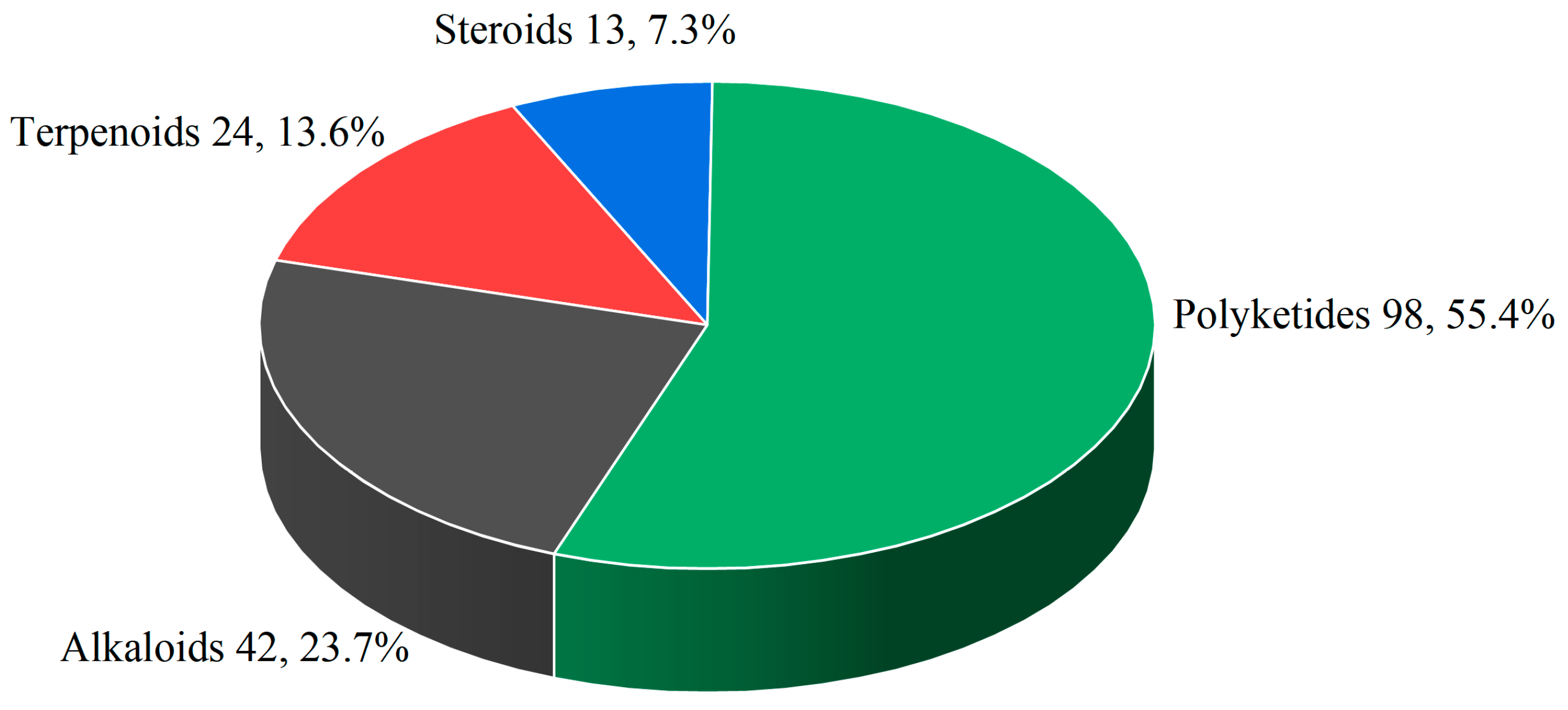
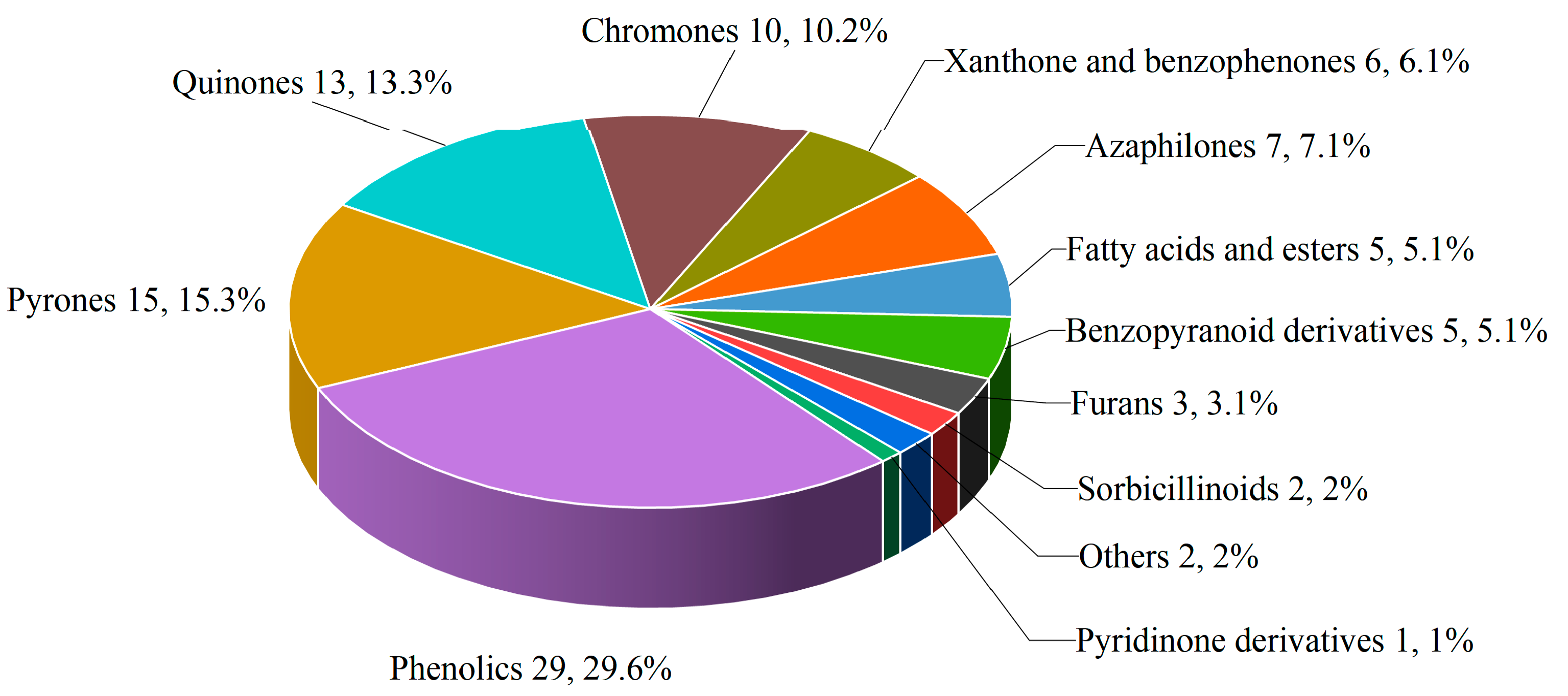
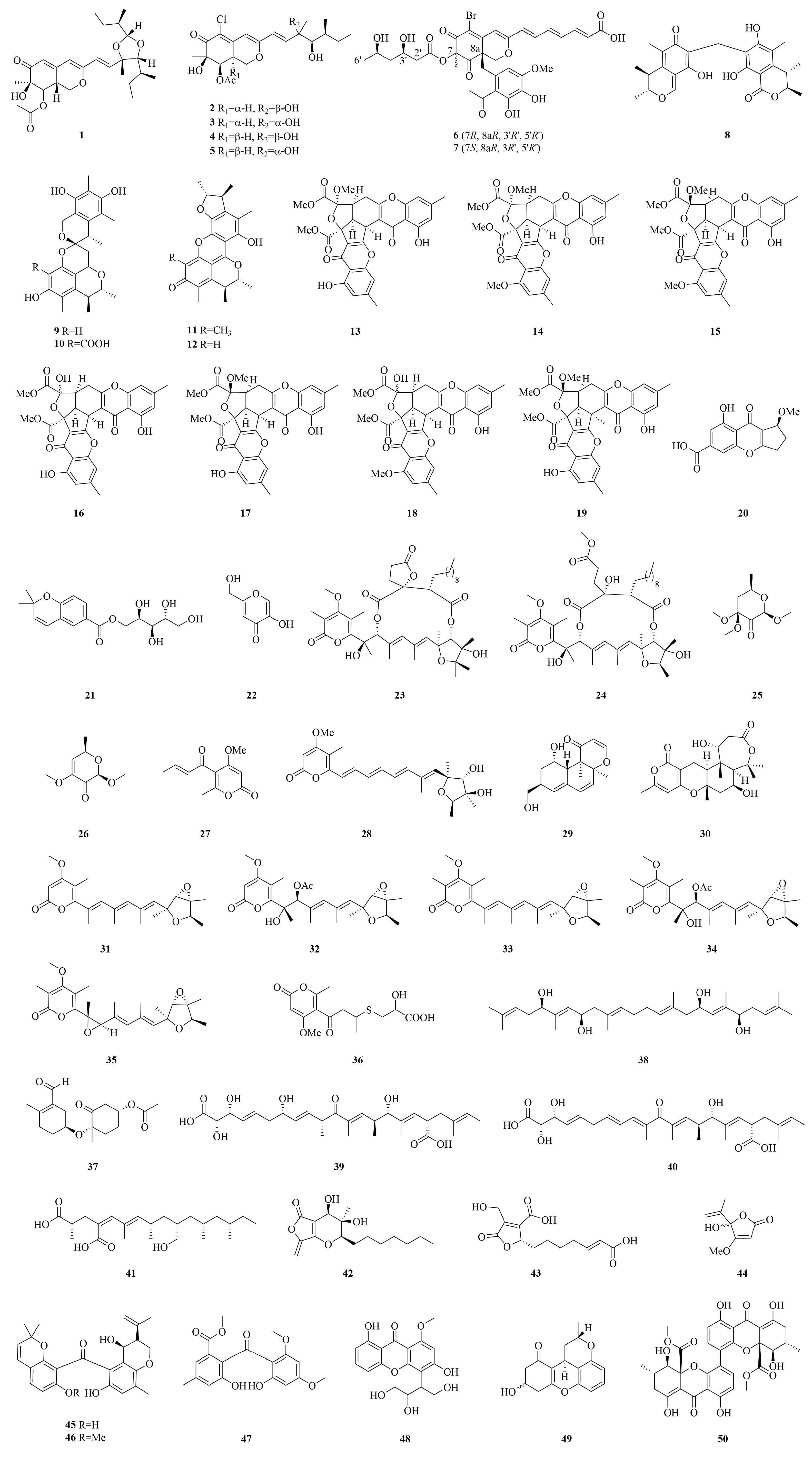

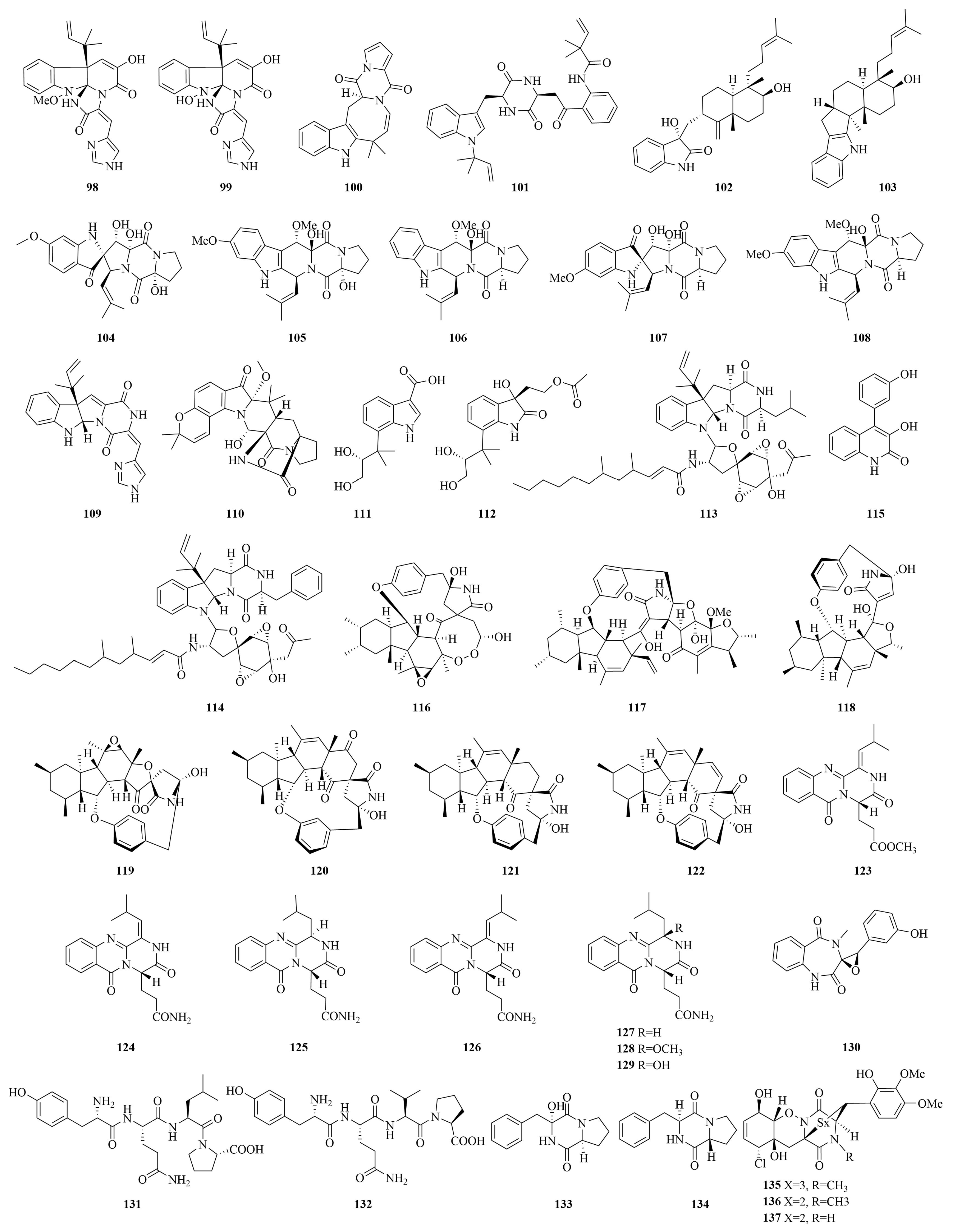
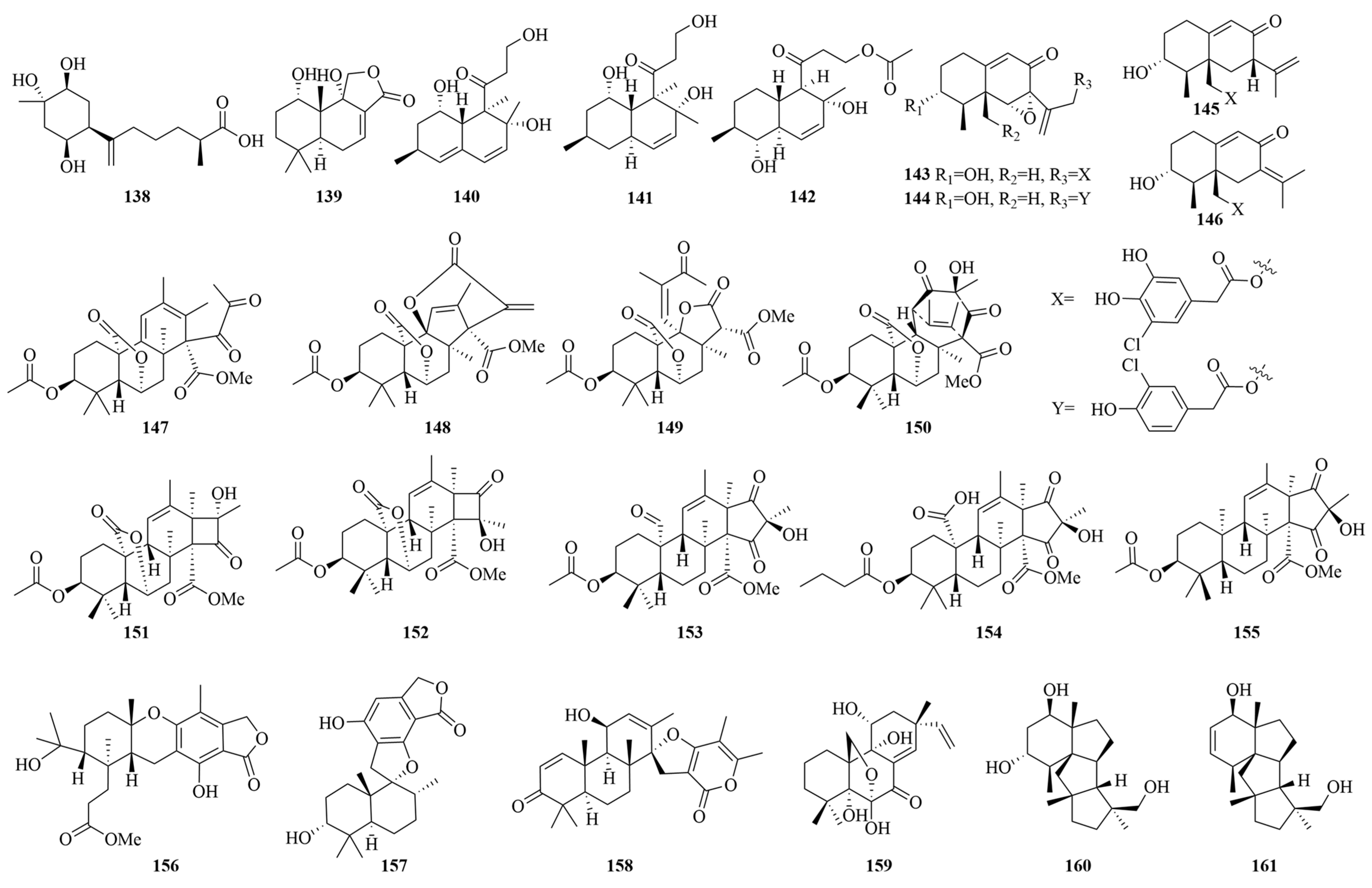

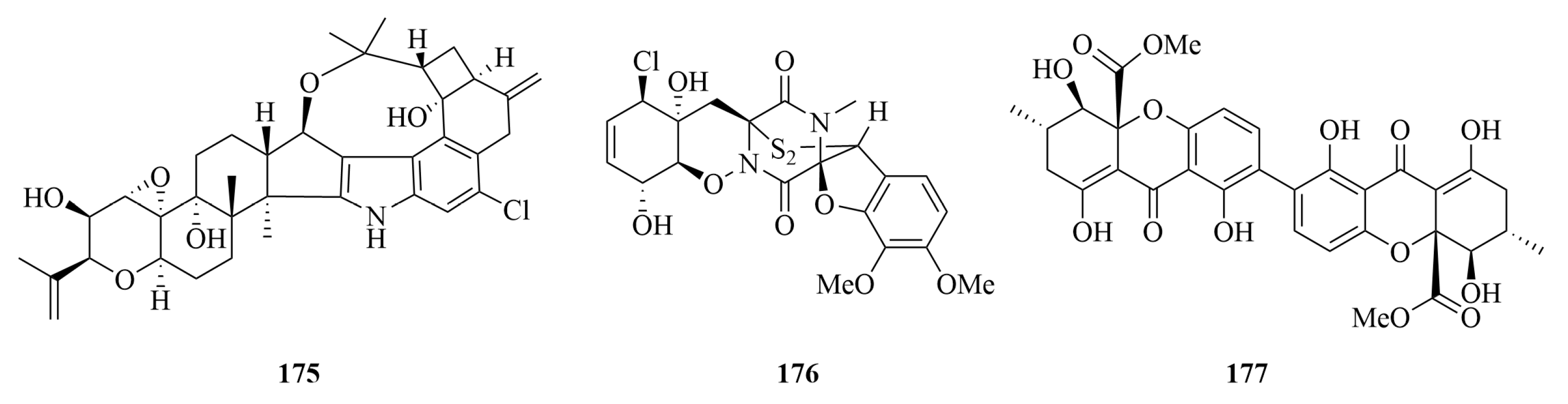
| Compounds | Source | Producing Strain | Cell Lines | IC50/GI50/Inhibition Rate/Mechanism | References |
|---|---|---|---|---|---|
| Penidioxolane C (1) | Marine sponge Holoxea sp. | Penicillium sclerotiorum E23 Y-1A | K562, BEL-7402, SGC-7901, A549, Hela | 23.94–60.66 µM | [46] |
| 8a-epi-hypocrellone A (2) | Marine alga Grateloupia sp. | Penicillium sclerotiorum | SH-SY5Y | 35.6 µM | [47] |
| 8 α-epi-eupenicilazaphilone C (3) | 73.2 µM | ||||
| Hypocrellone A (4) | >100 µM | ||||
| Eupenicilazaphilone C (5) | 95.2 µM | ||||
| Brominated azaphilone A (6) | Marine sponge Agelas oroides | Penicillium canescens | / | / | [48] |
| Brominated azaphilone B (7) | L5178Y, A2780 | 8.9, 2.7 µM | |||
| Dicitrinones G (8) | Starfish | Penicillium sp. GGF 16 -1-2 | BXPC-3, PANC-1 | 12.25, 24.33 µM | [50] |
| Xerucitrinin A (9) | Sponge Callyspongia sp. | Penicillium citrinum SCSIO 41017 | SF-268, MCF-7, HepG-2, A549 | 13.0–115.3 µM | [51] |
| Xerucitrinic acid A (10) | |||||
| Penicitrinol B (11) | |||||
| Penicitrinone A (12) | MCF-7 | 1.3 µM | |||
| Epiremisporine A (12) | Wastewater | Penicillium citrinum BCRC 09F458 | A549 | 49.15 µM | [52] |
| Epiremisporine C (13) | Wastewater | Penicillium citrinum BCRC 09F458 | A549 | >100 µM | [52] |
| Epiremisporine D (14) | |||||
| Epiremisporine E (15) | A549 | 43.82 µM | |||
| Epiremisporine B (16) | A549, HT-29 | 32.29, 50.88 µM | |||
| Epiremisporine F (17) | Wastewater | Penicillium citrinum BCRC 09F458 | HT-29, A549 | 44.77, 77.05 µM | [53] |
| Epiremisporine G (18) | 35.05, 52.30 µM | ||||
| Epiremisporine H (19) | 21.17, 31.43 µM | ||||
| Coniochaetone M (20) | Sponge Callypongia sp. | Penicillium citrinum SCSIO 41017 | SF-268, MCF-7, HepG-2, A549 | 43.0–16.0 µM | [51] |
| D-arabinitol-anofinicate (21) | Sea water | Penicillium sp. | / | Transcriptional activation of orphan nuclear receptor Nur77 | [54] |
| Kojicacid (22) | Deep-sea sediment | Penicillium chrysogenum strain S003 | A-549, HeLa, DU-145, HepG2, MCF-7 | >100 µM | [56] |
| Cyclopiumolide A (23) | Deep-sea sediment | Penicillium cyclopium SD-413 | SF126, FaDu, TE-1 | 5.86–17.05 µM | [57] |
| Cyclopiumolide B (24) | |||||
| Penisterine C (25) | Marine brown alga Sargassum cristaefolium | Penicillium sumatraense SC 29 | EPC | 28.5 µg/mL | [58] |
| Penisterine D (26) | / | Anti-angiogenic effects | |||
| Pyrenocine A (27) | Deep-sea sediment | Penicillium citreonigrum | HeLa | 5.4 µM | [59] |
| Citreoviridin (28) | 0.7 µM | ||||
| Peniciversiol A (29) | Deep-sea sediment | Penicillium chrysogenum | BIU-87 | 10.21 µM | [60] |
| Asperdemin (30) | ECA 109, BIU-87, BEL-7402 | >20, 12.75 µM | |||
| Nordeoxyverrucosidin (31) | Bohai Sea | Penicillium sp. XL-01 | HeLa, MDAMB-231, MGC-803 | 0.96–3.60 µM | [61] |
| Norverrucosidinol acetate (32) | NRK 52 E | 18.3 µM | |||
| Deoxyverrucosidin (33) | HeLa, MDAMB-231, MGC-803 | 1.14–6.37 µM | |||
| Verrucosidinol acetate (34) | NRK 52 E | 25.0 µM | |||
| Verrucosidin (35) | HeLa, MDA-MB-231, MCF-7, MGC-803, A-549, NRK 52 E | 1.91–3.91 and 23.1 µM | |||
| 2-hydroxyl-3-pyrenocine-thio propanoic acid (36) | Marine sediments | Penicillum citreonigrum XT20-134 | Bel 7402, HT 1080 | 7.63, 10.22 µM | [62] |
| Oxalihexane A (37) | Red algae Rhodomela confervoides | Penicillium oxalicum 2021CDF-3 | PATU 8988T | 93% (20 µM) | [63] |
| Penidifarnesylin A (38) | Marine mud | Penicillium sp. ZZ1750 | U87MG, U251 | 5.9, 27.6 µM | [64] |
| Penifellutins A (39) | Deep-sea sediment | Penicillium crustosum PR B-2, Penicillium fellutanum HDN 14 -323 | / | Suppressed zebrafish larval liver proliferation at 10 µM | [65] |
| Penifellutin B (40) | |||||
| Radiclonic acid (41) | Deep-sea sediment | Penicillium chrysogenum | ECA 109, BIU-87, BEL-7402 | 7.70–13.75 µM | [60] |
| Penicinones A (42) | Rhizospheric soil of mangrove ecosystem | Penicillium sp. LA 032 | HepG 2, MCF-7, B16 | 3.87, 30.01, 27.91 µM | [66] |
| Purpurogenic acid (43) | / | Penicillium purpurogenum G59 | K562, HL-60, HeLa, BGC-823 | 52.7%, 78.8%, 38.4%, 35.3% (100 μg/mL) | [67] |
| Penicillic acid (44) | Marine sponge | Penicillium canescens | L5178 Y | 8.9 µM | [48] |
| Chryxanthone A (45) | Red marine alga Grateloupia turuturu | Penicillium chrysogenum AD-1540 | BT-549, HeLa | 20.4, 23.5 µM | [68] |
| Chryxanthone B (46) | A549 | 20.4 µM | |||
| Spenibenzophenones B (47) | Mangrove | Penicillium citrinum HL-5126 | A549 | 15.7 μg/mL | [69] |
| 3,8-dihydroxy-4-(2,3-dihydroxy-1-hydroxymethylpropyl)-1-methoxYxanthone (48) | Deep-sea sediment | Penicillium chrysogenum | ECA 109, BIU-87, BEL-7402 | >20 and 15.94 µM | [60] |
| Penicixanthene E (49) | Mangrove | Penicillium sp. GXIMD 03101 | SW 1990 | 23.8 µM | [70] |
| 4,4′-bond secalonic acid D (4,4′-SAD) (50) | Deep-sea sediment | Penicillium pancreicum | BGC-823, SGC-7901, HGC-27, EC9706, KYSE450, CNE1, CNE2, SW620, SW480, LOVO, HuH-7, PLC/PRF/5, SK- HEP, Hela, A549, SK- MES-1, SPC-A1, 95D, Jeko-1, Raji, U937, A375, HFF, H22 | 0.484–1.384 µM | [71] |
| Averufin (51) | Marine sponge | Penicillium verruculosum XWSO1F60 | HL60 | 1.005 µM | [73] |
| Fumigatin chlorohydrin (52) | Marine sediments | Penicillium Brocae HDN-12- 143 | HL-60 | 18.63 µM | [74] |
| Iso-fumigatin chlorohydrin (53) | 24.83 µM | ||||
| Endocrocin methyl ester (54) | Red snail Strombus luhuanus Linnaeus | Penicillium sp. WP-13 | K562, BEL-7402, SGC-7901 | 31.49–57.10 µM | [75] |
| Emodin (55) | 56.78–87.67 µM | ||||
| Emodin (55) | Marine sponge | Penicillium sp. SCSIO 41015 | MGC 803 | 5.19 µM | [76] |
| Rugulosin A (56) | Sea water | Penicillium sp. | QGY7701, H1299, HCT 116 | 21.2, 18, 17.6 µM | [54] |
| Penithoketone (57) | Deep-sea sediment | Penicillium thomii YPGA 3 | MCF-7, MDAMB-468, C4-2B, C4-2B/ENZR | 4.9–9.1 µM | [77] |
| 3,5-dihydroxy-2-methoxy-1,4-naphthalenedione (58) | >50, 38, 41, >50 µM | ||||
| 2-methoxyjuglone (59) | 4.9–9.1 µM | ||||
| Peniquinone A (60) | Rhizosphere soil of Limonium sinense | Penicillium sp. L129 | MCF-7, U87, PC 3 | 12.39, 9.01, 14.59 µM | [78] |
| Peniquinone B (61) | 25.32, 13.45, 19.93 µM | ||||
| Embelin A (62) | Rhizosphere sediment of mangrove Aegiceras corniculatum | Penicillium sp. SCSIO 41411 | PC-3, LNCaP | 18.69, 31.62 µM | [79] |
| Questiomycin A (63) | Marine mud | Penicillium sp. ZZ1750 | U87 M, U251 | 14.13–22.56 µM | [80] |
| β-resoanaparticine A (64) | Marine brown alga Sargassum miyabei | Penicillium anacriticum KMM 4685 | LNCaP, DU 145, 22 Rv 1 | 31–82.5 µM | [85] |
| 8-dehydro-β-resoantarctine A (65) | |||||
| β-resoanaparticine B (66) | |||||
| Pensulfonoxy (67) | Red alga Laurencia obtusa | Penicillium aculeatum | HCT-116 | 5.23 µM | [86] |
| Pensulfonamide (68) | MCF-7, HCT-116 | 2.18, 6.12 µM | |||
| Peniresorcinoside A (69) | Marine mud | Penicillium sp. ZZ1750 | U87 MG, U251 | 4.0, 14.1 µM | [64] |
| Peniresorcinoside B (70) | 5.6, 9.8 µM | ||||
| Peniresorcinoside C (71) | U87MG | 53 µM | |||
| Peniresorcinoside D (72) | 19.4 µM | ||||
| Peniresorcinoside E (73) | 22.1 µM | ||||
| Sorbicillin (74) | Sea water | Penicillium allii-sativi | HT-29 | 5 µM | [87] |
| Resorcinosides A (75) | Deep-sea sediment | Penicillium janthinellum 168CLC-17 | NUGC-3 | 9.3 µM | [88] |
| 3,3’-dihydroxy-5,5’-dimethyldiphenyl ether (76) | Deep-sea sediment | Penicillium chrysogenum | ECA 109, BEL-7402, BIU-87 | >20 and 16.41 µM | [60] |
| Violaceol-II (77) | BIU-87, BEL-7402, ECA 109 | >20 and 8.95 µM | |||
| Dicitrinone F (78) | Marine sediment | Penicillium citrinum VM6 | A549, MCF 7, MDA-MB-231, Hela, AGS | 6.7–29.6 µg/mL | [89] |
| Phenol acid (79) | MCF 7 | 98.1 µg/mL | |||
| Spirolaxine (80) | Arthropod (Dardanus scutellatus) | Penicillium sp. ZYX-Z-143 | BEL-7402 | 34.35 µM | [90] |
| Xanthocillin X (81) | Marine mud | Penicillium sp. ZZ1750 | U87 M, U251 | 13.65–16.33 µM | [80] |
| (Z)-N-(4-hydroxystyryl)formamide (82) | Marine sediments | Penicillium sp. SY2107 | U251, U87 MG | 17.0, 39.8 µM | [91] |
| Halociline (83) | / | Penicillium griseofulvum. | SGC-7901, HeLa | 0.870, 1.442 µM | [92] |
| (2’R)-westerdijkin A (84) | Marine red alga | Penicillium chrysogenum LD-201810 | HepG 2 | 22.0 µM | [93] |
| (S)-(+)-11-dehydrosydonic acid (85) | A549, THP-1 | 21.2, 18.2 µM | |||
| Penicacid F (86) | Marine sediments | Penicillium parvum HDN17-478 | HCT-116, BEL-7402, MGC-803, SH-SY 5 Y, HO-8910, HL-60 | 12.61–26.38 µM | [94] |
| Penicacid G (87) | |||||
| Mycophenolic acid (88) | 1.69–12.98 µM | ||||
| Mycophenolic methyl ester (89) | |||||
| 2-(2-aminopropanamido)benzoic acid (90) | Soft coral | Penicillium chrysogenum VH 17 | MCF 7, HepG 2 | 87.17, 97.32 µM | [95] |
| Dehydrocurvularin (91) | Marine mangrove Bruguiera sexangular | Penicillium sumatrense MA-325 | HCT 116, 786-O, 5673, Hela | 3.5–14.9 µM | [96] |
| 5,5-dichloro-1-(3,5-dimethoxyphenyl)-1,4-dihydroxypentan-2-one (92) | Marine sediments | Penicillum citreonigrum XT20-134 | Bel 7402, HT 1080 | 13.14, 16.53 µM | [62] |
| Sorbicillfuran B (93) | Marine alga Coelarthorum sp. | Penicillium citrinum SCSIO 41402 | HL-60 | 9.6 µM | [97] |
| Sorbicatechol D (94) | Sea water | Penicillium allii-sativi | HT-29 | 30 µM | [87] |
| Penipyridinone B (95) | Marine mud | Penicillium sp. ZZ1750 | U87 M, U251 | 2.45, 11.40 µM | [80] |
| Terrein (96) | Marine sediments | Penicillium citreonigrum | HeLa | 11.3 µM | [59] |
| LAMA (97) | Marine mud | Penicillium chrysogenum strain S003 | A-549, HeLa, DU-145, HepG2, MCF-7 | >100 µM | [56] |
| Meleagrin (98) | Marine sediments | Penicillium sp. YPGA11 | EC109, EC9706, KYSE70, KYSE450, HepG2 | 25.03–36.93 and 7.0 µM | [101] |
| Glandicoline B (99) | EC109, EC9706, KYSE70, KYSE450 | 30.11–55.379 μM | |||
| Deoxy-14,15-dehydroisoaustamide (100) | Soft coral | Penicillium dimorphosporum KMM 4689 | / | Enhancing AR-targeted therapy efficacy | [105] |
| Peniokaramine (101) | Marine sediments | Penicillium sp. LSH-3-1 | A549 | 53.43% (50 μM) | [106] |
| Penicindopene A (102) | Deep-sea water | Penicillium sp. YPCMAC1 | A549, HeLa | 15.2, 20.5 µM | [107] |
| Emindole SB (103) | Bivalve mollusk (Meretrix lusoria) | Penicillium sp. KFD 28 | K562 | 18.8 µM | [108] |
| Spirotryprostatin G (104) | Bohai Sea | Penicillium brasilianum HBU-136 | HL-60 | 6 µM | [109] |
| Cyclotryprostatin F (105) | MCF-7 | 7.6 µM | |||
| Cyclotryprostatin G (106) | MCF-7 | 10.8 µM | |||
| Spirocyclic diketopiperazine alkaloid (107) | HL-60 | 7.9 µM | |||
| Cyclotryprostatin B (108) | MCF-7 | 5.1 µM | |||
| Roquefortine J (109) | Marine sediments | Penicillium granulatum | HepG3 | 19.5 µM | [102] |
| Asperinamide B (110) | Marine red alga | Penicillium pesticum 2021 CDF-3 | FADU | 0.43 µM | [110] |
| Peniochroloid A (111) | A549 | 29.84 µM | |||
| Peniochroloid B (112) | A549 | 15.30 µM | |||
| Penicimutanin C (113) | Bohai Sea | Penicillium purpurogenum G59 | K562, HL-60, HeLa, BGC-823, MCF-7 | 11.9, 5.0, 8.6, 8.7,6.0 µM 10.7, 6.1, 7.0, 8.3, 7.3 µM | [111] |
| Penicimutanin A (114) | |||||
| Viridicatol (115) | Marine mud | Penicillium solitum | PANC-1, Hela, A549 | 18, 19, 24 µM | [112] |
| Perpyrrospirone A (116) | / | Penicillium citrinum DY180712 | MGC 803, HepG 2, MDA-MB-231, MCF-7, Bel-7402, HeLa | 2.5–38.9 µM | [113] |
| Penicillione G (117) | |||||
| Penicipyrroether A (118) | Marine crab Pachygrapsus crassipes | Penicillium sp. ZZ380 | U87 MG, U251 | 1.64, 5.50 µM | [114] |
| Pyrrospirone J (119) | 10.52, 17.92 µM | ||||
| Pyrrospirone G (120) | Marine crab Pachygrapsus crassipes | Penicillium sp. ZZ380 | U87MG, U251, SHG44, C6 | 1.06–8.52 µM | [115] |
| Pyrrospirone H (121) | 7.44–26.64 µM | ||||
| Pyrrospirone I (122) | |||||
| Polonimide A (123) | Bohai Sea | Penicillium polonicum MN 623481 | A549, HGC-27, UMUC-3 | >10 µM | [116] |
| Polonimide B (124) | |||||
| Polonimide C (125) | |||||
| Aurantiomide C (126) | |||||
| Anacine (127) | 6.0, 6.2, 7.2 µM | ||||
| Aurantiomide A (128) | >10 µM | ||||
| Aurantiomide B (129) | |||||
| Cyclopenol (130) | Marine sediments | Penicillium chrysogenum | BIU-87, BEL-7402 | 8.34, 7.81 µM | [60] |
| Compound (131) | Marine sponge Petrosia sp. | Penicillium citrinum 2015 PF 07 | HCT 115, MCF-7 | 20 μg/mL | [117] |
| Compound (132) | |||||
| Penicillatide B (133) | Red Sea tunicate Didemnum sp. | Penicillium sp. | HCT-116 | 6 µM | [118] |
| Cyclo(R-Pro–S-Phe) (134) | 9.57 µM | ||||
| Adametizine C (135) | Mangrove sediment | Penicillium ludwiglium SCSIO 41408 | 22 Rv 1, PC-3 | 13.9, 44.0 µM | [119] |
| Adametizine A (136) | 22 Rv 1 | 13.0 µM | |||
| DC1149B (137) | 22 Rv 1, PC-3 | 13.6, 5.1 µM | |||
| Inonotic acid C (138) | Marine alga | Penicillium puericum MZY-202312-521 | MCF-7 | 7.7 µM | [123] |
| Purpuride G (139) | Deep-sea sediment | Penicillium minioluteum ZZ 1657 | U251, U87 MG | 4.49, 10.9 µM | [124] |
| Decumbenone A (140) | Marine sediments | Penicillium chrysogenum | ECA 109 | 12.41 µM | [60] |
| Decumbenone B (141) | 15.60 µM | ||||
| 1-acetylpallidopenilline A (142) | Marine grass | Penicillium yezoense KMM 4679 | MCF-7 | 0.66 µM | [125] |
| Copteremophilane D (143) | Sponge of Xestospongia testudinaria | Penicillium copticola WZXY-m122-9 | HCT-8 | 5.4 µM | [126] |
| Copteremophilane E (144) | HCT-8 | 7.3 µM | |||
| Copteremophilane G (145) | A549 | >10 µM | |||
| Copteremophilane H (146) | A549 | 3.23 µM | |||
| Meroanapartine A (147) | Marine Brown alga Sargassum miyabei | Penicillium anacuticum KMM 4685 | / | P-gp-inhibitory activity | [127] |
| Meroanapartine B (148) | |||||
| Meroanapartine C (149) | |||||
| Penicimeroterpenoid A (150) | Soft coral | Penicillium sp. SCSIO 41512 | CDC 25 B | 20 µM | [128] |
| Penicimeroterpenoid B (151) | |||||
| Penicimeroterpenoid C (152) | |||||
| Penimeroterpenoid A (153) | Marine sediments | Penicillium sp. A18 | A549, HCT 116, SW 480 | 82.61, 78.63, 95.54 µM | [129] |
| Penisimplinoid F (154) | Sponge | Penicillium simplicissimum 19 XS 15 ZM-3 | NCI-H446 | 6.49 µM | [130] |
| Andrastone A (155) | Deep-sea water | Penicilliumallii-sativi | HepG 2 | 7.8 µM | [131] |
| Austalide Y (156) | Sea water | Penicillium thomii YPGA 3 | MDA-MB-468 | 38.9 μM | [132] |
| Stachybotrylactone B (157) | Soft coral | Penicillium sp. SCSIO 41201 | HL-60, K562, MOLT-4, ACHN, 786-O, OS-RC-2 | 4.12–23.55 µM | [133] |
| Breviones I (158) | Soft coral | Penicillium sp. TJ403 -1 | HL-60, A-549, HEP 3B | 4.92, 8.60, 5.50 µM | [134,135] |
| Diaporthein B (159) | Intestinal tract of Onchidium struma | Penicillium sclerotiorum GZU-XW 03 -2 | HCT 116, LOVO | 1.5, 3 µM | [136] |
| Conidiogenol D (160) | Marine sediments | Penicillium sp. SY2107 | EC109, EC9706, KYSE30, KYSE70, KYSE450 | 36.80–54.7 µM | [101] |
| Conidiogenone C (161) | 27.05–42.13 µM | ||||
| 22-triene-3,5-diol (162) | Marine grass | Penicillium leveling N33.2 | Hep-G2, A549, MCF-7 | 2.89–18.51 µg/mL | [137] |
| Solitumergosterol A (163) | Marine sediments | Penicillium solarum | MB231 | 44.1% (20 μM) | [138] |
| Ergosterol (164) | Marine sediments | Penicillium chrysogenum strain S003 | A-549, DU-14, MCF-7, HepG 2 | 21.26, 1.50, 16.95, 2.89 µM | [56] |
| Epidioxyergosterol (165) | 19.3, 6.10, 13.6, 3.07 µM | ||||
| 16a-methylpregna-17a,19-dihydroxy-(9,11)-epoxy-4-ene-3,18-dione-20-acetoxy (166) | Sponge | Penicillium citrinum SCSIO 41017 | SF-268, MCF-7, HepG-2, A549 | 13.5–18.0 µM | [51] |
| Penicisteroid E (167) | Marine sediments | Penicillium granulatum | A549, BIU-87, BEL-7402, ECA-109, Hela-S3, PANC-1 | 14.4–4.1 µM | [139] |
| Penicisteroid G (168) | |||||
| Penicisteroid H (169) | |||||
| Penicisteroid A (170) | |||||
| Penicisteroid C (171) | |||||
| Isonuatigenin I (172) | Marine sediments | Penicillium granulatum | HepG2 | 8.6 µM | [102] |
| Penicisteroid A (173) | 8.2 µM | ||||
| 5 α, 6 α-epoxy-(22 E, 24 R)-ergosta-8(14), 22-diene-3 β,7 α-diol (174) | Soft coral | Penicillium chrysogenum VH17 | HepG2, A549, MCF 7 | 29.43, 33.02, 36.72 µM | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, H.; Sai, C.; Wang, Y.; Cheng, Z.; Zhang, Z. The Cytotoxic Activity of Secondary Metabolites from Marine-Derived Penicillium spp.: A Review (2018–2024). Mar. Drugs 2025, 23, 197. https://doi.org/10.3390/md23050197
Zhang S, Wang H, Sai C, Wang Y, Cheng Z, Zhang Z. The Cytotoxic Activity of Secondary Metabolites from Marine-Derived Penicillium spp.: A Review (2018–2024). Marine Drugs. 2025; 23(5):197. https://doi.org/10.3390/md23050197
Chicago/Turabian StyleZhang, Shuncun, Huannan Wang, Chunmei Sai, Yan Wang, Zhongbin Cheng, and Zhen Zhang. 2025. "The Cytotoxic Activity of Secondary Metabolites from Marine-Derived Penicillium spp.: A Review (2018–2024)" Marine Drugs 23, no. 5: 197. https://doi.org/10.3390/md23050197
APA StyleZhang, S., Wang, H., Sai, C., Wang, Y., Cheng, Z., & Zhang, Z. (2025). The Cytotoxic Activity of Secondary Metabolites from Marine-Derived Penicillium spp.: A Review (2018–2024). Marine Drugs, 23(5), 197. https://doi.org/10.3390/md23050197





