Discovery of MK8383s with Antifungal Activity from Mangrove Endophytic Fungi Medicopsis sp. SCSIO 40440 Against Fusarium Wilt of Banana
Abstract
1. Introduction
2. Results
2.1. Antifungal Activity and Isolation
2.2. Structure Elucidation
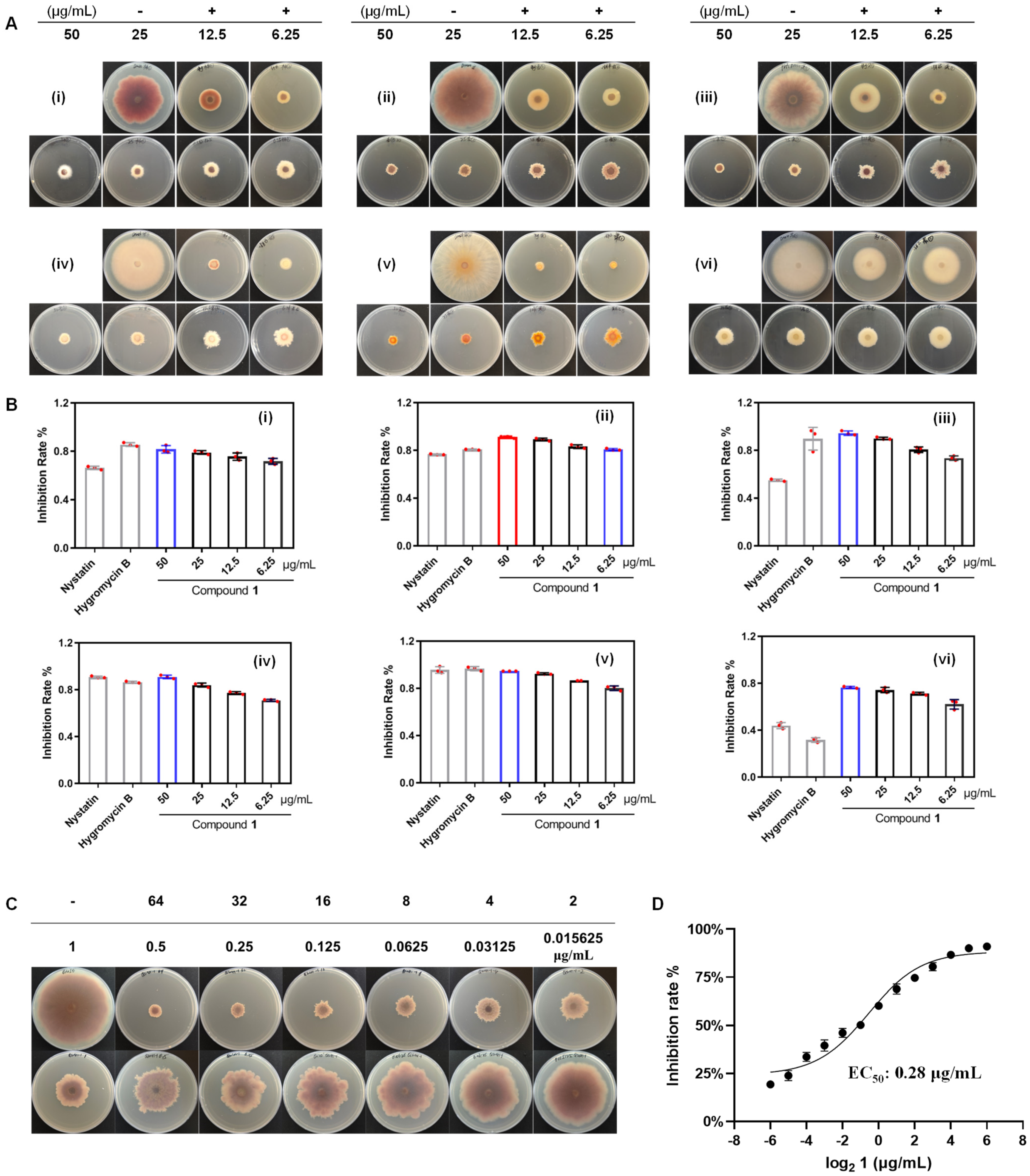
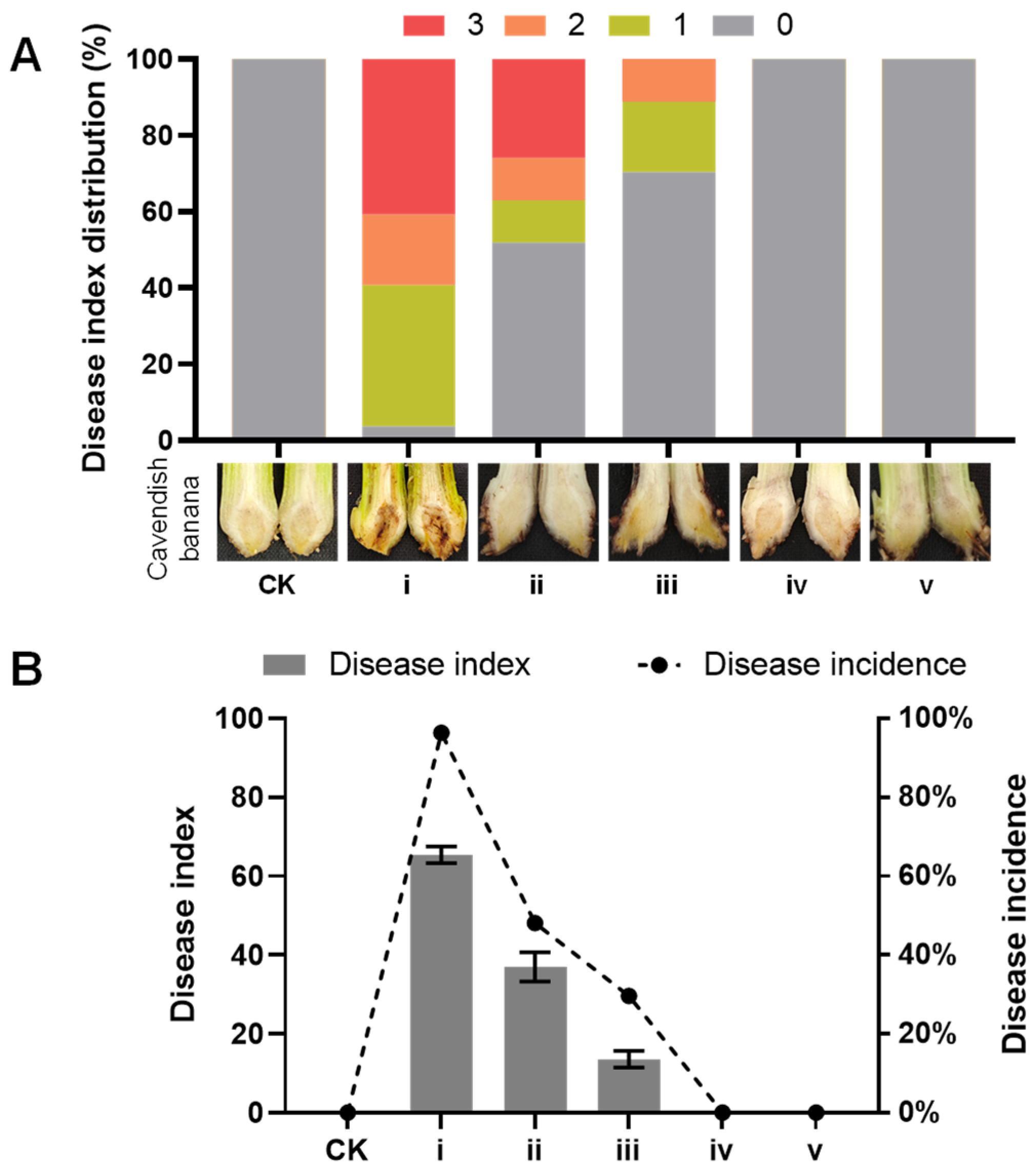
2.3. Activity of Isolated Compounds Against Fusarium Species
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. X-Ray Crystallographic Analysis
3.5. DFT Calculations
3.6. Antifungal Activity of Extracts of Medicopsis sp. SCSIO 40440
3.7. Antifungal Activity of Isolated Compounds Against Fusarium Species
3.8. Virulence Assays
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, A.; Pretorius, Z.; Hammond, K.; Dipietro, A.; Spanu, P.; Rudd, J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant. Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Usman, S.; Ge, X.; Xu, Y.; Qin, Q.; Xie, J.; Wang, B.; Jin, C.; Fang, W. Loss of phosphomannose isomerase impairs growth, perturbs cell wall integrity, and reduces virulence of Fusarium oxysporum f. sp. cubense on banana plants. J. Fungi 2023, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.W.; Mostert, D.; Yu, H.L.; Zhuo, M.X.; Li, G.T.; Zuo, C.W.; Haridas, S.; Webster, K.; Li, M.H.; et al. Virulence of banana wilt-causing fungal pathogen Fusarium oxysporum tropical race 4 is mediated by nitric oxide biosynthesis and accessory genes. Nat. Microbiol. 2024, 9, 2232–2243. [Google Scholar] [CrossRef]
- Tinzaara, W.; Mutambuka, M.; Oyesigye, E.; Blomme, G.; Dita, M.; Gold, C.S.; Rouard, M.; Karamura, E. Banana wilt diseases: Current status and future research strategies for their management. Int. J. Pest Manag. 2024, 70, 290–309. [Google Scholar] [CrossRef]
- Staver, C.; Pemsl, D.E.; Scheerer, L.; Perez Vicente, L.; Dita, M. Ex Ante Assessment of Returns on Research Investments to Address the Impact of Fusarium Wilt Tropical Race 4 on Global Banana Production. Front. Plant Sci. 2020, 11, 844. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, W.; Yang, C.; Zhang, L.; Huang, H.; Zhu, Y.; Ratnasekera, D.; Zhang, C. Discovery of kebanmycins with antibacterial and cytotoxic activities from the mangrove-derived Streptomyces sp. SCSIO 40068. J. Nat. Prod. 2024, 87, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wen, S.; Ding, M.; Guo, H.; Huang, C.; Zhu, X.; Huang, J.; She, Z.; Long, Y. The purification, characterization, and biological activity of new polyketides from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002. Mar. Drugs 2019, 17, 414. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-X.; Yan, X.; Fu, W.-H.; Yi, L.-Q.; Tang, B.-W.; Yu, L.-B.; Fang, M.-J.; Wu, Z.; Qiu, Y.-K. New β-Lactone with tea pathogenic fungus inhibitory effect from marine-derived fungus MCCC3A00957. J. Agric. Food Chem. 2019, 67, 2877–2885. [Google Scholar] [CrossRef]
- Wang, L.; Ye, W.X.; Zhao, M.R.; Zhang, Q.B.; Zhang, L.P.; Zhang, C.S.; Zhang, W.J. Diversity of culturable endophytic fungi from Zhanjiang mangroves and secondary metabolites of the isolate Stemphyliums sp. SCSIO 40436. Microbiol. China 2022, 49, 2995–3007. [Google Scholar]
- Wakui, F.; Harimaya, K.; Iwata, M.; Sashita, R.; Chiba, N.; Mikawa, T. Dicyclic carboxylic acid MK8383, its manufacture with Phoma spp., and the use as agrochemical microbicides. Jpn. Kokai Tokkyo Koho JP 1995, 7. [Google Scholar]
- Namikoshi, M.; Kobayashi, H.; Yoshimoto, T.; Hosoya, T. Phomopsidin, a new inhibitor of microtubule assembly produced by Phomopsis sp. isolated from coral reef in Pohnpei. J. Antibiot. 1997, 50, 890–892. [Google Scholar] [CrossRef]
- Kobayashi, H.; Meguro, S.; Yoshimoto, T.; Namikoshi, M. Absolute structure, biosynthesis, and anti-microtubule activity of phomopsidin, isolated from a marine-derived fungus Phomopsis sp. Tetrahedron 2003, 59, 455–459. [Google Scholar] [CrossRef]
- Tabekoueng, G.; Li, H.; Goldfuss, B.; Schnakenburg, G.; Dickschat, J.S. Skeletal rearrangements in the enzyme-catalysed biosynthesis of coral-type diterpenes from Chitinophaga pinensis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202413860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, B.; Fu, Z.; Mao, J.; Liu, L.; Chen, X.; Zheng, M.; Wang, C.-Y.; Wang, C.; Guo, Y.-W.; et al. Discovery of a terpene synthase synthesizing a nearly non-flexible eunicellane reveals the basis of flexibility. Nat. Commun. 2024, 15, 5940. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Rep, M.; Wang, B.; Ashton, A.; Dodds, P.; Ellis, J. Variation in potential effector genes distinguishing Australian and non-Australian isolates of the cotton wilt pathogen Fusarium oxysporum f.sp. vasinfectum. Plant Pathol. 2011, 60, 232–243. [Google Scholar] [CrossRef]
- Smith, S.N.; Snyder, W.C. Persistence of Fusarium oxysporum f. sp. vasinfectum. Phytopathology 1975, 65, 190–196. [Google Scholar] [CrossRef]
- Xiao, C.; Yu, X.; He, Y.; Deng, X.; Tang, E. Studies on the biological characteristics of the pathogen of cucurbit wilt. Plant Prot. 2008, 34, 83–86. [Google Scholar]
- Lan, C.; Ruan, H.; Yang, X.; Yao, J.; Jiang, J. Development of a loop-mediated isothermal amplification assay for sensitive and specific detection of Fusarium oxysporum f. sp. cucumerinum owen. Phytoparasitica 2018, 46, 283–293. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kim, Y.T.; Yoo, S.J.; Kim, H.G. Mycological characteristics of Fusarium solani f. sp. pisi isolated from pea, ginseng and soybean in Korea. Plant Pathol. J. 1999, 15, 44–47. [Google Scholar]
- Edgington, L.V.; Khew, K.L.; Barron, G.L. Fungitoxic spectrum of benzimidazole compounds. Phytopathology 1971, 61, 42–44. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Mennucci and G. Petersson, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- Tao, P.; Wu, C.; Hao, J.; Gao, Y.; He, X.; Li, J.; Shang, S.; Song, Z.; Song, J. Antifungal application of rosin derivatives from renewable pine resin in crop protection. J. Agric. Food Chem. 2020, 68, 4144–4154. [Google Scholar] [CrossRef]
- Kyselka, J.; Bleha, R.; Dragoun, M.; Bialasová, K.; Horáčková, Š.; Schätz, M.; Sluková, M.; Filip, V.; Synytsya, A. Antifungal polyamides of hydroxycinnamic acids from sunflower bee pollen. J. Agric. Food Chem. 2018, 66, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, J.; Xie, X.; Leng, Y.; Wang, H.; Xi, P.; Zhou, J.; Zhong, S.; Jiang, Z. Identification and application of a unique genetic locus in diagnosis of Fusarium oxysporum f. sp. cubense tropical race 4. Can. J. Plant Pathol. 2013, 35, 482–493. [Google Scholar] [CrossRef]
- Li, M.H.; Xie, X.L.; Lin, X.F.; Shi, J.X.; Ding, Z.J.; Ling, J.F.; Xi, P.G.; Zhou, J.N.; Leng, Y.; Zhong, S.; et al. Functional characterization of the gene FoOCH1 encoding a putative α-1,6-mannosyltransferase in Fusarium oxysporum f. sp. cubense. Fungal Genet. Biol. 2014, 65, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Y.; Cai, B.; Zhou, D.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; Wedge, D.E.; Pan, Z.; et al. Discovery of Niphimycin C from Streptomyces yongxingensis sp. nov. as a Promising Agrochemical Fungicide for Controlling Banana Fusarium Wilt by Destroying the Mitochondrial Structure and Function. J. Agric. Food Chem. 2022, 70, 12784–12795. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, R.; Chu, Y.; Liu, S.; Xiang, D.; Li, C. Mechanistic Insights into Stereospecific Antifungal Activity of Chiral Fungicide Prothioconazole against Fusarium oxysporum F. sp. cubense. Int. J. Mol. Sci. 2022, 23, 2352. [Google Scholar] [CrossRef] [PubMed]

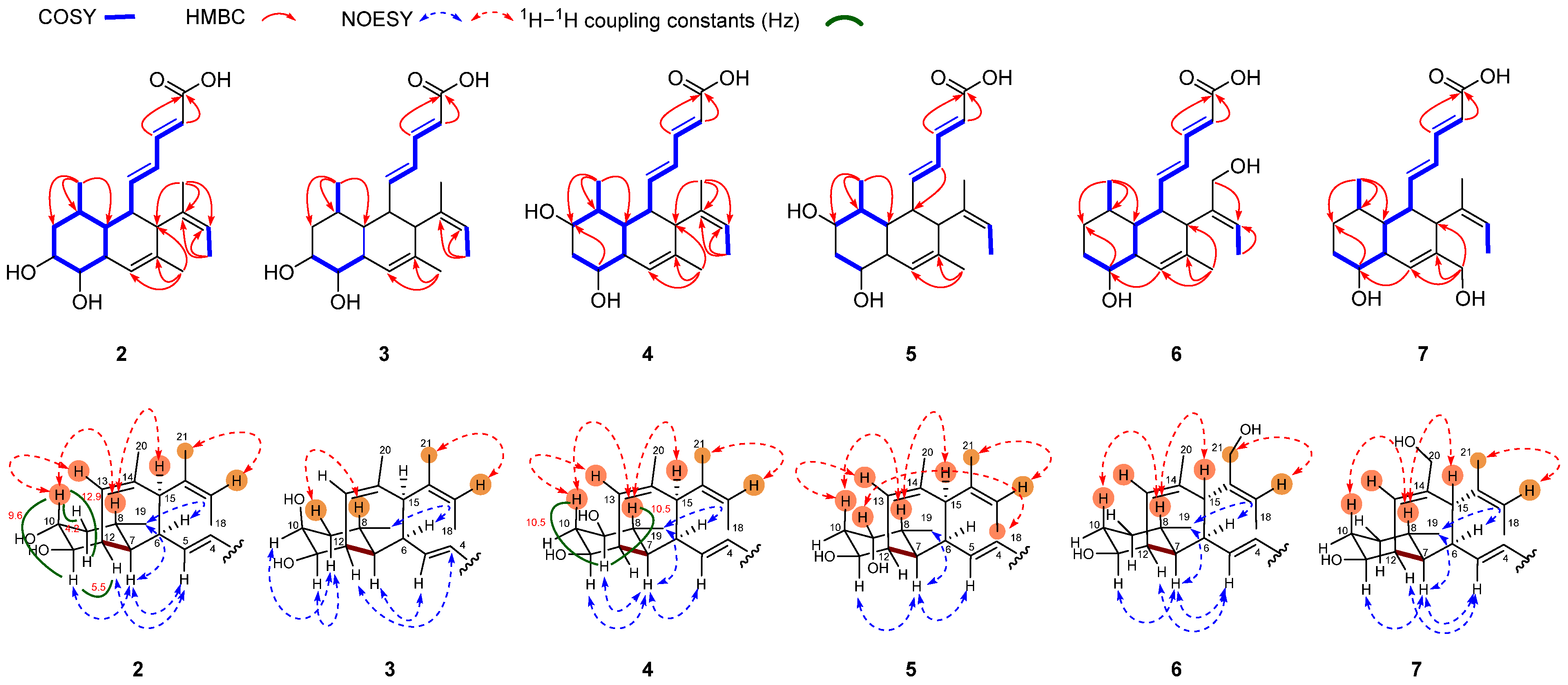
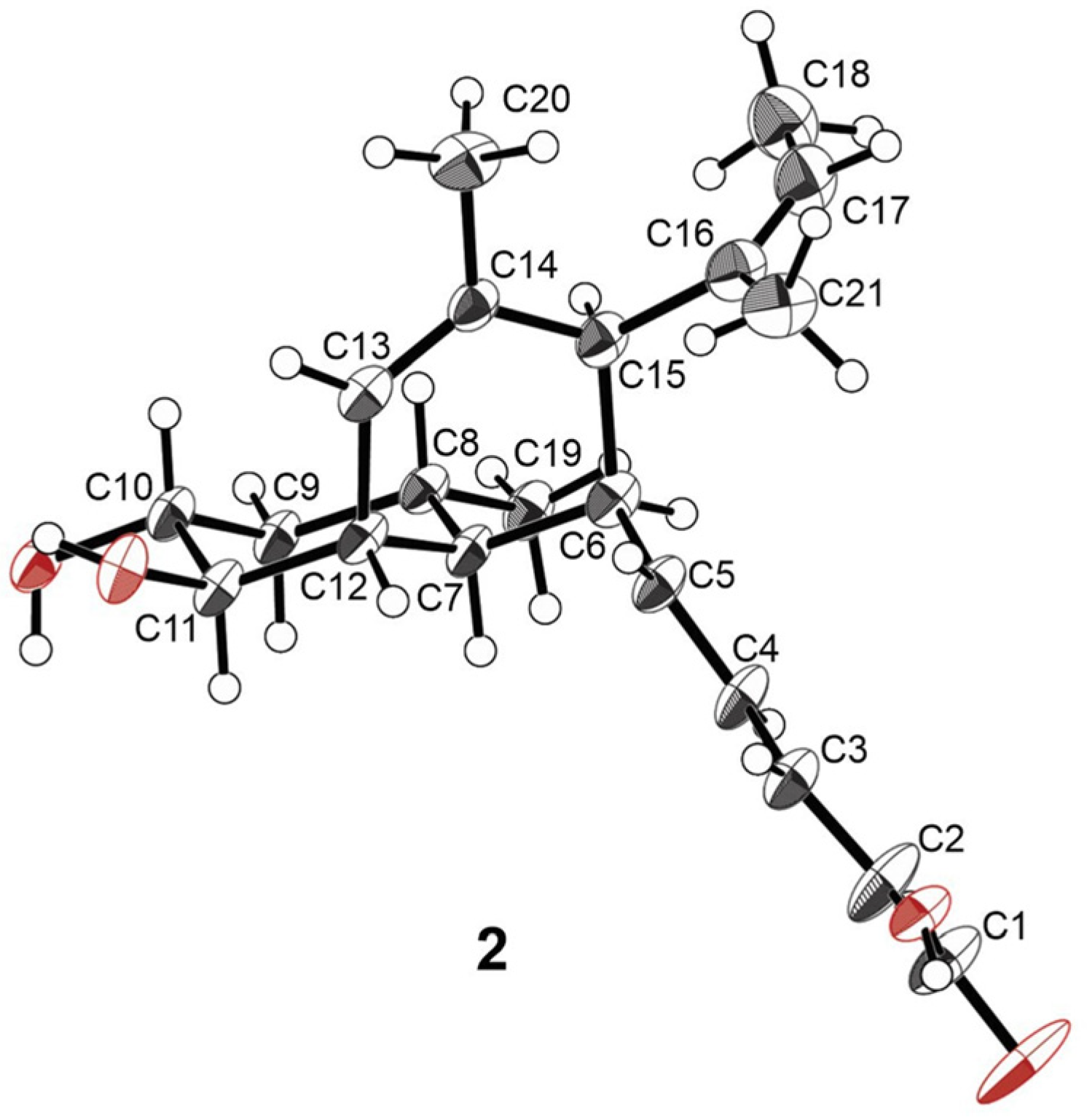
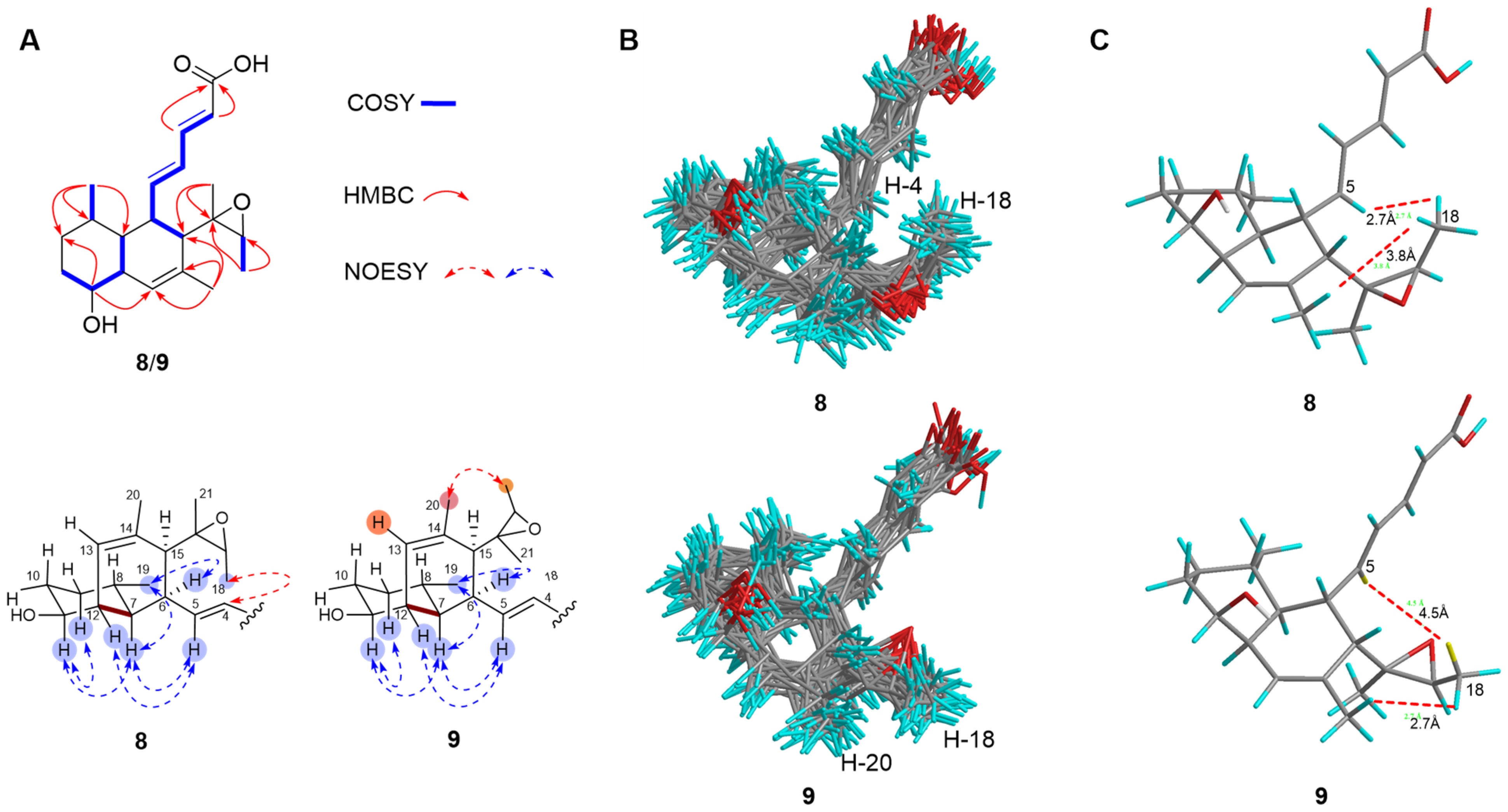
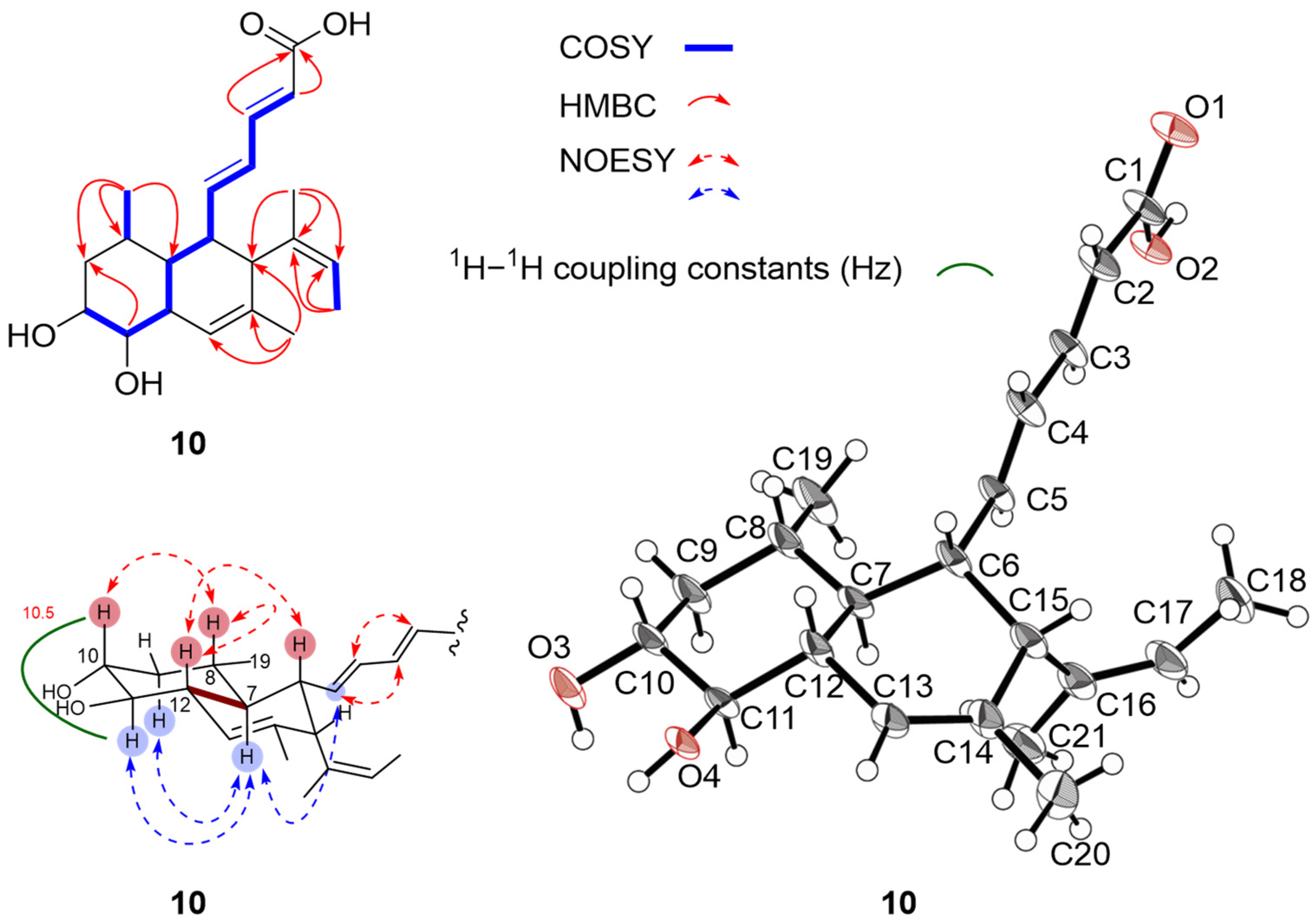

| No. | 2 a,c | 3 a,c | 4 a,c | 5 b,d | 6 a,c | 7 a,c | 8 a,c | 9 b,d | 10 b,d |
|---|---|---|---|---|---|---|---|---|---|
| δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | δH, mult. (J in Hz) | |
| 2 | 5.84, d (15.0) | 5.79, d (15.5) | 5.81, overlap | 5.84, d (15.4) | 5.79, d (15.3) | 5.84, d (15.5) | 5.88, d (15.5) | 5.84, d (15.4) | 5.82, d (15.3) |
| 3 | 7.31, dd (15.4, 11.4) | 7.11, dd (14.5, 10.8) | 7.30, dd (15.0, 11.2) | 7.27, dd (15.0, 10.5) | 7.24, dd (13.8, 11.5) | 7.28, dd (11.4, 15.5) | 7.25, dd (15.5, 10.2) | 7.17, dd (15.1, 11.1) | 7.27, dd (15.3, 10.6) |
| 4 | 6.27, dd (14.9, 11.3) | 6.21, dd (15.0, 11.5) | 6.27, dd (14.3, 10.7) | 6.27, dd (14.3, 11.0) | 6.26, dd (14.9, 11.4) | 6.28, dd (10.5, 15.0) | 6.35, dd (15.5, 9.8) | 6.32, dd (14.9, 11.1) | 6.26, overlap |
| 5 | 6.47, dd (15.0, 10.4) | 6.01, br s | 6.47, dd (14.9, 10.5) | 6.49, br s | 6.40, br s | 6.37, m | 6.37, dd (15.5, 9.8) | 6.46, dd (15.3, 10.7) | 6.26, overlap |
| 6 | 2.80, s | n | 2.83, br s | 2.83, overlap | 2.97, s | 2.95, br s | 2.87, m | 2.73, m | 2.55, m |
| 7 | 1.32, m | 1.34, br s | 1.29, br s | 1.85, br s | 1.34, m | 1.40, br s | 1.22, m | 1.15, m | 1.41, overlap |
| 8 | 1.70, s | 1.85, br s | 1.42, br s | 1.63, overlap | 1.39, o | 1.59, br s | 1.35, m | 1.21, overlap | 1.40, overlap |
| 9 | 1.06, m | 1.09 br s; | 3.11, dt (4.2, 10.5) | 3.89, br s | 1.02, m; | n | 1.64, overlap | 1.52, m; | 1.17, m |
| 9 | 1.82, dt (12.9, 3.9) | 1.66 br s | 1.64, m | 1.01, overlap | 0.93 m | 1.83, m | |||
| 10 | 3.42, ddd (12.9, 9.6, 4.2) | 3.64, br s | 1.46, q (10.5) | 1.79, overlap | 1.39, m; | n | 1.60, overlap | 1.48, overlap | 3.39, ddd (11.2, 8.3, 4.2) |
| 1.95, dtd (10.6, 4.0, 1.0) | 1.58, overlap | 1.64, m | 1.33, overlap | 1.16, dd (12.5, 3.7) | |||||
| 11 | 3.36, dd (5.5, 9.6) | 3.57, br s | 3.70, dt (10.5, 3.7) | 4.09, br s | 3.64, m | 3.71, m | 3.61, td (11.2, 4.5) | 3.43 | 2.96, dd (10.8, 8.6) |
| 12 | 2.86, s | 2.42, br s | 2.72, s | 2.72, br s | 2.78, m | 2.76, br s | 2.71, s | 2.60, m | 1.78, m |
| 13 | 5.85, s | 5.67, br s | 5.84, br s | 5.74, br s | 5.76, s | 6.13, br s | 5.81, br s | 5.72, br s | 5.98, m |
| 15 | n | n | 3.43, br s | 3.41, br s | 3.02, m | n | 2.17, s | 2.05, s | 3.24, d (4.3) |
| 17 | 5.36, m | 5.34, br s | 5.38, brs | 5.38, | 5.53, m | 5.38, q (6.4) | 2.82, q (6.0) | 2.79, q (5.4) | 5.44, dq (6.3. 1.3) |
| 18 | 1.65, d (6.6) | 1.49, br s | 1.65, d (7.5) | 1.62, overlap | 1.64, overlap | 1.63, overlap | 1.41, d (5.7) | 1.23, d (6.5) | 1.48, d (6.2) |
| 19 | 1.01, d (6.6) | 0.94, d (6.59) | 1.09, d (6.2) | 1.00, d (5.94) | 0.97, d (5.7) | 1.00, d (6.0) | 0.91, d (6.3) | 0.81, d (6.4) | 1.00, d (5.9) |
| 20 | 1.59, s | 1.49, overlap | 1.61, s | 1.79, overlap | 1.64, overlap | 3.94, d (13.0) | 1.82, s | 1.68, s | 1.52, s |
| 21 | 1.55, s | 1.53, overlap | 1.56, s | 1.59, overlap | 3.91, br s | 3.87, d (13.0) | 1.19, s | 1.10, s | 1.67, m |
| No. | 2 a,c | 3 a,c | 4 a,c | 5 b,d | 6 a,c | 7 a,c | 8 a,c | 9 b,d | 10 b,d |
|---|---|---|---|---|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | |
| 1 | 167.1, C | 168.4, C | 167.1, C | 169.4, C | 168.2, C | 167.7, C | 171.2, C | 168.6, C | 168.0, C |
| 2 | 119.8, CH | 120.8, CH | 119.8, CH | 121.8, CH | 120.3, CH | 120.3, CH | 122.6, CH | 122.4, CH | 120.0, CH |
| 3 | 145.2, CH | 144.8, CH | 145.2, CH | 146.3, CH | 146.2, CH | 144.8, CH | 145.6, CH | 144.3, CH | 146.0, CH |
| 4 | 128.3, CH | 128.7, CH | 128.3, CH | 130.1, CH | 129.4, CH | 128.4, CH | 129.9, CH | 129.0, CH | 127.2, CH |
| 5 | 146.7, CH | 147.7, CH | 146.9, CH | 148.0, CH | 147.8, CH | 146.8, CH | 147.3, CH | 147.0, CH | 150.5, CH |
| 6 | 43.2, CH | 43.0, CH | 43.1, CH | 44.9, CH | 44.0, CH | 43.1, CH | 42.3, CH | 41.4, CH | 49.7, CH |
| 7 | 47.9, CH | 44.7, CH | 45.1, CH | 43.1, CH | 48.5, CH | 47.2, CH | 49.9, CH | 48.6, CH | 35.7, CH |
| 8 | 28.1, CH | 34.0, CH | 36.9, CH | 35.1, CH | 29.8, CH | 28.5, CH | 29.9, CH | 29.2, CH | 45.7, CH |
| 9 | 41.4, CH2 | 37.5, CH2 | 72.5, CH | 73.1, CH | 34.2, CH2 | 38.5, CH2 | 34.2, CH2 | 33.1, CH2 | 44.4, CH2 |
| 10 | 70.4, CH | 67.3, CH | 40.2, CH2 | 39.4, CH2 | 31.5, CH2 | 38.9, CH2 | 31.1, CH2 | 31.3, CH2 | 75.1, CH |
| 11 | 76.8, CH | 72.0, CH | 69.0, CH | 67.8, CH | 72.5, CH | 70.9, CH | 73.3, CH | 71.1, CH | 78.4, CH |
| 12 | 37.5, CH | 36.4, CH | 37.7, CH | 39.3, CH | 38.6, CH | 37.3, CH | 38.9, CH | 38.2, CH | 47.6, CH |
| 13 | 122.7, CH | 122.8, CH | 122.7, CH | 126.3, CH | 122.4, CH | 123.2, CH | 125.0, CH | 124.2, CH | 125.1, CH |
| 14 | 133.3, C | 135.5, C | 134.1, C | 135.0, C | 136.0, C | 139.3, C | 134.9, C | 132.1, C | 134.4, C |
| 15 | 41.1, CH | n | 41.3, CH | n | 41.9, CH | n | 45.5, CH | 40.8, CH | 47.1, CH |
| 16 | 135.6, C | 135.5, C | 135.7, C | 137.0, C | 139.4, C | 135.0, CH | 62.7, C | 60.9, C | 134.8, C |
| 17 | 122.5, CH | 125.8, CH | 122.6, CH | 124.4, CH | 123.3, CH | 122.7, CH | 59.4, CH | 62.0, CH | 124.3, CH |
| 18 | 12.5, CH3 | 13.6, CH3 | 12.4, CH3 | 14.3, CH | 13.1, CH3 | 12.7, CH3 | 14.7, CH3 | 15.0, CH3 | 13.7, CH3 |
| 19 | 18.7, CH3 | 20.3, CH3 | 14.2, CH3 | 16.6, CH3 | 19.5, CH3 | 18.7, CH3 | 19.6, CH3 | 19.1, CH3 | 23.2, CH3 |
| 20 | 21.2, CH3 | 22.4, CH3 | 21.3, CH3 | 23.1, CH3 | 22.2, CH3 | 64.6, CH2 | 22.5, CH3 | 22.2, CH3 | 21.5, CH3 |
| 21 | 21.6, CH3 | 22.7, CH3 | 21.3, CH3 | 23.2, CH3 | 68.9, CH2 | 22.1, CH3 | 22.7, CH3 | 21.9, CH3 | 22.9, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Qiao, Y.; Wang, L.; Li, Z.; Zhang, H.; Zhang, L.; Liao, S.; Li, M.; Zhang, C.; Zhang, W. Discovery of MK8383s with Antifungal Activity from Mangrove Endophytic Fungi Medicopsis sp. SCSIO 40440 Against Fusarium Wilt of Banana. Mar. Drugs 2025, 23, 88. https://doi.org/10.3390/md23020088
Zhou T, Qiao Y, Wang L, Li Z, Zhang H, Zhang L, Liao S, Li M, Zhang C, Zhang W. Discovery of MK8383s with Antifungal Activity from Mangrove Endophytic Fungi Medicopsis sp. SCSIO 40440 Against Fusarium Wilt of Banana. Marine Drugs. 2025; 23(2):88. https://doi.org/10.3390/md23020088
Chicago/Turabian StyleZhou, Tianyu, Yulei Qiao, Lu Wang, Zifeng Li, Haibo Zhang, Liping Zhang, Shengrong Liao, Minhui Li, Changsheng Zhang, and Wenjun Zhang. 2025. "Discovery of MK8383s with Antifungal Activity from Mangrove Endophytic Fungi Medicopsis sp. SCSIO 40440 Against Fusarium Wilt of Banana" Marine Drugs 23, no. 2: 88. https://doi.org/10.3390/md23020088
APA StyleZhou, T., Qiao, Y., Wang, L., Li, Z., Zhang, H., Zhang, L., Liao, S., Li, M., Zhang, C., & Zhang, W. (2025). Discovery of MK8383s with Antifungal Activity from Mangrove Endophytic Fungi Medicopsis sp. SCSIO 40440 Against Fusarium Wilt of Banana. Marine Drugs, 23(2), 88. https://doi.org/10.3390/md23020088








