Ark Shell-Derived Peptides AWLNH (P3) and PHDL (P4) Mitigate Foam Cell Formation by Modulating Cholesterol Metabolism and HO-1/Nrf2-Mediated Oxidative Stress in Atherosclerosis
Abstract
1. Introduction
2. Results
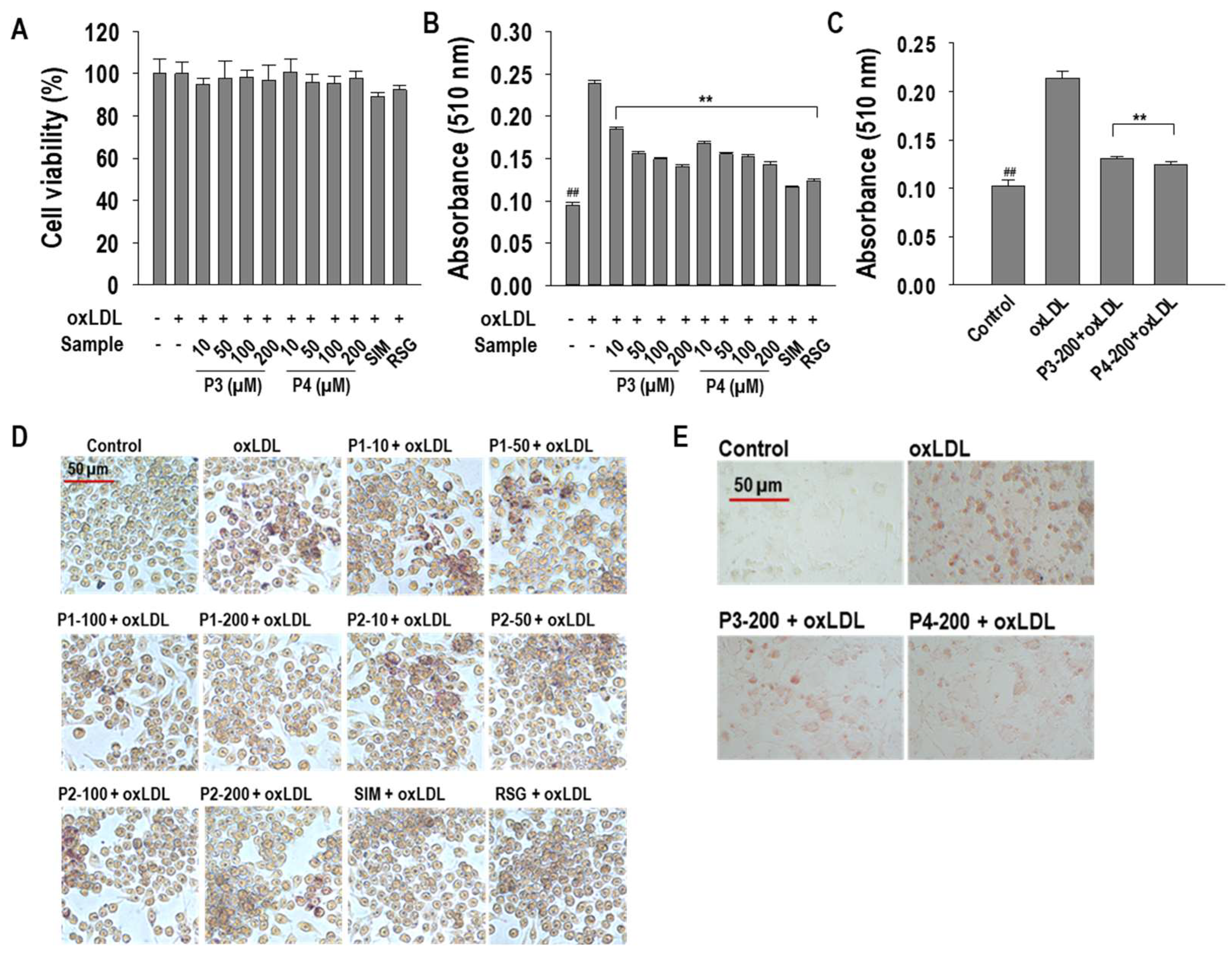
2.1. P3 and P4 Peptides Inhibit oxLDL-Induced Lipid Accumulation in RAW264.7 Macrophages and hASMCs
2.2. P3 and P4 Peptides Ameliorate Intracellular Cholesterol Levels in oxLDL-Treated RAW264.7 Macrophages
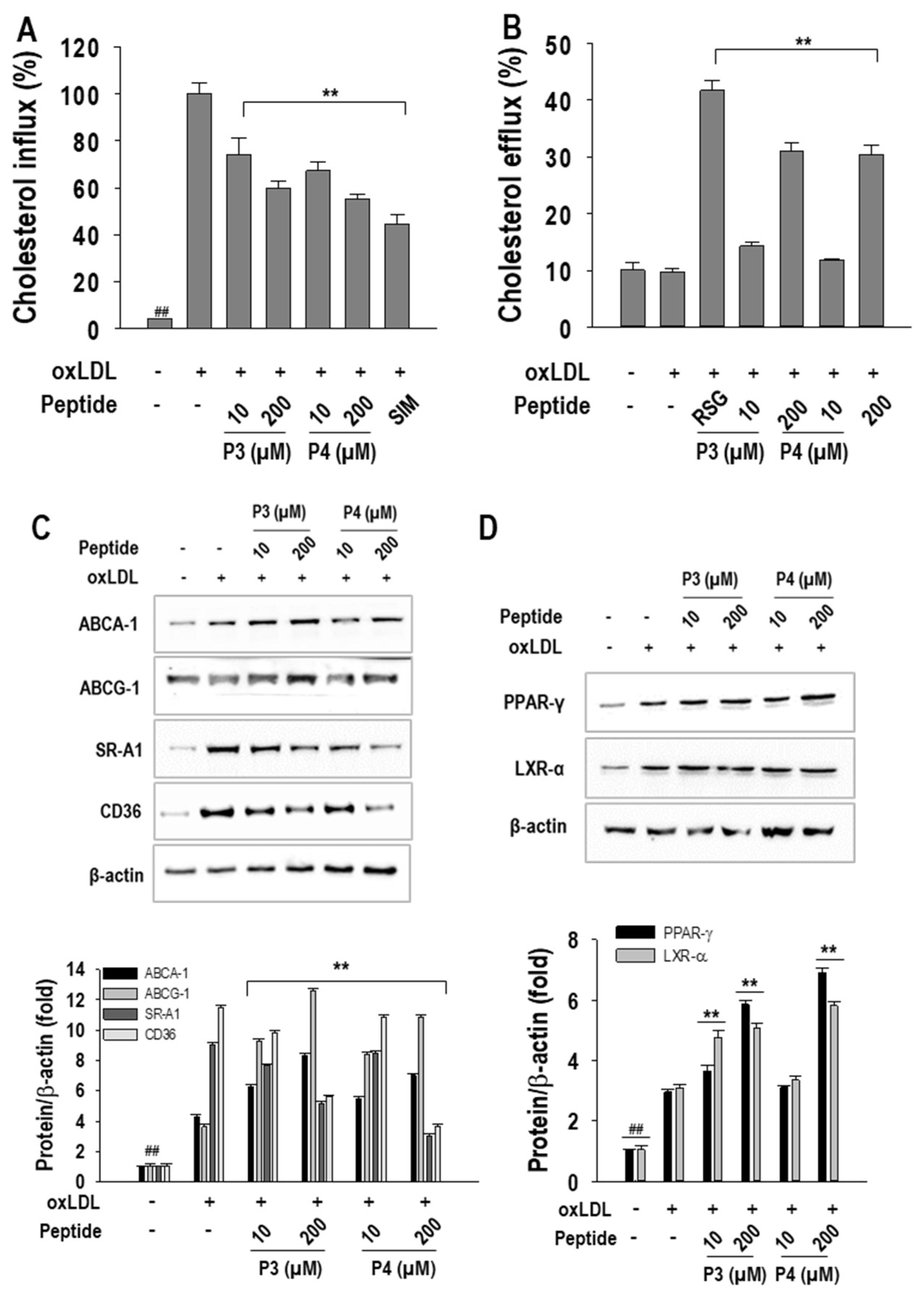
2.3. P3 and P4 Peptides’ Effect on Cholesterol Influx and Cholesterol Efflux and Related Transcription Factor Expressions in oxLDL-Treated RAW264.7 Macrophages
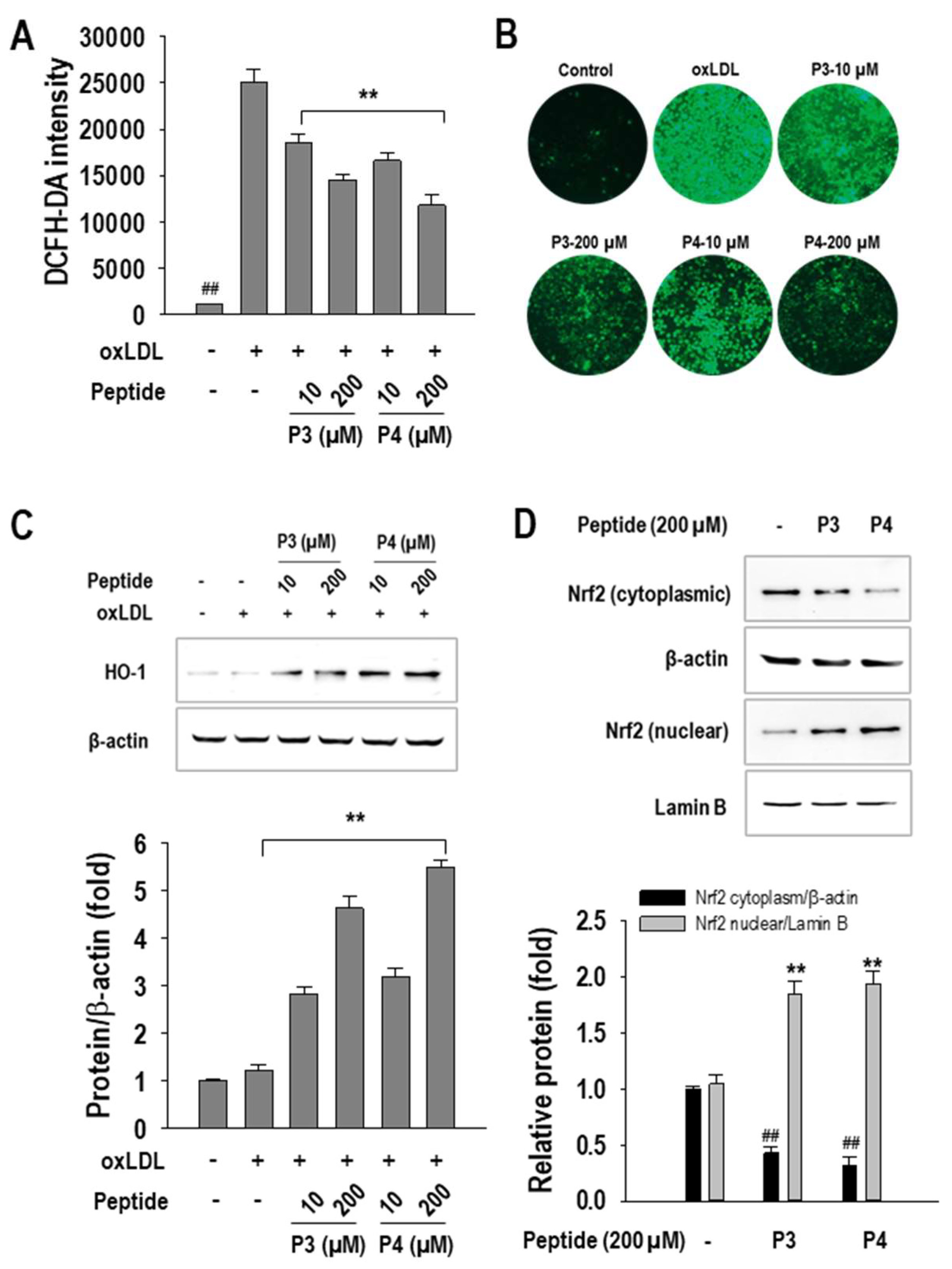
2.4. P3 and P4 Peptides Suppressed Oxidative Stress in oxLDL-Treated RAW264.7 Macrophages
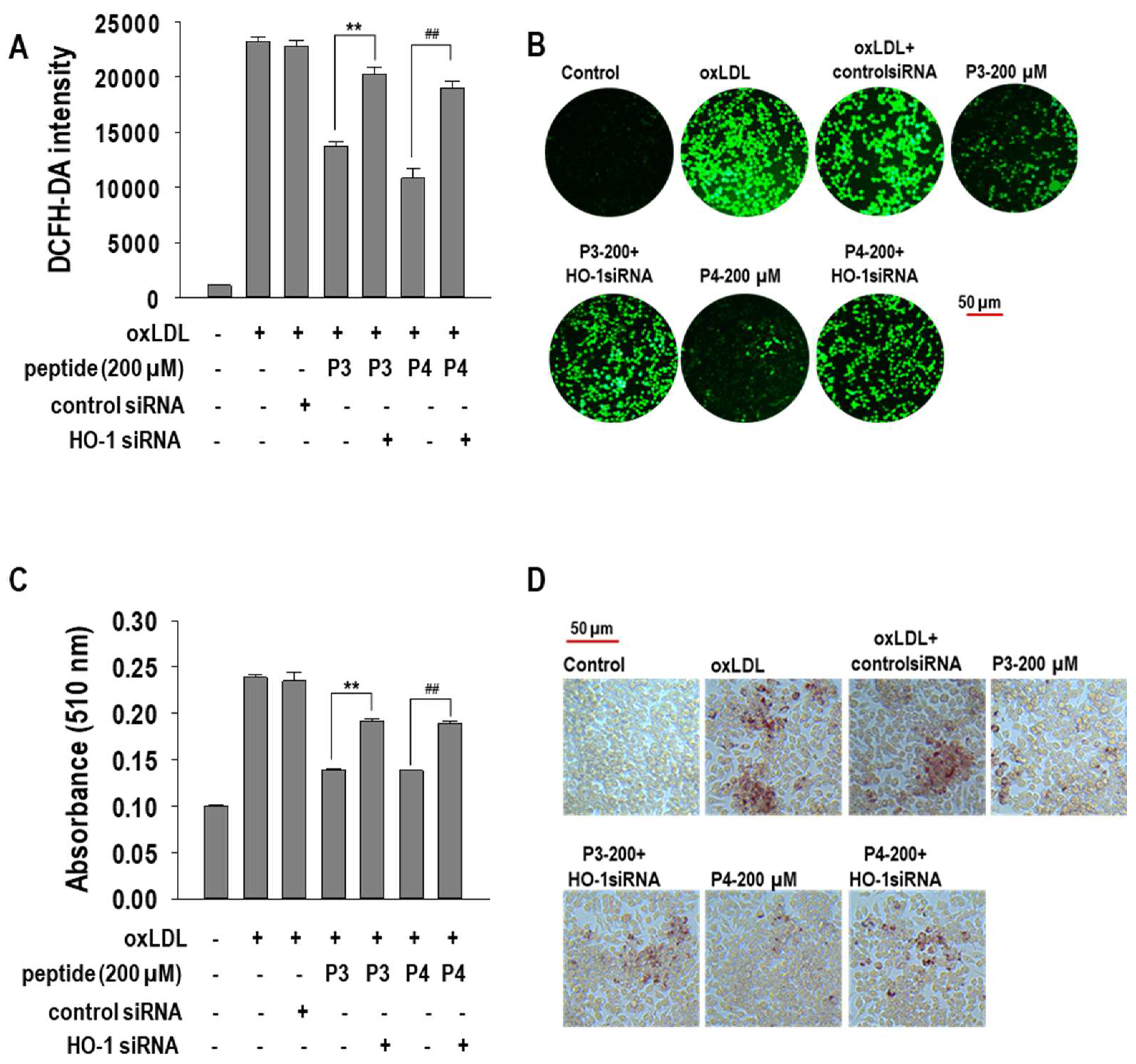
2.5. Effects of HO-1 siRNA Transfection on oxLDL-Induced Foam Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Oxidation of LDLs and Determination of Thiobarbituric Acid-Reactive Substances (TBARSs)
4.3. Cell Culture and Treatment
4.4. MTT Assay
4.5. ORO Staining Assay
4.6. Determination of TC, FC, CE, and TG Content
4.7. Determination of Cholesterol Influx and Efflux
4.8. Western Blot Analysis
4.9. siRNA Transfection
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, C.; Wu, P.; Dou, Y.; Li, Q.; Zhang, J. Bioresponsive nanoplatforms for imaging and therapy of cardiovascular diseases. View 2022, 3, 20200137. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Karuthedath, S. Cardiovascular Diseases; Unity Publishers: Austin, TX, USA, 2024. [Google Scholar]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Shashkin, P.; Dragulev, B.; Ley, K. Macrophage differentiation to foam cells. Curr. Pharm. Des. 2005, 11, 3061–3072. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Debnath, M.; Paul, A.; Bisen, P. Natural bioactive compounds and biotechnological potential of marine bacteria. Curr. Pharm. Biotechnol. 2007, 8, 253–260. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Cunha, N.B.D.; Carneiro, J.A.; Costa, R.A.D.; Alencar, S.A.D.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine organisms as a rich source of biologically active peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.-B.; Hyung, J.-H.; Je, J.-Y. Two novel peptides from ark shell protein stimulate osteoblast differentiation and rescue ovariectomy-induced bone loss. Toxicol. Appl. Pharmacol. 2019, 385, 114779. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.B.; Je, J.Y. Ark shell protein-derived bioactive peptides promote osteoblastic differentiation through upregulation of the canonical Wnt/β-catenin signaling in human bone marrow-derived mesenchymal stem cells. J. Food Biochem. 2020, 44, e13440. [Google Scholar] [CrossRef]
- Marasinghe, C.K.; Jung, W.K.; Je, J.Y. Anti-inflammatory action of ark shell (Scapharca subcrenata) protein hydrolysate in LPS-stimulated RAW264. 7 murine macrophages. J. Food Biochem. 2022, 46, e14493. [Google Scholar] [CrossRef] [PubMed]
- Marasinghe, C.K.; Yoon, S.D.; Je, J.Y. Two peptides LLRLTDL and GYALPCDCL inhibit foam cell formation through activating PPAR-γ/LXR-α signaling pathway in oxLDL-treated RAW264. 7 macrophages. BioFactors 2024, 50, 1161–1175. [Google Scholar] [CrossRef]
- Marasinghe, C.K.; Jung, W.-K.; Je, J.-Y. OxLDL-induced foam cell formation inhibitory activity of pepsin hydrolysate of ark shell (Scapharca subcrenata (Lischke, 1869)) in RAW264. 7 macrophages. J. Food Biochem. 2023, 2023, 6905673. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Je, J.-Y. Anti-adipogenic peptides from ark shell protein hydrolysate: Purification, identification and anti-adipogenic effect. Process Biochem. 2021, 109, 143–147. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Lei, Y.; Tzvetkov, N.T.; Liu, X.; Yeung, A.W.K.; Xu, S.; Atanasov, A.G.; Ma, Q. Targeting foam cell formation in atherosclerosis: Therapeutic potential of natural products. Pharmacol. Rev. 2019, 71, 596–670. [Google Scholar]
- Ouyang, Z.; Zhong, J.; Shen, J.; Zeng, Y. The cell origins of foam cell and lipid metabolism regulated by mechanical stress in atherosclerosis. Front. Physiol. 2023, 14, 1179828. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam cells in atherosclerosis: Novel insights into its origins, consequences, and molecular mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class AI/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Fu, Y.-C.; Zhang, D.-W.; Yin, K.; Tang, C.-K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-F.; Zhu, N.; Zhao, T.-J.; Li, H.-F.; Gu, J.; Liao, D.-F.; Qin, L. Involvement of LDL and ox-LDL in cancer development and its therapeutical potential. Front. Oncol. 2022, 12, 803473. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Gleaves, L.A.; Carter, K.J.; Suzuki, H.; Kodama, T.; Fazio, S.; Linton, M.F. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2593–2599. [Google Scholar] [CrossRef]
- Febbraio, M.; Podrez, E.A.; Smith, J.D.; Hajjar, D.P.; Hazen, S.L.; Hoff, H.F.; Sharma, K.; Silverstein, R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Investig. 2000, 105, 1049–1056. [Google Scholar] [CrossRef]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013, 93, 1317–1542. [Google Scholar] [CrossRef]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef]
- Ricote, M.; Valledor, A.F.; Glass, C.K. Decoding transcriptional programs regulated by PPARs and LXRs in the macrophage: Effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 230–239. [Google Scholar] [CrossRef]
- Chawla, A.; Boisvert, W.A.; Lee, C.-H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Pang, L.; Huang, G.; Huang, J.; Shi, M.; Sun, X.; Wang, Y. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-γ/LXR-α signaling pathway. Biochem. Biophys. Res. Commun. 2013, 441, 321–326. [Google Scholar] [CrossRef]
- Lazar, M.A. Progress in cardiovascular biology: PPAR for the course. Nat. Med. 2001, 7, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166 (Suppl. S1), S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The protective role of heme oxygenase-1 in atherosclerotic diseases. Int. J. Mol. Sci. 2019, 20, 3628. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.-H.; Ahn, C.-B.; Kim, B.I.; Kim, K.; Je, J.-Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur. J. Pharmacol. 2016, 793, 43–48. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Hyung, J.-H.; Ahn, C.-B.; Je, J.-Y. Ark shell protein hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through the down-regulation of transcriptional factors. RSC Adv. 2017, 7, 6223–6228. [Google Scholar] [CrossRef]
- Marasinghe, C.K.; Yoon, S.-D.; Je, J.-Y. Blue mussel (Mytilus edulis) hydrolysates attenuate oxidized-low density lipoproteins (ox-LDL)-induced foam cell formation, inflammation, and oxidative stress in RAW264. 7 macrophages. Process Biochem. 2023, 134, 131–140. [Google Scholar] [CrossRef]




| Peptide Sequences | Originally Observed Bioactivity | Foam Cell Formation Inhibitory Action |
|---|---|---|
| AWLNH (P3) PHDL (P4) | Osteogenesis and anti-osteoporotic activity [13] | HO-1/Nrf2 signaling |
| LLRLTDL (Bu1) GYALPCDCL (Bu2) | Anti-adipogenesis [18] | PPAR-γ/LXR-α signaling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasinghe, C.K.; Je, J.-Y. Ark Shell-Derived Peptides AWLNH (P3) and PHDL (P4) Mitigate Foam Cell Formation by Modulating Cholesterol Metabolism and HO-1/Nrf2-Mediated Oxidative Stress in Atherosclerosis. Mar. Drugs 2025, 23, 111. https://doi.org/10.3390/md23030111
Marasinghe CK, Je J-Y. Ark Shell-Derived Peptides AWLNH (P3) and PHDL (P4) Mitigate Foam Cell Formation by Modulating Cholesterol Metabolism and HO-1/Nrf2-Mediated Oxidative Stress in Atherosclerosis. Marine Drugs. 2025; 23(3):111. https://doi.org/10.3390/md23030111
Chicago/Turabian StyleMarasinghe, Chathuri Kaushalya, and Jae-Young Je. 2025. "Ark Shell-Derived Peptides AWLNH (P3) and PHDL (P4) Mitigate Foam Cell Formation by Modulating Cholesterol Metabolism and HO-1/Nrf2-Mediated Oxidative Stress in Atherosclerosis" Marine Drugs 23, no. 3: 111. https://doi.org/10.3390/md23030111
APA StyleMarasinghe, C. K., & Je, J.-Y. (2025). Ark Shell-Derived Peptides AWLNH (P3) and PHDL (P4) Mitigate Foam Cell Formation by Modulating Cholesterol Metabolism and HO-1/Nrf2-Mediated Oxidative Stress in Atherosclerosis. Marine Drugs, 23(3), 111. https://doi.org/10.3390/md23030111






