Bioprospecting Marine Fungi from the Plastisphere: Osteogenic and Antiviral Activities of Fungal Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Mineralogenic and Osteogenic Activities
2.1.1. In Vitro Mineralogenic Assay
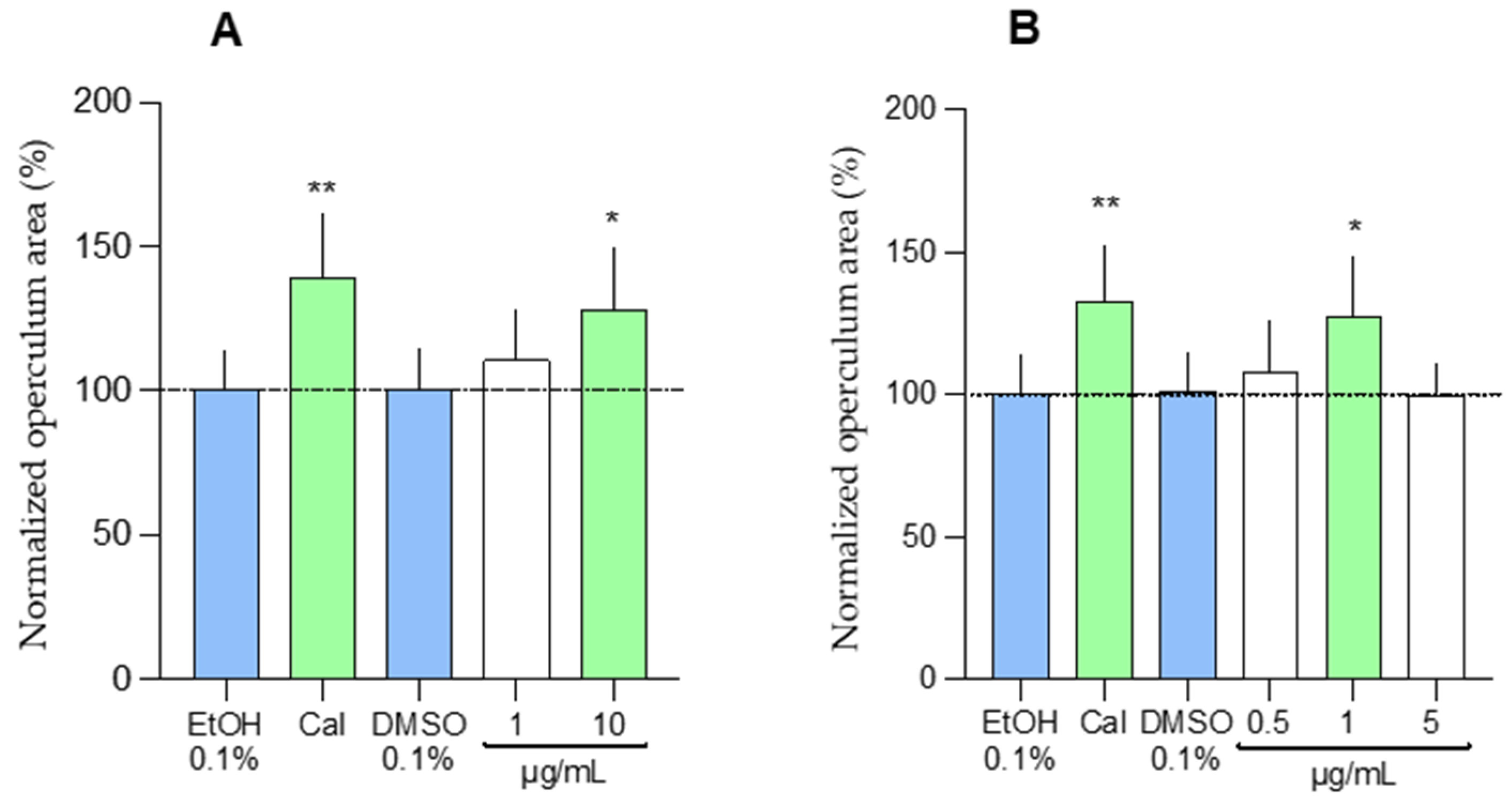
2.1.2. In Vivo Osteogenic Assay
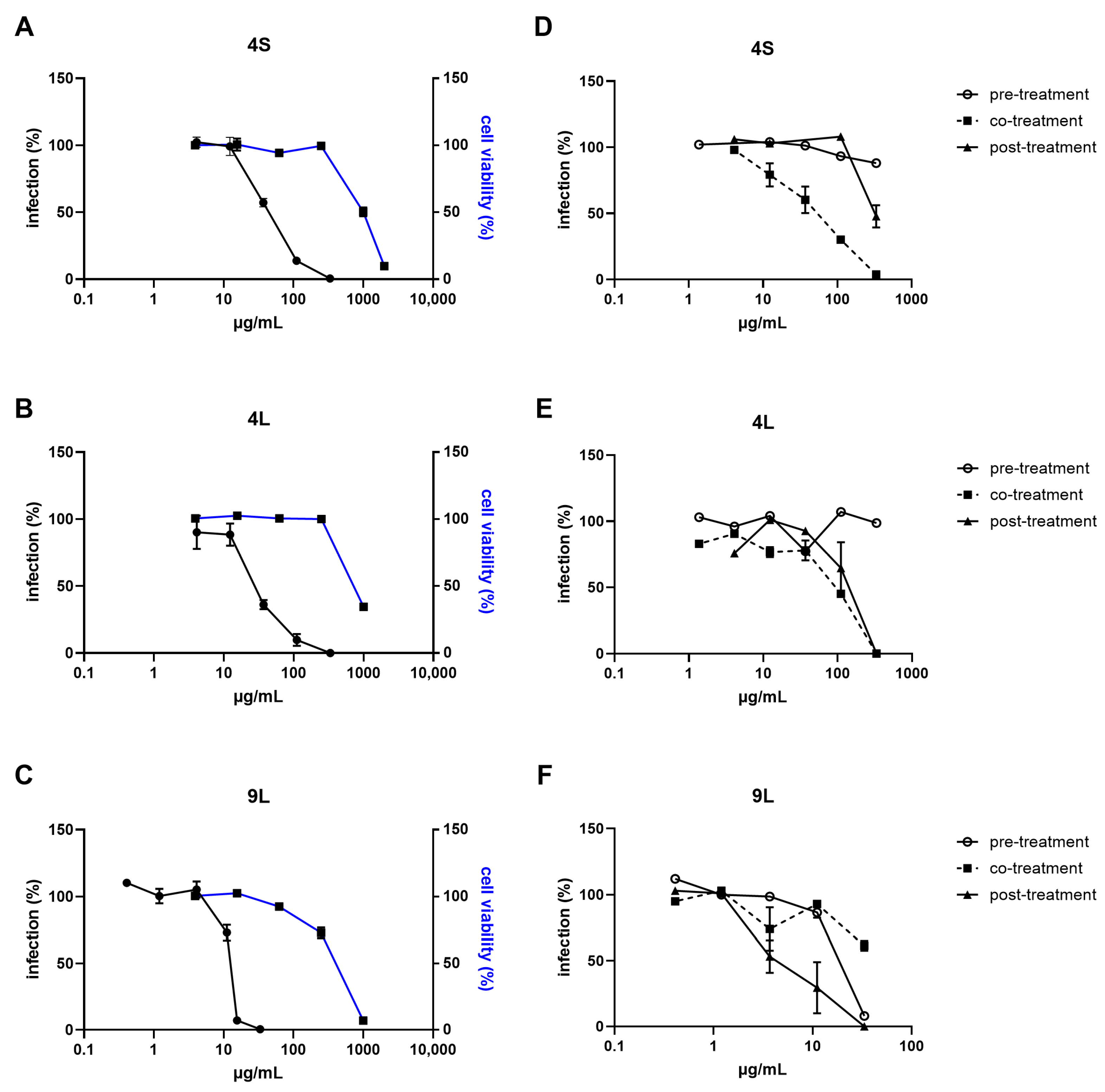
2.2. Antiviral Potential
2.3. Extraction and Purification of Bioactives
3. Materials and Methods
3.1. Fungal Strains
3.2. Growth of the Fungal Strains and Preparation of the Crude Extracts
3.3. Assessment of Mineralogenic Activity
3.4. Assessment of Osteogenic Activity
3.5. Assessment of Antiviral Activity
3.6. Isolation of Secondary Metabolites from A. Jensenii Extract 9L
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| RSV | Respiratory Syncytial Virus |
| HSV-1 | Herpes Simplex Virus Type 1 |
| HSV-2 | Herpes Simplex Virus Type 2 |
| SSF | Solid State Fermentation |
| SmF | Submerged Fermentation |
| ECM | Extracellular Matrix |
| PMA-Ca | Calcium Polymalate |
| EtOH | Ethanol |
| SI | Selectivity Indices |
| MEA | Malt Extract Agar |
| PDB | Potato Dextrose Broth |
| MM | Mineral Medium |
| TMS | Trace Metal Solution |
| MS | Mineral Solution |
| EtOAc | Ethyl Acetate |
| RM | Rice Media |
| DMSO | Dimethylsulfoxide |
| AR-S | Alizarin Red S |
| EC50 | Concentration of a compound that reduces viral infectivity by 50% |
| EC90 | Concentration of a compound that reduces viral infectivity by 90% |
| CC50 | Half maximal cytotoxic concentration |
| IC50 | Concentration of a compound that inhibit viral infectivity by 50% |
| CI | Confidence Interval |
| dpf | Days Post-Fertilization |
| FBS | Fetal Bovine Serum |
| DMEM | Dulbecco’s Modified Eagle medium |
References
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Rajendren, N.; Kathiresan, K.; Wang, M.H. Medicinal Drug-Related Bioactive Agents from Marine Fungi. Encycl. Mar. Biotechnol. 2020, 4, 2173–2190. [Google Scholar] [CrossRef]
- Newton, G.G.F.; Abraham, E.P. Cephalosporin C, a New Antibiotic Containing Sulphur and D-α-Aminoadipic Acid [1]. Nature 1955, 175, 548. [Google Scholar] [CrossRef]
- Lv, F.; Zeng, Y. Novel Bioactive Natural Products from Marine-Derived Penicillium Fungi: A Review (2021–2023). Mar. Drugs 2024, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qader, M.; Wang, Y.; Kong, F.; Wang, Q.; Wang, C. Progress in the Discovery of New Bioactive Substances from Deep-Sea Associated Fungi during 2020–2022. Front. Mar. Sci. 2023, 10, 1232891. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current Status and Future Prospects of Marine Natural Products (MNPs) as Antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef]
- Negi, B.; Kumar, D.; Rawat, D.S. Marine Peptides as Anticancer Agents: A Remedy to Mankind by Nature. Curr. Protein Pept. Sci. 2016, 18, 885–904. [Google Scholar] [CrossRef]
- Corzo, L.; Fernández-Novoa, L.; Carrera, I.; Martínez, O.; Rodríguez, S.; Alejo, R.; Cacabelos, R. Nutrition, Health, and Disease: Role of Selected Marine and Vegetal Nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Florio Furno, M.; Poli, A.; Ferrero, D.; Tardelli, F.; Manzini, C.; Oliva, M.; Pretti, C.; Campani, T.; Casini, S.; Fossi, M.C.; et al. The Culturable Mycobiota of Sediments and Associated Microplastics: From a Harbor to a Marine Protected Area, a Comparative Study. J. Fungi 2022, 8, 927. [Google Scholar] [CrossRef]
- Roager, L.; Sonnenschein, E.C. Bacterial Candidates for Colonization and Degradation of Marine Plastic Debris. Environ. Sci. Technol. 2019, 53, 11636–11643. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Neelam, D.K. Understanding Challenges Associated with Plastic and Bacterial Approach toward Plastic Degradation. J. Basic. Microbiol. 2023, 63, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of Plastics and Plastic-Degrading Bacteria in Cold Marine Habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef]
- Beloe, C.J.; Browne, M.A.; Johnston, E.L. Plastic Debris as a Vector for Bacterial Disease: An Interdisciplinary Systematic Review. Environ. Sci. Technol. 2022, 56, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.P.; Sneed, J.M.; Ritson-Williams, R.; Young, R. Marine Chemical Ecology in Benthic Environments. Nat. Prod. Rep. 2019, 36, 410–429. [Google Scholar] [CrossRef]
- Kamat, S.; Kumar, S.; Philip, S.; Kumari, M. Secondary Metabolites from Marine Fungi: Current Status and Application. In Microbial Biomolecules: Emerging Approach in Agriculture, Pharmaceuticals and Environment Management; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Hay, M.E. Marine Chemical Ecology: Chemical Signals and Cues Structure Marine Populations, Communities, and Ecosystems. Ann. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef]
- Sekurova, O.N.; Schneider, O.; Zotchev, S.B. Novel Bioactive Natural Products from Bacteria via Bioprospecting, Genome Mining and Metabolic Engineering. Microb. Biotechnol. 2019, 12, 574–575. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 1–38. [Google Scholar] [CrossRef]
- Brakhage, A.A.; Schroeckh, V. Fungal Secondary Metabolites—Strategies to Activate Silent Gene Clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Bishnoi, N.R. Evaluation of Cellulase Production from Aspergillus Niger and Aspergillus Heteromorphus under Submerged and Solid-state Fermentation. Environ. Sustain. 2021, 4, 437–442. [Google Scholar] [CrossRef]
- Asther, M.; Haon, M.; Roussos, S.; Record, E.; Delattre, M.; Lesage-Meessen, L.; Labat, M.; Asther, M. Feruloyl Esterase from Aspergillus Niger a Comparison of the Production in Solid State and Submerged Fermentation. Process Biochem. 2002, 38, 685–691. [Google Scholar] [CrossRef]
- Vintila, T.; Dragomirescu, M.; Jurcoane, S.; Vintila, D.; Caprita, R.; Maniu, M. Production of Cellulase by Submerged and Solid-State Cultures and Yeasts Selection for Conversion of Lignocellulose to Ethanol. Rom. Biotechnol. Lett. 2009, 14, 4275–4281. [Google Scholar]
- Ravichandran, S. Solid State and Submerged Fermentation for the Production of Bioactive Substances: A Comparative Study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological Advantages of Laboratory-Scale Solid-State Fermentation with Fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.T.; Tarasco, M.; Gavaia, P.J.; Cancela, M.L.; Laizé, V. Screening of Mineralogenic and Osteogenic Compounds in Zebrafish—Tools to Improve Assay Throughput and Data Accuracy. Pharmaceuticals 2022, 15, 983. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.A.; Nelson, J.; Cancela, M.L.; Laizé, V.; Gavaia, P.J.; Rae, M.; Heesch, S.; Verzin, E.; Maggs, C.; Gilmore, B.F.; et al. Red Algal Extracts from Plocamium Lyngbyanum and Ceramium Secundatum Stimulate Osteogenic Activities in Vitro and Bone Growth in Zebrafish Larvae. Sci. Rep. 2018, 8, 7725. [Google Scholar] [CrossRef]
- Laizé, V.; Gavaia, P.J.; Tarasco, M.; Viegas, M.N.; Caria, J.; Luis, N.; Cancela, M.L. Osteotoxicity of 3-Methylcholanthrene in Fish. Ecotoxicol. Environ. Saf. 2018, 161, 721–728. [Google Scholar] [CrossRef]
- Marchese, P.; Garzoli, L.; Gnavi, G.; O’Connell, E.; Bouraoui, A.; Mehiri, M.; Murphy, J.M.; Varese, G.C. Diversity and Bioactivity of Fungi Associated with the Marine Sea Cucumber Holothuria Poli: Disclosing the Strains Potential for Biomedical Applications. J. Appl. Microbiol. 2020, 129, 612–625. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, L.; Luo, X.; Cai, J.; Huang, L.; Tao, H.; Zhou, X.; Tan, Y.; Liu, Y. Identifying Marine-Derived Tanzawaic Acid Derivatives as Novel Inhibitors against Osteoclastogenesis and Osteoporosis via Downregulation of NF-ΚB and NFATc1 Activation. J. Med. Chem. 2024, 67, 2602–2618. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, J.; Wang, W.; Lee, Y.; Oh, E.; Park, K.H.; Park, C.; Woo, G.E.; Son, Y.J.; Kang, H. Austalide k from the Fungus Penicillium Rudallense Prevents Lps-Induced Bone Loss in Mice by Inhibiting Osteoclast Differentiation and Promoting Osteoblast Differentiation. Int. J. Mol. Sci. 2021, 22, 5493. [Google Scholar] [CrossRef]
- Cai, J.; Gao, L.; Wang, Y.; Zheng, Y.; Lin, X.; Zhou, P.; Chen, C.; Liu, K.; Tang, L.; Liu, Y.; et al. Discovery of a Novel Anti-Osteoporotic Agent from Marine Fungus-Derived Structurally Diverse Sirenins. Eur. J. Med. Chem. 2024, 265, 116068. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Choi, B.K.; Trinh, P.T.H.; Lee, H.S.; Kang, J.S.; Van, T.T.T.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Lee, J. Suppression of RANKL-Induced Osteoclastogenesis by the Metabolites from the Marine Fungus Aspergillus Flocculosus Isolated from a Sponge Stylissa Sp. Mar. Drugs 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, H.; Shi, T.; Wang, B. The Genus Cladosporium: A Prospective Producer of Natural Products. Int. J. Mol. Sci. 2024, 25, 1652. [Google Scholar] [CrossRef]
- Yu, H.Y.; Chen, Y.S.; Wang, Y.; Zou, Z.B.; Xie, M.M.; Li, Y.; Li, L.S.; Meng, D.L.; Wu, L.Q.; Yang, X.W. Anti-Necroptosis and Anti-Ferroptosis Compounds from the Deep-Sea-Derived Fungus Aspergillus Sp. MCCC 3A00392. Bioorg Chem. 2024, 144, 107175. [Google Scholar] [CrossRef]
- Zhu, G.; Kong, F.; Wang, Y.; Fu, P.; Zhu, W. Cladodionen, a Cytotoxic Hybrid Polyketide from the Marine-Derived Cladosporium Sp. OUCMDZ-1635. Mar. Drugs 2018, 16, 71. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Taktaz, F.; Sousa, E.; Fernandes, C.; Kijjoa, A. Peptides from Marine-Derived Fungi: Chemistry and Biological Activities†. Mar. Drugs 2023, 21, 510. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped Potential of Marine-Associated Cladosporium Species: An Overview on Secondary Metabolites, Biotechnological Relevance, and Biological Activities. Mar. Drugs 2021, 19, 645. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The Genus Cladosporium: A Rich Source of Diverse and Bioactive Natural Compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.R.S.; El-Sayed, H. Molecular Identification and Antimicrobial Activities of Some Wild Egyptian Mushrooms: Bjerkandera Adusta as a Promising Source of Bioactive Antimicrobial Phenolic Compounds. J. Genet. Eng. Biotechnol. 2021, 19, 106. [Google Scholar] [CrossRef]
- Nicoletti, R.; Trincone, A. Bioactive Compounds Produced by Strains of Penicillium and Talaromyces of Marine Origin. Mar. Drugs 2016, 14, 37. [Google Scholar] [CrossRef]
- Marchese, P.; Mahajan, N.; O’Connell, E.; Fearnhead, H.; Tuohy, M.; Krawczyk, J.; Thomas, O.P.; Barry, F.; Murphy, M.J. A Novel High-Throughput Screening Platform Identifies Itaconate Derivatives from Marine Penicillium Antarcticum as Inhibitors of Mesenchymal Stem Cell Differentiation. Mar. Drugs 2020, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Du, Q.; Wu, X.M.; Chen, Y.; Tan, R.X. Citrisorbicillinol, an Undescribed Hybrid Sorbicillinoid with Osteogenic Activity from Penicillium Citrinum ZY-2. Fitoterapia 2024, 173, 105836. [Google Scholar] [CrossRef]
- Li, F.; Xie, X.; Xu, X.; Zou, X. Water-Soluble Biopolymers Calcium Polymalate Derived from Fermentation Broth of Aureobasidium Pullulans Markedly Alleviates Osteoporosis and Fatigue. Int. J. Biol. Macromol. 2024, 268, 132013. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kusano, K.; Kondo, N.; Nishikawa, K.; Kuge, T.; Ohno, N. Biological Activity of High-Purity β-1,3-1,6-Glucan Derived from the Black Yeast Aureobasidium Pullulans: A Literature Review. Nutrients 2021, 13, 242. [Google Scholar] [CrossRef]
- Shin, H.D.; Yang, K.J.; Park, B.R.; Son, C.W.; Jang, H.J.; Ku, S.K. Antiosteoporotic Effect of Polycan, β-Glucan from Aureobasidium, in Ovariectomized Osteoporotic Mice. Nutrition 2007, 23, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Steinbüchel, A. Investigation of Poly(β-L-Malic Acid) Production by Strains of Aureobasidium Pullulans. Appl. Microbiol. Biotechnol. 1996, 46, 273–278. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Dong, J.; Zhang, D.; Huang, L.; Xu, Z.; Cen, P. High-Level Production of Poly (β-L-Malic Acid) with a New Isolated Aureobasidium Pullulans Strain. Appl. Microbiol. Biotechnol. 2011, 92, 295–303. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, Y.; Yang, S.T. Production of Polymalic Acid and Malic Acid by Aureobasidium Pullulans Fermentation and Acid Hydrolysis. Biotechnol. Bioeng. 2013, 110, 2105–2113. [Google Scholar] [CrossRef]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J.; et al. Genome Sequencing of Four Aureobasidium Pullulans Varieties: Biotechnological Potential, Stress Tolerance, and Description of New Species. BMC Genom. 2014, 15, 1–29. [Google Scholar] [CrossRef]
- Li, Y.; Chi, Z.; Wang, G.Y.; Wang, Z.P.; Liu, G.L.; Lee, C.F.; Ma, Z.C.; Chi, Z.M. Taxonomy of Aureobasidium Spp. and Biosynthesis and Regulation of Their Extracellular Polymers. Crit. Rev. Microbiol. 2015, 41, 132013. [Google Scholar] [CrossRef]
- Lorenz, P.; Jensen, P.R.; Fenical, W. Mactanamide, a New Fungistatic Diketopiperazine Produced by a Marine Aspergillus Sp. Nat. Prod. Lett. 1998, 12, 55–60. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Pharmacokinetics, Pharmacodynamics, Toxicity, and Formulations of Daidzein: An Important Isoflavone. Phytother. Res. 2023, 37, 2578–2604. [Google Scholar] [CrossRef] [PubMed]
- Junnan, C.; Xiaoqing, T.; Chengqi, F.; Jinchang, H.; Yanan, L.; Qinghua, H. Secondary Metabolites from the Antarctic Fungi Cladosporium Sp. NJF4 and NJF6. Chin. J. Polar Res. 2020, 32, 60. [Google Scholar] [CrossRef]
- Carletti, A.; Gavaia, P.J.; Cancela, M.L.; Laizé, V. Metabolic Bone Disorders and the Promise of Marine Osteoactive Compounds. Cell. Mol. Life Sci. 2024, 81, 11. [Google Scholar] [CrossRef]
- Marcadet, L.; Bouredji, Z.; Argaw, A.; Frenette, J. The Roles of RANK/RANKL/OPG in Cardiac, Skeletal, and Smooth Muscles in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 903657. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qin, K.; Fan, J.; Zhao, G.; Zhao, P.; Zeng, W.; Chen, C.; Wang, A.; Wang, Y.; Zhong, J.; et al. The Evolving Roles of Wnt Signaling in Stem Cell Proliferation and Differentiation, the Development of Human Diseases, and Therapeutic Opportunities. Genes. Dis. 2024, 11, 101026. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Macías-Rubalcava, M.L.; Trujillo-Roldán, M.A. Overview of Fungal Terpene Synthases and Their Regulation. World J. Microbiol. Biotechnol. 2023, 39, 1–21. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Jiang, Y.; Jin, B.; Wang, L. Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global Estimates of Prevalent and Incident Herpes Simplex Virus Type 2 Infections in 2012. PLoS ONE 2015, 10, e114989. [Google Scholar] [CrossRef]
- Gupta, R.; Warren, T.; Wald, A. Genital Herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Zou, G.; Cao, S.; Gao, Z.; Yie, J.; Wu, J.Z. Current State and Challenges in Respiratory Syncytial Virus Drug Discovery and Development. Antiviral Res. 2024, 221, 105791. [Google Scholar] [CrossRef] [PubMed]
- Bechini, A.; Salvati, C.; Bonito, B.; Del Riccio, M.; Stancanelli, E.; Bruschi, M.; Ionita, G.; Iamarino, J.A.; Bentivegna, D.; Buscemi, P.; et al. Costs and Healthcare Utilisation Due to Respiratory Syncytial Virus Disease in Paediatric Patients in Italy: A Systematic Review. Public. Health 2024, 227, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Arduino, I.; Rittà, M.; Molinar, C.; Feyles, E.; Lembo, D.; Cavalli, R.; Donalisio, M. Enhanced Anti-Herpetic Activity of Valacyclovir Loaded in Sulfobutyl-Ether-β-Cyclodextrin-Decorated Chitosan Nanodroplets. Microorganisms 2023, 11, 2460. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Choi, S.H. Epidemiology and Disease Burden of Respiratory Syncytial Virus Infection in Adults. Infect. Chemother. 2024, 56, 1. [Google Scholar] [CrossRef]
- Yu, M.L.; Guan, F.F.; Cao, F.; Jia, Y.L.; Wang, C.Y. A New Antiviral Pregnane from a Gorgonian-Derived Cladosporium Sp. Fungus. Nat. Prod. Res. 2018, 32, 1260–1266. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, L.; Li, Y.Q.; Luo, J.H.; Li, W.; Li, L.F.; Zheng, C.J.; Cao, F. Oxalierpenes A and B, Unusual Indole-Diterpenoid Derivatives with Antiviral Activity from a Marine-Derived Strain of the Fungus Penicillium Oxalicum. J. Nat. Prod. 2022, 85, 1880–1885. [Google Scholar] [CrossRef]
- Tang, J.; Su, L.; He, X.; Liu, D.; Zhao, C.; Zhang, S.; Li, Q.; Li, R.; Li, H. Biotransformation of Patchouli Alcohol by Cladosporium Cladosporioides and the Anti-Influenza Virus Activities of Biotransformation Products. J. Agric. Food Chem. 2024, 72, 7991–8005. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Mierzwa, R.; He, L.; King, A.; Patel, M.; Pichardo, J.; Hart, A.; Butkiewicz, N.; Puar, M.S. Isolation and Structure of Sch 351633: A Novel Hepatitis C Virus (HCV) NS3 Protease Inhibitor from the Fungus Penicillium Griseofulvum. Bioorg. Med. Chem. Lett. 1999, 9, 1949–1952. [Google Scholar] [CrossRef]
- Ngamcharungchit, C.; Chaimusik, N.; Panbangred, W.; Euanorasetr, J.; Intra, B. Bioactive Metabolites from Terrestrial and Marine Actinomycetes. Molecules 2023, 28, 5915. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological Evaluation and in Silico Study of Benzoic Acid Derivatives from Bjerkandera Adusta Targeting Proteostasis Network Modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Lin, S.; Zhang, B.; Jin, H.; Ding, L. Antiviral Potential of Natural Products from Marine Microbes. Eur. J. Med. Chem. 2020, 207, 112738. [Google Scholar] [CrossRef]
- Chen, M.; Shao, C.L.; Meng, H.; She, Z.G.; Wang, C.Y. Anti-Respiratory Syncytial Virus Prenylated Dihydroquinolone Derivatives from the Gorgonian-Derived Fungus Aspergillus Sp. XS-20090B15. J. Nat. Prod. 2014, 77, 2720–2724. [Google Scholar] [CrossRef]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and Butyrolactone Derivatives from a Marine-Derived Fungus Aspergillus Terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.H. Antiviral Peptides from Marine Gorgonian-Derived Fungus Aspergillus Sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Fujii, Y.; Asahara, M.; Ichinoe, M.; Nakajima, H. Fungal Melanin Inhibitor and Related Compounds from Penicillium Decumbens. Phytochemistry 2002, 60, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, M.M.; Klykov, A.G. Metabolites of Terrestrial Plants and Marine Organisms as Potential Regulators of Growth of Agricultural Plants in the Russian Far East. J. Agric. Sci. 2014, 6, 1019–1022. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Vishchuk, O.S.; Denisenko, V.A.; Slinkina, N.N.; Smetanina, O.F. Decumbenone C, a New Cytotoxic Decaline Derivative from the Marine Fungus Aspergillus Sulphureus KMM 4640. Arch. Pharm. Res. 2012, 35, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, M.M.; Chaikina, E.L.; Afiyatullov, S.S.; Zhuravleva, O.I.; Klykov, A.G.; Kraskovskaja, N.A.; Aminin, D.L. Decumbenones A–C from Marine Fungus Aspergillus Sulphureus as Stimulators of the Initial Stages of Development of Agricultural Plants. Agric. Sci. 2012, 3, 1019–1022. [Google Scholar] [CrossRef][Green Version]
- Yang, L.J.; Peng, X.Y.; Zhang, Y.H.; Liu, Z.Q.; Li, X.; Gu, Y.C.; Shao, C.L.; Han, Z.; Wang, C.Y. Antimicrobial and Antioxidant Polyketides from a Deep-Sea-Derived Fungus Aspergillus Versicolor SH0105. Mar. Drugs 2020, 18, 636. [Google Scholar] [CrossRef]
- He, Y.P.; Zhang, Z.K.; Li, Z.J.; Wu, P.P.; Hu, J.S.; Fan, H.; Zhang, C.X. Two New Types of Structures from Soft Coral-Associated Epiphytic Fungus Aspergillus Versicolor CGF9-1-2. Fitoterapia 2024, 177, 106136. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.L.; Mansoor, T.A.; Hong, J.; Lee, C.O.; Kyung, S.B.; Jung, J.H. Polyketides from a Sponge-Derived Fungus, Aspergillus Versicolor. Nat. Product. Sci. 2007, 13, 90–96. [Google Scholar]
- Fu, Y.; Wu, P.; Xue, J.; Wei, X.; Li, H. Versicorin, a New Lovastatin Analogue from the Fungus Aspergillus Versicolor SC0156. Nat. Prod. Res. 2015, 29, 1363–1368. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Smetanina, O.F.; Kalinovsky, A.I.; Kirichuk, N.N.; Pivkin, M.V.; Ivanets, E.V.; Yurchenko, E.A.; Afiyatullov, S.S. New Metabolites from a Marine Sediment-Derived Fungus, Aspergillus Carneus. Nat. Prod. Commun. 2015, 10, 1247–1250. [Google Scholar] [CrossRef]
- Guo, H.; Feng, T.; Li, Z.H.; Liu, J.K. Five New Polyketides from the Basidiomycete Craterellus Odoratus. Nat. Prod. Bioprospect. 2012, 2, 170–173. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Primary High Throughput Screening by Co-Culture Imaging for Identification Hits as a Selective Cytotoxic Compound to Cancer Cells in an EMT-Like State. PubChem Bioassay Record for AID 1345086, Source: University of Iowa High-Throughput Screening Core (UIHTS). Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1345086 (accessed on 24 February 2025).
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Pivkin, M.V.; Afiyatullov, S.S. New Kipukasin from Marine Isolate of the Fungus Aspergillus Flavus. Chem. Nat. Compd. 2016, 52, 266–268. [Google Scholar] [CrossRef]
- Niu, S.; Xia, M.; Chen, M.; Liu, X.; Li, Z.; Xie, Y.; Shao, Z.; Zhang, G. Cytotoxic Polyketides Isolated from the Deep-Sea-Derived Fungus Penicillium Chrysogenum MCCC 3A00292. Mar. Drugs 2019, 17, 686. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Hu, L.; Liu, S.; Xing, R.; Li, P. Isolation and Identification of Antimicrobial Metabolites from Sea Anemone-Derived Fungus Emericella Sp. SMA01. J. Oceanol. Limnol. 2021, 39, 1010–1019. [Google Scholar] [CrossRef]
- Shea, A.; Wolcott, M.; Daefler, S.; Rozak, D.A. Biolog Phenotype Microarrays. Methods Mol. Biol. 2012, 881, 331–373. [Google Scholar] [CrossRef]
- Pombinho, A.R.; Laizé, V.; Molha, D.M.; Marques, S.M.P.; Cancela, M.L. Development of Two Bone-Derived Cell Lines from the Marine Teleost Sparus Aurata; Evidence for Extracellular Matrix Mineralization and Cell-Type-Specific Expression of Matrix Gla Protein and Osteocalcin. Cell Tissue Res. 2004, 315, 393–406. [Google Scholar] [CrossRef]
- Marques, C.L.; Rafael, M.S.; Cancela, M.L.; Laizé, V. Establishment of Primary Cell Cultures from Fish Calcified Tissues. Cytotechnology 2007, 55, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tarasco, M.; Laizé, V.; Cardeira, J.; Cancela, M.L.; Gavaia, P.J. The Zebrafish Operculum: A Powerful System to Assess Osteogenic Bioactivities of Molecules with Pharmacological and Toxicological Relevance. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2017, 197, 115480. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Francese, R.; Civra, A.; Feyles, E.; Argenziano, M.; Volante, M.; Cavalli, R.; Mougharbel, A.M.; Kortz, U.; Donalisio, M.; et al. Polyoxometalate Exerts Broad-Spectrum Activity against Human Respiratory Viruses Hampering Viral Entry. Antivir. Res. 2024, 226, 105897. [Google Scholar] [CrossRef] [PubMed]



| Taxa | Id. Crude Extract SSF | Productivity for SSF (mg) | Id. Crude Extract SmF | Productivity for SmF (mg) |
|---|---|---|---|---|
| Cystobasidium slooffiae MUT6565 | 1S | 121.9 | 1L | 5.5 |
| Cladosporium cladosporioides MUT6583 | 2S | 188.8 | 2L | 8.8 |
| Aspergillus domesticus MUT6603 | 3S | 131.7 | 3L | 3.1 |
| Bjerkandera adusta MUT6589 | 4S | 43.0 | 4L | 5.4 |
| Parengydontium album MUT6579 | 5S | 197.4 | 5L | 4.1 |
| Cladosporium ramotenellum MUT6556 | 6S | 159.4 | 6L | 2.7 |
| Sesquicillium microsporum MUT6592 | 7S | 37.4 | 7L | 4.2 |
| Cladosporium halotolerans MUT6558 | 8S | 132.1 | 8L | 3.8 |
| Aspergillus jensenii MUT6581 | 9S | 176.8 | 9L | 9.0 |
| Sakaguchia dacryoidea MUT6569 | 10S | 355.3 | 10L | 7.0 |
| Aureobasidium pullulans MUT6584 | 11S | 15.4 | 11L | 12.0 |
| Vishniacozyma carnescens MUT6591 | 12S | 374.6 | 12L | 6.9 |
| Kondoa aeria MUT6563 | 13S | 119.3 | 13L | 3.4 |
| Cladosporium pseudocladosporioides MUT6586 | 14S | 132.2 | 14L | 2.8 |
| Penicillium griseofulvum MUT6588 | 15S | 293.8 | 15L | 108.2 |
| Average productivity | 187.9 | 12.4 |
| Decumbenone A | |||||
|---|---|---|---|---|---|
| Organism | Source | Bioactivity | Bioassay | Activity | Ref. |
| Penicillium decumbens | Terrestrial | Melanin inhibition | - | Active | [77] |
| Aspergillus sulphureus | Marine | Cytotoxicity Antiproliferation Antitumoral | Cancer cell lines (SK-MEL-28, SK-MEL-5, HCT 116) | Cytotoxic against SK-MEL-5, SK-MEL-28 Inhibitor for all Antitumoral on SK-MEL-5 and HCT 116 | [78,79,80] |
| Stimulators of development of agricultural plants | - | Stimulating effect on root growth of spring wheat | |||
| Aspergillus versicolor | Marine | Antimicrobial | ATCC bacterial and yeasts | None | [81] |
| Antioxidant | DPPH and FRAP | None | |||
| Aspergillus versicolor | Marine | Cytotoxicity | A549, SKOV-3, SK-MEL-2, XF498, HTC15 cell lines | None | [82] |
| Antibacterial | meticillin-resistent clinical strains | None | |||
| Aspergillus versicolor | Marine | Antitumoral | TDP1 inhibition assay | None | [83] |
| Aspergillus versicolor | Marine | - | - | - | [84] |
| Aspergillus carneus | Marine | Cytotoxicity | MTT assay | None | [85] |
| Antiradical activities | DPPH assay | None | |||
| Influence on fertilized sea urchin ovum | - | None | |||
| Craterellus odoratus | Terrestrial | Glucocorticoid metabolism regulation | Inhibition of 11β-HSD1 | None | [86] |
| Cytotoxicity | Cancer cell lines | None | |||
| Synthesized molecule | - | Cytotoxicity | Cancer cell lines | None | [87] |
| Decumbenone B | |||||
| Penicillium decumbens | Terrestrial | Melanin inhibition | - | Inactive | [77] |
| Aspergillus sulphureus | Marine | Cytotoxicity, Antiproliferation Antitumoral | Cancer cell lines (SK-MEL-28, SK-MEL-5, HCT 116) | Cytotoxicity against SK-MEL-5, SK-MEL-28, | [78,79,80] |
| Antiproliferative for all | |||||
| Antitumoral on SK-MEL-5 and HCT 116. | |||||
| Stimulators of development of agricultural plants | - | Stimulatory growth effect on roots of buckwheat | |||
| Aspergillus versicolor | marine | Antimicrobial | ATCC bacterial and yeasts | None | [81] |
| Antioxidant | DPPH and FRAP | None | |||
| Aspergillus versicolor | marine | Cytotoxicity agains human solid tumor cell lines | A549, SKOV-3, SK-MEL-2, XF498, HTC15 cell lines | None | [82] |
| Antibacterial | 20 meticillin-resistent clinical strains | None | |||
| Aspergillus versicolor | Marine | Antitumoral | TDP1 inhibition assay | None | [83] |
| Aspergillus versicolor | Marine | - | - | - | [84] |
| Aspergillus carneus | Marine | Cytotoxicity | MTT assay | None | [85] |
| Antiradical | DPPH assay | None | |||
| Influence on fertilized sea urchin ovum | - | None | |||
| Aspergillus flavus | Marine | Antimicrobial | ATCC bacterial and yeasts | None | [88] |
| Craterellus odoratus | Terrestrial | Glucocorticoid metabolism regulation | Inhibition of 11β-HSD1 | None | [86] |
| Cytotoxicity | Cancer cell lines | None | |||
| 15 Strains Recovered from Marine Microplastics |
| Solid State Fermentation: 15 crude extracts |
| Submerged Fermentation: 15 crude extracts |
| Mineralogenic and Osteogenic Activities |
| In vitro mineralogic assay |
| Endpoint: extracellular matrix mineralization using gilthead seabream vertebra-derived cell line VSa13 |
| Conditions: highest non-toxic concentration (100 µg/mL for most extracts; 10 µg/mL for extracts 9S and 15S) |
| In vivo osteogenic assay |
| Endpoint: mineralized operculum area using 6-day post-fertilization zebrafish larvae |
| Conditions: highest non-toxic concentration (1, 10, and 100 µg/mL depending on the extracts) |
| Antiviral Activities Against RSV And HSV-2 |
| Endpoint #1: viral infectivity reduction assay, based on the number of infected cells exposed to increasing extract concentrations |
| Conditions: extracts from 0.1 to 1000 µg/mL covering all steps of infection |
| Endpoint #2: time-of-addition assay, exposing cells to extracts before, during, or after viral infection |
| Conditions: the most active extracts |
| Identification of Bioactive Molecules |
| Endpoint #1: HPLC-UV/ELSD analysis of the crude extract |
| Conditions: Extract of Aspergillus jensenii 9L with pro-osteogenic and antiviral activities |
| Endpoint #2: purification of predominant compounds in 9L extract by HPLC and evaluation of mineralogic, osteogenic, and antiviral activities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florio Furno, M.; Laizé, V.; Arduino, I.; Pham, G.N.; Spina, F.; Mehiri, M.; Lembo, D.; Gavaia, P.J.; Varese, G.C. Bioprospecting Marine Fungi from the Plastisphere: Osteogenic and Antiviral Activities of Fungal Extracts. Mar. Drugs 2025, 23, 115. https://doi.org/10.3390/md23030115
Florio Furno M, Laizé V, Arduino I, Pham GN, Spina F, Mehiri M, Lembo D, Gavaia PJ, Varese GC. Bioprospecting Marine Fungi from the Plastisphere: Osteogenic and Antiviral Activities of Fungal Extracts. Marine Drugs. 2025; 23(3):115. https://doi.org/10.3390/md23030115
Chicago/Turabian StyleFlorio Furno, Matteo, Vincent Laizé, Irene Arduino, Giang Nam Pham, Federica Spina, Mohamed Mehiri, David Lembo, Paulo J. Gavaia, and Giovanna Cristina Varese. 2025. "Bioprospecting Marine Fungi from the Plastisphere: Osteogenic and Antiviral Activities of Fungal Extracts" Marine Drugs 23, no. 3: 115. https://doi.org/10.3390/md23030115
APA StyleFlorio Furno, M., Laizé, V., Arduino, I., Pham, G. N., Spina, F., Mehiri, M., Lembo, D., Gavaia, P. J., & Varese, G. C. (2025). Bioprospecting Marine Fungi from the Plastisphere: Osteogenic and Antiviral Activities of Fungal Extracts. Marine Drugs, 23(3), 115. https://doi.org/10.3390/md23030115











