Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications
Abstract
1. Introduction
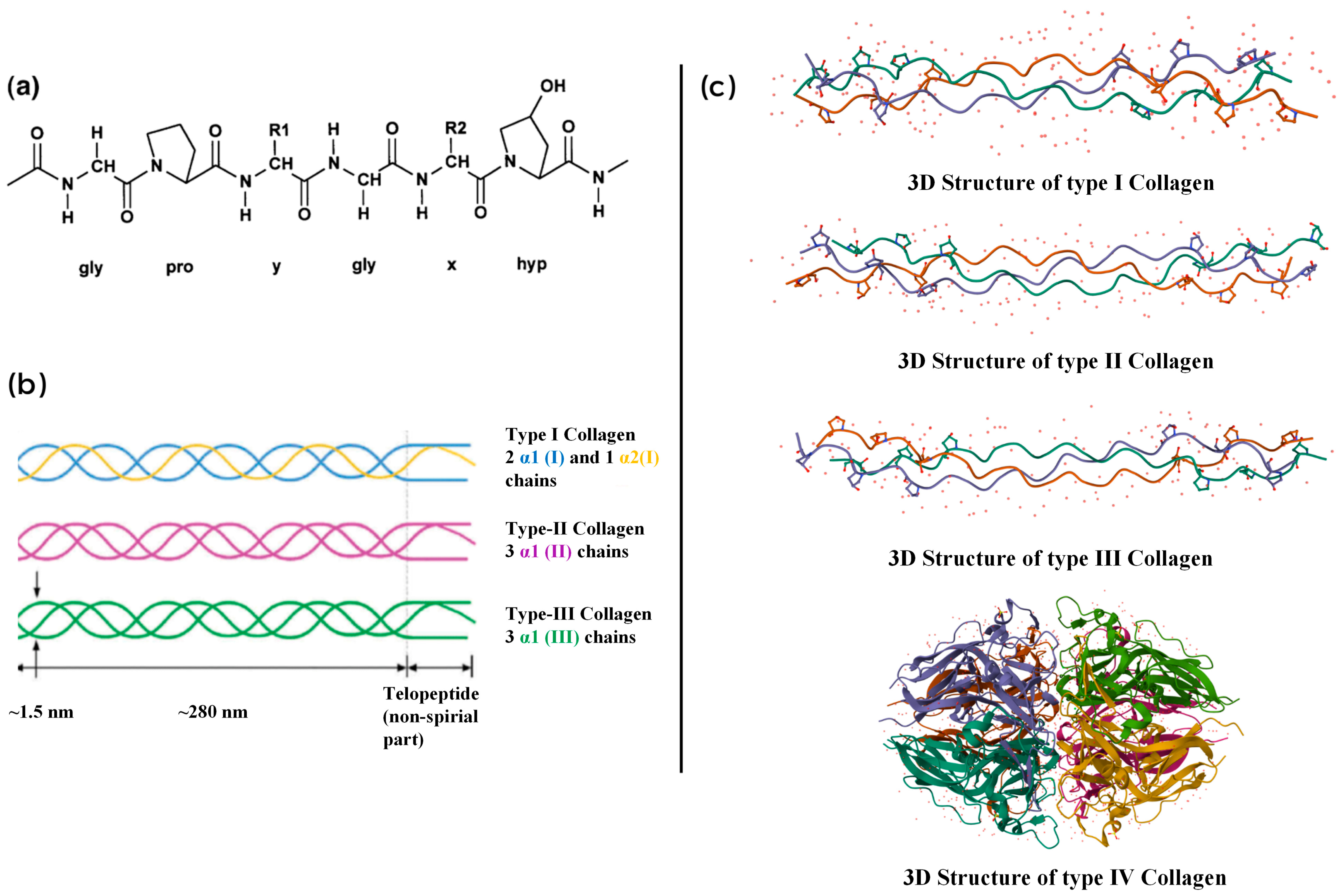
2. Chemical Structure and Composition
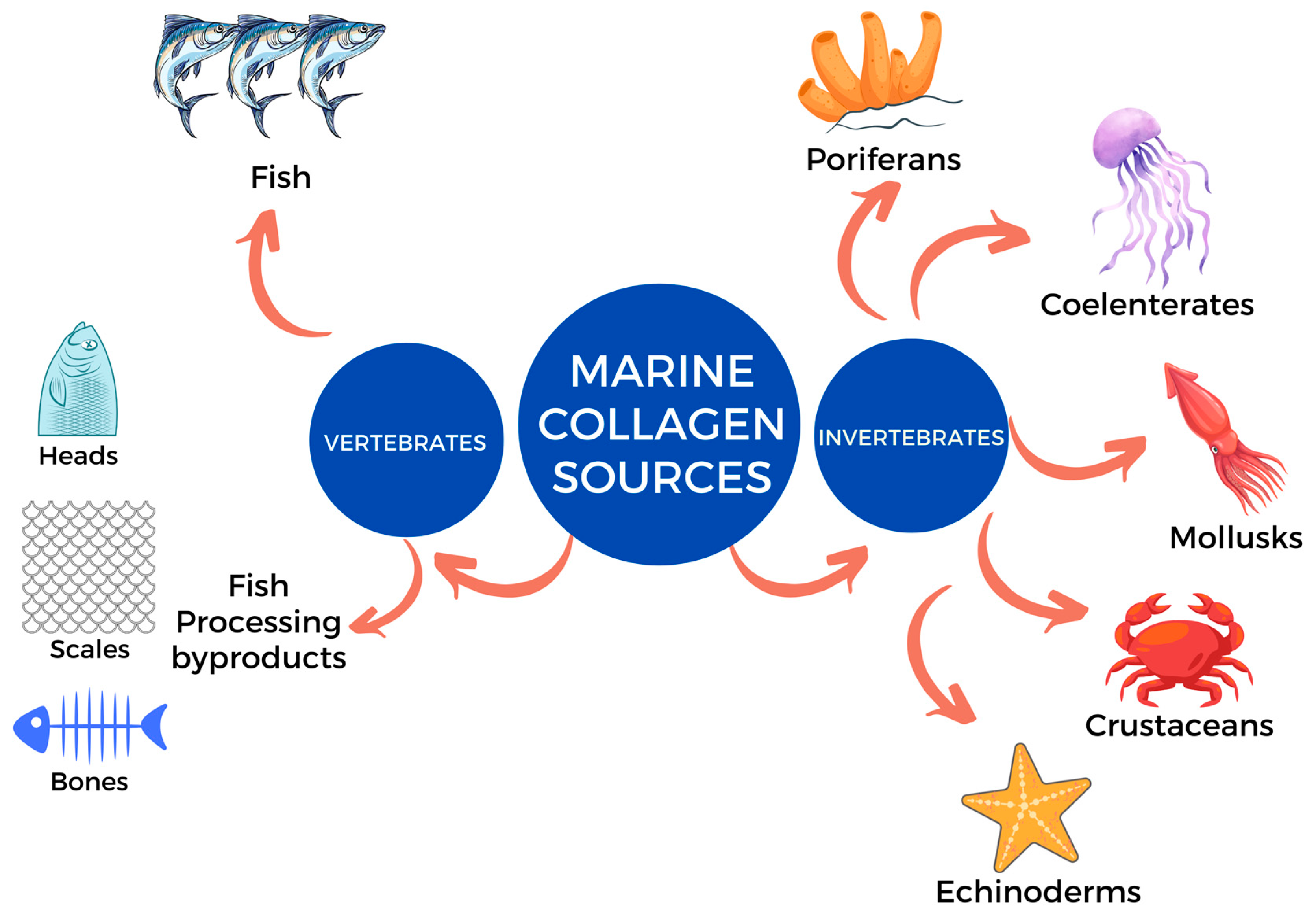
3. Source of Marine Collagen for Food and Dietary Supplements
3.1. Collagen from Marine Vertebrates
3.2. Collagen from Marine Invertebrates
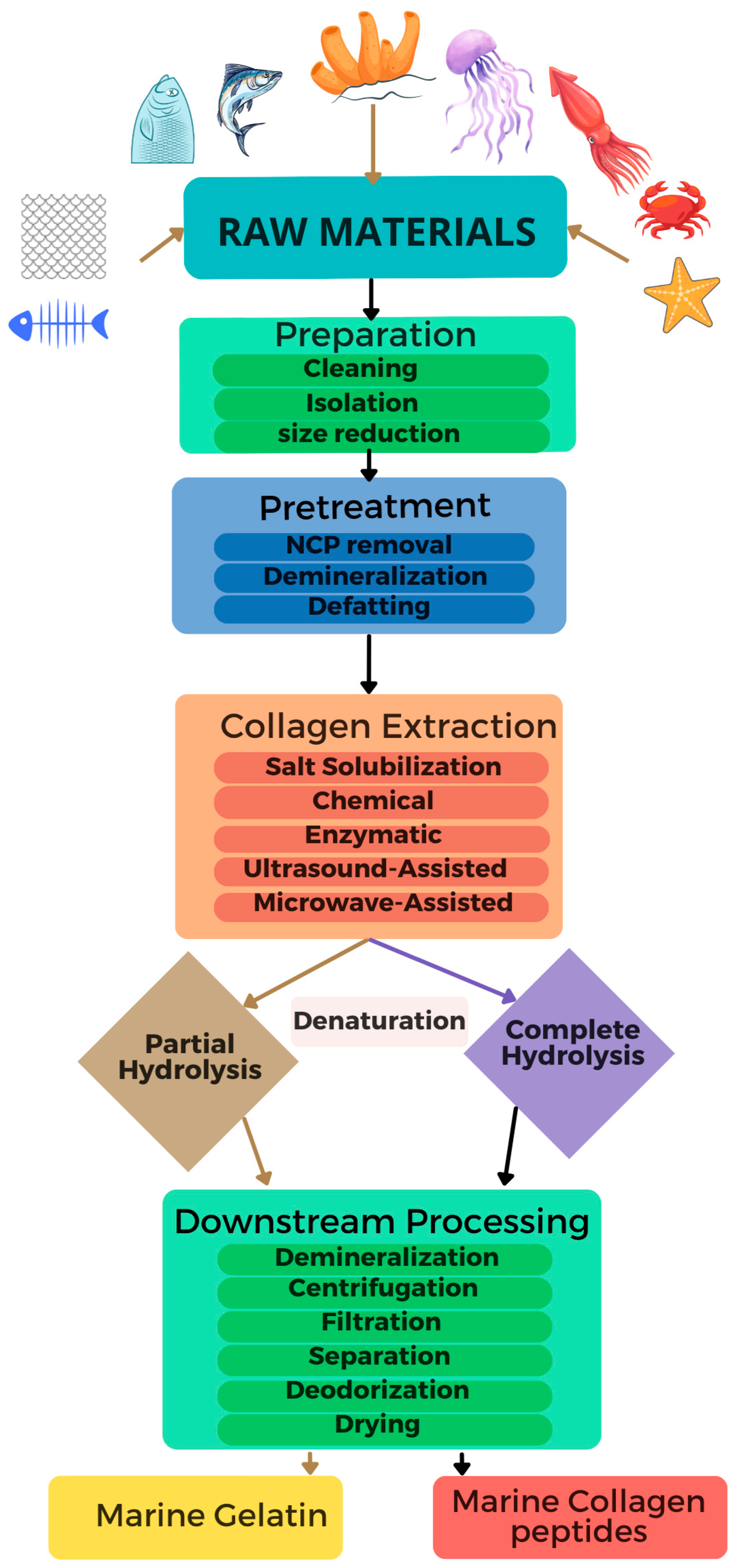
4. Production and Processing Technologies of Collagen and Collagen-Based Products
4.1. Preparation of Raw Materials and Pretreatment
4.2. Collagen Extraction
4.2.1. Salt-Solubilization Extraction (SSE)
4.2.2. Chemical Extraction
4.2.3. Enzymatic Extraction
4.2.4. Ultrasound-Assisted Extraction (UAE)
4.2.5. Microwave-Assisted Extraction
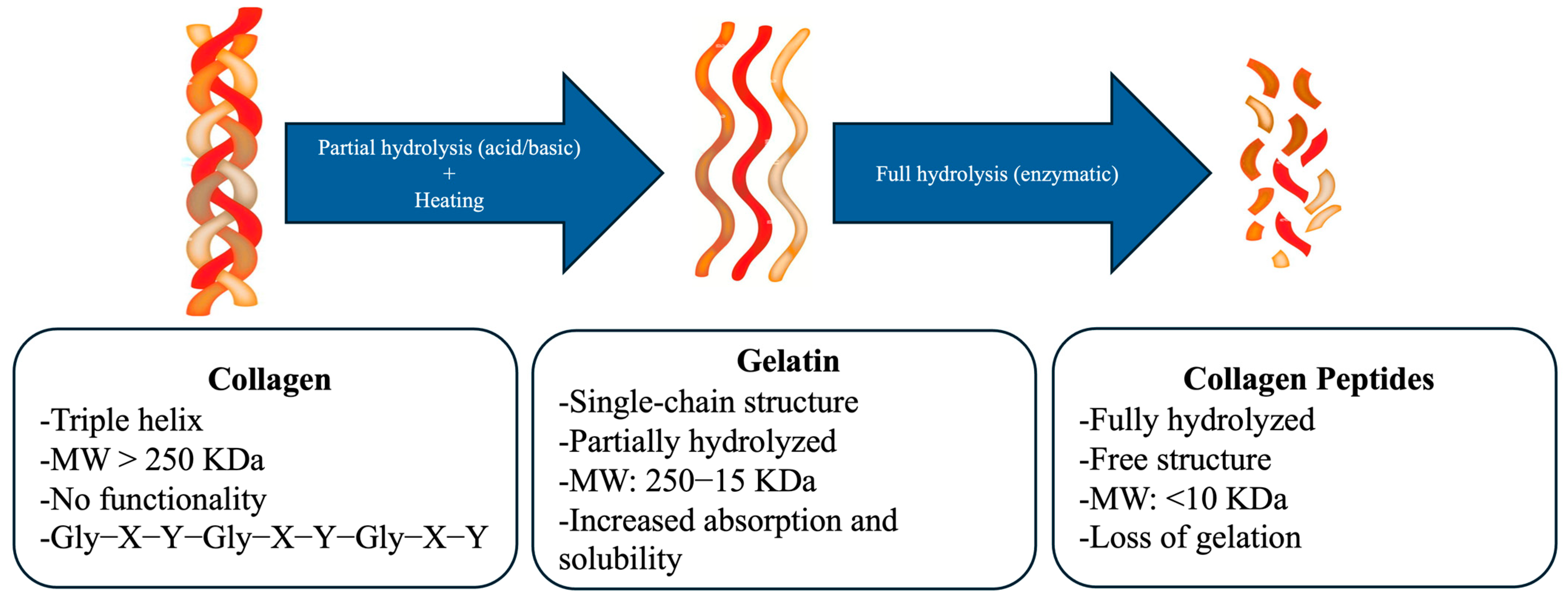
4.3. Denaturation of Marine Collagen
4.4. Downstream Processing
4.5. Emerging Analytical Techniques for Molecular Characterization of Collagen
5. Nutritional Composition and Health Benefits of Marine Collagen
6. Food Applications of Marine Collagen-Based Products
6.1. Collagen As a Viscosity, Texturing, and Stabilizing Agent
6.2. Collagen As a Functional Ingredient in Beverages
6.3. Collagen As a Film-Forming Agent
6.4. Collagen Peptides (CPs)
6.5. Other Applications
6.6. Case Study for Potential Marine Collagen Valorization: From Sourcing to Market Application
7. Safety and Quality Control
8. Consumer Acceptance and Market Trends
9. Challenges and Future Direction
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A Review on Recent Advances and Applications of Fish Collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef]
- Vital Protein the Many Benefits of Collagen. Available online: https://www.vitalproteins.com/blogs/stay-vital/benefits-of-collagen (accessed on 26 December 2023).
- Berillis, P. Marine Collagen: Extraction and Applications. In Research Trends in Biochemistry, Molecular Biology and Microbiology; SM Group: Dover, DE, USA, 2015. [Google Scholar]
- Chiarelli, P.G.; Suh, J.H.; Pegg, R.B.; Chen, J.; Mis Solval, K. The Emergence of Jellyfish Collagen: A Comprehensive Review on Research Progress, Industrial Applications, and Future Opportunities. Trends Food Sci. Technol. 2023, 141, 104206. [Google Scholar] [CrossRef]
- Paul, C.; Leser, S.; Oesser, S. Significant Amounts of Functional Collagen Peptides Can Be Incorporated in the Diet While Maintaining Indispensable Amino Acid Balance. Nutrients 2019, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- WebMD Collagen and Your Body. Available online: https://www.webmd.com/skin-problems-and-treatments/ss/slideshow-collagen-and-your-body (accessed on 25 January 2024).
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef]
- Hashim, P.; Ridzwan, M.S.M.; Bakar, J.; Hashim, D.M. Collagen in Food and Beverage Industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Pal, G.K.; Suresh, P.V. Sustainable Valorisation of Seafood By-Products: Recovery of Collagen and Development of Collagen-Based Novel Functional Food Ingredient. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Tan, A.; Salman, M.; Wagner, B.; McCluskey, B. The Role of Animal Health Components in a Biosurveillance System: Concept and Demonstration. Agriculture 2023, 13, 457. [Google Scholar] [CrossRef]
- Srikanya, A.; Dhanapal, K.; Sravani, K.; Madhavi, K.; Kumar, G.P. A Study on Optimization of Fish Protein Hydrolysate Preparation by Enzymatic Hydrolysis from Tilapia Fish Waste Mince. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3220–3229. [Google Scholar] [CrossRef]
- Bayón, B.; Berti, I.R.; Gagneten, A.M.; Castro, G.R. Biopolymers from Wastes to High-Value Products in Biomedicine. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 1–44. ISBN 978-981-10-7430-1. [Google Scholar]
- Grand View Research Collagen Market to Reach $19.9Bn by 2030|CAGR 10.2%. Available online: https://www.grandviewresearch.com/press-release/global-collagen-market (accessed on 26 December 2023).
- Fortune Business Insight Dietary Supplements Market Size & Trends|Growth, 2021–2028. Available online: https://www.fortunebusinessinsights.com/dietary-supplements-market-102082 (accessed on 26 December 2023).
- Fortune Business 2025 Marine Collagen Market Size, Trends|Global Report [2032]. Available online: https://www.fortunebusinessinsights.com/marine-collagen-market-102467 (accessed on 22 April 2025).
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Skinglo. Marine Collagen vs Bovine Collagen: Everything You Need to Know. 2023. Available online: https://www.skinglocollagen.com/blog/marine-collagen-vs-bovine-collagen-everything-you-need-to-know/?srsltid=AfmBOopSx0HTzwNWgZe23jmZipNonVXtNhUvxGqcS97KvoZvplqFgsm (accessed on 3 February 2025).
- Carvalho, A.; Marques, A.; Silva, T.; Reis, R. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, Z.; Li, Z.; Zhang, C.; Zhang, D. The Characterization of Acid and Pepsin Soluble Collagen from Ovine Bones (Ujumuqin sheep). J. Integr. Agric. 2018, 17, 704–711. [Google Scholar] [CrossRef]
- Friess, W. Collagen—Biomaterial for Drug delivery1Dedicated to Professor Dr. Eberhard Nürnberg, Friedrich-Alexander-Universität Erlangen-Nürnberg, on the Occasion of His 70th Birthday.1. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Gao, L.; Orth, P.; Cucchiarini, M.; Madry, H. Effects of Solid Acellular Type-I/III Collagen Biomaterials on in Vitro and in Vivo Chondrogenesis of Mesenchymal Stem Cells. Expert Rev. Med. Devices 2017, 14, 717–732. [Google Scholar] [CrossRef]
- RCSB RCSB PDB. Available online: https://www.rcsb.org/structure/7cwk (accessed on 31 January 2024).
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema Esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of Marine By-Products for the Recovery of Value-Added Products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Matarsim, N.N.; Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Type I Collagen from the Skin of Barracuda (Sphyraena sp.) Prepared with Different Organic Acids: Biochemical, Microstructural and Functional Properties. J. Funct. Biomater. 2023, 14, 87. [Google Scholar] [CrossRef]
- Ampitiya, A.G.D.M.; Gonapinuwala, S.T.; Fernando, C.A.N.; De Croos, M.D.S.T. Extraction and Characterisation of Type I Collagen from the Skin Offcuts Generated at the Commercial Fish Processing Centres. J. Food Sci. Technol. 2023, 60, 484–493. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Chun, B.-S. Characterization of Marine Derived Collagen Extracted from the By-Products of Bigeye Tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, K.; Tsubaki, K.; Hirata, M.; Yamamoto, K.; Biswas, A.; Moriyama, T.; Kawamura, Y. Isolation and Characterization of Acid-Soluble Bluefin Tuna (Thunnus orientalis) Skin Collagen. Fish. Aquat. Sci. 2018, 21, 7. [Google Scholar] [CrossRef]
- Yemisken, E.; Jiménez-Rosado, M.; Perez-Puyana, V.; Sancar, S.; Bektaş, S.; Yildiz, T.; Eryilmaz, L.; Romero, A. Alternative Sources of Marine Bioactive Compounds from the Black Sea: Isolation and Characterization of Fish Skin Collagen from Neogobius Melanostomus (Pallas 1814) (Perciformes: Gobiidae). Reg. Stud. Mar. Sci. 2023, 60, 102887. [Google Scholar] [CrossRef]
- Hukmi, N.M.M.; Sarbon, N. Isolation and Characterization of Acid Soluble Collagen (ASC) and Pepsin Soluble Collagen (PSC) Extracted from Silver Catfish (Pangasius sp.) Skin. Int. Food Res. J. 2018, 25, 2601–2607. [Google Scholar]
- Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Extraction and Characterization of Type I Collagen from Parrotfish (Scarus sordidus Forsskål, 1775) Scale Solubilized with the Aid of Acetic Acid and Pepsin. Int. J. Biomater. 2023, 2023, 7312447. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ge, B.; Wei, M.; Elango, J.; Wu, W. Isolation and Biochemical Properties of Type II Collagen from Blue Shark (Prionace glauca) Cartilage. Mar. Drugs 2023, 21, 260. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, L.; Sun, W.; Wang, Z.; Xu, J.; Ma, H. Isolation and Characterization of Collagen from the Cartilage of Amur Sturgeon (Acipenser schrenckii). Process. Biochem. 2014, 49, 318–323. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, Z. Physicochemical, Structural and Antioxidant Properties of Collagens from the Swim Bladder of Four Fish Species. Mar. Drugs 2022, 20, 550. [Google Scholar] [CrossRef]
- Abbas, A.A.; Shakir, K.A.; Walsh, M.K. Functional Properties of Collagen Extracted from Catfish (Silurus triostegus) Waste. Foods 2022, 11, 633. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia Reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef]
- Qi, H.; Li, N.; Zhao, X.; Xu, Z.; Qi, L. Physicochemical Properties and the Radical Scavenging Capacities of Pepsin-Solubilized Collagen from the Body Wall of Starfish (Asterina pectinifera). J. Aquat. Food Prod. Technol. 2017, 26, 376–389. [Google Scholar] [CrossRef]
- Han, S.-B.; Won, B.; Yang, S.; Kim, D.-H. Asterias Pectinifera Derived Collagen Peptide-Encapsulating Elastic Nanoliposomes for the Cosmetic Application. J. Ind. Eng. Chem. 2021, 98, 289–297. [Google Scholar] [CrossRef]
- Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Ko, W.-C.; Jung, C.-C.; Le Huynh, D.T.; Ji, Y.-X. Extraction and Characterization of Collagen from Sea Cucumber (Holothuria cinerascens) and Its Potential Application in Moisturizing Cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The Wound Healing Potential of Collagen Peptides Derived from the Jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Chiarelli, P.G.; Pegg, R.B.; Dev Kumar, G.; Mis Solval, K. Exploring the Feasibility of Developing Novel Gelatin Powders from Salted, Dried Cannonball Jellyfish (Stomolophus meleagris). Food Biosci. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Rastian, Z.; Pütz, S.; Wang, Y.; Kumar, S.; Fleissner, F.; Weidner, T.; Parekh, S.H. Type I Collagen from Jellyfish Catostylus mosaicus for Biomaterial Applications. ACS Biomater. Sci. Eng. 2018, 4, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, N.; González, G.; Troncoso, E.; Zúñiga, R.N. Acid and Enzyme-Aided Collagen Extraction from the Byssus of Chilean Mussels (Mytilus chilensis): Effect of Process Parameters on Extraction Performance. Food Biophys. 2014, 9, 322–331. [Google Scholar] [CrossRef]
- Hiransuchalert, R.; Oonwiset, N.; Imarom, Y.; Chindudsadeegul, P.; Laongmanee, P.; Arnupapboon, S. Extraction and Characterization of Pepsin-Soluble Collagen from Different Mantis Shrimp Species. Fish. Aquat. Sci. 2021, 24, 406–414. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of Food Waste, Fish Waste and Food Processing Waste for China’s Aquaculture Industry: Needs and Challenge. Sci. Total Environ. 2018, 613–614, 635–643. [Google Scholar] [CrossRef]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, Optimization and Characterization of Collagen from Sole Fish Skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Jaziri, A.A.; Shapawi, R.; Mohd Mokhtar, R.A.; Md Noordin, W.N.; Huda, N. Biochemical Analysis of Collagens from the Bone of Lizardfish (Saurida tumbil Bloch, 1795) Extracted with Different Acids. PeerJ 2022, 10, e13103. [Google Scholar] [CrossRef] [PubMed]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of Collagen Derived From Tropical Freshwater Carp Fish Scale Wastes and Its Amino Acid Sequence. Nat. Prod. Commun. 2019, 14, 1934578X1986628. [Google Scholar] [CrossRef]
- Cao, C.; Wang, H.; Zhang, J.; Kan, H.; Liu, Y.; Guo, L.; Tong, H.; Wu, Y.; Ge, C. Effects of Extraction Methods on the Characteristics, Physicochemical Properties and Sensory Quality of Collagen from Spent-Hens Bones. Foods 2023, 12, 202. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C. Isolation and Characterization of Collagen Extracted from Channel Catfish (Ictalurus punctatus) Skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern Pike (Esox lucius) Collagen: Extraction, Characterization and Potential Application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- He, G.; Yin, Y.; Yan, X.; Wang, Y. Semi-Bionic Extraction of Effective Ingredient from Fishbone by High Intensity Pulsed Electric Fields. J. Food Process. Eng. 2017, 40, e12392. [Google Scholar] [CrossRef]
- Bekhit, A.S.; Saeedi, P.; Alaa El-Din, A. Pulsed Electric Field Processing. In Innovative Technologies in Seafood Processing; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-32755-1. [Google Scholar]
- Tassara, E.; Orel, B.; Ilan, M.; Cavallo, D.; Dodero, A.; Castellano, M.; Vicini, S.; Giovine, M.; Pozzolini, M. Seasonal Molecular Difference in Fibrillar Collagen Extracts Derived from the Marine Sponge Chondrosia Reniformis (Nardo, 1847) and Their Impact on Its Derived Biomaterials. Mar. Drugs 2023, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and Characterization of Collagen from the Outer Skin of Squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Suresh, P.V.; Kudre, T.G.; Johny, L.C. Sustainable Valorization of Seafood Processing By-Product/Discard. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 111–139. ISBN 978-981-10-7431-8. [Google Scholar]
- Li, L.; Yu, Y.; Wu, W.; Wang, P. Extraction, Characterization and Osteogenic Activity of a Type I Collagen from Starfish (Asterias amurensis). Mar. Drugs 2023, 21, 274. [Google Scholar] [CrossRef]
- Vate, N.K.; Strachowski, P.; Undeland, I.; Abdollahi, M. Structural and Functional Properties of Collagen Isolated from Lumpfish and Starfish Using Isoelectric Precipitation vs Salting Out. Food Chem. X 2023, 18, 100646. [Google Scholar] [CrossRef]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and Its Derivatives: From Structure and Properties to Their Applications in Food Industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.; Benjakul, S.; Karim, A.A. Isolation and Characterisation of Collagen from the Ribbon Jellyfish (Chrysaora Sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. Collagen Fibers in Crocodile Skin and Teeth: A Morphological Comparison Using Light and Scanning Electron Microscopy. J Bionic Eng 2020, 17, 669–676. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen Extraction Process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Sulaiman, A.W.; Sarbon, N.M. Characterization of Acid Soluble Collagen (ASC) and Pepsin Soluble Collagen (PSC) Extracted from Shortfin Scad (Decapterus macrosoma) Waste. Food Res. 2020, 4, 2272–2280. [Google Scholar] [CrossRef]
- Li, L.-Y.; Zhao, Y.-Q.; He, Y.; Chi, C.-F.; Wang, B. Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Scales of Miiuy Croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 394. [Google Scholar] [CrossRef]
- Wei, P.; Zheng, H.; Shi, Z.; Li, D.; Xiang, Y. Isolation and Characterization of Acid-Soluble Collagen and Pepsin-Soluble Collagen from the Skin of Hybrid Sturgeon. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2019, 34, 950–959. [Google Scholar] [CrossRef]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of Alkaline Pretreatments and Acid Extraction Conditions on the Acid-Soluble Collagen from Grass Carp (Ctenopharyngodon idella) Skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef]
- Hadfi, N.H.; Sarbon, N.M. Physicochemical Properties of Silver Catfish (Pangasius Sp.) Skin Collagen Asinfluenced by Acetic Acid Concentration. Food Res. 2019, 3, 783–790. [Google Scholar] [CrossRef]
- Josephin, A.; Intan, N.; Basari, B.; Rahman, S.F. Extraction of Collagen and Hydroxyapatite from Fish for Bone Scaffold: A Review. In Proceedings of the 7th Biomedical Engineering’s Recent Progress in Biomaterials, Drugs Development, and Medical Devices: The 15th Asian Congress on Biotechnology in Conjunction with the 7th International Symposium on Biomedical Engineering (ACB-ISBE 2022), Bali, Indonesia, 2–6 October 2022; p. 090006. [Google Scholar]
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Dai, M.; Liu, X.; Wang, N.; Sun, J. Squid Type II Collagen as a Novel Biomaterial: Isolation, Characterization, Immunogenicity and Relieving Effect on Degenerative Osteoarthritis via Inhibiting STAT1 Signaling in pro-Inflammatory Macrophages. Mater. Sci. Eng. C 2018, 89, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Iswariya, S.; Velswamy, P.; Uma, T.S. Isolation and Characterization of Biocompatible Collagen from the Skin of Puffer Fish (Lagocephalus inermis). J. Polym. Env. 2018, 26, 2086–2095. [Google Scholar] [CrossRef]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Zhang, X.; Ookawa, M.; Tan, Y.; Ura, K.; Adachi, S.; Takagi, Y. Biochemical Characterisation and Assessment of Fibril-Forming Ability of Collagens Extracted from Bester Sturgeon Huso huso × Acipenser ruthenus. Food Chem. 2014, 160, 305–312. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Y.; Jin, H.; Zhao, Q.; Liu, C.; Li, R.; Yu, F.; Chen, Y.; Huang, F.; Yang, Z.; et al. Collagen Extracted from Bigeye Tuna (Thunnus obesus) Skin by Isoelectric Precipitation: Physicochemical Properties, Proliferation, and Migration Activities. Mar. Drugs 2019, 17, 261. [Google Scholar] [CrossRef]
- Ran, X.-G.; Wang, L.-Y. Use of Ultrasonic and Pepsin Treatment in Tandem for Collagen Extraction from Meat Industry By-Products: Ultrasound and Pepsin to Extract Collagen from Meat by-Products. J. Sci. Food Agric. 2014, 94, 585–590. [Google Scholar] [CrossRef]
- Yang, H.; Shu, Z. The Extraction of Collagen Protein from Pigskin. J. Chem. Pharm. Res. 2014, 6, 683–687. [Google Scholar]
- Isnaini, N.; Prajaputra, V.; Luthfanna, S.K.; Sari, I. An Update Review: Several Extraction Methods for Collagen Isolation in Vertebrate Fish. BIO Web Conf. 2024, 87, 03019. [Google Scholar] [CrossRef]
- Sukeri, N.; Sampath Kumar, N.S.; Shaik, M.I.; Sarbon, N.M. Extractability and Physicochemical Properties of Cobia (Rachycentron canadum) Skin Collagen as Influenced by Lactic Acid Concentration. J. Food Process. Preserv. 2021, 45, e15080. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.; Chen, T.; Wang, Z.; Xu, J.; Ma, H. Characterization of Collagen from the Skin of Amur Sturgeon (Acipenser schrenckii). Food Hydrocoll. 2014, 38, 104–109. [Google Scholar] [CrossRef]
- Prajaputra, V.; Isnaini, N.; Maryam, S.; Ernawati, E.; Deliana, F.; Haridhi, H.A.; Fadli, N.; Karina, S.; Agustina, S.; Nurfadillah, N.; et al. Exploring Marine Collagen: Sustainable Sourcing, Extraction Methods, and Cosmetic Applications. South Afr. J. Chem. Eng. 2024, 47, 197–211. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Song, L.; Farag, M.A. Maximizing Crustaceans (Shrimp, Crab, and Lobster) by-Products Value for Optimum Valorization Practices: A Comparative Review of Their Active Ingredients, Extraction, Bioprocesses and Applications. J. Adv. Res. 2023, 57, 59–76. [Google Scholar] [CrossRef]
- Delgado, L.M.; Shologu, N.; Fuller, K.; Zeugolis, D.I. Acetic Acid and Pepsin Result in High Yield, High Purity and Low Macrophage Response Collagen for Biomedical Applications. Biomed. Mater. 2017, 12, 065009. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Improved Collagen Extraction from Jellyfish (Acromitus hardenbergi) with Increased Physical-Induced Solubilization Processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-X.; Xu, H.-P.; Li, Y.; Zhang, Q.-W.; Xie, H. Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina molpadioides Obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef]
- Liu, Z.; Tuo, F.; Song, L.; Liu, Y.; Dong, X.; Li, D.; Zhou, D.; Shahidi, F. Action of Trypsin on Structural Changes of Collagen Fibres from Sea Cucumber (Stichopus japonicus). Food Chem. 2018, 256, 113–118. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and Characterization of Collagen from the Body Wall of Sea Cucumber Stichopus monotuberculatus. J. Food Sci. 2015, 80, C671–C679. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Chang, X.-N.; Bao, S.-S.; Song, L.; Zhu, B.-W.; Dong, X.-P.; Zong, Y.; Li, D.-M.; Zhang, M.-M.; Liu, Y.-X.; et al. Purification and Partial Characterisation of a Cathepsin L-like Proteinase from Sea Cucumber (Stichopus japonicus) and Its Tissue Distribution in Body Wall. Food Chem. 2014, 158, 192–199. [Google Scholar] [CrossRef]
- Yan, L.-J.; Zhan, C.-L.; Cai, Q.-F.; Weng, L.; Du, C.-H.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification, Characterization, cDNA Cloning and In Vitro Expression of a Serine Proteinase from the Intestinal Tract of Sea Cucumber (Stichopus japonicus) with Collagen Degradation Activity. J. Agric. Food Chem. 2014, 62, 4769–4777. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound Applications for the Extraction, Identification and Delivery of Food Proteins and Bioactive Peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Quarato, C.M.I.; Lacedonia, D.; Salvemini, M.; Tuccari, G.; Mastrodonato, G.; Villani, R.; Fiore, L.A.; Scioscia, G.; Mirijello, A.; Saponara, A.; et al. A Review on Biological Effects of Ultrasounds: Key Messages for Clinicians. Diagnostics 2023, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.I.; Chong, J.Y.; Sarbon, N.M. Effect of Ultrasound-Assisted Extraction on the Extractability and Physicochemical Properties of Acid and Pepsin Soluble Collagen Derived from Sharpnose Stingray (Dasyatis zugei) Skin. Biocatal. Agric. Biotechnol. 2021, 38, 102218. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Kishimura, H.; Benjakul, S. Extraction Efficiency and Characteristics of Acid and Pepsin Soluble Collagens from the Skin of Golden Carp (Probarbus jullieni) as Affected by Ultrasonication. Process. Biochem. 2018, 66, 237–244. [Google Scholar] [CrossRef]
- Pezeshk, S.; Rezaei, M.; Abdollahi, M. Impact of Ultrasound on Extractability of Native Collagen from Tuna By-Product and Its Ultrastructure and Physicochemical Attributes. Ultrason. Sonochem. 2022, 89, 106129. [Google Scholar] [CrossRef]
- Song, K.-M.; Jung, S.K.; Kim, Y.H.; Kim, Y.E.; Lee, N.H. Development of Industrial Ultrasound System for Mass Production of Collagen and Biochemical Characteristics of Extracted Collagen. Food Bioprod. Process. 2018, 110, 96–103. [Google Scholar] [CrossRef]
- Lee, J.E.; Noh, S.-K.; Kim, M.J. Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin. Antioxidants 2022, 11, 2112. [Google Scholar] [CrossRef]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of Fish Collagen in Tissue Regeneration and Drug Delivery. Eng. Regen. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- Noor, N.Q.I.M.; Razali, R.S.; Ismail, N.K.; Ramli, R.A.; Razali, U.H.M.; Bahauddin, A.R.; Zaharudin, N.; Rozzamri, A.; Bakar, J.; Shaarani, S.M. Application of Green Technology in Gelatin Extraction: A Review. Processes 2021, 9, 2227. [Google Scholar] [CrossRef]
- Liu, T.; Dai, H.; Ma, L.; Yu, Y.; Tang, M.; Li, Y.; Hu, W.; Feng, X.; Zhang, Y. Structure of Hyla Rabbit Skin Gelatin as Affected by Microwave-Assisted Extraction. Int. J. Food Prop. 2019, 22, 1594–1607. [Google Scholar] [CrossRef]
- Sultana, S.; Ali, M.E.; Ahamad, M.N.U. Gelatine, Collagen, and Single Cell Proteins as a Natural and Newly Emerging Food Ingredients. In Preparation and Processing of Religious and Cultural Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 215–239. ISBN 978-0-08-101892-7. [Google Scholar]
- Charoenchokpanich, W.; Muangrod, P.; Roytrakul, S.; Rungsardthong, V.; Vatanyoopaisarn, S.; Wonganu, B.; Thumthanaruk, B. Influence of Extraction Times on Physical and Functional Properties of Gelatin from Salted Jellyfish By-Products. E3S Web Conf. 2022, 355, 02014. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Liu, L.; Cui, W.; Regenstein, J.M. The Functional Properties and Application of Gelatin Derived from the Skin of Channel Catfish (Ictalurus punctatus). Food Chem. 2018, 239, 464–469. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Nasri, M. Protein Hydrolysates and Biopeptides. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 81, pp. 109–159. ISBN 978-0-12-811916-7. [Google Scholar]
- Chiarelli, P.G.; Chen, J.; Pegg, R.B.; Mis Solval, K. Demineralization Enhances the Physicochemical Properties of Hydrolyzed Collagen Powders Derived from Cannonball Jellyfish (Stomolophus meleagris). Food Biosci. 2023, 56, 103183. [Google Scholar] [CrossRef]
- Lueyot, A.; Wonganu, B.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wongsa-Ngasri, P.; Roytrakul, S.; Charoenlappanit, S.; Wu, T.; Thumthanaruk, B. Improved Jellyfish Gelatin Quality through Ultrasound-Assisted Salt Removal and an Extraction Process. PLoS ONE 2022, 17, e0276080. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Wijesekara, W.L.I.; Perera, P.R.D.; Senanayake, S.A.; Pathmalal, M.M.; Marapana, R.A.U.J. Nutritional Value and Potential Applications of Jellyfish. J. Aquat. Food Prod. Technol. 2022, 31, 445–482. [Google Scholar] [CrossRef]
- Wang, X.; Le, B.; Zhang, N.; Bak, K.H.; Zhang, Y.; Fu, Y. Off-flavour Compounds in Collagen Peptides from Fish: Formation, Detection and Removal. Int. J. Food Sci. Technol. 2023, 58, 1543–1563. [Google Scholar] [CrossRef]
- Dehnad, D.; Jafari, S.M.; Afrasiabi, M. Influence of Drying on Functional Properties of Food Biopolymers: From Traditional to Novel Dehydration Techniques. Trends Food Sci. Technol. 2016, 57, 116–131. [Google Scholar] [CrossRef]
- Kanwate, B.W.; Ballari, R.V.; Kudre, T.G. Influence of Spray-Drying, Freeze-Drying and Vacuum-Drying on Physicochemical and Functional Properties of Gelatin from Labeo Rohita Swim Bladder. Int. J. Biol. Macromol. 2019, 121, 135–141. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and Gel Properties of Gelatin from Goat Skin as Affected by Pretreatments Using Sodium Sulfate and Hydrogen Peroxide. J. Sci. Food Agric. 2016, 96, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Rasli, H.I.; Sarbon, N.M. Effects of Different Drying Methods on the Rheological, Functional and Structural Properties of Chicken Skin Gelatin Compared to Bovine Gelatin. Int. Food Res. J. 2015, 22, 584. [Google Scholar]
- Da Silva Araújo, C.; Pino-Hernández, E.; Souza Batista, J.T.; Sarkis Peixoto Joele, M.R.; De Arimateia Rodrigues Do Rego, J.; Henriques Lourenço, L.D.F. Optimization of Fish Gelatin Drying Processes and Characterization of Its Properties. Sci. Rep. 2021, 11, 20655. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Lou, Y.-Y.; Li, T.-H.; Liu, B.-Z.; Chen, K.; Zhang, D.; Li, T. Cross-Linking Methods of Type I Collagen-Based Scaffolds for Cartilage Tissue Engineering. Am. J. Transl. Res. 2022, 14, 1146–1159. [Google Scholar]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV Irradiation as a Binding Site Conserving Method for Crosslinking Collagen-Based Scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Q.; Zhou, A.; Yang, S. Research Progress of Collagen-based Three-dimensional Porous Scaffolds Used in Skin Tissue Engineering. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2015, 32, 924–928. [Google Scholar]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of Skeletal System with Genipin Crosslinked Biomaterials. J. Tissue Eng. 2020, 11, 204173142097486. [Google Scholar] [CrossRef]
- Chiarelli, P.G.; Fair, C.G.; Pegg, R.B.; Mis Solval, K. Modifying and Improving the Bloom Strength and Rheological Properties of Jellyfish Gelatin. Food Hydrocoll. 2025, 159, 110692. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gopinath, A.; Madhan, B.; Shanmugam, G. Vibrational circular dichroism spectroscopy as a probe for the detection of collagen fibril and fibrillation in solution. Biosens. Bioelectron. X 2022, 10, 100108. [Google Scholar] [CrossRef]
- Goldberga, I.; Li, R.; Duer, M.J. Collagen structure–function relationships from solid-state NMR spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Manrique, L.; Moussa, M.S.; Khan, M.T.; Tahboub, K.; Ritchie, R.O.; Asgari, M.; Zimmermann, E.A. Deformation of collagen-based tissues investigated using a systematic review and meta-analysis of synchrotron x-ray scattering studies. Cell Rep. Phys. Sci. 2024, 5, 102212. [Google Scholar] [CrossRef]
- Patterson, J.P.; Xu, Y.; Moradi, M.A.; Sommerdijk, N.A.; Friedrich, H. CryoTEM as an advanced analytical tool for materials chemists. Acc. Chem. Res. 2017, 50, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of Fish Collagen as a Scaffold for Regenerative Medicine. BioMed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Rodsuwan, U.; Thumthanaruk, B.; Kerdchoechuen, O.; Laohakunjit, N. Functional Properties of Type A Gelatin from Jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 507–514. [Google Scholar]
- Muralidharan, N.; Jeya Shakila, R.; Sukumar, D.; Jeyasekaran, G. Skin, Bone and Muscle Collagen Extraction from the Trash Fish, Leather Jacket (Odonus niger) and Their Characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-37291-4. [Google Scholar]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen Extraction and Recent Biological Activities of Collagen Peptides Derived from Sea-Food Waste: A Review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary Conservation of the Antimicrobial Function of Mucus: A First Defence against Infection. npj Biofilms Microbiomes 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- De Rinaldis, G.; Leone, A.; De Domenico, S.; Bosch-Belmar, M.; Slizyte, R.; Milisenda, G.; Santucci, A.; Albano, C.; Piraino, S. Biochemical Characterization of Cassiopea Andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea. Mar. Drugs 2021, 19, 498. [Google Scholar] [CrossRef]
- Del Sol Villalba-Urquidy, B.; Velazquez-Valdez, L.P.; Bracamontes-Picos, S.J.; Del Toro-Sánchez, C.L.; Chan-Higuera, J.E.; Ezquerra-Brauer, J.M. Conversion of Dry-Salted Cannonball Jellyfish (Stomolophus meleagris) Umbrella and Oral Arms to Cornmeal Snacks and Gelatin with Antioxidant Properties. Fishes 2022, 7, 277. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Foo, S.C.; Yau, S.K.; Chan, K.W.; Yusoff, F.M. Polarity Affects the Antioxidant and Antimicrobial Activities of Jellyfish (Acromitus hardenbergi) Extracts. Fish. Aquat. Sci. 2022, 25, 189–201. [Google Scholar] [CrossRef]
- Teng, L.; Wang, X.; Yu, H.; Li, R.; Geng, H.; Xing, R.; Liu, S.; Li, P. Jellyfish Peptide as an Alternative Source of Antioxidant. Antioxidants 2023, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Liu, D. Collagen Peptides and the Related Synthetic Peptides: A Review on Improving Skin Health. J. Funct. Foods 2021, 86, 104680. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 Levels—A Randomized, Double-Blind, Placebo-Controlled Trial. Mar. Drugs 2018, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxidative Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A Randomized, Triple-blind, Placebo-controlled, Parallel Study to Evaluate the Efficacy of a Freshwater Marine Collagen on Skin Wrinkles and Elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Meng, K.; Chen, L.; Xia, G.; Shen, X. Effects of Zinc Sulfate and Zinc Lactate on the Properties of Tilapia (Oreochromis niloticus) Skin Collagen Peptide Chelate Zinc. Food Chem. 2021, 347, 129043. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Bourdon, B.; Contentin, R.; Cassé, F.; Maspimby, C.; Oddoux, S.; Noël, A.; Legendre, F.; Gruchy, N.; Galéra, P. Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids. Int. J. Mol. Sci. 2021, 22, 580. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the Chondroprotective Effects of Glucosamine and Fish Collagen Peptide on a Rabbit ACLT Model Using Serum Biomarkers. J. Vet. Med. Sci. 2013, 75, 421–429. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M. Role of Gelatine in Foam Dynamics for Enhancing Food Texture, Phase Stability and Molecular Cohesion at Air-Water Interfaces. Food Hydrocoll. 2025, 163, 111139. [Google Scholar] [CrossRef]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline Hydroxylation in Collagen Supports Integrin Binding by Two Distinct Mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef]
- Yesiltas, B.; Gregersen, S.; Lægsgaard, L.; Brinch, M.L.; Olsen, T.H.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C.; García-Moreno, P.J. Emulsifier Peptides Derived from Seaweed, Methanotrophic Bacteria, and Potato Proteins Identified by Quantitative Proteomics and Bioinformatics. Food Chem. 2021, 362, 130217. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Kadharbasha, S.; Bajaj, M.; Das, J.; Chakraborty, T.; Bhat, C.; Banerjee, P. Contribution of Quasifibrillar Properties of Collagen Hydrolysates Towards Lowering of Interface Tension in Emulsion-Based Food Leading to Shelf-Life Enhancement. Food Bioprocess Technol. 2021, 14, 1566–1586. [Google Scholar] [CrossRef]
- Bilek, S.E.; Bayram, S.K. Fruit Juice Drink Production Containing Hydrolyzed Collagen. J. Funct. Foods 2015, 14, 562–569. [Google Scholar] [CrossRef]
- Ibrahim, F.N.; Ismail-Fitry, M.R.; Yusoff, M.M.; Shukri, R. Effects of Fish Collagen Hydrolysate (FCH) as Fat Replacer in the Production of Buffalo Patties. J. Adv. Res. Appl. Sci. Eng. Technol. 2018, 11, 108–117. [Google Scholar]
- Benjakul, S.; Chantakun, K.; Karnjanapratum, S. Impact of Retort Process on Characteristics and Bioactivities of Herbal Soup Based on Hydrolyzed Collagen from Seabass Skin. J. Food Sci. Technol. 2018, 55, 3779–3791. [Google Scholar] [CrossRef]
- Nurul Syahida, S.; Ismail-Fitry, M.R.; Ainun, Z.M.A.; Nur Hanani, Z.A. Effects of Palm Wax on the Physical, Mechanical and Water Barrier Properties of Fish Gelatin Films for Food Packaging Application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Nitsuwat, S.; Zhang, P.; Ng, K.; Fang, Z. Fish Gelatin as an Alternative to Mammalian Gelatin for Food Industry: A Meta-Analysis. LWT 2021, 141, 110899. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.; Joyner (Melito), H.S. Effect of Fish Gelatin-Gum Arabic Interactions on Structural and Functional Properties of Concentrated Emulsions. Food Res. Int. 2017, 102, 1–7. [Google Scholar] [CrossRef]
- Sow, L.C.; Kong, K.; Yang, H. Structural Modification of Fish Gelatin by the Addition of Gellan, κ-Carrageenan, and Salts Mimics the Critical Physicochemical Properties of Pork Gelatin. J. Food Sci. 2018, 83, 1280–1291. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Swedlund, P.J.; Hemar, Y. Physical and Rheological Properties of Fish Gelatin Gel as Influenced by κ-Carrageenan. Food Biosci. 2017, 20, 88–95. [Google Scholar] [CrossRef]
- Devi, A.F.; Buckow, R.; Hemar, Y.; Kasapis, S. Modification of the Structural and Rheological Properties of Whey Protein/Gelatin Mixtures through High Pressure Processing. Food Chem. 2014, 156, 243–249. [Google Scholar] [CrossRef]
- Yan, M.; Li, B.; Zhao, X.; Yi, J. Physicochemical Properties of Gelatin Gels from Walleye Pollock (Theragra chalcogramma) Skin Cross-Linked by Gallic Acid and Rutin. Food Hydrocoll. 2011, 25, 907–914. [Google Scholar] [CrossRef]
- Soutelino, M.E.M.; Rocha, R.D.S.; de Oliveira, B.C.R.; Mársico, E.T.; Silva, A.C.D.O. Technological aspects and health effects of hydrolyzed collagen and application in dairy products. Crit. Rev. in Food Sci. Nutr. 2024, 64, 6120–6128. [Google Scholar] [CrossRef]
- Liu, J.; Shibata, M.; Ma, Q.; Liu, F.; Lu, Q.; Shan, Q.; Hagiwara, T.; Bao, J. Characterization of Fish Collagen from Blue Shark Skin and Its Application for Chitosan- Collagen Composite Coating to Preserve Red Porgy (Pagrus major) Meat. J Food Biochem 2020, 44, e13265. [Google Scholar] [CrossRef]
- Al Hajj, W.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. Hydrolyzed collagen: Exploring its applications in the food and beverage industries and assessing its impact on human health – A comprehensive review. Heliyon 2024, 10, e36433. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Yang, H.; Won, M.; Song, K.B. Characterisation of Jellyfish Protein Films with Added Transglutaminase and Wasabi Extract. Int. J. Food Sci. Tech. 2015, 50, 1683–1689. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Senanayake, S.A.; Wijesekara, W.L.I.; Perera, P.R.D.; Pathmalal, M.M.; Marapana, R.A.U.J. Characterization of Biodegradable Films Prepared from Gelatin Extracted from Jellyfish Acromitus Flagellates Using Hot Water Extraction and Microwave-Assisted Extraction. Food Packag. Shelf Life 2024, 44, 101315. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Yang, H.-J.; Song, K.B. Application of a Puffer Fish Skin Gelatin Film Containing Moringa Oleifera Lam. Leaf Extract to the Packaging of Gouda Cheese. J. Food Sci. Technol. 2016, 53, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Nirmal, N.P.; Chuprom, J. Molecular Characteristics of Collagen Extracted from the Starry Triggerfish Skin and Its Potential in the Development of Biodegradable Packaging Film. RSC Adv. 2016, 6, 33868–33879. [Google Scholar] [CrossRef]
- Ben Slimane, E.; Sadok, S. Collagen from Cartilaginous Fish By-Products for a Potential Application in Bioactive Film Composite. Mar. Drugs 2018, 16, 211. [Google Scholar] [CrossRef]
- Palamutoğlu, R.; Kasnak, C. Antioxidant effect of fish collagen hydrolysate addition to meatballs. Mugla J. Sci. Technol. 2019, 5, 56–61. [Google Scholar] [CrossRef]
- Da Mata Rigoto, J.; Ribeiro, T.H.S.; Stevanato, N.; Sampaio, A.R.; Ruiz, S.P.; Bolanho, B.C. Effect of Açaí Pulp, Cheese Whey, and Hydrolysate Collagen on the Characteristics of Dairy Beverages Containing Probiotic Bacteria. J. Food Process. Eng. 2019, 42, e12953. [Google Scholar] [CrossRef]
- Mei, F.; Liu, J.; Wu, J.; Duan, Z.; Chen, M.; Meng, K.; Chen, S.; Shen, X.; Xia, G.; Zhao, M. Collagen Peptides Isolated from Salmo salar and Tilapia nilotica Skin Accelerate Wound Healing by Altering Cutaneous Microbiome Colonization via Upregulated NOD2 and BD14. J. Agric. Food Chem. 2020, 68, 1621–1633. [Google Scholar] [CrossRef]
- Song, H.; Meng, M.; Cheng, X.; Li, B.; Wang, C. The Effect of Collagen Hydrolysates from Silver Carp (Hypophthalmichthys molitrix) Skin on UV-Induced Photoaging in Mice: Molecular Weight Affects Skin Repair. Food Funct. 2017, 8, 1538–1546. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Song, H.; Li, B. Ingestion of Collagen Hydrolysates Alleviates Skin Chronological Aging in an Aged Mouse Model by Increasing Collagen Synthesis. Food Funct. 2020, 11, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Cryoprotective Effect of Gelatin Hydrolysate from Blacktip Shark Skin on Surimi Subjected to Different Freeze-Thaw Cycles. LWT 2012, 47, 437–442. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Solval, K.M.; Huang, J.; Bechtel, P.J.; Sathivel, S. Effects of Oil Extraction Methods on Physical and Chemical Properties of Red Salmon Oils (Oncorhynchus nerka). J. Am. Oil Chem. Soc. 2011, 88, 1641–1648. [Google Scholar] [CrossRef]
- Wan, Y.; Espinoza Rodezno, L.A.; Solval, K.M.; Li, J.; Sathivel, S. Optimization of Soluble Dietary Fiber Extraction from Defatted Rice Bran Using Response Surface Methodology. J. Food Process. Preserv. 2014, 38, 441–448. [Google Scholar] [CrossRef]
- Hazwani Oslan, S.N.; Shapawi, R.; Mohd Mokhtar, R.A.; Md Noordin, W.N.; Huda, N. Characterization of Acid- and Pepsin-Soluble Collagen Extracted from the Skin of Purple-Spotted Bigeye Snapper. Gels 2022, 8, 665. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.; Li, H.; Liu, J.; Cheng, J.; Qi, X.; Ye, S.; Gong, H.; Zhao, X.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef]
- Rahman, A.; Rehmani, R.; Pirvu, D.G.; Huang, S.M.; Puri, S.; Arcos, M. Unlocking the Therapeutic Potential of Marine Collagen: A Scientific Exploration for Delaying Skin Aging. Mar. Drugs 2024, 22, 159. [Google Scholar] [CrossRef]
- Kalic, T.; Kamath, S.D.; Ruethers, T.; Taki, A.C.; Nugraha, R.; Le, T.T.K.; Humeniuk, P.; Williamson, N.A.; Hira, D.; Rolland, J.M.; et al. Collagen—An Important Fish Allergen for Improved Diagnosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3084–3092.e10. [Google Scholar] [CrossRef]
- León-Campos, M.I.; Claudio-Rizo, J.A.; Rodriguez-Fuentes, N.; Cabrera-Munguía, D.A.; Becerra-Rodriguez, J.J.; Herrera-Guerrero, A.; Soriano-Corral, F. Biocompatible Interpenetrating Polymeric Networks in Hydrogel State Comprised from Jellyfish Collagen and Polyurethane. J. Polym. Res. 2021, 28, 291. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Madalena, D.A.; Vicente, A. Tackling Food Allergens—The Role of Food Processing on Proteins’ Allergenicity. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2023; Volume 106, pp. 317–351. ISBN 978-0-443-19304-0. [Google Scholar]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.; Ohno, S.; Yamaguchi, K. Oral Ingestion of Collagen Hydrolysate Leads to the Transportation of Highly Concentrated Gly-Pro-Hyp and Its Hydrolyzed Form of Pro-Hyp into the Bloodstream and Skin. J. Agric. Food Chem. 2017, 65, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z.; Ndagijimana, M.; Betti, M. Low Molecular Weight Bioactive Peptides Derived from the Enzymatic Hydrolysis of Collagen after Isoelectric Solubilization/Precipitation Process of Turkey by-Products. Poult. Sci. 2014, 93, 2347–2362. [Google Scholar] [CrossRef] [PubMed]
- Musayeva, F.; Özcan, S.; Kaynak, M.S. A Review on Collagen as a Food Supplement. J. Pharm. Technol. 2022, 3, 7–29. [Google Scholar] [CrossRef]
- Wang, S.; Hou, H.; Hou, J.; Tao, Y.; Lu, Y.; Yang, X.; Li, B. Characterization of Acid-Soluble Collagen From Bone of Pacific Cod (Gadus macrocephalus). J. Aquat. Food Prod. Technol. 2013, 22, 407–420. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Robins, S.P.; Tatakis, D.N.; Klaushofer, K.; Paschalis, E.P. Identification of Pyridinoline Trivalent Collagen Cross-Links by Raman Microspectroscopy. Calcif. Tissue Int. 2017, 100, 565–574. [Google Scholar] [CrossRef]
- Amyoony, J.; Gorman, M.; Dabas, T.; Moss, R.; McSweeney, M.B. Consumer Perception of Collagen from Different Sources: An Investigation Using Hedonic Scale and Check All That Apply. J. Food Sci. 2023, 88, 5236–5247. [Google Scholar] [CrossRef]
- Bhagwat, P.K.; Dandge, P.B. Isolation, Characterization and Valorizable Applications of Fish Scale Collagen in Food and Agriculture Industries. Biocatal. Agric. Biotechnol. 2016, 7, 234–240. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Ayseli, M.T. A Systematic Review on Nano-Delivery Systems Enriched with Aromatic Compounds: Flavor, Odor, and Chemical Quality Perspectives in Fish. Food Chem. Adv. 2024, 5, 100750. [Google Scholar] [CrossRef]
| Amino Acid | Bovine | Codfish (Cold Water) | Atlantic Salmon (Cold Water) | Sea Bass Scale (Warm Water) | Jellyfish (R. esculentum) |
|---|---|---|---|---|---|
| Alanine | 102.04 | 91.48 | 115.87 | 133 | 108.6 |
| Arginine | 32.86 | 30.45 | 39 | 52 | 76.9 |
| Aspartic acid | 36.65 | 38.82 | 51.36 | 44 | 68.3 |
| Cysteine | 1.24 | 1.28 | 0.82 | 0 | 2.8 |
| Glutamic acid | 59.43 | 56.08 | 73.82 | 71 | 85.8 |
| Glycine | 296.44 | 266.12 | 344.01 | 327 | 267.9 |
| Histidine | 3.11 | 5.01 | 7.68 | 7 | 5.7 |
| Isoleucine | 6.74 | 5.61 | 10.31 | 11 | 30.5 |
| Leucine | 17.5 | 16.51 | 20.61 | 21 | 41.9 |
| Lysine | 22.2 | 19.62 | 24.79 | 27 | 51.0 |
| Methionine | 7.81 | 15.04 | 16.43 | 15 | 11.6 |
| Phenylalanine | 11.58 | 12.7 | 16.07 | - | 29.6 |
| Proline | 89.89 | 62.69 | 96.82 | 108 | 72.9 |
| Serine | 32.03 | 53.87 | 46.38 | 28 | 44.4 |
| Threonine | 13.2 | 16.89 | 23.18 | 24 | 36.5 |
| Tyrosine | 1.48 | 2.25 | 3 | 5 | 18.3 |
| Valinine | 12.86 | 12.02 | 16.56 | 22 | 38.0 |
| Classification | Name | Part | Collagen Type | Extraction Method | Extraction Yield (%) | References |
|---|---|---|---|---|---|---|
| Alu-alu (Sphyraena sp.) | Skin | I | ASC | 6.7 | [28] | |
| Asian sea bass (Lates calcarifer) | Skin | I | ASC | 59.31 | [29] | |
| Bigeye tuna (Thunnus obesus) | Skin | I | ASC | 13.5 | [30] | |
| Bluefin tuna (Thunnus orientalis) | Skin | I | ASC | 2.1 | [31] | |
| Yellowfin tuna (Thunnus albacares) | Skin | I | ASC | 61.26 | [29] | |
| Vertebrates | Round goby (Neogobius melanostomus) | Skin | I | ASC | 10.0 | [32] |
| Silver catfish (Pangasius sp.) | Skin | I | ASC | 4.2 | [33] | |
| Lizardfish (Saurida tumbil) | Bone | I | ASC | - | [34] | |
| Blue shark (Prionace glauca) | Cartilage | II | PSC | - | [35] | |
| Parrotfish (Scarus sordidus) | Scale | I | ASC | 1.17 | [34] | |
| Amur sturgeon (Acipenser schrenckii) | Cartilage | I & II | ASC, PSC, SSC | 27.04, 55.92, 2.18 | [36] | |
| Grass carp (Ctenopharyngodon idella) | Swim bladder | I | PSC | 38.98 | [37] | |
| Catfish (Silurus triostegus) | Skin | I | ASC, PSC | 23.92 | [38] | |
| Sponge (C. reniformis) | Sponge tissue | IV | Enz | 19.2 | [39] | |
| Starfish (Asterina pectinifera) | Body wall | I | ASC | - | [40] | |
| Starfish (Asterias pectinifera) | Body wall | I | UAE | - | [41] | |
| Sea cucumber (H. cinerascens) | Body wall | I | ASC, PSC | 72.2 | [42] | |
| Jellyfish (Rhopilema esculentum) | Filament | I | PSC | 4.31 | [43] | |
| Invertebrates | Jellyfish (Stomolophus meleagris) | Tissue | I | Enzyme | - | [44] |
| Jellyfish (Catostylus mosaicus) | Tissue | I | ASC | - | [45] | |
| Byssus of Chilean mussels (Mytilus chilensis) | Mussels | I | ASC | 3.86–7.56 | [46] | |
| Mantis shrimp (Miyakella nepa) | Mussels | I | PSC | 0.47 | [47] |
| Extraction Method | Efficiency | Cost | Energy Requirement | Impact on Collagen Structure |
|---|---|---|---|---|
| Salt-Solubilization Extraction (SSE) | Low to moderate; often combined with other methods | Low | Low | Minimal when optimized; low yield |
| Acid Extraction | Moderate to high; depends on acid type and concentration | Moderate | Low | Can reduce molar mass; may enhance gel strength |
| Alkaline Extraction | Moderate; effective for pretreatment of rigid materials | Moderate | Low to moderate | Risk of degradation of specific amino acids at high temp/concentration |
| Enzymatic Extraction | High; specific and controlled hydrolysis | High (enzyme cost) | Low | Preserves native structure; minimal degradation |
| Ultrasound-Assisted Extraction (UAE) | High; enhances mass transfer and extraction yield | Moderate to high (equipment cost) | Moderate | Preserves triple helix with controlled parameters |
| Microwave-Assisted Extraction | High; enhances cell matrix breakdown when combined with enzymes | High (equipment and energy cost) | High | May degrade structure if overheating occurs |
| Collagen Source | Extraction Part | Collagen Forms | Function | Food Products | References |
|---|---|---|---|---|---|
| Fish processing byproducts (Tilapia) | Skin and bones | Peptides | Emulsifier | Butter and chocolate sauce | [152] |
| Fish collagen (commercial) | Skin and bone | Hydrolyzed collagen | Functional ingredient | Fruit juice | [153] |
| Fish collagen (Commercial) | - | Hydrolyzed collagen | Fat replacer | Buffalo patties | [154] |
| Seabass | Skin | Hydrolyzed collagen | Antioxidant | Herbal soup | [155] |
| Tilapia | Skin | Gelatin | Mechanical and water barrier | Food packaging | [156] |
| Jellyfish | Oral arm and umbrella | Gelatin | Ingredient | Cornmeal | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, J.; Mis Solval, K.E. Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications. Mar. Drugs 2025, 23, 190. https://doi.org/10.3390/md23050190
Islam J, Mis Solval KE. Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications. Marine Drugs. 2025; 23(5):190. https://doi.org/10.3390/md23050190
Chicago/Turabian StyleIslam, Joinul, and Kevin E. Mis Solval. 2025. "Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications" Marine Drugs 23, no. 5: 190. https://doi.org/10.3390/md23050190
APA StyleIslam, J., & Mis Solval, K. E. (2025). Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications. Marine Drugs, 23(5), 190. https://doi.org/10.3390/md23050190






