Structural Characterization and Anticoagulant Potential of Colochirus quadrangularis Fucosylated Glycosaminoglycan 5−12 Oligomers with Unusual Branches
Abstract
1. Introduction
2. Results and Discussion
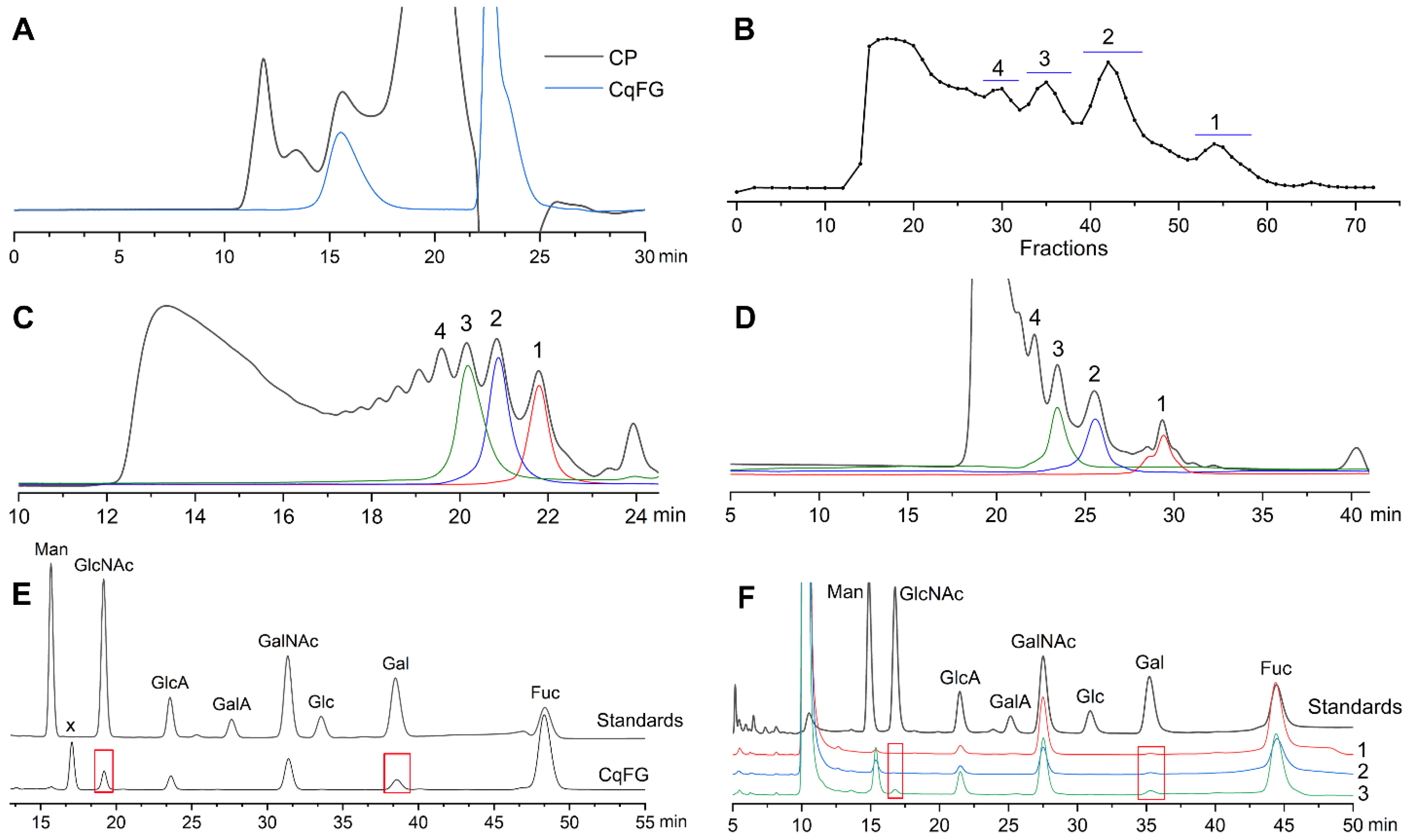
2.1. Preparation and Physicochemical Properties of CqFG, dCqFG, and Oligosaccharide Fractions
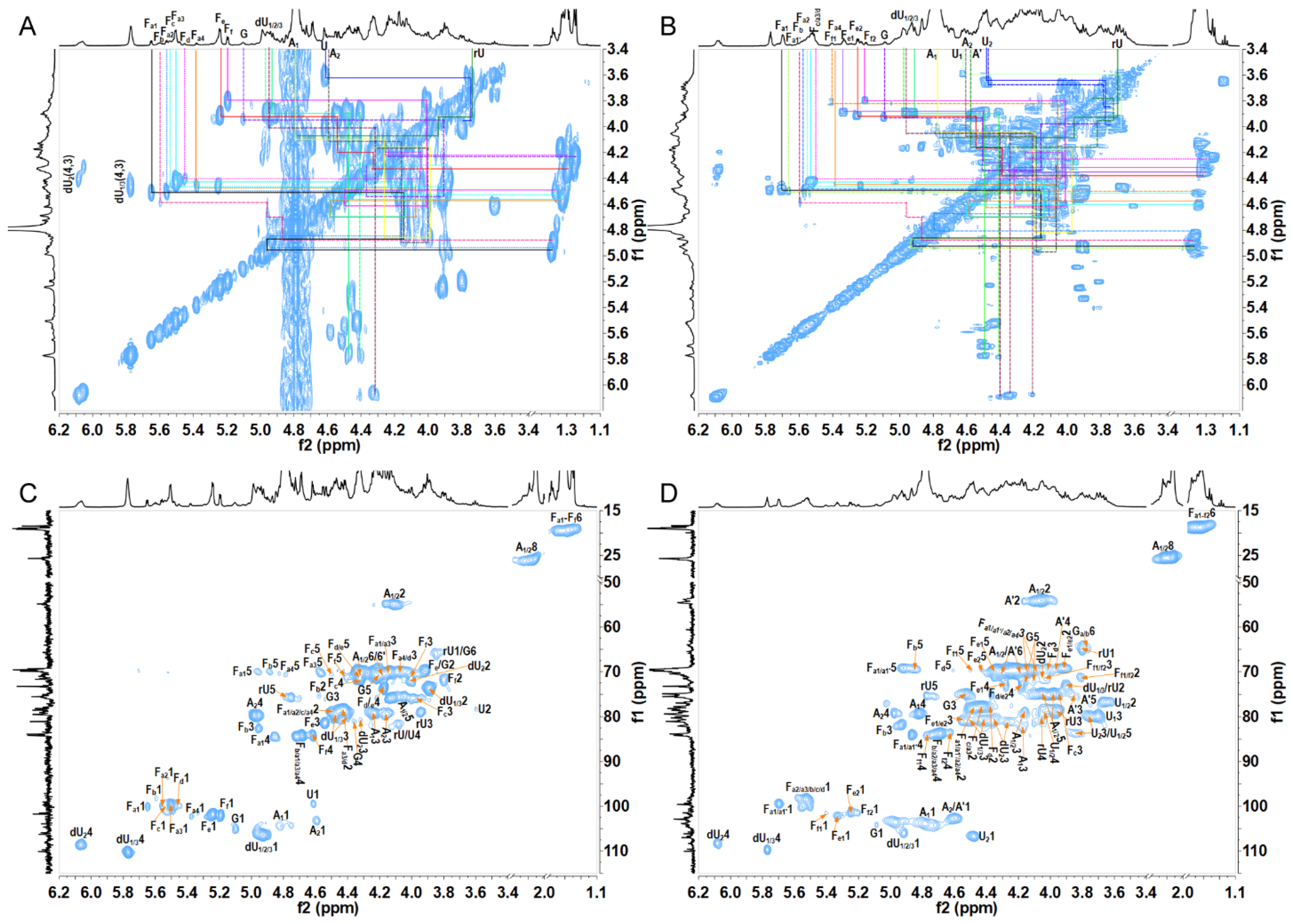
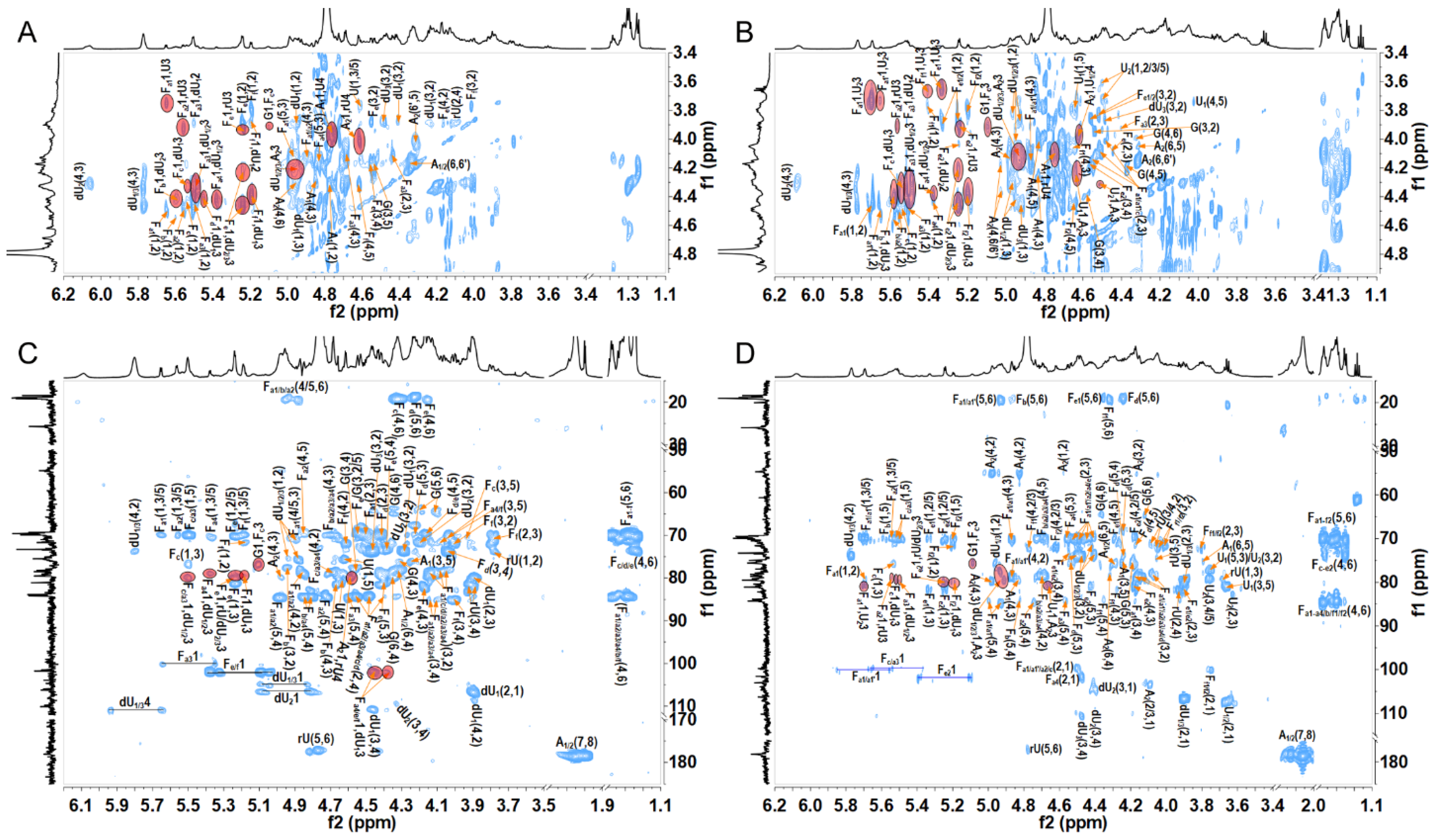
2.2. Structural Analysis of Oligosaccharide Fractions
2.3. Anticoagulant Activity of OF1–OF3 and Their Effects on Human Intrinsic Factor Tenase Complex (FXase)
2.4. Bleeding Risk of OF3
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction, Isolation, and Physicochemical Analysis of CqFG
3.3. Preparation of dCqFG and Isolation of Oligosaccharides
3.4. Nuclear Magnetic Resonance Analysis
3.5. Determination of Anticoagulant Activities
3.6. Determination of Effects of Oligosaccharide Fractions on Human Intrinsic FXase
3.7. Bleeding Tendency Determination of OF2 and OF3
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Masuko, S.; Takieddin, M.; Xu, H.; Liu, R.; Jing, J.; Mousa, S.A.; Linhardt, R.J.; Liu, J. Chemoenzymatic Synthesis of Homogeneous Ultralow Molecular Weight Heparins. Science 2011, 334, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chandarajoti, K.; Zhang, X.; Pagadala, V.; Dou, W.; Hoppensteadt, D.M.; Sparkenbaugh, E.M.; Cooley, B.; Daily, S.; Key, N.S.; et al. Synthetic Oligosaccharides Can Replace Animal-Sourced Low–Molecular Weight Heparins. Sci. Transl. Med. 2017, 9, eaan5954. [Google Scholar] [CrossRef] [PubMed]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, Synthesis, and Biomedical Applications of Synthetic Sulphated Polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, J.; Yuan, Q.; Lv, K.; Ma, H.; Li, T.; Liu, Y.; Mi, S.; Zhao, L. A Regular Chlorella Mannogalactan and Its Sulfated Derivative as a Promising Anticoagulant: Structural Characterization and Anticoagulant Activity. Carbohydr. Polym. 2023, 314, 120956. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, L.; Gao, N.; Yin, R.; Li, S.; Sun, H.; Zhou, L.; Zhao, G.; Purcell, S.W.; Zhao, J. From Multi-Target Anticoagulants to DOACs, and Intrinsic Coagulation Factor Inhibitors. Blood Rev. 2020, 39, 100615. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Q.; Lv, K.; Ma, H.; Gao, C.; Liu, Y.; Zhang, S.; Zhao, L. Low-Molecular-Weight Fucosylated Glycosaminoglycan and Its Oligosaccharides from Sea Cucumber as Novel Anticoagulants: A Review. Carbohydr. Polym. 2021, 251, 117034. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef]
- Zhao, L.; Lai, S.; Huang, R.; Wu, M.; Gao, N.; Xu, L.; Qin, H.; Peng, W.; Zhao, J. Structure and Anticoagulant Activity of Fucosylated Glycosaminoglycan Degraded by Deaminative Cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gao, N.; Li, Z.; Chen, J.; Chen, J.; Liu, J.; et al. Discovery of an Intrinsic Tenase Complex Inhibitor: Pure Nonasaccharide from Fucosylated Glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhou, L.; Gao, N.; Li, Z.; Zhao, L.; Shang, F.; Wu, M.; Zhao, J. Oligosaccharides from Depolymerized Fucosylated Glycosaminoglycan: Structures and Minimum Size for Intrinsic Factor Xase Complex Inhibition. J. Biol. Chem. 2018, 293, 14089–14099. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, H.; Gu, X.; Li, S.; Yang, S.; Zhang, L.; Mao, H.; Wang, P.; Yang, S.; Yin, R.; et al. Oligosaccharide-Assisted Resolution of Holothurian Fucosylated Chondroitin Sulfate for Fine Structure and P-Selectin Inhibition. Carbohydr. Polym. 2025, 351, 123145. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhou, L.; Gao, N.; Lin, L.; Sun, H.; Chen, D.; Cai, Y.; Zuo, Z.; Hu, K.; Huang, S.; et al. Unveiling the Disaccharide-Branched Glycosaminoglycan and Anticoagulant Potential of Its Derivatives. Biomacromolecules 2021, 22, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, W.; Pan, Y.; Lin, L.; Cai, Y.; Mao, H.; Zhang, T.; Li, S.; Chen, R.; Zhou, L.; et al. Structural Characterization and Anticoagulant Analysis of the Novel Branched Fucosylated Glycosaminoglycan from Sea Cucumber Holothuria Nobilis. Carbohydr. Polym. 2021, 269, 118290. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Cai, Y.; Li, S.; Sun, H.; Lin, L.; Pan, Y.; Yang, W.; He, Z.; Chen, R.; Zhou, L.; et al. A New Fucosylated Glycosaminoglycan Containing Disaccharide Branches from Acaudina Molpadioides: Unusual Structure and Anti-Intrinsic Tenase Activity. Carbohydr. Polym. 2020, 245, 118290. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, H.; Wang, Q.; Sun, S.; Fang, Z.; Tang, H.; Shi, X.; Wen, J.; Huang, L.; Bai, M.; et al. Deaminative-Cleaved, S. Monotuberculatus Fucosylated Glycosaminoglycan: Structural Elucidation and Anticoagulant Activity. Carbohydr. Polym. 2022, 298, 120072. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Zhang, J.; Shang, X.; Yu, L.; Xu, C.; Wang, P.; Cui, L.; Cheng, N.; Sun, H.; Ran, J.; et al. Branch Distribution Pattern and Anticoagulant Activity of a Fucosylated Chondroitin Sulfate from Phyllophorella Kohkutiensis. Carbohydr. Polym. 2023, 321, 121304. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Pan, Y.; Cai, Y.; Yang, F.; Gao, N.; Ruzemaimaiti, D.; Zhao, J. Re-Understanding of Structure and Anticoagulation: Fucosylated Chondroitin Sulfate from Sea Cucumber Ludwigothurea Grisea. Carbohydr. Polym. 2022, 294, 119826. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wen, J.; Lin, H.; Fan, S.; Sun, Y.; Yang, C.; Zhao, J.; Li, X. The Complete Mitochondrial Genome of Colochirus Quadrangularis (Dendrochirotida, Cucumariidae). Mitochondrial DNA Part. B 2020, 5, 1665–1666. [Google Scholar] [CrossRef]
- Gao, N.; Chen, R.; Mou, R.; Xiang, J.; Zhou, K.; Li, Z.; Zhao, J. Purification, Structural Characterization and Anticoagulant Activities of Four Sulfated Polysaccharides from Sea Cucumber Holothuria Fuscopunctata. Int. J. Biol. Macromol. 2020, 164, 3421–3428. [Google Scholar] [CrossRef]
- Qiu, P.; Wu, F.; Yi, L.; Chen, L.; Jin, Y.; Ding, X.; Ouyang, Y.; Yao, Y.; Jiang, Y.; Zhang, Z. Structure Characterization of a Heavily Fucosylated Chondroitin Sulfate from Sea Cucumber (H. Leucospilota) with Bottom-up Strategies. Carbohydr. Polym. 2020, 240, 116337. [Google Scholar] [CrossRef]
- Santos, G.R.C.; Porto, A.C.O.; Soares, P.A.G.; Vilanova, E.; Mourão, P.A.S. Exploring the Structure of Fucosylated Chondroitin Sulfate through Bottom-up Nuclear Magnetic Resonance and Electrospray Ionization-High-Resolution Mass Spectrometry Approaches. Glycobiology 2017, 27, 625–634. [Google Scholar] [CrossRef]
- Kariya, Y.; Watabe, S.; Hashimoto, K.; Yoshida, K. Occurrence of Chondroitin Sulfate E in Glycosaminoglycan Isolated from the Body Wall of Sea Cucumber Stichopus Japonicus. J. Biol. Chem. 1990, 265, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Peng, Y.; Zhou, L.; Zheng, W.; Liu, X.; Wang, P.; Yuan, Q.; Gao, N.; Zhao, L.; Zhao, J. Precise Structure and Anticoagulant Activity of Fucosylated Glycosaminoglycan from Apostichopus Japonicus: Analysis of Its Depolymerized Fragments. Mar. Drugs 2019, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, N.; Ren, L.; Liu, S.; Lin, L.; Zheng, W.; Zhou, L.; Yin, R.; Zhao, J. The Components and Activities Analysis of a Novel Anticoagulant Candidate DHG-5. Eur. J. Med. Chem. 2020, 207, 112796. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Anisimova, N.Y.; Dmitrenok, A.S.; Tsvetkova, E.A.; Kiselevskiy, M.V.; Nifantiev, N.E.; Usov, A.I. Depolymerization of a Fucosylated Chondroitin Sulfate from Cucumaria Japonica: Structure and Activity of the Product. Carbohydr. Polym. 2022, 281, 119072. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Ponce, N.M.A.; Stortz, C.A.; Nifantiev, N.E.; Usov, A.I. Fucosylated Chondroitin Sulfate from the Sea Cucumber Hemioedema Spectabilis: Structure and Influence on Cell Adhesion and Tubulogenesis. Carbohydr. Polym. 2020, 234, 115895. [Google Scholar] [CrossRef]
- Li, Q.; Cai, C.; Chang, Y.; Zhang, F.; Linhardt, R.J.; Xue, C.; Li, G.; Yu, G. A Novel Structural Fucosylated Chondroitin Sulfate from Holothuria Mexicana and Its Effects on Growth Factors Binding and Anticoagulation. Carbohydr. Polym. 2018, 181, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wen, D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.; Jiang, J.; Zhao, J. Anticoagulant and Antithrombotic Evaluation of Native Fucosylated Chondroitin Sulfates and Their Derivatives as Selective Inhibitors of Intrinsic Factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef]
- Wu, M.; Huang, R.; Wen, D.; Gao, N.; He, J.; Li, Z.; Zhao, J. Structure and Effect of Sulfated Fucose Branches on Anticoagulant Activity of the Fucosylated Chondroitin Sulfate from Sea Cucumber Thelenata Ananas. Carbohydr. Polym. 2012, 87, 862–868. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, N.; Sun, H.; Xiao, C.; Yang, L.; Lin, L.; Yin, R.; Li, Z.; Zhang, H.; Ji, X.; et al. Effects of Native Fucosylated Glycosaminoglycan, Its Depolymerized Derivatives on Intrinsic Factor Xase, Coagulation, Thrombosis, and Hemorrhagic Risk. Thromb. Haemost. 2020, 120, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, W.; Li, X.; Zhou, L.; Wang, Z.; Lin, L.; Chen, D.; Zhao, L.; Li, Z.; Liu, S.; et al. Precise Structures and Anti-Intrinsic Tenase Complex Activity of Three Fucosylated Glycosaminoglycans and Their Fragments. Carbohydr. Polym. 2019, 224, 115146. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Lu, F.; Xiao, C.; Yang, L.; Chen, J.; Zhou, K.; Wen, D.; Li, Z.; Wu, M.; Jiang, J.; et al. β-Eliminative Depolymerization of the Fucosylated Chondroitin Sulfate and Anticoagulant Activities of Resulting Fragments. Carbohydr. Polym. 2015, 127, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liang, R.; Lv, K.; Shi, X.; Leng, J.; Liu, Y.; Xiao, J.; Zhang, L.; Zhao, L. Structural Characterization of a Chlorella Heteropolysaccharide by Analyzing Its Depolymerized Product and Finding an Inducer of Human Dendritic Cell Maturation. Carbohydr. Polym. 2024, 333, 122000. [Google Scholar] [CrossRef]
- Xiao, C.; Zhao, L.; Gao, N.; Wu, M.; Zhao, J. Nonasaccharide Inhibits Intrinsic Factor Xase Complex by Binding to Factor IXa and Disrupting Factor IXa-Factor VIIIa Interactions. Thromb. Haemost. 2019, 119, 705–715. [Google Scholar] [CrossRef] [PubMed]
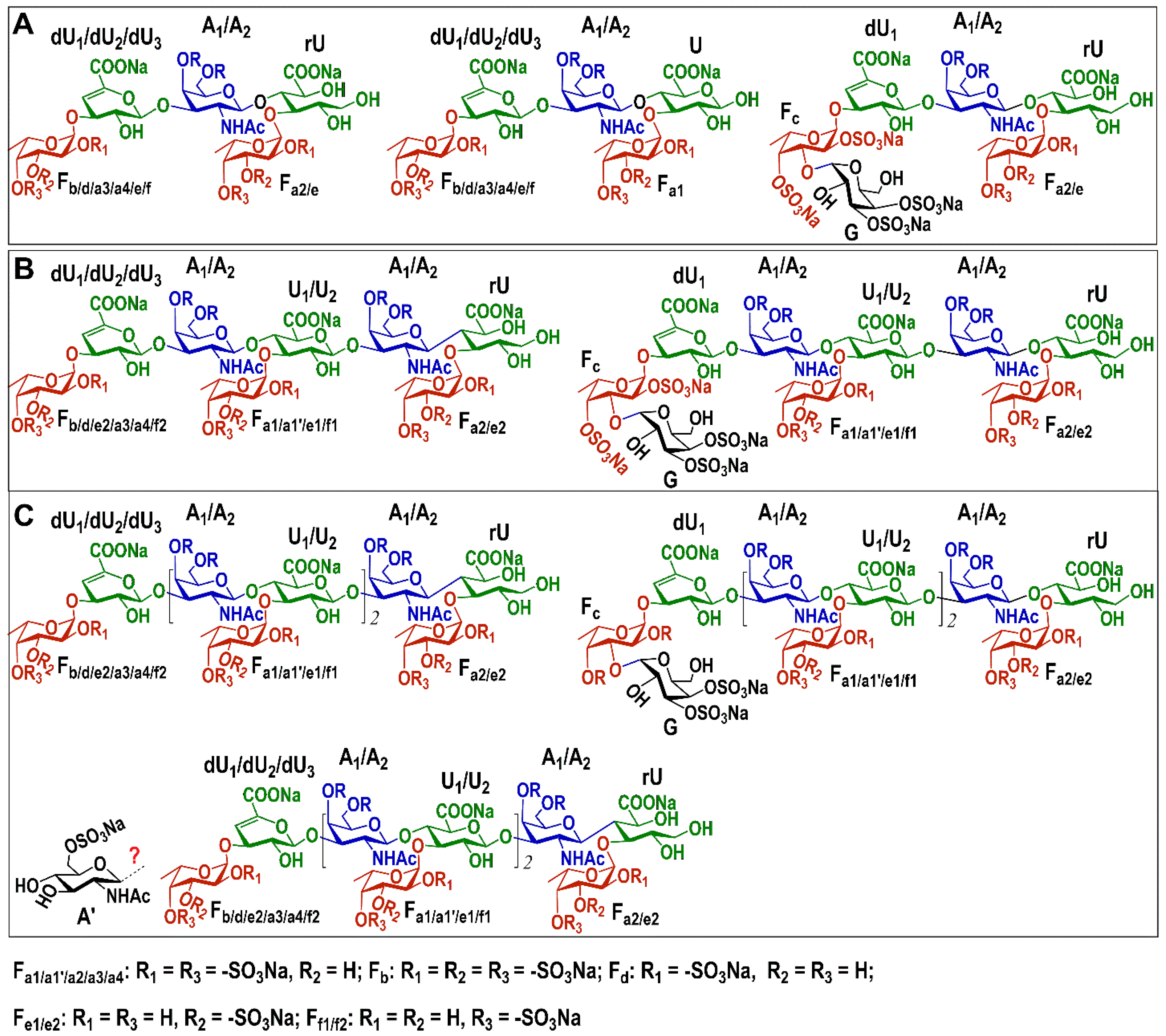

| Residues | H/C | Chemical Shifts (δ, ppm) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Connection Patterns | ||
| Fa1 | H | 5.65 | 4.51 | 4.15 | 4.88 | 4.96 | 1.38 | |||
| α-l-Fuc2S4S | C | 100.06 | 78.61 | 70.07 | 84.41 | 69.50 | 19.52 | Fa11-U3 | ||
| Fb | H | 5.60 | 4.58 | 4.96 | 4.70 | 4.89 | 1.37 | |||
| α-l-Fuc2S3S4S | C | 98.21 | 78.61 | 82.69 | 84.29 | 70.19 | 19.52 | Fb1-dU33 | ||
| Fa2 | H | 5.56 | 4.50 | 4.14 | 4.74 | 4.72 | 1.36 | |||
| α-l-Fuc2S4S | C | 100.06 | 78.61 | 70.07 | 84.41 | 69.29 | 19.52 | Fa21-rU3 | ||
| Fc | H | 5.54 | 4.49 | 3.92 | 4.31 | 4.50 | 1.32 | |||
| α-l-Fuc2S4S | C | 100.06 | 78.48 | 76.07 | 73.29 | 70.74 | 19.52 | Fc1-dU13 | ||
| Fa3 | H | 5.50 | 4.43 | 4.11 | 4.70 | 4.59 | 1.32 | |||
| α-l-Fuc2S4S | C | 100.06 | 78.48 | 70.07 | 84.41 | 70.36 | 19.52 | Fa31-dU1/23 | ||
| Fd | H | 5.45 | 4.40 | 4.09 | 4.18 | 4.23 | 1.25 | |||
| α-l-Fuc2S | C | 100.06 | 78.48 | 70.07 | 73.64 | 70.74 | 18.55 | Fd1-dU13 | ||
| Fa4 | H | 5.38 | 4.47 | 4.08 | 4.69 | 4.83 | 1.32 | |||
| α-l-Fuc2S4S | C | 102.29 | 78.73 | 70.07 | 84.16 | 70.36 | 19.52 | Fa41-dU1/23 | ||
| Fe | H | 5.24 | 3.92 | 4.56 | 4.17 | 4.38 | 1.30 | |||
| α-l-Fuc3S | C | 101.92 | 69.56 | 81.51 | 73.64 | 70.74 | 19.39 | Fe1-rU3/dU2/33 | ||
| Ff | H | 5.20 | 3.80 | 4.01 | 4.61 | 4.43 | 1.36 | |||
| α-l-Fuc4S | C | 102.29 | 71.79 | 71.04 | 84.16 | 70.49 | 19.52 | Ff1-dU13 | ||
| G | H | 5.10 | 3.93 | 4.54 | 4.36 | 4.14 | 3.80/3.98 | |||
| α-d-Gal3S4S | C | 105.07 | 69.81 | 75.98 | 80.96 | 70.83 | 63.84 | G1-Fc3 | ||
| dU1 | H | 4.95 | 3.89 | 4.34/4.42 | 5.78 | |||||
| α-l-Δ4,5GlcA | C | 106.12 | 73.64 | 79.66 | 110.33 | 149.76 | 172.11 | dU11-A23 | ||
| dU2 | H | 4.94 | 4.01 | 4.20/4.33/4.42 | 6.06 | |||||
| α-l-Δ4,5GlcA | C | 104.40 | 71.87 | 80.88 | 108.56 | 149.76 | 172.11 | dU21-A13 | ||
| dU3 | H | 4.93 | 3.91 | 4.48 | 5.77 | |||||
| α-l-Δ4,5GlcA | C | 106.50 | 73.64 | 79.66 | 110.33 | 149.76 | 172.11 | dU31-A23 | ||
| A1 | H | 4.79 | 4.10 | 4.27 | 4.85 | 4.01 | 4.17/4.31 | 2.06 | ||
| β-d-GalNAc4S6S | C | 104.52 | 54.41 | 79.41 | 79.62 | 75.75 | 70.78 | 177.98 | 25.96 | A11-U4 |
| U | 4.62 | 3.61 | 3.75 | 4.07 | 3.77 | |||||
| β-d-GlcA | 99.52 | 77.98 | 80.71 | 82.01 | 75.64 | 179.21 | ||||
| A2 | H | 4.59 | 4.09 | 4.17 | 4.98 | 4.09 | 4.17/4.31 | 2.06 | ||
| β-d-GalNAc4S6S | C | 103.22 | 54.83 | 80.84 | 79.66 | 75.62 | 70.78 | 177.98 | 25.96 | A21-rU4 |
| rU | H | 3.78/3.84 | 3.91 | 3.95 | 4.08 | 4.78 | ||||
| β-d-GlcA-ol | C | 65.68 | 72.68 | 79.11 | 81.72 | 75.96 | 177.21 | |||
| Sample | APTT a (μg/mL) | TT a (μg/mL) | PT a (μg/mL) | Anti-FXase b (ng/mL) |
|---|---|---|---|---|
| HP | 0.52 ± 0.18 | 0.22 ± 0.02 | 5.11 ± 0.10 | 18.58 ± 1.44 |
| LMWH | 3.28 ± 0.12 | 1.09 ± 0.10 | >128 | 78.76 ± 10.70 |
| CqFG | 2.24 ± 0.07 | 1.81 ± 0.04 | 40.73 ± 0.83 | 45.95 ± 7.02 |
| dCqFG | 3.55 ± 0.08 | 49.90 ± 1.11 | >128 | 51.22 ± 5.68 |
| OF1 | 34.95 ± 2.22 | >128 | >128 | >2000 |
| OF2 | 4.88 ± 0.29 | 100.76 ± 0.89 | >128 | 274.61 ± 25.30 |
| OF3 | 3.02 ± 0.61 | 45.02 ± 4.50 | >128 | 136.43 ± 18.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yan, G.; Liu, X.; Fu, J.; Shi, X.; Cao, P.; Sun, Y.; Zhong, S.; Nong, J.; Jiang, P.; et al. Structural Characterization and Anticoagulant Potential of Colochirus quadrangularis Fucosylated Glycosaminoglycan 5−12 Oligomers with Unusual Branches. Mar. Drugs 2025, 23, 64. https://doi.org/10.3390/md23020064
Zhang X, Yan G, Liu X, Fu J, Shi X, Cao P, Sun Y, Zhong S, Nong J, Jiang P, et al. Structural Characterization and Anticoagulant Potential of Colochirus quadrangularis Fucosylated Glycosaminoglycan 5−12 Oligomers with Unusual Branches. Marine Drugs. 2025; 23(2):64. https://doi.org/10.3390/md23020064
Chicago/Turabian StyleZhang, Xuedong, Guangwei Yan, Xinming Liu, Jiewen Fu, Xiang Shi, Pei Cao, Yuqian Sun, Shengping Zhong, Jiale Nong, Peiqi Jiang, and et al. 2025. "Structural Characterization and Anticoagulant Potential of Colochirus quadrangularis Fucosylated Glycosaminoglycan 5−12 Oligomers with Unusual Branches" Marine Drugs 23, no. 2: 64. https://doi.org/10.3390/md23020064
APA StyleZhang, X., Yan, G., Liu, X., Fu, J., Shi, X., Cao, P., Sun, Y., Zhong, S., Nong, J., Jiang, P., Liu, Y., Zhang, B., Yuan, Q., & Zhao, L. (2025). Structural Characterization and Anticoagulant Potential of Colochirus quadrangularis Fucosylated Glycosaminoglycan 5−12 Oligomers with Unusual Branches. Marine Drugs, 23(2), 64. https://doi.org/10.3390/md23020064








