Rational High-Throughput System for Screening Emodin High-Yielding Mutant from Marine Strains of Aspergillus flavipes HN4-13
Abstract
:1. Introduction
2. Results and Discussion
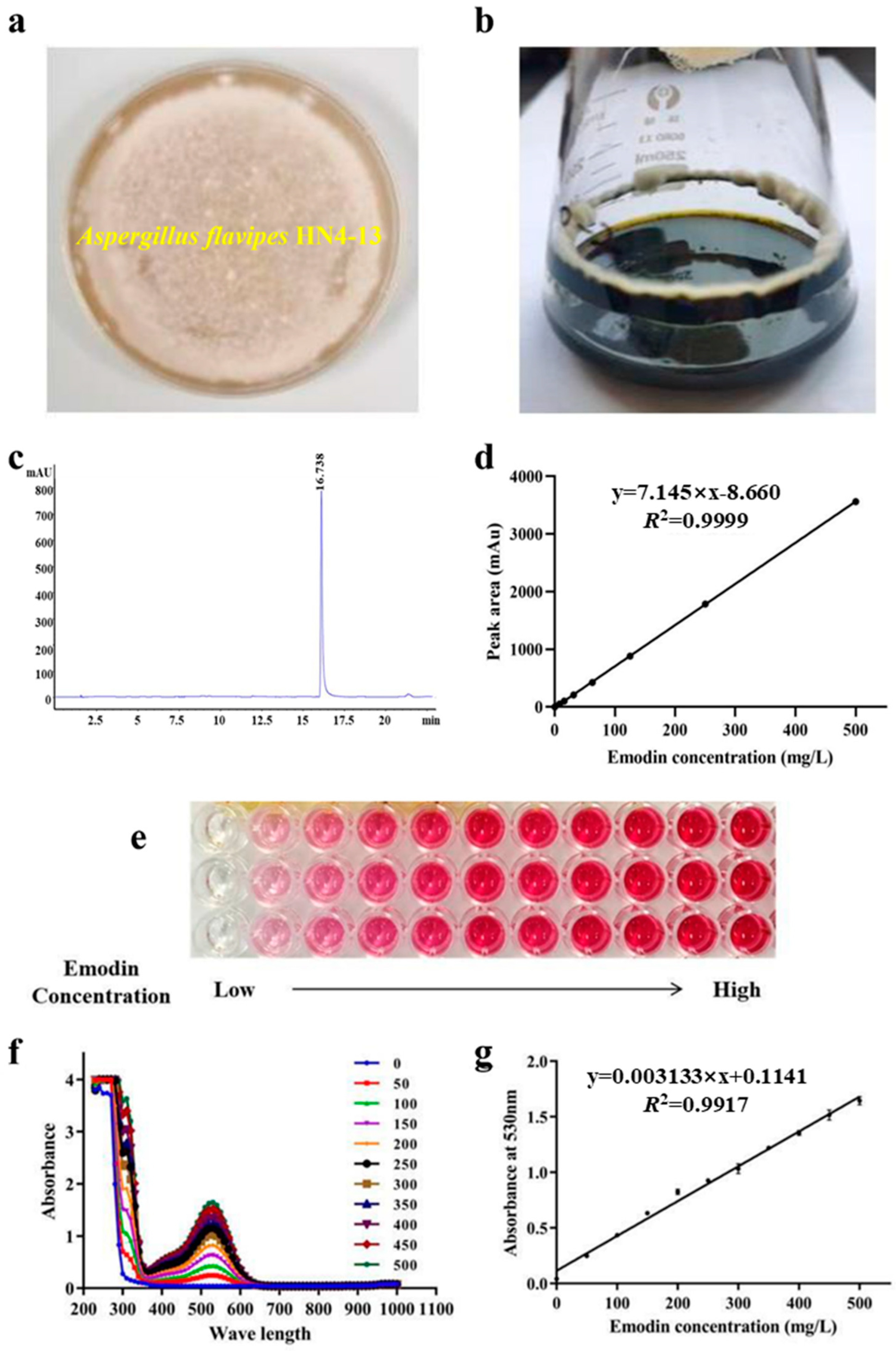
2.1. High-Throughput Detection Emodin Production Method
2.2. Screening the Optimum Fermentation Conditions for Emodin Product Strains
2.3. ARTP Mutagenesis Parameters and High-Through Selection
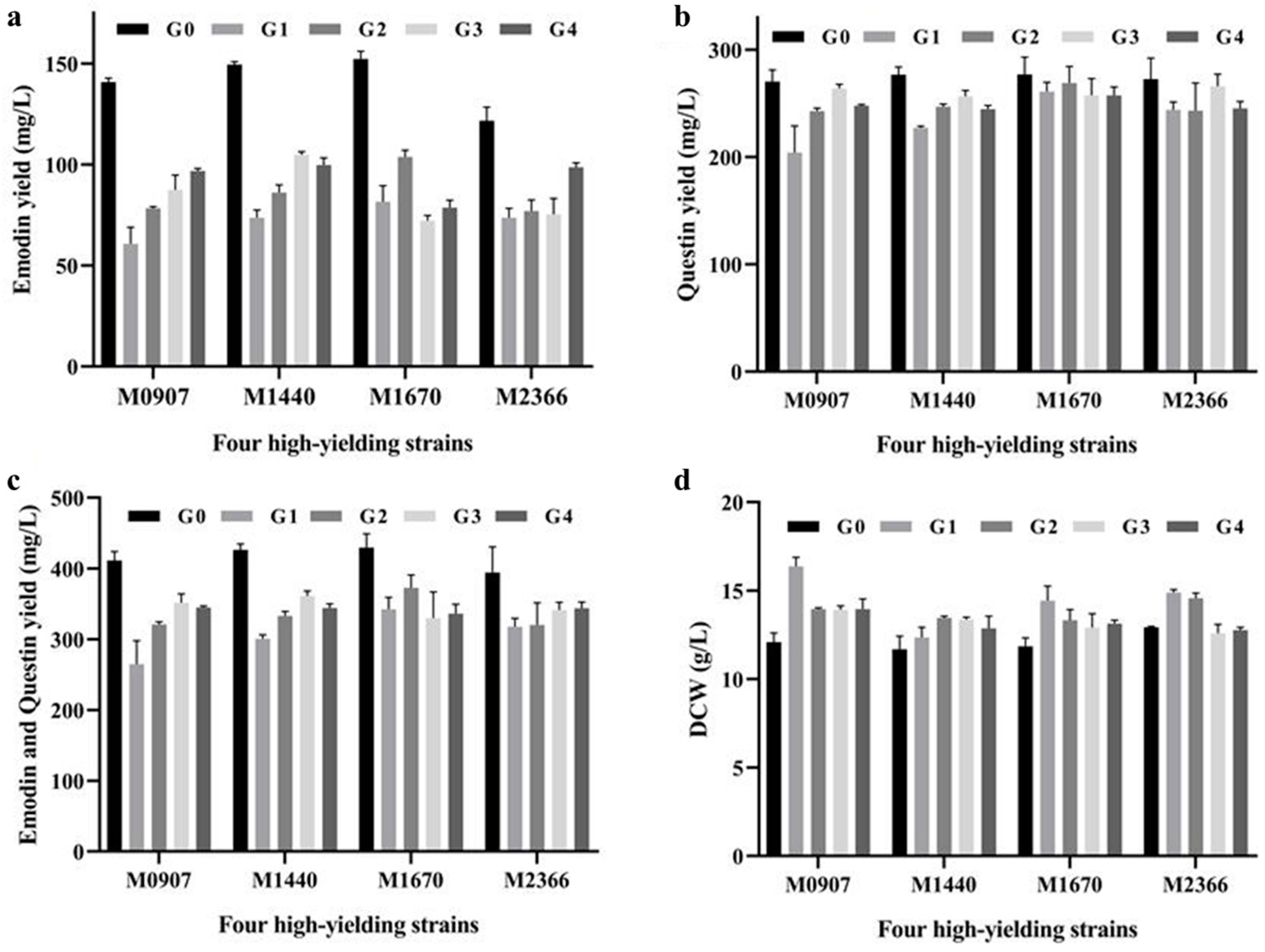
2.4. Genetic Stability Analysis of Four Emodin High-Yielding Mutants
2.5. Morphological Observation of Hyphae
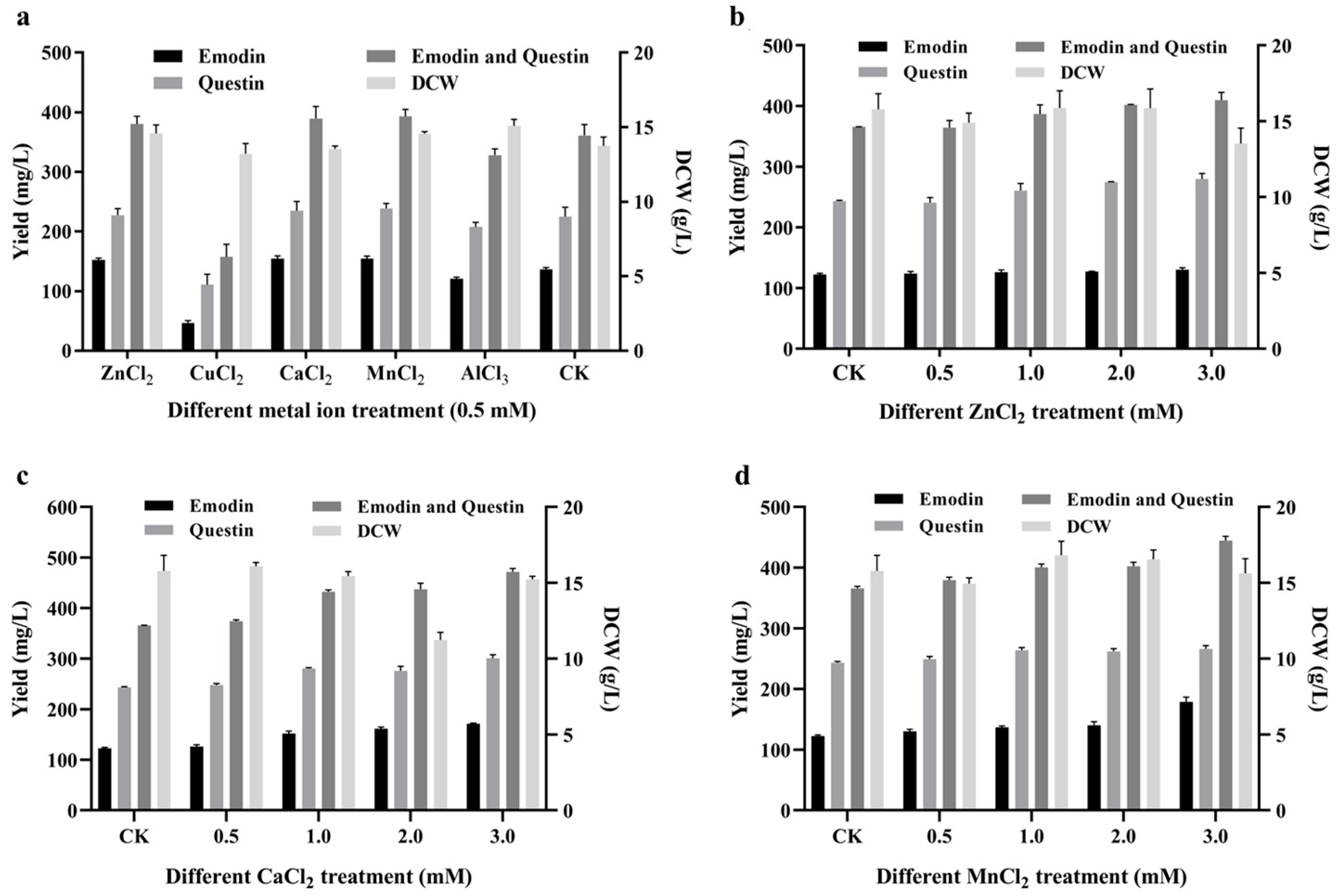
2.6. Effect of Metal Ion Addition on Emodin Production
3. Materials and Methods
3.1. Strain and Culture Conditions
3.2. Fermentation Product Extraction Method and DCW Detection
3.2.1. Emodin Quantification Using HPLC
3.2.2. Extraction Method of Emodin from Fermentation
3.2.3. Biomass Analysis of the Fermentation
3.3. Establishment of Emodin with High-Throughput Detection Method
3.4. Establishment of Emodin-Producing Culture Condition on Well Plate
3.5. ARTP Mutagenesis
3.6. Analysis of Genetic Stability of Emodin High-Yielding Mutants
3.7. Mycelial Appearance
3.8. Exogenous Metal Ion Addition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Emodin—A natural anthraquinone derivative with diverse pharmacological activities. Phytochemistry 2021, 190, 112854. [Google Scholar] [CrossRef] [PubMed]
- Waly, M.I.; Ali, B.H.; Al-Lawati, I.; Nemmar, A. Protective effects of emodin against cisplatin-induced oxidative stress in cultured human kidney (HEK 293) cells. Anal. Sci. J. 2013, 33, 626–630. [Google Scholar] [CrossRef]
- Li, H.L.; Chen, H.L.; Li, H.; Zhang, K.L.; Chen, X.Y.; Wang, X.W.; Kong, Q.Y.; Liu, J. Regulatory effects of emodin on NF-κB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int. J. Mol. Med. 2005, 16, 41–47. [Google Scholar] [CrossRef]
- Andersen, D.O.; Weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir. Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; de Souza, I.C.C.; Brasil, F.B. Mitochondrial Protection and Anti-inflammatory Effects Induced by Emodin in the Human Neuroblastoma SH-SY5Y Cells Exposed to Hydrogen Peroxide: Involvement of the AMPK/Nrf2 Signaling Pathway. Neurochem. Res. 2021, 46, 482–493. [Google Scholar] [CrossRef]
- Sakhri, A.; Chaouche, N.K.; Catania, M.R.; Ritieni, A.; Santini, A. Chemical Composition of Aspergillus creber Extract and Evaluation of its Antimicrobial and Antioxidant Activities. Pol. J. Microbiol. 2019, 68, 309–316. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, K.H.; Jeon, M.S.; Lee, Y.H.; Ko, Y.J.; Pang, C.; Kim, B.; Chung, K.H.; Kim, K.H. Hepatoprotective Potency of Chrysophanol 8-O-Glucoside from Rheum palmatum L. against Hepatic Fibrosis via Regulation of the STAT3 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 9044. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Eder, R.; Widmer, C. Untersuchungen über Derivate des β-Methylanthrachinons. III. Mitteilung. Synthese des Frangula-Emodins. Helv. Chim. Acta 1923, 6, 966–981. [Google Scholar] [CrossRef]
- Jacobson, R.A.; Adams, R. Trihydroxy-methylanthraquinones. III. synthesis of emodin. J. Am. Chem. Soc. 1924, 46, 1312–1316. [Google Scholar] [CrossRef]
- Graves, G.D.; Adams, R. Trihydroxy-methyl-anthraquinones. I. J. Am. Chem. Soc. 1923, 45, 2439–2455. [Google Scholar] [CrossRef]
- Li, X.; Ping, L. The Influence of Short Chain Fatty Acids on Biosynthesis of Emodin by Aspergillus ochraceus LP-316. In Proceedings of the Frontier and Future Development of Information Technology in Medicine and Education; Springer: Dordrecht, The Netherlands, 2014; pp. 2331–2336. [Google Scholar]
- Sun, L.; Liu, G.; Li, Y.; Jiang, D.; Guo, W.; Xu, H.; Zhan, R. Metabolic engineering of Saccharomyces cerevisiae for efficient production of endocrocin and emodin. Metab. Eng. 2019, 54, 212–221. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, X.; Cui, T. Production of emodin from Aspergillus ochraceus at preparative scale. Afr. J. Biotechnol. 2010, 9, 512–517. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Degl’Innocenti, D. Marine Migrastatics: A Comprehensive 2022 Update. Mar. Drugs 2022, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Z.; Xin, X.; An, F. Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells. Mar. Drugs 2024, 22, 227. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Xu, S.; Xin, X.; An, F. Perpyrrospirone A, an unprecedented hirsutellone peroxide from the marine-derived Penicillium citrinum. Chin. Chem. Lett. 2023, 34, 107562. [Google Scholar] [CrossRef]
- Qiu, X.; Gong, L.; Xin, X.; An, F. Enhancement of Emodin Production by Medium Optimization and KH2PO4 Supplementation in Submerged Fermentation of Marine-Derived Aspergillus favipes HN4-13. Mar. Drugs 2021, 19, 421. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wu, Y.; Qiu, X.; Xin, X.; An, F.; Guo, M. Adsorption Characteristics and Enrichment of Emodin from Marine-Derived Aspergillus flavipes HN4-13 Extract by Macroporous Resin XAD-16. Mar. Drugs 2022, 20, 231. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, X.; Jin, W.; Wang, F.; Tu, R.; Han, S.; Chen, H.; Chen, C.; Xie, G.-J.; Ma, F. Improving of lipid productivity of the oleaginous microalgae Chlorella pyrenoidosa via atmospheric and room temperature plasma (ARTP). Bioresour. Technol. 2017, 244, 1400–1406. [Google Scholar] [CrossRef]
- Qiang, W.; Ling-ran, F.; Luo, W.; Han-guang, L.; Lin, W.; Ya, Z.; Xiao-bin, Y. Mutation Breeding of Lycopene-Producing Strain Blakeslea Trispora by a Novel Atmospheric and Room Temperature Plasma (ARTP). Appl. Biochem. Biotechnol. 2014, 174, 452–460. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Ma, K.; Li, G.; Zhang, C.; Zhao, H.; Lai, Q.; Li, H.-P.; Xing, X.-H. Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem. Eng. J. 2011, 55, 17–22. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, Y.; Dabbour, M.; Mintah, B.K.; Zhang, Z.; Dai, C.; He, R.; Huang, G.; Ma, H. Metabolomic and genomic profiles of Streptomyces albulus with a higher ε-polylysine production through ARTP mutagenesis. Biochem. Eng. J. 2020, 162, 107720. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Jin, C.; Qu, Y.; Xiao, X. Development and validation of a UPLC method for quality control of rhubarb-based medicine: Fast simultaneous determination of five anthraquinone derivatives. J. Pharm. Biomed. Anal. 2008, 47, 765–770. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Khorana, M.L. Paper chromatographic separation of sennoside A and sennoside B. J. Chromatogr. A. 1961, 6, 537–538. [Google Scholar] [CrossRef]
- Dell’Arciprete, D.; Blow, M.L.; Brown, A.T.; Farrell, F.D.C.; Lintuvuori, J.S.; McVey, A.F.; Marenduzzo, D.; Poon, W.C.K. A growing bacterial colony in two dimensions as an active nematic. Nat. Commun. 2018, 9, 4190. [Google Scholar] [CrossRef]
- Zhu, X.; Arman, B.; Chu, J.; Wang, Y.; Zhuang, Y. Development of a method for efficient cost-effective screening of Aspergillus niger mutants having increased production of glucoamylase. Biotechnol. Lett. 2017, 39, 739–744. [Google Scholar] [CrossRef]
- An, J.; Shen, W.; Liu, H.; Yang, C.; Chen, K.; Yuan, Q.; Li, Z.; Xiao, D.; Wang, Z.; Lan, X.; et al. Comparison of the effects of rumen-protected and unprotected L-leucine on fermentation parameters, bacterial composition, and amino acids metabolism in in vitro rumen batch cultures. Front. Microbiol. 2023, 14, 1282767. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Q.; Chen, Y.-J.; Song, M.; Zhang, Y.-Y.; Chang, Z.-Y.; Gao, H.-L. Effects of Shear Force on Microbial Transglutaminase Fermentation. Acta Agric. Boreali-Sin. 2008, 23, 126–128. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; He, L.; Li, C.; Tao, H.; Ye, C.; Liu, L.; Zeng, X.; Ran, G. Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt. Fermentation 2022, 8, 489. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Chang, L. Effect of edible rose (Rosa rugosa cv. Plena) flower extract addition on the physicochemical, rheological, functional and sensory properties of set-type yogurt. Food Biosci. 2021, 43, 101249. [Google Scholar] [CrossRef]
- Jiang, Y.; Shang, Y.-P.; Li, H.; Zhang, C.; Pan, J.; Bai, Y.-P.; Li, C.-X.; Xu, J.-H. Enhancing transglutaminase production of Streptomyces mobaraensis by iterative mutagenesis breeding with atmospheric and room-temperature plasma (ARTP). Bioresour. Bioprocess. 2017, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Dong, S.; Yu, J.; Ning, X.; Xu, K.; Zhang, S.-J.; Xu, L.; Li, B.-Z.; Li, J.; Yuan, Y.-J.; et al. Stress-driven dynamic regulation of multiple tolerance genes improves robustness and productive capacity of Saccharomyces cerevisiae in industrial lignocellulose fermentation. Metab. Eng. 2020, 61, 160–170. [Google Scholar] [CrossRef]
- Ji, S.; Yang, H.; Xie, X.; Zhang, Y.; Li, X.; Wang, F. Combined fermentation and ARTP mutagenesis to enhance lipase activity of Penicillium camembertii and its application for high-purity 1,3-diacylglycerol preparation. Food Chem. 2025, 465, 142072. [Google Scholar] [CrossRef]
- Salam, N.; Xian, W.-D.; Asem, M.D.; Xiao, M.; Li, W.-J. From ecophysiology to cultivation methodology: Filling the knowledge gap between uncultured and cultured microbes. Mar. Life Sci. Technol. 2021, 3, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Kordjazi, T.; Tancredi, F.; Manini, P.; Lanzillo, F.; Raganati, F.; Marzocchella, A.; Porta, R.; Mariniello, L. Metal Ion Supplementation to Boost Melanin Production by Streptomyces nashvillensis. Int. J. Mol. Sci. 2025, 26, 416. [Google Scholar] [CrossRef]
- Xiao, B.; Hu, Y.; Feng, X.; Sui, Z. Breeding of New Strains of Gracilariopsis lemaneiformis with High Agar Content by ARTP Mutagenesis and High Osmotic Pressure Screening. Mar. Biotechnol. 2023, 25, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, E.; Yin, Y.; Yang, L.; Huang, Q.; Chen, S.; Ho, C.-T. Enhancing Activities of Salt-Tolerant Proteases Secreted by Aspergillus oryzae Using Atmospheric and Room-Temperature Plasma Mutagenesis. J. Agric. Food Chem. 2020, 68, 2757–2764. [Google Scholar] [CrossRef]
- Raza, W.; Yang, X.; Wu, H.; Huang, Q.; Xu, Y.; Shen, Q. Evaluation of metal ions (Zn2+, Fe3+ and Mg2+) effect on the production of fusaricidin-type antifungal compounds by Paenibacillus polymyxa SQR-21. Bioresour. Technol. 2010, 101, 9264–9271. [Google Scholar] [CrossRef]



| Strains | Emodin (mg/L) | Questin (mg/L) | Sum (mg/L) a | DCW (g/L) |
|---|---|---|---|---|
| HN4-13 | 76.6 | 212.9 | 287.5 | 11.3 |
| M0907 | 114.1 | 270.4 | 384.5 | 12.1 |
| M1440 | 124.6 | 276.9 | 401.5 | 11.7 |
| M1670 | 112.2 | 277.1 | 389.3 | 11.9 |
| M2366 | 108.5 | 272.6 | 381.1 | 12.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Li, Z.; Xu, M.; Zhou, Y.; Zhang, W.; Zhao, J.; Xin, X.; An, F. Rational High-Throughput System for Screening Emodin High-Yielding Mutant from Marine Strains of Aspergillus flavipes HN4-13. Mar. Drugs 2025, 23, 174. https://doi.org/10.3390/md23040174
Gong L, Li Z, Xu M, Zhou Y, Zhang W, Zhao J, Xin X, An F. Rational High-Throughput System for Screening Emodin High-Yielding Mutant from Marine Strains of Aspergillus flavipes HN4-13. Marine Drugs. 2025; 23(4):174. https://doi.org/10.3390/md23040174
Chicago/Turabian StyleGong, Lizhi, Zixuan Li, Meina Xu, Yushan Zhou, Wenqing Zhang, Jian Zhao, Xiujuan Xin, and Faliang An. 2025. "Rational High-Throughput System for Screening Emodin High-Yielding Mutant from Marine Strains of Aspergillus flavipes HN4-13" Marine Drugs 23, no. 4: 174. https://doi.org/10.3390/md23040174
APA StyleGong, L., Li, Z., Xu, M., Zhou, Y., Zhang, W., Zhao, J., Xin, X., & An, F. (2025). Rational High-Throughput System for Screening Emodin High-Yielding Mutant from Marine Strains of Aspergillus flavipes HN4-13. Marine Drugs, 23(4), 174. https://doi.org/10.3390/md23040174








