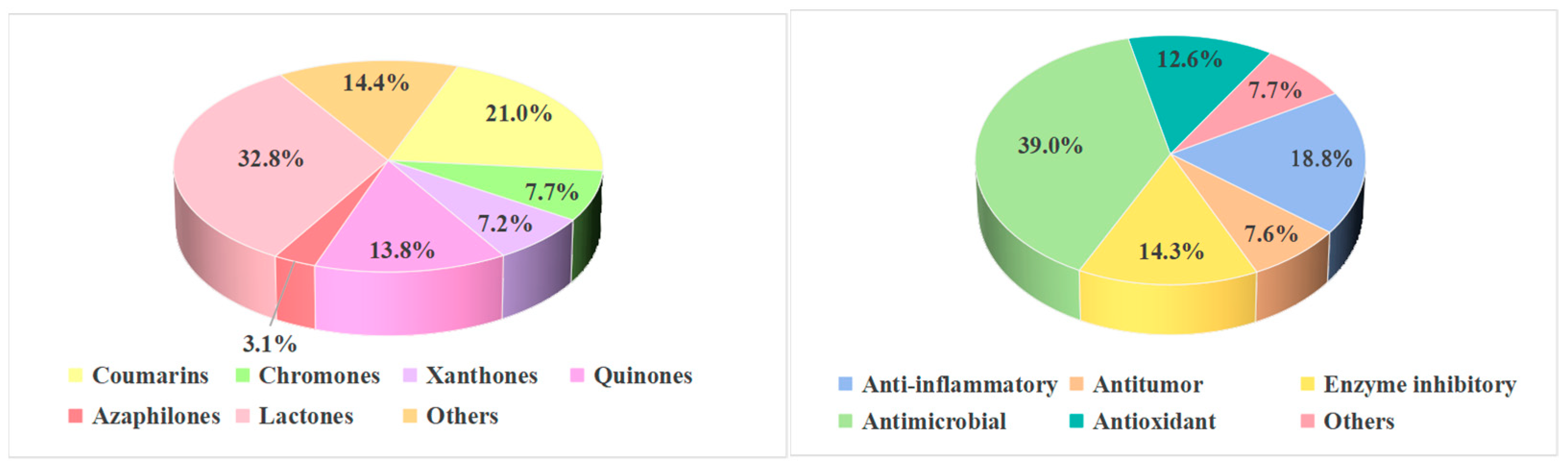
2.1. Coumarins and Isocoumarins
Coumarins and isocoumarins represent a significant class of bioactive compounds widely distributed in nature. A total of 96 coumarin and isocoumarin compounds have been isolated from mangrove-derived fungi, with their structures illustrated in
Figure 1. Among these, 37 compounds exhibit biological activities such as anti-inflammatory, antimicrobial, and antioxidant effects.
Two compounds, 3-methyl-6,8-dihydroxyisocoumarin (
1) and 6,8-dihydroxy-5-methoxy-3-methyl-1
H-isochromen-1-one (
2), were isolated from the mangrove fungus
Penicillium sp. SCSIO 41411. Activity screening revealed that compounds
1 and
2 exhibited weak inhibitory activity against PDE4, with inhibition rates of 27.42% and 27.39%, respectively, at a concentration of 10 µM [
8]. Five compounds, fusaraisochromenone (
3), 3
R-3,4-dihydro-6,8-dihydroxy-3-methylisocoumarin (
4), 2-acetyl-7-methoxybenzofuran (
5), 4,8-dimethoxy-1
H-isochromen-1-one (
6), and (+)-citreoisocoumarin (
7), were isolated from the mangrove endophytic fungus
Daldinia eschscholzii MCZ-18. Compounds
3 and
5 exhibited broad-spectrum inhibitory activity against five pathogenic strains,
Enterococcus faecalis, methicillin-resistant
Staphylococcus aureus (MRSA),
Escherichia coli,
Pseudomonas aeruginosa, and
Candida albicans, with IC
50 values ranging from 6.25 to 50 µM. Compound
7 demonstrated significant inhibitory activity against MRSA with an IC
50 value of 6.25 µM. Preliminary structure-activity relationship studies suggested that the oxygen-containing heterocyclic structure may enhance the compound’s effect against
P. aeruginosa,
E. faecalis, MRSA, and
E. coli. Furthermore, the position of -OH and -OCH
3 substitutions on the benzene ring or lactone moiety of the isocoumarin backbone appears to confer selectivity towards different pathogenic bacteria [
9]. Seven compounds, 8-hydroxy-6-methoxy-3-methyl-1
H-isochromen-1-one (
8), (
S)-8-hydroxy-3-(2-hydroxypropyl)-6-methoxy-1
H-isochromen-1-one (
9), (3
S,4
R)-4,8-dihydroxy-6-methoxy-3,4,5-trimethylisochroman-1-one (
10), (
S)-8-hydroxy-6-methoxy-4,5-dimethyl-3-methyleneisochroman-1-one (
11), (
S)-6,8-dihydroxy-3-(2-hydroxypropyl)-1
H-isochromen-1-one (
12), 6,8-dihydroxy-3-methyl-1
H-isochromen-1-one (
13), and 4,8-dihydroxy-6-methoxy-4,5-dimethyl-3-methyleneisochroman-1-one (
14), were isolated from the mangrove sediment-derived fungus
Roussoella sp. SCSIO 41427 [
10]. Two compounds, 4,6-dihydroxymellein (
15) and similanpyrone B (
16), were isolated from the mangrove-derived fungus
Talaromyces sp. WHUF0362 [
11]. One compound, (-)-mellein-5-carboxylic acid (
17), was isolated from the mangrove-derived fungus TBRC-BCC 64093 [
12]. One compound, tenuissimasatin (
18), was isolated from the mangrove-derived fungus
Mollisia sp. SCSIO41409 [
13]. In another study, Cai et al. isolated two compounds, (3
R,4
R)-cis-4-hydroxy-5-methylmellein (
19) and 3
S,4
R-4-hydroxy-mellein (
20), from the mangrove-derived fungus
Phomopsis sp. HYP11. Compounds
19 and
20 demonstrated significant antioxidant activity, with IC
50 values of 0.09 mM and 0.17 mM, respectively, which were stronger than that of the positive control Trolox (IC
50 = 0.29 mM) [
14].
One compound, 7-chloro-6-methoxymellein (
21), was isolated from the mangrove endophytic fungus
Aspergillus sp. GXNU-A9 [
15]. One new natural product, 7-chloro-3,4-dihydro-6,8-dihydroxy-3-methylisocoumarine (
22), along with seven known compounds, (
S)-5,7-dichloro-6-methoxy-2-methyl-2,3-dihydrobenzofuran-4-carboxylic acid (
23), pericochlorosin A (
24), palmaerones F-G (
25–
26), 5-chloro-6-hydroxymellein (
27), (
R)-6-hydroxymellein (
28), and 3-methyl-6-hydroxy-8-methoxy-3,4-dihydroisocoumarin (
29), were isolated from the mangrove endophytic fungus
Amorosia sp. SCSIO 41026. At non-toxic concentrations, compounds
22,
23,
27, and
29 inhibited the production of nitric oxide and pro-inflammatory cytokines in lipopolysaccharide (LPS)-induced RAW264.7 macrophages. Specifically, these compounds suppressed both the mRNA expression and release of the pro-inflammatory cytokines IL-6 and TNF-
α. Further in vivo studies demonstrated that compound
27 alleviated pathological lung injury in LPS-treated mice and protected RAW264.7 macrophages from LPS-induced inflammatory responses by inhibiting the PI3K/AKT pathway [
16].
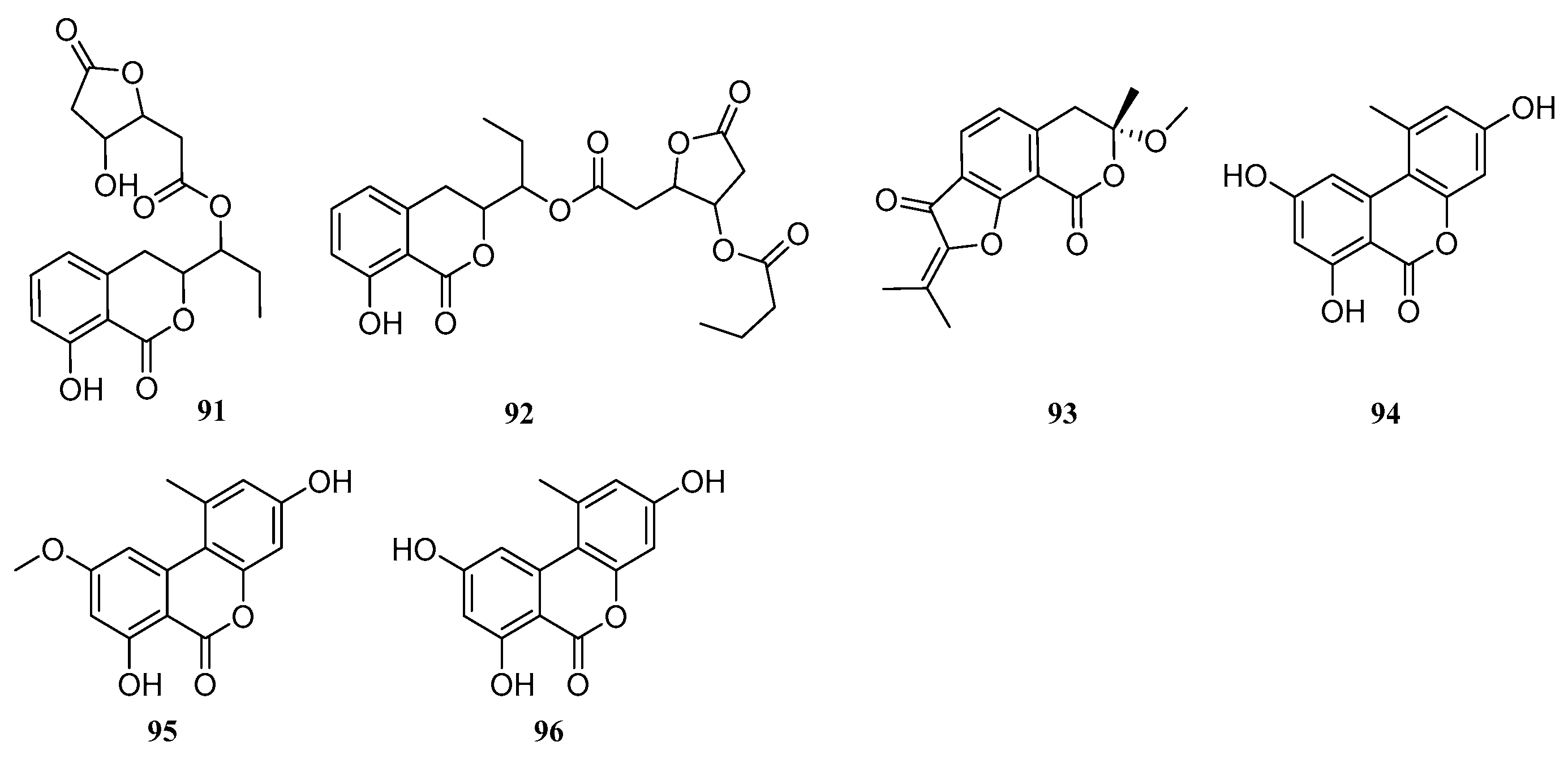
One compound, 6,8-dihydroxy-5-methoxy-3-methyl-1
H-isochromen-1-one (
30), was isolated from the mangrove endophytic fungus
Phyllosticta capitalensis. Compound
30 exhibited weak inhibitory activity against
Pseudomonas aeruginosa and
Staphylococcus aureus, with a MIC value of 225 μM [
17]. Four compounds, (-)-trans-axial-4-hydroxymellein (
31), (-)-cis-equatorial-4-hydroxymellein (
32), 4,8-dihydroxy-3-methylisochroman-1-one (5-hydroxymellein) (
33), and mellein (
34), were isolated from the mangrove fungus
Lasiodiplodia theobromae. Compounds
31–
34 showed significant inhibitory activity against
Trypanosoma brucei, with IC
50 values ranging from 1.20 to 4.10 μM [
18].
Xu et al. isolated one compound, 6,8-dihydroxy-5-methoxy-3-methyl-1
H-isochromen-1-one (
35), from the mangrove endophytic fungus
Aspergillus fumigatus HQD24 [
19]. In another study, Xu et al. isolated one new compound, pestalotiopisorin B (
36), and one known compound, (
R)-(-)-mellein methyl ether (
37), from the mangrove-derived fungus
Pestalotiopsis sp. HHL101. Compound
36 exhibited antibacterial activity against
Escherichia coli and
Pseudomonas aeruginosa, with IC
50 values of 56.31 and 225.23 μM, respectively [
20]. Three compounds, aspergillumarin C (
38), (3
R)-(7,8-dihydroxy-1-oxoisochroman-3-yl)propanoic acid (
39), and aspergillumarin B (
40), were isolated from the mangrove endophytic fungus
Talaromyces sp. SCNU-F0041 [
21]. One compound, dichlorodiaportin (
41), was isolated from the mangrove sediment-derived fungus
Trichoderma harzianum SCSIO 41051 [
22].
One new compound, cladosporin E (
42), along with two known compounds, cladosporin C (
43) and decarboxydihydrocitrinone (
44), were isolated from the mangrove sediment-derived fungus
Talaromyces sp. SCSIO 41428. Compound
43 exhibited significant inhibitory activity against prostate cancer cells PC-3 and 22Rv1, with IC
50 values of 6.10 and 9.25 µM, respectively [
23]. A new compound, penicimarin N (
45), was isolated from the mangrove endophytic fungus
Penicillium sp. TGM112. Compound
45 demonstrated strong antioxidant activity with an IC
50 value of 1.0 µM, and also showed moderate inhibitory activity against α-glucosidase with an IC
50 value of 620 µM [
24]. Two new compounds, penicimarins L-M (
46–
47), and seven known compounds, peniciisocoumarin E (
48), aspergillumarin A (
49), penicimarin I (
50), peniciisocoumarin F (
51), penicilloxalone B (
52), penicimarin G (
53), and penicimarin H (
54), were isolated from the mangrove endophytic fungus
Penicillium sp. MGP11. All compounds, except
50 and
51, exhibited antioxidant activity, with IC
50 values ranging from 4.6 to 40.5 µM. The activity of compound
53 (IC
50 = 4.6 µM) was stronger than that of the positive control Trolox (IC
50 = 12.9 µM). Compounds
50,
53, and
54 showed
α-glucosidase inhibitory activity, with IC
50 values of 776.5, 683.7, and 868.7 µM, respectively, compared to the positive control acarbose (IC
50 = 313.9 µM) [
25]. Two new compounds, penicillol A (
55) and penicillol B (
56), along with two known compounds, dichlorodiaportal (
57) and citreoviranol (
58), were isolated from the mangrove endophytic fungus
Penicillium sp. BJR-P2. Compound
56 inhibited NO production in LPS-induced RAW264.7 cells with an IC
50 value of 12.0 µM, which was stronger than the positive control indomethacin (IC
50 = 35.8 µM). Molecular docking studies were conducted to further investigate the mechanism by which compound
56 inhibits NO production. The results indicated that compound
56 interacts with the active site of inducible nitric oxide synthase (iNOS) by forming multiple characteristic hydrogen bonds. In contrast, the carbonyl group at position 4′ in compound
55 differs from the hydroxyl group in
56, resulting in a distinct conformation for
55 that prevents the formation of hydrogen bonds with key amino acid residues in the iNOS active region, thereby explaining its lack of inhibitory activity [
26]. Four new compounds, hypoxymarins A-D (
59–
62), and six known compounds, penicimarin (
63), aspergillumarin A (
64), aspergillumarin B (
65), 5-hydroxysescandelin (
66), sescandelin A (
67), and sescandelin B (
68), were isolated from the mangrove endophytic fungus
Hypoxylon sp. Compounds
61 and
65 exhibited DPPH radical scavenging activity, with IC
50 values of 15.36 and 3.69 µM, respectively [
27]. A new compound, 8-hydroxy-3-hydroxymethyl-6-methoxy-7-methylisocoumarin (
69), was isolated from the mangrove endophytic fungus
Botryosphaeria ramosa. Compound
69 exhibited inhibitory activity against
Fusarium oxysporum,
Fusarium graminearum,
Penicillium italicum, and
Colletotrichum musae, with IC
50 values ranging from 52.97 to 847.46 µM. Its activity against some pathogens was stronger than that of the positive control triadimefon [
28]. One compound, peniisocoumarin H (
70), was isolated from the mangrove-derived fungus
Trichoderma harzianum D13 [
29]. A new compound, cytospomarin (
71), was isolated from the mangrove-derived fungus
Cytospora sp. Compound
71 exhibited weak inhibitory activity against
Escherichia coli GIM1.201 and
Magnaporthe oryzae, with MIC values of 0.35 and 1.41 mM, respectively [
30].
Five new compounds, setosphamarins A-E (
72–
76), and three known compounds, 4,8-dihydroxy-3-((
R)-2-hydroxypentyl)-6,7-dimethoxyisochroman-1-one (
77), (3
R,4
R)-4,8-dihydroxy-3-(2-hydroxypentyl)-6,7-dimethoxyisochroman-1-one (
78), and (3
R,4
R)-4,6,8-trihydroxy-3-((
R)-2-hydroxypentyl)-7-methoxyisochroman-1-one (
79), were isolated from the mangrove-derived fungus
Setosphaeria rostrate [
31]. Three new compounds, phomochromenones D-F (
80–
82), and two known compounds, diaporchromanone C (
83) and diaporchromanone D
(84), were isolated from the mangrove sediment-derived fungus
Phomopsis asparagi DHS-48 [
32]. A new compound, incarxanthone E (
85), was isolated from the mangrove endophytic fungus
Peniophora incarnate Z4 [
33]. Three new compounds, spiromastol M (
86), (
P, 9′
R) spiromastol N (
87), (
M, 9′
R) spiromastol N (
88), and one known compound, palmaerin A (
89), were isolated from the mangrove-derived fungus
Spiromastix sp. SCSIO F190. Notably, compounds
87 and
88 were identified as a mixture of isomers. Compounds
86–
88 exhibited significant antibacterial activity against methicillin-resistant
Staphylococcus aureus (MRSA),
Enterococcus faecalis,
Micrococcus luteus,
Staphylococcus simulans,
Enterococcus faecium ATCC 29212,
Bacillus subtilis, and
Enterococcus gallinarum BS01. The MIC values for compound
86 ranging from 17.35 to 69.41 μM, while those for compounds
87–
88 the MIC values ranging from 3.92 to 62.75 μM [
34].
A new compound xylariachromanone A (
90), was isolated from the mangrove endophytic fungus
Xylaria arbuscula QYF [
35]. Two compounds, 1-(8-hydroxy-1-oxoisochroman-3-yl)propyl 4′-(6′-hydroxy-8′-oxotetrahydrofuran-5′-yl)acetate (
91) and 6′
α-(3′-(1′-(8-hydroxy-1-oxoisochroman-3-yl)propoxy)-3′-oxoethyl)-8′-oxotetrahydro-furan-6′-yl butyrate (
92), were isolated from the mangrove endophytic fungus
Bacillus amyloliquefaciens. Compounds
91 and
92 demonstrated anti-inflammatory activity, as determined by a 5-LOX inhibition assay, with IC
50 values of 1.23 and 1.11 mM, respectively [
36]. A new compound asperisocoumarin G (
93), was isolated from the mangrove endophytic fungus
Aspergillus sp. 085242. Compound
93 exhibited α-glucosidase inhibitory activity with an IC
50 value of 392.4 μM, which was superior to that of the positive control acarbose (IC
50 = 725.1 μM) [
37]. Two compounds, alternariol (
94) and alternariol 4-methyl ether (
95), were isolated from the mangrove endophytic fungus
Alternaria sp. R6 [
38]. One compound, alternariol (
96), was isolated from the mangrove rhizosphere sediment-derived fungus
Arthrinium sp. SCSIO 41305 [
39].
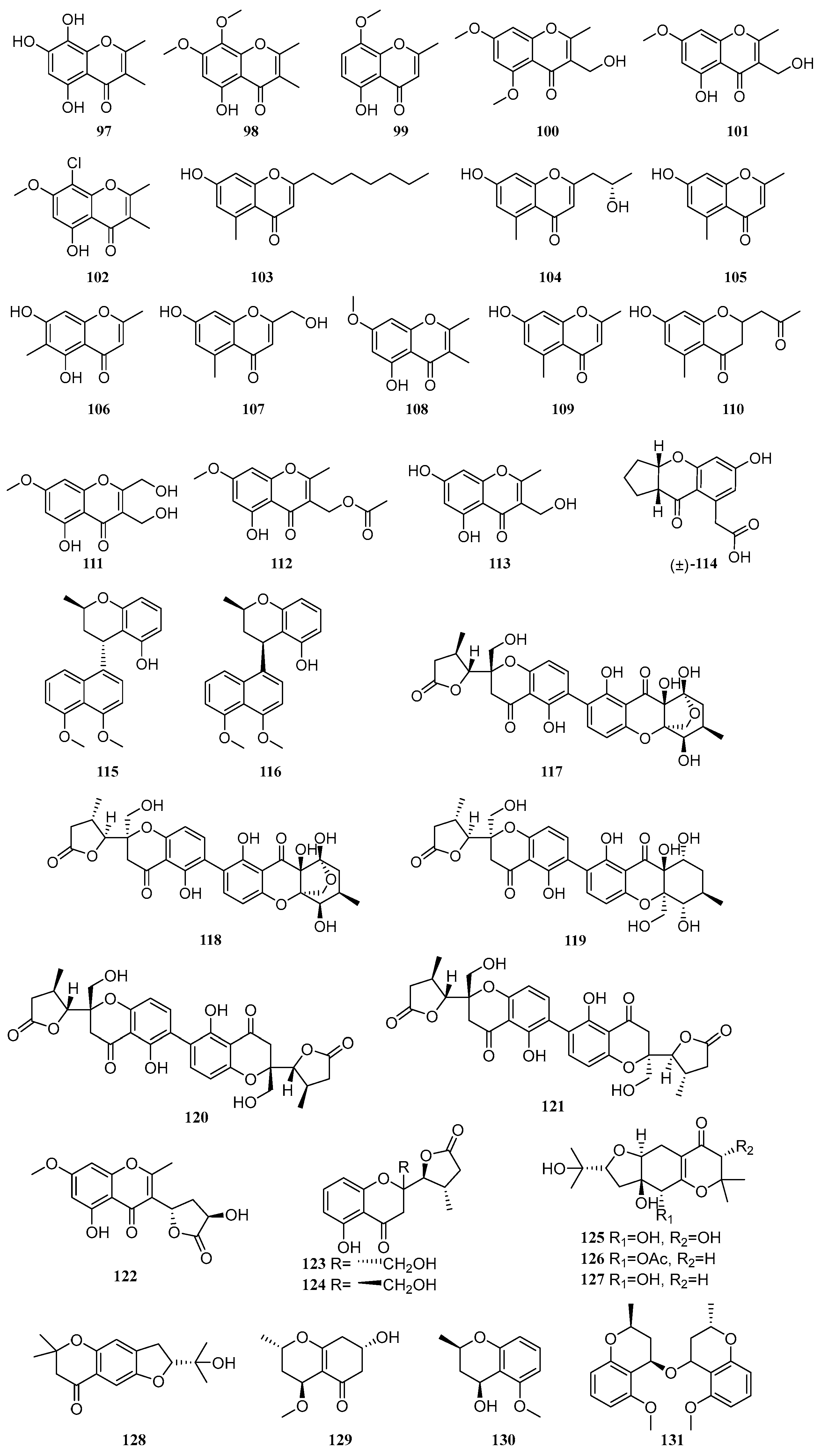
2.2. Chromones
Chromones are a class of natural products with benzo-γ-pyrone as the core scaffold, which are widely distributed in plants and microorganisms. From 2020 to 2025, a total of 35 chromone compounds were isolated and identified from mangrove-derived fungi. Their structures are shown in (
Figure 2), and 14 of these compounds exhibit biological activities such as antibacterial, antioxidant, and enzyme inhibitory effects.
Liu et al. employed the OSMAC strategy to isolate one new chromone, talamin E (
97), and one known compound, talamin B (
98), from the mangrove-derived fungus
Penicillium sp. HDN15-312. Compound
97 exhibited good DPPH free radical scavenging activity with an IC
50 value of 6.79 μM, which was more potent than that of the positive control, vitamin C [
40]. One known compound, 5-hydroxy-8-methoxy-2-methyl-4
H-1-benzopyran-4-one (
99), were isolated from the mangrove endophytic fungus
Daldinia eschscholzii MCZ-18 [
9]. A new compound 3-(hydroxymethyl)-5,7-dimethoxy-2-methyl-4
H-chromen-4-one (
100), along with a known compound, 5-hydroxy-3-(hydroxymethyl)-7-methoxy-2-methyl-4
H-chromen-4-one (
101), were isolated from the mangrove-derived fungus
Trichoderma lentiforme ML-P8-2. The IC
50 values of compounds
100 and
101 against acetylcholinesterase (AChE) were 33.7 µM and 20.6 µM, respectively. Additionally, compound
101 exhibited moderate inhibitory activity against
Candida albicans, with an MIC value of 25 µM [
41]. A new compound 8-chloro-5-hydroxy-2,3-dimethyl-7-methoxychromone (
102), was isolated from the mangrove-derived fungus
Mollisia sp. SCSIO41409 [
13]. A known compound, phomotone F (
103), was isolated from the mangrove-derived fungus
Phomopsis sp. QYM-13. Compound
103 demonstrated significant anti-inflammatory activity, with an IC
50 value of 25.0 µM, which was stronger than that of the positive control L-NMMA (IC
50 = 32.8 µM) [
42]. A compound 2-(2′-hydroxypropyl)-5-methyl-7-hydroxychromone (
104), was isolated from the co-culture fermentation products of two mangrove endophytic fungi,
Phomopsis asparagi DHS-48 and
Phomopsis sp. DHS-11. This compound showed weak inhibitory activity on ConA (T cell)- and LPS (B cell)-induced proliferation of mouse splenic lymphocytes, with IC
50 values of 111.01 and 123.84 µM, respectively [
43]. A known compound, 7-hydroxy-2,5-dimethylchromone (
105), was isolated from the mangrove endophytic fungus
Epicoccum sorghinum. Compound
105 significantly inhibited the growth of
Fusarium graminearum and
Fusarium oxysporum, both with an MIC value of 526.32 μM [
44]. A known compound, eugenitol (
106), was isolated from the mangrove endophytic fungus
Aspergillus sp. SCSIO41407. Compound
106 exhibited weak inhibitory activity against methicillin-resistant
Staphylococcus aureus (MRSA), with an MIC value of 485.4 µM [
45].
A compound, 7-hydroxy-2-(hydroxymethyl)-5-methyl-4
H-chromen-4-one (
107), was isolated from the mangrove-derived fungus
Penicillium janthinellum [
46]. A compound 5-hydroxy-2,3-dimethyl-7-methoxychromone (
108), was isolated from the mangrove sediment-derived fungus
Trichoderma harzianum SCSIO 41051 [
22]. Hu et al. isolated two compounds, altechromone A (
109) and aloesone (
110), from the mangrove soil-derived fungus
Arthrinium sp. SCSIO 41305 [
39]. In another study, Hu et al. identified three new compounds, 5-hydroxy-2,3-dihydroxymethyl-7-methoxychromone (
111), 5-hydroxy-3-acetoxymethyl-2-methyl-7-methoxychromone (
112), and 5,7-dihydroxy-3-hydroxymethyl-2-methylchromone (
113), from the mangrove endophytic fungus
Botryosphaeria ramose. Compounds
111–
113 exhibited antimicrobial activities against
Fusarium oxysporum,
Fusarium graminearum,
Penicillium italicum, and
Colletotrichum musae, with IC
50 values ranging from 24.8 to 793.65 µM. Some of the compounds showed stronger activity than the positive control triadimefon [
28]. A new compound, curvulanone (
114), featuring a rare 3-acetylchromone scaffold, was isolated from the mangrove endophytic fungus
Curvularia aeria. The structure of
114 was unequivocally determined by X-ray single-crystal diffraction. Biological evaluation revealed that compound
114 inhibited monoamine oxidase B (MAO-B) with an IC
50 of 55.8 µM, while exhibiting weaker inhibition against MAO-A (IC
50 = 117.9 µM) and sirtuin 1 (SIRT1, IC
50 = 107.9 µM). A putative biosynthetic pathway for
114 was also proposed [
47]. Two new compounds, cladonaphchroms A (
115) and B (
116), were isolated from the mangrove endophytic fungus
Cladosporium sp. JJM22. Compound
115 displayed significant antibacterial activity against
Staphylococcus albus ATCC 8799 with an MIC of 3.57 μM, and also inhibited
Escherichia coli ATCC 25922,
Bacillus subtilis ATCC 6633,
Micrococcus tetragenus ATCC 13623, and
Micrococcus luteus ATCC 9341, with MIC values ranging from 7.14 to 28.57 μM. Additionally, compounds
115 and
116 showed antifungal activities against
Alternaria brassicicola,
Phytophthora parasitica var.
nicotianae,
Colletotrichum capsici,
Bipolaris oryzae,
Diaporthe medusaea, and
Ceratocystis paradoxa, with MIC values between 71.43 and 285.71 μM [
48].
Guided by metabolomics, three new compounds, phomoxanthones L-N (
117–
119), along with two known compounds, phomopsis-H76A (
120) and diaporthochromone B (
121), were isolated from the co-culture fermentation products of two mangrove endophytic fungi,
Phomopsis asparagi DHS-48 and
Phomopsis sp. DHS-11 [
49]. A new compound 5-hydroxy-3-((3′
R,5′
S)-3′-hydroxy-2′-oxotetrahydrofuran-5′-yl)-7-methoxy-2-methyl-4
H-chromen-4-one (
122), was isolated from the mangrove-derived fungus
Trichoderma lentiforme ML-P8-2. Compound
122 exhibited moderate inhibitory activity against acetylcholinesterase (AChE) with an IC
50 value of 38.6 µM, as well as moderate anti-fungal activity against
Candida albicans, showing an MIC value of 50 µM [
41]. Two known compounds, mycochromone A (
123) and mycochromone B (
124), were isolated from the mangrove endophytic fungus
Mycosphaerella sp. L3A1. The absolute configurations of compounds
123 and
124 were determined using X-ray single-crystal diffraction with CuKα radiation and electronic circular dichroism (ECD) calculations [
50]. Two new compounds, pestalotheols P-Q (
125–
126), and two known compounds, pestalotheol A (
127) and pestalotheol D (
128), were isolated from the mangrove endophytic fungus
Pseudopestalotiopsis theae [
51]. A new chromone derivative, xylariaone A (
129), was isolated from the mangrove endophytic fungus
Xylaria arbuscula QYF. Its absolute configuration was established via Mosher’s ester method [
35]. Two new compounds, (2
R,4
S)-5-methoxy-2-methyl-2
H-1-benzopyran-4-ol (
130) and (2
S,2′
S,4
R,4′
R)-bis(5-methoxy-2-methyl-2
H-1-benzopyran)-4-ether (
131), were isolated from the mangrove endophytic fungus
Penicillium citrinum QJF-22 [
52].
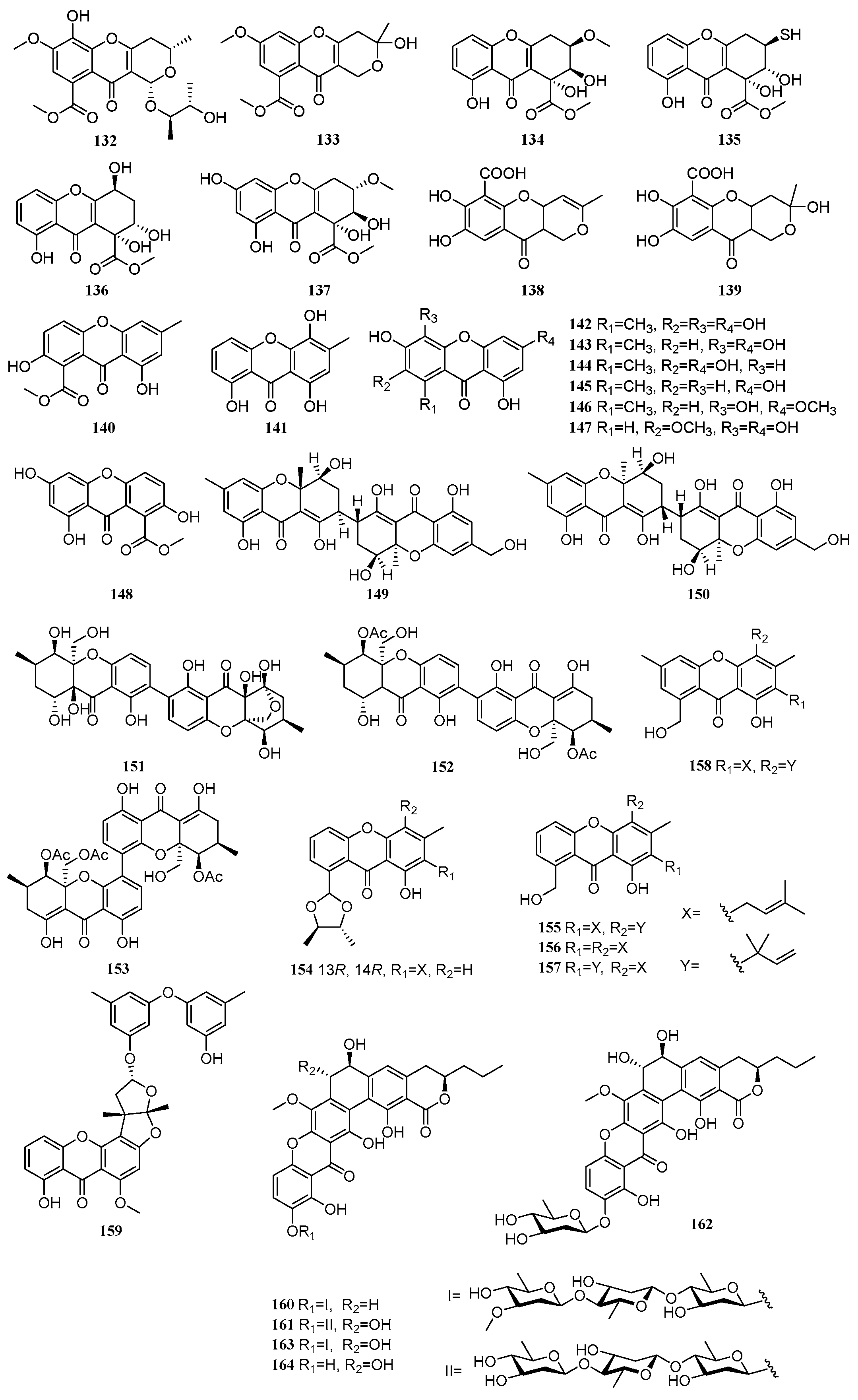
2.3. Xanthones
Between 2020 and 2025, a total of 33 xanthone derivatives were isolated and characterized from mangrove-derived fungi. Their structures are shown in
Figure 3. Among these, 25 compounds exhibited various biological activities, including antitumor, anti-inflammatory, and antimicrobial effects.
A new compound, phomochromenone G (
132), and one known compound, diaporchromone A (
133), were isolated from the mangrove sediment-derived fungus
Phomopsis asparagi DHS-48. Compound
133 exhibited moderate to weak immunosuppressive activity against T and B lymphocytes, with IC
50 values of 34 and 117 µM, respectively [
32]. Three new compounds, incarxanthones A–C (
134–
136), and one known compound, globosuxanthone B (
137), were isolated from the mangrove endophytic fungus
Peniophora incarnate Z4. Compound
135 showed inhibitory activity against three tumor cell lines: human melanoma cells (A375), human breast cancer cells (MCF-7), and human leukemia cells (HL-60), with IC
50 values of 8.6, 6.5, and 4.9 µM, respectively [
33]. Two known compounds, penialidin C (
138) and penialidin A (
139), were isolated from the mangrove-derived fungus
Penicillium javanicum. Compounds
138 and
139 exhibited moderate to strong inhibitory activities against four strains of
Staphylococcus aureus. Notably, compound
138 showed significant antibacterial activity against methicillin-resistant
S. aureus (MRSA) ATCC 43300, with an MIC value of 2.67 µM, comparable to the positive control vancomycin (0.54 µM). It was also active against three other MRSA strains (ATCC 33591, ATCC 25923, and ATCC 29213), with MIC values ranging from 21.40 to 85.62 µM. Compound
139 exhibited antibacterial activity against MRSA ATCC 43300 and
S. aureus ATCC 29213, with MIC values of 10.10 and 40.32 µM, respectively. At a concentration of 50 µg/mL, compound
139 also inhibited the growth of
Alternaria alternata, with an inhibition rate of 56.8% [
53]. One compound, pinselin (
140), were isolated from the mangrove sediment-derived fungus
Talaromyces sp. SCSIO 41428 [
23]. A known compound, ravenelin (
141), was isolated from the mangrove-derived fungus
Setosphaeria rostrata. Its anti-inflammatory activity was evaluated by measuring NO production in LPS-induced J774A.1 macrophage cells. Compound
141 demonstrated significant inhibitory activity with an IC
50 value of 6.27 µM. Mechanistic studies revealed that it suppressed the expression of iNOS and COX-2 [
31]. Six compounds, anomalin B (
142), 1,3,5,6-tetrahydroxy-8-methylxanthone (
143), anomalin A (
144), 1,3,6-trihydroxy-8-methylxanthone (
145), 3,4,8-trihydroxy-6-methoxy-1-methylxanthone (
146), and caloxanthone E (
147), were isolated from the mangrove soil-derived fungus
Arthrinium sp. SCSIO 41305. Compounds
142,
143,
145, and
147 showed moderate inhibitory activity against neuraminidase (NA), with inhibition rates of 83.30%, 91.46%, 75.72%, and 77.46% at 100 µg/mL, respectively. Further testing indicated that only compound
143 exhibited weak inhibition against AChE, with an inhibition rate of 52.14% at 50 µg. Compounds
143–
147 showed weak enzyme inhibitory activity against phosphatidylinositol 3-kinase (PI3K), with IC
50 values of 1.07, 4.41, 1.93, 2.90, and 3.32 µM, respectively [
39]. A new compound, 2,8-dihydroxyvertixanthone (
148), was isolated from the mangrove endophytic fungus
Peniophora incarnate Z4 [
33]. Two new compounds, aflaxanthones A (
149) and B (
150), were isolated from the mangrove endophytic fungus
Aspergillus flavus QQYZ. Compound
149 exhibited good inhibitory activity against
Colletotrichum gloeosporioides with an MIC of 3.13 µM (positive control ketoconazole, MIC = 0.1 µM), and moderate activity against
Fusarium oxysporum and
Candida albicans (MIC = 12.5 µM). Compound
150 showed moderate activity against
F. oxysporum and
Colletotrichum musae (MIC = 12.5 µM). Compound
149 also displayed moderate activity against MRSA (MIC = 12.5 µM) and inhibited
Bacillus subtilis ATCC 6633 (MIC = 25 µM, positive control ampicillin, MIC = 0.39 µM) [
54]. Three compounds, phomoxanthone D (
151), dicerandrol (
152), and 12-
O-deacetyl-phomoxanthone A (
153), were isolated from the co-culture fermentation products of two mangrove endophytic fungi,
Phomopsis asparagi DHS-48 and
Phomopsis sp. DHS-11. Compounds
152 and
153 exhibited significant cytotoxicity against human liver cancer cells (HepG-2), with IC
50 values ranging from 4.83 to 12.06 µM. Compound
151 showed weak immunosuppressive activity on ConA-induced (T cell) and LPS-induced (B cell) proliferation of mouse splenic lymphocytes [
49]. Five new compounds, staprexanthones A-E (
154–
158), were isolated from the mangrove endophytic fungus
Stachybotrys chartarum. Compounds
154,
155, and
158 significantly increased β-cell numbers in zebrafish. Compounds
155 and
158 enhanced β-cell mass by promoting cell cycle progression at the G1/S transition, suggesting their potential as novel anti-diabetic agents through stimulation of β-cell regeneration [
55]. A new compound, rhizoaspergillinol A (
159), was isolated from the mangrove endophytic fungus
Aspergillus sp. A1E3. Compound
159 exhibited potent anti-proliferative activity against three tumor cell lines, HepG2, LLC, and B16-F10, with IC
50 values of 8.83, 14.18, and 15.12 µM, respectively. It induced G2/M phase arrest in HepG2 cells in a dose-dependent manner [
56]. Three new compounds, kebanmycins A-C (
160–
162), and two known compounds, FD-594 (
163) and its aglycon (
164), were isolated from the mangrove-derived fungus
Streptomyces sp. SCSIO 40068. Compounds
160–
164 were active against a panel of Gram-positive bacteria, including
S. aureus ATCC 29213, MRSA shhs-A1, MRSA 1862, MRSA 669, MRSA 991,
B. subtilis 1064,
V. alginolyticus ATCC 13214, and Gram-negative bacteria,
A. baumannii 19606. Compound
160 showed remarkable antibacterial activity, particularly against
S. aureus ATCC 29213, MRSA shhs-A1, and MRSA 1862, with a uniform MIC of 0.125 µg/mL. It also exhibited more potent antitumor activity than compound
163, significantly inhibiting HepG2 and MCF-7 cells with IC
50 values of 0.25 µM and 0.12 µM, respectively, outperforming the positive control doxorubicin (IC
50 = 3.1 and 0.72 µM). This finding highlights the importance of the absence of the 7-OH group for enhancing antibacterial activity. Through in vitro biochemical characterization, the involvement of the methyltransferase KebMT2 was demonstrated, and a biosynthetic pathway for the compounds was proposed [
57].
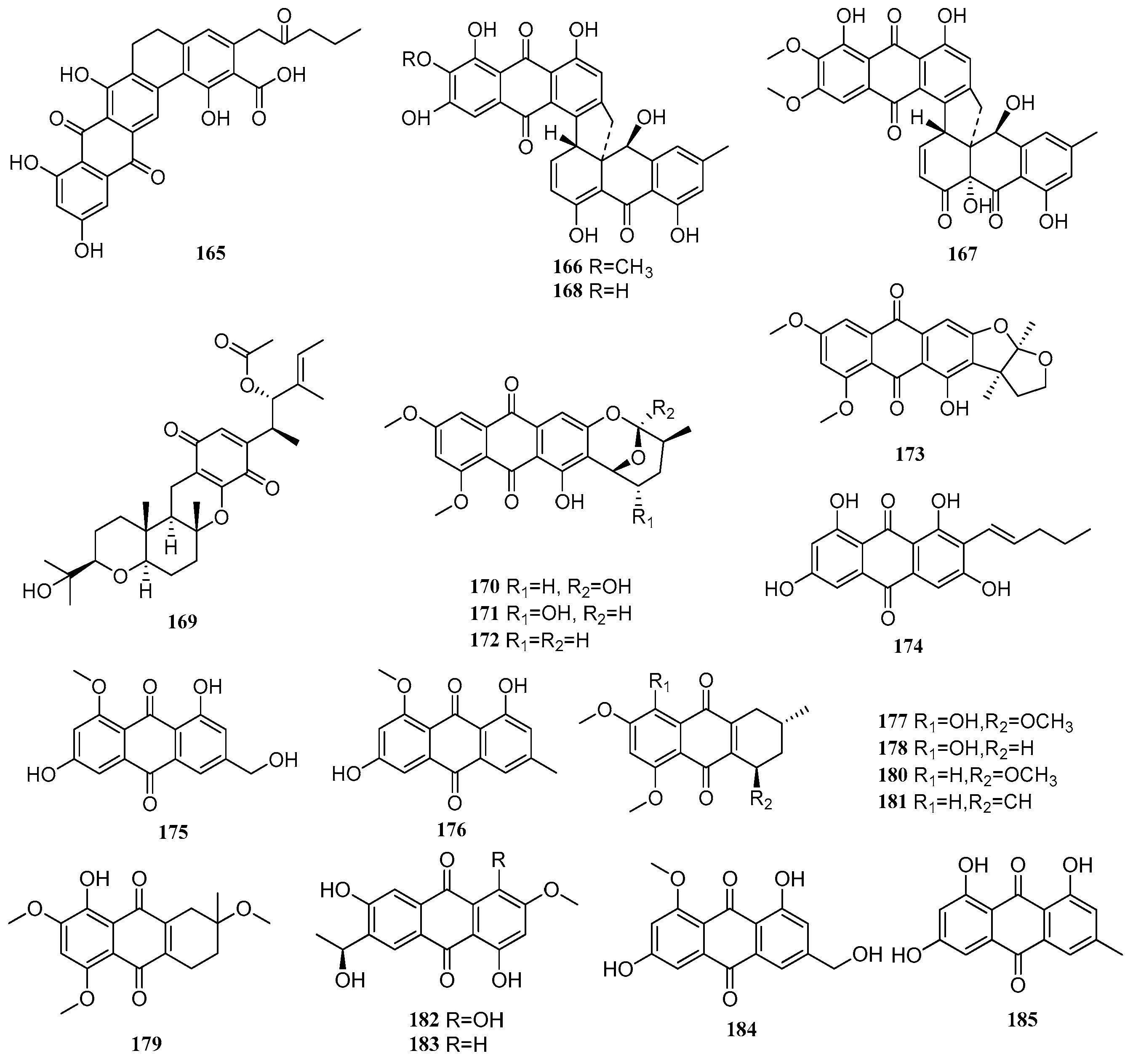
2.4. Quinones
Between 2020 and 2025, a total of 63 quinone compounds were isolated and identified from mangrove-derived fungi. Their structures are shown in
Figure 4. Among these, 37 compounds exhibited various biological activities, including anti-inflammatory, antitumor, and antimicrobial effects.
A new compound, kebanmycin D (
165), was isolated from the mangrove-derived fungus
Streptomyces sp. SCSIO 40068. Compound
165 showed antibacterial activity against a range of Gram-positive bacteria, including
S. aureus ATCC 29213, MRSA shhs-A1, MRSA 1862, MRSA 669, and MRSA 991, with MIC values ranging from 31.87 to 63.75 μM [
57]. Two new compounds, parengyomarin A (
166) and parengyomarin B (
167), along with one known compound, torrubiellin B (
168), were isolated from the mangrove endophytic fungus
Parengyodontium album. Compounds
166–
168 exhibited significant antibacterial activity against both
Staphylococcus aureus and methicillin-resistant
S. aureus (MRSA), with MIC values between 0.39 and 3.12 μM [
58]. A known compound, stemphone C (
169), was isolated from the mangrove-derived fungus
Mollisia sp. SCSIO 41409. The absolute configuration of
169 was determined for the first time via X-ray single-crystal diffraction analysis. The compound displayed varying degrees of antibacterial activity against
Erysipelothrix rhusiopathiae WH13013 and
Streptococcus suis SC19, with IC
50 values of 3.04 and 12.16 µM, respectively, comparable to the positive control penicillin (MIC = 19.53 µM). In addition, compound
169 exhibited broad-spectrum cytotoxicity against seven tumor cell lines (22Rv1, PC-3, HepG2, A549, HeLa, WPMY-1, and MC3T3-E1), with IC
50 values ranging from 2.11 to 11.68 µM. It showed particularly potent anti-proliferative activity against the human prostate cancer cell line PC-3 (IC
50 = 2.77 µM). Further studies revealed that
169 exerted its anti-proliferative effects by reducing colony formation, inducing apoptosis, and arresting the cell cycle in PC-3 cells [
13].
One new compound, asperquinone A (
170), and four known compounds, 6,8-di-
O-methylnidurufin (
171), 6,8-di-
O-methylaverufin (
172), aversin (
173), and averythrin (
174), were isolated from the mangrove endophytic fungus
Aspergillus sp. 16-5C. These compounds (
170–
174) were preliminarily screened for inhibitory activity against
Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB), but none showed significant inhibition (IC
50 > 60 µg/mL) [
59]. Two known compounds, questinol (
175) and questin (
176), were isolated from the mangrove endophytic fungus
Aspergillus sp. SCSIO 41407 [
45]. Two new compounds, 6-hydroxy-astropaquinone B (
177) and astropaquinone D (
178), and three known compounds, 3-
O-methyl-9-
O-methylfusarubin (
179), (1
R,3
S)-6-hydroxy-astropaquinone B (
180), and (1
R,3
S)-6-hydroxy-astropaquinone C (
181), from the mangrove endophytic fungus
Fusarium napiforme. Compounds
177–
179 exhibited antibacterial activity against
Staphylococcus aureus, with MIC values of 18.98, 41.39, and 18.98 μM, respectively. They also showed moderate antibacterial effects against
Pseudomonas aeruginosa, all with MIC values ranging from 18.98 to 20.86 μM [
60].
Two new compounds, (11
S)-1,4,6-trihydroxy-7-(1-hydroxyethyl)-3-methoxyanthracene-9,10-dione (
182) and (11
S)-1,6-dihydroxy-7-(1-hydroxyethyl)-3-methoxyanthracene-9,10-dione (
183), were isolated from the mangrove endophytic fungus
Fusarium sp. J3-2. Compound
182 exhibited weak to moderate antibacterial activity against five pathogenic strains,
Staphylococcus aureus ATCC 43300, ATCC 25923, ATCC 29213,
Enterococcus faecalis ATCC 51299, and
Enterococcus faecium ATCC 35667, with MIC values ranging from 75.76 to 151.52 μM. In addition, both compounds
182 and
183 demonstrated anti-fouling activity, completely inhibiting the attachment of barnacle larvae (attachment rate = 0%) [
61]. Three known compounds, questinol (
184), emodin (
185), and catenarin (
186), were isolated from the mangrove endophytic fungus
Aspergillus sp. WHUF0343. Compounds
184 and
185 exhibited antibacterial activity against
Staphylococcus aureus ATCC 25923 and methicillin-resistant
Staphylococcus aureus NRS271, with MIC values between 29.63 and 59.26 µM. Compound
186 also showed strong inhibitory activity against four strains of
Helicobacter pylori (26695, G27, 159, and 129), with MIC values ranging from 3.50 to 13.99 µM [
62]. A known compound, averufanin (
187), was isolated from the mangrove endophytic fungus
Aspergillus sp. A1E3. The absolute configuration of
187 was determined for the first time via ECD calculations [
56]. Two known compounds, questin (
188) and physcion (
189), were isolated from the mangrove endophytic fungus
Aspergillus fumigatus HQD24. Compound
188 exhibited inhibitory activity on LPS-induced B-cell proliferation (IC
50 = 108.67 μM) and ConA-induced T-cell proliferation (IC
50 = 41.67 μM) [
19]. In another study, Xu et al. isolated two new compounds, dalesconosides C-D (
190–
191), and one new natural product, dalesconoside E (
192), from the mangrove endophytic fungus
Daldinia eschscholzii MCZ-18. Compound
190 displayed broad-spectrum antibacterial activity against five pathogenic microorganisms,
Enterococcus faecalis, methicillin-resistant
Staphylococcus aureus,
Escherichia coli,
Pseudomonas aeruginosa, and
Candida albicans, with IC
50 values ranging from 12.5 to 50 μM [
9].
One new compound (6
R,7
R,8
R)-theissenone A (
193), and two known compounds, (6
S,7
R,8
R)-theissenone (
194) and arthrinone (
195), were isolated from the mangrove endophytic fungus
Arthrinium marii M-211. The IC
50 values of compounds
193–
195 against rat hepatoma H4IIE cells were 67.5, 46.6, and 13.4 μM, respectively (positive control staurosporine: IC
50 = 20.9 nM). Compounds
193 and
194 showed moderate antibacterial activity against both
Pseudomonas aeruginosa ATCC 15442 and
Staphylococcus aureus NBRC 13276, with a uniform MIC of 25 μM, while compound
195 exhibited moderate antibacterial activity against the same strains with an MIC of 12.5 μM [
63]. Four known compounds, anhydrofusarubin (
196), javanicin (
197), dihydrojavanicin (
198), and solaniol (
199), were isolated from the mangrove-derived fungus
Lasiodiplodia theobromae. Compounds
197–
199 displayed notable inhibitory activity against
Trypanosoma brucei, with MIC values of 0.60, 0.32, and 1.90 μM, respectively [
18].
Two new compounds, talanaphthoquinones A-B (
200–
201), along with ten known compounds, anhydrojavanicin (
202), 2,3-dihydro-5-hydroxy-4-hydroxymethyl-8-methoxy-2-methylnaphtho[1,2-b]furan-6,9-dione (
203), anhydrojavanicin (
204), anhydrofusarubin (
205), 2-acetonyl-3-methyl-5-hydroxy-7-methoxynaphthazarin (
206), 6-ethyl-2,7-dimethoxyjuglone (
207), 6-[1-(acetyloxy)ethyl]-5-hydroxy-2,7-dimethoxy-1,4-naphthalenedione (
208), 5-hydroxy-6-(1-hydroxyethyl)-2,7-dimethoxy-1,4-naphthalenedione (
209), solaniol (
210), and javanicin (
211), were isolated from the mangrove endophytic fungus
Talaromyces sp. SK-S009. All compounds except
201 inhibited NO production induced by LPS, with IC
50 values ranging from 3.9 to 22.6 µM, which were lower than that of the positive control indomethacin (26.3 µM). Compound
208 suppressed the mRNA expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in RAW264.7 macrophages. Furthermore, it reduced the mRNA levels of pro-inflammatory cytokines interleukin (IL-1β, IL-6) and tumor necrosis factor (TNF-α) [
64]. A known compound, stenocarpoquinone B (
212), were isolated from the mangrove endophytic fungus
Avicennia officinalis [
65]. A known compound,
trans-3,4-dihydro-3,4,8-trihydroxynaphthalen-1(2
H)-one (
213), was isolated from the mangrove endophytic fungus
Penicillium polonicum H175 [
66]. A known compound,
trans-3,4-dihydro-3,4,8-trihydroxynaphthalen-1(2
H)-one (
214), was isolated from the mangrove sediment-derived fungus
Roussoella sp. SCSIO 41427 [
10]. A known compound, (4
S)-4,8-dihydroxy-α-tetralone (
215), was isolated from the mangrove-derived fungus
Colletotrichum sp. J065 [
67]. A known compound, regiolone (
216), was isolated from the mangrove-derived fungus
Cytospora sp. Compound
216 exhibited weak antibacterial activity against
Bacillus subtilis,
Colletotrichum gloeosporioides, and
Magnaporthe oryzae, with a uniform IC
50 value of 561.6 µM [
68]. A known compound,
cis-(3
R,4
S)-3,4-dihydro-3,4,8-trihydroxynaphthalen-1(2
H)-one (
217), was isolated from the mangrove endophytic fungus
Penicillium citrinum QJF-22. Compound
217 exhibited moderate anti-inflammatory activity by inhibiting LPS-induced NO release in RAW264.7 cells, with an IC
50 value of 44.7 µM, and showed no cytotoxicity toward RAW264.7 cells at concentrations up to 50 µM [
52].
A new compound dalesconoside F (
218), and seven known compounds, regiolone (
219), nodulisporone (
220), nodulisporol (
221), xylariol A (
222), (4
R)-4,8-dihydroxy-3-hydro-5-methoxy-1-naphthalenone (
223), (4
R)-
O-methylsclerone (
224), and (4
R)-3,4-dihydro-4,5-dihydroxynaphthalen-1(2
H)-one (
225), were isolated from the mangrove endophytic fungus
Daldinia eschscholzii MCZ-18. Compounds
223–
225 exhibited antibacterial activity against five pathogenic bacteria, with IC
50 values ranging from 6.25 to 50 µM [
9]. A new natural product, embelin A (
226), was isolated from the mangrove-derived fungus
Penicillium sp. SCSIO 41411. Its absolute configuration was determined for the first time via X-ray single-crystal diffraction. Compound
226 displayed cytotoxic activity against prostate cancer cell lines PC-3 and LNCaP, with IC
50 values of 18.69 and 31.62 µM, respectively [
8]. A known compound anserinone A (
227), was isolated from the mangrove-derived fungus TBRC-BCC 64093 [
12].
2.5. Lactones
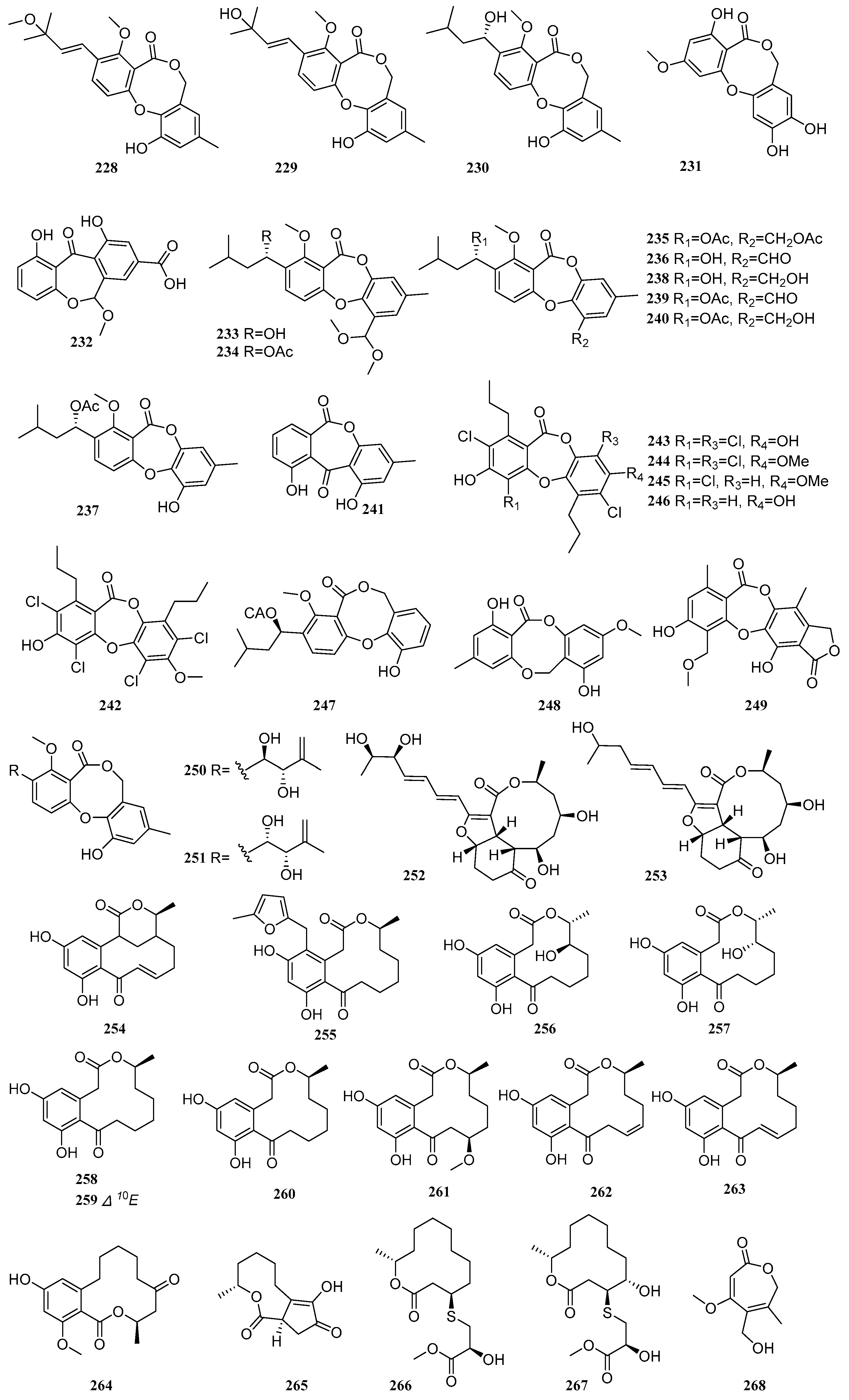
Lactones represent a major class of secondary metabolites from mangrove-derived fungi. These cyclic organic molecules, composed of carboxylate esters, are formed through the dehydration of lactic acid. Based on ring size, they can be categorized into macrolides, sesquiterpene lactones, among others. Macrolides often exhibit antibacterial properties, while sesquiterpene lactones are noted for their antimalarial and immunomodulatory activities. Between 2020 and 2025, a total of 150 lactone compounds were isolated and identified from mangrove-derived fungi. Their structures are shown in
Figure 5.
Four compounds, alterlactone (
228), penicillide (
229), dehydroisopenicillide (
230), and 3′-
O-methyldehydroisopenicillide (
231), were isolated from the mangrove-derived fungus
Talaromyces sp. Compounds
229–
231 exhibited antibacterial activity against
Staphylococcus aureus, with MIC values of 50, 50, and 25 µg/mL, respectively. Compound
228 showed DPPH free radical scavenging activity with an EC
50 value of 96.51 µM, which was weaker than that of the positive control vitamin C (EC
50 = 72.39 µM) [
69]. Liu et al., employing an OSMAC strategy, identified a new compound arugosinacid A (
232), from the mangrove-derived fungus
Penicillium sp. HDN15-312. Compound
232 exhibited moderate DPPH radical scavenging activity, with an IC
50 value of 56.92 μM [
40]. Four new compounds, talaronins A-D (
233–
236), and five known compounds, purpactin A (
237), talaromyone A (
238), purpactin C (
239), talaromyone B (
240), and alternaphenol B (
241), were isolated from the mangrove-derived fungus
Talaromyces sp. WHUF0362. Compounds
237 and
238 showed potent activity against four strains of
Helicobacter pylori (26695, G27, 159, and 129), with MIC values ranging from 2.42 to 36.04 μM [
11]. Five known compounds, spiromastixones L (
242), I (
243), J (
244), G (
245), and E (
246), were isolated from the mangrove-derived fungus
Spiromastix sp. SCSIO F190. Compounds
242-246 exhibited significant antibacterial activity against methicillin-resistant
Staphylococcus aureus (MRSA),
Enterococcus faecalis,
Micrococcus luteus,
Staphylococcus simulans,
Enterococcus faecium ATCC 29212,
Bacillus subtilis, and
Enterococcus gallinarum BS01, with MIC values ranging from 0.125 to 32 μg/mL. Compound
244 was particularly potent, with MIC values between 0.125 and 4 μg/mL. Structure-activity relationship studies indicated that the presence of both ester and ether bonds linking rings A and B in compound
244 was crucial for its high activity, suggesting that the absence of an ether bond leads to a marked reduction in antibacterial efficacy [
34]. A known compound, purpactin A (
247), were isolated from the mangrove endophytic fungus
Penicillium sp. TGM112. The compound exhibited moderate antioxidant activity, with an IC
50 value of 4.6 mM [
24]. A known compound, barceloneic lactone (
248), was isolated from the mangrove endophytic fungus
Epicoccum sorghinum [
44]. A new compound, guanxidone A (
249), was isolated from the mangrove endophytic fungus
Aspergillus sp. GXNU-A9. It significantly reduced NO production in LPS-induced RAW264.7 cells, with an IC
50 value of 8.22 μM [
15].
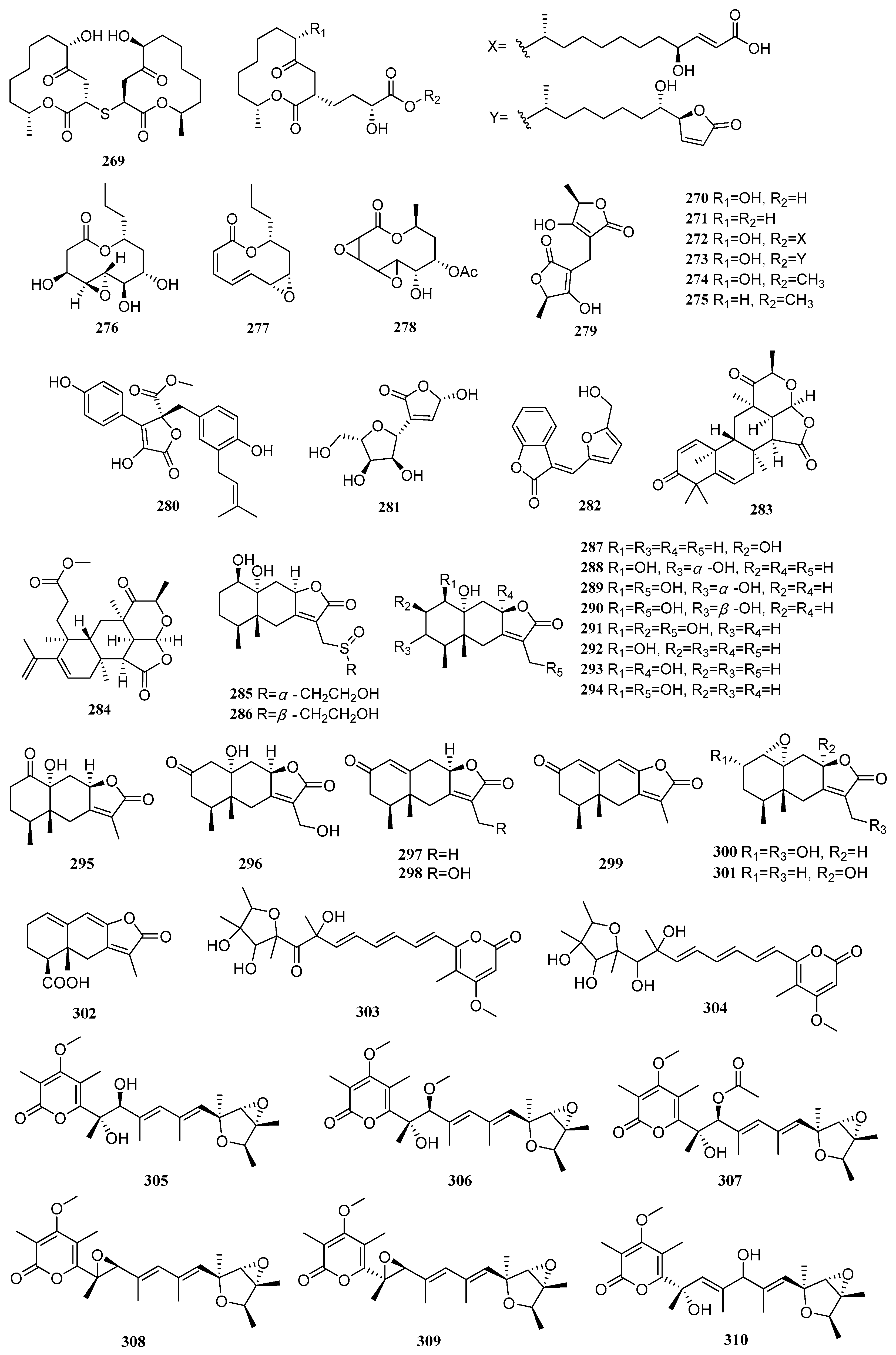
Two known compounds, pestalotiollides A-B (
250–
251), were isolated from the mangrove-derived fungus
Pestalotiopsis sp. HHL101 [
20]. Two new compounds, colletotrikalactones A and B (
252–
253), from the mangrove-derived fungus
Colletotrichum sp. J065 [
67]. A known compound, α,β-dehydrocurvularin (
254), was isolated from the mangrove endophytic fungus
Trichoderma sp. FM652. It significantly inhibited the TNF-α-induced NF-κB pathway with an IC
50 value of 14.63 µM. Compound
254 also exhibited moderate antibacterial activity against
Staphylococcus aureus ATCC 12600 and methicillin-resistant
Staphylococcus aureus ATCC 43300, with an MIC value of 33.11 µM, and inhibited
Bacillus subtilis ATCC 6633 with an MIC value of 66.22 µM [
70]. Three new compounds, sumalarins D, F-G (
255–
257), and two known compounds, curvularin (
258) and dehydrocurvularin (
259), were isolated from the mangrove-derived fungus
Penicillium sumatrense MA-325. Compounds
255 and
258–
259 exhibited inhibitory activity against the aquatic pathogens
Vibrio alginolyticus and
Vibrio harveyi, with MIC values ranging from 13.70 to 219.18 µM. Furthermore, compound
259 showed cytotoxic activity against tumor cell lines 5673, HCT 116, 786-O, and HeLa, with IC
50 values of 3.5, 10.6, 10.9, and 14.9 µM, respectively [
71]. Four known compounds, curvularin (
260), 11-β-methoxycurvularin (
261), β,γ-dehydrocurvularin (
262), and α,β-dehydrocurvularin (
263), were isolated from the mangrove endophytic fungus
Alternaria longipes, and proposed a plausible biosynthetic pathway for compounds
260–
263 [
72]. A known compound, 6-oxolasiodiplodin (
264), was isolated from the mangrove endophytic fungus
Trichoderma erinaceum F1-1 [
73].
Three new compounds cladocladosin A (
265) and thiocladospolides F-G (
266–
267), were isolated from the mangrove endophytic fungus
Cladosporium cladosporioides MA-299. Compound
265 features a novel carbon skeleton with a 5/9 bicyclic ring system, and a biosynthetic pathway for compounds
265–
267 was proposed. Compounds
265–
267 showed activity against the aquatic pathogens
Edwardsiella tarda and
Vibrio anguillarum, with MIC values ranging from 4.46 to 11.49 µM. Compound
265 was active against
Pseudomonas aeruginosa, and compound
266 showed activity against the plant pathogenic fungus
Helminthosporium maydis, both with MIC values of 17.86 and12.05 µM, respectively [
74]. A new compound, botroxepinone (
268), was isolated from the mangrove endophytic fungus
Botryosphaeria ramose. It exhibited antimicrobial activity against
Fusarium oxysporum,
Fusarium graminearum, and
Colletotrichum musae, with IC
50 values ranging from 25 to 200 µg/mL, some of which were stronger than the positive control triadimefon [
28]. Five new compounds, thiocladospolides F-J (
269–
273), and two known compounds, pandangolide (
274) and thiocladospolide A (
275), were isolated from the mangrove endophytic fungus
Cladosporium oxysporum. Compound
270 exhibited broad-spectrum antibacterial activity against multiple pathogens, including
Cytospora mandshurica Miura,
Colletotrichum gloeosporioides,
Fusarium oxysporum f. sp.
cucumerinum,
Edwardsiella tarda, and
Edwardsiella ictaluri, with MIC values ranging from 4 to 32 µg/mL [
75]. One new compound, asperlactone A (
276), and two known compounds, (6
Z,8
E)-3-propyl-4,11-dioxa-bicyclo[8.1.0]undeca-6,8-dien-5-one (
277) and 8-
O-acetyl-5,6-dihydro-5,6-epoxymultiplolide A (
278), were isolated from the mangrove endophytic fungus
Aspergillus sp. GXNU-A9. Compounds
276–
278 exhibited moderate anti-inflammatory activity by inhibiting LPS-induced NO production, with IC
50 values of 16.69, 15.87, and 30.48 µM, respectively [
76].
A known compound, (+)-(5
R,5′
R)-3,3′-methylenebistetronic acid (
279), was isolated from the mangrove endophytic fungus
Penicillium crustosum SCNU-F0006. It exhibited inhibitory activity against human pathogenic bacteria and plant pathogenic fungi, including
Pseudomonas aeruginosa (MIC = 0.5 mg/mL),
Salmonella typhimurium (MIC = 1.0 mg/mL),
Fusarium oxysporum (MIC = 0.25 mg/mL), and
Penicillium italicum (MIC = 0.25 mg/mL) [
77]. Two known compounds, butyrolactone I (
280) and polybotrin (
281), were isolated from the mangrove-derived fungus
Penicillium sp. SCSIO 41411. Compound
280 exhibited DPPH radical scavenging activity with an EC
50 of 16.21 µg/mL. Additionally, compounds
280 and
281 showed weak inhibitory activity against PDE4, with inhibition rates of 29.10% and 26.22%, respectively [
8]. A new lactone compound, (
E)-3-[5-(hydroxymethyl)furan-2-yl-methylene]benzofuran-2(3
H)-one (
282), was isolated from the mangrove endophytic fungus
Xylaria arbuscula QYF [
35]. Two new compounds, littoreanoids E-F (
283-284), were isolated from the mangrove endophytic fungus
Penicillium sp. HLLG-122. Compound
284 exhibited anti-inflammatory activity with an IC
50 value of 30.41 µM [
78]. Nine new compounds 13-(
R)-(2-hydroxyethyl)sulfinylmairetolide F (
285), 13-(
S)-(2-hydroxyethyl)sulfinylmairetolide F (
286), 2β,10α,13-trihydroxyeremophil-7(11)-en-12,8β-olide (
287), 1β,3α,10α-trihydroxyeremophil-7(11)-en-12,8β-olide (
288), 1β,3α,10α,13-tetrahydroxyeremophil-7(11)-en-12,8β-olide (
289), 1β,3β,10α,13-tetrahydroxyeremophil-7(11)-en-12,8β-olide (
290), 1β,2β,10α,13-tetrahydroxyeremophil-7(11)-en-12,8β-olide (
291), 1-oxo-10α-hydroxyeremophil-7(11)-en-12,8β-olide (
295), 2-oxo-10α,13-dihydroxyeremophil-7(11)-en-12,8β-olide (
296), and nine known compounds, mairetolides F-G (
292–
293), 13-hydroxymairetolide F (
294), xylareremophil (
297), 13-hydroxyxylareremophil (
298), 2-oxo-eremophil-1(10),7(11),8-trien-12,8-olide (
299), 2α,13-dihydroxymairetolide A (
300), mairetolide B (
301), and eremophil-1(10),7(11),8-trien-12,8-olide-15-oic acid (
302), were isolated from the mangrove-derived fungus TBRC-BCC 64093. Compounds
285 and
294 exhibited weak cytotoxicity against the Vero (African green monkey kidney) cell line, with IC
50 values of 49.44 and 186.09 µM, respectively [
12]. Two new compounds, citreoviridin H (
303) and citreoviridin I (
304), were isolated from the mangrove endophytic fungus
Penicillium sp. BJR-P2 [
26]. Six known compounds, verrucosidinol (
305), methyl verrucosidinol (
306), verrucosidinol acetate (
307), normethylverrucosidin (
308), verrucosidin (
309), and penicyrone A (
310), were isolated from the mangrove endophytic fungus
Penicillium polonicum H175 [
66].
One new compound 2,3-dihydro-2-hydroxyvertinolide (
311), and two known compounds, 5-hydroxyvertinolide (
312) and vertinolide (
313), were isolated from the mangrove endophytic fungus
Trichoderma sp. FM652. Compound
311 significantly inhibited TNF-α-induced NF-κB activation with an IC
50 value of 13.83 µM [
70]. A known compound, (
R)-striatisporolide A (
314), was isolated from the mangrove endophytic fungus
Eupenicillium sp. [
79]. Three known compounds, (4
S,5
S,11
R)-iso-cladospolide B (
315), (4
S,5
S,11
S)-iso-cladospolide B (
316), and (4
R,5
S,11
R)-iso-cladospolide B (
317), were isolated from the mangrove endophytic fungus
Cladosporium sp. HNWSW-1 [
80].
Three new compounds, qinlactones A-C (
318–
320), were isolated from the mangrove endophytic fungus
Streptomyces qinglanensis 172205. Compounds
318–
319 exhibited weak cytotoxic activity against the human breast cancer cell line MCF-7 and the human cervical cancer cell line HeLa, with IC
50 values ranging from 129 to 207 µM [
81]. A known compound, iso-cladospolide B (
321), was isolated from the mangrove endophytic fungus
Cladosporium oxysporum HDN13-314. It exhibited antibacterial activity against multiple pathogens, including
Cytospora mandshurica Miura,
Colletotrichum gloeosporioides,
Bipolaris sorokiniana,
Fusarium oxysporum f. sp.
cucumerinum,
Edwardsiella tarda, and
Edwardsiella ictaluri, with MIC values ranging from 35.09 to 140.35 μM [
75]. A new compound, (4
S,5
S,6
S,7
R)-4-(3-chloro-1,2-dihydroxybutyl)-butyrolactone (
322), was isolated from the mangrove endophytic fungus
Neofusicoccum parvum Y2NBKZG1016. At concentrations ≥ 6.25 µM, it showed weak anti-inflammatory activity (NO inhibition), with a maximum inhibition rate of 28.9% [
82]. Four new compounds, penipyrols C-F (
323–
326), were isolated from the mangrove-derived fungus
Penicillium sp. HDN-11-131. These compounds feature a rare skeleton in which a γ-butyrolactone is linked via a double bond to an α-pyrrole ring. At 10 µM, compound
323 induced pancreatic β-cell regeneration in zebrafish (45.20 ± 2.359%), exceeding the effect of the positive control prednisolone (39.86 ± 1.773%), indicating promising anti-diabetic potential [
83]. Two known compounds, asperteretal G (
327) and 3-(2-hydroxypropyl)-4-(hexa-2
E,4
E-dien-6-yl)furan-2(5
H)-one (
328), were isolated from the mangrove sediment-derived fungus
Trichoderma harzianum SCSIO 41051. Compound
327 exhibited moderate inhibitory activity against acetylcholinesterase (AChE) with an IC
50 of 2.49 µM and against pancreatic lipase (PL) with an IC
50 of 2.34 µM. Molecular docking studies indicated interactions between compound
327 and the AChE protein [
22]. Four new compounds, asperbutenolides B-C (
329–
330) and asperbutenolides E-F (
331–
332), along with ten known compounds, butyrolactone III (
333), (+)-3′,3′-di-(dimethylallyl)-butyrolactone II (
334), 3-hydroxy-5-(4-hydroxybenzyl)-4-(4-hydroxyphenyl)furan-2(5
H)-one (
335), butyrolactone II (
336), versicolactone B (
337), asperlide B (
338), 7″
R-methoxy-8″
S-hydroxy-aspernolide E (
339), asperlide A (
340), butyrolactone IV (
341), and aspernolide E (
342), were isolated from the mangrove-derived fungus
Aspergillus terreus SCAU011. Compounds
331 and
336 showed COX-2 inhibitory activity superior to the positive control celecoxib. Compounds
334 and
335 exhibited significant α-glucosidase inhibitory activity with IC
50 values of 56.1 and 12.9 µM, respectively. Meanwhile, compounds
329,
333–
336, and
340–
342 demonstrated antioxidant activity similar to or better than the positive control curcumin, with IC
50 values ranging from 0.7 to 23.3 µM. Compounds
334 and
342 showed moderate antibacterial activity against
Staphylococcus aureus, with IC
50 values of 17.4 and 36.6 µM, respectively [
84]. A known compound, xenofuranone B (
343), was isolated from the mangrove endophytic fungus
Phyllosticta capitalensis [
17]. Two new compounds, (8″
S,9′)-dihydroxy-dihydrobutyrolactone I (
344) and asperbutenolide A (
345), were isolated from the mangrove endophytic fungus
Aspergillus terreus SCAU011. At 20 µM, compounds
344 and
345 inhibited cyclooxygenase-2 (COX-2) by 91.8% and 100%, respectively. Compound
345 also exhibited α-glucosidase inhibitory activity (IC
50 = 10.5 µM) and antibacterial effects against
Staphylococcus aureus and
Vibrio splendidus, with IC
50 values of 1.3 and 3.7 µM, respectively [
85]. Two new compounds, (±)-isoepicolactone (±)-
346, and two known compounds, aepicoccone F (
347) and 4,5,6-trihydroxy-7-methylphthalide (
348), were isolated from the mangrove endophytic fungus
Epicoccum nigrum SCNU-F0002. Compounds (+)-
346 and (−)-
346 showed weak inhibitory activity against COX-2, with inhibition rates of 28.8% and 31.2%, respectively [
86].
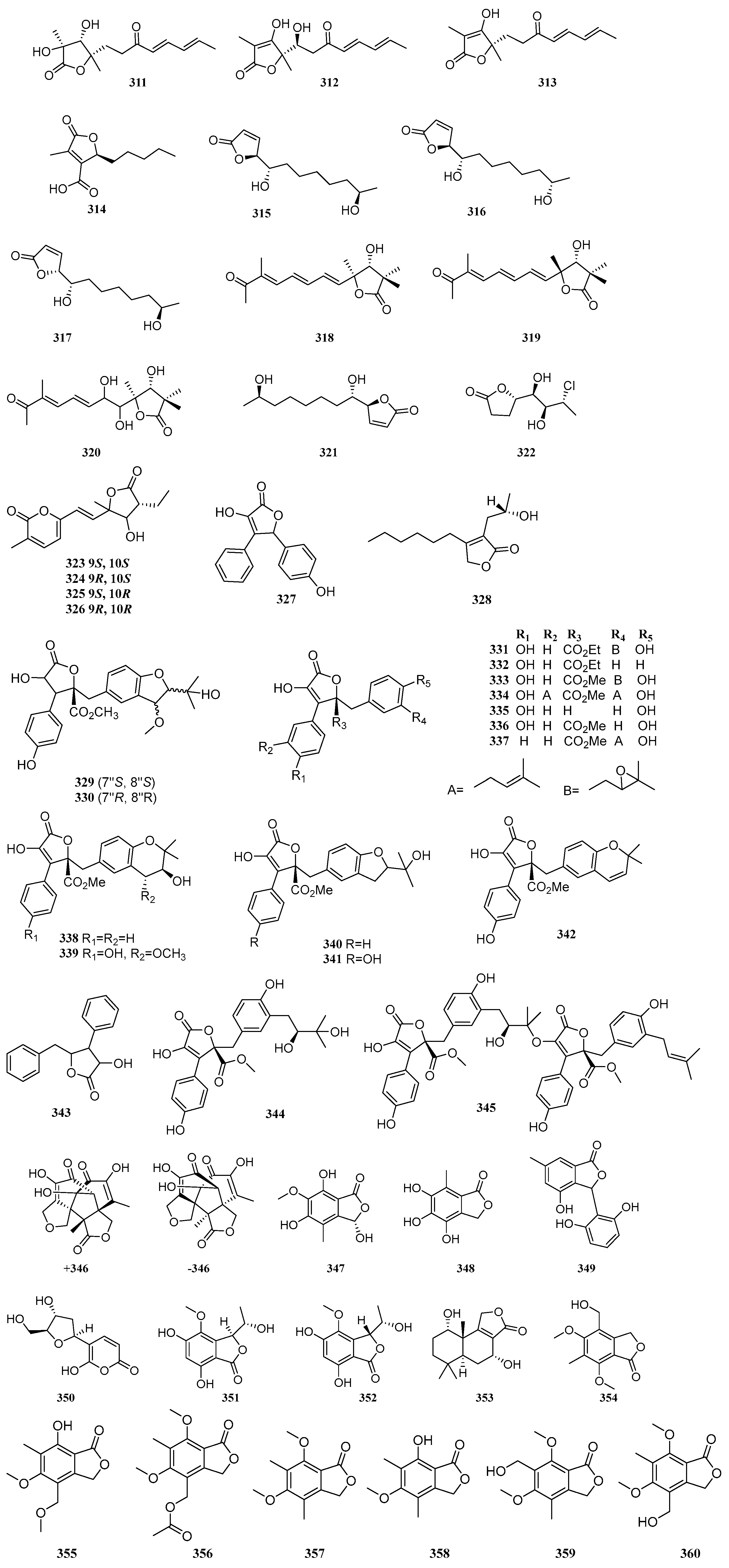
Two known compounds, 3-(2,6-dihydroxyphenyl)-4-hydroxy-6-methyl-isobenzofuran-1(3
H)-one (
349) and 3-(2-deoxy-β-erythro-pentofuranosyl)-6-hydroxy-2
H-pyran-2-one (
350), were isolated from the co-culture fermentation products of two mangrove endophytic fungi,
Phomopsis asparagi DHS-48 and
Phomopsis sp. DHS-11 [
43]. One new compound, embeurekol D (
351), and one known compound, embeurekol C (
352), were isolated from the mangrove-derived fungus
Penicillium sp. SCSIO 41411. The absolute configurations of
351 and
352 were determined by Mosher’s ester method and ECD calculations. At a concentration of 10 µM, compounds
351 and
352 exhibited weak inhibitory activity against PDE4, with inhibition rates of 18.62% and 14.95%, respectively [
8]. Using an OSMAC strategy, Liu et al. identified a known compound, astalaminoid C (
353), from the mangrove-derived fungus
Penicillium sp. HDN15-312. It exhibited moderate DPPH radical scavenging activity with an IC
50 value of 32.11 μM [
40]. A known compound, 4-(hydroxymethyl)-5,7-dimethoxy-6-methylisobenzofuran-1(3
H)-one (
354), was isolated from the mangrove endophytic fungus
Aspergillus sp. GXNU-Y85 [
87]. Two new compounds, pestalotiophthalides A-B (
355–356), and four known compounds, 5,7-dimethoxy-4,6-dimethylisobenzofuran-1(3
H)-one (
357), 7-hydroxy-5-methoxy-4,6-dimethylisobenzofuran-1(3
H)-one (
358), 6-(hydroxymethyl)-5,7-dimethoxy-4-methylisobenzofuran-1(3
H)-one (
359), and 4-(hydroxymethyl)-5,7-dimethoxy-6-methylisobenzofuran-1(3
H)-one (
360), were isolated from the mangrove endophytic fungus
Pestalotiopsis sp. SAS4 [
88]. One new compound, 3-hydroxyepicoccone B (
361), and three known compounds, 4,6-dihydroxy-5-methoxy-7-methylphthalide (
362), 4,5,6-trihydroxy-7-methyl-3
H-isobenzofuran-1-one (
363), and sparalide C (
364), were isolated from the mangrove endophytic fungus
Epicoccum nigrum MLY-3. At 10 µg/mL, compounds
361 and
363 exhibited DPPH radical scavenging activity with IC
50 values of 29.3 and 16.5 µM, respectively, and ABTS radical scavenging activity with IC
50 values of 23.7 and 23.3 µM, respectively, outperforming the positive control acarbose (IC
50 = 33.6 ± 0.8 µM) [
89]. Two known compounds, pestaphthalide A (
365) and (
S)-3-[(
S)-1-hydroxyethyl]-5,7-dimethoxy-6-methylisobenzofuran-1(3
H)-one (
366), were isolated from the mangrove endophytic fungus
Botryosphaeria ramose. Compounds
365 and
366 exhibited inhibitory activity against
Penicillium italicum, with IC
50 values of 223.21 and 99.21 µM, respectively, which were stronger than the positive control triadimefon (IC
50 = 170.65 µM) [
28].
A known compound, dimethoxyphtalide (
367), was isolated from the mangrove-derived fungus
Cytospora sp. [
30]. Five new compounds, (±)-epicoccone C (±
368), epicoccone D (
369), epicoccone E (
370), epicolactone A (
371), and one known compound, epicolactone (
372), were isolated from the mangrove endophytic fungus
Epicoccum nigrum SCNU-F0002. Compounds (+)-
368 and
370 exhibited strong α-glucosidase inhibitory activity with IC
50 values of 43.2 and 53.2 µM, respectively, stronger than the positive control acarbose. Compounds (-)-
368,
369, and
371 showed moderate inhibitory activity, with IC
50 values ranging from 130.2 to 252.4 µM. In addition, compounds (±)-
368 demonstrated antioxidant activity stronger than the positive controls gallic acid and vitamin C, with IC
50 values of 11.1 and 10.2 µM, respectively [
90]. Four new compounds, trichoderolides C-F (
373–
376), and one known compound, (3
R,5
R)-harzialactone A (
377), were isolated from the mangrove endophytic fungus
Trichoderma erinaceum F1-1 [
73].
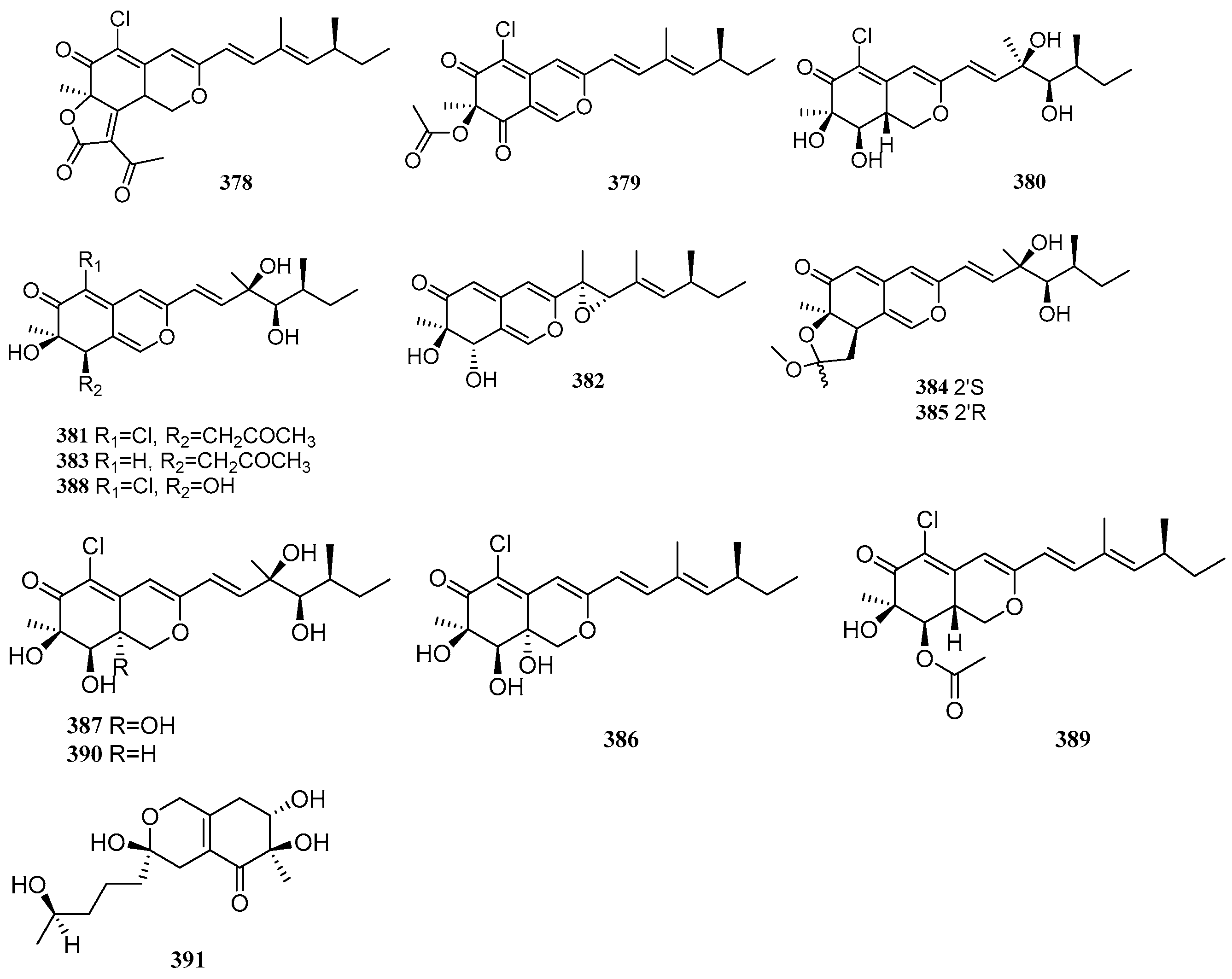
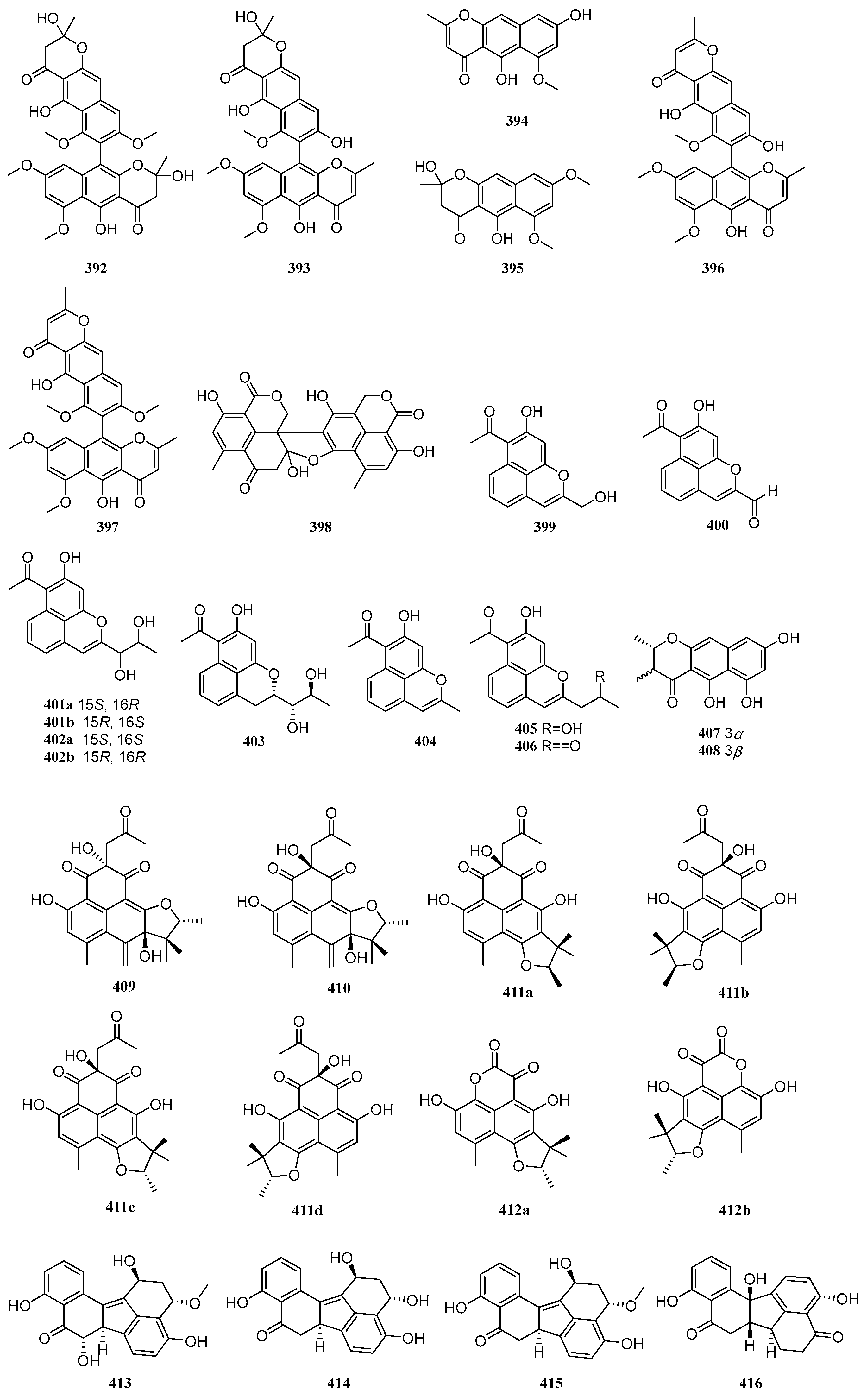
2.7. Others
Polyketides exhibit diverse structural types beyond those mentioned above, including various other skeletons. Between 2020 and 2025, a total of 66 other polyketide compounds were isolated and identified from mangrove-derived fungi. Their structures are shown in
Figure 7.
Six known compounds, aurasperone B (
392), aurasperone F (
393), TMC-256A1 (
394), fonsecin B (
395), dianhydroaurasperone C (
396), and aurasperone A (
397), were isolated from three mangrove-derived fungi,
Aspergillus sp. IQ-503,
Aspergillus sp. IQ-548, and
Talaromyces sp. I-567. Compounds
392–
394 exhibited inhibitory effects on bacterial growth, with IC
50 values ranging from 6.9 to 9.9 µg/mL. Through in vitro evaluation of molecular interactions with the
Acinetobacter baumannii filamenting temperature-sensitive mutant Z (AbFtsZ) protease to identify anti-
A. baumannii agents, it was found that compounds
392,
393, and
395 enhanced AbFtsZ activity under interaction, whereas compound
394, as the sole inhibitor of AbFtsZ, suppressed bacterial growth [
94]. A known compound, bacillisporin C (
398), was isolated from the mangrove-derived fungus
Talaromyces sp. WHUF0362 [
11]. Five new compounds, RM18c-RM18g (
399–
403), and three known compounds, RM18b (
404), wailupemycin K (
405), and RM18 (
406), were isolated from the mangrove endophytic fungus
Streptomyces sp. SCSIO 40069. Among these, compounds
401 and
402 constitute a pair of racemates. Compounds
399–
401,
402b, and
406 exhibited antibacterial activity against
Acinetobacter baumannii ATCC 19606,
Vibrio alginolyticus ATCC 13214,
Staphylococcus aureus ATCC 29213,
Klebsiella pneumonia ATCC 13883, and
Micrococcus luteus SCSIO ML01, with MIC values ranging from 8 to 64 μg/mL [
95]. Two new compounds, peninaphones A (
407) and B (
408), were isolated from the mangrove endophytic fungus
Penicillium sp. HK1-22. Compounds
407–
408 showed weak antibacterial activity against
Staphylococcus aureus, with inhibition zone diameters ranging from 10.4 to 21.0 mm [
96]. Four new compounds, aceneoherqueinones A and B (
409–
410), (+)-aceatrovenetinone A (
411a), and (+)-aceatrovenetinone B (
411d), along with four known compounds, (-)-aceatrovenetinone A (
411b), (-)-aceatrovenetinone B (
411c), (-)-scleroderolide (
412a), and (+)-scleroderolide (
412b), were isolated from the mangrove endophytic fungus
Penicillium herquei MA-370. Compounds
409 and
410 inhibited angiotensin-converting enzyme (ACE) with IC
50 values of 3.10 and 11.28 µM, respectively. Molecular docking analysis elucidated the intermolecular interactions and potential binding sites of
409 and
410 with ACE, indicating that compound
409 binds favorably via hydrogen interactions with residues Ala261, Gln618, Trp621, and Asn624, while compound
410 interacts with residues Asp358 and Tyr360 [
97]. One new compound, guhypoxylonol A (
413), and three known compounds, hypoxylonol C (
414), hypoxylonol B (
415), and daldinone C (
416), were isolated from the mangrove endophytic fungus
Aspergillus sp. GXNU-Y45. Compounds
413 and
415 inhibited LPS-induced NO production with IC
50 values of 14.42 and 21.05 µM, respectively, compared to the positive control dexamethasone (IC
50 = 16.12 µM) [
98].
Zou et al. activated silent biosynthetic genes by modifying culture medium components and adding sodium bromide/sodium chloride, leading to the isolation and identification of 12 new compounds, (±)-6′-hydroxy-7-dechlorogriseofulvin [(±)-
417], (±)-6′-hydroxy-7-dechloroepigriseofulvin [(±)-
418], (+)-6′-hydroxygriseofulvin [(+)-
419], (±)-6′-hydroxyepigriseofulvin [(±)-
420], 6-
O-desmethyl-7-bromogriseofulvin (
426), 5-bromo-6-
O-desmethyl-7-dechlorogriseofulvin (
427), 5,7-dibromo-6-
O-desmethylgriseofulvin (
428), 3′,4′-dihydroeupenigriseofulvin (
430), 4′-demethoxy-7-dechloroisogriseofulvin (
431), along with two new natural products, 7-bromogriseofulvin (
425) and 4′-demethoxyisogriseofulvin (
432), and six known analogs, (−)-6′-hydroxygriseofulvin [(−)-
419], 7-dechlorogriseofulvin (
421), griseofulvin (
422), 6-
O-desmethyl-7-dechlorogriseofulvin (
423), 6-
O-desmethylgriseofulvin (
424), and eupenigriseofulvin (
429), from the mangrove-derived fungus
Nigrospora sp. QQYB1. Compounds
422 and
425 exhibited significant antifungal activity against
Colletotrichum truncatum,
Microsporum gypseum, and
Trichophyton mentagrophytes, with inhibition zone diameters ranging from 28 to 41 mm (10 μg/disk). Structure-activity relationship studies revealed that substitutions at C-6, C-7, and C-6′, as well as the positions of carbonyl groups and double bonds, significantly influenced antifungal potency. Comparison of compounds
422–
424 and
429–
431 (or
425–
428) showed that a 6-methyl group enhanced antifungal activity, while substitution with a 6-hydroxyl group markedly reduced activity. Evaluation of compounds
421–
422 and
425 indicated that halogen atoms at C-7 contributed to antifungal efficacy, with bromine substitution at C-7 causing substantial changes in activity. Furthermore, comparing compounds
417–
420 with
422 demonstrated that hydroxylation at C-6′ significantly diminished antifungal activity [
99]. A known compound griseofulvin (
433), was isolated from the mangrove endophytic fungus
Arthrinium sp. SCSIO 41306. It inhibited LPS-induced NF-κB activation in RAW264.7 macrophages with an IC
50 value of 22.21 µM and showed no significant cytotoxicity in bone marrow-derived macrophages (BMMs) [
100]. Li et al. identified three new compounds, 14-hydroxybislongiquinolide (
434), 20-hydroxybislongiquinolide (
435), and 14,20-dihydroxybislongiquinolide (
436), along with four known compounds, bislongiquinolide (
437), bisorbicillinolide (
438), saturnispol B (
439), and bisvertinolone (
440), from the mangrove-derived fungus
Trichoderma reesei SCNU-F0042. Compound
435 exhibited moderate SARS-CoV-2 inhibitory activity with an EC
50 value of 29.0 µM [
101]. A known compound bisvertinol (
441), was isolated from the mangrove endophytic fungus
Hypocrea jecorina H8 [
102].
Three known compounds, isobisvertinol (
442), bisvertinol (
443), and trichodimerol (
444), were isolated from the mangrove endophytic fungus
Trichoderma sp. FM652. Compounds
442 and
443 inhibited TNF-α-induced NF-κB pathway activation with IC
50 values of 24.40 and 14.63 µM, respectively. Compound
444 showed moderate antibacterial activity against
Staphylococcus aureus and methicillin-resistant
S. aureus with an MIC value of 40.32 µM [
70]. Three new compounds, asperisocoumarin H (
445) and (±)-asperisocoumarin I [(±)-
446], and one known compound, pergillin (
447), were isolated from the mangrove endophytic fungus
Aspergillus sp. 085242. Compound
447 exhibited α-glucosidase inhibitory activity with an IC
50 value of 428.1 µM, stronger than the positive control acarbose (IC
50 = 725.1 µM) [
37]. Using an OSMAC strategy, Liu et al. identified two new compounds, furantides A-B (
448–
449), from the mangrove-derived fungus
Penicillium sp. HDN15-312 [
40]. A known compound, penicyclone A (
450), was isolated from the mangrove sediment-derived fungus
Penicillium sp. N-5. It was evaluated for cytotoxicity against SNB-19, MDA-MB-231, MDA-MB-435, and HCT-116 cell lines but showed no cytotoxic activity [
103]. A known compound (3
S)-3,8-dihydroxy-6,7-dimethyl-α-tetralone (
451), was isolated from the mangrove endophytic fungus
Daldinia eschscholzii MCZ-18. It exhibited broad-spectrum antibacterial activity against five pathogens, Enterococcus
faecalis, methicillin-resistant
Staphylococcus aureus,
Escherichia coli,
Pseudomonas aeruginosa, and
Candida albicans, with IC
50 values ranging from 6.25 to 50 µM [
9]. A known compound, 2-benzylpyrone (
452), was isolated from the mangrove endophytic fungus
Mycosphaerella sp. L3A1 [
50]. Two new compounds, phomasparapyrones A (
453) and B (
454), and one known compound, kojic acid (
455), were isolated from the mangrove endophytic fungus
Phomopsis asparagi LSLYZ-87. Compound
454 showed dose-dependent inhibition of LPS-induced NO accumulation in BV-2 cells at 30, 40, and 50 µM, with no cytotoxicity observed at 50.0 µM [
104]. Two new compounds, aspermicrone B (
456) and aspermicrone C (
457), were isolated from the mangrove endophytic fungus
Epicoccum nigrum SCNU-F0002 [
86].