The Mediterranean Sea on the Bench: Unveiling the Marine Invertebrate Sidnyum elegans as a Source of Novel Promising Therapeutic Tools Against Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Extraction and Fractionation of S. elegans
2.2. SEB1–9 Fractions Inhibit Proliferation of Human TNBC Cells
2.3. LC-HRMS/MS and NMR Analyses of SEB-5
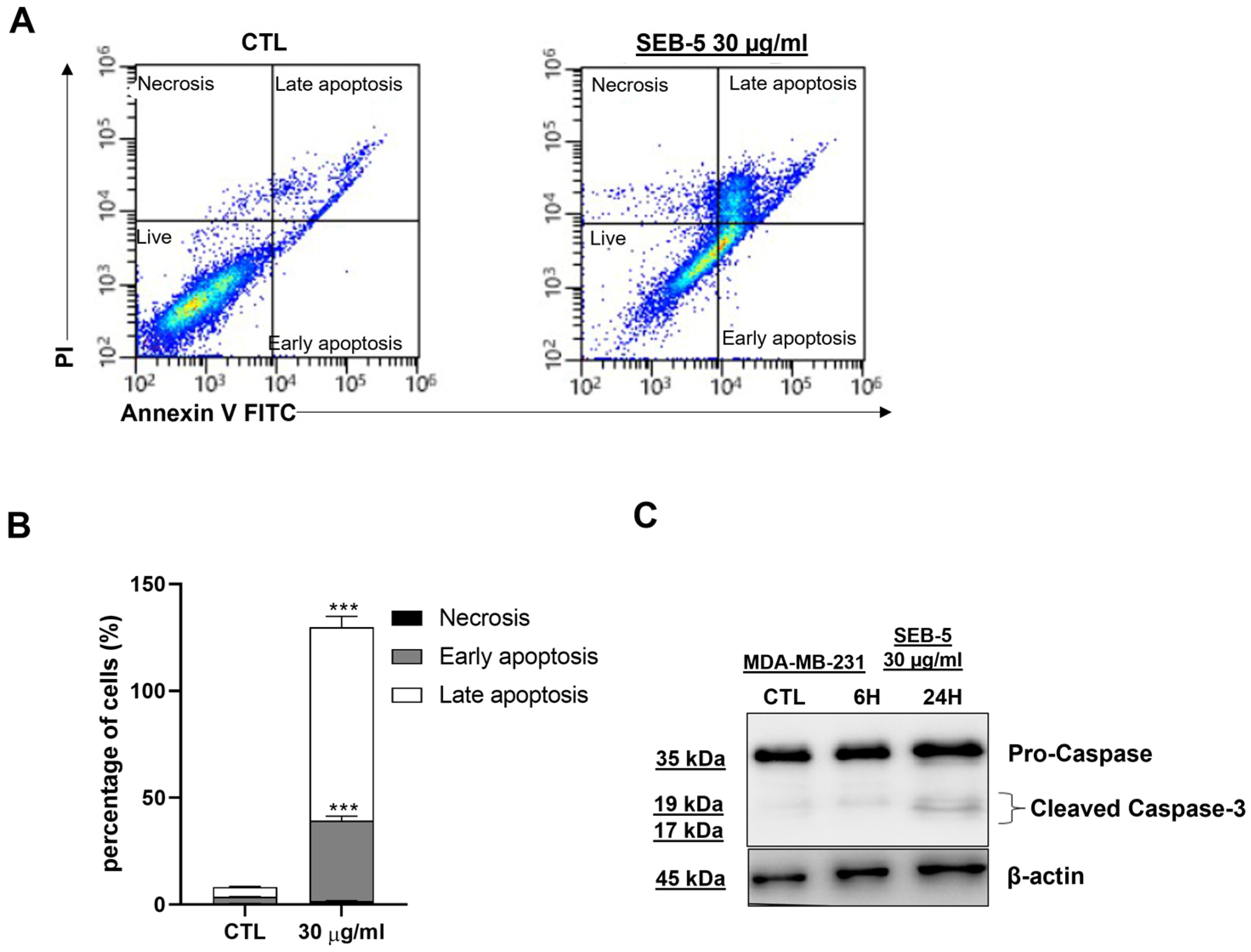
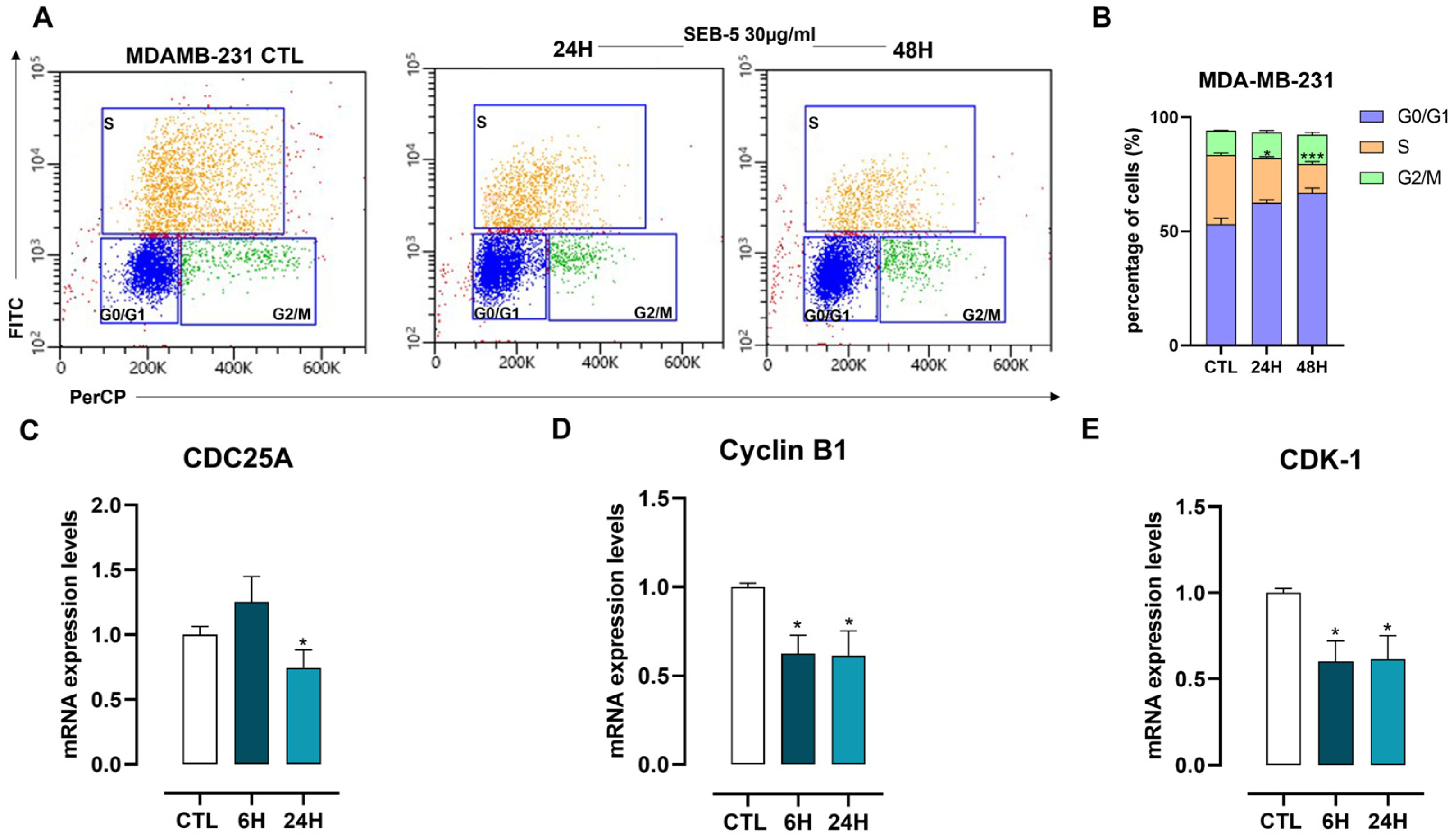
2.4. SEB-5 Induced Apoptotic Cell Death and Cell Cycle Arrest in Human TNBC Cells
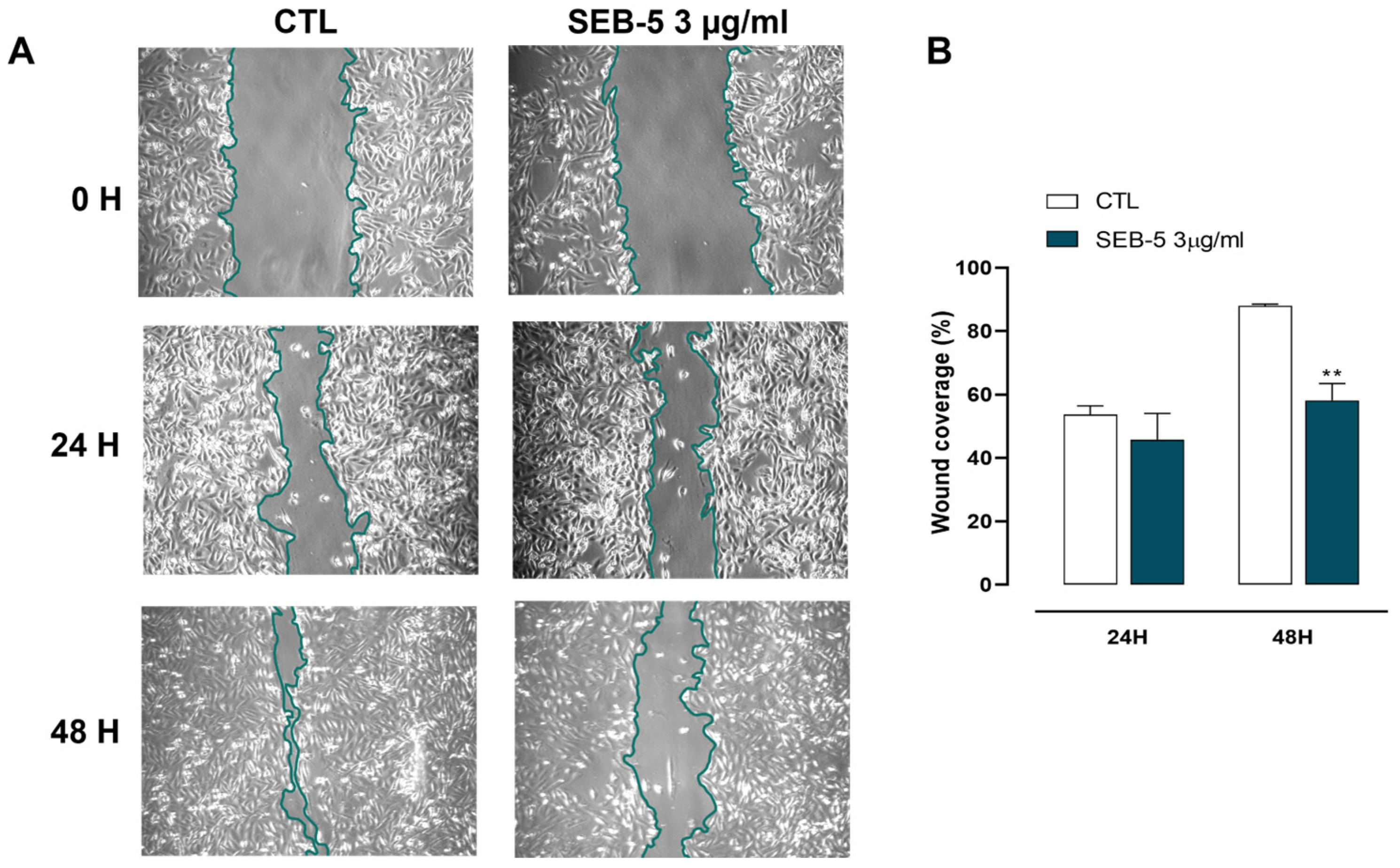
2.5. SEB-5 Reduces Cell Migration in Human TNBC Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Collection, Extraction, and Fractionation of the Ascidian S. elegans
4.3. LC-HRMS/MS Profiling of S. elegans
4.4. Isolation of the Novel Metabolites 3–5
4.5. Cell Cultures
4.6. Western Blot Analysis
4.7. RNA Purification and Quantitative Real-Time PCR
4.8. Proliferation Assay
4.9. Flow Cytometry
4.10. Cell Cycle Analysis
4.11. Wound-Healing Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Riaz, F. New Strategies for the Management of Triple-Negative Breast Cancer. Curr. Opin. Obstet. Gynecol. 2024, 36, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Papalexis, P.; Georgakopoulou, V.E.; Drossos, P.V.; Thymara, E.; Nonni, A.; Lazaris, A.C.; Zografos, G.C.; Spandidos, D.A.; Kavantzas, N.; Thomopoulou, G.E. Precision Medicine in Breast Cancer (Review). Mol. Clin. Oncol. 2024, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Pajewska, M.; Partyka, O.; Czerw, A.; Deptała, A.; Sygit, K.; Gąska, I.; Porada, S.; Drobnik, J.; Pobrotyn, P.; Grata-Borkowska, U.; et al. Advanced and Metastatic Triple Negative Breast Cancer-Potential New Treatment. Cancers 2025, 17, 1183. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Huang, Y. Triple-Negative Breast Cancer Treatment Advancements: A Review of Evolving Strategies. Eur. J. Cancer Care 2024, 2024, 8299502. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development. Nat. Prod. Bioprospect. 2017, 7, 1–111. [Google Scholar] [CrossRef]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive Peptides from Marine Ascidians and Future Drug Development–A Review. Int. J. Pept. Res. Ther. 2018, 24, 13–18. [Google Scholar] [CrossRef]
- Casertano, M.; Genovese, M.; Paoli, P.; Santi, A.; Aiello, A.; Menna, M.; Imperatore, C. Insights into Cytotoxic Behavior of Lepadins and Structure Elucidation of the New Alkaloid Lepadin L from the Mediterranean Ascidian Clavelina Lepadiformis. Mar. Drugs 2022, 20, 65. [Google Scholar] [CrossRef]
- Cooreman, K.; De Spiegeleer, B.; Van Poucke, C.; Vanavermaete, D.; Delbare, D.; Wynendaele, E.; De Witte, B. Emerging Pharmaceutical Therapies of Ascidian-Derived Natural Products and Derivatives. Environ. Toxicol. Pharmacol. 2023, 102, 104254. [Google Scholar] [CrossRef]
- Casertano, M.; Esposito, E.; Bello, I.; Indolfi, C.; Putra, M.Y.; Di Cesare Mannelli, L.; Ghelardini, C.; Menna, M.; Sorrentino, R.; Cirino, G.; et al. Searching for Novel Sources of Hydrogen Sulfide Donors: Chemical Profiling of Polycarpa Aurata Extract and Evaluation of the Anti-Inflammatory Effects. Mar. Drugs 2023, 21, 641. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Luciano, P.; Aiello, A.; Vitalone, R.; Irace, C.; Santamaria, R.; Li, J.; Guo, Y.-W.; Menna, M. Structure and Configuration of Phosphoeleganin, a Protein Tyrosine Phosphatase 1B Inhibitor from the Mediterranean Ascidian Sidnyum Elegans. J. Nat. Prod. 2016, 79, 1144–1148. [Google Scholar] [CrossRef]
- Imperatore, C.; Aiello, A.; D’Aniello, F.; Luciano, P.; Vitalone, R.; Meli, R.; Raso, G.M.; Menna, M. New Bioactive Alkyl Sulfates from Mediterranean Tunicates. Molecules 2012, 17, 12642–12650. [Google Scholar] [CrossRef] [PubMed]
- Luciano, P.; Imperatore, C.; Senese, M.; Aiello, A.; Casertano, M.; Guo, Y.-W.; Menna, M. Assignment of the Absolute Configuration of Phosphoeleganin via Synthesis of Model Compounds. J. Nat. Prod. 2017, 80, 2118–2123. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of Marine Natural Products in Drug Research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luo, D.; Luesch, H. Advances in Exploring the Therapeutic Potential of Marine Natural Products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Refaey, M.S.; Elias, N.; El-Mallah, M.F.; Albaqami, F.M.K.; Dergaa, I.; Du, M.; Salem, M.F.; Tahir, H.E.; Dagliaa, M.; et al. Marine Natural Products as a Source of Novel Anticancer Drugs: An Updated Review (2019–2023). Nat. Prod. Bioprospect. 2025, 15, 13. [Google Scholar] [CrossRef]
- Deng, R.; Zong, G.-F.; Wang, X.; Yue, B.-J.; Cheng, P.; Tao, R.-Z.; Li, X.; Wei, Z.-H.; Lu, Y. Promises of Natural Products as Clinical Applications for Cancer. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189241. [Google Scholar] [CrossRef]
- Montuori, E.; Hyde, C.A.C.; Crea, F.; Golding, J.; Lauritano, C. Marine Natural Products with Activities against Prostate Cancer: Recent Discoveries. Int. J. Mol. Sci. 2023, 24, 1435. [Google Scholar] [CrossRef]
- Casertano, M.; Genovese, M.; Piazza, L.; Balestri, F.; Del Corso, A.; Vito, A.; Paoli, P.; Santi, A.; Imperatore, C.; Menna, M. Identifying Human PTP1B Enzyme Inhibitors from Marine Natural Products: Perspectives for Developing of Novel Insulin-Mimetic Drugs. Pharmaceuticals 2022, 15, 325. [Google Scholar] [CrossRef]
- Genovese, M.; Imperatore, C.; Casertano, M.; Aiello, A.; Balestri, F.; Piazza, L.; Menna, M.; Del Corso, A.; Paoli, P. Dual Targeting of PTP1B and Aldose Reductase with Marine Drug Phosphoeleganin: A Promising Strategy for Treatment of Type 2 Diabetes. Mar. Drugs 2021, 19, 535. [Google Scholar] [CrossRef]
- Maccari, R.; Del Corso, A.; Paoli, P.; Adornato, I.; Lori, G.; Balestri, F.; Cappiello, M.; Naß, A.; Wolber, G.; Ottanà, R. An Investigation on 4-Thiazolidinone Derivatives as Dual Inhibitors of Aldose Reductase and Protein Tyrosine Phosphatase 1B, in the Search for Potential Agents for the Treatment of Type 2 Diabetes Mellitus and Its Complications. Bioorg. Med. Chem. Lett. 2018, 28, 3712–3720. [Google Scholar] [CrossRef]
- Kousaxidis, A.; Petrou, A.; Lavrentaki, V.; Fesatidou, M.; Nicolaou, I.; Geronikaki, A. Aldose Reductase and Protein Tyrosine Phosphatase 1B Inhibitors as a Promising Therapeutic Approach for Diabetes Mellitus. Eur. J. Med. Chem. 2020, 207, 112742. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and Insulin Resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Spacho, N.; Casertano, M.; Imperatore, C.; Papadopoulos, C.; Menna, M.; Eleftheriadis, N. Investigating the Catalytic Site of Human 15-Lipoxygenase-1 via Marine Natural Products. Chem. A Eur. J. 2024, 30, e202402279. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.Y.; Shoichet, B.K. Synergy and Antagonism of Promiscuous Inhibition in Multiple-Compound Mixtures. J. Med. Chem. 2006, 49, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-Target Therapeutics: When the Whole Is Greater than the Sum of the Parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef]
- Senger, M.R.; Fraga, C.A.M.; Dantas, R.F.; Silva, F.P. Filtering Promiscuous Compounds in Early Drug Discovery: Is It a Good Idea? Drug Discov. Today 2016, 21, 868–872. [Google Scholar] [CrossRef]
- Imperatore, C.; Valadan, M.; Tartaglione, L.; Persico, M.; Ramunno, A.; Menna, M.; Casertano, M.; Dell’Aversano, C.; Singh, M.; d’Aulisio Garigliota, M.L.; et al. Exploring the Photodynamic Properties of Two Antiproliferative Benzodiazopyrrole Derivatives. Int. J. Mol. Sci. 2020, 21, 1246. [Google Scholar] [CrossRef]
- Panza, E.; Bello, I.; Smimmo, M.; Brancaleone, V.; Mitidieri, E.; Bucci, M.; Cirino, G.; Sorrentino, R.; D Emmanuele di Villa Bianca, R. Endogenous and Exogenous Hydrogen Sulfide Modulates Urothelial Bladder Carcinoma Development in Human Cell Lines. Biomed. Pharmacother. 2022, 151, 113137. [Google Scholar] [CrossRef] [PubMed]
- Panza, E.; De Cicco, P.; Armogida, C.; Scognamiglio, G.; Gigantino, V.; Botti, G.; Germano, D.; Napolitano, M.; Papapetropoulos, A.; Bucci, M.; et al. Role of the Cystathionine γ Lyase/Hydrogen Sulfide Pathway in Human Melanoma Progression. Pigment Cell Melanoma Res. 2015, 28, 61–72. [Google Scholar] [CrossRef] [PubMed]
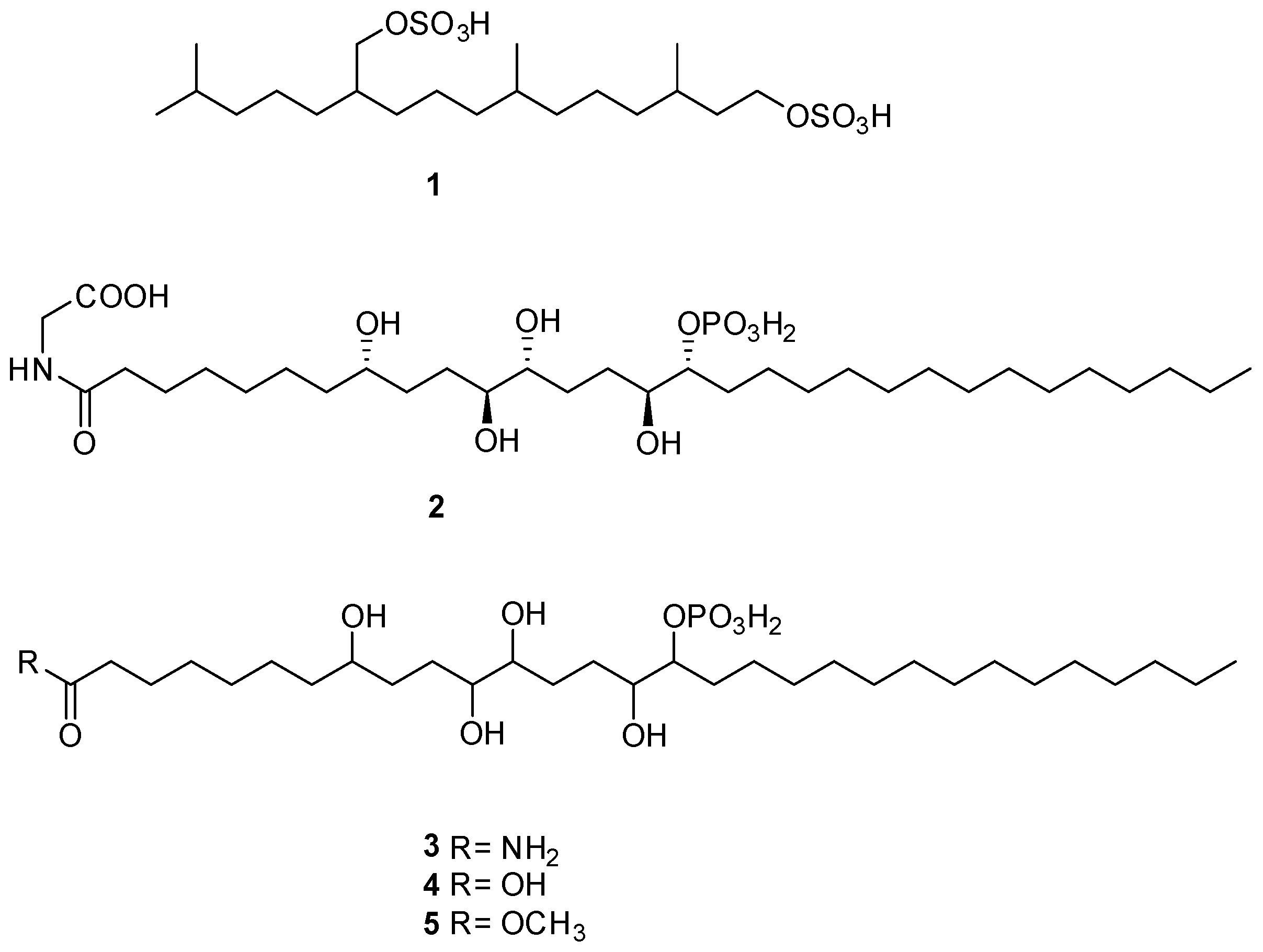


| Proposed Structure | Exp. MS (m/z) | Formula | Δ ppm, RDB | RT |
|---|---|---|---|---|
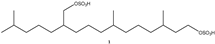 | 473.2249 | C20H41O8S2− | 2.460, 0.5 | 14.01 |
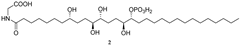 | 668.4150 | C32H63O11NP− | 2.507, 2.5 | 19.62 |
 | 610.4092 | C30H61O9NP− | 2.219, 1.5 | 19.94 |
 | 611.3931 | C30H60O10P− | 2.027, 1.5 | 21.20 |
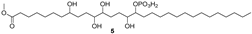 | 625.4091 | C31H62O10P− | 2.541, 1.5 | 23.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casertano, M.; Esposito, C.; Bello, I.; Barile, M.; Izzo, L.; Mitidieri, E.; Sorrentino, R.; Menna, M.; Panza, E.; Imperatore, C.; et al. The Mediterranean Sea on the Bench: Unveiling the Marine Invertebrate Sidnyum elegans as a Source of Novel Promising Therapeutic Tools Against Triple-Negative Breast Cancer. Mar. Drugs 2025, 23, 195. https://doi.org/10.3390/md23050195
Casertano M, Esposito C, Bello I, Barile M, Izzo L, Mitidieri E, Sorrentino R, Menna M, Panza E, Imperatore C, et al. The Mediterranean Sea on the Bench: Unveiling the Marine Invertebrate Sidnyum elegans as a Source of Novel Promising Therapeutic Tools Against Triple-Negative Breast Cancer. Marine Drugs. 2025; 23(5):195. https://doi.org/10.3390/md23050195
Chicago/Turabian StyleCasertano, Marcello, Camilla Esposito, Ivana Bello, Martina Barile, Luana Izzo, Emma Mitidieri, Raffaella Sorrentino, Marialuisa Menna, Elisabetta Panza, Concetta Imperatore, and et al. 2025. "The Mediterranean Sea on the Bench: Unveiling the Marine Invertebrate Sidnyum elegans as a Source of Novel Promising Therapeutic Tools Against Triple-Negative Breast Cancer" Marine Drugs 23, no. 5: 195. https://doi.org/10.3390/md23050195
APA StyleCasertano, M., Esposito, C., Bello, I., Barile, M., Izzo, L., Mitidieri, E., Sorrentino, R., Menna, M., Panza, E., Imperatore, C., & d’Emmanuele di Villa Bianca, R. (2025). The Mediterranean Sea on the Bench: Unveiling the Marine Invertebrate Sidnyum elegans as a Source of Novel Promising Therapeutic Tools Against Triple-Negative Breast Cancer. Marine Drugs, 23(5), 195. https://doi.org/10.3390/md23050195












