Abstract
The Red Sea is the home of a rich diversity of sponge species with unique ecological adaptations that thrive in its saline, warm, and nutrient-poor waters. Red Sea sponges offer potential as sources of bioactive compounds and novel drugs. The organic extract of the Red Sea sponge Agelas sp. aff. marmarica was investigated for its antimicrobial constituents. Through bioassay-guided fractionation of the antimicrobial fraction of the extract on SiO2 and Sephadex LH-20, as well as HPLC purification, three bioactive compounds, marmaricines A-C (1–3), were isolated. Structural elucidation of the compounds was performed using 1D (1H and 13C) and 2D (COSY, HSQC, HMBC, and NOESY) NMR, as well as (+)-HRESIMS, leading to the identification of the compounds. The antimicrobial activities of the compounds were assessed through evaluation of their inhibition zones, MIC, MBC, and MFC, against Methicillin-Resistant Staphylococcus aureus (MRSA), Escherichia coli, and Candida albicans. Marmaricines A and B exhibited the strongest antibacterial effects against MRSA, with inhibition zones ranging from 14.00 to 15.00 mm, MIC values of 8 µg/mL, and MBC values of 16 µg/mL. In comparison, marmaracine C showed slightly weaker activity (inhibition zone: 12 mm, MIC: 16 µg/mL, MBC: 32 µg/mL). In terms of antifungal activity, marmaricines B and C demonstrated the greatest effect against C. albicans, with inhibition zones of 14–15 mm, MIC values of 8 µg/mL, and MFCs of 16 µg/mL. Interestingly, none of the compounds showed any inhibitory effect against E. coli. The results indicate that marmaricines A-C are selectively active against MRSA, and marmaricines B and C demonstrate potential against C. albicans, making them promising candidates for the development of novel antimicrobial agents targeting resistant pathogens.
1. Introduction
The Red Sea is renowned for its exceptional biodiversity [1] and endemism [2], hosting over 1000 fish species and more than 50 genera of hermatypic corals. Recent inventories have expanded the knowledge of the region’s biodiversity, identifying 635 polychaete, 79 ascidians, and 211 echinoderm species [2]. However, despite this remarkable richness, the Red Sea and the broader Arabian region remain relatively understudied compared to other worldwide coral reef systems, particularly with respect to sponge diversity [2,3]. One challenge in understanding the Red Sea ecosystem is that much of the existing research has been concentrated on a small stretch of coastline in the far northern Red Sea, specifically the Gulf of Eilat/Aqaba (~6 km in length) [4]. Nevertheless, the Red Sea is increasingly attracting attention from climate change researchers due to its distinctive environmental conditions, including high and variable water temperatures and high salinity levels (40.0 psu in the northern Red Sea) [5]. These unique conditions are believed to reflect the near-future state of oceans in other parts of the world [6]. While coral research in the Red Sea has received significant attention, knowledge of its sponge biodiversity remains limited in comparison to that for other regions in the world [7,8]. Early studies of Red Sea sponges primarily focused on cataloging regional biodiversity, with little exploration of their ecological roles or chemical properties. The reported sponge fauna of the Red Sea is primarily composed of species from the classes Demospongiae (225 species) and Calcarea (32 species). In contrast, much less is understood about Homoscleromorpha (2 species) and Hexactinellida (glass sponges), which are typically found in deeper waters and are currently represented by only two described species. It is likely that these two classes harbor many undiscovered species in the Red Sea [9]. Worldwide, marine sponges (Porifera) have become a primary focus of research due to their biologically active secondary metabolites. Red Sea sponges are particularly diverse in terms of their morphologies and the range of bioactive compounds they produce. These compounds not only play crucial roles in marine ecosystems, such as facilitating symbiosis with microorganisms, but also have potential pharmaceutical applications.
Marine sponges from the genus Agelas (class Demospongiae, order Agelasida, family Agelasidae) are among the most common sponges found in tropical and subtropical regions worldwide, with 36 valid species currently recognized. Ongoing research continues to expand our understanding of these species and their ecological and biochemical significance. Currently, only two species of Agelas have been reported in the Red Sea including Agelas marmarica and Agelas mauritiana [9]. The secondary metabolites isolated from Agelas sponges, since their initial discovery, represent a fascinating area of research that has driven significant advancements in the field of marine natural products [10]. Over five decades (1971–2021), more than 355 compounds have been reported from several members of the genus Agelas [10]. The largest producers of the compounds among the Agelas species are A. oroides (15%), A. nakamurai (13%), and A. mauritiana (11%), while the remainder was reported from unclassified Agelas species [10]. Members of the genus Agelas exhibit notable structural diversity in their pyrrole and terpenoidal alkaloids [11]. Following the isolation of specific bromopyrrole derivatives from Agelas oroides in 1971 [12] and the identification of agelasine, a quaternary 9-methyladenine derivative of an unidentified diterpene from Agelas dispar in 1975 [13], in addition to pyrrole and terpenoidal alkaloids, numerous bioactive metabolites of varying biogenetic origins, such as glycosphingolipids, sterols, carotenoids, and many others have been discovered within the genus Agelas [11,14,15,16,17,18]. Pyrrole alkaloids of this genus typically possess a backbone consisting of a bromo- or debromo-pyrrole-2-carboxamide structure, which is associated with various side chains and cyclic formations [19,20]. In contrast, the less common diterpene alkaloids primarily include those containing a 9-N-methyladeninium group (such as agelines, agelasines, and nemoechines) [21,22,23], as well as diterpenes related to hypotaurocyamine (for example, agelasidines) [24]. Secondary metabolites of the genus Agelas show a wide range of biological activities, including antimicrobial [25,26], antihistaminic [27], antimalarial [28], antileukemic [29], cytotoxic [26,30], antifouling [30,31], Na+,K+-adenosine triphosphatase (ATPase) inhibitory [21,24], and antiangiogenic matrix metalloproteinase inhibitory effects [32].
In our continuous effort to identify bioactive compounds from Red Sea marine sponges [33,34], we investigated the sponge Agelas sp. aff. marmarica. Bioassay-guided partitioning of the antimicrobial fraction of the organic extract of the sponge and final HPLC purification afforded three new brominated pyrrole-derived alkaloids, marmaricines A-C. The current study describes the isolation, structural elucidation, and antimicrobial activities of these compounds.
2. Results and Discussion
2.1. Purification of Compounds 1–3
Fractionation of the antimicrobial fraction of the organic extract of the Red Sea sponge Agelas sp. aff. marmarica on normal silica gel, Sephadex LH 20, and purification of the active fraction on HPLC afforded compounds 1–3 (Figure 1).
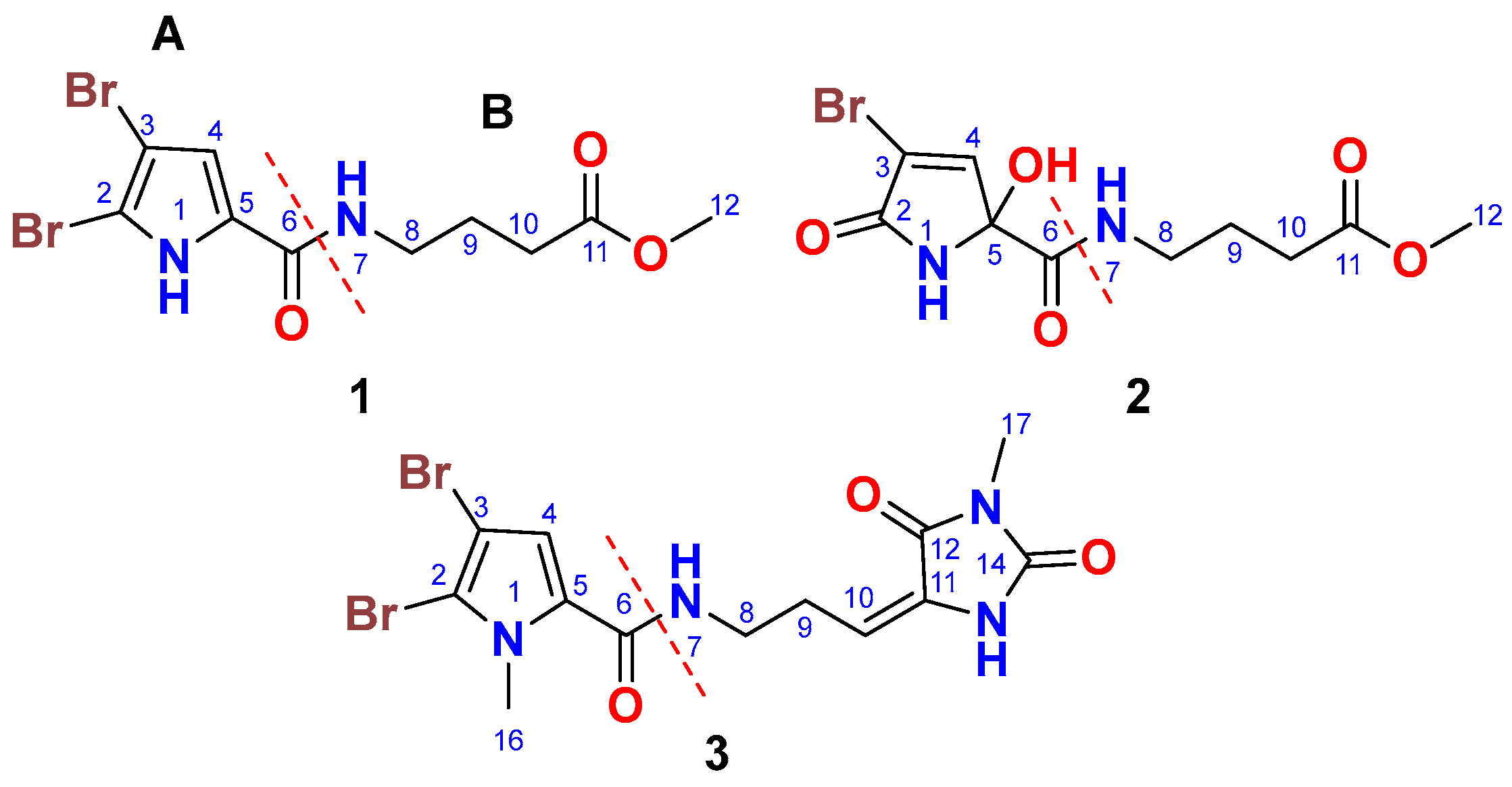
Figure 1.
Chemical structures of compounds 1–3.
2.2. Structure of Compound 1
Compound 1 (Figure 1) was isolated as a yellowish powder with the molecular formula C10H12Br2N2O3, determined from the (+)-HRESIMS ion peak at m/z 388.9111 [M + Na]+. The presence of two bromine atoms in compound 1 was confirmed by the observation of three ion peaks at m/z 388.9, 390.9, and 392.9 in a 1:2:1 ratio. The structure of 1 was elucidated through the interpretation of its 1D and 2D NMR spectra. The 13C NMR spectrum showed signals for 10 carbon atoms (Table 1). Analysis of the 13C NMR spectrum, supported by the HSQC experiment, led to the assignment of five non-protonated carbons, one methine, three methylene groups, and one methyl group. The 13C NMR chemical shifts suggested the presence of two distinct parts (A and B) in compound 1, including a 4,5-dibromo-2-carboxylic acid moiety (A) linked to a 4-amino-1-methylbutanoate (B) unit via an amidic linkage (CO-NH) at C-6/NH-7. The 13C NMR spectrum exhibited two carbonyl signals at δC 159.4 and 173.6, which were attributed to carboxamide (C-6) and carboxylic acid ester (C-11) groups, respectively. The 1H/13C signals at δH/C 12.65 (s) (NH-1), 104.9 (C, C-2), 98.2 (C, C-3), 6.90 (s)/112.9 (CH, C-4), and 128.6 (C, C-5) were consistent with the structure of an amidic derivative of 4,5-dibromo-pyrrole-2-carboxylic acid (substructure A).

Table 1.
NMR data of compounds 1–3.
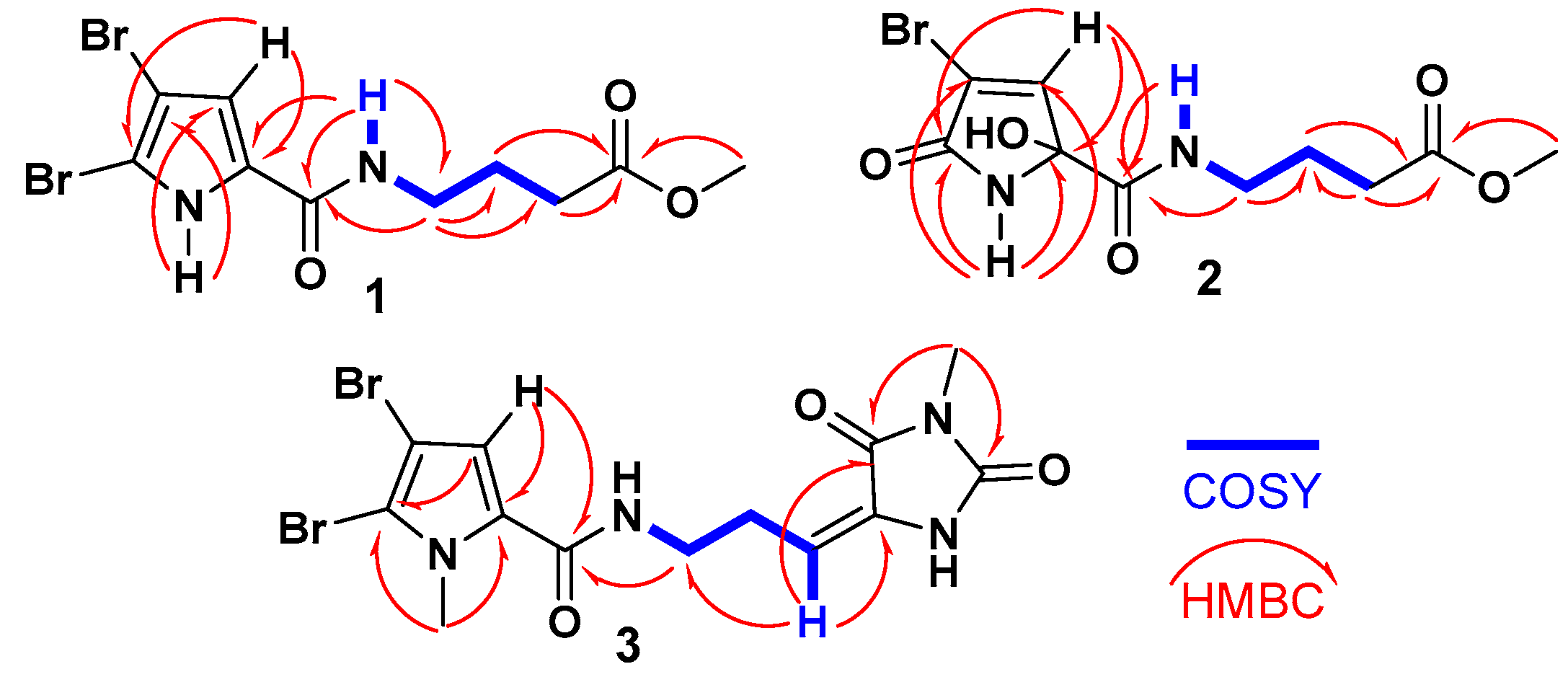
The 1H-1H COSY spectrum revealed a single spin-coupling system from NH-7 through H2-8 to H2-10 (Figure 2). The signals at δH/C 8.14 (t, J = 5.6 Hz, NH-7), 3.21 (q, J = 6.5 Hz)/38.3 (CH2, C-8), 1.73 (quin., J = 7.0 Hz)/25.0 (CH2, C-9), and 2.34 (t, J = 7.4 Hz)/31.1 (CH2, C-10) confirmed this coupling system and this portion of the molecule.
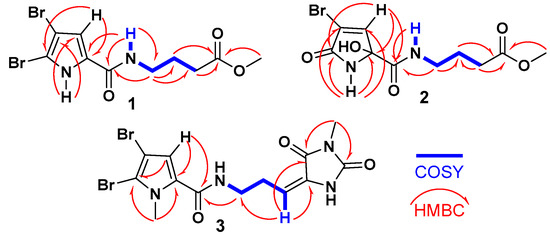
Figure 2.
1H-1H COSY and 1H-13C HMBC correlations of compounds 1–3.
Further HMBC correlations from the three-proton singlet at δH 3.57 (s) to C-11 (δC 173.6) supported the presence of a methyl ester group. HMBC couplings from H2-9 and H2-10 to C-11 (δC 174.9) further confirmed this assignment. The connectivity between the two parts of the compound was established through HMBC correlations (Figure 2) from H-4 (δH 6.90) to C-6 (δC 159.4), from NH-7 (δH 12.65) to C-6 (δC 159.4), and from H2-8 (δH 3.21) to C-6 (δC 159.4). Additional HMBC correlations from NH-1 (δH 12.65) to C-3 (δC 98.2) and C-4 (δC 112.9), and from H-4 (δH 6.90) to C-2 (δC 104.9) and C-5 (δC 128.6), confirmed the assignments of these carbons. Therefore, compound 1 was identified as 4-(4,5-dibromo-1H-pyrrole-2-carboxamido)-1-methylbutanoate. It is worth noting that compound 1 exists as a commercial product with the CAS number 1706460-02-7 and is sold by several chemical companies. In addition, a racemic mixture of a 9-hydroxy derivative of 1, named agesasine B, was previously reported from the marine sponge Agelas spp., collected off the Kerama Islands, Okinawa [35]. Agesasine B was inactive against human cervical carcinoma (HeLa), lung carcinoma (A549), and breast cancer (MCF7) cell lines, with IC50 values > 100 µM [35]. Recently, both enantiomers of agesasine B were synthesized [36]. The (S)-agesasine B displayed moderate cytotoxic activity against breast cancer cell line (MCF7), with an IC50 value of 18 μM, while the (R)-agesasine B was inactive against this cell line. When tested against liver cancer cells (HEPG2), (R)-agesasine B was weakly active with IC50 of 52 μM, while (S)-agesasine B was inactive. Finally, both enantiomers were highly weakly active towards colorectal adenocarcinoma (CACO2) (IC50 = 72–74 μM) [36]. Conclusively, this is the first report of the isolation of compound 1 from a natural source, making it a newly identified natural product. The generic name marmaricine A was given to compound 1.
2.3. Structure of Compound 2
Compound 2 (Figure 1) was isolated as an optically inactive yellowish powder ([α]D 0°, c 0.10, MeOH) with the molecular formula C10H13BrN2O5, as determined from the (+)-HRESIMS ion peak at m/z 342.9906 [M + Na]+. The observation of two ion peaks at m/z 342.9 and 344.9 in a 1:1 ratio confirmed the presence of a single bromine atom in compound 2. The structure of compound 2 was determined through the analysis of its 1D and 2D NMR spectra. The 13C NMR spectrum showed signals for 10 carbon atoms (Table 1). Interpretation of the 13C NMR data, combined with the HSQC experiment, allowed for the assignment of the carbons as five non-protonated carbons, i.e., one methine, three methylene groups, and one methyl group. The COSY, 13C NMR, HSQC, and HMBC data facilitated the identification of two main parts (A and B) in compound 2, including a 4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamide moiety (A) linked to a 4-amino-1-methylbutanoate unit (B) via an amidic bond (C-6/NH-7).
When compared to compound 1, which contains a 4,5-dibromo-1H-pyrrole-2-carboxamide moiety as part A, compound 2 features a 4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamide moiety. The assignment of this substructure in compound 2 was supported by the 1H/13C NMR signals at δH/C 9.05 (NH-1), 167.5 (C, C-2), 120.3 (C, C-3), 7.24 (d, J = 1.6 Hz)/147.0 (CH, C-4), 87.8 (C, C-5), and 167.3 (C, C-6). HMBC correlations (Figure 2) from NH-1 to C-2, C-3, C-5, and C-6, as well as from H-4 to C-2 and C-5, supported this assignment. The chemical shifts of the 1H and 13C NMR signals for the second part of compound 2 (B) are like those in compound 1, suggesting that the same substructure is present in both molecules (Table 1). The connection between the two parts of compound 2 was further confirmed by HMBC correlations (Figure 2) from H-4 to C-6, from NH-7 to C-6, and from H2-8 to C-6 (δC 167.3). The racemic nature of compound 2 was confirmed by the lack of any optical activity and the absence of any Cotton effects (CE) in the experimental ECD spectrum. Therefore, compound 2 was determined to be a racemic mixture and assigned as (±)-4-(4-bromo-2-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxamido)-1-methylbutanoate. Compound 2 is reported here as a new natural product and named marmaricine B.
2.4. Structure of Compound 3
Compound 3 (Figure 1) was purified and obtained as a yellowish powder with the molecular formula C13H14Br2N4O3, as indicated by the (+)-HRESIMS ion peak at m/z 454.9327 [M + Na]+, which suggests the presence of eight degrees of unsaturation. The detection of three ion peaks at m/z 454.9, 456.9, and 458.9 in a 1:2:1 ratio further corroborates the dibrominated nature of compound 3. The structure for compound 3 was determined through analysis of its 1D (1H and 13C) and 2D (COSY, HSQC, HMBC, and NOESY) NMR spectra. The NMR data (Table 1) supports the presence of two substructures (A and B). Except for the absence of the NH-1 signal, compound 3 exhibited similar 1H and 13C NMR signals to the substructure A of compound 1. Notably, the 1H/13C NMR signals at 3.90 (3H, s, H3-16)/36.4 (CH3, C-16) in the 1H and 13C NMR spectra of compound 3, along with the HMBC correlations (Figure 2) from H3-16 (δH = 3.90) to C-2 (δC = 111.9) and C-5 (δC = 129.4), and from H-4 (δH = 6.24) to C-2, C-5, and C-6 (δC = 161.4), support the assignment of substructure A as the 4,5-dibromo-1-methyl-1H-pyrrole-2-carboxamide moiety.
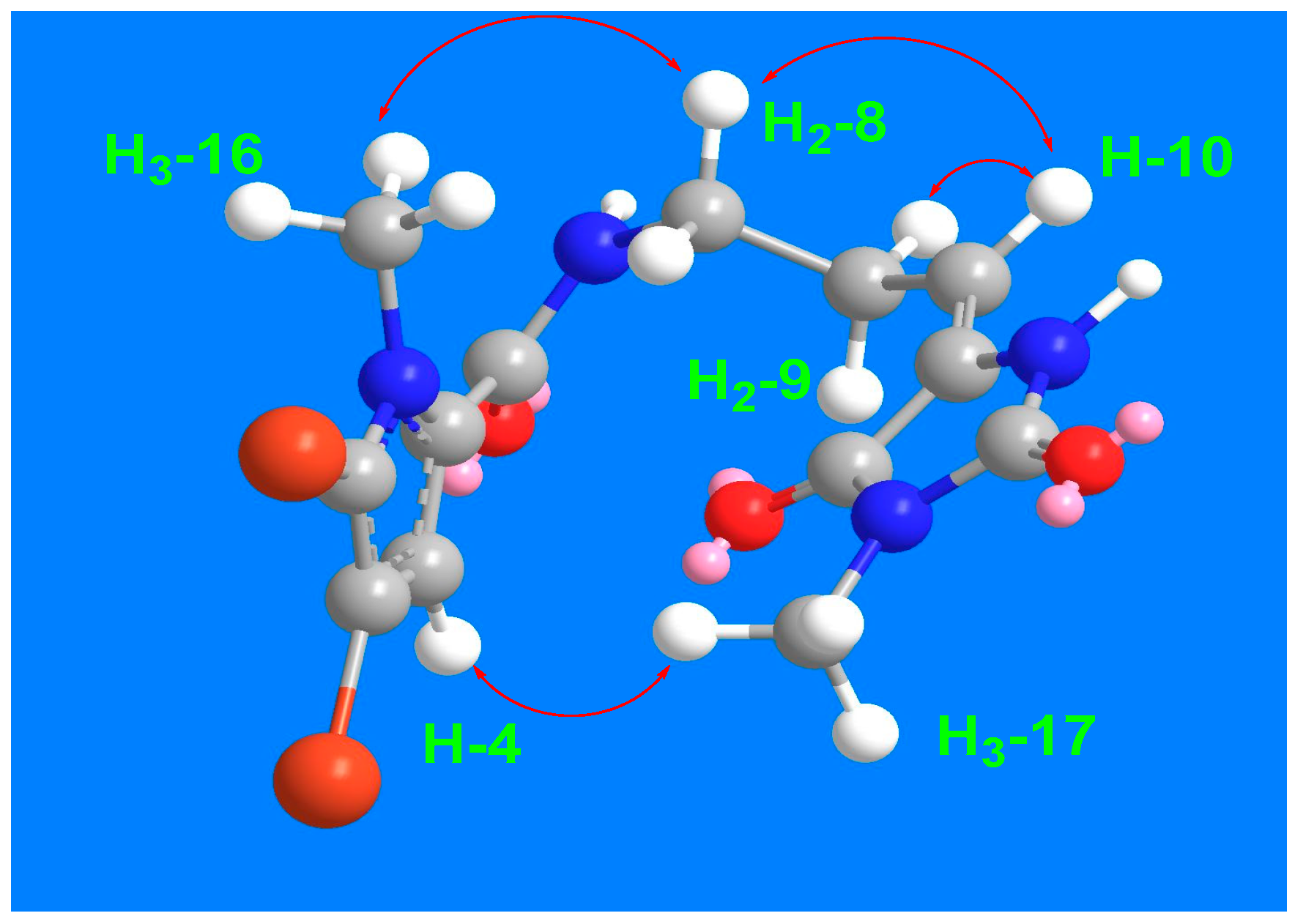
The remaining signals for compound 3 were attributed to the 5-(3-aminopropylidene)-3-methylimidazolidine-2,4-dione moiety, based on the 1H and 13C NMR signals at δH/C 3.45 (t, J = 7.5 Hz)/39.2 (CH2, C-8), 2.61 (q, J = 7.5 Hz)/28.8 (CH2, C-9), 6.24 (q, J = 7.5 Hz)/120.5 (CH, C-10), 130.0 (C, C-11), 163.1 (C, C-12), 154.0 (C, C-14), and 3.20 (3H, s, H3-17)/26.5 (CH3, C-17). This assignment is further validated by the COSY correlations (Figure 2) from H2-8 to H-10 and the HMBC correlations (Figure 2) from H-10 to C-8 (δC = 39.2), C-11 (δC = 130.0), and C-12 (δC = 163.1), as well as from CH3-17 to C-12 (δC = 163.1) and C-14 (δC = 154.0). The interconnection between the substructures in compound 3 is also supported by the HMBC correlation (Figure 2) from H2-8 to C-6. Additionally, the assignment of the 1H and 13C NMR signals for substructure B was confirmed through the COSY (Figure 2) and HMBC correlations. The E configuration at Δ10,11 is supported by NOESY correlations between H-10 and H2-8 and between H3-17 and H-4, as well as between H2-8 and H3-16 (Figure 3).
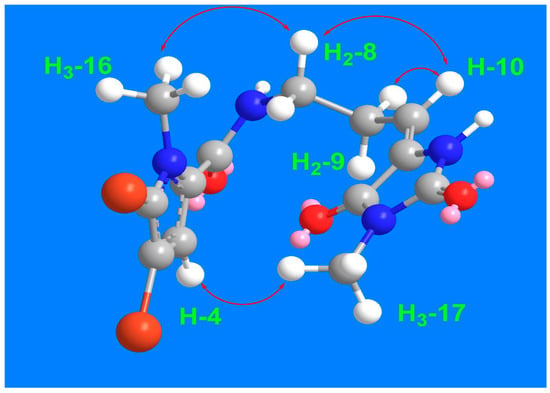
Figure 3.
Significant 1H-1H NOESY correlations of compound 3.
Previously, compound 3 was synthesized as a mixture of E and Z isomers in a 1:3 ratio [37]. To compare the 13C NMR data of compound 3 with the previously published data of the synthetic compound, the NMR spectra of 3 were acquired in DMSO-d6. The 13C NMR data for compound 3 closely resemble those reported for the E isomer of (E/Z)-4,5-dibromo-N-[3-(1-methyl-2,5-dioxo-imidazolidin-4-ylidene)propyl]-1-methylpyrrole-2-carboxamide [37]. In conclusion, compound 3 was assigned as (E)-4,5-dibromo-1-methyl-N-(3-(1-methyl-2,5-dioxoimidazolidin-4-ylidene)propyl)-1H-pyrrole-2-carboxamide and is being reported here as a new natural product, named marmaricine C.
The antimicrobial activities of marmaricines A-C were evaluated using the inhibition zones, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) against Methicillin-Resistant S. aureus (MRSA), E. coli, and C. albicans. Marmaricines A and B showed the highest antibacterial activity against MRSA, with inhibition zones ranging from 14.00 to 15.00 mm, MIC values of 8 µg/mL, and MBC values of 16 µg/mL (Table 2), indicating potent activity. In comparison, marmaracine C showed weaker antibacterial effects, with a 12 mm inhibition zone, an MIC of 16 µg/mL, and an MBC of 32 µg/mL, highlighting its lower potency against MRSA. When tested against C. albicans, marmaricines B and C demonstrated significant antifungal activity, with inhibition zones of 14–15 mm, MIC values of 8 µg/mL, and MFC values of 16 µg/mL (Table 2). However, none of the compounds showed activity against E. coli, indicating that they are more effective against Gram-positive bacteria and fungi. These results underline the selective antimicrobial properties of marmaricines A-C, particularly their effectiveness against MRSA and C. albicans. Marmaricines A and B are strong candidates for further development as antibacterial agents, especially against resistant strains like MRSA, while marmaracine C, though less potent, still holds potential. The antifungal activity of marmaricines B and C against C. albicans also makes them promising agents for fungal infections. Further, the lack of activity against E. coli indicates selectivity of these compounds against MRSA and C. albicans.

Table 2.
Antimicrobial activities of compounds 1–3.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded using a JASCO DIP-370 digital polarimeter (Jasco Co., Tokyo, Japan) at 25 °C, with measurements taken at the sodium D line (589 nm). The (+)-LRESIMS mass spectra of the compounds were evaluated using an Agilent 1200 system connected to an Agilent 6320 Ion Trap LC-ESI-M. The HPLC included a solvent delivery module, a quaternary pump, an auto sampler, and a column compartment (Agilent Technologies Deutschland GmbH, Waldbronn, Germany). The column effluent was connected to an Agilent 6320 Ion Trap LC-ESI-MS. The column heater was set at 25 ± 2 °C, and the HPLC system control and data processing were carried out using ChemStation (Rev. B.01.03-SR2, 204) and 6300 Series Trap Control version 6.2, Build No. 62.24 (Bruker Daltonik GmbH, Bremen, Germany). The compounds were run on an Agilent Zorbax Eclipse XDB-C18 column (250 × 4.6 mm, 5 μm particle diameter), protected with an Agilent Zorbax XDB-C18 pre-column. The mobile phase was programmed to be a gradient from 10% acetonitrile to 0% acetonitrile in 30 min, at a flow rate of 0.5 mL/min. The ESI-MS ion trap conditions included a smart target ion up to 1500 m/z, a nebulizer at 36 psi, dry gas at 12 L/min, and dry temperature at 350 °C. The (+)-HRESIMS mass spectral data were obtained with a Micromass Q-tof equipped with a lockspray mass spectrometer, using Leucine Enkaphalin at m/z 556.2771 [M + H]+ as a reference mass. One-dimensional and two-dimensional NMR spectra (chemical shifts in ppm and coupling constants in Hz) were acquired on Bruker Avance DRX 800 MHz (800 MHz for 1H and 200 MHz for 13C) or 500 MHz (500 MHz for 1H and 125 MHz for 13C) spectrometers (Bruker, Rheinstetten, Germany), using DMSO-d6 or CD3OD as the solvent. HPLC separation was carried out on a C18 column (Atlantis®, 150 × 4.6 mm, 2.5 μm, Waters Corporation, Milford, MA, USA), with a CH3CN:H2O gradient as the mobile phase, monitored at 220 nm, and a flow rate of 2.0 mL/min.
3.2. Biological Materials
The Red Sea sponge Agelas sp. aff. marmarica (Figure 4) was collected off the Saudi Red Sea coast (N 021°39′17.5′′, E 038°52′26.3′′). The sponge belongs to kingdom: Animalia, phylum: Porifera, class: Demospongiae, subclass: Heteroscleromorpha, order: Agelasida, family: Agelasidae, genus: Agelas, and species: Agelas sp. aff. marmarica. The sponge was kindly identified by Rob van Soest. A specimen of the sponge was kept at the collection of the Naturalis Biodiversity Center at Leiden, The Netherlands, under registration number RMNHPOR 9165. Another specimen was stored at the Red Sea Invertebrates Collection at King Abdulaziz University, under code No. DY-16.

Figure 4.
A photograph of the Red Sea sponge Agelas sp. aff. marmarica.
3.3. Purification of the Compounds
The freeze-dried sponge materials (0.35 Kg) were macerated in a mixture of CH2Cl2:CH3OH (1:1) (3 × 2000 mL) at room temperature. The combined extracts were dried under reduced pressure to yield a brown residue. The dried residue (17.5 g) was subjected to partitioning on a VLC silica gel column using n-hexane-CH2Cl2-MeOH gradients, affording 12 main fractions (Fr. 1–12). The antimicrobial fraction eluted with 100% CH2Cl2, Fr. 6 (0.41 g) (inhibition zone = 8 mm against C. albicans) was subjected to partitioning on Sephadex LH-10 using MeOH to afford five factions (Fr. A-E). The antimicrobial fraction (Fr. C) (126 mg) (inhibition zone = 10 mm against C. albicans) was purified on a reversed-phase HPLC column (XDB-C18, 250 × 9.4 mm 5 µm, Agilent) using CH3CN:H2O gradients at 2 mL/min, starting from 20% CH3CN to 0% CH3CN in 50 min to yield compounds 1 (2.5 mg, tR = 11 min), 2 (3.9 mg, tR = 20.5 min), and 3 (4.7 mg, tR = 17.5 min).
3.4. Spectral Data of 1–3
3.4.1. Marmaricine A (1)
Yellowish powder; HRESIMS m/z 388.9111 (calcd for C10H12Br2N2O3Na [M + Na]+, 388.9106); 1H NMR (800 MHz, DMSO-d6): 12.65 (1H, s, NH-1), 6.90 (1H, s, H-4), 8.14 (1H, t, J = 5.6 Hz, NH-7), 3.21 (2H, q, J = 6.5 Hz, H2-8), 1.73 (2H, quin., J = 7.1 Hz, H2-9), 2.34 (2H, t, J = 7.4 Hz, H2-10), 3.57 (3H, s, H3-12); 13C NMR (200 MHz, DMSO-d6): 104.9 (C, C-2), 98.2 (C, C-3), 112.9 (CH, C-4), 128.6 (C, C-5), 159.4 (C, C-6), 38.3 (CH2, C-8), 25.0 (CH2, C-9), 31.1 (CH2, C-10), 173.6 (C, C-11), 51.7 (CH3, C-12).
3.4.2. Marmaricine B (2)
Yellowish powder; HRESIMS m/z 342.9906 (calcd for C10H13BrN2O5Na [M + Na]+, 342.9900); 1H NMR (800 MHz, DMSO-d6): 9.05 (1H, s, NH-1), 7.24 (1H, t, J = 1.6, H-4), 8.21 (1H, t, J = 5.9 Hz, NH-7), 3.07 (2H, q, J = 7.8 Hz, H2-8), 1.65 (2H, quin., J = 7.2 Hz, H2-9), 2.27 (2H, t, J = 7.5 Hz, H2-10), 3.55 (3H, s, H3-12); 13C NMR (200 MHz, DMSO-d6): 167.5 (C, C-2), 120.3 (C, C-3), 147.0 (CH, C-4), 87.8 (C, C-5), 167.3 (C, C-6), 38.8 (CH2, C-8), 24.7 (CH2, C-9), 31.0 (CH2, C-10), 173.6 (C, C-11), 51.7 (CH3, C-12).
3.4.3. Marmaricine C (3)
Yellowish powder; [α]D 0° (c 0.1, MeOH); HRESIMS m/z 454.9327 (calcd for C13H14Br2N4O3Na [M + Na]+, 454.9324); NMR data: 1H NMR (500 MHz, CD3OD): 6.82 (1H, s, H-4), 3.45 (2H, t, J = 7.5 Hz, H2-8), 2.61 (2H, q, J = 7.5 Hz, H2-9), 6.24 (1H, t, J = 7.5 Hz, H-10), 3.90 (3H, s, H3-16), 3.20 (3H, s, H3-17); 13C NMR (125 MHz, CD3OD): 111.9 (C, C-2), 99.2 (C, C-3), 116.1 (CH, C-4), 129.4 (C, C-5), 161.4 (C, C-6), 39.2 (CH2, C-8), 28.8 (CH2, C-9), 120.5 (CH, C-10), 130.0 (C, C-11), 163.1 (C, C-12), 154.0 (C, C-14), 36.4 (CH3, C-16)., 26.5 (CH3, C-17); 1H NMR (800 MHz, DMSO-d6): 7.08 (s, H-4), 8.19 (t, J = 7.6 Hz, H-7), 3.26 (q, J = 7.6, H2-8), 2.37 (q, J = 7.5, H2-9), 5.96 (t, J = 7.3 Hz, H-10), 10.93 (brs, H-15), 3.87 (s, H3-16), 3.03 (s, H3-17); 13C NMR (200 MHz, DMSO-d6): 110.3, 96.7, 114.8, 127.8, 159.7, 38.2, 25.9, 109.9, 128.9, 163.0, 153.5, 35.2, 23.6.
3.5. Antimicrobial Activities of the Compounds
3.5.1. Disk Diffusion Assay
The in vitro antimicrobial activity was evaluated using the disc diffusion method, as previously described [38,39,40,41,42,43]. A variety of test microorganisms were employed, including Staphylococcus aureus (ATCC 43300, methicillin-resistant), Escherichia coli (ATCC 35218), and Candida albicans (ATCC 76615). Each microorganism was inoculated to a turbidity matching a 0.5 McFarland standard and evenly streaked over the surface of Muller–Hinton agar plates using sterile swabs. Sterile 6 mm filter paper discs were impregnated with 50 μg of each compound and placed onto the inoculated agar. The plates were then incubated at 37 °C for 24 h. Solvent control discs were included to assess any potential solvent effects. Ciprofloxacin (5 μg/disc) served as the antibacterial reference, while clotrimazole (10 μg/disc) was used as the antifungal reference. The antimicrobial activity of each compound was determined by measuring the diameter of the inhibition zones in millimeters. The experiment was performed in duplicate, and the mean diameter of each inhibition zone was recorded.
3.5.2. Evaluation of the Minimum Inhibitory Concentrations (MICs)
The minimum inhibitory concentration (MIC) of the compounds was determined using the broth microdilution method in accordance with the CLSI standards (CLSI M02 and CLSI M07) [38,39]. Two-fold serial dilutions of the compounds were prepared in Muller–Hinton broth (Sigma-Aldrich, St. Louis, MO, USA), with final concentrations ranging from 1.0 to 1000 μg/mL. A volume of 100 μL from each dilution was added to the wells of a 96-well plate in duplicate, followed by 100 μL of the bacterial inoculum. The inoculum was prepared by diluting 0.5 McFarland suspensions 150-fold in Muller–Hinton broth, achieving a density of 5 × 105; CFU/mL. Growth control (without compound) and sterility control (without compound and inoculum) were also included. Ciprofloxacin and clotrimazole, used as a reference antibiotic and antifungal, respectively, was tested at concentrations ranging from 0.125 to 64 μg/mL. The prepared plates were incubated at 35 ± 1 °C for 18 ± 1 h. MIC was defined as the lowest concentration of the compound that inhibited bacterial growth, measured spectrophotometrically using a microplate reader (OD600, Boeco BMR-100) (Boeckel + Co (GmbH + Co), Hamburg, Germany)
3.5.3. Determination of the Minimum Bactericidal Concentrations (MBCs)
After incubating the microplates and determining the MIC values of the compounds against MRSA, the MBCs of the compounds were determined by sub-culturing 25 μL of the test solution onto Mueller–Hinton agar and were considered as the lowest concentration that killed 99.99% of the initial inoculum.
3.5.4. Assessment of the Minimum Fungicidal Concentrations (MFCs)
After incubating the microplates and determining the MIC values against C. albicans, the minimum fungicidal concentrations (MFCs) of the compounds were assessed. To do this, a 20 µL aliquot of the culture medium was taken from a well where no growth (no turbidity) was observed and plated onto Sabouraud dextrose agar plates. These plates were then incubated at 37 °C for 24–48 h. The MFC value was defined as the lowest concentration at which no more than one colony grew.
4. Conclusions
The organic extract derived from the Red Sea sponge Agelas sp. aff. marmarica has demonstrated remarkable promise as a source of antimicrobial agents. This study undertook a detailed investigation aimed at identifying and characterizing the bioactive constituents of this sponge through a rigorous bioassay-guided fractionation process. Utilizing chromatographic techniques, including silica gel column chromatography, Sephadex LH-20, and HPLC, three distinct bioactive compounds were isolated, designated as marmaricines A-C (1–3). The structural characterization of these compounds was carried out by interpreting their 1D and 2D NMR and (+)-HRESIMS. These methodologies provided comprehensive insights into the molecular structures of the isolated compounds, facilitating the identification of their unique chemical features. Marmaricines A and B demonstrated the most potent antibacterial effects against methicillin-resistant S. aureus (MRSA), with inhibition zones of 14.00–15.00 mm, MIC values of 8 µg/mL, and MBC values of 16 µg/mL. Marmaracine C, while exhibiting weaker antibacterial activity, also showed efficacy against MRSA (inhibition zone: 12 mm, MIC: 16 µg/mL, MBC: 32 µg/mL). In addition, marmaricines B and C showed significant antifungal activity against C. albicans, with inhibition zones of 14–15 mm, MICs of 8 µg/mL, and MFCs of 16 µg/mL. Notably, none of the compounds demonstrated activity against E. coli. These findings suggest that marmaricines A-C possess selectivity towards MRSA and C. albicans and are promising candidates for developing new antimicrobial agents, particularly against resistant pathogens such as MRSA and C. albicans.
Future investigations should focus on the isolation of larger amounts of the natural marmaricines and the preparation of related compounds through derivatization or total synthesis to understand the mechanisms of action of this class of compounds and their related analogs, particularly their specificity towards MRSA and C. albicans. Expanding the antimicrobial spectrum of these compounds to include other resistant pathogens would be an important next step. Further pharmacological studies, including in vivo testing and toxicity assessments, are essential to evaluate the clinical potential of these compounds. Additionally, exploring the possible synergistic effects of marmaricines in combination with existing antibiotics could enhance their efficacy and provide novel therapeutic strategies for combating drug-resistant infections.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/md23020080/s1, Figures S1–S24: (+)-LRESIMS, (+)-HRESIMS, 1D (1H and 13C), and 2D (COSY, HSQC, HMBC, NOESY) NMR spectra of compounds 1–3.
Author Contributions
Conceptualization, D.T.A.Y. and L.A.S.; methodology, D.T.A.Y., A.S.A., T.A. and L.A.S.; formal analysis, D.T.A.Y., A.S.A., T.A., A.M.A. and L.A.S.; investigation, D.T.A.Y., A.S.A., A.M.A., T.A. and L.A.S.; resources, D.T.A.Y.; data curation, D.T.A.Y. and L.A.S.; writing—D.T.A.Y. and L.A.S.; writing—review and editing, D.T.A.Y.; supervision, D.T.A.Y.; project administration, D.T.A.Y.; funding acquisition, D.T.A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University under grant no. (GPIP: 1370-166-2024).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data related to this manuscript are available in the manuscript and its related Supplementary Materials.
Acknowledgments
The authors, therefore, acknowledge with thanks the DSR for providing technical and financial support. We would like to thank Rob van Soest for the identification of the sponge material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stehli, F.G.; Wells, J.W. Diversity and Age Patterns in Hermatypic Corals. Syst. Zool. 1971, 20, 115–126. [Google Scholar] [CrossRef]
- DiBattista, J.D.; Roberts, M.B.; Bouwmeester, J.; Bowen, B.W.; Coker, D.J.; Lozano-Cortés, D.F.; Choat, J.H.; Gaither, M.R.; Hobbs, J.P.A.; Khalil, M.T.; et al. A Review of Contemporary Patterns of Endemism for Shallow Water Reef Fauna in the Red Sea. J. Biogeogr. 2016, 43, 423–439. [Google Scholar] [CrossRef]
- Vaughan, G.O.; Burt, J.A. The changing dynamics of coral reef science in Arabia. Mar. Pollut. Bull. 2016, 105, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Spaet, J.L.; Thorrold, S.R.; Berumen, M.L. A Review of Elasmobranch Research in the Red Sea. J. Fish Biol. 2012, 80, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Edwards, F.J. Climate and Oceanography. In Red Sea; Edwards, A.J., Head, S., Eds.; Pergamon Press: Oxford, UK, 1987; pp. 45–68. [Google Scholar]
- Voolstra, C.R.; Miller, D.J.; Ragan, M.A.; Hoffmann, A.; Hoegh-Guldberg, O.; Bourne, D.; Ball, E.; Ying, H.; Foret, S.; Takahashi, S.; et al. The ReFuGe 2020 Consortium—Using “Omics” Approaches to Explore the Adaptability and Resilience of Coral Holobionts to Environmental Change. Front. Mar. Sci. 2015, 2, 68. [Google Scholar]
- van Soest, R.W.M.; Beglinger, E.J. Tetractinellid and Hadromerid Sponges of the Sultanate of Oman. Zool. Meded. 2008, 82, 749–790. [Google Scholar]
- Berumen, M.L.; Hoey, A.S.; Bass, W.H.; Bouwmeester, J.; Catania, D.; Cochran, J.E.; Khalil, M.T.; Miyake, S.; Mughal, M.R.; Spät, J.L.; et al. The Status of Coral Reef Ecology Research in the Red Sea. Coral Reefs 2013, 32, 737–748. [Google Scholar] [CrossRef]
- Wooster, M.K.; Voigt, O.; Erpenbeck, D.; Wörheide, G.; Berumen, M.L. Sponges of the Red Sea. In Coral Reefs of the Red Sea. Coral Reefs of the World; Voolstra, C., Berumen, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 11. [Google Scholar]
- Chu, M.-J.; Li, M.; Ma, H.; Li, P.-L.; Li, G.-Q. Secondary metabolites from marine sponges of the Genus Agelas: A Comprehensive Update Insight on Structural Diversity and Bioactivity. RSC Adv. 2022, 12, 7789–7820. [Google Scholar] [CrossRef]
- Forenza, S.; Minale, L.; Riccio, R.; Fattorusso, E. New Bromopyrrole Derivatives from the Sponge Agelas oroides. Chem. Commun. 1971, 18, 1129–1130. [Google Scholar] [CrossRef]
- Cullen, E.; Devlin, J.P. Agelasine: A Novel Quaternary 9-Methyladenine from the Sponge Agelas dispar. Can. J. Chem. 1975, 53, 1690–1691. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Mangoni, A.; Taglialatela-Scafati, O. Clathramides, Unique Bromopyrrole Alkaloids from the Caribbean Sponge Agelas clathrodes. Tetrahedron 1996, 52, 13713–13720. [Google Scholar] [CrossRef]
- Assmann, M.; Lichte, E.; van Soest, R.W.M.; Köck, M. New Bromopyrrole Alkaloid from the Marine Sponge Agelas wiedenmayeri. Org. Lett. 1999, 1, 455–457. [Google Scholar] [CrossRef]
- Kusama, T.; Tanaka, N.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Bromopyrrole Alkaloids from a Marine Sponge Agelas sp. Chem. Pharm. Bull. 2014, 62, 499–503. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Agelasines O–U, New Diterpene Alkaloids with a 9-N-Methyladenine Unit from a Marine Sponge Agelas sp. Tetrahedron 2012, 68, 9738–9744. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tang, Y.; Zhang, Q.; Bourne, G.T.; Christoph, C.A.; Leet, J.E.; Knight, J.C.; Pettit, R.K.; Chapuis, J.-C.; Doubek, D.L.; et al. Isolation and Structures of Axistatins 1–3 from the Republic of Palau Marine Sponge Agelas axifera Hentschel. J. Nat. Prod. 2013, 76, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, H.; Tsuda, M.; Kobayashi, J. Mukanadins A–C, New Bromopyrrole Alkaloids from Marine Sponge Agelas nakamurai. J. Nat. Prod. 1999, 62, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Schroif-Gregoire, C.; Appenzeller, J.; Debitus, C.; Zaparucha, A.; Al-Mourabit, A. Debromokeramadine from the Marine Sponge Agelas cf. mauritiana: Isolation and Short Regioselective and Flexible Synthesis. Tetrahedron 2015, 71, 3609–3613. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Gu, B.-B.; Yang, F.; Jiao, W.-H.; Hu, G.-H.; Yu, H.-B.; Han, B.-N.; Zhang, W.; Shen, Y.; et al. Antifungal Bromopyrrole Alkaloids from the South China Sea Sponge Agelas sp. Tetrahedron 2016, 72, 2964–2971. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, Novel Bicycle Diterpenoids with a 9-Methylladeninium Unit Possessing Inhibitory Effects on Na-K-ATPase from the Okinawan Sea Sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Capon, R.J.; Faulkner, D.J. Antimicrobial Metabolites from a Pacific Sponge, Agelas sp. J. Am. Chem. Soc. 1984, 106, 1819–1822. [Google Scholar] [CrossRef]
- Li, T.; Wang, B.; de Voogd, N.J.; Tang, X.-L.; Wang, Q.; Chu, M.-J.; Li, P.-L.; Li, G.-Q. Two New Diterpene Alkaloids from the South China Sea Sponge Agelas aff. Nemoechinata. Chin. Chem. Lett. 2016, 27, 1048–1051. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Kobayashi, J.; Kobayashi, M.; Ohizumi, Y.; Hirata, Y. Agelasidines, Novel Hypotaurocyamine Derivatives from the Okinawan Sea Sponge Agelas nakamurai Hoshinot. J. Org. Chem. 1985, 50, 2494–2497. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Tanner, R.S.; Kelly-Borges, M. Agelasines H and I, 9-Methyladenine-Containing Diterpenoids from an Agelas Sponge. J. Nat. Prod. 1998, 61, 548–550. [Google Scholar] [CrossRef]
- Vik, A.; Hedner, E.; Charnock, C.; Samuelsen, Ø.; Larsson, R.; Gundersen, L.-L.; Bohlin, L. (+)-Agelasine D: Improved Synthesis and Evaluation of Antibacterial and Cytotoxic Activities. J. Nat. Prod. 2006, 69, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Taglialatela-Scafati, O. Two Novel Pyrrole-Imidazole Alkaloids from the Mediterranean Sponge Agelas oroides. Tetrahedron Lett. 2000, 41, 9917–9922. [Google Scholar] [CrossRef]
- Appenzeller, J.; Mihci, G.; Martin, M.-T.; Gallard, J.-F.; Menou, J.-L.; Boury-Esnault, N.; Hooper, J.; Petek, S.; Chevalley, S.; Valentin, A.; et al. Agelasines J, K, and L from the Solomon Islands Marine Sponge Agelas cf. mauritiana. J. Nat. Prod. 2008, 71, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Ishibashi, M.; Shigemori, H.; Sasaki, T.; Kobayashi, J. Agelasine G, A New Antileukemic Alkaloid from the Okinawan Marine Sponge Agelas sp. Chem. Pharm. Bull. 1992, 40, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From Anti-Fouling to Biofilm Inhibition: New Cytotoxic Secondary Metabolites from Two Indonesian Agelas Sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Adachi, K.; Shizuri, Y. New Agelasine Compound from the Marine Sponge Agelas mauritiana as an Antifouling Substance Against Macroalgae. J. Nat. Prod. 1997, 60, 411–413. [Google Scholar] [CrossRef]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoh, Y.; Yamashita, J.; van Soest, R.W.M.; Fusetani, N. Ageladine A: An Antiangiogenic Matrixmetalloproteinase Inhibitor from the Marine Sponge Agelas nakamurai. J. Am. Chem. Soc. 2003, 125, 15700–15701. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A. Hemimycalins C–E; Cytotoxic and Antimicrobial Alkaloids with Hydantoin and 2-Iminoimidazolidine-4-one Backbones from the Red Sea Marine Sponge Hemimycale sp. Mar. Drugs 2021, 19, 691. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica. Mar. Drugs 2020, 18, 525. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tanaka, N.; Takahashi, S.; Tsuji, D.; Kim, S.-Y.; Kojoma, M.; Itoh, K.; Kobayashi, J.; Kashiwada, Y. Agesasines A and B, Bromopyrrole Alkaloids from Marine Sponges Agelas spp. Mar. Drugs 2020, 18, 455. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yuan, T.; Lan, P.; White, L.V.; Chen, J.; Banwell, M.G. Syntheses and Preliminary Biological Evaluations of the Dibromopyrrole-Containing Marine Natural Products Agesasine A, Agesasine B, Longamide E and Various Congeners. Eur. J. Org. Chem. 2023, 26, e202300003. [Google Scholar] [CrossRef]
- Lindel, L.; Hoffmann, H. Synthesis of rac-Midpacamide and the spiro-C yclization of Its Precursor. Liehigs Ann. 1997, 1997, 1525–1528. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Shaala, L.A.; Genta-Jouve, G. Asperopiperazines A and B: Antimicrobial and cytotoxic dipeptides from a tunicate-derived fungus Aspergillus sp. DY001. Mar. Drugs 2022, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.F. The Disc Susceptibility Test. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; Williams & Wilkins: Philadelphia, PA, USA, 1980; pp. 24–54. [Google Scholar]
- Youssef, D.T.A.; Asfour, H.Z.; Genta-Jouve, G.; Shaala, L.A. Magnificines A and B, Antimicrobial Marine Alkaloids Featuring a tetrahydrooxazolo [3,2-a]azepine-2,5(3H, 6H)-dione Backbone from the Red Sea Sponge Negombata magnifica. Mar. Drugs 2021, 19, 214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).