Abstract
Microalgae are small, single-celled, or simple multicellular organisms that contain Chlorophyll a, allowing them to efficiently convert CO2 and water into organic matter through photosynthesis. They are valuable in producing a range of products such as biofuels, food, pharmaceuticals, and cosmetics, making them economically and environmentally significant. Currently, CO2 is delivered to microalgae cultivation systems mainly through aeration with CO2-enriched gases. However, this method demonstrates limited CO2 absorption efficiency (13–20%), which reduces carbon utilization effectiveness and significantly increases carbon-source expenditure. To overcome these challenges, innovative CO2 supplementation technologies have been introduced, raising CO2 utilization rates to over 50%, accelerating microalgae growth, and reducing cultivation costs. This review first categorizes CO2 supplementation technologies used in photobioreactor systems, focusing on different mechanisms for enhancing CO2 mass transfer. It then evaluates the effectiveness of these technologies and explores their potential for scaling up. Among these strategies, membrane-based CO2 delivery systems and the incorporation of CO2 absorption enhancers have shown the highest efficiency in boosting CO2 mass transfer and microalgae productivity. Future efforts should focus on integrating these methods into large-scale photobioreactor systems to optimize cost-effective, sustainable production.
1. Introduction
Microalgae have the capability to fix CO2 through photosynthesis, producing a range of valuable chemicals. Certain species, such as Botryococcus braunii, can generate hydrocarbons that constitute 15% to 75% of their dry weight. Other species accumulate glycogen or glycerol, and many have lipid contents exceeding 30% of their dry weight [1,2]. The pyrolysis of microalgal biomass can produce biofuels with an average calorific value of up to 33 MJ/kg [3]. Moreover, microalgae can be cultivated in seawater, alkaline water, or semi-alkaline water, which helps avoid competition with crops for arable land and freshwater. They can also utilize nitrogen-rich wastewater, making them a valuable resource in areas with limited freshwater and degraded land [4,5]. Thus, microalgae present a promising future source of energy and chemicals.
The carbon content in microalgal cells represents about half of their dry weight. During growth, microalgae fix CO2 into their cellular components through photosynthesis, necessitating a continuous supply of carbon sources in the cultivation medium [6]. In the medium, inorganic carbon exists in three forms: HCO3−, CO32−, and free CO2. The concentration and ratio of these forms depend on the total inorganic carbon concentration and pH [7]. For most economically valuable microalgal species, the optimal growth pH ranges from 6 to 8, during which the primary forms of inorganic carbon in the medium are free CO2 and HCO3−. In contrast, alkaliphilic species, such as Spirulina, thrive at a pH of around 9.0, where the dominant carbon species are HCO3− and CO32−. When sodium bicarbonate (NaHCO3) is used, the medium’s pH increases due to the dissociation of HCO3− and CO2 consumption. This process can convert more than half of the NaHCO3 into Na2CO3, which is not usable by the microalgae and complicates medium recycling due to the elevated pH [8]. Using CO2 directly as a carbon source is more effective for microalgae, as it avoids the issue of rising pH and helps maintain an optimal cultivation environment, allowing for extended or repeated use of the medium.
Microalgal cultivation methods are generally divided into open and closed systems [9]. Both typically involve bubbling CO2-enriched gas into the cultivation medium, but this method is inefficient due to low CO2 absorption rates (13–20%), leading to high carbon-source costs [10,11]. Since carbon accounts for approximately half of the dry weight of microalgal cells, an increase of 1 g/L in cell concentration requires the assimilation of around 2 g/L of CO2. However, the solubility of pure CO2 in water is relatively low, at only 1.45 g/L at 25 °C, and even lower when CO2 is sourced from air, with a solubility of just 0.58 mg/L under the same conditions. This limited solubility poses a significant challenge for maintaining sufficient inorganic carbon availability in the cultivation medium. Therefore, enhancing the concentration of inorganic carbon species and improving CO2 absorption efficiency are critical for supporting rapid microalgal growth and minimizing cultivation costs.
Recent advancements have led to the development of innovative CO2 supplementation technologies designed to meet the rapid growth requirements of microalgae while reducing cultivation costs. Examples include in situ CO2 supplementation devices in raceway ponds, which increase gas–liquid contact time and surface area, and methods that improve CO2 absorption and conversion using immobilized carbonic anhydrase [7,12,13]. This review categorizes these CO2 supplementation technologies based on their mechanisms for enhancing CO2 mass transfer, assesses their effectiveness, and explores the potential for scaling up these technologies. By systematically addressing these objectives, this review aims to offer a comprehensive understanding of CO2 management in microalgal cultivation and highlight innovative strategies to overcome the limitations of conventional carbon-supplementation methods.
2. Methodologies and Devices for Enhancing CO2 Mass Transfer in Microalgal Systems
2.1. CO2 Mass-Transfer Process
Under photoautotrophic growth conditions, microalgae use inorganic carbon sources to synthesize organic compounds and convert light energy into chemical energy. Microalgae can absorb both CO2 and HCO3− but cannot utilize CO32− [14]. CO2 enters the cells through diffusion and is used directly, while HCO3−, being a polar and negatively charged ion, requires active transport across the cell membrane, a process that consumes energy [15]. Consequently, the absorption of HCO3− is slower compared to CO2, though some algae species that thrive in high pH environments, such as Spirulina, exhibit better HCO3− absorption.
During CO2 transfer in the cultivation medium, it can react with OH− and CO32−, though these reactions have minimal impact on CO2 transfer efficiency [16]. In microalgal culture media, CO2 transfer is a multi-step process involving several stages: from the gas phase to the gas film, diffusion within the gas film, transfer from the gas film to the liquid film, diffusion through the liquid film, movement from the liquid film into the liquid phase, diffusion within the liquid phase, transfer from the liquid phase to the liquid film at the cell-wall surface, and finally, cellular absorption. According to the two-membrane theory, the primary resistance to CO2 transfer occurs within the liquid film, which serves as the main barrier limiting the efficiency of gas–liquid mass transfer [17,18]. The mass-transfer rate is proportional to the driving force and the area available for mass transfer. The rate of CO2 transfer can be expressed as:
where KL,a is the overall liquid volumetric mass-transfer coefficient for the absorption of CO2, dependent on factors like phase contact area and the intensity of gas–liquid mixing. represents the CO2 concentration in the liquid phase at equilibrium with the gas-phase concentration.
2.2. In Situ CO2 Supplementation
To enhance CO2 absorption in algal medium, several in situ CO2 supplementation devices have been developed. Kumar et al. improved CO2 mass transfer by using hollow-fiber membranes, which provide a significantly larger interphase contact area compared to traditional bubbling methods, resulting in a mass-transfer coefficient approximately ten times greater (Figure 1A) [19]. This technology enhances CO2 absorption efficiency and facilitates CO2 recycling, thereby reducing cultivation costs [20]. Ketheesan et al. introduced a novel airlift raceway-pond design where CO2 is injected into an ascending channel, increasing CO2 and liquid contact time and achieving a 50% absorption rate [21]. Our research team implemented an in situ CO2 supplementation trap device in an open raceway pond for Spirulina platensis cultivation (Figure 1B) [22]. This device, featuring a trap container, partition, and gas distributor, effectively extended the gas–liquid contact time from 3 s to 8 s and enhanced CO2 utilization efficiency to over 90% (Table 1). Chen et al. utilized a leak-proof cover over the cultivation layer, which collected CO2 and created a large gas–liquid exchange area (Figure 1C), though challenges included limited gas–liquid exchange surface area, the accumulation of oxygen and nitrogen, and reduced light transmittance [23]. Our research team also developed a submerged cover-type CO2 supplementation device installed at the bottom of open ponds. Transparent glass covers above the aeration points and below the liquid surface allowed bubbles to have extended contact time with the liquid, reducing CO2 escape and improving CO2 absorption efficiency [24].
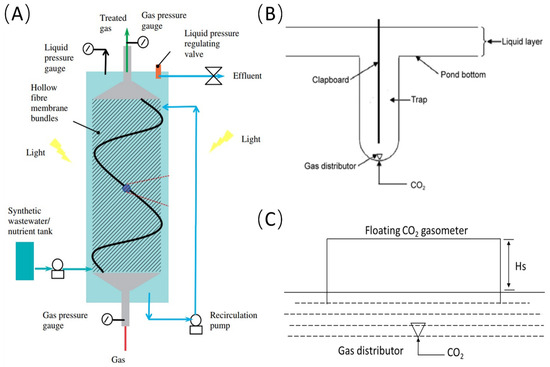
Figure 1.
In situ carbon-supplementation devices in microalgae cultivation systems; (A) hollow fiber membrane module; (B) trap-type carbon-supplementation device; (C) leak-proof cover device.
In closed photobioreactors, Bergmann et al. enhanced bubble residence time by modifying flat-panel reactors to multiple chambers, achieving over 80% CO2 absorption (Figure 2A) [25]. Huang et al. further enhanced gas–liquid mixing in flat-panel reactors by incorporating disturbance columns or inclined baffles (Figure 2B). This modification increased mixing intensity by up to 52%, significantly boosting the CO2 mass-transfer coefficient [26]. The tubular photobioreactor, currently the most widely used closed photobioreactor, has evolved through multiple generations into a structure comprising light absorption units, gas–liquid exchange units, and circulation pumps. CO2 can be introduced into the gas–liquid exchange unit or before the culture liquid enters the light absorption unit, or at a specific position within the light absorption unit. Under the action of the circulation pump, the gas moves with the liquid and is gradually absorbed, resulting in high CO2 absorption rates (Figure 2C) [27].
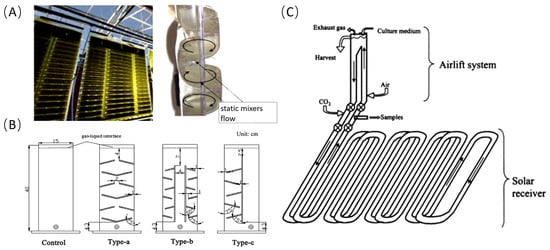
Figure 2.
Closed photobioreactor and structural modifications for enhanced CO2 mass transfer; (A) multi-chamber structure of flexible flat-panel reactor; (B) different types of baffle structures in flat-panel reactors; (C) tubular reactor and CO2 supplementation positions.

Table 1.
Effect of methodologies and devices on microalgae growth and CO2 utilization efficiency.
Table 1.
Effect of methodologies and devices on microalgae growth and CO2 utilization efficiency.
| Microalgal Culture System | Methodologies or Devices | Species | Biomass | CO2 Utilization Efficiency | Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Photobioreactor | Hollow fiber membrane | Spirulina platensis | 2131 mg/ L ↑ | 85% ↑ | Increase the interfacial contact area available for gas transfer | [19,20] |
| Raceway pond | An ascending channel | Scenedesmus sp. | 0.16 ± 0.03 g/(L·d) ↑ | 50% ↑ | Increase mixing intensity | [21] |
| Raceway pond | CO2 supplementation trap device | Spirulina platensis | 3.45–6.04 g/(m2·d) ↑ | 90% ↑ | Prolong gas–liquid contact time | [22] |
| Open pond | Leak-proof cover | Cyanobacterium sp. | 2.5 g/L ↑ | 80% ↑ | Create a large gas–liquid exchange area | [23] |
| Open pond | Submerged cover-type | Spirulina platensis | 13.3 g/(m2·d) ↑ | 92% ↑ | Prolong gas–liquid contact time | [24] |
| Photobioreactor | Multiple chambers | Nannochloropsis salina | 0.12 g/(L·d) ↑ | 80% ↑ | Enhance bubble residence time | [25] |
| Flat-plate PBRs | Inclined baffles | Chlorella pyrenoidosa. | 1.3 g/ L | No data | Increase mixing intensity | [26] |
| Raceway pond | Vertical absorption tower | Chlorella pyrenoidosa. | 20 g/(m2·d) ↑ | 83% ↑ | Prolong gas–liquid contact time | [28] |
| Open pond | Absorption tank | Spirulina platensis | 6–12 g/(m2·d) | >50% | Increase mixing intensity | [4,29] |
↑ indicates that the indicators of the experimental group have improved compared with the control group.
2.3. Ex Situ CO2 Supplementation
In addition to in situ carbon-supplementation devices in photobioreactor systems, some ex situ carbon-supplementation devices have also been developed. Putt et al. set up a vertical absorption tower outside the raceway pond, with a dynamic pump driving the culture medium to circulate between the vertical absorption tower and the raceway pond, achieving a CO2 absorption rate of 83% [28]. Trench-type carbon supplementation involves excavating a deep trench adjacent to the cultivation pond, allowing the culture medium to flow through it, with aeration pipes installed at the trench bottom to supply CO2 [30]. In practical applications, these carbon-supplementation trenches are often designed in a funnel or conical shape to enhance flow dynamics. However, this method disrupts the conventional spatial configuration of open ponds, and over time, CO2 can accumulate at the trench bottom, creating a mass-transfer dead zone and reducing the system’s overall effectiveness. Increasing the aeration rate can mitigate the formation of dead zones, but it inevitably shortens bubble residence time, leading to greater CO2 escape into the atmosphere. During large-scale Spirulina cultivation, the culture liquid, after being enriched with CO2 in a carbon-supplementation tank, is returned to the cultivation pond for photosynthetic production [4,29]. The development and application of these carbon-supplementation technologies have increased the annual production of Spirulina by 20%, reduced annual sodium bicarbonate usage by 66%, and lowered carbon-source costs by 58% [31] (Figure 3).
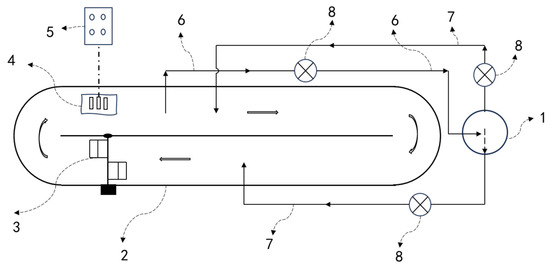
Figure 3.
Microalgae cultivation system equipped with ex situ CO2 supplementation devices. (1) vertical column CO2 absorption tower, or CO2 absorption tank, (2) open raceway pond, (3) paddle wheel, (4) pH/O2 electrode, (5) control system, (6) medium outlet pipe, (7) medium inlet pipe, (8) circulation pump.
In summary, CO2 supplementation strategies that extend gas–liquid contact time or enhance gas–liquid mixing intensity often come at the cost of increased energy consumption. For instance, the incorporation of CO2 supplementation trenches in raceway ponds prolongs bubble residence time; however, maintaining the same liquid flow rate results in an 80% increase in the energy consumption of the paddlewheel system [21]. In contrast, membrane-based technologies enhance CO2 mass-transfer efficiency without significantly increasing energy demand. This approach diffuses CO2 into the liquid medium through a nonporous hollow-fiber membrane, eliminating macroscopic bubbles. It achieves three times the CO2 mass-transfer efficiency of conventional sparging while avoiding the shear forces associated with micro- and nano-bubbles that can damage microalgal cells.
3. Strategies for Enhancing CO2 Mass Transfer Using Chemical Solvents
According to the two-membrane theory and the CO2 mass-transfer model in the culture medium (Equation (1)), the primary resistance to CO2 transfer occurs at the liquid membrane interface. In addition to increasing the overall volumetric mass-transfer coefficient by enhancing gas–liquid mixing intensity, increasing the gas–liquid contact specific area, and extending the gas–liquid contact time, the efficiency of CO2 mass transfer in the culture medium can also be improved by introducing chemical reactions and altering the physical properties of the culture medium [32,33].
3.1. Novel Enhancing Mechanism of Introducing Chemical Reaction
During CO2 transfer in the culture medium, it reacts with OH− and CO32− present in the medium. However, it has been indicated that these chemical reactions have minimal impact on CO2 transfer and absorption, primarily because the optimal pH for most microalgae is close to neutral, resulting in low concentrations of OH− and CO32− in the medium under such conditions. If a high-concentration alkaline solution is thoroughly contacted with CO2-containing gas in an ex situ absorption tower (Figure 3), the resulting carbon-rich solution can be used as a carbon source for microalgae cultivation, ensuring high CO2 absorption rates and meeting the substantial carbon-source demand of microalgae. Zhu et al. used NaOH and Na2CO3 solutions as absorbents, and after convective mass transfer with CO2-containing gas, the primary inorganic carbon species in the resulting CO2-enriched solution was HCO3−. This solution was used as a carbon source to achieve high-density cultivation of Spirulina, alkali-resistant Oscillatoria, and Isochrysis galbana, resulting in high CO2 utilization rates in closed floating photobioreactors [34,35,36]. However, as previously mentioned, the continuous proliferation of microalgal cells leads to an increase in the pH of the culture medium, promoting the conversion of HCO3− into CO32−. Since CO32− is not a bioavailable form of inorganic carbon for microalgae, this results in carbon-source wastage. Moreover, the use of NaOH or Na2CO3 solutions as absorbents elevates the Na+ ion concentration in the culture medium, increasing extracellular osmotic pressure and adversely affecting microalgal growth. For instance, Chlorella, a typical freshwater microalga, can tolerate salinity levels of only 5–10‰. In addition to inhibiting growth, elevated salinity complicates the recycling and reuse of the culture medium, further reducing the efficiency of the cultivation process [37,38].
Our research team has explored the strategy of using “ammonium hydroxide” as a nitrogen source to enhance microalgae growth and CO2 absorption. The primary product of CO2 absorption by ammonia water is ammonium bicarbonate, which can be directly used by microalgae cells as both a carbon and nitrogen source without introducing additional metal ions. This strategy can significantly reduce nutrient costs in microalgae cultivation [39]. By combining pH-feedback CO2 supplementation strategies with the metabolic kinetics of microalgae cells regarding nitrogen sources, stable control of low-concentration ammonium salts in the culture medium can be achieved, avoiding the inhibition of algal-cell growth and nitrogen-source loss due to ammonia volatilization [40]. Furthermore, using a closed gas-lift reactor for cultivating Chlorella sp., CO2 utilization rates reached 87.8%. In open raceway ponds, using a simple bottom-bubbling method for CO2 supplementation resulted in a CO2 utilization rate of 35.58%, which is an increase of 14.46% compared to the control group. However, 1 mol of ammonia water absorbs approximately 0.3–0.8 mol of CO2. Based on the elemental composition of microalgae cells (CH1.911O0.496N0.196P0.007S0.005), the required C/N ratio for the culture medium is approximately 5 [41,42]. Thus, when using the “ammonium hydroxide” strategy for microalgae cultivation, a substantial amount of CO2 must still be supplemented to meet the fast growth requirements for carbon sources.
Amines are commonly used CO2 absorbents in carbon capture, storage, and utilization (CCUS) applications [43]. These include monoethanolamine (MEA), diethanolamine (DEA), triethanolamine (TEA), and N-methyldiethanolamine (MDEA), which react reversibly with CO2 as follows:
The impact of adding amine-based CO2 absorbents to microalgae cultivation systems on carbon-source utilization and microalgae growth have been investigated by our team and the other researchers (Table 2) [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. The introduction of chemical reactions increases the CO2 mass-transfer rate (with a chemical absorption enhancement factor β > 1.0) [32]. Additionally, the resulting carbamate (RNHCOO−) acts as a “CO2 carrier”, becoming the fourth form of carbon species in the culture medium. Under near-neutral pH conditions, as microalgae cells consume CO2 and HCO3−, HCO3− is gradually released from the carbamate, acting as a slow-release carbon reservoir. It is indicated that the addition of amine-based CO2 absorbents can enhance the biomass productivity of Spirulina, and Chlorella. In column reactors, CO2 utilization efficiency increased from 44.5% to 76.1% [46,47,48,49,50]. It is worth noting that most amine molecules are not easily metabolized by microalgae cells, allowing them to act as a repeated CO2 capture agent in the culture medium [48]. However, Rosa et al. found that high concentrations of chemical absorbents can inhibit microalgae growth. For instance, when MEA concentrations exceed 150 mg/L, microalgae biomass productivity decreases, and cell growth and intracellular metabolic activity are suppressed [51,52]. This phenomenon may be attributed to the corrosive nature of high-concentration amine solutions. Therefore, selecting an appropriate “CO2 carrier” requires careful consideration of its biocompatibility.

Table 2.
Effect of adding chemical absorbents or immobilized enzymes on microalgae growth and CO2 utilization efficiency.
3.2. Novel Enhancing Mechanism of Altering the Medium’s Physical Properties
In industrial applications, methods for CO2 capture from flue gases also include low-temperature methanol methods (Restisol process), ethylene glycol ether methods, propylene carbonate methods (Flour process), and N-methyl-2-pyrrolidone methods [53,54]. These compounds do not chemically react with CO2 but enhance CO2 solubility in the liquid phase by altering the physicochemical properties of the absorbent, such as reducing surface tension. Since CO2 solubility in solvents follows Henry’s Law, these absorbents generally have lower Henry’s coefficients compared to aqueous solvents. Therefore, adding such absorbents to microalgae cultivation systems effectively increases the equilibrium concentration of CO2 in the liquid phase, thereby promoting CO2 absorption according to the mass-transfer model (Equation (1)) [50,55,56].
Using gas-lift photobioreactors to cultivate Chlorella. sp., our research indicated that the addition of four types of absorption enhancers—methanol, NHD, PC, and NMP—can significantly increase CO2 utilization rates during cultivation, with an optimal condition improving CO2 utilization by 71%, without significantly affecting the biochemical composition of the microalgae [55]. A method is utilized where a water-immiscible solvent is directly added to a microalgal culture, simultaneously allowing for increased CO2 absorption by the algae while also extracting lipids from the cells in a single process, eliminating the need for separate cultivation and extraction steps; often, n-heptane is used as the water-immiscible solvent due to its ability to act as a “CO2 sink” and readily extract lipids without significantly harming the algal cells [50].
4. Carbonic Anhydrase-Assisted CO2 Absorption and Conversion
CO2 absorption and conversion in microalgae culture can be broadly divided into two stages: gas–liquid mass transfer and biological conversion (Figure 4). After the gas–liquid mass transfer, the dissolved inorganic carbon sources include CO2 and HCO3−. The latter is further absorbed by algal cells through two main pathways: active transport via membrane carrier proteins or conversion into CO2 molecules under the action of extracellular carbonic anhydrase, which then rapidly diffuses into the cell [57,58,59]. Thus, highly active extracellular carbonic anhydrase facilitates CO2 biological conversion, reducing the concentration of inorganic carbon sources in the liquid phase and promoting CO2 gas–liquid mass transfer (Table 2). However, most microalgae exhibit low carbonic anhydrase activity under near-neutral conditions [60,61]. The addition of exogenous carbonic anhydrase may enhance microalgae’s ability to absorb and convert CO2, thereby influencing cell growth rates. However, the stability and durability of carbonic anhydrase in photobioreactors are currently poor [62]. In bubbling gas–liquid environments, shear forces significantly degrade enzyme activity, increasing the cost of microalgae cultivation beyond nutrient input and limiting the application of this strategy in microalgae cultivation technologies. The setup of efficient methods for enzyme immobilization makes carbonic anhydrase utilization in continuous bioreactors increasingly attractive and opens up new opportunities for the industrial use of carbonic anhydrase [12,63].
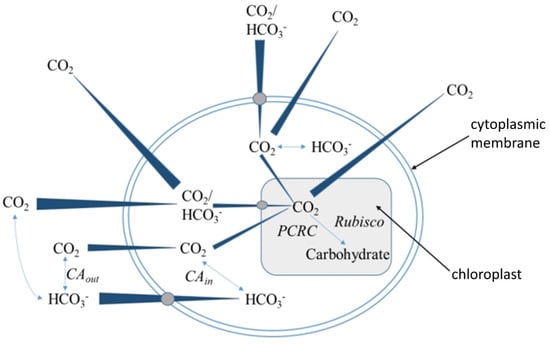
Figure 4.
Diagrammatic representation of CO2 absorption and conversion pathways in microalgae cells.
Xu et al. proposed a technique involving the addition of immobilized carbonic anhydrase microbeads to microalgae culture, which effectively addressed the issue of carbon limitation when cultivating microalgae with air CO2 as the sole carbon source [13]. Jun et al. fixed carbonic anhydrase onto electrospun polymer nanofibers using enzyme deposition coating. This approach increased the microalgae growth rate by 134% in a bubbling reactor [64]. Yang et al. developed carbonic anhydrase-coated nylon fiber membranes and proposed a novel photobioreactor that improves CO2 solubility and absorption rates in the microalgae solution. Testing revealed that CO2 conversion rates increased by 62.7% with the use of this immobilized exogenous carbonic anhydrase [65]. Our team has isolated mineralizing bacteria from soil environments that symbiotically coexist with Chlorella sp. These symbiotic bacteria can extensively express extracellular carbonic anhydrase. By optimizing algal–bacterial inoculation ratios and other parameters, we have developed a microalgae cultivation system that enhances CO2 absorption through immobilized mineralizing bacteria [66]. Wang et al. utilized bamboo cellulose as a renewable porous scaffold to immobilize carbonic anhydrase through oxidation-induced aldehyde formation, followed by Schiff base linkage. The cellulose-immobilized enzyme significantly enhanced microalgal growth and biomass accumulation [67].
5. Challenges and Future Prospects
In the large-scale cultivation of alkaliphilic microalgae such as Spirulina (with an optimal pH range of 9.0–11.0), the combined use of CO2 supplementation devices and pH-feedback CO2 supplementation strategies can achieve carbon-source utilization rates exceeding 80%, effectively reducing cultivation costs. This is primarily due to higher CO2 absorption rates under alkaline conditions and the higher total inorganic carbon concentration in the culture medium (maintained by adding large amounts of NaHCO3), which supports rapid microalgal growth and carbon-source demands. In near-neutral (pH 6.0–8.0) cultivation systems (e.g., Chlorella, Scenedesmus, Microcystis, Euglena, and other economically significant algae), it is necessary to control the total inorganic carbon concentration in the carbon-rich culture medium from the supplementation device to prevent the loss of free CO2 during circulation. This results in a lower total inorganic carbon concentration that only supports short-distance flow, depleting the carbon source and affecting algal biomass productivity.
For example, in a typical microalgae cultivation process with pH = 7, a liquid layer depth of 20 cm, an area productivity of 15 g/(m2·d), and 30 °C, if the CO2 in the culture medium is in equilibrium with the air upon leaving the supplementation point (meaning no CO2 loss to the air), calculations show that the total inorganic carbon concentration drops to zero after 2 min of flow (although, in practice, microalgae growth is limited before reaching zero). At a flow velocity of 20 cm/s, this equates to a distance of 24 m. Thus, in fixed-scale raceway ponds, such as those with a perimeter of over 200 m in large-scale production, multiple carbon-supplementation points are needed to reduce CO2 loss, ensure high CO2 utilization rates, and maintain high algal-cell productivity. For a raceway pond with a single carbon-supplementation point and a perimeter of 200 m, the low concentration of available carbon sources quickly leads to carbon-source limitation after leaving the supplementation point, impacting algal biomass productivity. Increasing the carbon-source concentration at the supplementation point forces CO2 to escape, exacerbating losses.
Thus, CO2 supplementation in microalgal photobioreactor systems must achieve both high CO2 absorption rates at supplementation points and continuously increase the total inorganic carbon concentration in the culture medium. Future research should focus on multi-scale studies and the precise application of various strategies to optimize the CO2 absorption process for microalgae.
Firstly, the kinetics of microalgae CO2 absorption and conversion should be comprehensively studied. Utilize visual experimental methods to explore the kinetics of CO2 dissolution and mass transfer, identify rate-limiting steps, and establish a balance between CO2 supplementation, dissolution, transfer, and conversion processes. Precise control strategies and models should be developed. Secondly, create new and efficient microalgae cultivation systems by integrating in situ or ex situ setups with photobioreactor systems. Investigate low-energy methods for bubble nanonization, combine gas–liquid mixing with membrane technologies, and improve CO2 mass-transfer efficiency under near-neutral pH conditions. Finally, select compounds or enzyme preparations with good biocompatibility that increase CO2 solubility through chemical reactions or alterations in the physicochemical properties of the culture medium. This will enhance the CO2 sink in microalgae cultivation systems, meet the rapid carbon-source demands of microalgae growth, reduce the frequency of carbon supplementation, and save energy.
6. Conclusions
This review highlights the various CO2 supplementation strategies aimed at enhancing the efficiency of microalgae cultivation for biotechnological applications. Traditional aeration methods often result in low CO2 absorption rates, which hinder microalgal growth and biomass productivity. To address these limitations, several innovative approaches have been developed. Direct CO2 injection and advanced open-pond designs, such as trap-type carbon-replenishing devices and membrane-based systems, significantly enhance CO2 mass-transfer efficiency. Modifications to closed photobioreactors further improve CO2 utilization. Additionally, CO2 absorption enhancer or immobilized carbonic anhydrase plays a crucial role in optimizing CO2 absorption and promoting algal growth. Among these strategies, membrane-based CO2 delivery systems and the incorporation of CO2 absorption enhancers have shown the highest efficiency in boosting CO2 mass transfer and microalgae productivity. Future efforts should focus on integrating these methods into large-scale photobioreactor systems to optimize cost-effective, sustainable production.
Author Contributions
Conceived the study, C.B., Z.S. and L.S.; analyzed the data, S.C. and Z.S.; wrote the paper, C.B. and Z.S.; revised the manuscript, L.S. and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Shandong Province, grant number ZR2021MC097; National Natural Science Foundation of China, grant number 32102819.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Ağbulut, Ü.; Sirohi, R.; Lichtfouse, E.; Chen, W.-H.; Len, C.; Show, P.L.; Le, A.T.; Nguyen, X.P.; Hoang, A.T. Microalgae bio-oil production by pyrolysis and hydrothermal liquefaction: Mechanism and characteristics. Bioresour. Technol. 2023, 376, 128860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Hong, Y.; He, Y.T.; Liu, Y. Growth and high-valued products accumulation characteristics of microalgae in saline-alkali leachate from Inner Mongolia. Environ. Sci. Pollut. Res. 2019, 26, 36985–36992. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Agbebi, T.V.; Ojo, E.O.; Watson, I.A. Towards optimal inorganic carbon delivery to microalgae culture. Algal Res. 2022, 67, 102841. [Google Scholar] [CrossRef]
- Su, Z.F.; Kang, R.J.; Shi, S.Y.; Cong, W.; Cai, Z.L. An effective device for gas-liquid oxygen removal in enclosed microalgae culture. Appl. Biochem. Biotechnol. 2010, 160, 428–437. [Google Scholar] [CrossRef]
- Su, Z.F.; Kang, R.J.; Shi, S.Y.; Cong, W.; Cai, Z.L. Study on the destabilization mixing in the flat plate photobioreactor by means of CFD. Biomass Bioenergy 2010, 34, 1879–1884. [Google Scholar] [CrossRef]
- Vasumathi, K.K.; Premalatha, M.; Subramanian, P. Parameters influencing the design of photobioreactor for the growth of microalgae. Renew. Sustain. Energy Rev. 2012, 16, 5443–5450. [Google Scholar] [CrossRef]
- Demirbas, A. Production economics of high-quality microalgae. Energy Sources Part B Econ. Plan. Policy 2017, 12, 395–401. [Google Scholar] [CrossRef]
- Soares, F.R.; Martins, G.; Seo, E.S.M. An assessment of the economic aspects of CO2 sequestration in a route for biodiesel production from microalgae. Environ. Technol. 2013, 34, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. Immobilization of carbonic anhydrase for CO2 capture and utilization. Appl. Microbiol. Biotechnol. 2022, 106, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kentish, S.E.; Martin, G.J.O. Direct air capture of CO2 by microalgae with buoyant beads encapsulating carbonic anhydrase. ACS Sustain. Chem. Eng. 2021, 9, 9698–9706. [Google Scholar] [CrossRef]
- Valdés, F.J.; Hernández, M.R.; Catalá, L.; Marcilla, A. Estimation of CO2 stripping/CO2 microalgae consumption ratios in a bubble column photobioreactor using the analysis of the pH profiles. Application to Nannochloropsis oculata microalgae culture. Bioresour. Technol. 2012, 119, 1–6. [Google Scholar] [CrossRef]
- Ughetti, A.; Roncaglia, F.; Anderlini, B.; D’Eusanio, V.; Russo, A.L.; Forti, L. Integrated Carbonate-Based CO2 Capture-Biofixation through Cyanobacteria. Appl. Sci. 2023, 13, 10779. [Google Scholar] [CrossRef]
- Fu, J.W.; Huang, Y.; Liao, Q.; Xia, A.; Fu, Q.; Zhu, X. Photo-bioreactor design for microalgae: A review from the aspect of CO2 transfer and conversion. Bioresour. Technol. 2019, 292, 121947. [Google Scholar] [CrossRef]
- Ndiaye, M.; Gadoin, E.; Gentric, C. CO2 gas-liquid mass transfer and K_L,a estimation: Numerical investigation in the context of airlift photobioreactor scale-up. Chem. Eng. Res. Des. 2018, 133, 90–102. [Google Scholar] [CrossRef]
- Thobie, C.; Gadoin, E.; Blel, W.; Pruvost, J.; Gentric, C. Global characterization of hydrodynamics and gas-liquid mass transfer in a thin-gap bubble column intended for microalgae cultivation. Chem. Eng. Process. 2017, 122, 76–89. [Google Scholar] [CrossRef]
- Kumar, A.; Yuan, X.; Sahu, A.K.; Dewulf, J.; Ergas, S.J.; Van Langenhove, H. A hollow fiber membrane photo-bioreactor for CO2 sequestration from combustion gas coupled with wastewater treatment: A process engineering approach. J. Chem. Technol. Biotechnol. 2010, 85, 387–394. [Google Scholar] [CrossRef]
- Ferreira, B.S.; Fernandes, H.L.; Reis, A.; Mateus, M. Microporous hollow fibres for carbon dioxide absorption: Mass transfer model fitting and the supplying of carbon dioxide to microalgal cultures. J. Chem. Technol. Biotechnol. 1998, 71, 61–70. [Google Scholar] [CrossRef]
- Ketheesan, B.; Nirmalakhandan, N. Development of a new airlift-driven raceway reactor for algal cultivation. Appl. Energy 2011, 88, 3370–3376. [Google Scholar] [CrossRef]
- Bao, Y.L.; Liu, M.; Wu, X.; Cong, W.; Ning, Z.X. In situ carbon supplementation in large-scale cultivations of Spirulina platensis in open raceway pond. Biotechnol. Bioprocess Eng. 2012, 17, 93–99. [Google Scholar] [CrossRef]
- Chen, Z.S.; Li, T.; Yang, B.J.; Jin, X.J.; Wu, H.L.; Wu, J.Y.; Lu, Y.D.; Xiang, W.Z. Isolation of a novel strain of Cyanobacterium sp. with good adaptation to extreme alkalinity and high polysaccharide yield. J. Oceanol. Limnol. 2021, 39, 1131–1142. [Google Scholar] [CrossRef]
- Cong, W. Horizontal Submerged Hood Carbon Supplementation Device for Open Pond Microalgae Cultivation and Its Carbon Supplementation Method. CN201210138845.8, 30 April 2014. [Google Scholar]
- Bergmann, P.; Ripplinger, P.; Beyer, L.; Trösch, W. Disposable Flat Panel Airlift Photobioreactors. Chem. Ing. Tech. 2013, 85, 202–205. [Google Scholar]
- Huang, J.; Li, Y.; Wan, M.; Yan, Y.; Feng, F.; Qu, X.; Wang, J.; Shen, G.; Li, W.; Fan, J.; et al. Novel flat-plate photobioreactors for microalgae cultivation with special mixers to promote mixing along the light gradient. Bioresour. Technol. 2014, 159, 8–16. [Google Scholar] [CrossRef]
- Razzak, S.A.; Al-Aslani, I.; Hossain, M.M. Hydrodynamics and mass transfer of CO2 in water in a tubular photobioreactor. Eng. Life Sci. 2016, 16, 355–363. [Google Scholar] [CrossRef]
- Putt, R.; Singh, M.; Chinnasamy, S.; Das, K.C. An efficient system for carbonation of high-rate algae pond water to enhance CO2 mass transfer. Bioresour. Technol. 2011, 102, 3240–3245. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Benemann, J.R.; Zhang, X.; Hu, H.; Qin, S. Microalgal industry in China: Challenges and prospects. J. Appl. Phycol. 2016, 28, 715–725. [Google Scholar] [CrossRef]
- Yan, C.H.; Wang, Z.; Wu, X.; Wen, S.M.; Yu, J.; Cong, W. Outdoor cultivation of Chlorella sp. in an improved thin-film flat-plate photobioreactor in desertification areas. J. Biosci. Bioeng. 2020, 129, 619–623. [Google Scholar] [CrossRef]
- Gao, F.Z.; Ge, B.S.; Xiang, W.Z.; Qin, S. Development of microalgal industries in the past 60 years due to biotechnological research in China: A review. Sci. Sin. Vitae 2021, 51, 26–39. [Google Scholar]
- Basheer, C.; Kamran, M.; Ashraf, M.; Lee, H.K. Enhancing liquid-phase microextraction efficiency through chemical reactions. TrAC Trends Anal. Chem. 2019, 118, 426–433. [Google Scholar] [CrossRef]
- Morales, D.O.; Regalado-Méndez, A.; Pérez-Alonso, C.; Natividad, R. Physical and reactive absorption of CO2 in capillaries: Mass transfer, modelling, and produced chemical species. Chem. Eng. Res. Des. 2023, 198, 247–258. [Google Scholar] [CrossRef]
- Zhu, C.B.; Chen, S.L.; Ji, Y.; Schwaneberg, U.; Chi, Z.Y. Progress toward a bicarbonate-based microalgae production system. Trends Biotechnol. 2022, 40, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Zhang, R.L.; Cheng, L.Y.; Chi, Z.Y. A recycling culture of Neochloris oleoabundans in a bicarbonate-based integrated carbon capture and algae production system with harvesting by auto-flocculation. Biotechnol. Biofuels 2018, 11, 204. [Google Scholar] [CrossRef]
- Zhu, C.B.; Zhu, H.; Cheng, L.Y.; Chi, Z.Y. Bicarbonate-based carbon capture and algal production system on ocean with floating inflatable-membrane photobioreactor. J. Appl. Phycol. 2018, 30, 875–885. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.Z.; Hu, Q.A.; Sommerfeld, M.; Chen, Y.S. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef]
- Guieysse, B.; Béchet, Q.; Shilton, A. Variability and uncertainty in water demand and water footprint assessments of fresh algae cultivation based on case studies from five climatic regions. Bioresour. Technol. 2013, 128, 317–323. [Google Scholar] [CrossRef]
- Sun, Z.L.; Sun, L.Q.; Liu, Y.H. The potential impact of replacing nitrate with ammonium hydroxide in microalgae production on the biomass productivity and CO2 utilization efficiency. Algal Res. 2022, 67, 102870. [Google Scholar] [CrossRef]
- Bao, Y.L.; Wen, S.M.; Cong, W.; Wu, X.; Ning, Z.X. An Optical-Density-Based Feedback Feeding Method for Ammonium Concentration Control in Spirulina platensis Cultivation. J. Microbiol. Biotechnol. 2012, 22, 967–974. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wyman, K.; Falkowski, P.G.; Morel, F.M.M. The elemental composition of some marine phytoplankton. J. Phycol. 2003, 39, 1145–1159. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Sun, Z.L.; Xue, S.Z.; Yan, C.H.; Cong, W.; Kong, D.Z. Utilisation of tris(hydroxymethyl)aminomethane as a gas carrier in microalgal cultivation to enhance CO2 utilisation and biomass production. RSC Adv. 2016, 6, 2703–2711. [Google Scholar] [CrossRef]
- Sun, Z.L.; Zhang, D.M.; Yan, C.H.; Cong, W.; Lu, Y.M. Promotion of microalgal biomass production and efficient use of CO2 from flue gas by monoethanolamine. J. Chem. Technol. Biotechnol. 2015, 90, 730–738. [Google Scholar] [CrossRef]
- Cardias, B.B.; Morais, M.G.d.; Costa, J.A.V. CO2 conversion by the integration of biological and chemical methods: Spirulina sp. LEB 18 cultivation with diethanolamine and potassium carbonate addition. Bioresour. Technol. 2018, 267, 77–83. [Google Scholar] [CrossRef]
- Yin, Q.; Mao, W.; Chen, D.; Song, C. Effect of adding tertiary amine TMEDA and space hindered amine DACH on the CO2 chemical absorption-microalgae conversion system. Energy 2023, 263, 125726. [Google Scholar] [CrossRef]
- Kim, G.; Choi, W.; Lee, C.-H.; Lee, K. Enhancement of dissolved inorganic carbon and carbon fixation by green alga Scenedesmus sp. in the presence of alkanolamine CO2 absorbents. Biochem. Eng. J. 2013, 78, 18–23. [Google Scholar] [CrossRef]
- Rosa, G.M.d.; Morais, M.G.d.; Costa, J.A.V. Fed-batch cultivation with CO2 and monoethanolamine: Influence on Chlorella fusca LEB 111 cultivation, carbon biofixation, and biomolecules production. Bioresour. Technol. 2019, 273, 627–633. [Google Scholar] [CrossRef]
- Bai, L.; Lu, S.J.; Qiu, S.; Li, J.; Chen, S.M. Single-step integrated technology for enhanced CO2 biofixation and efficient lipid extraction in microalgal system including a water-immiscible solvent. Chem. Eng. J. 2022, 432, 134374. [Google Scholar] [CrossRef]
- Rosa, G.M.d.; Moraes, L.; de Souza, M.d.R.A.Z.; Costa, J.A.V. Spirulina cultivation with a CO2 absorbent: Influence on growth parameters and macromolecule production. Bioresour. Technol. 2016, 200, 528–534. [Google Scholar] [CrossRef]
- Rosa, G.M.d.; Morais, M.G.d.; Costa, J.A.V. Green alga cultivation with monoethanolamine: Evaluation of CO2 fixation and macromolecule production. Bioresour. Technol. 2018, 261, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Gao, S.; Ma, Q.; Wei, Y.; Li, G. Techno-economic analysis of carbon capture and utilization technologies and implications for China. Renew. Sustain. Energy Rev. 2024, 199, 114550. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kus, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Sun, Z.L.; Xin, M.R.; Li, P.; Sun, L.Q.; Wang, S.K. Enhancing CO2 utilization by a physical absorption-based technique in microalgae culture. Bioprocess Biosyst. Eng. 2021, 44, 1901–1912. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Chang, J.-S.; Miao, X. A Two-Stage Culture Strategy for Scenedesmus sp. FSP3 for CO2 Fixation and the Simultaneous Production of Lutein under Light and Salt Stress. Molecules 2022, 27, 7497. [Google Scholar] [CrossRef]
- Wang, Y.J.; Stessman, D.J.; Spalding, M.H. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J.; Giordano, M. Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth. Res. 2014, 121, 111–124. [Google Scholar] [CrossRef]
- Yao, D.; Wu, L.; Tan, D.; Yu, Y.; Jiang, Q.; Wu, H.; Wang, Y.; Liu, Y. Enhancing CO2 fixation by microalgae in a Photobioreactor: Molecular mechanisms with exogenous carbonic anhydrase. Bioresour. Technol. 2024, 408, 131176. [Google Scholar] [CrossRef]
- Swarnalatha, G.V.; Hegde, N.S.; Chauhan, V.S.; Sarada, R. The effect of carbon dioxide rich environment on carbonic anhydrase activity, growth and metabolite production in indigenous freshwater microalgae. Algal Res. 2015, 9, 151–159. [Google Scholar] [CrossRef]
- van Hille, R.; Fagan, M.; Bromfield, L.; Pott, R. A modified pH drift assay for inorganic carbon accumulation and external carbonic anhydrase activity in microalgae. J. Appl. Phycol. 2014, 26, 377–385. [Google Scholar] [CrossRef]
- González, J.M.; Fisher, S.Z. Carbonic Anhydrases in Industrial Applications. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Frost, S.C., McKenna, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 405–426. [Google Scholar]
- Lin, J.Y.; Sri Wahyu Effendi, S.; Ng, I.S. Enhanced carbon capture and utilization (CCU) using heterologous carbonic anhydrase in Chlamydomonas reinhardtii for lutein and lipid production. Bioresour. Technol. 2022, 351, 127009. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.H.; Yang, J.; Jeon, H.; Kim, H.S.; Pack, S.P.; Jin, E.; Kim, J. Stabilized and immobilized carbonic anhydrase on electrospun nanofibers for enzymatic CO2 conversion and utilization in expedited microalgal growth. Environ. Sci. Technol. 2020, 54, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Li, M.J.; Hung, T.C. Enhancing CO2 dissolution and inorganic carbon conversion by metal–organic frameworks improves microalgal growth and carbon fixation efficiency. Bioresour. Technol. 2024, 407, 131113. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.R. Study on the Effect of Exogenous Carbonic Anhydrase in Promoting Carbon Absorption in Microalgae Cells. Master’s Thesis, Yantai University, Yantai, China, 2022. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Liu, Z.; Shi, Z.; Yu, D.; Ge, B.; Huang, F. Carbonic anhydrase encapsulation using bamboo cellulose scaffolds for efficient CO2 capture and conversion. Int. J. Biol. Macromol. 2024, 277, 134410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).