In Vitro Digestion and Gut Microbiota Fermentation of the Anticancer Marine Drug BG136: Stability and Biotransformation Investigation
Abstract
1. Introduction
2. Results
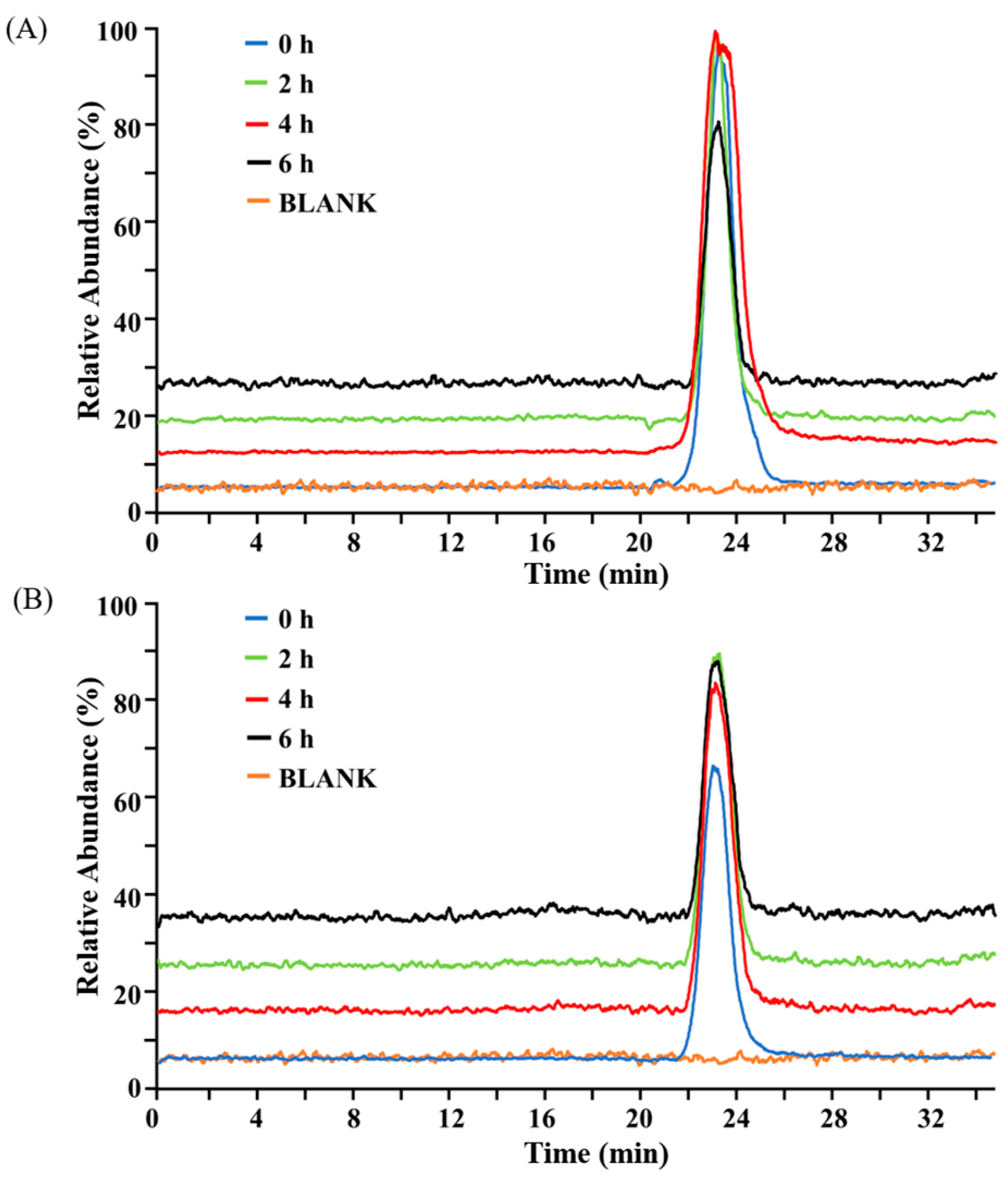
2.1. Structural Stability of BG136 During In Vitro Digestion
2.2. Saccharides Dynamics During In Vitro Fermentation
2.3. Structural Modifications of BG136 During In Vitro Fermentation
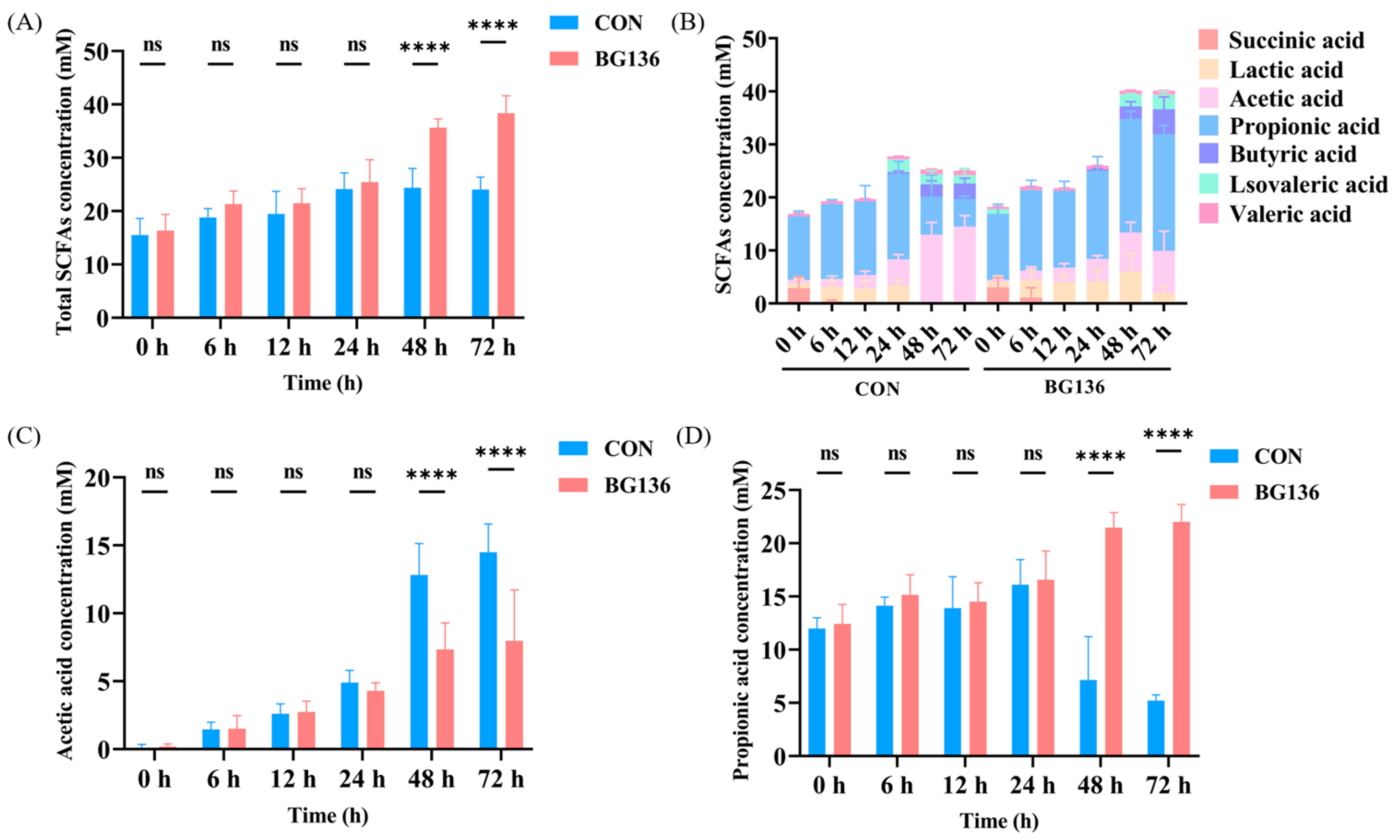
2.4. BG136 Fermentation Promoted the Production of SCFAs
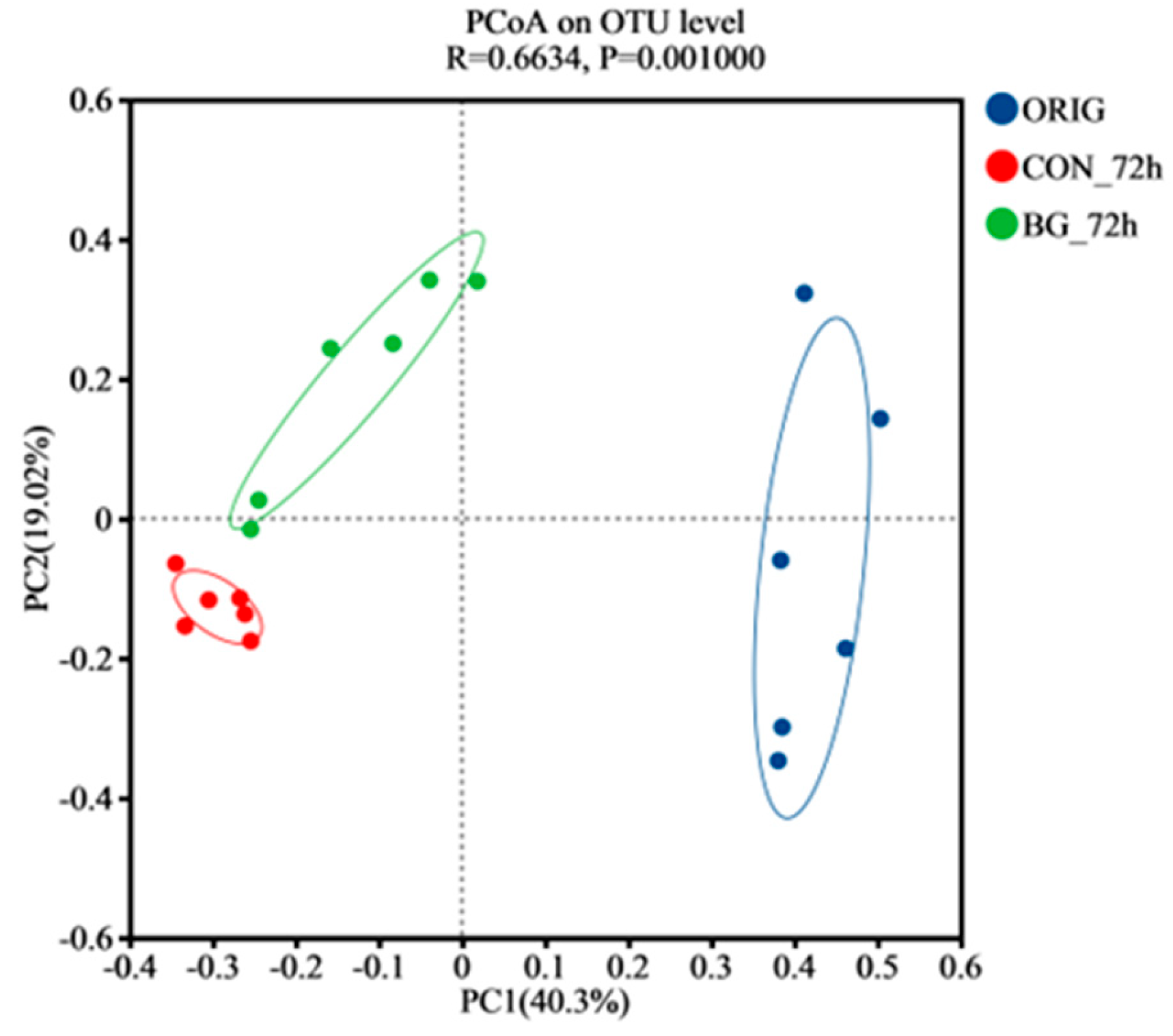
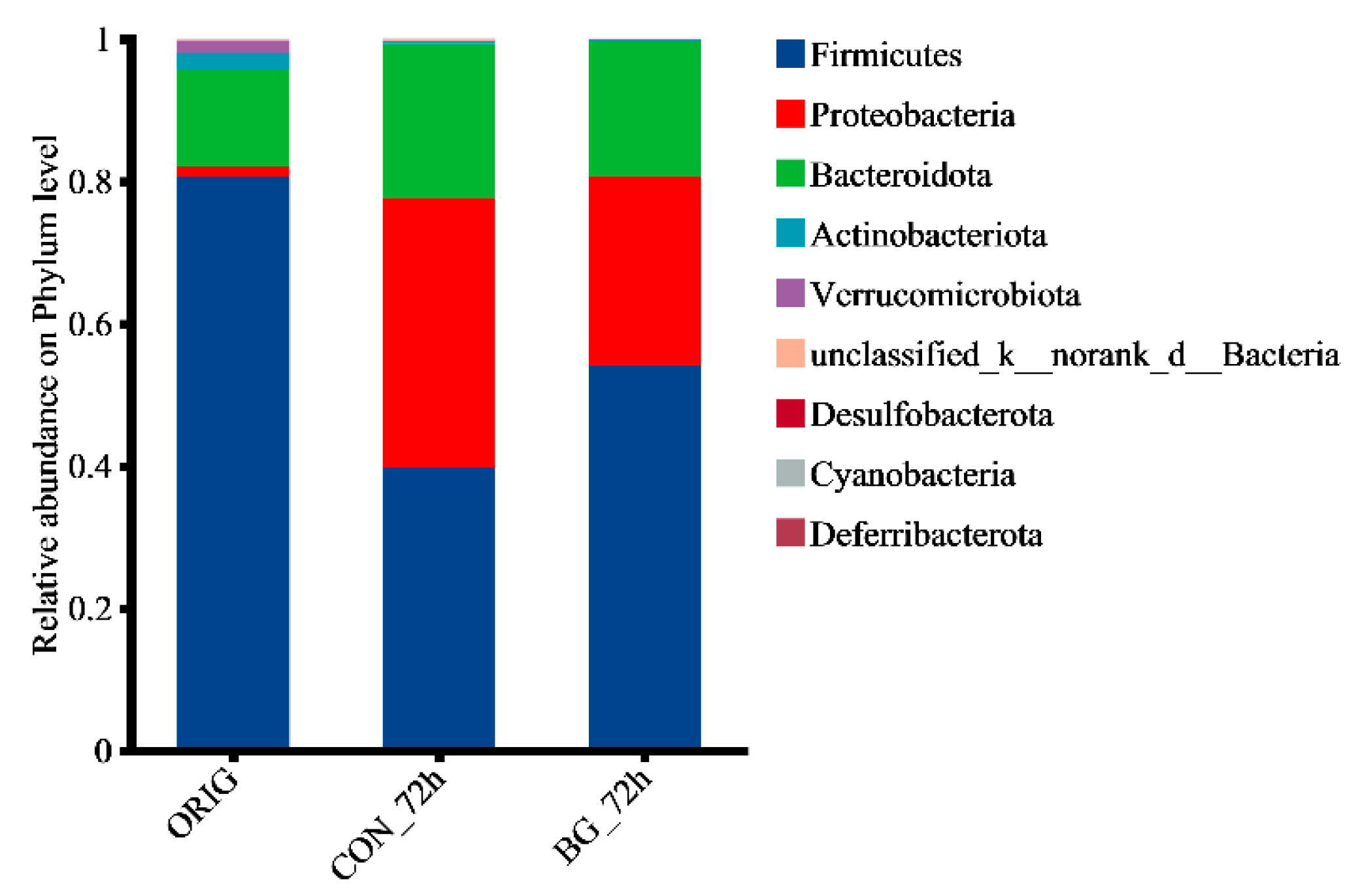
2.5. BG136 Fermentation Reshaped the Composition of Rat Gut Microbiota
3. Materials and Methods
3.1. Materials and Chemicals
3.2. In Vitro Simulated Digestion of BG136
3.2.1. Design for In Vitro Simulated Gastric Fluid Digestion
3.2.2. Design for In Vitro Simulated Intestinal Fluid Digestion
3.2.3. HPSEC-MS Detection of BG136 After Digestion
3.3. In Vitro Simulated Fermentation of BG136
3.3.1. Preparation of Fecal Fermentation Media
3.3.2. Preparation of Fecal Suspension and Fermentation
3.3.3. TLC Analysis of BG136 Degradation
3.3.4. Determination of Total Sugar, Reducing Sugar, and Calculation of Fermentability
3.3.5. HPSEC-MS Detection of BG136 After Fermentation
3.3.6. Determination of Short-Chain Fatty Acids
3.3.7. Analysis of Gut Microbiota
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lesco, K.C.; Williams, S.K.R.; Laurens, L.M.L. Marine algae polysaccharides: An overview of characterization techniques for structural and molecular elucidation. Mar. Drugs 2025, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Li, J.; Jiang, H.; Shan, X.; Wang, Y.; Ma, W.; Hao, J.; Yu, G. A β-glucan from Durvillaea antarctica has immunomodulatory effects on RAW264.7 macrophages via toll-like receptor 4. Carbohydr. Polym. 2018, 191, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xu, Y.; Zhang, M.; Wu, L.; Liu, S.; Lv, Y.; Hu, T.; Zhao, J.; Zhang, X.; Xu, X.; et al. A β-1,3/1,6-glucan enhances anti-tumor effects of PD1 antibody by reprogramming tumor microenvironment. Int. J. Biol. Macromol. 2024, 279, 134660. [Google Scholar] [CrossRef]
- Su, F.; Song, Q.; Zhang, C.; Xu, X.; Li, M.; Yao, D.; Wu, L.; Qu, X.; Guan, H.; Yu, G.; et al. A β-1,3/1,6-glucan from Durvillaea antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohydr. Polym. 2019, 222, 114993. [Google Scholar] [CrossRef]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, X.; Luo, L.; Zhang, Q.; Huang, X.; Liu, Y.; Wang, K.; Zhang, Y. Advances in oral absorption of polysaccharides: Mechanism, affecting factors, and improvement strategies. Carbohydr. Polym. 2022, 282, 119110. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zhao, N.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J. Soluble polysaccharide derived from Laminaria japonica attenuates obesity-related nonalcoholic fatty liver disease associated with gut microbiota regulation. Mar. Drugs 2021, 19, 699. [Google Scholar] [CrossRef]
- Zou, S.; Duan, B.; Xu, X. Inhibition of tumor growth by β-glucans through promoting CD4+ T cell immunomodulation and neutrophil-killing in mice—ScienceDirect. Carbohydr. Polym. 2019, 213, 370–381. [Google Scholar] [CrossRef]
- Xiao, H.; Feng, J.; Peng, J.; Wu, P.; Chang, Y.; Li, X.; Wu, J.; Huang, H.; Deng, H.; Qiu, M.; et al. Fuc-S-a new ultrasonic degraded sulfated alpha-l-fucooligosaccharide-alleviates DSS-inflicted colitis through reshaping gut microbiota and modulating host-microbe tryptophan metabolism. Mar. Drugs 2022, 21, 16. [Google Scholar] [CrossRef]
- Ma, M.; Fu, T.; Wang, Y.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. Polysaccharide from Edible Alga Enteromorpha clathrata improves ulcerative colitis in association with increased abundance of Parabacteroides spp. in the gut microbiota of dextran sulfate sodium-fed mice. Mar. Drugs 2022, 20, 764. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory effects of Lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, Y.; Shi, W.; Liu, Y.; Kan, Y.; Chen, J.; Hu, J.; Pang, W. Use of fluorescent 2-AB to explore the bidirectional transport mechanism of Pseudostellaria heterophylla polysaccharides across Caco-2 cells. Molecules 2022, 27, 3192. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef]
- Gullon, B.; Gullon, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. 2015, 6, 525–531. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Fu, X.; You, L.; Abbasi, A.M.; Liu, R.H. The digestibility of mulberry fruit polysaccharides and its impact on lipolysis under simulated saliva, gastric and intestinal conditions. Food Hydrocoll. 2016, 58, 171–178. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, C.; Zheng, Q.; Wu, J.; Zhu, K.; Shen, X.; Cao, J. Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity, molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota). Food Hydrocoll. 2019, 89, 735–741. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef]
- Li, L.; Yao, H.; Li, X.; Zhang, Q.; Wu, X.; Wong, T.; Zheng, H.; Fung, H.; Yang, B.; Ma, D.; et al. Destiny of Dendrobium officinale polysaccharide after oral administration: Indigestible and nonabsorbing, ends in modulating gut microbiota. J. Agric. Food. Chem. 2019, 67, 5968–5977. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. B-Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, Q.; Li, G.; Wang, X.; Yu, G. Degradation of marine algae-derived carbohydrates by Bacteroidetes isolated from human gut microbiota. Mar. Drugs 2017, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ai, X.; Fu, T.; Ren, L.; Shang, Q.; Li, G.; Yu, G. In vitro fermentation of hyaluronan by human gut microbiota: Changes in microbiota community and potential degradation mechanism. Carbohydr. Polym. 2021, 269, 118313. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, Y.; Luo, S.; Zhou, F.; Wu, Q.; Lu, Z. Short-chain fatty acids and cancer. Trends Cancer 2025, 11, 154–168. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.; Pires, E.; et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Ma, M.; Quan, M.; Zhang, J.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. In vitro fermentation of polysaccharide from Edible Alga Enteromorpha clathrata by the gut microbiota of patients with ulcerative colitis. Nutrients 2023, 15, 4122. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, S.; Wang, Z.; Fu, X.; Huang, Q.; Yuan, Y.; Wang, K.; Zhang, B. In vitro fecal fermentation of propionylated high-amylose maize starch and its impact on gut microbiota. Carbohydr. Polym. 2019, 223, 115069. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Chen, F.; Cui, D.; Wang, M. Advances in the in vitro digestion and fermentation of polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 4970–4982. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Gu, S.; Wu, N.; Wang, K.; Zhang, Y. Size exclusion chromatography and asymmetrical flow field-flow fractionation for structural characterization of polysaccharides: A comparative review. Int. J. Biol. Macromol. 2024, 277, 134236. [Google Scholar] [CrossRef]
- Conchie, J.; Macdonald, D.C. Glycosidases in the mammalian alimentary tract. Nature 1959, 184, 1233. [Google Scholar] [CrossRef]
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. J. Funct. Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118–121. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, R.; You, L.; Ma, Y.; Liao, L.; Pedisić, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. Food. Chem. Toxicol. 2021, 151, 112145. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Cheng, Y.; Guo, Y.; Hu, B.; Yao, W.; Zhu, X.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.A.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef]
- Procházková, N.; Laursen, M.F.; La Barbera, G.; Tsekitsidi, E.; Jørgensen, M.S.; Rasmussen, M.A.; Raes, J.; Licht, T.R.; Dragsted, L.O.; Roager, H.M. Gut physiology and environment explain variations in human gut microbiome composition and metabolism. Nat. Microbiol. 2024, 9, 3210–3225. [Google Scholar] [CrossRef]
- Yu, L.; Gao, Y.; Ye, Z.; Duan, H.; Zhao, J.; Zhang, H.; Narbad, A.; Tian, F.; Zhai, Q.; Chen, W. Interaction of beta-glucans with gut microbiota: Dietary origins, structures, degradation, metabolism, and beneficial function. Crit. Rev. Food. Sci. Nutr. 2024, 64, 9884–9909. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The therapeutic effect of SCFA-mediated regulation of the intestinal environment on obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-Del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Wu, D.T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.R.; Wang, S.P.; Gan, R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Ge, X.; Xu, W.; Chen, Y.; Li, F.; Cheng, D.; Shao, R. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from Helicteres angustifolia L. Int. J. Biol. Macromol. 2019, 141, 1065–1071. [Google Scholar] [CrossRef]
- Huang, T.; Staniak, M.; Veiga, L.F.; Figueroa-Navedo, A.M.; Ivanov, A.R.; Nesvizhskii, A.I.; Choi, M.; Vitek, O. Statistical detection of differentially abundant proteins in experiments with repeated measures designs and isobaric labeling. J. Proteome Res. 2023, 22, 2641–2659. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Buttner, L.; de Lima, R.E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Wang, J.; Xie, B.; Sun, Z. The improvement of carboxymethyl β-glucan on the antibacterial activity and intestinal flora regulation ability of lotus seedpod procyanidins. Lwt 2021, 137, 110441. [Google Scholar] [CrossRef]
- Lam, K.; Keung, H.; Ko, K.; Kwan, H.; Cheung, P.C. In vitro fermentation of beta-glucans and other selected carbohydrates by infant fecal inoculum: An evaluation of their potential as prebiotics in infant formula. Bioact. Carbohydr. Diet. Fibre 2018, 14, 20–24. [Google Scholar] [CrossRef]
- Diaz, A.M.; Almozni, B.; Molina, M.A.; Sparo, M.D.; Manghi, M.A.; Canellada, A.M.; Castro, M.S. Potentiation of the humoral immune response elicited by a commercial vaccine against bovine respiratory disease by Enterococcus faecalis CECT7121. Benef. Microbes 2018, 9, 553–562. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kamiya, S.; Hanawa, T.; Fukuda, M.; Kawakami, H.; Takahashi, H.; Yokota, H. Effect of probiotic bacterial strains of Lactobacillus, Bifidobacterium, and Enterococcus on enteroaggregative Escherichia coli. J. Infect. Chemother. 2010, 16, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yuan, M.; Chang, S.; Wang, C. N-carbamylglutamate supplementation regulates hindgut microbiota composition and short-chain fatty acid contents in Charollais and Small Tail Han crossbred sheep. Front. Vet. Sci. 2023, 10, 1230190. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.; et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef]
- Liu, T.; Asif, I.M.; Liu, L.; Zhang, M.; Li, B.; Wang, L. Laminarin ameliorates iodoacetamide-induced functional dyspepsia via modulation of 5-HT(3) receptors and the gut microbiota. Int. J. Biol. Macromol. 2024, 268, 131640. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.H.; Kim, E.; Kim, S.I.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef]
- Ren, P.; Liu, M.; Wei, B.; Tang, Q.; Wang, Y.; Xue, C. Fucoidan exerts antitumor effects by regulating gut microbiota and tryptophan metabolism. Int. J. Biol. Macromol. 2025, 300, 140334. [Google Scholar] [CrossRef]
- Shi, H.; Chang, Y.; Gao, Y.; Wang, X.; Chen, X.; Wang, Y.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017, 8, 3383–3393. [Google Scholar] [CrossRef]
- Huang, T.W.; Ho, Y.C.; Tsai, T.N.; Tseng, C.L.; Lin, C.; Mi, F.L. Enhancement of the permeability and activities of epigallocatechin gallate by quaternary ammonium chitosan/fucoidan nanoparticles. Carbohydr. Polym. 2020, 242, 116312. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native kappa-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary kappa-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, S.; Chen, B.; Gao, P.; Lv, Y.; Shang, Q.; Yu, G.; Li, G. In Vitro Digestion and Gut Microbiota Fermentation of the Anticancer Marine Drug BG136: Stability and Biotransformation Investigation. Mar. Drugs 2025, 23, 156. https://doi.org/10.3390/md23040156
Li X, Xu S, Chen B, Gao P, Lv Y, Shang Q, Yu G, Li G. In Vitro Digestion and Gut Microbiota Fermentation of the Anticancer Marine Drug BG136: Stability and Biotransformation Investigation. Marine Drugs. 2025; 23(4):156. https://doi.org/10.3390/md23040156
Chicago/Turabian StyleLi, Xintong, Shuying Xu, Baiyuan Chen, Pengcheng Gao, Youjing Lv, Qingsen Shang, Guangli Yu, and Guoyun Li. 2025. "In Vitro Digestion and Gut Microbiota Fermentation of the Anticancer Marine Drug BG136: Stability and Biotransformation Investigation" Marine Drugs 23, no. 4: 156. https://doi.org/10.3390/md23040156
APA StyleLi, X., Xu, S., Chen, B., Gao, P., Lv, Y., Shang, Q., Yu, G., & Li, G. (2025). In Vitro Digestion and Gut Microbiota Fermentation of the Anticancer Marine Drug BG136: Stability and Biotransformation Investigation. Marine Drugs, 23(4), 156. https://doi.org/10.3390/md23040156








