Marine-Derived Bioactive Compounds: A Promising Strategy for Ameliorating Skeletal Muscle Dysfunction in COPD
Abstract
1. Introduction
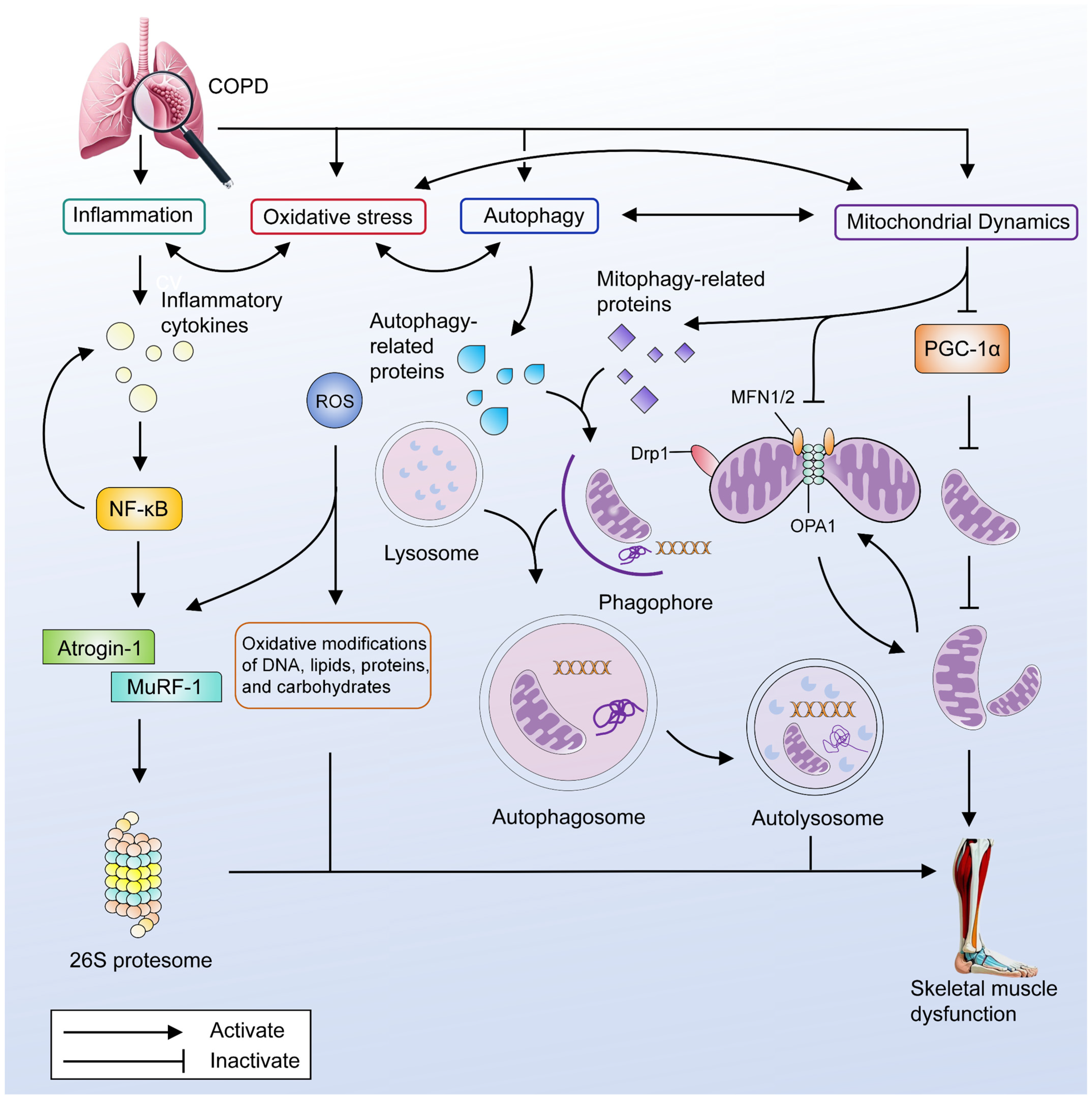
2. COPD Skeletal Muscle Dysfunction
3. The Main Pathogenesis of Skeletal Muscle Dysfunction in COPD
3.1. Inflammation
3.2. Oxidative Stress
3.3. Autophagy
3.4. Mitochondrial Dysfunction
3.4.1. Decreased Mitochondrial Biogenesis
3.4.2. Mitophagy
3.4.3. Mitochondrial Dynamics
4. Potential of Marine-Derived Bioactive Compounds on COPD Skeletal Muscle Dysfunction
4.1. Polysaccharides
4.1.1. Fucoidan
4.1.2. Chitosan

| Ref. | Object, Sample Size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Yang, C. (2024) [52] | C57BL/6J mice (16/16) | Fucoidan | Undaria pinnatifida | Incorporate a non-caloric sweetener for oral use | 400 mg/kg/d | 10 weeks | Running Distance ↑; Muscle mass ↑; COX4 mRNA ↑; MYH1 mRNA ↑; PGC-1α mRNA ↑; PPAR-γ mRNA ↑; IGF-1 mRNA ↑ |
| McFadden, B. A. (2023) [54] | Healthy adult (8/8) | Fucoidan | Undaria pinnatifida | Oral | 1 g/day | 12 days | IL-6 and IL-10 concentrations 30 min post-exercise ↑ |
| McBean, S.E. (2021) [53] | C57BL/6J mice (8/10) | Fucoidan | Undaria pinnatifida and Fucus vesiculosus | Oral gavage | 400 mg/kg/d | 4 weeks | CSA of EDL and soleus fibers ↑; TA force ↑; MHC-2x of gastrocnemius ↑ |
| Chen, Y.M. (2014) [51] | ICR mice (8/8) | Fucoidan | Laminaria japonica | Oral gavage | 310 mg/kg/d 620 mg/kg/d | 21 days | Forelimb Grip Strength ↑; Weight-loaded swimming test time ↑; Serum lactate levels after acute exercise challenge ↓; Serum ammonia levels after acute exercise challenge ↓; Serum glucose levels after acute exercise challenge ↑; Serum levels of total protein ↑; Serum levels of blood urea nitrogen ↓; Serum levels of triacylglycerol ↓ |
| Ha, B.G. (2016) [55] | C2C12 myotube | COS | Commercial sources | - | 2 mg/mL | 1 h | Glucose uptake ↑ |
| Teodoro, J.S (2016) [58] | Wistar rat (ND) | COS | Commercial sources | Feed the water with 0.5% COS | 0.5% (w/w) | 6 weeks | SDH activity ↓; COX activity ↓; ATPSynthase activity ↓ |
| Jeong, H.W. (2012) [56] | Sprague-Dawley rat (12/12)/C2C12 myotube | COS | Commercial sources | Feed the chow with 0.5% (w/w) COS/- | 0.5% (w/w)/10 μg/mL; 100 μg/mL; 500 μg/mL | 6 weeks/ 24 h or 12 h | Mitochondrial content of soleus ↑; PGC1α mRNA ↑; Nrf1 mRNA ↑; CPT1b mRNA ↑; TFAM mRNA ↑/Mitochondrial content ↑; NDFUA9 protein ↑; SDHA protein ↑; UQCRC2 protein ↑; COX1 protein ↑; ATP5a protein ↑ |
| Cho, S.Y. (2010) [57] | BALB/c mice | COS lactate | Commercial sources | Oral | 500 mg/kg | 2 weeks | Immobility time in a forced swimming test ↑; Cortisol ↓; LDH ↓; SOD activity ↑; MDA ↓ Mitochondrial mass ↑; Membrane potential ↑; PGC-1α ↑; Cyt C ↑ |
4.2. Lipids
4.2.1. Long-Chain Omega-3 Polyunsaturated Fatty Acids
4.2.2. Sterols
| Ref. | Object, Sample Size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Engelen M (2024) [63] | Patients with COPD of grade II–IV (16/16) | n-3 LC-PUFAs | ND | Oral | 1.68 g EPA + 522 mg DHA/d | 10 weeks | Lean soft tissue ↑ |
| Fekete M (2022) [61] | Patients with COPD (19/381) | n-3 LC-PUFAs | ND | Oral | 0.5 g/day | 6 months | BMI ↑; CAT ↑; Inhaled short-acting bronchodilators use ↓; Number of exacerbations in the previous half year ↓; 6MWD ↑ |
| Ogasawara T (2018) [106] | Patients with COPD of exacerbation (24/21) | EPA + pulmonary rehabilitation | ND | Oral | 1 g/day | 12 days | Insignificant increase in LBMI and SMI |
| Calder, P C (2018) [97] | COPD patients with cachexia (20/19) | Nutrients rich in n-3 LC-PUFAs, and 25-hydroxy-vitamin D3 + pulmonary rehabilitation | ND | Oral | 200 mL/unit, 2 units/d; ~230 kcal, 10 g whey protein concentrate, 2.0 g DHA + EPA, and 10 μg 25-hydroxy-vitamin D3 per unit | 12 weeks | FM ↑; Dyspnea ↓; Anti-fatigue ↑ |
| van de Bool C (2017) [92] | COPD patients with low muscle mass (38/42) | Nutrients riche leucine, n-3 LC-PUFAs, and vitamin D | ND | Oral | 125 mL/unit, 2~3 units/d, 187.5 kCal in a distribution of 20 energy percent protein, 60 energy percent carbohydrate, and 20 energy percent fat, and is enriched with leucine, n-3 PUFAs, and vitamin D per unit | 4 months | Inspiratory muscle strength ↑ |
4.3. Polyphenols
4.3.1. Diphlorethohydroxycarmalol
4.3.2. Dieckol and 2,7″-phloroglucinol-6,6′-bieckol
4.3.3. Miscellaneous Polyphenols

| Ref. | Object, Sample Size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Ryu B (2022) [65] | ICR mice with skeletal muscle atrophy (10) | DPHC/Ishige okamuraede extract | Ishige okamuraede | Oral gavage | DPHC: 2.41 mg/kg/day Ishige okamuraede extract: 50 mg/kg/d; 100 mg/kg/d; 200 mg/kg/d | 38 days | Grip strength ↑; Time of ladder climbing ↑; Lean mass of calf muscle ↑; Thickness of calf muscle↑; Gastrocnemius thickness ↑; CSA of gastrocnemius fiber ↑; Soleus muscle thickness ↑; Fiber diameter of soleus muscle ↑; MuRF-1 mRNA of gastrocnemius ↓; Atrogin-1 mRNA of gastrocnemius ↓; PI3K mRNA of gastrocnemius ↑; Akt mRNA of gastrocnemius ↑; TRPV4 mRNA of gastrocnemius ↑; A1R mRNA of gastrocnemius ↑; Myostatin mRNA of gastrocnemius ↑ |
| Kim SY (2020) [110] | Inflammatory C2C12 myotube | DPHC | Ishige okamuraede | - | 1.56 μg/mL; 3.125 μg/mL; 6.25 μg/mL; 12.5 μg/mL | 1 h | MuRF-1 protein ↓; Atrogin-1 protein ↓ |
| Kim, S.Y (2021) [66] | C2C12 myotube | DK | Ecklonia cava | - | 5 nM; 10 nM; 20 nM | 24 h | CK activity ↑; p-Smad2/3↓; Smad4 protein ↓; p-Akt protein ↑; p-FoxO protein ↑; MyoD ↑; |
| Kim, S.Y (2021) [66] | C2C12 myotube | PHB | Ecklonia cava | - | 5 nM; 10 nM; 20 nM | 24 h | CK activity ↑; p-Smad2/3 protein ↓; Smad4 protein ↓; p-Akt protein ↑; p-FoxO protein ↑; MyoD ↑ |
| Kwon, I.S. (2021) [115] | Elderly Individuals with sarcopenia (10/10) | MOPs | Brown algae | Oral | One spoon (0.7 g) of Mannas™/d, Mannas™ with 1% MOPs | 4 weeks | SSM ↑; %FFMI ↑; The 2.4 m up and go test ↓ |
4.4. Peptides
4.4.1. Oyster Peptides
4.4.2. Pyropia Yezoensis Peptides
4.4.3. Hippocampus Peptides
| Ref. | Object, Sample size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Lin, S (2024) [117] | Kunming mice (10/10) | Oyster peptides | Crassostrea plicatula Gmelin | Gavage | 0.4 mg/(g·d) | 14 days | Swimming time ↑; Liver glycogen content ↑; BUN ↓; LDH ↓; lactic acid ↓; AMPK ↑; PGC-1α ↑; HO-1↑ |
| Xiao, M. (2020) [67] | Kunming mice (20/20) | Oyster polypeptides | Oyster | Gavage | 200 mg/kg/d; 400 mg/kg/d; 600 mg/kg/d | 4 weeks | Swimming time ↑; lactic acid ↓; BUN ↓; SOD activity ↑; GSH-Px activity ↑; PEPCK mRNA ↑; AMPK mRNA ↑ |
| Lee MK (2019) [119] | Atrophic C2C12 myotubes | Pyropia yezoensis peptide | Pyropia yezoensis Ueda | - | 500 ng/mL | 24 h | p-IGF-IR ↑; p-IRS-1 ↑; p-Akt ↑; p-mTOR ↑; Raptor protein ↑; p70S6K ↑; p-4EBP1 ↑; p-p70S6K ↑; p-S6 ↑; eIF4E ↑; FoxO3a ↓; FoxO1 ↓; p-FoxO3a ↑; p-FoxO1 ↑; conversion of LC3-I to LC3- II ↓; cathepsin-L ↓ |
| Lee MK (2017) [68] | Atrophic C2C12 myotubes | Pyropia yezoensis peptide | Synthesis | - | 500 ng/mL | 24 h | Myotubes diameter ↑; MuRF-1 mRNA ↓; MuRF-1 protein ↓; Atrogin-1 mRNA ↓; Atrogin-1 protein ↓; 20S proteasome activity ↓ |
| Guo, Z [69] | ICR mice (20/20) | Hippocampus peptides | Hippocampus | Gavage | 0.15 mg/g/d; 0.5 mg/g/d; 1.5 mg/g/d | 4 weeks | Exhausted swimming time ↑; Concentration of hepatic glycogen ↑; blood lactate ↓; BUN ↓ |
4.5. Carotenoids
4.5.1. Astaxanthin
4.5.2. Fucoxanthin
4.5.3. β-Carotene
4.5.4. β-Cryptoxanthin
4.5.5. Lutein and Zeaxanthin
| Ref. | Object, Sample Size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Yue, H (2025) [72] | Skeletal muscle atrophy Male C57Bl/6J mice (8) | AST | Haematococcus pluvialis | Intraperitoneal injection | 30 mg/kg/d, 60 mg/kg/d, 120 mg/kg/d | 4 weeks | Body weight ↑; Time and distance exercise fatigue experiment ↑; Forelimb grip strength ↑; Hanging time ↑; Skeletal muscle mass ↑; CSA of myofibres ↑; MuRF-1 protein ↓; MyoD1 ↑ |
| Yu X (2024) [71] | C26 tumor-bearing cancer cachexia male BALB/c mice (8) | AST | ND | ND | 30 mg/kg/day, 60 mg/kg/day, 120 mg/kg/day | 4 weeks | Body weight ↑; Tumor-free body weight ↑; Food intake ↑; Skeletal muscle mass ↑; skeletal muscle weight/tumor-free body weight ↑; CSA of myofibres ↑; Myosin heavy chain ↑; MuRF-1 mRNA ↓; MuRF-1 protein ↓; Atrogin-1mRNA ↓; Atrogin-1 protein ↓ |
| Mano Y (2022) [70] | C57BL/6J COPD mice (10) | AST | ND | Feed the CE-2 diet containing AST | 0.02% | 8 weeks | Proportion of type I fibers ↑; CSA of type I fibers ↑; CSA of type IIA fibers ↑; Atrogin-1protein ↓ |
| Shibaguchi, T. (2016) [73] | Wistar rat with atrophy | AST | Haematococcus pluvialis | Feed the CE-2 diet containing AST | 0.04%, 0.2% | 10 days | Degree of atrophy↓; SOD1 protein ↑; Cathepsin L ↓; Ubiquitin ↓ |
| Deng, M. (2023) [131] | C57BL/6J mice with COPD (12/12)/Human bronchial epithelial cells with CSE | AST | Commercial sources | Ganage | 10 mg/kg/d, 50 mg/kg/d, and 100 mg/kg/d/ 50 μM, 100 μM | 29 days/24 h | MLI ↓; MAA ↓; Body weight ↑; MMP-9 ↓; TIMP-1 ↑; Airway inflammation ↓; Peribronchiolar collagen deposition ↓; E-cadherin ↑; α-SMA ↓ SIRT1 protein ↑/MDA ↓; T-AOC ↑; SOD ↑; GSH ↑; Nrf2 protein↑; HO-1 protein↑; TNF-α↓; IL-6 ↓; p-NF-κBp65 protein ↓; SIRT1 protein ↑ |
| Zhiyin, L. (2021) [74] | C2C12 myotube with atrophy | FX | Commercial sources | - | 10 μM | 24 h | Atrogin-1 protein ↓; MuRF1 protein ↓; Diameter of myotube ↑; MHC protein ↑ ATP production ↑; SIRT1 protein ↑; p-Akt/Akt ↑; FoxO3a protein ↑; p-FoxO3a/FoxO3a ↑; AC-FoxO3a ↑; PGC-1αprotein ↑; Nrf1 protein ↑; TFAM protein ↑; Bax/Bcl-2 ↓; Cleaved caspase-3 protein ↓; Apoptosis cells ↓ |
| Yoshikawa, M. (2021) [75] | ICR mice with atrophy (7/8) | FX | Commercial sources | Feed a mixture containing 0.2% Fx | 0.2% | 27 days | TA weight ↑; MDA ↑; p-AMPK/AMPK ↓; COX4 protein ↑; p-mTOR/Mtor ↑ |
| Yoshikawa, M. (2020) [76] | C2C12 myotube with H2O2/3T3-L1 adipocytes | FXOH | Commercial sources | - | 5 μM/2.5 μM, 5 μM and 10 μM | 24 h/72 h | Area of myotubes ↑; MYC protein ↑; ROS ↓;/TG ↓; Glycerol release ↑; Fatty acid release ↑; lipolysis-associated proteins (ATGL, p-HSL, Perilipin and CGI-58) ↓; p-AMPK/AMPK ↑; p-ACC/ACC ↑; FAS ↓ |
| Kim, Y. (2023) [77] | BALB/c with cancer cachexia | β-carotene | Commercial sources | Oral | 0.5 mg/kg 2 mg/kg 2 t/w | 5 weeks | Muscle mass ↑; Fat weight ↑; Adipocytes size ↑; lipolysis markers (ATGL and HSL) mRNA ↓; Brown adipocyte-specific genes (UCP1, PDK4, PGC-1α) mRNA ↓; Serum of IL-6 ↓; Serum of TNF-α ↓; Altered fecal microbiota structure; Intestinal flora diversity ↑ |
| Kitakaze, T. (2015) [79] | Kwl:ddY mice (5/5) | β-carotene | Commercial sources | Gavage | 0.5 mg/day | 2 weeks | Muscle mass ↑; MVC ↑; IGF-1 mRNA ↑; IGF-Ea mRNA ↑ |
| Ogawa, M. (2013) [78] | C2C12 myotube with H2O2/Denervated Kwl:ddY mice (15/15) | β-carotene | Commercial sources | -/Gavage | 10 μM/0.5 mg/day | 12 h/2 weeks | MHC ↑; Tropomyosin ↑; Atrogin-1 mRNA ↓; MuRF1 mRNA ↓; USP14 mRNA ↓; USP19 mRNA ↓; nuclear localization of FoxO3a ↓/Related soleus muscle mass ↑; TBARS ↓; ubiquitin conjugates ↓; Atrogin-1 mRNA ↓; MuRF1 mRNA ↓; USP14 mRNA ↓; USP19 mRNA ↓ |
| Noguchi, M. (2020) [80] | SAMP1 mice (6~8/6~8) | β-cryptoxanthin | Satsuma mandarin | Gavage | 0.5 mg/day | 15 weeks | Grip Strength ↑; EDL muscle mass/body weight ↑; CSA of EDL muscle fiber ↑; MHC I ↑; Autophagy-related factors (Beclin-1, p62, LC3-I, and LC3-II) protein ↓; p62-positive Fiber ↓; Ubiquitin conjugates ↓ |
| Ogawa, M. (2013) [78] | C2C12 myotube with H2O2 | β-cryptoxanthin | ND | - | 10 μM | 12 h | MHC ↑; Tropomyosin ↑ |
| Thomson, R.L. (2014) [142] | Older adults (20/19) | Lutein and Zeaxanthin | ND | Oral | Lutein: 21 mg/day; Zeaxanthin: 0.9 mg/day | 4 weeks | Plasma lutein ↑; Plasma zeaxanthin ↑; Plasma lutein is negatively associated with sedentary time; Plasma lutein is positively associated with daily activity counts |
4.6. Combinatorial Antioxidants
4.7. Miscellaneous
4.7.1. Gloiopeltis Tenax
| Ref. | Object, Sample Size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Kawamura, A. (2020) [144] | ICR mice with atrophy | Astaxanthin, β-carotene, and resveratrol | Commercial sources | Oral | 0.06% (w/w) | 5 weeks | Muscle mass ↑; p-mTOR protein ↑; p-p70S6K protein ↑; carbonylation ↓ |
| Kawamura, A. (2021) [146] | Healthy men (13/13) | Astaxanthin, β-carotene, and resveratrol | Salmon flakes, green and yellow vegetable juice, and lingonberry jam | Oral | ND | 10 weeks | MVC ↑; oxygen consumption ↑; respiratory quotient ↓ |
4.7.2. Undaria Pinnatifida
4.7.3. Codium Fragile
4.7.4. Oysters
5. Therapeutic Opportunities and Challenges of Marine-Derived Bioactive Compounds in COPD Skeletal Muscle Dysfunction
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes, D.O.M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [PubMed]
- Chen, S.; Kuhn, M.; Prettner, K.; Yu, F.; Yang, T.; Bärnighausen, T.; Bloom, D.E.; Wang, C. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020–50: A health-augmented macroeconomic modelling study. Lancet Glob. Health 2023, 11, e1183–e1193. [Google Scholar]
- Wang, C.; Xu, J.; Yang, L.; Xu, Y.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; Wen, F.; et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 2018, 391, 1706–1717. [Google Scholar]
- Jiang, M.; Li, P.; Wang, Y.; Cao, Y.; Han, X.; Jiang, L.; Liu, X.; Wu, W. Role of Nrf2 and exercise in alleviating COPD-induced skeletal muscle dysfunction. Ther. Adv. Respir. Dis. 2023, 17, 1611179335. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Orozco-Levi, M.; Barreiro, E.; Ferrer, A.; Broquetas, J. Structural and functional changes in the skeletal muscles of COPD patients: The “compartments” theory. Monaldi Arch. Chest Dis. 2001, 56, 214–224. [Google Scholar] [PubMed]
- Raja, K.; Suresh, K.; Anbalagan, S.; Ragini, Y.P.; Kadirvel, V. Investigating the nutritional viability of marine-derived protein for sustainable future development. Food Chem. 2024, 448, 139087. [Google Scholar]
- Lobine, D.; Rengasamy, K.; Mahomoodally, M.F. Functional foods and bioactive ingredients harnessed from the ocean: Current status and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 5794–5823. [Google Scholar]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar]
- Shahidi, F.; Santhiravel, S. Novel marine bioactives: Application in functional foods, nutraceuticals, and pharmaceuticals. J. Food Bioact. 2022, 19, 4–96. [Google Scholar]
- Celli, B.R.; Decramer, M.; Wedzicha, J.A.; Wilson, K.C.; Agustí, A.A.; Criner, G.J.; MacNee, W.; Make, B.J.; Rennard, S.I.; Stockley, R.A.; et al. An official American Thoracic Society/European Respiratory Society statement: Research questions in COPD. Eur. Respir. Rev. 2015, 24, 159–172. [Google Scholar] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, Q.; Yan, L.; Bian, Y.; Li, R.; Gao, J.; Wang, Y.; Miao, J.; Li, J.; Zhou, X.; et al. Glycyl-l-histidyl-l-lysine-Cu2+ rescues cigarette smoking-induced skeletal muscle dysfunction via a sirtuin 1-dependent pathway. J. Cachexia Sarcopenia Muscle 2023, 14, 1365–1380. [Google Scholar]
- Benz, E.; Trajanoska, K.; Lahousse, L.; Schoufour, J.D.; Terzikhan, N.; De Roos, E.; de Jonge, G.B.; Williams, R.; Franco, O.H.; Brusselle, G.; et al. Sarcopenia in COPD: A systematic review and meta-analysis. Eur. Respir. Rev. 2019, 28, 190049. [Google Scholar] [CrossRef]
- McDonald, M.L.; Diaz, A.A.; Ross, J.C.; San, J.E.R.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Spositon, T.; Oliveira, J.M.; Rodrigues, A.; Fonseca, J.; Santin, L.; Machado, F.; Hernandes, N.A.; Marques, A.; Pitta, F.; Furlanetto, K.C. Quadriceps weakness associated with mortality in individuals with chronic obstructive pulmonary disease. Ann. Phys. Rehabil. Med. 2022, 65, 101587. [Google Scholar]
- Decker, S.T.; Kwon, O.S.; Zhao, J.; Hoidal, J.R.; Heuckstadt, T.; Richardson, R.S.; Sanders, K.A.; Layec, G. Skeletal muscle mitochondrial adaptations induced by long-term cigarette smoke exposure. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E80E89. [Google Scholar]
- van den Borst, B.; Slot, I.G.; Hellwig, V.A.; Vosse, B.A.; Kelders, M.C.; Barreiro, E.; Schols, A.M.; Gosker, H.R. Loss of quadriceps muscle oxidative phenotype and decreased endurance in patients with mild-to-moderate COPD. J. Appl. Physiol. 2013, 114, 1319–1328. [Google Scholar] [CrossRef]
- Gea, J.G.; Pasto, M.; Carmona, M.A.; Orozco-Levi, M.; Palomeque, J.; Broquetas, J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 17, 939–945. [Google Scholar] [CrossRef]
- Gosselink, R.; Troosters, T.; Decramer, M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2000, 20, 353–360. [Google Scholar]
- Passey, S.L.; Hansen, M.J.; Bozinovski, S.; McDonald, C.F.; Holland, A.E.; Vlahos, R. Emerging therapies for the treatment of skeletal muscle wasting in chronic obstructive pulmonary disease. Pharmacol. Ther. 2016, 166, 56–70. [Google Scholar] [CrossRef] [PubMed]
- van Bakel, S.; Gosker, H.R.; Langen, R.C.; Schols, A. Towards Personalized Management of Sarcopenia in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Man, S.F.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Man, S.F. Skeletal muscle weakness, reduced exercise tolerance, and COPD: Is systemic inflammation the missing link? Thorax 2006, 61, 1–3. [Google Scholar]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar]
- Jimenez-Gutierrez, G.E.; Martínez-Gómez, L.E.; Martínez-Armenta, C.; Pineda, C.; Martínez-Nava, G.A.; Lopez-Reyes, A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar]
- Powers, S.K.; Radak, Z.; Ji, L.L.; Jackson, M. Reactive oxygen species promote endurance exercise-induced adaptations in skeletal muscles. J. Sport. Health Sci. 2024, 13, 780–792. [Google Scholar] [CrossRef]
- Duranti, G. Oxidative Stress and Skeletal Muscle Function. Int. J. Mol. Sci. 2023, 24, 10227. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [PubMed]
- Hussain, S.N.; Sandri, M. Role of autophagy in COPD skeletal muscle dysfunction. J. Appl. Physiol. 2013, 114, 1273–1281. [Google Scholar]
- Puig-Vilanova, E.; Rodriguez, D.A.; Lloreta, J.; Ausin, P.; Pascual-Guardia, S.; Broquetas, J.; Roca, J.; Gea, J.; Barreiro, E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free. Radic. Biol. Med. 2015, 79, 91–108. [Google Scholar] [CrossRef]
- Guo, Y.; Gosker, H.R.; Schols, A.M.; Kapchinsky, S.; Bourbeau, J.; Sandri, M.; Jagoe, R.T.; Debigaré, R.; Maltais, F.; Taivassalo, T.; et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1313–1320. [Google Scholar]
- Balnis, J.; Drake, L.A.; Singer, D.V.; Vincent, C.E.; Korponay, T.C.; D’Armiento, J.; Lee, C.G.; Elias, J.A.; Singer, H.A.; Jaitovich, A. Deaccelerated Myogenesis and Autophagy in Genetically Induced Pulmonary Emphysema. Am. J. Respir. Cell Mol. Biol. 2022, 66, 623–637. [Google Scholar]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar]
- Liu, B.H.; Xu, C.Z.; Liu, Y.; Lu, Z.L.; Fu, T.L.; Li, G.R.; Deng, Y.; Luo, G.Q.; Ding, S.; Li, N.; et al. Mitochondrial quality control in human health and disease. Mil. Med. Res. 2024, 11, 32. [Google Scholar] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar]
- Lazaar, A.L.; Greenhaff, P.L. Impaired muscle mitochondrial density and/or function: A COPD-specific mitochondropathy or simply deconditioning? Eur. Respir. J. 2012, 40, 1070–1071. [Google Scholar] [PubMed]
- Remels, A.H.; Schrauwen, P.; Broekhuizen, R.; Willems, J.; Kersten, S.; Gosker, H.R.; Schols, A.M. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur. Respir. J. 2007, 30, 245–252. [Google Scholar] [CrossRef]
- Leermakers, P.A.; Schols, A.; Kneppers, A.; Kelders, M.; de Theije, C.C.; Lainscak, M.; Gosker, H.R. Molecular signalling towards mitochondrial breakdown is enhanced in skeletal muscle of patients with chronic obstructive pulmonary disease (COPD). Sci. Rep. 2018, 8, 15007. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics. 2023, 13, 736–766. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hashimoto, M.; Tanihata, J.; Matsubayashi, S.; Sasaki, R.; Fujimoto, S.; Kawamoto, H.; Hosaka, Y.; Ichikawa, A.; Kadota, T.; et al. Involvement of Parkin-mediated mitophagy in the pathogenesis of chronic obstructive pulmonary disease-related sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 1864–1882. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Tan, Z.; Li, S.; Zhu, S.; Yao, X.; Li, J.; Gao, X.; Yang, S. Effect of cigarette smoke extract on mitochondrial division in mouse quadriceps femoris cells. Ann. Transl. Med. 2021, 9, 1699. [Google Scholar]
- Chen, Y.M.; Tsai, Y.H.; Tsai, T.Y.; Chiu, Y.S.; Wei, L.; Chen, W.C.; Huang, C.C. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 2014, 7, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Dwan, C.; Wimmer, B.C.; Wilson, R.; Johnson, L.; Caruso, V. Fucoidan from Undaria pinnatifida Enhances Exercise Performance and Increases the Abundance of Beneficial Gut Bacteria in Mice. Mar. Drugs 2024, 22, 485. [Google Scholar] [CrossRef] [PubMed]
- McBean, S.E.; Church, J.E.; Thompson, B.K.; Taylor, C.J.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y.; van der Poel, C. Oral fucoidan improves muscle size and strength in mice. Physiol. Rep. 2021, 9, e14730. [Google Scholar] [PubMed]
- McFadden, B.A.; Vincenty, C.S.; Chandler, A.J.; Cintineo, H.P.; Lints, B.S.; Mastrofini, G.F.; Arent, S.M. Effects of fucoidan supplementation on inflammatory and immune response after high-intensity exercise. J. Int. Soc. Sport. Nutr. 2023, 20, 2224751. [Google Scholar]
- Ha, B.G.; Park, J.E.; Shon, Y.H. Stimulatory Effect of Balanced Deep-Sea Water Containing Chitosan Oligosaccharides on Glucose Uptake in C2C12 Myotubes. Mar. Biotechnol. 2016, 18, 475–484. [Google Scholar] [CrossRef]
- Jeong, H.W.; Cho, S.Y.; Kim, S.; Shin, E.S.; Kim, J.M.; Song, M.J.; Park, P.J.; Sohn, J.H.; Park, H.; Seo, D.B.; et al. Chitooligosaccharide induces mitochondrial biogenesis and increases exercise endurance through the activation of Sirt1 and AMPK in rats. PLoS ONE 2012, 7, e40073. [Google Scholar]
- Cho, S.Y.; Lee, J.H.; Song, M.J.; Park, P.J.; Shin, E.S.; Sohn, J.H.; Seo, D.B.; Lim, K.M.; Kim, W.G.; Lee, S.J. Effects of chitooligosaccharide lactate salt on sleep deprivation-induced fatigue in mice. Biol. Pharm. Bull. 2010, 33, 1128–1132. [Google Scholar]
- Teodoro, J.S.; Gomes, A.P.; Varela, A.T.; Duarte, F.V.; Rolo, A.P.; Palmeira, C.M. Hepatic and skeletal muscle mitochondrial toxicity of chitosan oligosaccharides of normal and diabetic rats. Toxicol. Mech. Method 2016, 26, 650–657. [Google Scholar]
- Flórez-Fernández, N.; Rodríguez-Coello, A.; Latire, T.; Bourgougnon, N.; Torres, M.D.; Buján, M.; Muíños, A.; Muiños, A.; Meijide-Faílde, R.; Blanco, F.J.; et al. Anti-inflammatory potential of ulvan. Int. J. Biol. Macromol. 2023, 253, 126936. [Google Scholar]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Lehoczki, A.; Tarantini, S.; Varga, J.T. Effects of omega-3 supplementation on quality of life, nutritional status, inflammatory parameters, lipid profile, exercise tolerance and inhaled medications in chronic obstructive pulmonary disease. Ann. Palliat. Med. 2022, 11, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- de Batlle, J.; Sauleda, J.; Balcells, E.; Gómez, F.P.; Méndez, M.; Rodriguez, E.; Barreiro, E.; Ferrer, J.J.; Romieu, I.; Gea, J.; et al. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J. Nutr. Biochem. 2012, 23, 817–821. [Google Scholar] [CrossRef]
- Engelen, M.; Simbo, S.Y.; Ruebush, L.E.; Thaden, J.J.; Ten, H.G.; Harrykissoon, R.I.; Zachria, A.J.; Calder, P.C.; Pereira, S.L.; Deutz, N. Functional and metabolic effects of omega-3 polyunsaturated fatty acid supplementation and the role of β-hydroxy-β-methylbutyrate addition in chronic obstructive pulmonary disease: A randomized clinical trial. Clin. Nutr. 2024, 43, 2263–2278. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Ryu, B.; Oh, S.; Yang, H.W.; Sosorburam, B.; Chung, D.M.; Seo, M.; Park, S.J.; Byun, K.; Jeon, Y.J. Diphlorethohydroxycarmalol Derived from Ishige okamurae Improves Behavioral and Physiological Responses of Muscle Atrophy Induced by Dexamethasone in an In-Vivo Model. Pharmaceutics 2022, 14, 719. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.H.; Kang, N.; Kim, K.N.; Jeon, Y.J. The Effects of Marine Algal Polyphenols, Phlorotannins, on Skeletal Muscle Growth in C2C12 Muscle Cells via Smad and IGF-1 Signaling Pathways. Mar. Drugs 2021, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lin, L.; Chen, H.; Ge, X.; Huang, Y.; Zheng, Z.; Li, S.; Pan, Y.; Liu, B.; Zeng, F. Anti-fatigue property of the oyster polypeptide fraction and its effect on gut microbiota in mice. Food Funct. 2020, 11, 8659–8669. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, Y.M.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide PYP1-5 protects against dexamethasone-induced muscle atrophy through the downregulation of atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes. Mol. Med. Rep. 2017, 15, 3507–3514. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, D.; Guo, J.; Zhang, Y.; Zheng, B. In Vitro Antioxidant Activity and In Vivo Anti-Fatigue Effect of Sea Horse (Hippocampus) Peptides. Molecules 2017, 22, 482. [Google Scholar] [CrossRef]
- Mano, Y.; Tsukamoto, M.; Wang, K.Y.; Nabeshima, T.; Kosugi, K.; Tajima, T.; Yamanaka, Y.; Suzuki, H.; Kawasaki, M.; Nakamura, E.; et al. Oxidative stress causes muscle structural alterations via p38 MAPK signaling in COPD mouse model. J. Bone Miner. Metab. 2022, 40, 927–939. [Google Scholar]
- Yu, X.; Ren, P.; Yang, R.; Yue, H.; Tang, Q.; Xue, C. Astaxanthin Ameliorates Skeletal Muscle Atrophy in Mice with Cancer Cachexia. Nutr. Cancer 2024, 76, 529–542. [Google Scholar]
- Yue, H.; Huan, Y.; Ren, P.; Yu, X.; Tang, Q.; Xue, C.; Xu, J. Astaxanthin ameliorates dexamethasone-induced skeletal muscle atrophy and disorders of glucolipid metabolism. Biochem. Bioph Res. Commun. 2025, 743, 151138. [Google Scholar]
- Shibaguchi, T.; Yamaguchi, Y.; Miyaji, N.; Yoshihara, T.; Naito, H.; Goto, K.; Ohmori, D.; Yoshioka, T.; Sugiura, T. Astaxanthin intake attenuates muscle atrophy caused by immobilization in rats. Physiol. Rep. 2016, 4, e12885. [Google Scholar]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin rescues dexamethasone induced C2C12 myotubes atrophy. Biomed. Pharmacother. 2021, 139, 111590. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Nishino, H.; Hashimoto, T. Effects of Fucoxanthin on the Inhibition of Dexamethasone-Induced Skeletal Muscle Loss in Mice. Nutrients 2021, 13, 1079. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Fujita, T.; Nishino, H.; Hashimoto, T. Fucoxanthinol attenuates oxidative stress-induced atrophy and loss in myotubes and reduces the triacylglycerol content in mature adipocytes. Mol. Biol. Rep. 2020, 47, 2703–2711. [Google Scholar] [PubMed]
- Kim, Y.; Jung, S.; Park, G.; Shin, H.; Heo, S.C.; Kim, Y. β-Carotene suppresses cancer cachexia by regulating the adipose tissue metabolism and gut microbiota dysregulation. J. Nutr. Biochem. 2023, 114, 109248. [Google Scholar]
- Ogawa, M.; Kariya, Y.; Kitakaze, T.; Yamaji, R.; Harada, N.; Sakamoto, T.; Hosotani, K.; Nakano, Y.; Inui, H. The preventive effect of β-carotene on denervation-induced soleus muscle atrophy in mice. Br. J. Nutr. 2013, 109, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Harada, N.; Imagita, H.; Yamaji, R. β-Carotene Increases Muscle Mass and Hypertrophy in the Soleus Muscle in Mice. J. Nutr. Sci. Vitaminol. 2015, 61, 481–487. [Google Scholar]
- Noguchi, M.; Kitakaze, T.; Kobayashi, Y.; Mukai, K.; Harada, N.; Yamaji, R. β-Cryptoxanthin Improves p62 Accumulation and Muscle Atrophy in the Soleus Muscle of Senescence-Accelerated Mouse-Prone 1 Mice. Nutrients 2020, 12, 2180. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Dufour, A.B.; Fielding, R.A.; Newman, A.B.; Kiel, D.P.; Hannan, M.T.; Jacques, P.F. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am. J. Clin. Nutr. 2021, 113, 437–445. [Google Scholar]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar]
- Chen, T.; Sun, C.; Tian, X.; Jiang, X.; Zhang, M. Natural Polysaccharide: Modification and Application. Pap. Biomater. 2021, 6, 43. [Google Scholar]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar]
- Tsvetkov, Y.E.; Paulovičová, E.; Paulovičová, L.; Farkaš, P.; Nifantiev, N.E. Synthesis of Biotin-Tagged Chitosan Oligosaccharides and Assessment of Their Immunomodulatory Activity. Front. Chem. 2020, 8, 554732. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jin, Y.; Hou, X.; Zhu, Y.; Chen, D.; Tai, J.; Chen, Q.; Shi, C.; Ye, J.; Wu, M.; et al. Application of Marine Natural Products against Alzheimer’s Disease: Past, Present and Future. Mar. Drugs 2023, 21, 43. [Google Scholar] [CrossRef]
- Van Dael, P. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: Review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22: 5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Yum, H.W.; Na, H.K.; Surh, Y.J. Anti-inflammatory effects of docosahexaenoic acid: Implications for its cancer chemopreventive potential. Semin. Cancer Biol. 2016, 40–41, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Lacombe, R.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Aspects Med. 2018, 64, 109–134. [Google Scholar] [CrossRef]
- van de Bool, C.; Rutten, E.; van Helvoort, A.; Franssen, F.; Wouters, E.; Schols, A. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Mazurak, V.C. Fish oil supplementation and maintaining muscle mass in chronic disease: State of the evidence. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 164–173. [Google Scholar] [CrossRef]
- Bird, J.K.; Troesch, B.; Warnke, I.; Calder, P.C. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: A scoping systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 46, 73–86. [Google Scholar] [CrossRef]
- Troosters, T.; Janssens, W.; Demeyer, H.; Rabinovich, R.A. Pulmonary rehabilitation and physical interventions. Eur. Respir. Rev. 2023, 32, 220222. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, R.; Wouters, E.F.; Creutzberg, E.C.; Weling-Scheepers, C.A.; Schols, A.M. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005, 60, 376–382. [Google Scholar]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachexia Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar]
- Hannan, M.A.; Sohag, A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [PubMed]
- PubChem. Fucosterol | C29H48O | CID 5281328. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fucosterol (accessed on 6 March 2025).
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar]
- Kirindage, K.; Jayasinghe, A.; Han, E.J.; Jee, Y.; Kim, H.J.; Do, S.G.; Fernando, I.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Sanjeewa, K.; Lee, H.G.; Nagahawatta, D.P.; Yang, H.W.; Kang, M.C.; Jeon, Y.J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-κB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef]
- El, O.N.; Bakrim, S.; Khalid, A.; Abdalla, A.N.; Iesa, M.; El, K.K.; Tang, S.Y.; Goh, B.H.; Bouyahya, A. Unveiling the molecular mechanisms: Dietary phytosterols as guardians against cardiovascular diseases. Nat. Product. Bioprosp. 2024, 14, 27. [Google Scholar]
- Li, Y.; Li, X.; Liu, G.; Sun, R.; Wang, L.; Wang, J.; Wang, H. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 195, 515–521. [Google Scholar] [CrossRef]
- Fernando, I.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.; De Silva, H.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Ogasawara, T.; Marui, S.; Miura, E.; Sugiura, M.; Matsuyama, W.; Aoshima, Y.; Kasamatsu, N.; Ogiku, M.; Ikematsu, Y. Effect of eicosapentaenoic acid on prevention of lean body mass depletion in patients with exacerbation of chronic obstructive pulmonary disease: A prospective randomized controlled trial. Clin. Nutr. ESPEN 2018, 28, 67–73. [Google Scholar] [PubMed]
- Pereira, L.; Cotas, J. Therapeutic Potential of Polyphenols and Other Micronutrients of Marine Origin. Mar. Drugs 2023, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.; Hsieh, Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Cha, S.H.; Lee, S.H.; Kang, D.H.; Jung, W.K.; Affan, A.; Oh, C.; Jeon, Y.J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ahn, G.; Kim, H.S.; Je, J.G.; Kim, K.N.; Jeon, Y.J. Diphlorethohydroxycarmalol (DPHC) Isolated from the Brown Alga Ishige okamurae Acts on Inflammatory Myopathy as an Inhibitory Agent of TNF-α. Mar. Drugs. 2020, 18, 529. [Google Scholar]
- Irfan, M.; Kwon, T.H.; Kwon, H.W.; Rhee, M.H. Pharmacological actions of dieckol on modulation of platelet functions and thrombus formation via integrin α(IIb)β(3) and cAMP signaling. Pharmacol. Res. 2022, 177, 106088. [Google Scholar]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar]
- Sim, H.H.; Shiwakoti, S.; Lee, J.H.; Lee, I.Y.; Ok, Y.; Lim, H.K.; Ko, J.Y.; Oak, M.H. 2,7-Phloroglucinol-6,6’-bieckol from Ecklonia cava ameliorates nanoplastics-induced premature endothelial senescence and dysfunction. Sci. Total Environ. 2024, 949, 175007. [Google Scholar]
- Jacobsen, C.; Sørensen, A.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar]
- Kwon, I.S.; Park, D.S.; Shin, H.C.; Seok, M.G.; Oh, J.K. Effects of marine oligomeric polyphenols on body composition and physical ability of elderly individuals with sarcopenia: A pilot study. Phys. Act. Nutr. 2021, 25, 1–7. [Google Scholar]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, Peptidomimetics, and Polypeptides from Marine Sources: A Wealth of Natural Sources for Pharmaceutical Applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Y.; Ye, P.; Zhao, H.; Yang, K.; Hao, G. Oyster (Ostrea Plicatula Gmelin) Peptides Improve Exercise Endurance Capacity via Activating AMPK and HO-1. J. Am. Nutr. Assoc. 2024, 43, 437–451. [Google Scholar] [PubMed]
- Lee, H.A.; Kim, I.H.; Nam, T.J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [PubMed]
- Lee, M.K.; Choi, J.W.; Choi, Y.H.; Nam, T.J. Protective Effect of Pyropia yezoensis Peptide on Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes. Mar. Drugs 2019, 17, 284. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Alipanah, N.; Varadhan, R.; Sun, K.; Ferrucci, L.; Fried, L.P.; Semba, R.D. Low serum carotenoids are associated with a decline in walking speed in older women. J. Nutr. Health Aging 2009, 13, 170–175. [Google Scholar]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Dayhoff-Brannigan, M.; Giacomini, V.; Corsi, A.M.; Guralnik, J.M.; Ferrucci, L. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 376–383. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, X.; Xia, Z.; Guo, Y.; Dai, Y. The Associations Between Serum Vitamins and Carotenoids with Chronic Obstructive Pulmonary Disease: Results from the NHANES. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2985–2997. [Google Scholar] [CrossRef]
- Jun, L.; Root, M. Association of Carotenoid Intake with Pulmonary Function. J. Am. Coll. Nutr. 2021, 40, 708–712. [Google Scholar] [CrossRef]
- Si, P.; Zhu, C. Biological and neurological activities of astaxanthin (Review). Mol. Med. Rep. 2022, 26, 300. [Google Scholar] [PubMed]
- Cheng, J.; Eroglu, A. The Promising Effects of Astaxanthin on Lung Diseases. Adv. Nutr. Int. Rev. J. 2021, 12, 850–864. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [PubMed]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar]
- Deng, M.; Tong, R.; Bian, Y.; Hou, G. Astaxanthin attenuates cigarette smoking-induced oxidative stress and inflammation in a sirtuin 1-dependent manner. Biomed. Pharmacother. 2023, 159, 114230. [Google Scholar]
- Tang, H.; Zhang, Y.; Wang, Q.; Zeng, Z.; Wang, X.; Li, Y.; Wang, Z.; Ma, N.; Xu, G.; Zhong, X.; et al. Astaxanthin attenuated cigarette smoke extract-induced apoptosis via decreasing oxidative DNA damage in airway epithelium. Biomed. Pharmacother. 2023, 167, 115471. [Google Scholar]
- Kubo, H.; Asai, K.; Kojima, K.; Sugitani, A.; Kyomoto, Y.; Okamoto, A.; Yamada, K.; Ijiri, N.; Watanabe, T.; Hirata, K.; et al. Astaxanthin Suppresses Cigarette Smoke-Induced Emphysema through Nrf2 Activation in Mice. Mar. Drugs 2019, 17, 673. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar]
- Olson, J.A. Provitamin A function of carotenoids: The conversion of beta-carotene into vitamin A. J. Nutr. 1989, 119, 105–108. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar]
- Olmedilla, B.; Granado, F.; Southon, S.; Wright, A.J.; Blanco, I.; Gil-Martinez, E.; Berg, H.; Corridan, B.; Roussel, A.M.; Chopra, M.; et al. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br. J. Nutr. 2001, 85, 227–238. [Google Scholar]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, occurrence, and potential health benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nishita, Y.; Ando, F.; Shimokata, H.; Otsuka, R. Low Serum Total Carotenoids and β-Cryptoxanthin Are Associated with Low Lean Body Mass in Older Community-Dwellers in the National Institute for Longevity Sciences-Longitudinal Study of Aging: A 4-Y Longitudinal Study. J. Nutr. 2024, 154, 3042–3047. [Google Scholar]
- Cooke, M.C.; Coates, A.M.; Buckley, E.S.; Buckley, J.D. Lutein Intake and Blood Lutein Concentration Are Positively Associated with Physical Activity in Adults: A Systematic Review. Nutrients 2018, 10, 1186. [Google Scholar] [CrossRef]
- Thomson, R.L.; Coates, A.M.; Howe, P.R.; Bryan, J.; Matsumoto, M.; Buckley, J.D. Increases in plasma lutein through supplementation are correlated with increases in physical activity and reductions in sedentary time in older adults. Nutrients 2014, 6, 974–984. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hagio, M.; Inoue, R.; Mitani, T.; Yajima, M.; Hara, H.; Yajima, T. Long-term oral feeding of lutein-fortified milk increases voluntary running distance in rats. PLoS ONE 2014, 9, e93529. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Higashi, A. Combined intake of astaxanthin, β-carotene, and resveratrol elevates protein synthesis during muscle hypertrophy in mice. Nutrition 2020, 69, 110561. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.F.; Yang, Y.C.; Li, R.Y.; Jiao, Y.H.; Mou, J.H.; Yang, W.D.; Lin, C.; Li, H.Y.; Wang, X. Synergistic and stepwise treatment of resveratrol and catechol in Haematococcus pluvialis for the overproduction of biomass and astaxanthin. Biotechnol. Biofuels Bioprod. 2024, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Kuwahata, M.; Higashi, A. Astaxanthin-, β-Carotene-, and Resveratrol-Rich Foods Support Resistance Training-Induced Adaptation. Antioxidants 2021, 10, 113. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, Y.; Yao, F.; Chen, W.; Shi, G. Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar. Drugs 2012, 10, 2634–2647. [Google Scholar] [CrossRef]
- Ahn, J.; Ha, T.Y.; Ahn, J.; Jung, C.H.; Seo, H.D.; Kim, M.J.; Kim, Y.S.; Jang, Y.J. Undaria pinnatifida extract feeding increases exercise endurance and skeletal muscle mass by promoting oxidative muscle remodeling in mice. FASEB J. 2020, 34, 8068–8081. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, M.J.; Yoo, A.; Ahn, J.; Ha, T.Y.; Jung, C.H.; Seo, H.D.; Jang, Y.J. Identifying Codium fragile extract components and their effects on muscle weight and exercise endurance. Food Chem. 2021, 353, 129463. [Google Scholar] [CrossRef]
- Oh, S.; Choi, C.H.; Lee, B.J.; Park, J.H.; Son, K.H.; Byun, K. Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress. Molecules 2021, 26, 7128. [Google Scholar] [CrossRef]
- Kim, S.H.; Leem, Y.E.; Park, H.E.; Jeong, H.I.; Lee, J.; Kang, J.S. The Extract of Gloiopeltis tenax Enhances Myogenesis and Alleviates Dexamethasone-Induced Muscle Atrophy. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Lu, Y.; Zheng, Y.; Pan, L.; Wu, X.; Yuan, Y.; Shen, Z.; Ma, S.; Zhang, X.; et al. BNIP3L/NIX-mediated mitophagy: Molecular mechanisms and implications for human disease. Cell Death Dis. 2021, 13, 14. [Google Scholar] [PubMed]
- Kabir, A.; Muth, A. Polypharmacology: The science of multi-targeting molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 1242–1269. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.; Da, S.G.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological properties of polyphenols: Bioavailability, mechanisms of action, and biological effects in in vitro studies, animal models, and humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef]
- Maiani, G.; Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S194–S218. [Google Scholar] [CrossRef]
- Wang, S.; Huang, C.; Chen, C.; Chang, C.; Huang, C.; Dong, C.; Chang, J. Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity. Biochem. Eng. J. 2021, 165, 107798. [Google Scholar]

| Family | Compound | Origin | Bioactivity | Ref. |
|---|---|---|---|---|
| Polysaccharides | Fucoidan | Brown algae and Undaria pinnatifida | Anti-fatigue; Promote muscle synthesis; Enhance the contractility of muscle fibers; Enhance mitochondrial biogenesis; Promote the formation of an inflammatory environment after high-intensity exercise | [51,52,53,54] |
| Chitosan | Chitin produced from the exoskeletons of arthropods | Anti-fatigue; Antioxidant; Promote glucose uptake, mitochondrial biogenesis; Alter mitochondrial respiratory chain complex | [55,56,57,58] | |
| Ulvan | Green algae | Antioxidant; Anti-inflammation | [59,60] | |
| Lipids | DHA | Fish oil | Promote muscle synthesis; Anti-inflammation | [61,62,63] |
| EPA | Fish oil | Promote muscle synthesis; Anti-inflammation | [61,62,63] | |
| FST | Brown algae | Antioxidant; Anti-inflammation; | [64] | |
| Polyphenols | DPHC | Ishige okamurae | Promote muscle synthesis; Promote contraction capacity | [65] |
| DK | Ecklonia cava | Promote muscle synthesis; Inhibit muscle degradation; | [66] | |
| PHB | Ecklonia cava | Promote muscle synthesis; Inhibit muscle degradation; | [66] | |
| Peptides | Oyster peptides | Oyster | Antioxidant; Anti-fatigue; Anti-inflammation; Enhance energy metabolism; Modulate gut microbiota composition | [67] |
| Pyropia yezoensis peptides | Pyropia yezoensis | Inhibit skeletal muscle atrophy; Inhibit autophagy; Promote muscle protein synthesis; Inhibit muscle protein degradation | [68] | |
| Hippocampus Peptides | Hippocampus | Anti-fatigue; Regulate metabolism; Antioxidant; | [69] | |
| Carotenoids | AST | Haematococcus pluvialis | Antioxidant; Anti-inflammation; Promote muscle hypertrophy; Inhibit protein degradation; Regulate mitochondrial fission and fusion | [70,71,72,73] |
| FX | Brown algae | Reduced protein degradation; Improves mitochondrial count and function; Apoptosis and autophagy; Antioxidant; Promote lipolysis; Inhibit lipogenesis | [74,75,76] | |
| β-carotene | Marine microorganisms and algae | Antioxidant; Anti-inflammation; Promote skeletal muscle hypertrophy; Inhibit skeletal muscle atrophy, lipolysis and fat browning; Modulate gut microbiota and diversity | [77,78,79] | |
| β-cryptoxanthin | Promote muscle synthesis; Inhibit autophagy | [78,80] | ||
| Lutein | Algae | Antioxidant; Promote muscle synthesis; | [81] | |
| Zeaxanthin | Algae | Antioxidant; Promote muscle synthesis; | [81,82] |
| Ref. | Object, Sample Size | Intervention | Origin | Administration Route | Dose | Duration | Primary Results |
|---|---|---|---|---|---|---|---|
| Kim, S H (2024) [152] | C57Bl/6 mice with skeletal muscle atrophy | Gloiopeltis tenax extract | Gloiopeltis tenax | Oral | 8 mg/kg/d | 7 days | Myotube size ↑; Isometric strength of forelimb muscles ↑; Aerobic endurance ↑ Weight of the EDL ↑; Diameter of muscle fibers ↑; Atogin-1 mRNA ↓; MuRF-1 mRNA ↓ |
| Ahn, J. (2020) [149] | C57BL/6 mice (10/10) | Undaria pinnatifida extract | Commercial sources | Add to the diet | 0.25% | 8 weeks | Running distance ↑; Maximum speed ↑ Total running time ↑; EDL weight ↑ Gastrocnemius weight ↑; CSA of gastrocnemius ↑; MHC1 protein ↑; MEF2 mRNA ↑; MEF2C mRNA ↑; Cyt C mRNA ↑; COX5a mRNA↑; Fatty acid uptake-related genes (FATP1, Apoe, Fabp4) ↑; Fatty acid oxidation-related genes (Acadm, PDK4, UCP3, CPT1a, CPT1b, and CPT2) ↑; Glucose uptake-related genes (GULT3, GULT4) ↑; CD31 intensity ↑; Angiogenesis markers genes (VEGFa, VEGFb, FGF1, ANGPT1, and ANGPT2) ↑; Mitochondria area ↑; Mitochondrial OXPHOS respiratory complex-related genes (NDUFS8, UQCRC1, ATP5a, SDHb, and COX5b) ↑; Complex I, III-V protein ↑; Nrf2 mRNA ↑; TFAM mRNA ↑; Nrf2 protein ↑; ERRγ protein ↑; ERRα protein ↑; SIRT1 protein ↑ |
| Li, Y (2021) [153] | ICR mice with muscle atrophy | Oyster extracts | Crassostrea gigas | Oral | 50 mg/kg/d, 100 mg/kg/d, 200 mg/kg/d | 4 weeks | Body weight ↑; Muscle thickness of gastrocnemius ↑; Weight of gastrocnemius CSA of gastrocnemius fibers ↑; Strength of gastrocnemius ↑ |
| Ahn, J. (2021) [150] | Old C57BL/6 mice | CF extracts | Commercial sources | AIN-93 M diet with CF | 0.1% (w/w) | 10 weeks | Running time ↑; Maximum speed ↑; Total running time ↑; Quadriceps weight, ↑; Soleus muscle ↑; CSA of soleus muscle fiber ↑; Skeletal muscle growth-related protein of quadriceps (T-MHC, p-AKT, p-mTOR, p70-S6K1, and p-4EBP1) ↑; Myf5 protein ↑; MyoD protein ↑ MHC1 protein ↑; MHC2a protein ↑; Complex I-V ↑; MEF2A ↑; MEF2C ↑; ERRγ protein ↑; PPARδ protein ↑; PGC-1α protein ↑; SIRT1 protein ↑; ERRαprotein ↑; Nrf1 protein ↑; Myoglobin protein ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Li, P.; Han, X.; Jiang, L.; Han, L.; He, Q.; Yang, C.; Sun, Z.; Wang, Y.; Cao, Y.; et al. Marine-Derived Bioactive Compounds: A Promising Strategy for Ameliorating Skeletal Muscle Dysfunction in COPD. Mar. Drugs 2025, 23, 158. https://doi.org/10.3390/md23040158
Jiang M, Li P, Han X, Jiang L, Han L, He Q, Yang C, Sun Z, Wang Y, Cao Y, et al. Marine-Derived Bioactive Compounds: A Promising Strategy for Ameliorating Skeletal Muscle Dysfunction in COPD. Marine Drugs. 2025; 23(4):158. https://doi.org/10.3390/md23040158
Chicago/Turabian StyleJiang, Meiling, Peijun Li, Xiaoyu Han, Linhong Jiang, Lihua Han, Qinglan He, Chen Yang, Zhichao Sun, Yingqi Wang, Yuanyuan Cao, and et al. 2025. "Marine-Derived Bioactive Compounds: A Promising Strategy for Ameliorating Skeletal Muscle Dysfunction in COPD" Marine Drugs 23, no. 4: 158. https://doi.org/10.3390/md23040158
APA StyleJiang, M., Li, P., Han, X., Jiang, L., Han, L., He, Q., Yang, C., Sun, Z., Wang, Y., Cao, Y., Liu, X., & Wu, W. (2025). Marine-Derived Bioactive Compounds: A Promising Strategy for Ameliorating Skeletal Muscle Dysfunction in COPD. Marine Drugs, 23(4), 158. https://doi.org/10.3390/md23040158





