Isolation and Characterization of L-Asparaginase-Producing Bacteria from the Arabian–Persian Gulf Region: First Report on Bacillus xiamenensis ASP-J1-4 as a Producer and Its Potential Application
Abstract
1. Introduction
2. Results
2.1. Isolation and Screening of Marine Bacteria for L-ASNase Production
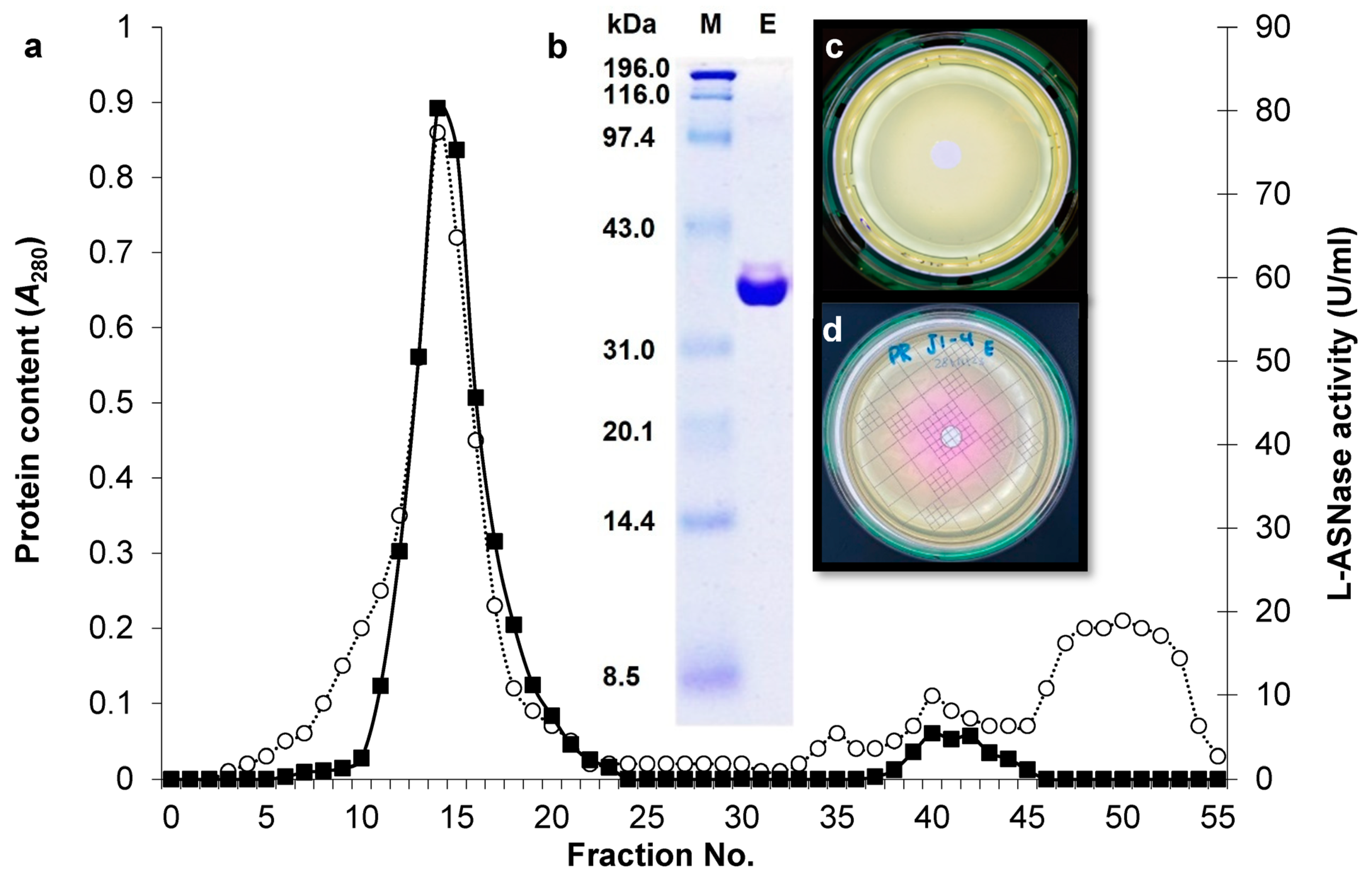
2.2. Purification of L-ASNase from B. xiamenensis Isolate ASP-J1-4
2.3. Effect of pH and Temperature on ASP-J1-4 L-ASNase Activity and Stability
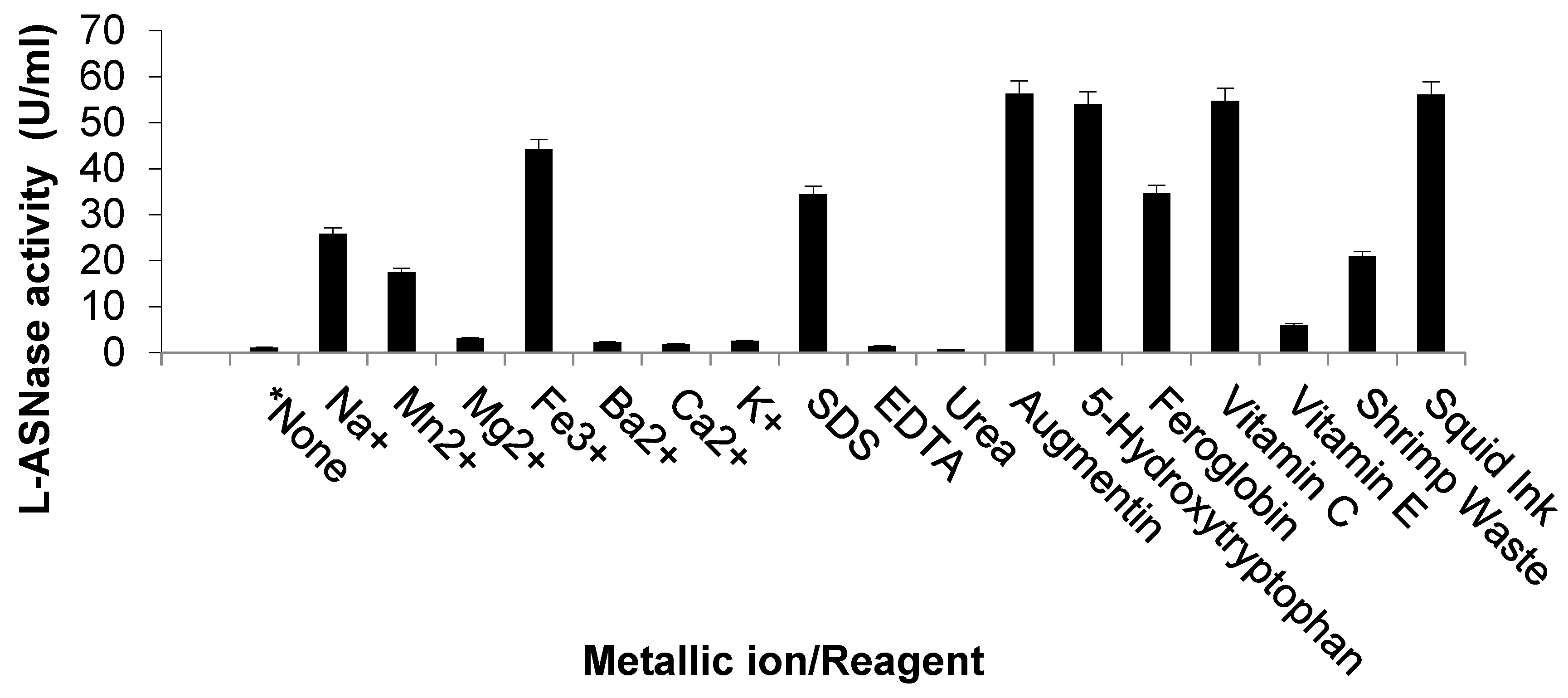
2.4. Effect of Metal Ions and Reagents on ASP-J1-4 L-ASNase Activity
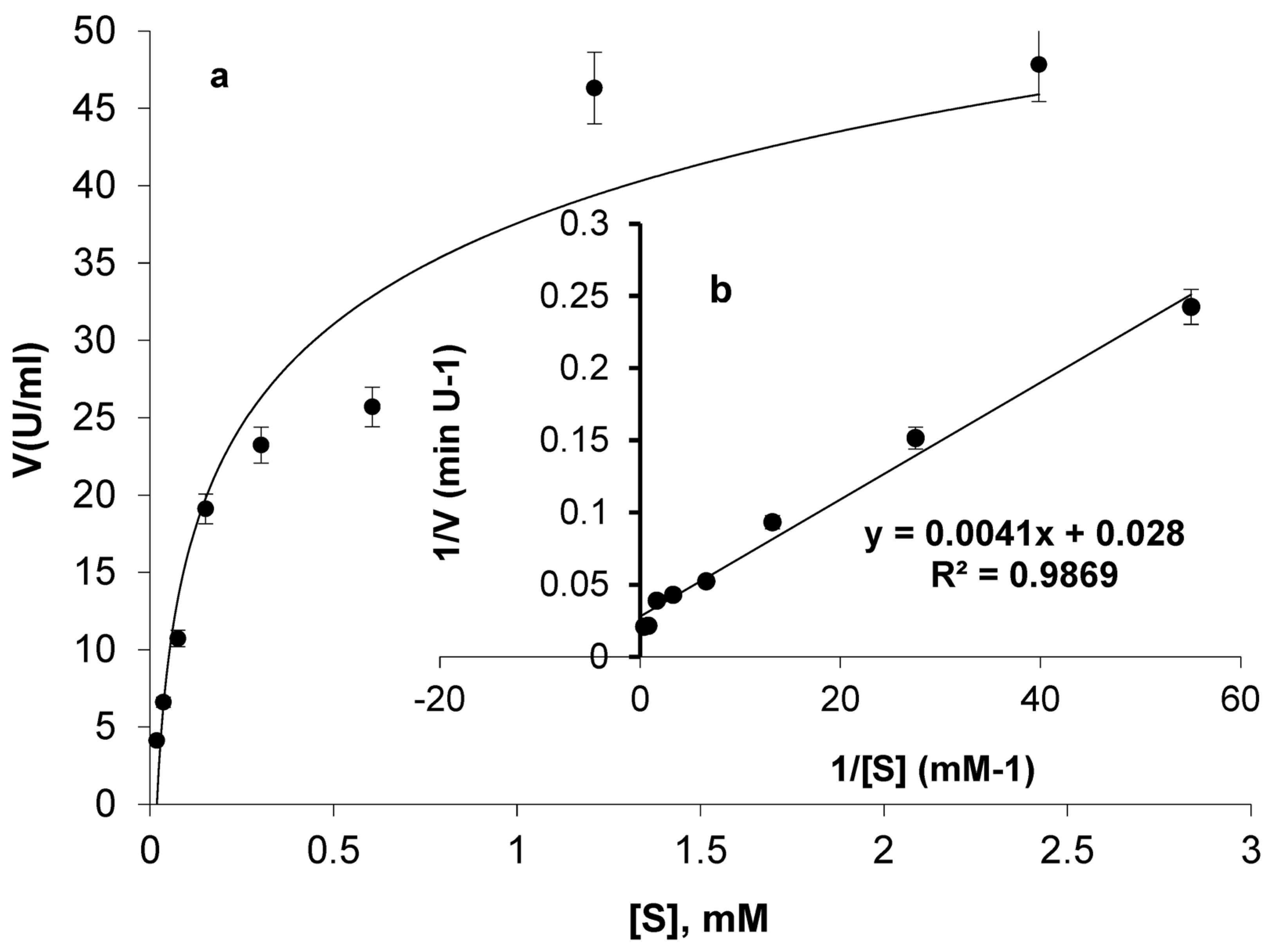
2.5. Evaluation of ASP-J1-4 L-ASNase Kinetics
2.6. Antitumor Activity
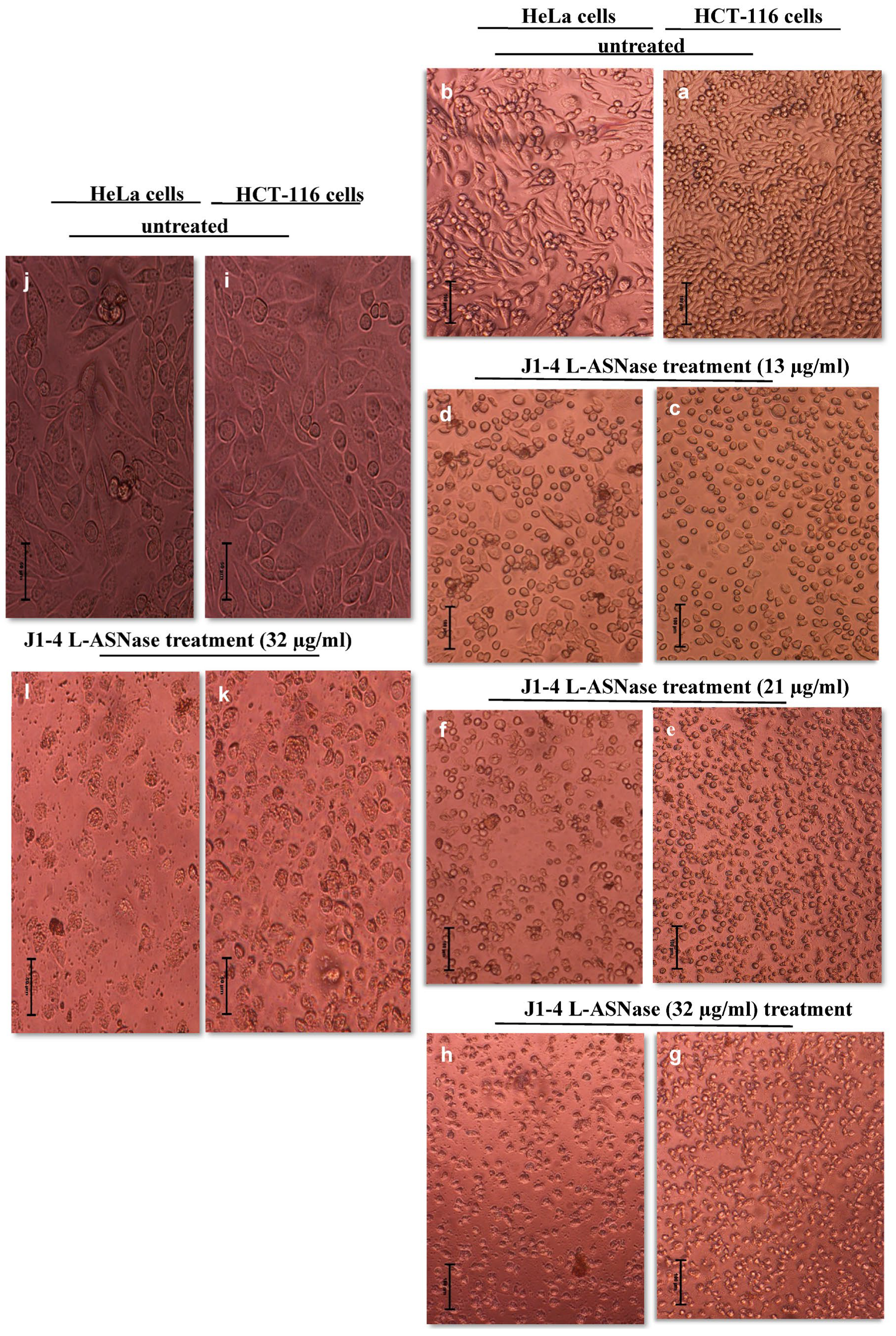
2.6.1. Dose-Dependent Reduction of HeLa and HCT-116 Cell Proliferation and Viability
2.6.2. ASP-J1-4 L-ASNase Treatment-Induced Nucleus Morphological Changes
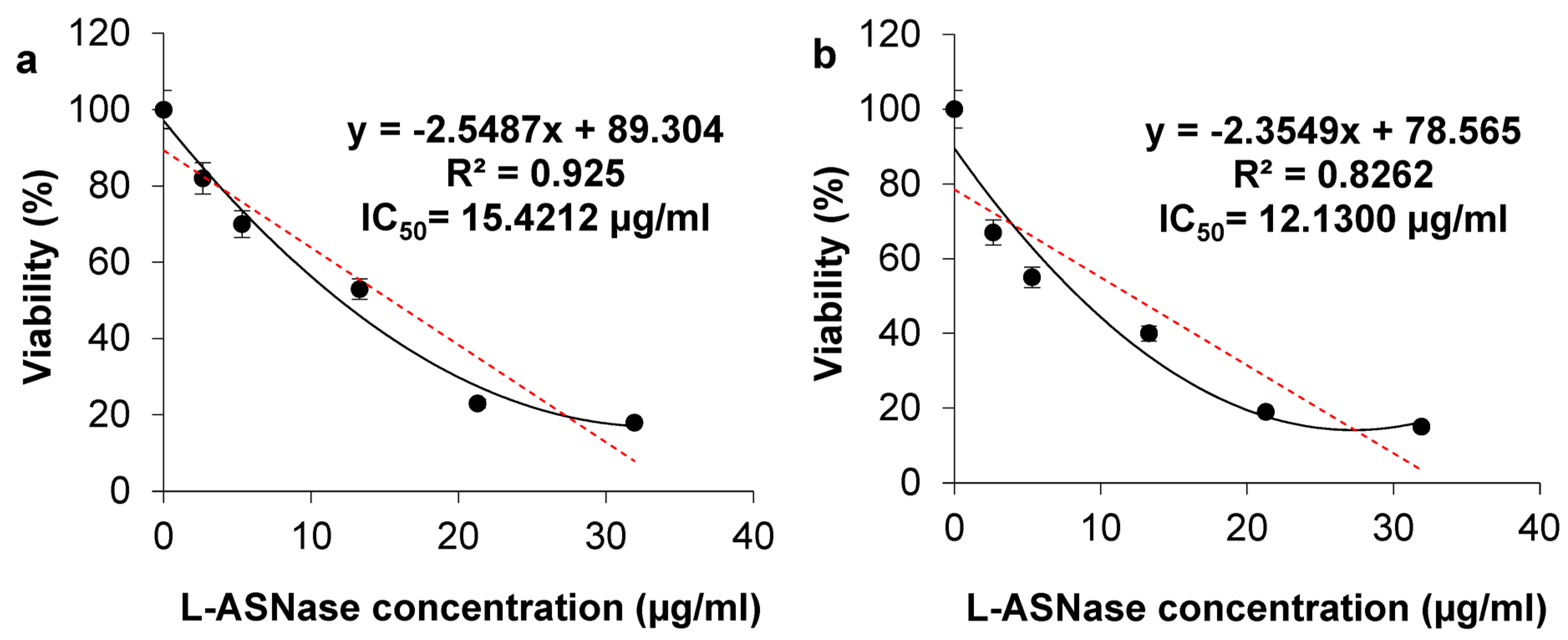
2.6.3. Viability of HCT-116 and HeLa Cells After Treatment with ASP-J1-4 and IC50 Calculation
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Collection and Isolation of l-ASNase-Producing Bacteria
4.3. Identification of Potential Isolates
4.4. Production and Quantitative Assay of L-ASNase
4.5. Purification of L-ASNase
4.6. Characterization of L-ASNase
4.6.1. Impact of Temperature and pH on Enzyme Stability and Activity
4.6.2. Effect of Metal Ions and Reagents on Enzyme Activity
4.6.3. Determination of Kinetic Constants
4.7. Antitumor Activity
4.7.1. Cell Culture of Human Cell Lines
4.7.2. Cell Proliferation and Cell Viability Assessment
4.7.3. Nuclear Morphology Evaluation Using DAPI Staining and Fluorescent Microscopy
4.7.4. Cytotoxicity and Anti-Proliferative Determination of ASP-J1-4 L-ASNase on HCT-116 and HeLa Using MTT Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute type of Lymphoblastic Leukemia |
| DAPI | 4′,6-diamidino-2-phenylindole dihydrochloride |
| DEAE | Diethylaminoethyl |
| DMEM | Dulbecco’s Modified Eagles medium |
| DMSO | Dimethyl sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| HCT | Human colon carcinoma |
| HeLa | Henrietta Lacks carcinoma |
| IC50 | Half-maximal inhibitory concentration |
| Km | Michaelis–Menten constant |
| L-ASN | L-asparagine |
| L-ASNase | L-asparaginase |
| L-GTNase | L-glutaminase |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| PBS | Physiological buffered saline |
| RPMI | Roswell Park Memorial Institute |
| SDS | Sodium dodecyl-sulphate |
| SDS-PAGE | Sodium dodecyl-sulphate polyacrylamide gel electrophoresis |
| T1/2 | Half-life time |
| Tm | Midpoint temperature |
| Vmax | Maximum velocity of reaction |
References
- Kotb, E. Improvement of uricase production from Bacillus subtilis RNZ-79 by solid state fermentation of shrimp shell wastes. Biologia 2016, 71, 229–238. [Google Scholar] [CrossRef]
- Kotb, E. Production, purification, and partial characterization for an oxalate decarboxylase (OxDcase) from the probiote Lactobacillus plantarum KSK-II. Curr. Sci. 2018, 114, 835–844. [Google Scholar] [CrossRef]
- Al-Dhuayan, I.; Kotb, E.; Alqosaibi, A.; Mahmoud, A. Histological studies on a newly isolated Bacillus subtilis D10 protease in the debridement of burn wound eschars using mouse model. Pharmaceutics 2021, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- AlShaikh-Mubarak, G.A.; Kotb, E.; Alabdalall, A.H.; Aldayel, M.F. A survey of elastase-producing bacteria and characteristics of the most potent producer, Priestia megaterium gasm32. PLoS ONE 2023, 18, e0282963. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Hegde, K.; Veeranki, V.D. Production and characterization of novel glutaminase free recombinant L-asparaginase II of Erwinia carotovora subsp. Atroseptica SCRI 1043 in E. coli BL21 (DE3). Br. Microbiol. Res. J. 2015, 6, 95–112. [Google Scholar] [CrossRef]
- Alhazzani, W.; Matar, A.; Alharbi, I.; Alghamdi, H.; Aljuhani, M.; Alghamdi, M. Impact of a clinical pathway on the management of acute coronary syndrome: A retrospective study. Saudi Med. J. 2025, 46, 316–324. [Google Scholar]
- Delgado-Andrade, C.; Mesías, M.; Morales, F.J. Introduction to the special issue: New frontiers in acrylamide study in foods—Formation, analysis and exposure assessment. Foods 2020, 9, 1506. [Google Scholar] [CrossRef]
- Tosta Pérez, M.; Herrera Belén, L.; Letelier, P.; Calle, Y.; Pessoa, A.; Farías, J.G. L-asparaginase as the gold standard in the treatment of acute lymphoblastic leukemia: A comprehensive review. Med. Oncol. 2023, 40, 150. [Google Scholar] [CrossRef]
- Tamzi, N.N.; Rahman, M.M.; Das, S. Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy. Inter-Natl. J. Transl. Med. 2024, 4, 740–781. [Google Scholar] [CrossRef]
- Hosseini, K.; Zivari-Ghader, T.; Akbarzadehlaleh, P.; Ebrahimi, V.; Sharafabad, B.E.; Dilmaghani, A. A Comprehensive Review of L-Asparaginase: Production, Applications and Therapeutic Potential in Cancer Treatment. Appl. Biochem. Microbiol. 2024, 60, 599–613. [Google Scholar] [CrossRef]
- Alrumman, S.A.; Mostafa, Y.S.; Al-izran, K.A.; Alfaifi, M.Y.; Taha, T.H.; Elbehairi, S.E. Production and anticancer activity of an L-asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci. Rep. 2019, 9, 3756. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, Y.; Alrumman, S.; Alamri, S.; Hashem, M.; Al-izran, K.; Alfaifi, M.; Elbehairi, S.; Taha, T. Enhanced production of glutaminase-free L-asparaginase by marine Bacillus velezensis and cytotoxic activity against breast cancer cell lines. Electron. J. Biotechnol. 2019, 42, 6–15. [Google Scholar] [CrossRef]
- Lai, Q.; Liu, Y.; Shao, Z. Bacillus xiamenensis sp. nov., isolated from intestinal tract contents of a flathead mullet (Mugil cephalus). Antonie Van Leeuwenhoek 2014, 105, 99–107. [Google Scholar] [CrossRef]
- Watanabe, M.; Sekino, Y.; Kuramochi, K.; Furuyama, Y. Bacillus xiamenensis inhibits the growth of Moraxella osloensis by producing indole-3-carboxaldehyde. Microbiologyopen 2024, 13, e70009. [Google Scholar] [CrossRef]
- Lailaja, V.P.; Hari, V.; Sumithra, T.G.; Anusree, V.N.; Suresh, G.; Sanil, N.K.; Sharma, S.R.K.; Gopalakrishnan, A. In vitro and in silico analysis unravelled clinically desirable attributes of Bacillus altitudinis L-asparaginase. J. Appl. Microbiol. 2024, 135, lxae062. [Google Scholar] [CrossRef] [PubMed]
- Hadi, W.A.; Edwin, B.T.; Jayakumaran, N.A. Isolation and identification of marine Bacillus altitudinis KB1 from coastal Kerala: Asparaginase producer. J. Mar. Bio. Assoc. India 2021, 63, 43–48. [Google Scholar] [CrossRef]
- Hozoorbakhsh, F.; Ghiasian, M.; Ghandehari, F.; Emami-Karvani, Z.; Khademi Dehkordi, M. An immunoinformatic approach employing molecular docking and molecular dynamics simulation for evaluation of L-asparaginase produced by Bacillus velezensis. JBSD 2022, 41, 9057–9071. [Google Scholar] [CrossRef]
- Chand, S.; Mihooliya, K.N.; Sahoo, D.K.; Prasad, J.P.; Sharma, G. L-asparaginase from Bacillus flexus strain SS: Isolation, screening, production process optimization, purification, and anticancer activity. Appl. Biochem. Microbiol. 2022, 58, 416–427. [Google Scholar] [CrossRef]
- Abdelrazek, N.A.; Elkhatib, W.F.; Raafat, M.M.; Aboulwafa, M.M. Experimental and bioinformatics study for production of L-asparaginase from Bacillus licheniformis: A promising enzyme for medical application. AMB Express 2019, 9, 39. [Google Scholar] [CrossRef]
- Mahajan, R.V.; Saran, S.; Kameswaran, K.; Kumar, V.; Saxena, R.K. Efficient production of L-asparaginase from Bacillus licheniformis with low-glutaminase activity: Optimization, scale up and acrylamide degradation studies. Bioresour. Technol. 2012, 125, 11–16. [Google Scholar] [CrossRef]
- Arredondo-Nuñez, A.; Monteiro, G.; Flores-Fernández, C.N.; Antenucci, L.; Permi, P.; Zavaleta, A.I. Characterization of a type II L-asparaginase from the halotolerant Bacillus subtilis CH11. Life 2023, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Darnal, S.; Patial, V.; Kumar, V.; Kumar, S.; Kumar, V.; Padwad, Y.S.; Singh, D. Biochemical characterization of extremozyme L-asparaginase from Pseudomonas sp. PCH199 for therapeutics. AMB Express 2023, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Khushoo, A.; Pal, Y.; Singh, B.N.; Mukherjee, K.J. Extracellular expression and single step purification of recombinant Escherichia coli L-asparaginase II. Protein Expr. Purif. 2004, 38, 29–36. [Google Scholar] [CrossRef]
- Radha, R.; Arumugam, N.; Gummadi, S.N. Glutaminase free L-asparaginase from Vibrio cholerae: Heterologous expression, purification and biochemical characterization. Int. J. Biol. Macromol. 2018, 111, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Singh, H.R.; Jha, S.K. Production, purification and kinetic characterization of glutaminase free anti-leukemic L-asparaginase with low endotoxin level from novel soil isolate. Prep. Biochem. Biotechnol. 2020, 50, 260–271. [Google Scholar] [CrossRef]
- Badoei-Dalfard, A. Purification and characterization of L-asparaginase from Pseudomonas aeruginosa strain SN004: Production optimization by statistical methods. Biocatal. Agric. Biotechnol. 2015, 4, 388–397. [Google Scholar] [CrossRef]
- Kumar, S.; Darnal, S.; Patial, V.; Kumar, V.; Kumar, V.; Kumar, S.; Singh, D. Molecular cloning, characterization, and in-silico analysis of L-asparaginase from Himalayan Pseudomonas sp. PCH44. 3 Biotech 2022, 12, 162. [Google Scholar] [CrossRef]
- Amena, S.; Vishalakshi, N.; Prabhakar, M.; Dayanand, A.; Lingappa, K. Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Braz. J. Microbiol. 2010, 41, 173–178. [Google Scholar] [CrossRef]
- Jia, M.; Xu, M.; He, B.; Rao, Z. Cloning, expression, and characterization of L-asparaginase from a newly isolated Bacillus subtilis B11-06. J. Agric. Food Chem. 2013, 61, 9428–9434. [Google Scholar] [CrossRef]
- Sanghvi, G.; Bhimani, K.; Vaishnav, D.; Oza, T.; Dave, G.; Kunjadia, P. Mitigation of acrylamide by L-asparaginase from Bacillus subtilis KDPS1 and analysis of degradation products by HPLC and HPTLC. Springerplus 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Pokrovskaya, M.V.; Aleksandrova, S.S.; Pokrovsky, V.S.; Omeljanjuk, N.M.; Borisova, A.A.; Anisimova, N.Y.; Sokolov, N.N. Cloning, expression and characterization of the recombinant Yersinia pseudotuberculosis L-asparaginase. Protein Expr. Purif. 2012, 82, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.; Kim, M. Purification and characterization of thermostable L-asparaginase from Bacillus amyloliquefaciens MKSE in Korean soybean paste. LWT 2019, 109, 415–421. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, S.; Jiao, Y.; Wang, Y.; Wang, M.; Du, G. Gene cloning and expression of the L-asparaginase from Bacillus cereus BDRD-ST26 in Bacillus subtilis WB600. J. Biosci. Bioeng. 2019, 127, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Sharma, A.; Kumar, S.; Malik, F. Purification and characterization of glutaminase free asparaginase from Enterobacter cloacae: In vitro evaluation of cytotoxic potential against human myeloid leukemia HL-60 cells. PLoS ONE 2016, 11, e0148877. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liu, Y.; Mu, Q.; Jiang, Z.; Yang, S. Biochemical characterization of a novel L-asparaginase from Paenibacillus barengoltzii being suitable for acrylamide reduction in potato chips and mooncakes. Int. J. Biol. Macromol. 2017, 96, 93–99. [Google Scholar] [CrossRef]
- Singh, Y.; Gundampati, R.K.; Jagannadham, M.V.; Srivastava, S.K. Extracellular L-asparaginase from a protease-deficient Bacillus aryabhattai ITBHU02: Purification, biochemical characterization, and evaluation of antineoplastic activity in vitro. Appl. Biochem. Biotechnol. 2013, 171, 1759–1774. [Google Scholar] [CrossRef]
- Roy, M.P.; Das, V.; Patra, A. Isolation, purification and characterization of an extracellular L-asparaginase produced by a newly isolated Bacillus megaterium strain MG1 from the water bodies of Moraghat forest, Jalpaiguri, India. J. Gen. Appl. Microbiol. 2019, 65, 137–144. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Zhang, C.; Bie, X.; Zhao, H.; Lu, F.; Lu, Z. Biochemical characterization of a novel L-asparaginase from Bacillus megaterium H-1 and its application in French fries. Food Res. Int. 2015, 77, 527–533. [Google Scholar] [CrossRef]
- Kumar, S.; Venkata Dasu, V.; Pakshirajan, K. Purification and characterization of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour. Technol. 2011, 102, 2077–2082. [Google Scholar] [CrossRef]
- Warangkar, S.C.; Khobragade, C.N. Purification, characterization, and effect of thiol compounds on activity of the Erwinia carotovora L-asparaginase. Enzym. Res. 2010, 2010, 165878. [Google Scholar] [CrossRef]
- Costa, I.M.; Schultz, L.; De Araujo Bianchi Pedra, B.; Leite, M.S.M.; Farsky, S.H.P.; De Oliveira, M.A.; Pessoa, A.; Monteiro, G. Recombinant L-asparaginase 1 from Saccharomyces cerevisiae: An allosteric enzyme with antineoplastic activity. Sci. Rep. 2016, 6, 36239. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, S.R.; Costa-Silva, T.A.; Pessoa, A., Jr.; Madeira, P.; Monteiro, G. Effect of osmolytes on the activity of anti-cancer enzyme L-asparaginase II from Erwinia chrysanthemi. Process Biochem. 2019, 81, 123–131. [Google Scholar] [CrossRef]
- Beckett, A.; Gervais, D. What makes a good new therapeutic L-asparaginase? World J. Microbiol. Biotechnol. 2019, 35, 152. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Kaur, A.; Chadha, B.S. Single step purification of asparaginase from endophytic bacteria Pseudomonas oryzihabitans exhibiting high potential to reduce acrylamide in processed potato chips. Food Bioprod. Process. 2016, 99, 222–230. [Google Scholar] [CrossRef]
- El-Fakharany, E.; Orabi, H.; Abdelkhalek, E.; Sidkey, N. Purification and biotechnological applications of L-asparaginase from newly isolated Bacillus halotolerans OHEM18 as antitumor and antioxidant agent. J. Biomol. Struct. Dyn. 2022, 40, 3837–3849. [Google Scholar] [CrossRef]
- Einsfeldt, K.; Baptista, I.C.; Pereira, J.C.; Bruno, R.E.; Mello, F.V.; Costa-Amaral, I.C.; Costa, E.S.; Ribeiro, M.C.; Land, M.G.; Alves, T.L.; et al. Recombinant L-asparaginase from Zymomonas mobilis: A potential new antileukemic agent produced in Escherichia coli. PLoS ONE 2016, 11, e0163203. [Google Scholar] [CrossRef]



| Isolate Id. | Accession No. | Source | Coordinate’s Location | L-ASNase Activity (U/mL) |
|---|---|---|---|---|
| B. altitudinis J1-2 | PQ593940 | Seablite Suaeda maritima | 4HCC+FMC Al Jubail | 49.07 ± 3.26 |
| B. xiamenensis ASP-J1-4 | PQ593941 | 53.20 ± 2.31 | ||
| B. velezensis J3-1 | PQ593943 | Shoreline soil | 4HCC+FMC Al Jubail | 12.26 ± 0.85 |
| Paenibacillus lautus J6-1 | PQ593936 | 13.06 ± 0.76 | ||
| Psychrobacter phenylpyruvicus STF-1 | PQ593945 | Seastar Asteroidea #1 | C5V3+H4X Dammam | 38.49 ± 2.24 |
| Proteus mirabilis A-2 | PQ593938 | Marine alga Eucheuma | 26.3249619, 50.2285868 | 31.34 ± 2.19 * |
| Psychrobacter sanguinis K2-2 | PQ593953 | Sea squids | 26.182416, 50.165275 | 5.09 ± 0.22 |
| Macrococcus caseolyticum K-3 | PQ593933 | Planaria | 66QC+8PAL Khobar | 8.79 ± 0.54 |
| B. velezensis SPBR-6 | PQ593947 | Sponge #1 | 4XHV+FR6, unnamed road, Dhahran 34814 | 11.54 ± 0.85 * |
| B. paralicheniformis SPF-4 | PQ593949 | Sponge #2 | GH74+HP5Khafji | 1.65 ± 0.09 |
| B. subtilis STH-5 | PQ593951 | Seastar Asteroidea #2 | 4XHV+FR6, unnamed road, Dhahran 34814 | 2.47 ± 0.08 |
| Priestia flexa MR-25 | PQ593935 | Mangrove sediment #1 | PXMV+Q7Ras Tanura | 24.74 ± 1.15 |
| B. licheniformis MR-7 | PQ593957 | 5.64 ± 0.33 | ||
| B. subtilis MQ1-1 | PQ593959 | Mangrove sediment #2 | 26.590850, 50.044333 | 3.16 ± 0.12 |
| Staphylococcus epidermidis HOR-4 | PQ593965 | Seahorse Hippocampus | 4XHV+FR6, unnamed road, Dhahran 34814 | 8.25 ± 0.43 |
| B. paralicheniformis HOR-5 | PQ593967 | 9.62 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harbi, G.M.; Kotb, E.; Almiman, A.A.; Berekaa, M.M.; Alhamad, S.; Alahmady, N.F.; Aljafary, M.A.; Alqazlan, N.M.; Alyami, R.I.; Alqarni, J.M.; et al. Isolation and Characterization of L-Asparaginase-Producing Bacteria from the Arabian–Persian Gulf Region: First Report on Bacillus xiamenensis ASP-J1-4 as a Producer and Its Potential Application. Mar. Drugs 2025, 23, 194. https://doi.org/10.3390/md23050194
Al-Harbi GM, Kotb E, Almiman AA, Berekaa MM, Alhamad S, Alahmady NF, Aljafary MA, Alqazlan NM, Alyami RI, Alqarni JM, et al. Isolation and Characterization of L-Asparaginase-Producing Bacteria from the Arabian–Persian Gulf Region: First Report on Bacillus xiamenensis ASP-J1-4 as a Producer and Its Potential Application. Marine Drugs. 2025; 23(5):194. https://doi.org/10.3390/md23050194
Chicago/Turabian StyleAl-Harbi, Ghofran M., Essam Kotb, Abeer A. Almiman, Mahmoud M. Berekaa, Salwa Alhamad, Nada F. Alahmady, Meneerah A. Aljafary, Nadiyah M. Alqazlan, Reem I. Alyami, Joud M. Alqarni, and et al. 2025. "Isolation and Characterization of L-Asparaginase-Producing Bacteria from the Arabian–Persian Gulf Region: First Report on Bacillus xiamenensis ASP-J1-4 as a Producer and Its Potential Application" Marine Drugs 23, no. 5: 194. https://doi.org/10.3390/md23050194
APA StyleAl-Harbi, G. M., Kotb, E., Almiman, A. A., Berekaa, M. M., Alhamad, S., Alahmady, N. F., Aljafary, M. A., Alqazlan, N. M., Alyami, R. I., Alqarni, J. M., & Al-Suhaimi, E. A. (2025). Isolation and Characterization of L-Asparaginase-Producing Bacteria from the Arabian–Persian Gulf Region: First Report on Bacillus xiamenensis ASP-J1-4 as a Producer and Its Potential Application. Marine Drugs, 23(5), 194. https://doi.org/10.3390/md23050194