The Benthic Dinoflagellate Coolia malayensis (Dinophyceae) Produces an Array of Compounds with Antineoplastic Activity in Cells of Tumor Origin
Abstract
1. Introduction
1.1. The Benthic Dinoflagellate Coolia Malayensis
1.2. Cancer and the Search for New Molecules in the Marine Environment
2. Results
2.1. Maceration Times and Yields of Extracts
| Maceration | Extract/Phase | Weight (mg) | Yield (%) | Origin |
|---|---|---|---|---|
| 72 h 3677 mg dry biomass | MET | 785 | 21.35 | Biomass |
| DCM | 177 | - | MET | |
| AQU | 170 | - | MET | |
| HYD | 32 | 0.87 | Biomass (extracted pellet) | |
| 120 h 2817 mg dry biomass | MET | 865 | 30.72 | Biomass |
| DCM | 100 | - | MET | |
| AQU | 239 | - | MET | |
| HYD | 96 | 3.41 | Biomass (Pellet) |
2.2. Effect of Crude Extract (MET); Secondary Extract (HYD) and Phases (DCM, AQU) on Cell Viability
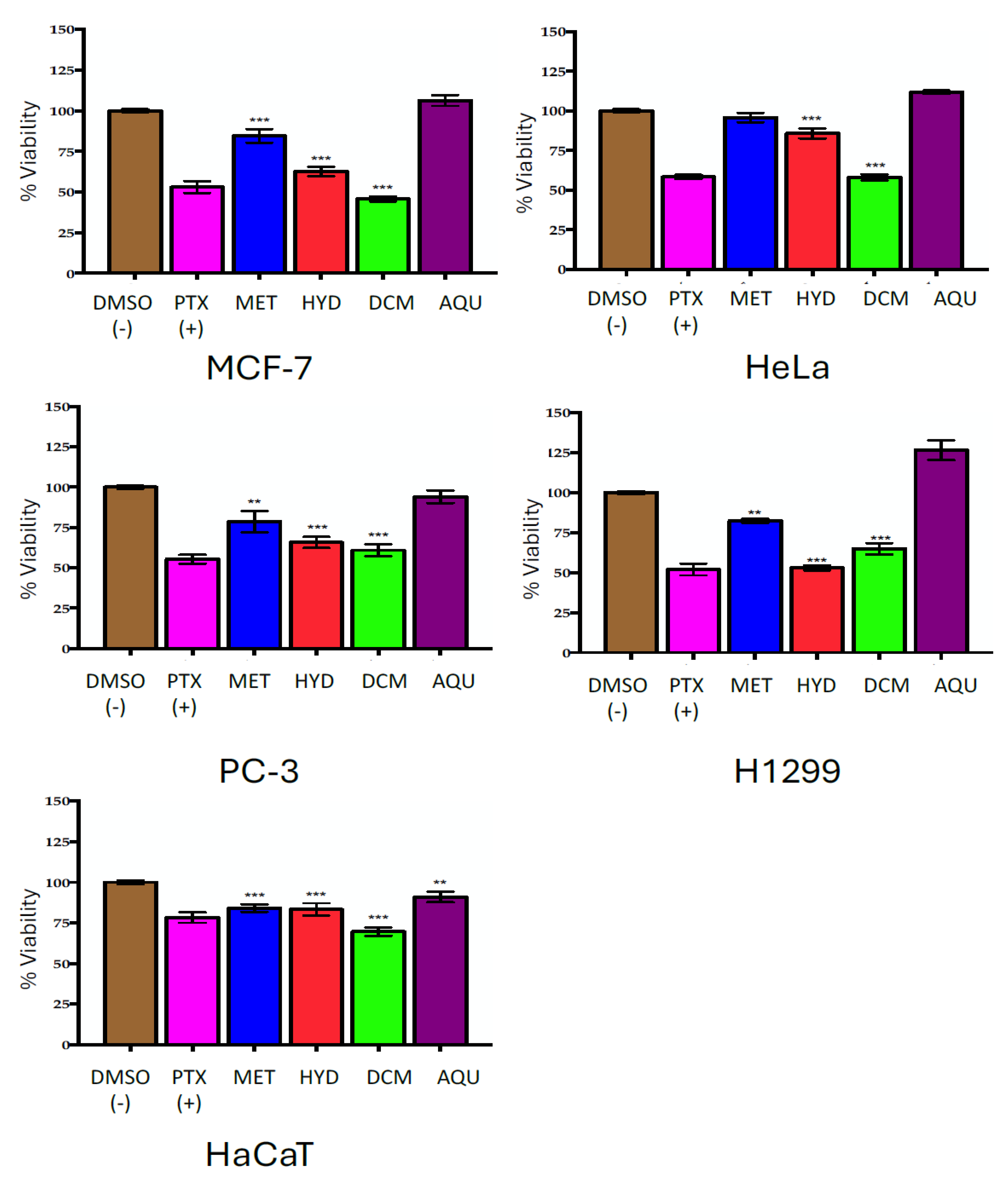
| Cell Line/% Inhibition ± SD | |||||
|---|---|---|---|---|---|
| Treatment | MCF-7 | HeLa | PC-3 | H1299 | HaCaT |
| MET | −15.51 ± 4.15 | 4.19 ± 3.00 | 21.40 ± 6.61 | 17.70 ± 1.23 | 15.85 ± 2.36 |
| HYD | 37.34 ± 2.99 | 14.21 ± 3.09 | 31.31 ± 3.46 | 47.03 ± 1.61 | 16.55 ± 3.80 |
| DCM | 54.25 ± 1.59 | 41.92 ± 1.96 | 39.14 ± 3.64 | 35.19 ± 3.48 | 30.40 ± 2.57 |
| AQU | −6.29 ± 3.31 | −11.77 ± 1.02 | 5.93 ± 3.95 | −26.50 ± 6.21 | 9.03 ± 3.26 |
| PTX (+) | 46.94 ± 3.64 | 41.68 ± 1.35 | 44.73 ± 2.73 | 45.04 ± 3.73 | 21.75 ± 3.22 |
2.3. Effect of Sub-Extracts on Cell Viability
2.4. Chromatographic Fractionation of the Methanolic Crude Extract
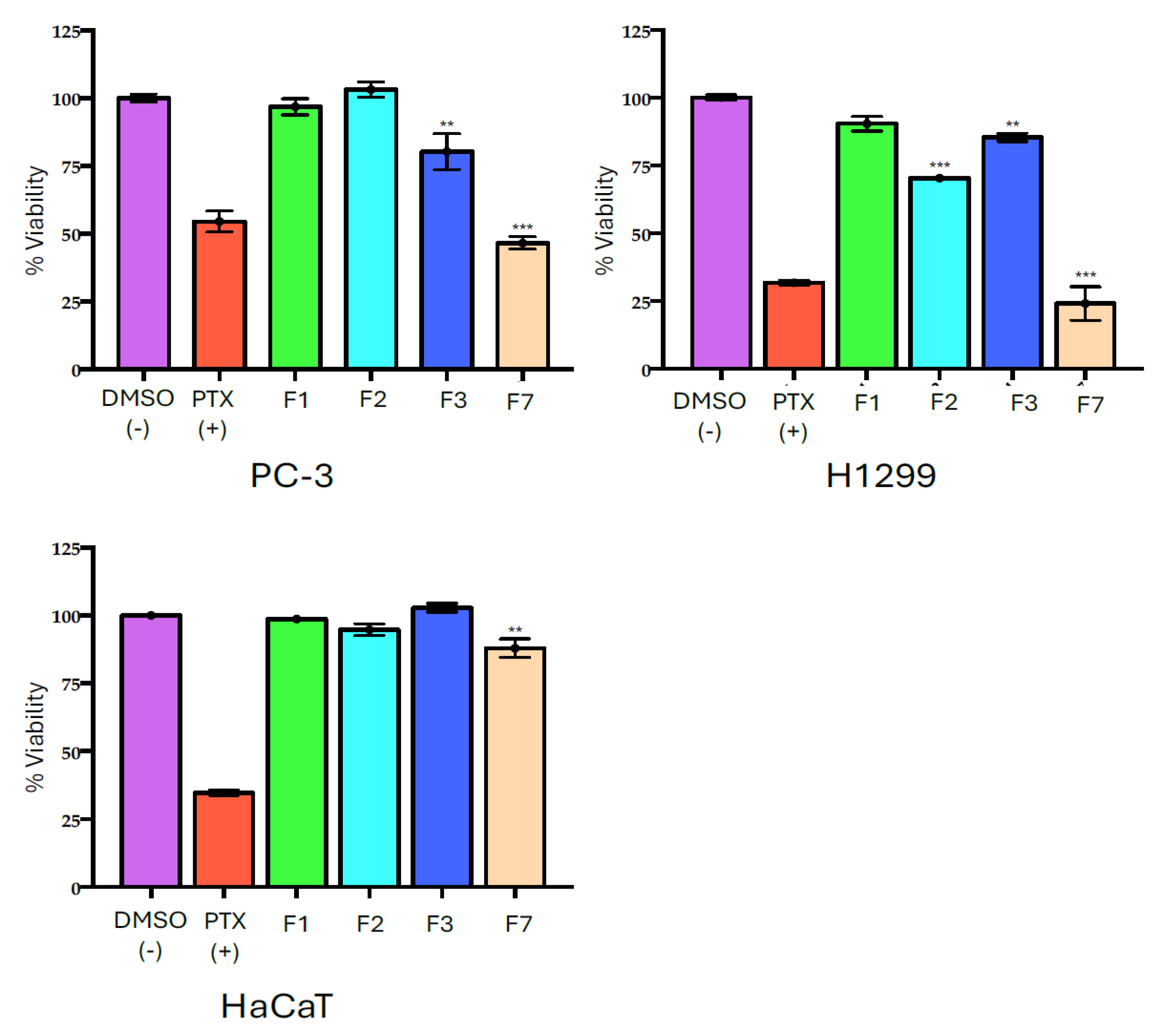
2.5. Effect of MET Fractions on Cell Viability
2.6. Half-Maximal Inhibitory Concentration of Active Sub-Extract and Fractions
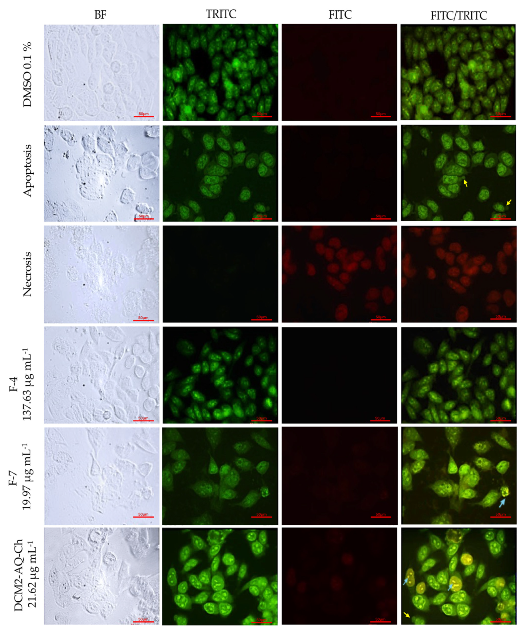
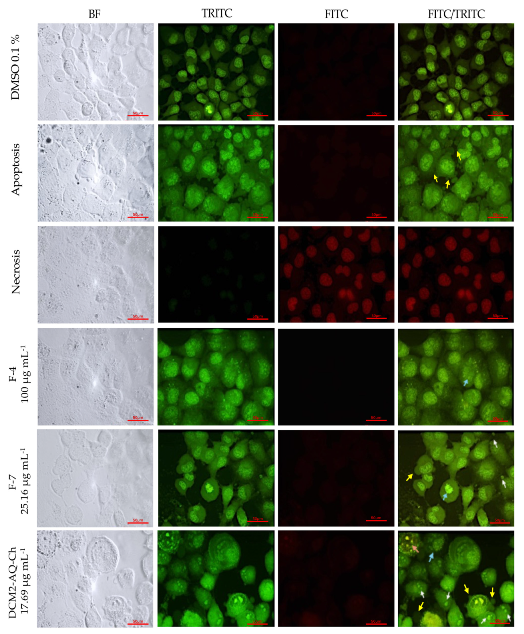
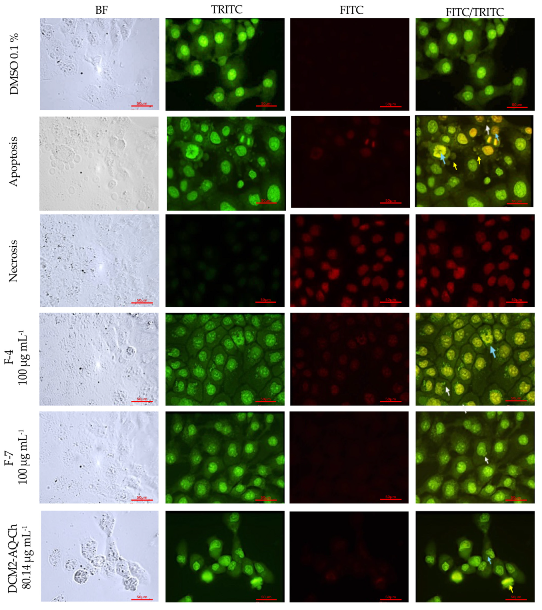
2.7. Acridine Orange (AO)-Ethidium Bromide (EB) Double Staining Cell Morphological Analysis
2.7.1. PC-3 Cell Line
2.7.2. H1299 Cell Line
2.7.3. HaCaT Cell Line
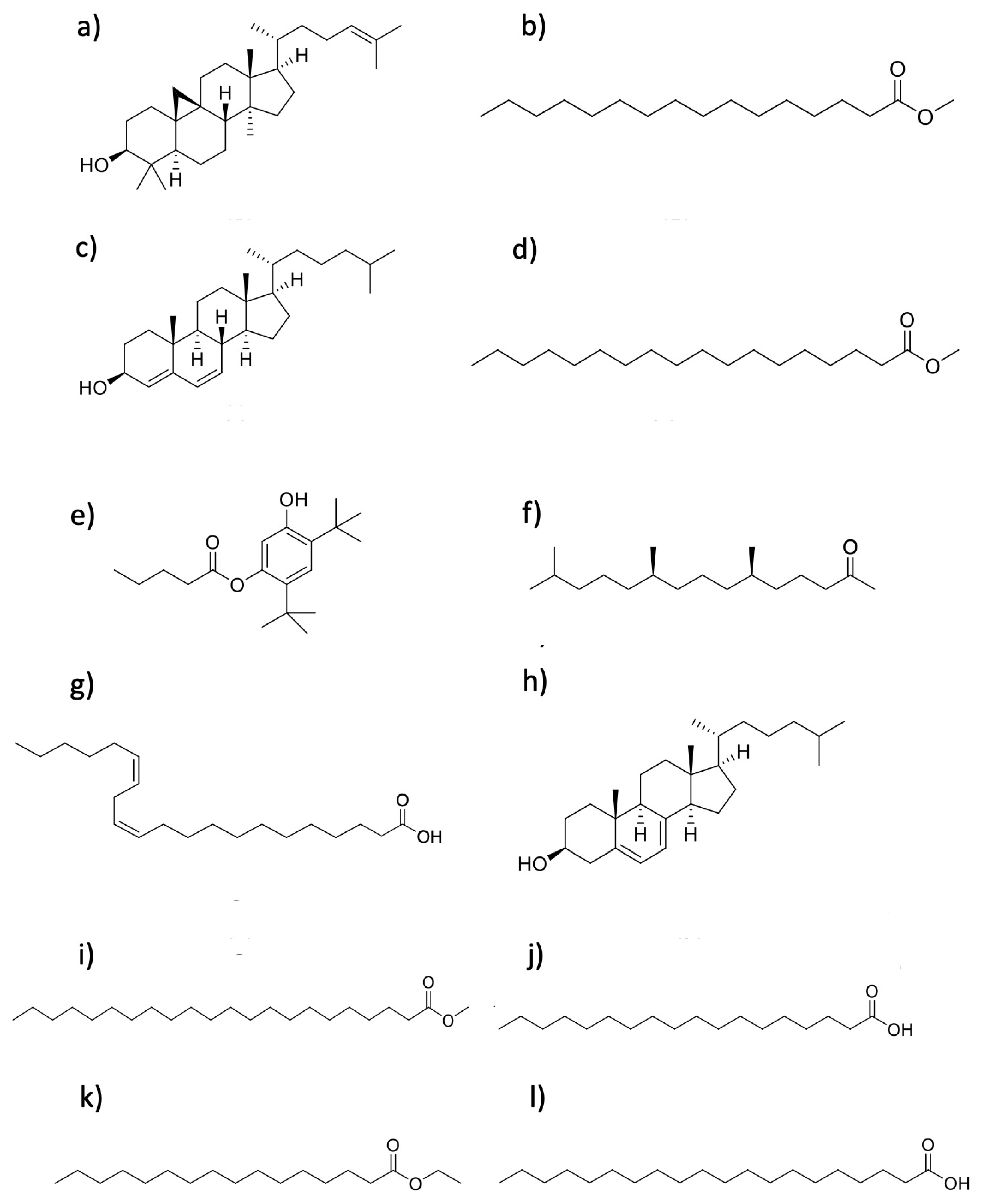
2.8. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Active Dichloromethane Phase
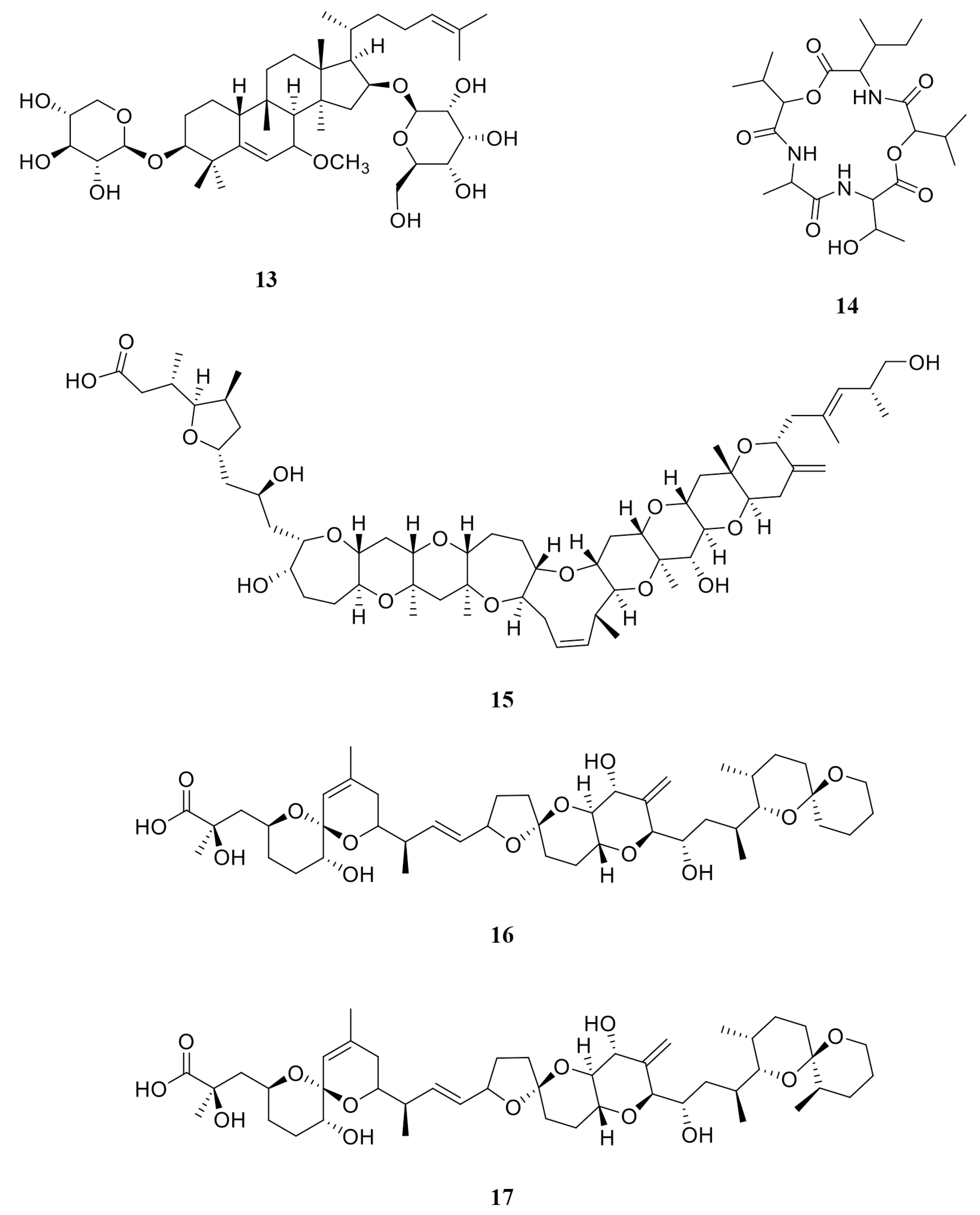
| Figure 7 | Compound | MW | RT (Min) | LRI (Calculated) | LRI (Reported) | Relative Abundance % | SI % (Similarity Index) |
|---|---|---|---|---|---|---|---|
| a | Cycloartenol | 426.70 | 121.088 | 3487 | 3465 [18] | 24.72 | 99 |
| b | Methyl palmitate | 270.50 | 61.233 | 1870 | 1927 [19] | 24.63 | 98 |
| c | (3β)-Cholesta-4,6-dien-3-ol | 384.63 | 97.423 | 2742 | NR | 15.16 | 95 |
| d | Methyl stearate | 298.50 | 70.426 | 2064 | 2106 [20] | 11.51 | 99 |
| e | Pentanoic acid, 5-hydroxy-2,4-di-t-butylphenyl ester | 306.45 | 40.05 | 1481 | 1512 [21] | 6.91 | 99 |
| f | Phytone | 268.48 | 57.229 | 1790 | 1836 [22] | 4.86 | 98 |
| g | cis-13,16-Docosadienoic acid | 336.55 | 87.715 | 2478 | 2566 * | 3.76 | 97 |
| h | (3β)-Cholesta-5,7-dienol | 384.63 | 108.088 | 3067 | 3160 * | 2.62 | 93 |
| i | Docosanoic acid methyl ester | 354.61 | 89.666 | 2529 | 2530 [20] | 2.20 | 95 |
| j | Stearic acid | 284.48 | 74.558 | 2157 | 2161 [23] | 1.88 | 92 |
| k | Ethyl palmitate | 284.47 | 64.586 | 1939 | 1978 [24] | 1.26 | 98 |
| l | Eicosanoic acid | 312.53 | 81.915 | 2331 | 2359 [25] | 0.60 | 96 |
2.9. Nuclear Magnetic Resonance (NMR) Analysis of the Active Extracts and Fractions
2.9.1. Dichloromethane Phase (DCM)
2.9.2. Sub Extract DCM2-AQ-Ch
2.9.3. Fraction F4
2.9.4. Fraction F7
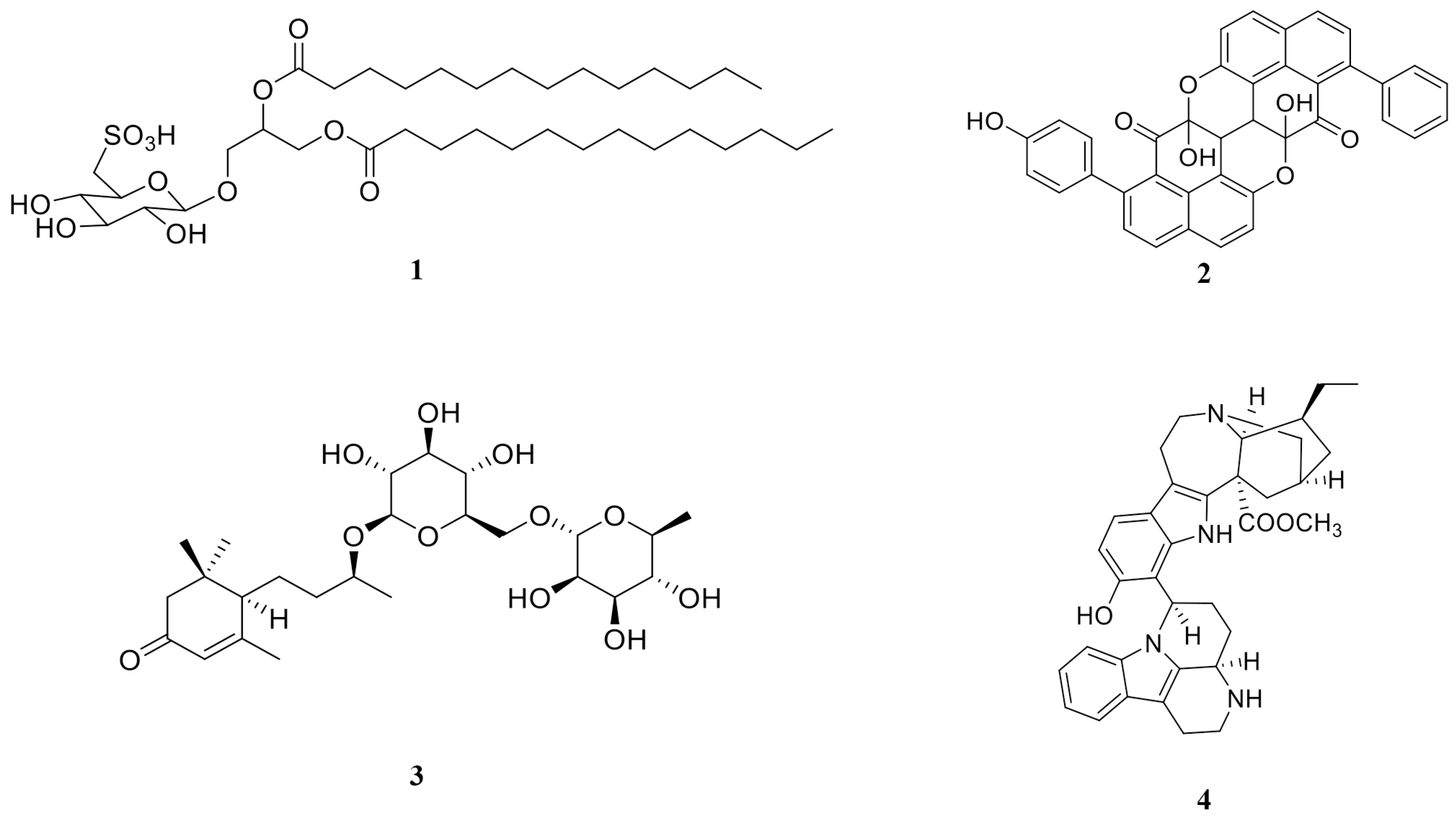
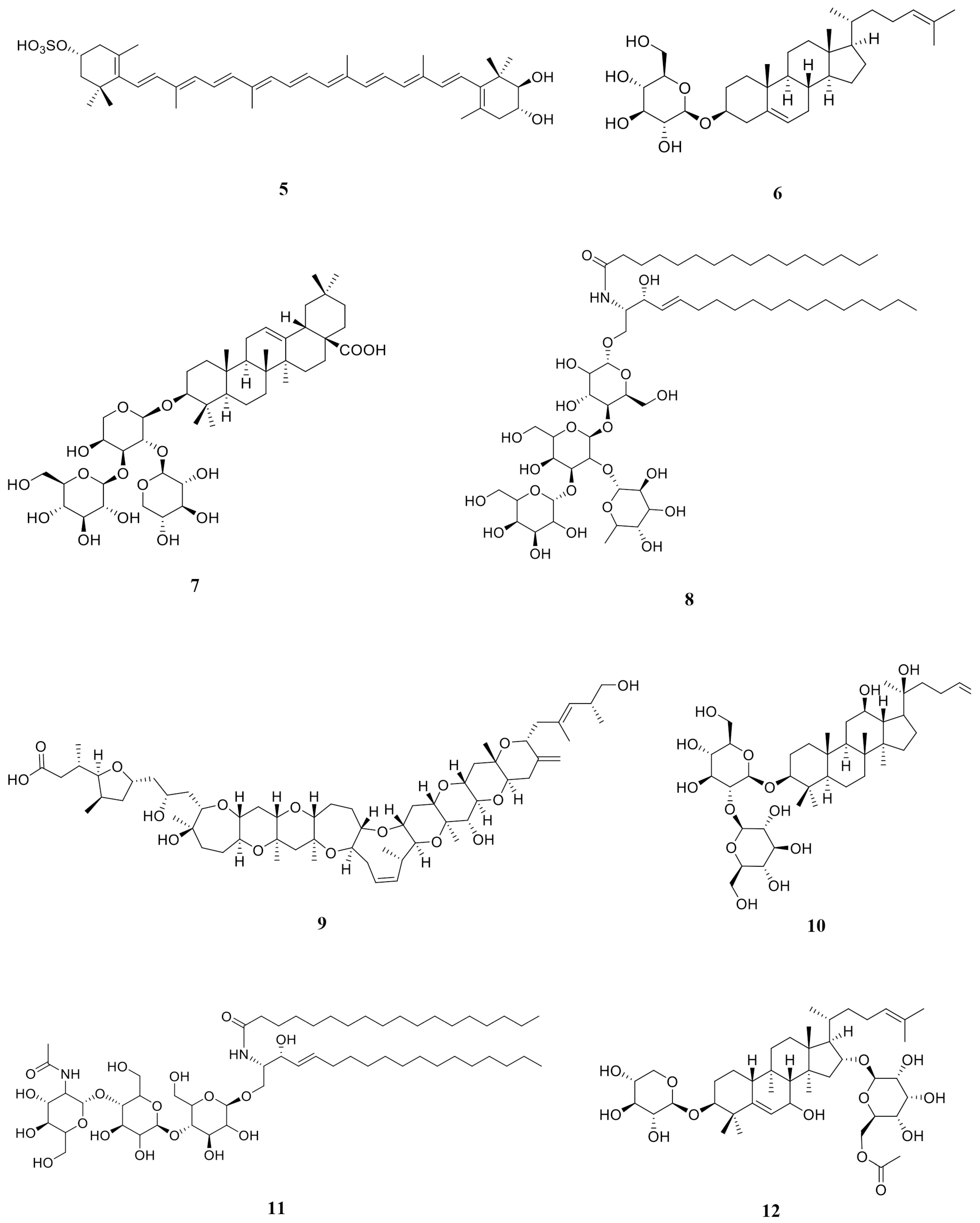
2.10. HPLC-QTOF-MS Analysis of DCM Phase, DCM2-AQ-Ch Sub-Extract, and Fraction F4
| Ref. Num. Figure 9 | Compound | DCM | DCM2-AQ-Ch | F4 | Reported Biological Activity |
|---|---|---|---|---|---|
| 1 | Sulfoquinovosyl diacylglycerol [SQDG] | ✓ | Anticancer [28,29] | ||
| 2 | 4′- Hydroxyanigorootin | ✓ | Antioxidant and analgesic [30] | ||
| 3 | Blumenol-C-O-[rhamnosyl-(1→6)-glucoside] | ✓ | Anticancer [31] | ||
| 4 | Bonafousine | ✓ | Cytotoxic [32] | ||
| 5 | Caloxanthin sulfate | ✓ | Anticancer, antiviral [33] | ||
| 6 | Stigmasterol glucoside | ✓ | Anticancer [34] | ||
| 7 | Elatoside E | ✓ | Cytotoxic [35] | ||
| 8 | Galalpha1–3(Fucalpha1-2)Galbeta1-4Glcbeta-Cer(d18:1/16:0) [globotriaosylceramide] | ✓ | Inhibitor angiogenesis [36] | ||
| 9 | Gambieric acid B (GAB) | ✓ | Fungicide [37] | ||
| 10 | Ginsenoside Rg3 | ✓ | Carcinoma [38] | ||
| 11 | GlcNAcbeta1-4Manbeta1-4Glcbeta-Cer(d18:1/18:0) [Lactosylceramide (d18:1/12:0)] | ✓ | Anticancer [39] | ||
| 12 | Hebevinoside III | ✓ | Cytotoxic [40] | ||
| 13 | Hebevinoside X | ✓ | Cytotoxic [41] | ||
| 14 | Sarcodon scabrosus Depsipeptide | ✓ | Anticancer [42] | ||
| 15 | Gambieric acid A (GAA) | ✓ | Cytotoxic [43] | ||
| 16 | Okadaic acid (OA) | ✓ | ✓ | Anticancer [44] | |
| 17 | Dinophysistoxin-1 (DTX-1) | ✓ | Cytotoxic [45] |
3. Discussion
3.1. Effects of Extracts, Sub-Extracts, and Fractions on Cell Viability
3.1.1. Effect of Crude Extracts on Cell Viability
3.1.2. Effect of Sub-Extracts on Cell Viability
3.1.3. Effect of Methanolic Fractions on Cell Viability
3.2. Acridine Orange (AO)-Ethidium Bromide (EB) Double Staining Cell Morphological Analysis
3.3. Bioactive Compounds Revealed by Gas Chromatography-Mass Spectrometry of the Dichloromethane Phase
3.4. Nuclear Magnetic Resonance Analysis of the Active Extracts and Fractions
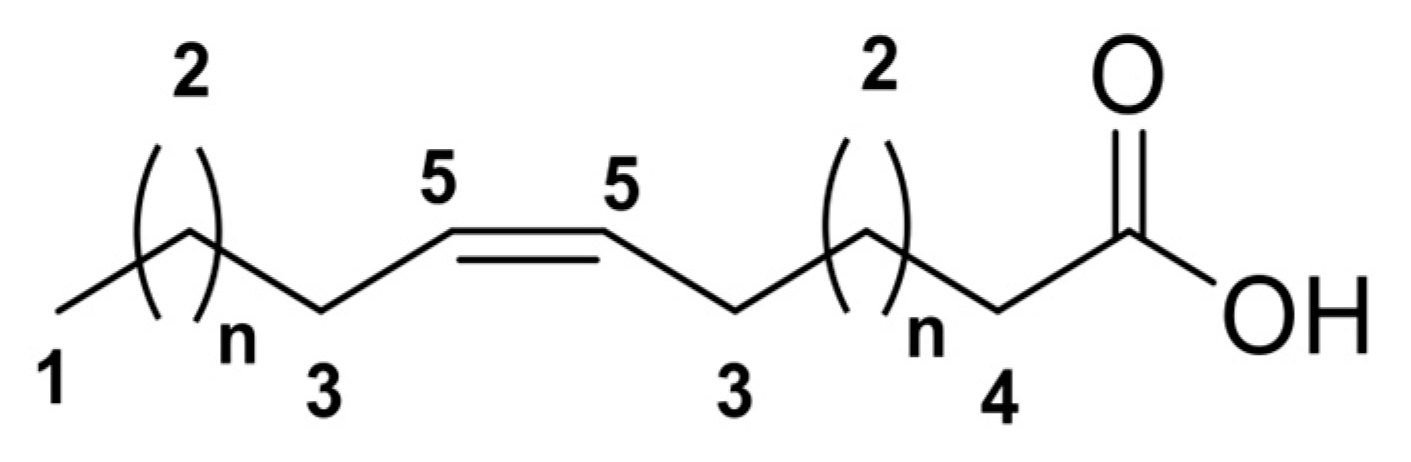
3.5. Fatty Acids from F7
3.6. HPLC-QTOF-MS Analysis of DCM Phase, DCM2-AQ-Ch Sub-Extract, and Fraction F4
4. Materials and Methods
4.1. Coolia Malayensis Isolation and Biomass Production
4.2. Human Cell Cultures and Controls
4.3. Extraction and Fractionation
4.3.1. Methanolic Crude Extract
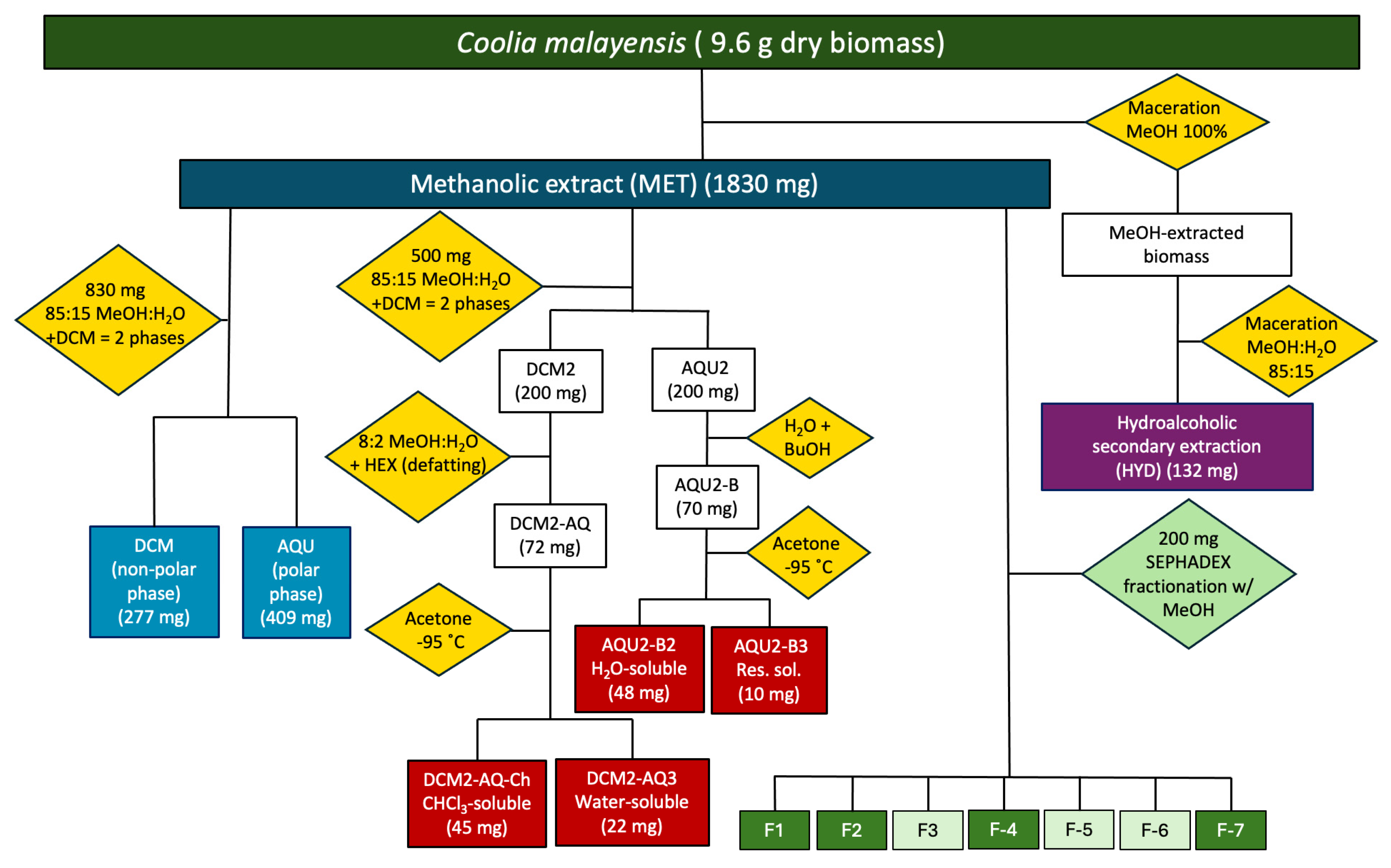
4.3.2. Dichloromethane and Aqueous Phases
4.3.3. Hydroalcoholic Secondary Extract
4.3.4. Sub-Extracts from the Methanolic Crude Extract
4.3.5. Sephadex Fractionation of the MET Extract
4.4. Chromatographic Fractionation of the Methanolic Crude Extract
4.5. In Vitro Cell Viability Assays and Morphological Analysis
4.5.1. Cell Viability Assays
4.5.2. Half-Maximal Inhibitory Concentration
4.5.3. Acridine Orange (AO)-Ethidium Bromide (EB) Double Staining Cell Morphological Analysis
4.6. Gas Chromatography-Mass Spectrometry Analysis
4.7. Nuclear Magnetic Resonance Analysis
4.8. High-Performance Liquid Chromatography-Quadrupole-Time-of-Flight Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, J.-Q.; Shen, Q.-H.; Han, Y.-Y.; Wu, Y.; He, X.-F.; Li, D.-W.; Huang, Y. Analysis of Research Status and Trends on Marine Benthic Dinoflagellate Toxins: A Bibliometric Study Based on Web of Science Database and VOSviewer. Environ. Res. 2023, 238, 117179. [Google Scholar] [CrossRef] [PubMed]
- Okolodkov, Y.B.; Durán-Riveroll, L.M.; Band-Schmidt, C.J.; Leyva-Valencia, I.; Gárate-Lizárraga, I.; Cembella, A.D.; Okolodkov, Y.B.; Durán-Riveroll, L.M.; Band-Schmidt, C.J.; Leyva-Valencia, I.; et al. A Review on Marine Benthic Dinoflagellates in Mexico. Hidrobiológica 2022, 32, 183–210. [Google Scholar] [CrossRef]
- Li, X.; Yan, M.; Gu, J.; Lam, V.T.T.; Wai, T.-C.; Baker, D.M.; Thompson, P.D.; Yiu, S.K.F.; Lam, P.K.S.; Leung, P.T.Y. The Effect of Temperature on Physiology, Toxicity and Toxin Content of the Benthic Dinoflagellate Coolia malayensis from a Seasonal Tropical Region. Water Res. 2020, 185, 116264. [Google Scholar] [CrossRef] [PubMed]
- Abdennadher, M.; Zouari, A.B.; Medhioub, W.; Penna, A.; Hamza, A.; Abdennadher, M.; Zouari, A.B.; Medhioub, W.; Penna, A.; Hamza, A. Characterization of Coolia spp. (Gonyaucales, Dinophyceae) from Southern Tunisia: First Record of Coolia malayensis in the Mediterranean Sea. Algae 2021, 36, 175–193. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2024. Available online: https://www.algaebase.org (accessed on 17 December 2024).
- Hoppenrath, M.; Chomerat, N.; Horiguchi, T.; Murray, S.A.; Rhodes, L. Marine Benthic Dinoflagellates—Their Relevance for Science and Society. Available online: https://www.schweizerbart.de/publications/detail/isbn/9783510614240/Marine_benthic_Dinoflagellates_2nd_Edit (accessed on 23 December 2024).
- Murray, J.S.; Finch, S.C.; Puddick, J.; Rhodes, L.L.; Harwood, D.T.; van Ginkel, R.; Prinsep, M.R. Acute Toxicity of Gambierone and Quantitative Analysis of Gambierones Produced by Cohabitating Benthic Dinoflagellates. Toxins 2021, 13, 333. [Google Scholar] [CrossRef]
- Varela, A.T.; Neves, R.A.F.; Nascimento, S.M.; Oliveira, P.J.; Pardal, M.A.; Rodrigues, E.T.; Moreno, A.J. Mitochondrial Impairment and Cytotoxicity Effects Induced by the Marine Epibenthic Dinoflagellate Coolia malayensis. Environ. Toxicol. Pharmacol. 2020, 77, 103379. [Google Scholar] [CrossRef]
- Lim, A.S.; Jeong, H.J. Benthic Dinoflagellates in Korean Waters. Algae 2021, 36, 91–109. [Google Scholar] [CrossRef]
- Morales-Amador, A.; Souto, M.L.; Hertweck, C.; Fernández, J.J.; García-Altares, M. Rapid Screening of Polyol Polyketides from Marine Dinoflagellates. Anal. Chem. 2022, 94, 14205–14213. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, K.; Hu, Z.; Hu, Q.; Tang, Y.Z. Toxic and Non-Toxic Dinoflagellates Host Distinct Bacterial Communities in Their Phycospheres. Commun. Earth Environ. 2023, 4, 263. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Chaudhry, G.-S.; Md Akim, A.; Sung, Y.Y.; Sifzizul, T.M.T. Cancer and Apoptosis: The Apoptotic Activity of Plant and Marine Natural Products and Their Potential as Targeted Cancer Therapeutics. Front. Pharmacol. 2022, 13, 842376. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development. Nat. Prod. Bioprospect. 2017, 7, 1–111. [Google Scholar] [CrossRef]
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-Derived Drugs: Recent Advances in Cancer Therapy and Immune Signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef] [PubMed]
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef]
- Pinto, K.B.; dos Santos, P.H.B.; Krause, L.C.; Caramão, E.B.; Bjerk, T.R. Preliminary Prospection of Phytotherapic Compounds from the Essential Oils from Barks and Leaves of Umburana (Commiphora leptophloeos). Braz. J. Pharm. Sci. 2022, 58, e21609. [Google Scholar] [CrossRef]
- Sani, M.S.A.; Bakar, J.; Rahman, R.A.; Abas, F. Effects of Coated Capillary Column, Derivatization, and Temperature Programming on the Identification of Carica papaya Seed Extract Composition Using GC/MS Analysis. J. Anal. Test. 2020, 4, 23–34. [Google Scholar] [CrossRef]
- Vedernikov, D.; Shabanova, N.; Roshchin, V. Change in the Chemical Composition of the Crust and Inner Bark of the Betula pendula Roth. Birch (Betulaceae) with Tree Height. Russ. J. Bioorg. Chem. 2011, 37, 877–882. [Google Scholar] [CrossRef]
- Shang, C.; Yaoming, H.; Deng, C.; Hu, K. Rapid Determination of Volatile Constituents of Michelia alba Flowers by Gas Chromatography-Mass Spectrometry with Solid-Phase Microextraction. J. Chromatogr. A 2002, 942, 283–288. [Google Scholar] [CrossRef]
- Bi, K.; Bedi, G.; Koffi, A.; Chalchat, J.-C.; Guessan, T. Volatiles Constituents from Leaves of Morinda morindoïdes (Rubiaceae): A Medicinal Plant from the Ivory Coast. Open Nat. Prod. J. 2010, 3, 6–9. [Google Scholar] [CrossRef]
- Radulović, N.; Đorđević, N.; Marković, M.; Palić, R. Volatile Constituents of Glechoma hirsute Waldst. & Kit. and G. hederacea L. (Lamiaceae). Bull. Chem. Soc. Ethiop. 2010, 24, 67–76. [Google Scholar] [CrossRef]
- Demetzos, C.; Angelopoulou, D.; Perdetzoglou, D. A Comparative Study of the Essential Oils of Cistus salviifolius in Several Populations of Crete (Greece). Biochem. Syst. Ecol. 2002, 30, 651–665. [Google Scholar] [CrossRef]
- Xu, L.-L.; Han, T.; Wu, J.-Z.; Zhang, Q.-Y.; Zhang, H.; Huang, B.-K.; Rahman, K.; Qin, L.-P. Comparative Research of Chemical Constituents, Antifungal and Antitumor Properties of Ether Extracts of Panax ginseng and Its Endophytic Fungus. Phytother. Res. 2009, 16, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kwak, H.-S.; Choi, E.; Hong, T.E.; Yoon, H.; Lee, Y.; Baik, K.; Uhm, H.S. Traces of Isotopic Reactive Species Produced from a Non-Thermal Plasma Jet in Bio-Molecules. New J. Phys. 2015, 17, 113031. [Google Scholar] [CrossRef]
- Tibiriçá, C.E.J.d.A.; Sibat, M.; Fernandes, L.F.; Bilien, G.; Chomérat, N.; Hess, P.; Mafra, L.L., Jr. Diversity and Toxicity of the Genus Coolia meunier in Brazil, and Detection of 44-Methyl Gambierone in Coolia tropicalis. Toxins 2020, 12, 327. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Ghobeishavi, S.; Yegdaneh, A. Cytotoxic Sulfoquinovosyl Glycerols from the Seaweed Sargassum angustifolium from Persian Gulf. Adv. Biomed. Res. 2024, 13, 22. [Google Scholar] [CrossRef]
- Yuan, Y.; Zeng, W. An Overview of Multifaceted Applications and the Future Prospects of Glyceroglycolipids in Plants. J. Agric. Food Chem. 2024, 72, 22420–22432. [Google Scholar] [CrossRef]
- Saha, S.; Shilpi, J.A.; Mondal, H.; Gofur, R.; Billah, M.; Nahar, L.; Sarker, S.D. Bioactivity Studies on Musa seminifera Lour. Pharmacogn. Mag. 2013, 9, 315–322. [Google Scholar] [CrossRef]
- Hu, J.; Qi, Q.; Zhu, Y.; Wen, C.; Olatunji, O.J.; Jayeoye, T.J.; Eze, F.N. Unveiling the Anticancer, Antimicrobial, Antioxidative Properties, and UPLC-ESI-QTOF-MS/GC–MS Metabolite Profile of the Lipophilic Extract of Siam Weed (Chromolaena odorata). Arab. J. Chem. 2023, 16, 104834. [Google Scholar] [CrossRef]
- Naidoo, C.M.; Naidoo, Y.; Dewir, Y.H.; Murthy, H.N.; El-Hendawy, S.; Al-Suhaibani, N. Major Bioactive Alkaloids and Biological Activities of Tabernaemontana Species (Apocynaceae). Plants 2021, 10, 313. [Google Scholar] [CrossRef]
- Zahran, E.M.; Albohy, A.; Khalil, A.; Ibrahim, A.H.; Ahmed, H.A.; El-Hossary, E.M.; Bringmann, G.; Abdelmohsen, U.R. Bioactivity Potential of Marine Natural Products from Scleractinia-Associated Microbes and In Silico Anti-SARS-CoV-2 Evaluation. Mar. Drugs 2020, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Abdel Bar, F.M.; Galala, A.A.; El-Sokkary, M.M.A.; Khalil, A.T.; Sallam, A. In Vitro Cytotoxicity and Secondary Metabolites of Talaromyces wortmannii Isolated from Arundo donax L.: Identification of a New Phytoceramide. Sci. Afr. 2025, 27, e02600. [Google Scholar] [CrossRef]
- Thu, H.; Duyen, N.H.H.; Dieu, T.; Ngoc, H.; Van, H.; Thị Ngọc, H.Á.; Xuan, H.; Khanh, P.; Cuong, N.; Toan, P. In Vitro and in Silico Cytotoxic Activities of Triterpenoids from the Leaves of Aralia dasyphylla Miq. and the Assessment of Their ADMET Properties. J. Biomol. Struct. Dyn. 2022, 41, 5863–5871. [Google Scholar] [CrossRef]
- Birklé, S.; Desselle, A.; Chaumette, T.; Gaugler, M.-H.; Cochonneau, D.; Fleurence, J.; Dubois, N.; Hulin, P.; Aubry, J.; Paris, F. Inhibition of Tumor Angiogenesis by Globotriaosylceramide Immunotargeting. Oncoimmunology 2013, 2, e23700. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Murata, M.; Torigoe, K.; Satake, M.; Yasumoto, T. Gambieric Acids, New Potent Antifungal Substances with Unprecedented Polyether Structures from a Marine Dinoflagellate Gambierdiscus toxicus. J. Org. Chem. 1992, 57, 5448–5453. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Chen, T.; Wu, W.; Yan, Z.; Li, X. Anticancer Effect and Molecular Mechanism of Ginsenoside Rg3 in Various Cancer Types. Intell. Pharm. 2023, 1, 52–63. [Google Scholar] [CrossRef]
- Martin, S.F.; Williams, N.; Chatterjee, S. Lactosylceramide Is Required in Apoptosis Induced by N-Smase. Glycoconj. J. 2006, 23, 147–157. [Google Scholar] [CrossRef]
- Liu, D.-Z. A Review of Ergostane and Cucurbitane Triterpenoids of Mushroom Origin. Nat. Prod. Res. 2014, 28, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kang, S.; Zhao, L.; Gao, J.; Liu, C. Natural and Engineered Xylosyl Products from Microbial Source. Nat. Prod. Bioprospect. 2024, 14, 13. [Google Scholar] [CrossRef]
- Tiago, R.E.S.; Eugenio, V.D.S.N.; Ana, L.T.G.R.; Mary, A.F.; Caio, H.F.; Carolina, B.M.; Valéria, M.D.O. Antimicrobial, Antiparasitic and Antiproliferative Effects of the Extract of Bacillus safensis SG-32 Isolated from a Brazilian Oil Reservoir. Afr. J. Microbiol. Res. 2018, 12, 897–907. [Google Scholar] [CrossRef]
- Murray, J.S.; Passfield, E.M.F.; Rhodes, L.L.; Puddick, J.; Finch, S.C.; Smith, K.F.; van Ginkel, R.; Mudge, E.M.; Nishimura, T.; Funaki, H.; et al. Targeted Metabolite Fingerprints of Thirteen Gambierdiscus, Five Coolia and Two Fukuyoa Species. Mar. Drugs 2024, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Ozel, B.; Kipcak, S.; Biray Avci, C.; Gunduz, C.; Saydam, G.; Aktan, C.; Selvi Gunel, N. Combination of Dasatinib and Okadaic Acid Induces Apoptosis and Cell Cycle Arrest by Targeting Protein Phosphatase PP2A in Chronic Myeloid Leukemia Cells. Med. Oncol. 2022, 39, 46. [Google Scholar] [CrossRef]
- Huguet, A.; Drapeau, O.; Rousselet, F.; Quenault, H.; Fessard, V. Differences in Toxic Response Induced by Three Variants of the Diarrheic Shellfish Poisoning Phycotoxins in Human Intestinal Epithelial Caco-2 Cells. Toxins 2020, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Phua, Y.H.; Roy, M.C.; Lemer, S.; Husnik, F.; Wakeman, K.C. Diversity and Toxicity of Pacific Strains of the Benthic Dinoflagellate Coolia (Dinophyceae), with a Look at the Coolia canariensis Species Complex. Harmful Algae 2021, 109, 102120. [Google Scholar] [CrossRef]
- Mohammad, I.K.; Mansur, S.A.; Razak, A.H.A.; Fauzi, N.A.M.; Kassim, A.S.M. Comparison Study on Antioxidant Capacity of Chromolaena odorata Leaves Extract Obtained from Maceration and Ultrasound-Assisted Maceration. Prog. Eng. Appl. Technol. 2022, 3, 330–340. [Google Scholar]
- Rubini, S.; Albonetti, S.; Menotta, S.; Cervo, A.; Callegari, E.; Cangini, M.; Dall’Ara, S.; Baldini, E.; Vertuani, S.; Manfredini, S. New Trends in the Occurrence of Yessotoxins in the Northwestern Adriatic Sea. Toxins 2021, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Sigwart, J.D.; Blasiak, R.; Jaspars, M.; Jouffray, J.-B.; Tasdemir, D. Unlocking the Potential of Marine Biodiscovery. Nat. Prod. Rep. 2021, 38, 1235–1242. [Google Scholar] [CrossRef]
- Hiraga, Y.; Kaku, K.; Omoda, D.; Sugihara, K.; Hosoya, H.; Hino, M. A New Digalactosyl Diacylglycerol from a Cultured Marine Dinoflagellate Heterocapsa circularisquama. J. Nat. Prod. 2002, 65, 1494–1496. [Google Scholar] [CrossRef]
- Alves, E.; Dias, M.; Lopes, D.; Almeida, A.; Domingues, M.d.R.; Rey, F. Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects. Antibiotics 2020, 9, 441. [Google Scholar] [CrossRef]
- Orefice, I.; Balzano, S.; Romano, G.; Sardo, A. Amphidinium spp. as a Source of Antimicrobial, Antifungal, and Anticancer Compounds. Life 2023, 13, 2164. [Google Scholar] [CrossRef]
- Louzao, M.C.; Vilariño, N.; Vale, C.; Costas, C.; Cao, A.; Raposo-Garcia, S.; Vieytes, M.R.; Botana, L.M. Current Trends and New Challenges in Marine Phycotoxins. Mar. Drugs 2022, 20, 198. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Serrano, F.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Ibarguren, M.; López, D.J.; Terés, S.; Alonso-Sande, M.; Higuera, M.; Torres, M.; Busquets, X.; et al. The Triacylglycerol, Hydroxytriolein, Inhibits Triple Negative Mammary Breast Cancer Cell Proliferation through a Mechanism Dependent on Dihydroceramide and Akt. Oncotarget 2019, 10, 2486–2507. [Google Scholar] [CrossRef]
- Heaney, M.L.; Gardner, J.R.; Karasavvas, N.; Golde, D.W.; Scheinberg, D.A.; Smith, E.A.; O’Connor, O.A. Vitamin C Antagonizes the Cytotoxic Effects of Antineoplastic Drugs. Cancer Res. 2008, 68, 8031–8038. [Google Scholar] [CrossRef]
- Tartaglione, L.; Loeffler, C.R.; Miele, V.; Varriale, F.; Varra, M.; Monti, M.; Varone, A.; Bodi, D.; Spielmeyer, A.; Capellacci, S.; et al. Dereplication of Gambierdiscus balechii Extract by LC-HRMS and In Vitro Assay: First Description of a Putative Ciguatoxin and Confirmation of 44-Methylgambierone. Chemosphere 2023, 319, 137940. [Google Scholar] [CrossRef]
- McMillan, J.P.; Hoffman, P.A.; Granade, H.R. Gambierdiscus toxicus from the Caribbean: A Source of Toxins Involved in Ciguatera. Mar. Fish. Rev. 1986. [Google Scholar]
- Juranovic, L.R.; Park, D.L.; Fremy, J.-M.; Nielsen, C.A.; Ah, S.S.; Ayala, C.E. Isolation and Separation of Toxins Produced by Gambierdiscus toxicus and Prorocentrum concavum. J. Aquat. Food Prod. Technol. 1997, 6, 5–25. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, H.; Krock, B.; Lu, S.; Yang, W.; Gu, H. Morphology, Molecular Phylogeny and Okadaic Acid Production of Epibenthic Prorocentrum (Dinophyceae) Species from the Northern South China Sea. Algal Res. 2017, 22, 14–30. [Google Scholar] [CrossRef]
- Séchet, V.; Sibat, M.; Billien, G.; Carpentier, L.; Rovillon, G.-A.; Raimbault, V.; Malo, F.; Gaillard, S.; Perrière-Rumebe, M.; Hess, P.; et al. Characterization of Toxin-Producing Strains of Dinophysis spp. (Dinophyceae) Isolated from French Coastal Waters, with a Particular Focus on the D. acuminata-Complex. Harmful Algae 2021, 107, 101974. [Google Scholar] [CrossRef]
- Bagnis, R.; Chanteau, S.; Chungue, E.; Hurtel, J.M.; Yasumoto, T.; Inoue, A. Origins of Ciguatera Fish Poisoning: A New Dinoflagellate, Gambierdiscus toxicus Adachi and Fukuyo, Definitively Involved as a Causal Agent. Toxicon 1980, 18, 199–208. [Google Scholar] [CrossRef]
- Cho, K.; Heo, J.; Han, J.; Hong, H.D.; Jeon, H.; Hwang, H.-J.; Hong, C.-Y.; Kim, D.; Han, J.W.; Baek, K. Industrial Applications of Dinoflagellate Phycotoxins Based on Their Modes of Action: A Review. Toxins 2020, 12, 805. [Google Scholar] [CrossRef]
- Ferrara, L. Dinoflagellates Important Marine Producers of Natural Bio-Compounds with High Biotechnological and Pharmacological Potential. J. Food Chem. Nanotechnol. 2020, 6, 138–149. [Google Scholar] [CrossRef]
- Du, A.; Wang, Z.; Huang, T.; Xue, S.; Jiang, C.; Qiu, G.; Yuan, K. Fatty Acids in Cancer: Metabolic Functions and Potential Treatment. MedComm–Oncol. 2023, 2, e25. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, G. Mechanisms and Therapeutic Regulation of Pyroptosis in Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2020, 21, 1456. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E. Apoptosis (Programmed Cell Death) and Its Signals—A Review. Braz. J. Biol. 2020, 81, 1133–1143. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Microalgae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ye, G.; Li, R.; Li, T.; Huang, S. Extraction and Characterization of Cycloartenol Isolated from Stems and Leaves of Coix Lacryma-Jobi L. and Its Potential Cytotoxic Activity. Res Sq. 2021, V1, 1–16. [Google Scholar] [CrossRef]
- El-Dakroury, Y.M.; Abou Zeid, A.H.; El Hela, A.A.; Temraz, A.; Mohammed, R.S. GC-MS Profiling, In-Vitro Anti-Oxidant, Anti-Inflammatory and Anti-Diabetic Activities of Petroleum Ether Extract of Dypsis decaryi and Dypsis leptocheilos Leaves Cultivated in Egypt: Experimental and Computational Studies. Azhar Int. J. Pharm. Med. Sci. 2025, 5, 157–176. [Google Scholar] [CrossRef]
- Hernández-Flandes, A.; Hernández-Ortega, S.; Ramírez-Apan, T.; Rocha-Zavaleta, L.; Silva-Jimenez, N.; Martínez-Vázquez, M. Synthesis of Cycloartan-16β-Ol from 16β 24R-Epoxy-Cycloartane and Their Cytotoxicity Evaluation against Human Cancer Cell Lines. Chem. Biodivers. 2024, 21, e202301346. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Wang, A.; Yang, L.; Gan, Q.; Yi, L.; Summons, R.E.; Volkman, J.K.; Lu, Y. Widespread Sterol Methyltransferase Participates in the Biosynthesis of Both C4α- and C4β-Methyl Sterols. J. Am. Chem. Soc. 2022, 144, 9023–9032. [Google Scholar] [CrossRef]
- Nian, Y.; Wang, H.-Y.; Su, J.; Zhou, L.; Qiu, M.-H. A Cytotoxic 4α-Methyl Steroid from the Aerial Parts of Cimicifuga foetida L. Fitoterapia 2012, 83, 293–297. [Google Scholar] [CrossRef]
- Breeta, R.D.I.E.; Grace, V.M.B.; Wilson, D.D. Methyl Palmitate-A Suitable Adjuvant for Sorafenib Therapy to Reduce In Vivo Toxicity and to Enhance Anti-Cancer Effects on Hepatocellular Carcinoma Cells. Basic Clin. Pharmacol. Toxicol. 2021, 128, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Martyasari, N.W.R.; Ardiana, N.; Ilhami, B.T.K.; Padmi, H.; Abidin, A.; Sunarwidhi, A.L.; Sunarpi, H.; Nikmatullah, A.; Widyastuti, S.; Prasedya, E.S. The Effect of Extraction Solvent Polarity on Cytotoxic Properties of Sargassum crassifolium Against B16-F10 Melanoma Cancer Cell Model. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012105. [Google Scholar] [CrossRef]
- Moreira-González, A.R.; Rosa, K.M.S.; Mafra, L.L., Jr. Prevalence of Okadaic Acid in Benthic Organisms Associated Prorocentrum lima Complex in a Sub-Tropical Estuary. Food Addit. Contam. Part A 2022, 39, 382–396. [Google Scholar] [CrossRef]
- Morton, S.L.; Moeller, P.D.R.; Young, K.A.; Lanoue, B. Okadaic Acid Production from the Marine Dinoflagellate Prorocentrum belizeanum Faust Isolated from the Belizean Coral Reef Ecosystem. Toxicon 1998, 36, 201–206. [Google Scholar] [CrossRef]
- Dickey, R.W.; Bobzin, S.C.; Faulkner, D.J.; Bencsath, F.A.; Andrzejewski, D. Identification of Okadaic Acid from a Caribbean Dinoflagellate, Prorocentrum concavum. Toxicon 1990, 28, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Cembella, A.D.; Durán-Riveroll, L.M.; Tarazona-Janampa, U.I.; Okolodkov, Y.B.; García-Sandoval, R.; Krock, B.; Hörstmann, C.; John, U. Phylogeography and Diversity Among Populations of the Toxigenic Benthic Dinoflagellate Prorocentrum from Coastal Reef Systems in Mexico. Front. Mar. Sci. 2021, 8, 716669. [Google Scholar] [CrossRef]
- Tarazona-Janampa, U.I.; Cembella, A.D.; Pelayo-Zárate, M.C.; Pajares, S.; Márquez-Valdelamar, L.M.; Okolodkov, Y.B.; Tebben, J.; Krock, B.; Durán-Riveroll, L.M. Associated Bacteria and Their Effects on Growth and Toxigenicity of the Dinoflagellate Prorocentrum lima Species Complex From Epibenthic Substrates Along Mexican Coasts. Front. Mar. Sci. 2020, 7, 569. [Google Scholar] [CrossRef]
- Lee, S.Y.; Woo, S.Y.; Tian, F.; Park, J.B.; Choi, K.-S.; Chun, H.S. Simultaneous Determination of Okadaic Acid, Dinophysistoxin-1, Dinophysistoxin-2, and Dinophysistoxin-3 Using Liquid Chromatography-Tandem Mass Spectrometry in Raw and Cooked Food Matrices. Food Control 2022, 139, 109068. [Google Scholar] [CrossRef]
- Park, J.B.; Cho, S.; Lee, S.Y.; Park, S.M.; Chun, H.S. Occurrence and Risk Assessment of Okadaic Acid, Dinophysistoxin-1, Dinophysistoxin-2, and Dinophysistoxin-3 in Seafood from South Korea. Environ. Sci. Pollut. Res. 2024, 31, 6243–6257. [Google Scholar] [CrossRef]
- Ayache, N.; Bill, B.D.; Brosnahan, M.L.; Campbell, L.; Deeds, J.R.; Fiorendino, J.M.; Gobler, C.J.; Handy, S.M.; Harrington, N.; Kulis, D.M.; et al. A Survey of Dinophysis spp. and Their Potential to Cause Diarrhetic Shellfish Poisoning in Coastal Waters of the United States. J. Phycol. 2023, 59, 658–680. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, L.; Wei, Y.; Ma, F. PP2A as a Potential Therapeutic Target for Breast Cancer: Current Insights and Future Perspectives. Biomed. Pharmacother. 2024, 173, 116398. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.H.; Pimentel, J.M.; Chatterjee, M.; Allen, J.E.; Zhuang, Z.; Wu, G.S. Targeting PP2A Inhibits the Growth of Triple-Negative Breast Cancer Cells. Cell Cycle 2020, 19, 592–600. [Google Scholar] [CrossRef]
- Brautigan, D.L.; Farrington, C.; Narla, G. Targeting Protein Phosphatase PP2A for Cancer Therapy: Development of Allosteric Pharmaceutical Agents. Clin. Sci. 2021, 135, 1545–1556. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, J.-H.; Jung, H.J.; Hwang, J.H.; Chun, H.S.; Yoon, Y.S.; Oh, S.H. Okadaic Acid Is at Least as Toxic as Dinophysistoxin-1 after Repeated Administration to Mice by Gavage. Toxins 2023, 15, 587. [Google Scholar] [CrossRef]
- Figueroa, D.; Signore, A.; Araneda, O.; Contreras, H.R.; Concha, M.; García, C. Toxicity and Differential Oxidative Stress Effects on Zebrafish Larvae Following Exposure to Toxins from the Okadaic Acid Group. J. Toxicol. Environ. Health Part A 2020, 83, 573–588. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Komori, A.; Suganuma, M. Cancer Progression by the Okadaic Acid Class of Tumor Promoters and Endogenous Protein Inhibitors of PP2A, SET and CIP2A. J. Cancer Res. Clin. Oncol. 2023, 149, 9425–9433. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gong, J.; Hu, Y.; Tan, Q.-L.; Liu, B.; Yu, X.-W.; Hao, X.-L.; Guo, Q.-N. Long-Term Exposure to Low Levels of Okadaic Acid Accelerates Cell Cycle Progression in Colonic Epithelial Cells via P53 and Jak/Stat3 Signaling Pathways. Heliyon 2022, 8, e10444. [Google Scholar] [CrossRef]
- Song, Q.; Wang, H.; Jiang, D.; Xu, C.; Cui, J.; Zhang, Q.; Wang, H.; Huang, J.; Su, J.; Wu, G.S.; et al. Pharmacological Inhibition of PP2A Overcomes Nab-Paclitaxel Resistance by Downregulating MCL1 in Esophageal Squamous Cell Carcinoma (ESCC). Cancers 2021, 13, 4766. [Google Scholar] [CrossRef]
- Johnson, H.; Narayan, S.; Sharma, A.K. Altering Phosphorylation in Cancer through PP2A Modifiers. Cancer Cell Int. 2024, 24, 11. [Google Scholar] [CrossRef]
- Biswas, P.; Datta, C.; Rathi, P.; Bhattacharjee, A. Fatty Acids and Their Lipid Mediators in the Induction of Cellular Apoptosis in Cancer Cells. Prostaglandins Other Lipid Mediat. 2022, 160, 106637. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Ferreri, R.; Amézaga, J.; Tueros, I. Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways. Metabolites 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Asefy, Z.; Tanomand, A.; Hoseinnejhad, S.; Ceferov, Z.; Oshaghi, E.A.; Rashidi, M. Unsaturated Fatty Acids as a Co-Therapeutic Agents in Cancer Treatment. Mol. Biol. Rep. 2021, 48, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Natural Polyether Ionophores and Their Pharmacological Profile. Mar. Drugs 2022, 20, 292. [Google Scholar] [CrossRef]
- Blanco, J.; Moroño, Á.; Arévalo, F.; Correa, J.; Lamas, J.P. Yessotoxins in Mollusks of the Galician Coast from 2014 to 2022: Variability, Biotransformation, and Resistance to Alkaline Hydrolysis. Toxins 2023, 15, 661. [Google Scholar] [CrossRef] [PubMed]
- Tudó, À.; Rambla-Alegre, M.; Flores, C.; Sagristà, N.; Aguayo, P.; Reverté, L.; Campàs, M.; Gouveia, N.; Santos, C.; Andree, K.B.; et al. Identification of New CTX Analogues in Fish from the Madeira and Selvagens Archipelagos by Neuro-2a CBA and LC-HRMS. Mar. Drugs 2022, 20, 236. [Google Scholar] [CrossRef]
- Cano, M.; Otálvaro, F. Total Synthesis of (±)-Anigorootin. Org. Lett. 2024, 26, 8752–8755. [Google Scholar] [CrossRef]
- Pogam, P.L.; Beniddir, M.A. Structural Diversity and Chemical Logic Underlying the Assembly of Monoterpene Indole Alkaloids Oligomers. Nat. Prod. Rep. 2024, 41, 1723–1765. [Google Scholar] [CrossRef]
- Huang, W.-C.; Huang, T.-H.; Yeh, K.-W.; Chen, Y.-L.; Shen, S.-C.; Liou, C.-J. Ginsenoside Rg3 Ameliorates Allergic Airway Inflammation and Oxidative Stress in Mice. J. Ginseng Res. 2021, 45, 654–664. [Google Scholar] [CrossRef]
- Walter, M.H. C13 α-Ionol (Blumenol) Glycosides and C14 Mycorradicin: Apocarotenoids Accumulating in Roots during the Arbuscular Mycorrhizal Symbiosis. In Biology, Chemistry and Applications of Apocarotenoids; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-34420-6. [Google Scholar]
- Endo, Y.; Minowa, A.; Kanamori, R.; Araya, H. A Rare α-Pyrone from Bitter Tooth Mushroom, Sarcodon scabrosus (Fr.) Karst. Biochem. Syst. Ecol. 2012, 44, 286–288. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.; Bolch, C.; Haskard, K.; Hallegraeff, G. Reproductive Compatibility among Four Global Populations of the Toxic Dinoflagellate Gymnodinium catenatum (Dinophyceae). Phycologia 2001, 40, 78–87. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Tudó, A.; Bravo, I.; Díaz, P.A.; Diogène, J.; Riobó, P. Toxicity Characterisation of Gambierdiscus Species from the Canary Islands. Toxins 2020, 12, 134. [Google Scholar] [CrossRef]
- Carlin, M.; Pelin, M.; Ponti, C.; Sosa, S.; Tubaro, A. Functional and Structural Biological Methods for Palytoxin Detection. J. Mar. Sci. Eng. 2022, 10, 916. [Google Scholar] [CrossRef]
- Mejía-Camacho, A.L.; Durán-Riveroll, L.M.; Cembella, A.D. Toxicity Bioassay and Cytotoxic Effects of the Benthic Marine Dinoflagellate Amphidinium operculatum. J. Xenobiot. 2021, 11, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qiu, J.; Zhang, J.; Li, A.; Wang, G. Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2. Toxins 2024, 16, 381. [Google Scholar] [CrossRef]
- Moon, S.; Choi, D.H.; Lee, Y.; Rho, J.R. Three New Okadaic Acid Derivatives Isolated from a Benthic Dinoflagellate Prorocetrum lima. J. Korean Magn. Reson. Soc. 2024, 28, 25–31. [Google Scholar]
- Li, Y.-Y.; Tian, X.-Q.; Lu, Y.-N.; Han, Q.-H.; Ma, L.-Y.; Fan, C.-Q. Toxins and Other Chemical Constituents from Prorocentrum lima. Biochem. Syst. Ecol. 2020, 89, 104015. [Google Scholar] [CrossRef]
- Babich, H.; Borenfreund, E. Applications of the Neutral Red Cytotoxicity Assay to In Vitro Toxicology. Altern. Lab. Anim. 1990, 18, 129–144. [Google Scholar] [CrossRef]
- Ude, A.; Afi-Leslie, K.; Okeke, K.; Ogbodo, E.; Ude, A.; Afi-Leslie, K.; Okeke, K.; Ogbodo, E. Trypan Blue Exclusion Assay, Neutral Red, Acridine Orange and Propidium Iodide. In Cytotoxicity—Understanding Cellular Damage and Response; IntechOpen: London, UK, 2022; ISBN 978-1-80356-246-9. [Google Scholar]
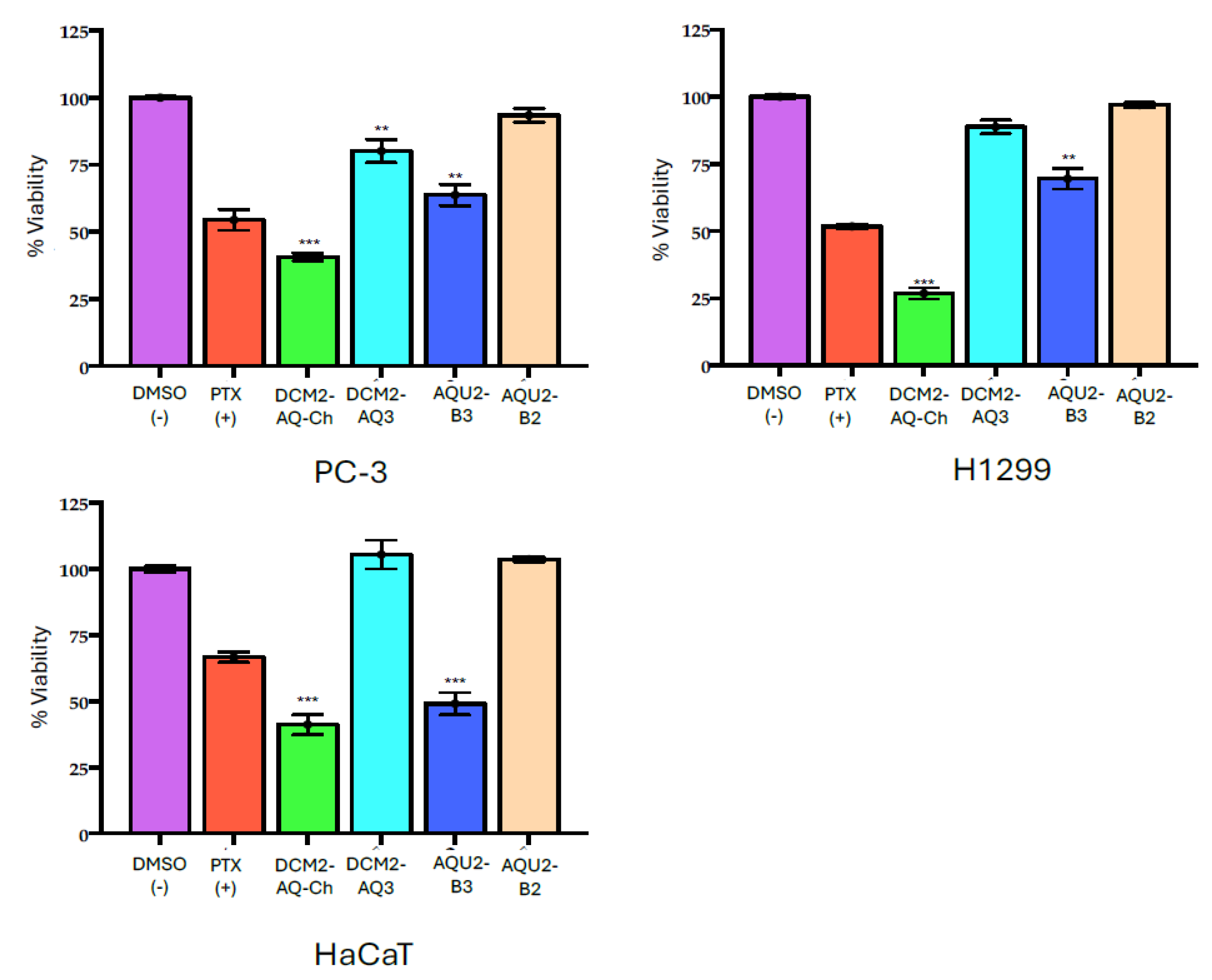
| Cell Line/% Inhibition ± SD | |||
|---|---|---|---|
| Sub-Extract | PC-3 | H1299 | HaCaT |
| DCM2-AQ-Ch | 59.35 ± 2.25 | 73.16 ± 2.35 | 58.83 ± 3.75 |
| DCM2-AQ3 | 19.86 ± 4.38 | 11.19 ± 2.51 | −5.46 ± 3.19 |
| AQU2-B2 | 6.58 ± 2.59 | 3.05 ± 0.95 | −3.68 ± 0.90 |
| AQU2-B3 | 36.22 ± 4.27 | 30.48 ± 3.88 | 50.93 ± 4.25 |
| PTX (+) | 54.49 ± 3.84 | 51.76 ± 0.91 | 66.68 ± 1.96 |
| 200 mg of MET Extract | ||
|---|---|---|
| Fraction (F) | Weight (mg) | Yield (%) |
| F1 | 88 | 44 |
| F2 | 20 | 10 |
| F3 | 24 | 12 |
| F4 | 7 | 3.5 |
| F5 | 15 | 7.5 |
| F6 | 7 | 3.5 |
| F7 | 4 | 2 |
| Total yield | 165 | 82.5 |
| Cell line/% Inhibition ± SD | |||
|---|---|---|---|
| Fraction | PC-3 | H1299 | HaCaT |
| F1 | 3.18 ± 1.92 | 9.63 ± 4.90 | 1.12 ± 0.29 |
| F2 | −3.05 ± 2.64 | 29.60 ± 0.11 | 6.25 ± 2.17 |
| F4 | 20.73 ± 6.61 | 14.65 ± 1.55 | −2.84 ± 1.56 |
| F7 | 53.46 ± 2.28 | 75.88 ± 6.19 | 13.05 ± 3.46 |
| PTX | 45.50 ± 3.29 | 68.23 ± 0.88 | 66.32 ± 0.96 |
| IC50 ± SD (μg mL−1) | SI Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | DCM2-AQ-Ch | F4 | F7 | PTX | DCM2-AQ-Ch | F4 | F7 | PTX |
| H1299 | 17.69 ± 2.38 | >100 | 25.16 ± 2.38 | 1.28 × 10−2 | 4.53 | - | 21.41 | 2.0 |
| PC-3 | 21.62 ± 5.73 | 137.63 | 19.97 ± 1.32 | 1.25 × 10−2 | 3.70 | 0.83 | 26.96 | 2.04 |
| HaCaT | 80.18 ± 4.90 | >100 | >100 | 2.56 × 10−2 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Montesinos, I.B.; Rios, M.Y.; Ocampo-Acuña, Y.D.; Esquivel-Rodríguez, B.; Bustos-Brito, C.; Osorio-Ramírez, M.d.C.; Durán-Riveroll, L.M.; González-Maya, L. The Benthic Dinoflagellate Coolia malayensis (Dinophyceae) Produces an Array of Compounds with Antineoplastic Activity in Cells of Tumor Origin. Mar. Drugs 2025, 23, 127. https://doi.org/10.3390/md23030127
Morales-Montesinos IB, Rios MY, Ocampo-Acuña YD, Esquivel-Rodríguez B, Bustos-Brito C, Osorio-Ramírez MdC, Durán-Riveroll LM, González-Maya L. The Benthic Dinoflagellate Coolia malayensis (Dinophyceae) Produces an Array of Compounds with Antineoplastic Activity in Cells of Tumor Origin. Marine Drugs. 2025; 23(3):127. https://doi.org/10.3390/md23030127
Chicago/Turabian StyleMorales-Montesinos, Itzel B., Maria Yolanda Rios, Yordin D. Ocampo-Acuña, Baldomero Esquivel-Rodríguez, Celia Bustos-Brito, María del Carmen Osorio-Ramírez, Lorena M. Durán-Riveroll, and Leticia González-Maya. 2025. "The Benthic Dinoflagellate Coolia malayensis (Dinophyceae) Produces an Array of Compounds with Antineoplastic Activity in Cells of Tumor Origin" Marine Drugs 23, no. 3: 127. https://doi.org/10.3390/md23030127
APA StyleMorales-Montesinos, I. B., Rios, M. Y., Ocampo-Acuña, Y. D., Esquivel-Rodríguez, B., Bustos-Brito, C., Osorio-Ramírez, M. d. C., Durán-Riveroll, L. M., & González-Maya, L. (2025). The Benthic Dinoflagellate Coolia malayensis (Dinophyceae) Produces an Array of Compounds with Antineoplastic Activity in Cells of Tumor Origin. Marine Drugs, 23(3), 127. https://doi.org/10.3390/md23030127









