Optimization of Nanoencapsulation of Codium tomentosum Extract and Its Potential Application in Yogurt Fortification
Abstract
1. Introduction
2. Results and Discussion
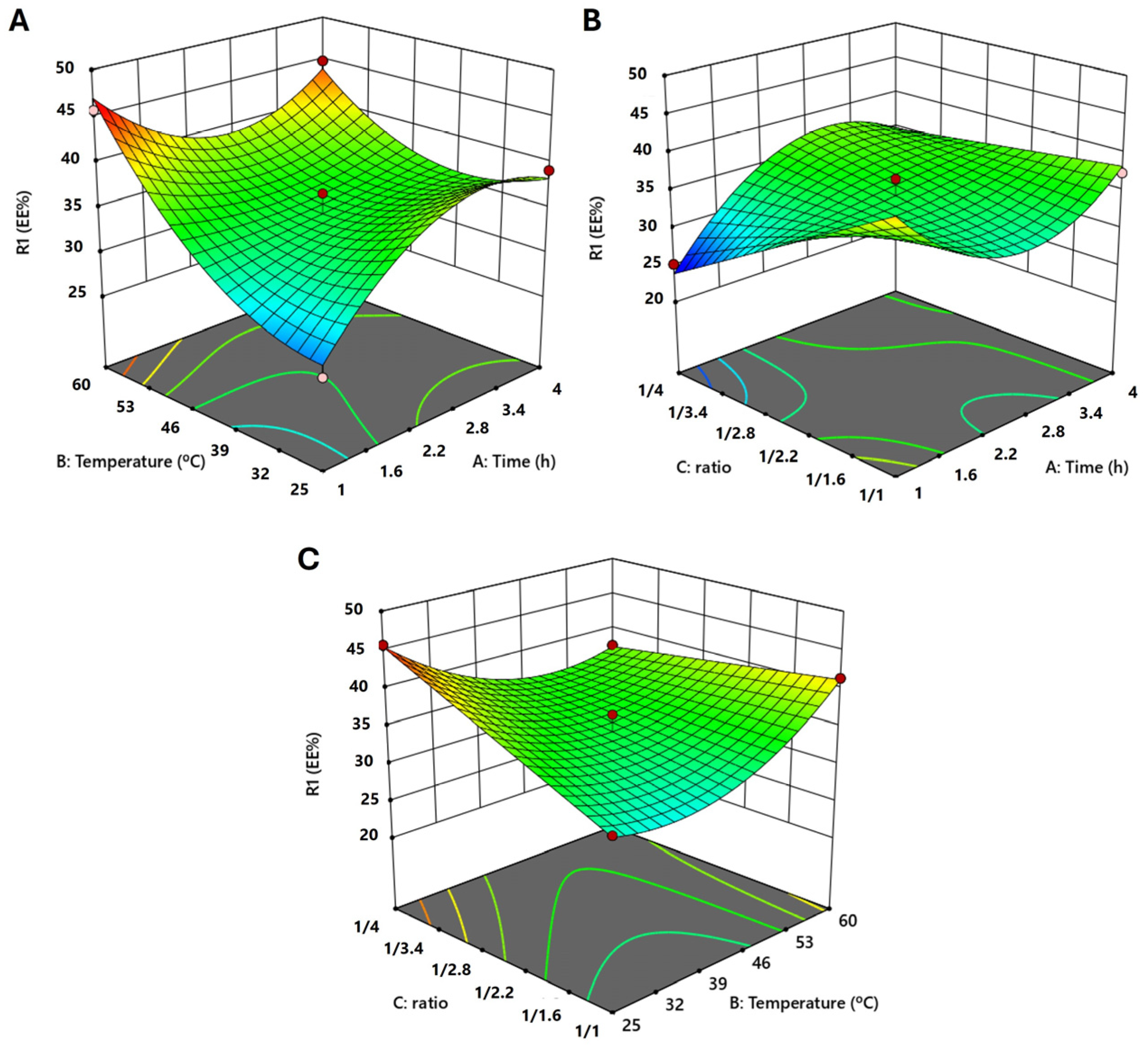
2.1. Box–Behnken Design
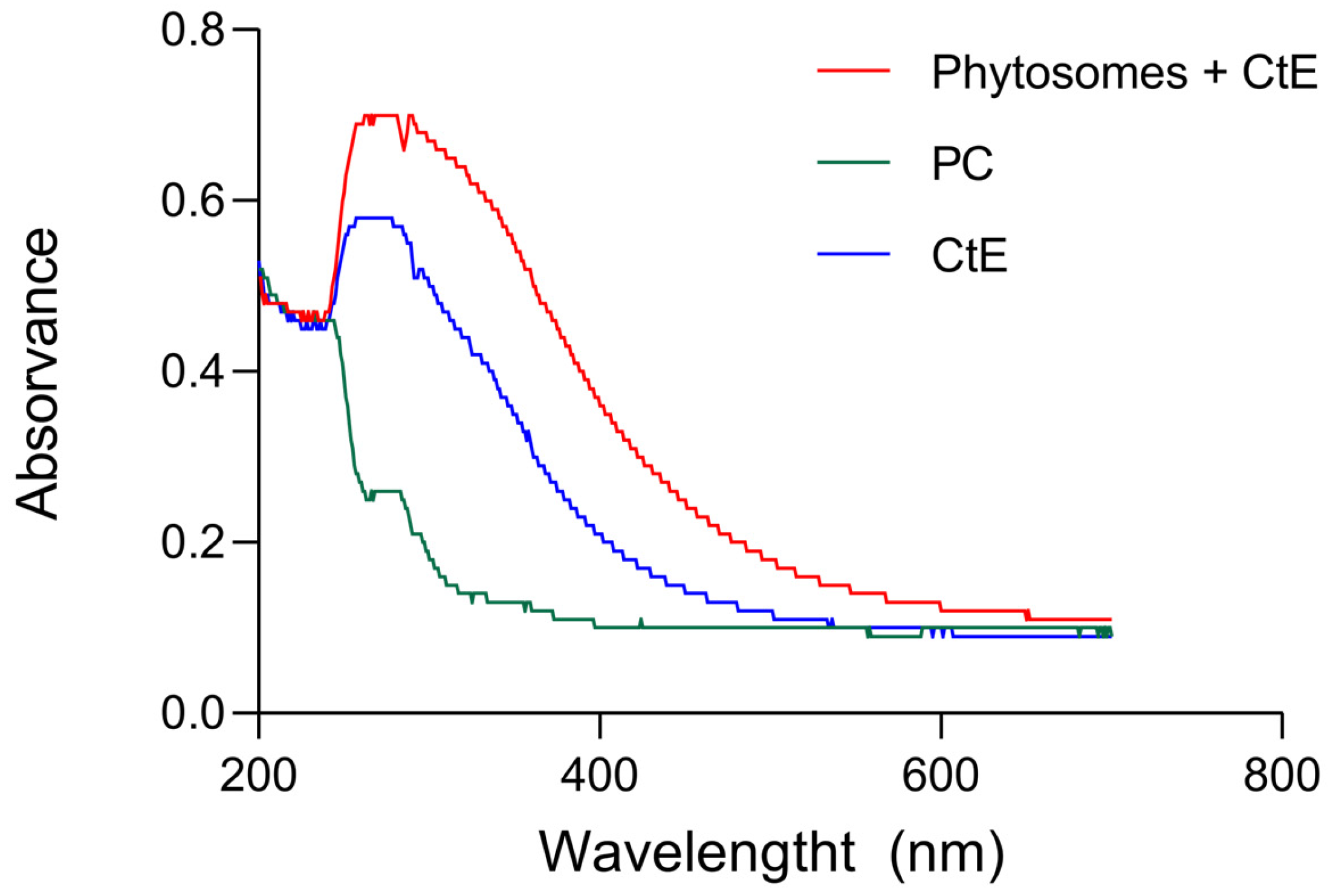
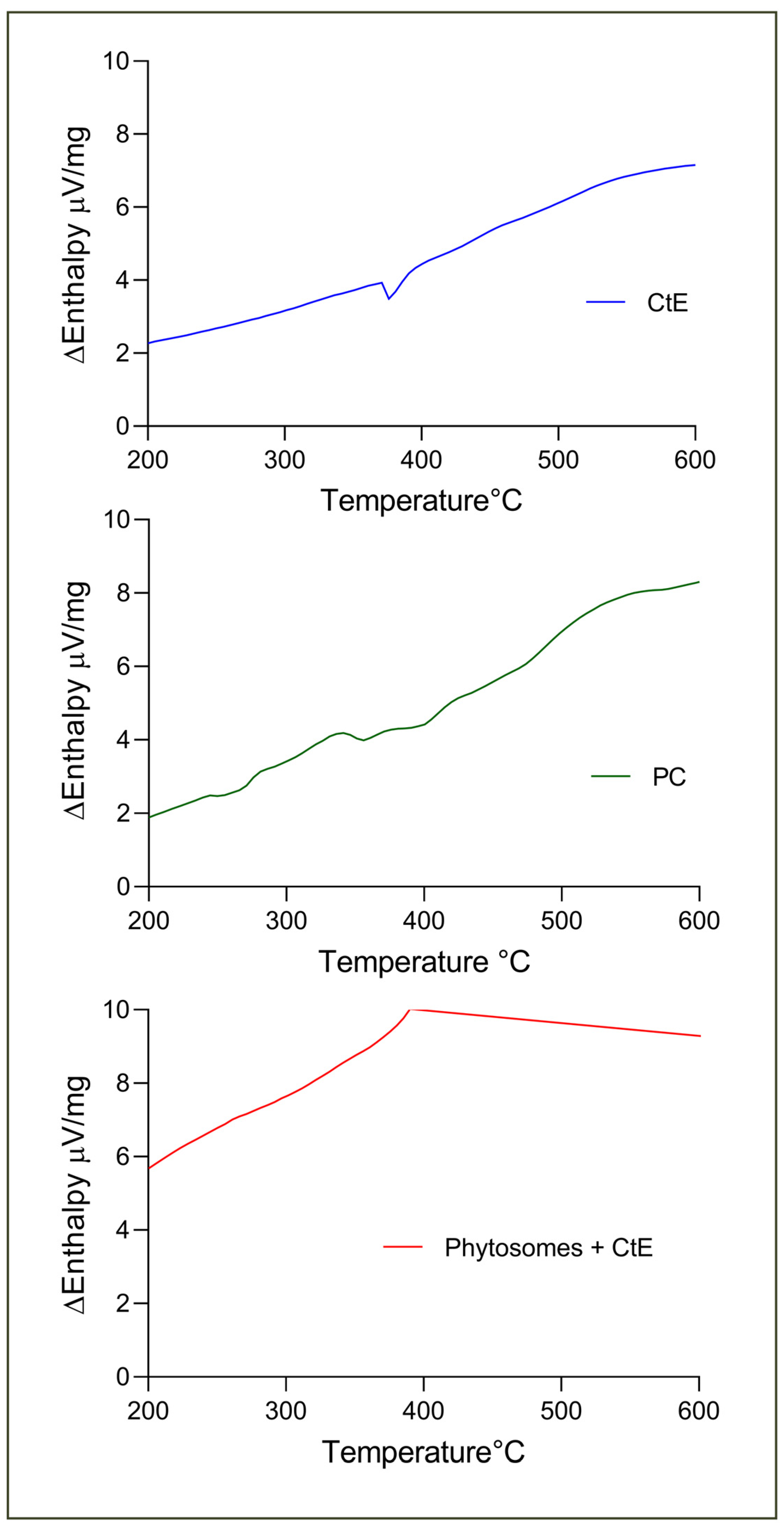
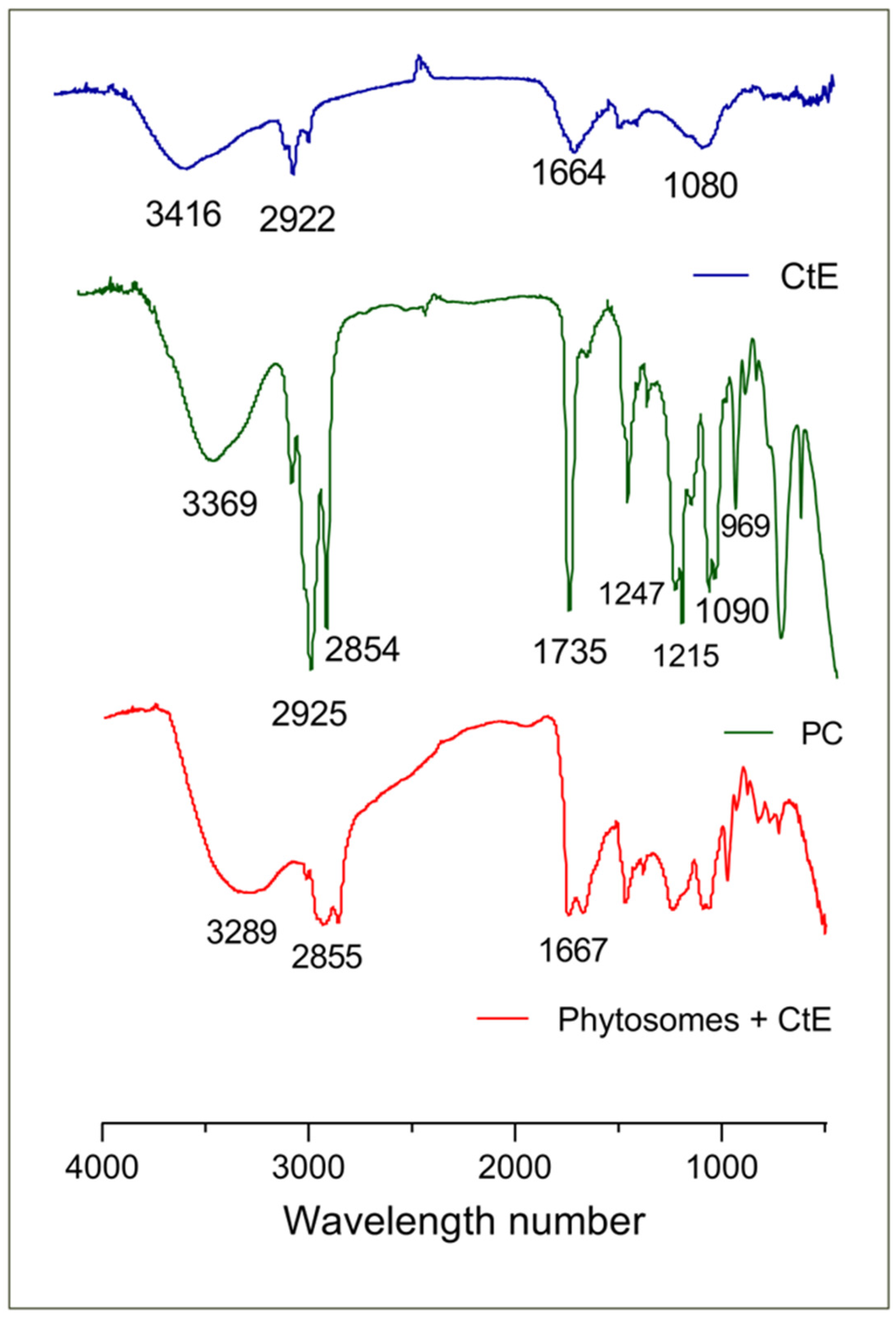
2.2. Characterization of Phytosomes
2.3. Characterization of Fortified Yogurt
2.3.1. Sensorial Analysis and Selection of Optimal Formulations for Further Analysis
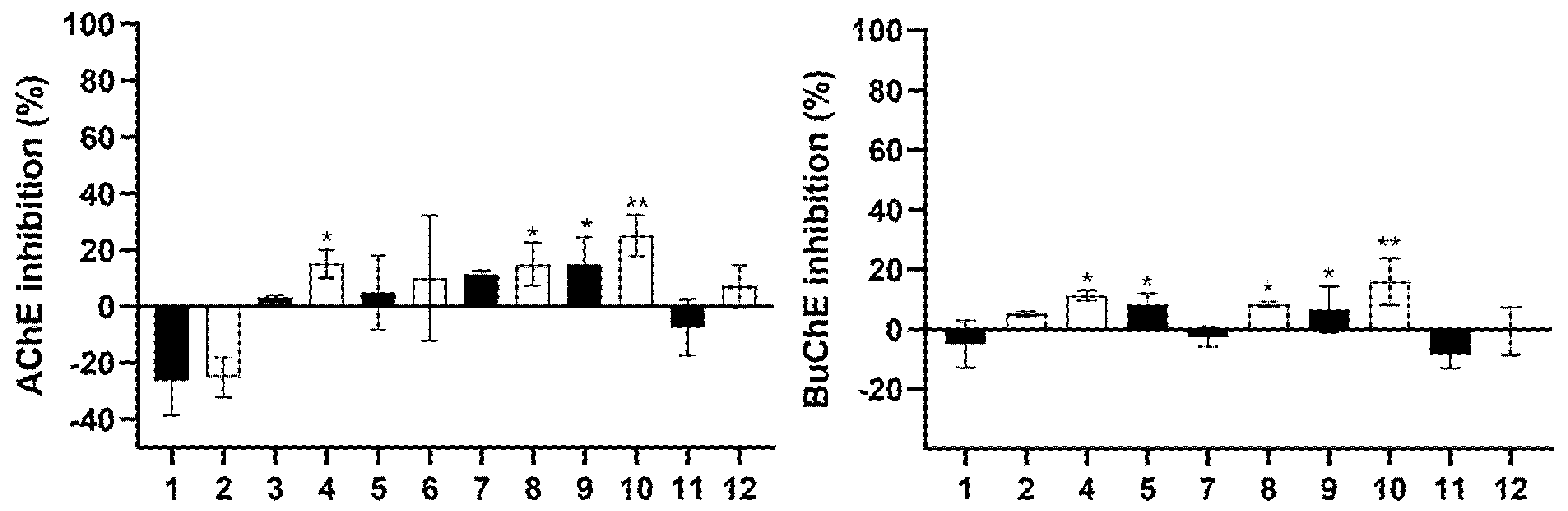
2.3.2. Inhibition Capacity of Brain Enzymes Cholinesterases (AChE and BuChE)
2.3.3. Antioxidant Activity
2.3.4. pH, Titratable Acidity, and Peroxide Value
2.3.5. Syneresis, Moisture, Total Solids, Organic, and Ash Contents in Yogurts
2.3.6. Mineral Composition (Ca, K, Mg and Na)
2.3.7. Total Lipids, Proteins, Total Sugars, and Energetic Value
3. Material and Methods
3.1. Reagents
3.2. Samples and Extraction
3.3. Box–Behnken Factorial Design
3.4. Phytosome Production and Characterization
3.5. Functional Yogurt Preparation and Characterization
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Mar. Drugs 2024, 22, 496. [Google Scholar] [CrossRef] [PubMed]
- Jilani, M.G.; Hoque, M.; Tabrez, S.; Ali, S. The Genetic Basis of Neurodegenerative Disorders: Implications in Alzheimer’s Disease. In A New Era in Alzheimer’ s Research; Singh, S.K., Maccioni, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 67–90. [Google Scholar]
- Alzheimer’s Society. Factsheet Risk Factors for Dementia; Alzheimer’s Society: Belfast, UK, 2021. [Google Scholar]
- NIH-NINDS. Parkinson’s Disease: Challenges, Progress, and Promise; National Institute of Neurological Disorders and Stroke, National Institutes of Health: Bethesda, MD, USA, 2025. [Google Scholar]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Kim, D.-O.; Kim, G.-H.; Heo, H.J. Mixture of Phlorotannin and Fucoidan from Ecklonia Cava Prevents the Aβ-Induced Cognitive Decline with Mitochondrial and Cholinergic Activation. Mar. Drugs 2021, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Van Staden, J. Acetylcholinesterase Inhibitory Activity of Phlorotannins Isolated from the Brown Alga, Ecklonia Maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Jia, C.; Chai, J.; Zhang, S.; Sun, Y.; He, L.; Sang, Z.; Chen, D.; Zheng, X. The Advancements of Marine Natural Products in the Treatment of Alzheimer’s Disease: A Study Based on Cell and Animal Experiments. Mar. Drugs 2025, 23, 91. [Google Scholar] [CrossRef]
- Lu, J.; Pan, Q.; Zhou, J.; Weng, Y.; Chen, K.; Shi, L.; Zhu, G.; Chen, C.; Li, L.; Geng, M.; et al. Pharmacokinetics, Distribution, and Excretion of Sodium Oligomannate, a Recently Approved Anti-Alzheimer’s Disease Drug in China. J. Pharm. Anal. 2022, 12, 145–155. [Google Scholar] [CrossRef]
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362. [Google Scholar] [CrossRef]
- Bosch, M.E.; Dodiya, H.B.; Michalkiewicz, J.; Lee, C.; Shaik, S.M.; Weigle, I.Q.; Zhang, C.; Osborn, J.; Nambiar, A.; Patel, P.; et al. Sodium Oligomannate Alters Gut Microbiota, Reduces Cerebral Amyloidosis and Reactive Microglia in a Sex-Specific Manner. Mol. Neurodegener. 2024, 19, 18. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Silva, J.; Martins, A.; Alves, C.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Natural Approaches for Neurological Disorders—The Neuroprotective Potential of Codium Tomentosum. Molecules 2020, 25, 5478. [Google Scholar] [CrossRef]
- Soares, C.; Paíga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simões, P.; Costa, L.; Bernardo, A.; Fernández, N.; et al. Multi-Step Subcritical Water Extracts of Fucus vesiculosus L. and Codium tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Mancini, S.; Nardo, L.; Gregori, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, C. Functionalized Liposomes and Phytosomes Loading Annona muricata L. Aqueous Extract: Potential Nanoshuttles for Brain-Delivery of Phenolic Compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Deleanu, M.; Sanda, G.M.; Barbălată, T.; Niculescu, L.Ş.; Sima, A.V.; Stancu, C.S. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 4162. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M.; Afonso, C. Seaweed Bioactives Potential as Nutraceuticals and Functional Ingredients: A Review. J. Food Compos. Anal. 2024, 133, 106453. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of Seaweeds to Develop New Food Products with Enhanced Shelf-Life, Quality and Health-Related Beneficial Properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the Formation of New Antioxidants in Natural Samples after Subcritical Water Extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Karangwa, E. Inhibition Effects of Maillard Reaction Products Derived from L-Cysteine and Glucose on Enzymatic Browning Catalyzed by Mushroom Tyrosinase and Characterization of Active Compounds by Partial Least Squares Regression Analysis. RSC Adv. 2016, 6, 65825–65836. [Google Scholar] [CrossRef]
- Bagoudou, A.F.; Matsumoto, K.; Chawalitpong, S.; Park, H.Y.; Vattem, D.A.; Nakamura, S.; Katayama, S. Maillard Reaction Products Derived from Heat-Dried Green Tomato Increase Longevity and Neuroprotection in Caenorhabditis Elegans. Food Sci. Technol. Res. 2021, 27, 747–757. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Deng, L. The Mechanism of Acrylamide-Induced Neurotoxicity: Current Status and Future Perspectives. Front. Nutr. 2022, 9, 859189. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Gong, Z.; Jiao, W.; Li, L.; Dong, M.; Xiang, T.; Feng, N.; Wu, Q. Inhibition of Polyphenols on Maillard Reaction Products and Their Induction of Related Diseases: A Comprehensive Review. Phytomedicine 2024, 128, 155589. [Google Scholar] [CrossRef]
- Takeuchi, M. Toxic AGEs (TAGE) Theory: A New Concept for Preventing the Development of Diseases Related to Lifestyle. Diabetol. Metab. Syndr. 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Deleanu, M.; Toma, L.; Sanda, G.M.; Barbălată, T.; Niculescu, L.Ş.; Sima, A.V.; Deleanu, C.; Săcărescu, L.; Suciu, A.; Alexandru, G.; et al. Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics 2023, 15, 1066. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Reis, C.; Roque, L.; Gonçalves, M.; Sanches-Silva, A.; Gaspar, M.M.; Pinto, R.; Rocha, J.; Sepodes, B.; Rosário Bronze, M.; et al. Phytosomes with Persimmon (Diospyros kaki L.) Extract: Preparation and Preliminary Demonstration of In Vivo Tolerability. Pharmaceutics 2019, 11, 296. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-Phytosome as a Potential Food-Grade Delivery System. Food Biosci. 2016, 15, 126–135. [Google Scholar] [CrossRef]
- Kumar, S.; Baldi, A.; Sharma, D.K. Characterization and In Vitro Investigation of Antiscabietic Effect of Phytosomes Assimilating Quercetin and Naringenin Rich Fraction of Pistacia Integerrima Galls Extract against Sarcoptes Scabiei. J. Drug Deliv. Sci. Technol. 2022, 67, 102851. [Google Scholar] [CrossRef]
- Telange, D.R.; Sohail, N.K.; Hemke, A.T.; Kharkar, P.S.; Pethe, A.M. Phospholipid Complex-Loaded Self-Assembled Phytosomal Soft Nanoparticles: Evidence of Enhanced Solubility, Dissolution Rate, Ex Vivo Permeability, Oral Bioavailability, and Antioxidant Potential of Mangiferin. Drug Deliv. Transl. Res. 2021, 11, 1056–1083. [Google Scholar] [CrossRef]
- Semalty, A.; Tanwar, Y.S.; Singh, D.; Rawat, M.S.M. Phosphatidylcholine Complex in Improving Oral Drug Delivery of Epicatechin: Preparation and Characterization. J. Drug Discov. Dev. Deliv. 2014, 1, 46–55. [Google Scholar]
- Singh, D.; Rawat, M.S.M.; Semalty, A.; Semalty, M. Chrysophanol–Phospholipid Complex. J. Therm. Anal. Calorim. 2013, 111, 2069–2077. [Google Scholar] [CrossRef]
- Goh, K.K.T.; Sarkar, A.; Singh, H. Milk Protein–Polysaccharide Interactions. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2014; pp. 387–419. [Google Scholar]
- O’Sullivan, A.M.; O’Grady, M.N.; O’Callaghan, Y.C.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. Seaweed Extracts as Potential Functional Ingredients in Yogurt. Innov. Food Sci. Emerg. Technol. 2016, 37, 293–299. [Google Scholar] [CrossRef]
- Abdulqahar, F.W.; El-Messery, T.M.; Zaky, A.A.; El-Said, M.M. In Vitro Digestibility of Aucklandia Costus-Loaded Nanophytosomes and Their Use in Yoghurt as a Food Model. Food Biosci. 2022, 50, 102106. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-Encapsulation of Fish Oil in Nano-Liposomes and Its Application in Fortification of Yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and Healthy Yogurts Fortified with Probiotics and Fruit Peel Powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- Nuñez, M.; Picon, A. Seaweeds in Yogurt and Quark Supplementation: Influence of Five Dehydrated Edible Seaweeds on Sensory Characteristics. Int. J. Food Sci. Technol. 2017, 52, 431–438. [Google Scholar] [CrossRef]
- Ramadan, M.M.; El-Said, M.M.; El-Messery, T.M.; Mohamed, R.S. Development of Flavored Yoghurt Fortified with Microcapsules of Triple Omega 3-6-9 for Preventing Neurotoxicity Induced by Aluminum Chloride in Rats. J. Food Process. Preserv. 2021, 45, e15759. [Google Scholar] [CrossRef]
- Dewi, E.N.; Purnamayati, L. Characterization of Caulerpa Racemosa Yogurt Processed Using Lactobacillus Bulgaricus and Streptococcus Thermophilus. Food Res. 2021, 5, 54–61. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent Advances of Fermented Fruits: A Review on Strains, Fermentation Strategies, and Functional Activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef]
- Savaghebi, D.; Ghaderi-Ghahfarokhi, M.; Barzegar, M. Encapsulation of Sargassum Boveanum Algae Extract in Nano-Liposomes: Application in Functional Mayonnaise Production. Food Bioprocess Technol. 2021, 14, 1311–1325. [Google Scholar] [CrossRef]
- Gaspar, P.; Carvalho, A.L.; Vinga, S.; Santos, H.; Neves, A.R. From Physiology to Systems Metabolic Engineering for the Production of Biochemicals by Lactic Acid Bacteria. Biotechnol. Adv. 2013, 31, 764–788. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Standard for Fermented Milks (CODEX STAN 243-2003) Milk and Milk Products; FAO: Rome, Italy, 2015. [Google Scholar]
- Clarke, H.J.; McCarthy, W.P.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods 2021, 10, 2315. [Google Scholar] [CrossRef] [PubMed]
- Marín, D.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Encapsulation of Food Waste Compounds in Soy Phosphatidylcholine Liposomes: Effect of Freeze-Drying, Storage Stability and Functional Aptitude. J. Food Eng. 2018, 223, 132–143. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Taneja, A.; Zhu, X.; Singh, H. Evaluation of Processed Cheese Fortified with Fish Oil Emulsion. Food Res. Int. 2009, 42, 1093–1098. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Aydın, S.; Sözeri, D.; Demirci, T.; Sert, D.; Akın, N. Fortification of Set-Type Yoghurts with Elaeagnus angustifolia L. Flours: Effects on Physicochemical, Textural, and Microstructural Characteristics. LWT 2018, 90, 620–626. [Google Scholar] [CrossRef]
- Górnicki, K.; Choińska, A.; Kaleta, A. Effect of Variety on Rehydration Characteristics of Dried Apples. Processes 2020, 8, 1454. [Google Scholar] [CrossRef]
- Greis, M.; Sainio, T.; Katina, K.; Nolden, A.A.; Kinchla, A.J.; Seppä, L.; Partanen, R. Physicochemical Properties and Mouthfeel in Commercial Plant-Based Yogurts. Foods 2022, 11, 941. [Google Scholar] [CrossRef]
- Amal, A.; Eman, A.; Nahla, S.Z. Fruit Flavored Yogurt: Chemical, Functional and Rheological Properties. Int. J. Environ. Agric. Res. 2016, 2, 57–66. [Google Scholar]
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Salehi, F. Quality, Physicochemical, and Textural Properties of Dairy Products Containing Fruits and Vegetables: A Review. Food Sci. Nutr. 2021, 9, 4666–4686. [Google Scholar] [CrossRef]
- Sánchez-Segarra, P. Influence of the Addition of Fruit on the Mineral Content of Yoghurts: Nutritional Assessment. Food Chem. 2000, 71, 85–89. [Google Scholar] [CrossRef]
- Souza, S.O.; Santos, V.S.; Santos, E.S.; Ávila, D.V.L.; Nascimento, C.C.; Costa, S.S.L.; Garcia, C.A.B.; Araujo, R.G.O. Evaluation of the Mineral Content in Milk and Yogurt Types Using Chemometric Tools. Microchem. J. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent Developments in the Application of Seaweeds or Seaweed Extracts as a Means for Enhancing the Safety and Quality Attributes of Foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Correia, H.; Soares, C.; Morais, S.; Pinto, E.; Marques, A.; Nunes, M.L.; Almeida, A.; Delerue-Matos, C. Seaweeds Rehydration and Boiling: Impact on Iodine, Sodium, Potassium, Selenium, and Total Arsenic Contents and Health Benefits for Consumption. Food Chem. Toxicol. 2021, 155, 112385. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Pannerchelvan, S.; Rios-Solis, L.; Wasoh, H.; Sobri, M.Z.M.; Faizal Wong, F.W.; Mohamed, M.S.; Mohamad, R.; Halim, M. Functional Yogurt: A Comprehensive Review of Its Nutritional Composition and Health Benefits. Food Funct. 2024, 15, 10927–10955. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Sloan, A.E. Top 10 Functional Food Trends: Reinventing Wellness. Food Technol. 2004, 58, 28–51. [Google Scholar]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Research, G.V. Functional Foods Market Size & Trends Analysis Report, 2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/functional-food-market (accessed on 3 March 2025).
- Saeed, M.; Ali, S.W.; Ramzan, S. Physicochemical Analysis of Mango Flavored Yogurt Supplemented with Moringa Oleifera Leaf Powder. J. Food Sci. Technol. 2021, 58, 4805–4814. [Google Scholar] [CrossRef]
- Rabbani, M.; Pezeshki, A.; Ahmadi, R.; Mohammadi, M.; Tabibiazar, M.; Ahmadzadeh Nobari Azar, F.; Ghorbani, M. Phytosomal Nanocarriers for Encapsulation and Delivery of Resveratrol- Preparation, Characterization, and Application in Mayonnaise. LWT 2021, 151, 112093. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, L 304, 18–63.
- Ramalhosa, M.J.; Paíga, P.; Morais, S.; Rui Alves, M.; Delerue-Matos, C.; Oliveira, M.B.P.P. Lipid Content of Frozen Fish: Comparison of Different Extraction Methods and Variability during Freezing Storage. Food Chem. 2012, 131, 328–336. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Tenreiro Machado, J.A.; Lopes, A.M.; Vieira, E.; Delerue-Matos, C. Electrochemical Impedance Spectroscopy Characterization of Beverages. Food Chem. 2020, 302, 125345. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mendes, M.; Carvalho, A.P.; Magalhães, J.M.C.S.; Moreira, M.; Guido, L.; Gomes, A.M.; Delerue-Matos, C. Response Surface Evaluation of Microwave-Assisted Extraction Conditions for Lycium Barbarum Bioactive Compounds. Innov. Food Sci. Emerg. Technol. 2016, 33, 319–326. [Google Scholar] [CrossRef]
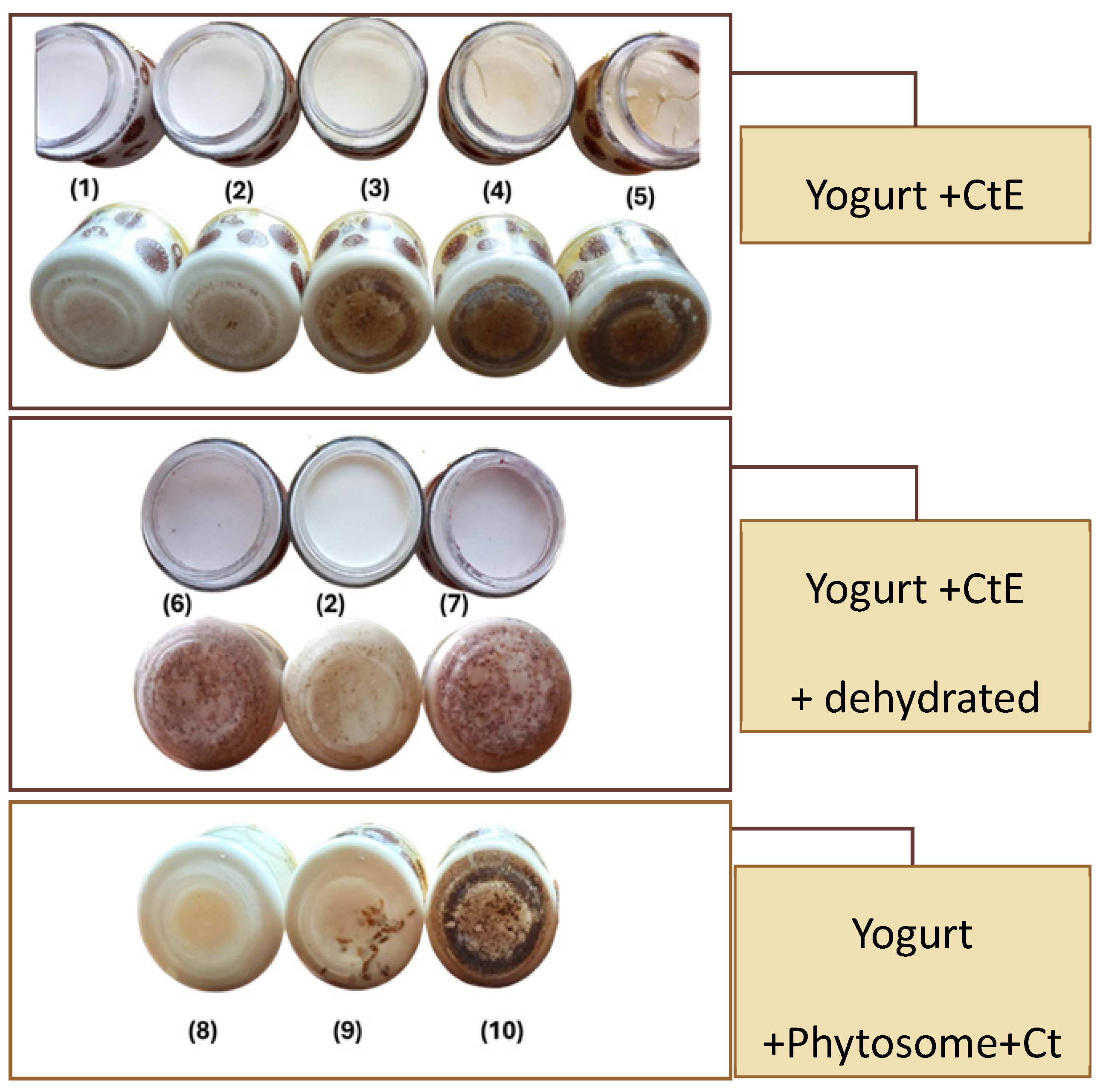
| Samples | ABTS (mg TE/100 g) | ORAC (μmol TE/100 g) |
|---|---|---|
| Control (0%) | 13.5 ± 0.3 a | 62.1 ± 0.3 a |
| 0.01% CtE | 11.1 ± 0.1 a | 118 ± 1 b |
| 0.01% CtE + DF | 24.2 ± 1.0 c | 245 ± 1 e |
| 0.15% CtE | 18.4 ± 1.6 b | 162 ± 3 c |
| 0.15% CtE + DF | 27.0 ± 0.7 d | 167 ± 1 d |
| 0.01% Phytosomes | 12.9 ± 1.2 a | 60.0 ± 0.1 a |
| Samples | pH0 | pH7 | TA0 (g LA/100 g Yogurt) | TA7 (g LA/100 g Yogurt) | PV7 (meqO2/100 g Yogurt) |
|---|---|---|---|---|---|
| Control (0%) | 4.36 ± 0.01 B,d | 4.31 ± 0.01 A,d | 0.708 ± 0.003 A,b | 0.850 ± 0.029 B,b | n.d. |
| 0.01% CtE | 4.37 ± 0.01 A,d | 4.33 ± 0.01 A,e | 0.683 ± 0.028 A,b | 0.773 ± 0.089 A,a,b | 0.0267 ± 0.0012 b |
| 0.01% CtE + DF | 4.26 ± 0.01 B,a | 4.23 ± 0.01 A,a | 0.820 ± 0.004 B,c | 0.757 ± 0.022 A,a,b | 0.0254 ± 0.0015 b |
| 0.15% CtE | 4.31 ± 0.01 B,c | 4.29 ± 0.01 A,c | 0.816 ± 0.001 B,c | 0.801 ± 0.001 A,b | 0.0418 ± 0.0033 c |
| 0.15% CtE + DF | 4.28 ± 0.01 A,b | 4.24 ± 0.01 B,b | 0.828 ± 0.001 B,c | 0.800 ± 0.001 A,b | 0.0483 ± 0.0044 c |
| 0.01% Phytosomes | 4.40 ± 0.01 B,e | 4.37 ± 0.01 A,f | 0.541 ± 0.002 A,a | 0.679 ± 0.001 B,a | 0.00635 ± 0.00032 a |
| Samples | Syneresis (%) | Moisture (%) | Total Solids (%) | Organic Content (%) | Ash (%) |
|---|---|---|---|---|---|
| Control (0%) | 19.8 ± 1.4 a | 90.1 ± 0.1 c | 9.92 ± 0.08 a | 94.8 ± 0.2 c | 5.20 ± 0.25 a |
| 0.01% CtE | 30.1 ± 2.4 b | 90.4 ± 0.3 c | 9.63 ± 0.30 a | 94.1 ± 0.2 b | 5.87 ± 0.24 b |
| 0.01% CtE + DF | 18.2 ± 0.7 a | 87.9 ± 0.1 a | 12.1 ± 0.1 c | 92.6 ± 0.4 a | 7.43 ± 0.38 c |
| 0.15% CtE | 52.5 ± 3.2 d | 88.9 ± 0.1 b | 11.1 ± 0.1 b | 92.6 ± 0.2 a | 7.38 ± 0.15 c |
| 0.15% CtE + DF | 39.6 ± 3.6 c | 87.9 ± 0.1 a | 12.1 ± 0.1 c | 92.3 ± 0.3 a | 7.73 ± 0.27 c |
| 0.01% Phytosomes | 17.2 ± 1.0 a | 88.2 ± 0.1 a | 11.8 ± 0.1 c | 92.2 ± 0.1 a | 7.82 ± 0.01 c |
| Samples | Ca (mg/100 g Yogurt) | K (mg/100 g Yogurt) | Mg (mg/100 g Yogurt) | Na (mg/100 g Yogurt) | Salt (mg/100 g Yogurt) |
|---|---|---|---|---|---|
| Control (0%) | 96.8 ± 3.9 a | 104 ± 19 a,b | 21.6 ± 1.1 a | 87.5 ± 16.9 a | 219 |
| 0.01% CtE | 96.2 ± 3.0 a | 93.4 ± 4.3 a | 22.6 ± 2.3 a | 85.3 ± 1.9 a | 213 |
| 0.01% CtE + DF | 96.4 ± 3.7 a | 144 ± 3 c | 22.9 ± 0.2 a | 76.7 ± 1.8 a | 192 |
| 0.15% CtE | 106 ± 1 a,b | 121 ± 4 b,c | 22.1 ± 0.3 a | 84.7 ± 4.5 a | 212 |
| 0.15% CtE + DF | 103 ± 4 a,b | 137 ± 2 c | 22.7 ± 0.4 a | 75.7 ± 0.8 a | 189 |
| 0.01% Phytosomes | 113 ± 10 b | 99.4 ± 7.4 a,b | 19.3 ± 2.0 a | 75.9 ± 6.4 a | 190 |
| Samples | Total Lipids (%) | Total Sugars (%) | Total Protein (%) | Caloric Content (kcal) |
|---|---|---|---|---|
| Control (0%) | 1.05 ± 0.09 a,b | 2.10 ± 0.19 b | 3.17 ± 0.29 a | 30.6 |
| 0.01% CtE | 0.794 ± 0.056 a | 1.51 ± 0.11 a | 3.54 ± 0.25 a,b | 27.3 |
| 0.01% CtE + DF | 0.921 ± 0.037 a,b | 1.27 ± 0.05 a | 3.75 ± 0.15 a,b,c | 28.4 |
| 0.15% CtE | 1.49 ± 0.10 b,c | 1.37 ± 0.10 a | 4.28 ± 0.30 b,c | 36.0 |
| 0.15% CtE + DF | 1.89 ± 0.09 c | 2.14 ± 0.11 b | 4.40 ± 0.22 c | 43.2 |
| 0.01% Phytosomes | 5.35 ± 0.48 d | 3.00 ± 0.27 c | 4.45 ± 0.40 c | 78.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.; Soares, C.; Silva, A.; Barroso, M.F.; Simões, P.; Ferreira, M.; Gameiro, P.; Grosso, C.; Delerue-Matos, C. Optimization of Nanoencapsulation of Codium tomentosum Extract and Its Potential Application in Yogurt Fortification. Mar. Drugs 2025, 23, 147. https://doi.org/10.3390/md23040147
Costa M, Soares C, Silva A, Barroso MF, Simões P, Ferreira M, Gameiro P, Grosso C, Delerue-Matos C. Optimization of Nanoencapsulation of Codium tomentosum Extract and Its Potential Application in Yogurt Fortification. Marine Drugs. 2025; 23(4):147. https://doi.org/10.3390/md23040147
Chicago/Turabian StyleCosta, Micaela, Cristina Soares, Aurora Silva, Maria Fátima Barroso, Pedro Simões, Mariana Ferreira, Paula Gameiro, Clara Grosso, and Cristina Delerue-Matos. 2025. "Optimization of Nanoencapsulation of Codium tomentosum Extract and Its Potential Application in Yogurt Fortification" Marine Drugs 23, no. 4: 147. https://doi.org/10.3390/md23040147
APA StyleCosta, M., Soares, C., Silva, A., Barroso, M. F., Simões, P., Ferreira, M., Gameiro, P., Grosso, C., & Delerue-Matos, C. (2025). Optimization of Nanoencapsulation of Codium tomentosum Extract and Its Potential Application in Yogurt Fortification. Marine Drugs, 23(4), 147. https://doi.org/10.3390/md23040147














