Insights into the Bioactivities and Mechanism of Action of the Microbial Diketopiperazine Cyclic Dipeptide Cyclo(L-leucyl-L-prolyl)
Abstract
1. Introduction
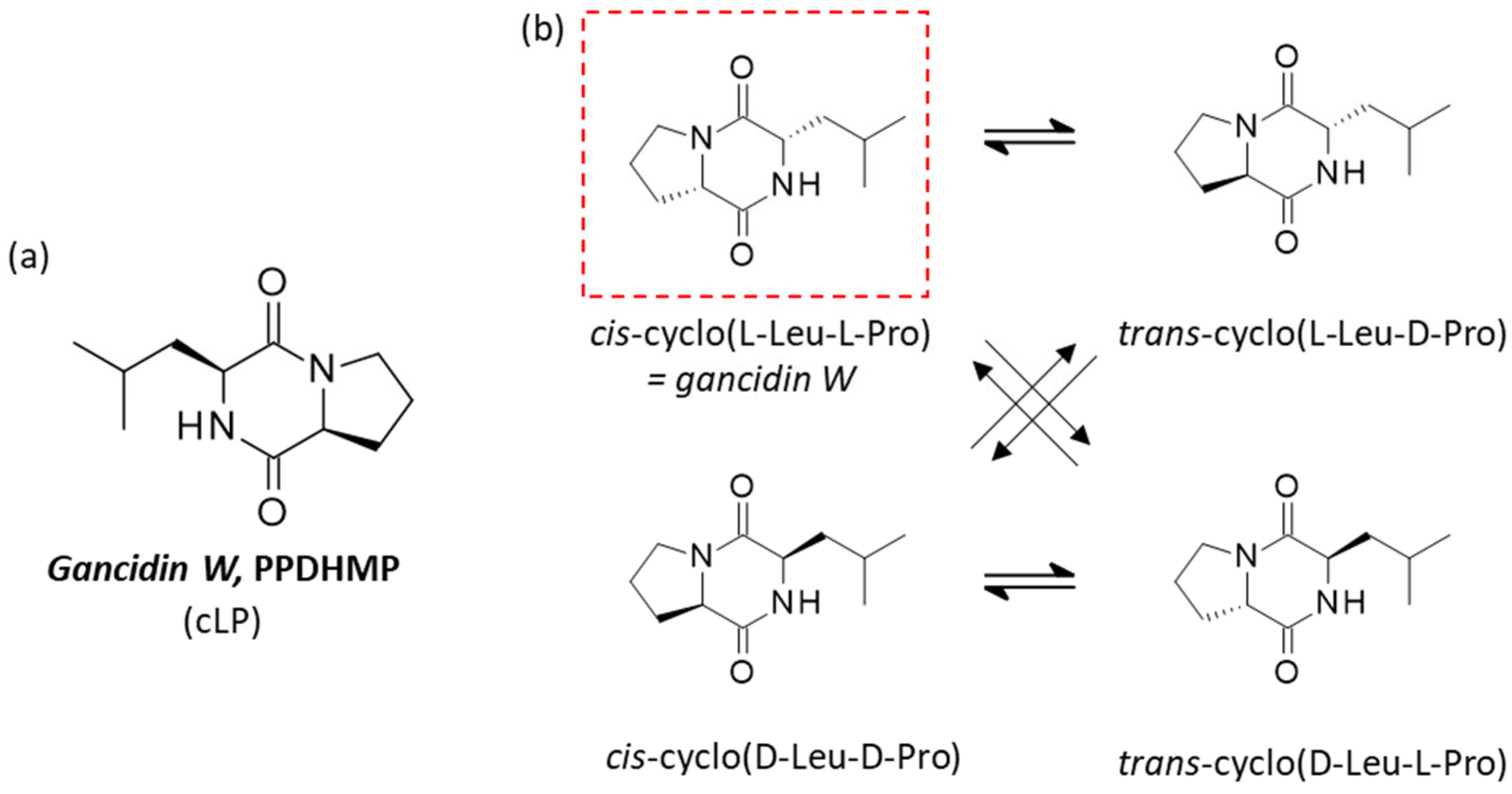
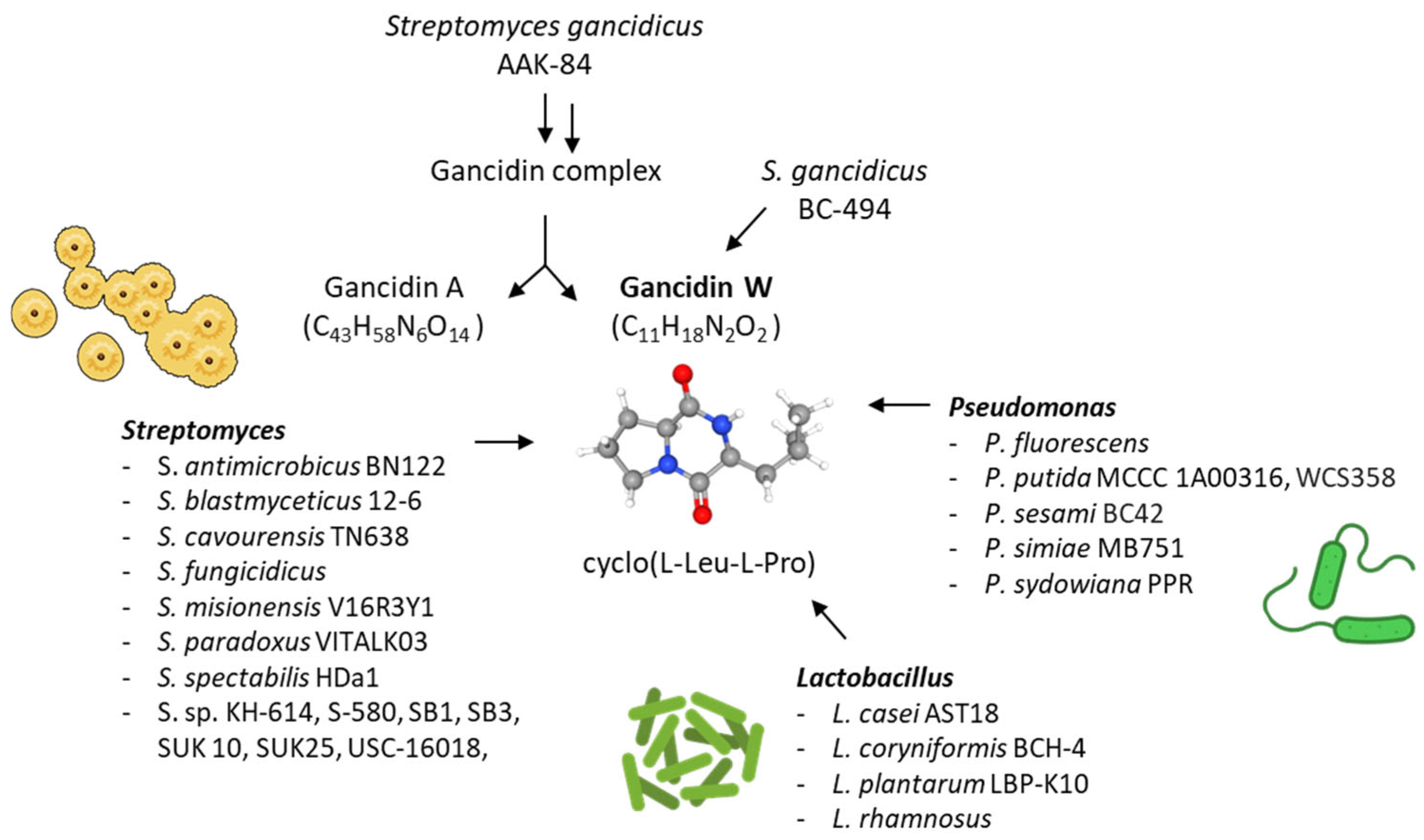
2. cLP as a Natural Product
| Microorganisms 1 | Org. 2 | Bioactivities | Refs |
|---|---|---|---|
| Achromobacter xylosoxidans NFRI-A1 | B (g−) | Inhibition of fungal growth by cLP isolated from this endophytic bacterium. cLP repressed transcription of the aflatoxin-related genes. | [62] |
| Aspergillus aculeatus F027 | F | Isolation of cyclo(L-Pro-L-Leu) and cyclo(L-Pro-L-Phe) and their antibacterial activities. | [63] |
| Bacillus amyloliquefaciens MMS-50 | B (g+) | Characterization of cLP and its activity against Streptococcus mutans, responsible for dental caries. | [64] |
| Antibiofilm activity of cLP against Listeria monocytogenes (MIC = 512 μg/mL). | [45] | ||
| Bacillus baekryungensis AMHSU | B (g+) | Isolation of PPDHMP (cLP) and characterization of its anti-inflammatory activity. | [65] |
| Bacillus pumilus GL0057 | B (g+) | Two DKPs, cyclo-(L-Leu-L-Pro) and cyclo-(L-Phe-L-Pro), identified from this marine bacterium isolated from the black coral Antipathes sp. | [66] |
| Bacillus sp. strain N | B (g+) | Marked activity of cyclo(l-Pro-l-Leu) against the fungus pathogen Penicillium expansum. | [67] |
| Bacillus sp. VITLTMJ4. | B (g+) | Identification of PPDHMP (cLP) isolated from this bacterial species endophyte of Citrus limon (Kaji nemu) and its antibacterial properties. | [50] |
| Bacillus vallismortis BS07 | B (g+) | Characterization of cyclic dipeptides, including cyclo(L-Leu-L-Pro), and their role in disease resistance in Arabidopsis against Pseudomonas syringae infection. | [68] |
| Bacillus velezensis Ea73 | B (g+) | Cyclo(L-Leu-L-Pro) and cyclo(L-Pro-L-Val) extracted from this endophytic bacterium in the poisonous weed Ageratina adenophora. | [69] |
| Brevibacillus laterosporus | B (g+) | Identification of cyclo(Leu-Pro) and its activity against several pathogenic microorganisms. | [70] |
| Corollospora pulchella | F | Production of gancidin W by the marine fungus C. pulchella. | [33,34] |
| Cronobacter sakazakii | B (g−) | The role of cyclo(l-Pro-l-Leu) as a quorum-sensing signal between C. sakazakii and Bacillus cereus. | [71] |
| Exiguobacterium acetylicum S01 | B (g+) | Four DKPs identified, including cLP, capable of inducing cell growth arrest and apoptosis of HT-29 cancer cells. The four DKPs inhibited tumor progression in a zebrafish xenograft model. | [72] |
| Exiguobacterium R2567 | B (g+) | In this bacterium, cyclo(Leu-Pro) activates the rice strigolactone signaling pathway by binding to the SL receptor OsD14, so as to regulate tillering. | [73] |
| Galactomyces geotrichum | F | Identification of cyclo(Leu-Pro) as a metabolite in this species from Laminaria japonica. | [74] |
| Haemophilus influenzae Rd KW20 | B (g−) | Identification of cyclo(Leu-Pro) as a metabolite in this species via a genome-scale metabolic model. | [75] |
| Lactiplantibacillus plantarum CCFM8724 | B (g+) | Identification of cyclo(leu-pro) and cyclo(phe-pro) and their roles as biofilm inhibitors. | [76] |
| Lactobacillus casei AST18 | B (g+) | Identification of cyclo(Leu-Pro) and its synergistic antifungal effect with lactic acid against Penicillium sp. | [77] |
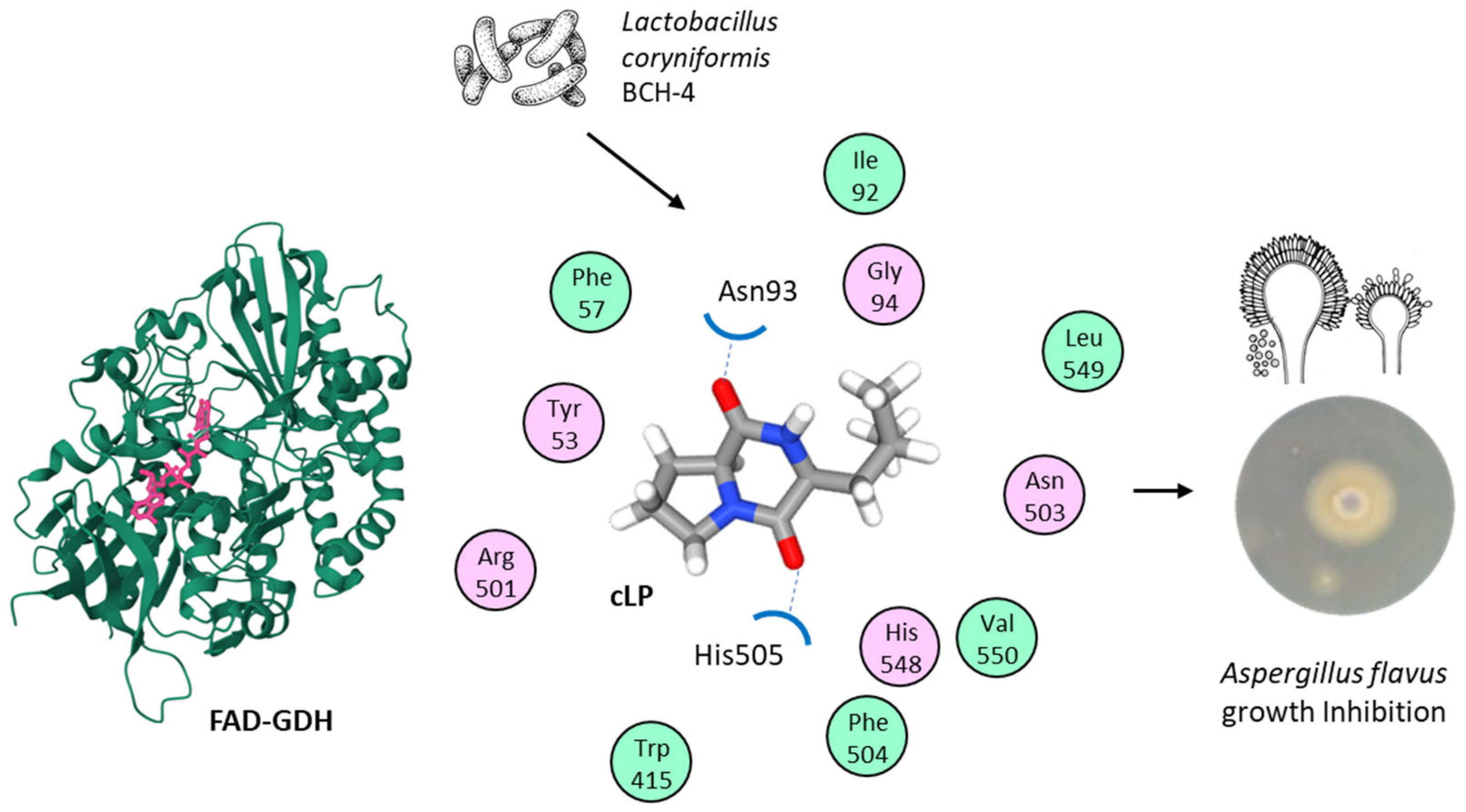
| Lactobacillus coryniformis BCH-4 (Loigolactobacillus coryniformis BCH-4) | B (g+) | Identification of cLP and characterization of its antifungal action against Aspergillus flavus and potential target proteins. Bioprotective activity. | [59] |
| Lactobacillus plantarum LBP-K10 | B (g+) | Identification of five DKPs, including cyclo(l-Leu-l-Pro), and their inhibitory effects against Ganoderma boninense. | [54,55] |
| Antiviral activity of fractions containing cis-cyclo(L-Leu-L-Pro) against Influenza virus. | [56] | ||
| Antimicrobial activity of cyclo(L-Leu-L-Pro) against multidrug-resistant bacteria, alone and in combination with a microbial fraction (Q9). | [78] | ||
| Lactobacillus rhamnosus | B (g+) | Identification of cyclo(L-Leu-L-Pro) and its antibiofilm activity. | [79] |
| Lactococcus lactis subsp. cremoris | B (g+) | Identification of cLP in this species. | [80] |
| Lasiodiplodia iranensis F0619 | F | Identification of cLP in a fractionated extract of L. iranensis isolated from the Panamean mangrove Avicennia germinans. | [81] |
| Leuconostoc mesenteroides LBP-K06 | B (g+) | Bacteria found in fermented food kimchi and characterization of cyclo(Leu-Pro) and its activity against different Gram-positive/negative bacteria. | [61] |
| Optimization of culture conditions to produce DKPs in this system, notably with co-culture of Lb. plantarum LBP-K10 and Leu. mesenteroides LBP-K06. | [82] | ||
| Limosilactobacillus reuteri LR-9 (Lactobacillus reuteri) | B (g+) | Identification of cyclo(L-Pro-L-Leu), cyclo(L-Pro-L-Phe), and the antifungal activity of bacterial fractions. | [83] |
| Lysobacter capsici AZ78 | B (g−) | Isolation of cyclo(l-Pro-l-Leu) and other DKPs, and their activity against Gram-positive bacterium Rhodococcus fascians LMG 3605. | [84] |
| Marchantia polymorpha | L | Identification of cLP and cyclo(l-Phe-l-Pro) produced by endophytes from M. polymorpha. | [85] |
| Nocardia ignorata | B (g+) | Identification of cyclo(l-Pro-l-Leu) and other DKPs from this actinobacterium isolated from the terrestrial lichen Collema auriform. | [86] |
| Nocardiopsis sp. GRG 1 | B (g+) | Activity of a bacterial extract against biofilm forming uropathogens and identification of PPDHMP (cLP) from this species. | [53] |
| Nocardiopsis sp. HT88 | B (g+) | Identification of 8 DKPs, including cyclo(L-Pro-L-Leu), from the endophytic bacterium of Mallotus nudiflorus L. | [87] |
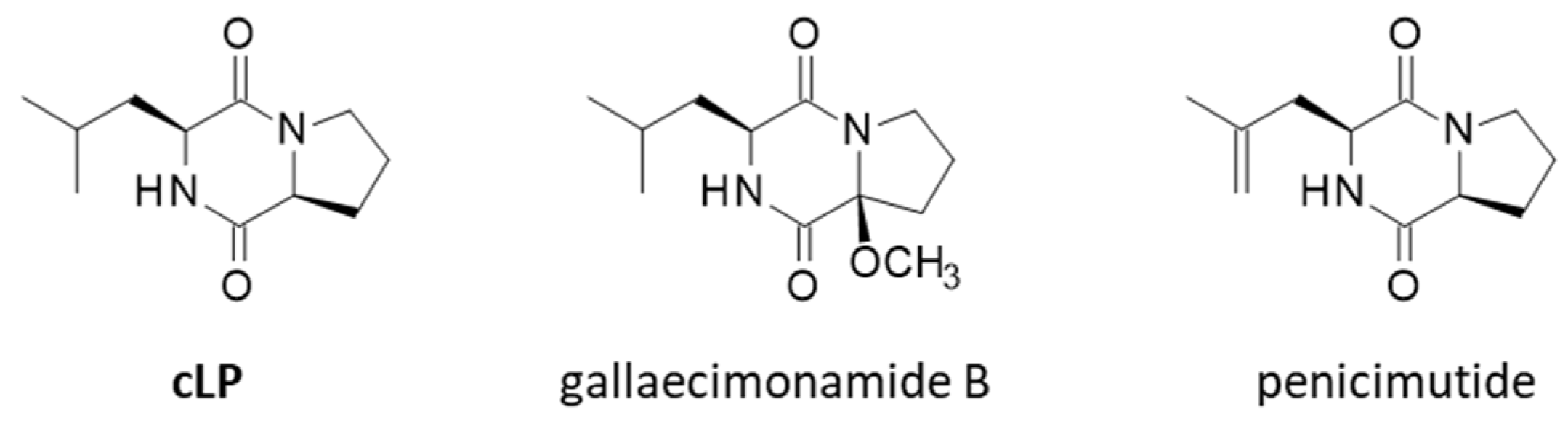
| Penicillium purpurogenum G59 | F | Different Pro-containing DKPs, including cLP, were isolated from a neomycin-resistant mutant of this marine-derived fungus, together with penicimutide. | [88] |
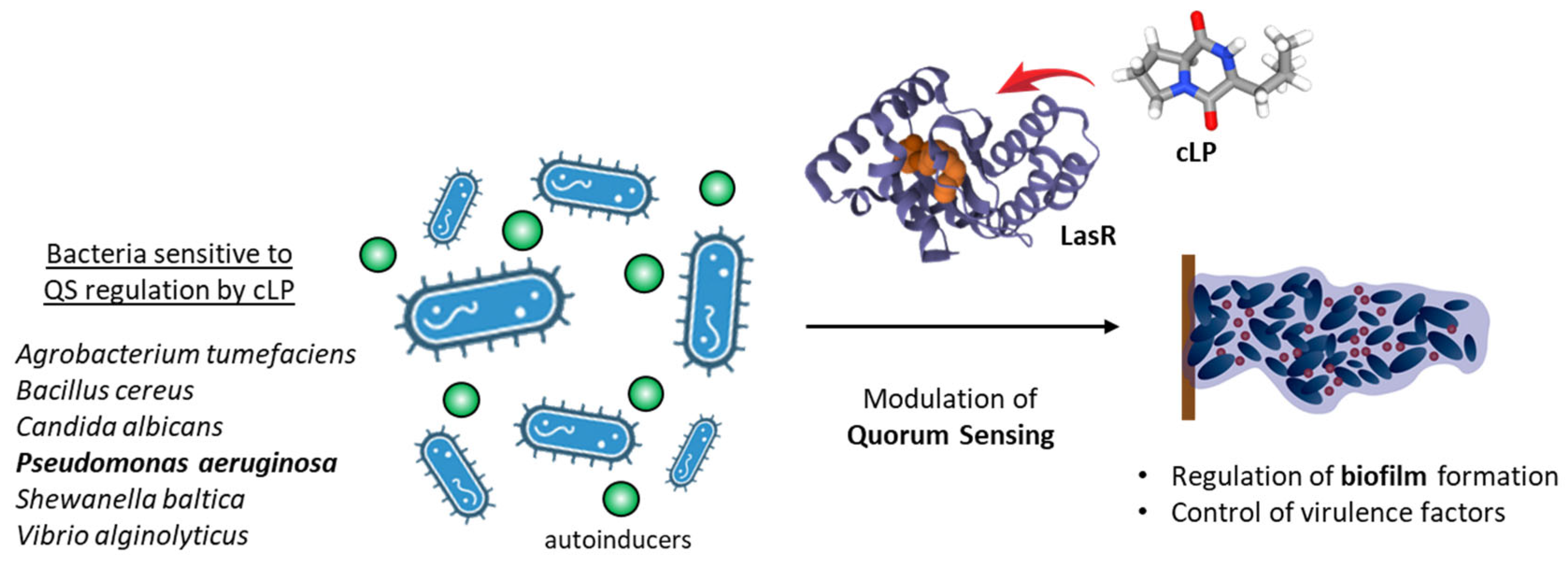
| Pestalotiopsis sydowiana PPR | F | cLP from the marine fungal P. sydowiana inhibits biofilm formation by Pseudomonas aeruginosa PAO1 at sub-toxic concentrations. Anti-QS activity at sub-MIC concentrations of cLP. | [89] |
| Pithomyces sacchari | F | cLP isolated with two other DKPs from the endophytic fungus Pithomyces sacchari of the Laurencia sp. collected in the South China sea. | [90] |
| Pseudofusicoccum sp. | F | Isolation and characterization of cyclo(L-Pro-L-Val) and cyclo(L-Leu-L-Pro) in this fungus, which produces the burgundy pigment upon fermentation. | [91] |
| Pseudomonas fluorescens | B (g−) | Isolation of cyclo(L-Leu-L-Pro) and bactericidal activity against S. aureus and P. aeruginosa. | [92] |
| Pseudomonas putida MCCC 1A00316 | B (g−) | Identification of cyclo(l-Pro-l-Leu) and characterization of its activity against the nematode Meloidogyne incognita. The product increased the mortality rates of second-stage juveniles (J2) of M. incognita. | [93] |
| Pseudomonas putida WCS358 | B (g−) | Identification of four DKPs, including cyclo(l-Leu-l-Pro), and characterization of their capacity to activate the quorum-sensing biosensors of the plant pathogen Agrobacterium tumefaciens. | [94] |
| Pseudomonas sesami BC42 | B (g−) | Identification of three isomers of cyclo(Leu-Pro), including cyclo(l-Leu-l-Pro), which potently reduced conidia germination and leaf lesion size caused by the fungal pathogen Colletotrichum orbiculare. | [95] |
| Pseudomonas simiae MB751 | B (g−) | Isolation of cyclo(L-Pro-L-Leu) from this nematicidal bacterium and its capacity to kill the root-knot nematode Meloidogyne incognita. | [96] |
| Pseudomonas sp. PTR-08 | B (g−) | Antioxidant and anti-glycation activities of the bacterial extract that contains PPDHMP (cLP). | [97] |
| Rheinheimera japonica KMM 9513T | B (g−) | cLP and other DKPs identified from this marine bacterium. No antibacterial activity observed with cLP. | [98] |
| Rosellinia necatrix | F | Identification of three DKPs, including cyclo(Leu-Pro), and their capacity to inhibit the growth of plant seedlings and plant roots. | [99] |
| Ruegeria sp. | B (g−) | Identification and structural characterization of cyclo(Leu-Pro) in an extract of Ruegeria sp. from Indonesia. | [100] |
| Sceloporus virgatus (lizard) | A | Identification of cyclo(L-Leu-L-Pro) and cyclo(L-Pro-L-Pro) in the femoral gland secretions of the lizard. | [101] |
| Shewanella baltica SA02 | B (g−) | Production of cyclic dipeptides, including cyclo(L-Pro-L-Leu), and their role in the production of biofilm matrixes. | [102] |
| Staphylococcus xylosus VITURAJ10 | B (g+) | Isolation of PPDHMP (cLP) from the strain VITURAJ10 isolated from goat milk and antimicrobial activity of the bacterial extract. | [46] |
| Staphylococcus sp. MB30 | B (g+) | Isolation of PPDHMP from this marine bacterium and characterization of its antiproliferative and pro-apoptotic properties using lung (A549) and cervical (HeLa) cancer cells. | [52] |
| Streptomyces antimicrobicus BN122. | B (g+) | Cyclo-(L-Pro-L-Xxx) DKPs, including cLP, identified from the Streptomyces strain, which is an endophyte in Oryza sativa. | [103] |
| Streptomyces blastmyceticus 12-6 | B (g+) | Identification of cyclo-(Leu-Pro) and characterization of its activity against several pathogenic fungi, notably the spores of Colletotrichum acutatum responsible for anthracnose in plants. | [104] |
| Streptomyces cavourensis TN638 | B (g+) | cLP identified as one of the DKPs present in the studied extracts, and its antibacterial activities. | [105] |
| Streptomyces fungicidicus | B (g+) | Isolation of cyclo(l-Leu-l-Pro) and four other DKPs from a culture of this deep-sea actinomycete bacterium, and their activities against the larvae of the barnacle Balanus amphitrite. | [106] |
| Streptomyces gancidicus BC-494 | B (g+) | The first strain from which gancidin W (cLP) was identified and structurally characterized. | [32] |
| Streptomyces griseorubens K5 | B (g+) | Metabolite profiling of the bacterial extract and identification of PPDHMP (cLP). | [107] |
| Streptomyces lavendulae No. 314. | B (g+) | Isolation of Pro-containing DKP, including cLP, from a culture filtrate of this species. | [108] |
| Streptomyces misionensis V16R3Y1 | B (g+) | Identification of cyclo(l-Leu-l-Pro) in fractions active against human pathogenic bacteria. | [58] |
| Streptomyces paradoxus VITALK03 | B (g+) | Production of gancidin W by this marine strain. Evaluation of its cytotoxic properties (IC50 = 1.56 μg/mL against MCF7 breast cancer cells) and potential binding to protein targets using molecular modeling (binding to Kras). | [35,36] |
| Streptomyces sp. KH-614 | B (g+) | Marked activity against vancomycin-resistant enterococci, notably E. faecalis (strains K-99-34, K-00-184, and K-00-221); MIC = 12.5 m g/mL. | [37] |
| Potent activity of cyclo(l-Leu-l-Pro) against the phytopathogenic fungus Pyricularia oryzae IFO5994 (MIC = 2.5 mg/mL). | [109] | ||
| Cyclo(l-Leu-l-Pro) inhibits the growth of different pathogenic microorganisms and displays anti-mutagenic effects in Salmonella strains. | [110] | ||
| Streptomyces sp. S2A | B (g+) | Antibacterial activities of extracts from this species and characterization of PPDHMP (cLP) as a main bioactive product. | [111] |
| Streptomyces sp. S-580 | B (g+) | Isolation of l-Leucyl-l-Proline anhydride and the formation mechanism of l-prolyl diketopiperazines. | [29,112,113] |
| Streptomyces sp. SB1 and SB3 | B (g+) | Identification of cLP and other DKPs produced by Streptomyces species SB1 and SB3. | [114] |
| Streptomyces sp. SUK 10 | B (g+) | Gancidin W was produced by bacteria Streptomyces, sp. SUK10, identified from the bark of the Shorea ovalis tree. The dipeptide was shown to inhibit the growth of Plasmodium berghei PZZ1/100 in mice. | [38,39] |
| Streptomyces sp. SUK 25 | B (g+) | Identification of five DKPs, including cLP, and their activities against methicillin-resistant S. aureus and Enterococcus raffinosus. | [115] |
| Streptomyces sp. USC-16018 | B (g+) | Antiplasmodial activity of cyclo(l-Pro-l-Leu), but not cyclo(l-Pro-l-Phe), cyclo(l-Pro-l-Val), and cyclo(l-Pro-l-Ty), against Plasmodium falciparum strains 3D7 and Dd2, without cytotoxicity. | [116] |
| Streptomyces sp. VITMK1 | B (g+) | Isolation of PPDHMP (cLP) and characterization of its free radical scavenging activity. | [117] |
| Streptomyces spectabilis HDa1 | B (g+) | Isolation of three DKPs, including cyclo-(L-Leu-l-Pro). No acetylcholinesterase inhibitory activity observed with this DKP. | [118] |
| Veillonella tobetsuensis | B (g−) | Characterization of cLP and its capacity to inhibit Streptococcus gordonii biofilm development. | [119] |
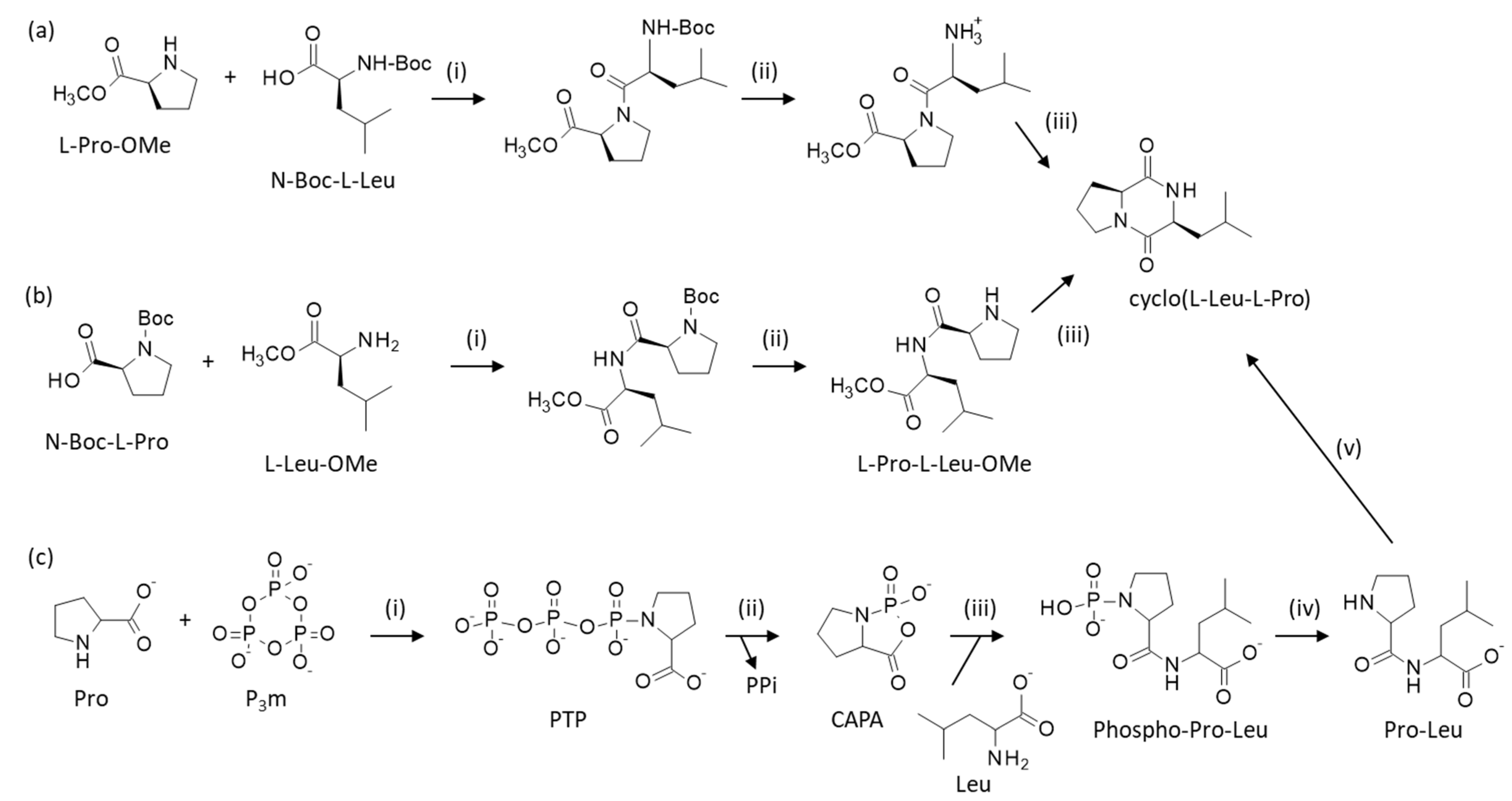
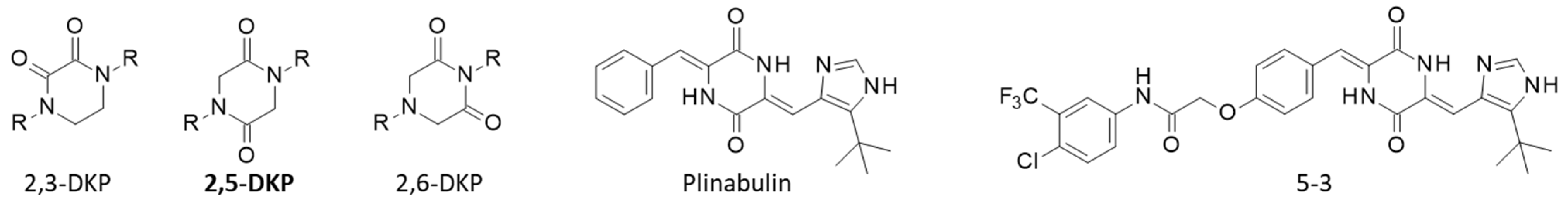
3. Chemical Synthesis of cLP
4. Bioactivities of cLP
4.1. Antioxidant and Bioprotective Activities
4.2. Antibacterial Activities
4.3. Anticariogenic Activity
4.4. Antifungal Activity
4.5. Antiparasitic Activity
4.6. Anticancer Activity
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osorio-Nieto, U.; Salas, C.O.; Mendez-Alvarez, D.; Rivera, G.; Moreno-Rodriguez, A.; Perez-Cervera, Y.; Castillo-Real, L.M. Espinosa-Bustos C.2,3-Diketopiperazine as potential scaffold to develop new anti-Chagasic agents. Med. Chem. Res. 2023, 32, 176–188. [Google Scholar] [CrossRef]
- Du, R.R.; Wang, R.Y.; Zhou, J.C.; Gao, H.H.; Qin, W.J.; Duan, X.M.; Yang, Y.N.; Zhang, X.W.; Zhang, P.C. Heteryunine A, an amidated tryptophan-catechin-spiroketal hybrid with antifibrotic activity from Heterosmilax yunnanensis. Bioorganic Chem. 2024, 151, 107618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Pan, G.; Li, Y.; Ma, G.; Hou, Y.; Zhu, N.; Xu, X. Orychophragvioline A, a Novel Alkaloid Isolated from Orychophragmus violaceus with Anti-Cervical Cancer Activity. Molecules 2025, 30, 1759. [Google Scholar] [CrossRef] [PubMed]
- Dawidowski, M.; Herold, F.; Chodkowski, A.; Kleps, J.; Szulczyk, P.; Wilczek, M. Synthesis and anticonvulsant activity of novel 2,6-diketopiperazine derivatives. Part 1: Perhydropyrrole[1,2-a]pyrazines. Eur. J. Med. Chem. 2011, 46, 4859–4869. [Google Scholar] [CrossRef]
- Dawidowski, M.; Herold, F.; Chodkowski, A.; Kleps, J. Synthesis and anticonvulsant activity of novel 2,6-diketopiperazine derivatives. Part 2: Perhydropyrido[1,2-a]pyrazines. Eur. J. Med. Chem. 2012, 48, 347–353. [Google Scholar] [CrossRef]
- Dawidowski, M.; Turło, J. Multicomponent synthesis and anticonvulsant activity of monocyclic 2,6-diketopiperazine derivatives. Med. Chem. Res. 2014, 23, 2007–2018. [Google Scholar] [CrossRef]
- Fytas, G.; Zoidis, G.; Taylor, M.C.; Kelly, J.M.; Tsatsaroni, A.; Tsotinis, A. Novel 2,6-diketopiperazine-derived acetohydroxamic acids as promising anti-Trypanosoma brucei agents. Future Med. Chem. 2019, 11, 1259–1266. [Google Scholar] [CrossRef]
- Fytas, G.; Zoidis, G.; Drakopoulos, A.; Taylor, M.C.; Kelly, J.M.; Tsatsaroni, A.; Tsotinis, A. New Lipophilic Hydroxamates as Promising Trypanocidal Agents: Design, Synthesis, SAR, and Conformational Behavior Studies. ACS Med. Chem. Lett. 2024, 15, 1041–1048. [Google Scholar] [CrossRef]
- Garrido González, F.P.; Macías Pérez, M.E.; Rodríguez Cortés, O.; Mera Jiménez, E.; Mancilla Percino, T. Selective Antiproliferative and Apoptotic Effects of 2,6-Diketopiperazines on MDA-MB-231 Triple-Negative Breast Cancer. Chem. Biol. Drug Des. 2025, 105, e70098. [Google Scholar] [CrossRef]
- Khong, Q.T.; Smith, E.A.; Wendt, K.L.; Dalilian, M.; Goncharova, E.I.; Brownell, I.; Cichewicz, R.H.; Henrich, C.J.; Beutler, J.A.; O’Keefe, B.R.; et al. Chemoreactive 2,5-Diketopiperazines from a Penicillium sp., Structure Revision of Reported Analogues and Proposed Facile Transformation Pathways. J. Nat. Prod. 2024, 87, 1826–1837. [Google Scholar] [CrossRef]
- Walker, K.L.; Loach, R.P.; Movassaghi, M. Total synthesis of complex 2,5-diketopiperazine alkaloids. Alkaloids Chem. Biol. 2023, 90, 159–206. [Google Scholar]
- Wang, S.; Zhong, C.; Li, F.; Ding, Z.; Tang, Y.; Li, W. Design, synthesis, and structure-activity relationship study of novel plinabulin derivatives as anti-tumor agents based on the co-crystal structure. Mol. Divers. 2025, 29, 3877–3898. [Google Scholar] [CrossRef]
- Han, B.; Feinstein, T.; Shi, Y.; Chen, G.; Yao, Y.; Hu, C.; Shi, J.; Feng, J.; Wu, H.; Cheng, Y.; et al. Plinabulin plus docetaxel versus docetaxel in patients with non-small-cell lung cancer after disease progression on platinum-based regimen (DUBLIN-3): A phase 3, international, multicentre, single-blind, parallel group, randomised controlled trial. Lancet Respir. Med. 2024, 12, 775–786. [Google Scholar] [CrossRef]
- Bi, S.; Cao, Y.; Fang, S.; Chu, Y.; Zhang, Z.; Li, M.; Yu, R.; Yang, J.; Tang, Y.; Qiu, P. The Novel Diketopiperazine Derivative, Compound 5-3, Selectively Inhibited the Proliferation of FLT3-ITD Mutant Acute Myeloid Leukemia (AML) Cells. Mar. Drugs 2025, 23, 289. [Google Scholar] [CrossRef]
- Goher, S.S.; Abdrabo, W.S.; Veerakanellore, G.B.; Elgendy, B. 2,5-Diketopiperazines (DKPs): Promising Scaffolds for Anticancer Agents. Curr. Pharm. Des. 2024, 30, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Harken, L.; Li, S.M. Modifications of diketopiperazines assembled by cyclodipeptide synthases with cytochrome P(450) enzymes. Appl. Microbiol. Biotechnol. 2021, 105, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef]
- Turkez, H.; Cacciatore, I.; Arslan, M.E.; Fornasari, E.; Marinelli, L.; Di Stefano, A.; Mardinoglu, A. Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates. Biomolecules 2020, 10, 737. [Google Scholar] [CrossRef]
- Kumar, S.N.; Mohandas, C.; Nambisan, B. Purification, structural elucidation and bioactivity of tryptophan containing diketopiperazines, from Comamonas testosteroni associated with a rhabditid entomopathogenic nematode against major human-pathogenic bacteria. Peptides 2014, 53, 48–58. [Google Scholar] [CrossRef]
- Mosetti, V.; Rosetti, B.; Pierri, G.; Bellotto, O.; Adorinni, S.; Bandiera, A.; Adami, G.; Tedesco, C.; Crosera, M.; Magnano, G.C.; et al. Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity. Biomedicines 2022, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Aiso, K.; Arai, T.; Suzuki, M.; Takamizawa, Y. Gancidin, An Antitumor Substance Derived from Streptomyces sp. I. J. Antibiot. Ser. A 1956, 9, 97–101. [Google Scholar]
- Wakaki, S.; Marumo, H.; Tomioka, K.; Shimizu, M.; Kato, E.; Kamada, H.; Kudo, S.; Fujimoto, Y. Purification and Isolation Study on Gancidins. J. Antibiot. Ser. A 1958, 11, 150–155. [Google Scholar]
- Suzuki, M. Studies on an antitumor substance, gancidin. Mycological study on the strain AAK-84 and production, purification of active fractions. J. Chiba Med. Soc. 1957, 33, 535–542. [Google Scholar]
- Suzuki, M. Studies on Antibacterial Activity of Gancidin (Rept. 2.). J. Chiba Med. Soc. 1957, 33, 528–535. [Google Scholar]
- Arai, T.A.; Suzuki, M. A rapid agar dilution technique for the estimation of antitumor-cell activity. J. Antibiot. Ser. A 1956, 9, 169–171. [Google Scholar]
- Johnson, J.L.; Jackson, W.G.; Eble, T.E. Isolation of L-leucyl-Lproline anhydride from microbiological fermentations. J. Am. Chem. Soc. 1951, 3, 2947–2948. [Google Scholar] [CrossRef]
- Kosuge, T.; Kamiya, H. L-Leucyl-L-Proline from peptone. Chem. Pharm. Bull. 1962, 10, 154–155. [Google Scholar] [CrossRef]
- Koaze, Y. Isolation of l-Leucyl-l-Proline Anhydride from the Culture Filtrate of Streptomyces sp. S-580. J. Agric. Chem. Soc. Japan 1960, 24, 530–531. [Google Scholar]
- Vickery, H.B.; Osborne, T.B. A review of hypotheses of the structure of proteins. Phys. Rev. 1928, 8, 393–446. [Google Scholar]
- Inagaki, N. Isolation and Properties of Helmintin (C11H18N2O2), an Antifungal Substance produced by Helminthosporium siccans. Chem. Pharm. Bull. 1962, 10, 152–154. [Google Scholar] [CrossRef]
- Jain, T.C.; Dingerdissen, J.; Weisbach, J.A. Isolation and Structure Elucidation of Gancidin W. Heterocycles 1977, 7, 341–346. [Google Scholar] [CrossRef]
- Furuya, K.; Okudaira, M.; Shindo, T.; Sato, A. Corollospora pulchella, a marine fungus producing antibiotics, melinacidins III, IV and gancidin W. Annu. Rep. Sankyo Res. Lab. 1985, 37, 140–142. [Google Scholar]
- Biabani, M.A.F.; Laatsch, H. Advances in chemical studies on low-molecular weight metabolites of marine fungi. J. Prakt. Chem./Chem.-Ztg. 1998, 340, 589–607. [Google Scholar] [CrossRef]
- Ravi, L.; Ragunathan, A.; Krishnan, K. Marine Streptomyces paradoxus VITALK03 derived gancidin W mediated cytotoxicity through Ras-Raf-MEK-ERK signalling pathway. Indian J. Biotechnol. 2017, 16, 164–175. [Google Scholar]
- Ravi, L.; Ragunathan, A.; Krishnan, K. Antidiabetic and Antioxidant Potential of GancidinW from VITALK03. Open Bioact. Compd. J. 2017, 5, 31–42. [Google Scholar] [CrossRef]
- Rhee, K.H. Isolation and characterization of Streptomyces sp. KH-614 producing anti-VRE (vancomycin-resistant enterococci) antibiotics. J. Gen. Appl. Microbiol. 2002, 48, 321–327. [Google Scholar] [CrossRef]
- Baba, M.S.; Zin, N.M.; Hassan, Z.A.; Latip, J.; Pethick, F.; Hunter, I.S.; Edrada-Ebel, R.; Herron, P.R. In vivo antimalarial activity of the endophytic actinobacteria, Streptomyces SUK 10. J. Microbiol. 2015, 53, 847–855. [Google Scholar] [CrossRef]
- Zin, N.M.; Baba, M.S.; Zainal-Abidin, A.H.; Latip, J.; Mazlan, N.W.; Edrada-Ebel, R. Gancidin W, a potential low-toxicity antimalarial agent isolated from an endophytic Streptomyces SUK10. Drug Des. Dev. Ther. 2017, 11, 351–363. [Google Scholar] [CrossRef]
- Ravi, L.; Kannabiran, K. Bioactivity-Guided Extraction and Identification of Antibacterial Compound from Marine Actinomycetes Strains Isolated from Costal Soil Samples of Rameswaram and Dhanushkodi, Tamil Nadu, India. Asian J. Pharm. 2016, 10, 504–509. [Google Scholar]
- Ravi, L.; Kannabiran, K. Extraction and Identification of Gancidin W from Marine Streptomyces sp. VITLGK012. Indian J. Pharm. Sci. 2018, 80, 1093–1099. [Google Scholar] [CrossRef]
- Khan, M.S.; Gao, J.; Munir, I.; Zhang, M.; Liu, Y.; Moe, T.S.; Xue, J.; Zhang, X. Characterization of Endophytic Fungi, Acremonium sp., from Lilium davidii and Analysis of Its Antifungal and Plant Growth-Promoting Effects. Biomed. Res. Int. 2021, 2021, 9930210. [Google Scholar] [CrossRef]
- Mangamuri, U.K.; Muvva, V.; Poda, S.; Manavathi, B.; Bhujangarao, C.; Yenamandra, V. Chemical characterization & bioactivity of diketopiperazine derivatives from the mangrove derived Pseudonocardia endophytica. Egypt. J. Aquat. Res. 2016, 42, 169–175. [Google Scholar] [CrossRef]
- Jamal, Q.; Cho, J.Y.; Moon, J.H.; Munir, S.; Anees, M.; Kim, K.Y. Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita. Molecules 2017, 22, 1839. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Sivaranjani, M.; Kamaladevi, A.; Ravi, A.V.; Balamurugan, K.; Karutha Pandian, S. Cyclic dipeptide cyclo(l-leucyl-l-prolyl) from marine Bacillus amyloliquefaciens mitigates biofilm formation and virulence in Listeria monocytogenes. Pathog. Dis. 2016, 74, ftw017. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, S.; Zhu, C.; Zhao, F.; Yu, Y.; Yue, Z.; Liu, B.; Yang, Y.; Dai, J.; Shi, J. Studies on constituents of cultures of fungus Phellinus igniarius. Zhongguo Zhong Yao Za Zhi 2011, 36, 874–880. [Google Scholar] [PubMed]
- Mangrolia, U.; Osborne, W.J. Staphylococcus xylosus VITURAJ10: Pyrrolo [1,2alpha] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) (PPDHMP) producing, potential probiotic strain with antibacterial and anticancer activity. Microb. Pathog. 2020, 147, 104259. [Google Scholar] [CrossRef]
- Al-Askar, A.; Al-Otibi, F.O.; Abo-Zaid, G.A.; Abdelkhalek, A. Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl), as the primary secondary metabolite of Bacillus spp., could be an effective antifungal agent against the soil-borne fungus, Sclerotium bataticola. Egypt. J. Chem. 2024, 67, 1009–1022. [Google Scholar] [CrossRef]
- Manam, M.; Srivatsa, S.; Osborne, W.J. Endophytic bacteria of Gracilaria edulis in combating human bacterial pathogens by PPDHMP—A crude to single molecule product development approach. Microb. Pathog. 2025, 202, 107431. [Google Scholar] [CrossRef]
- Buragohain, T.; Dey, P.; Osborne, W.J. In vitro studies on the inhibition of microbial pathogens by PPDHMP synthesized by Bacillus sp.; an endophyte of Citrus limon (Kaji nemu). Food Biosci. 2023, 55, 103003. [Google Scholar] [CrossRef]
- Prasad, J.K.; Pandey, P.; Anand, R.; Raghuwanshi, R. Drought Exposed Burkholderia seminalis JRBHU6 Exhibits Antimicrobial Potential Through Pyrazine-1,4-Dione Derivatives Targeting Multiple Bacterial and Fungal Proteins. Front. Microbiol. 2021, 12, 633036. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Veena, V.; Vidhyapriya, P.; Lakshmi, P.; Krishna, R.; Sakthivel, N. Anticancer potential of pyrrole (1, 2, a) pyrazine 1, 4, dione, hexahydro 3-(2-methyl propyl) (PPDHMP) extracted from a new marine bacterium, Staphylococcus sp. strain MB30. Apoptosis 2016, 21, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.; Vijayan, R.; Maruthupandy, M.; Vaseeharan, B.; Manoharan, N. Antibiofilm effect of Nocardiopsis sp. GRG 1 (KT235640) compound against biofilm forming Gram negative bacteria on UTIs. Microb. Pathog. 2018, 118, 190–198. [Google Scholar] [CrossRef]
- Kwak, M.K.; Liu, R.; Kwon, J.O.; Kim, M.K.; Kim, A.H.; Kang, S.O. Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza A virus. J. Microbiol. 2013, 51, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Liu, R.; Kim, M.K.; Moon, D.; Kim, A.H.; Song, S.H.; Kang, S.O. Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 2014, 52, 64–70. [Google Scholar] [CrossRef]
- Son, J.; Hong, Y.; Seong, H.; Oh, Y.S.; Kwak, M.K. The high-throughput solid-phase extraction of cis-cyclo(L-Leu-L-Pro) and cis-cyclo(L-Phe-L-Pro) from Lactobacillus plantarum demonstrates efficacy against multidrug-resistant bacteria and influenza A (H3N2) virus. Front. Mol. Biosci. 2024, 11, 1346598, Erratum in Front. Mol. Biosci. 2025, 12, 1605848. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.C.; Sang, M.K. Identification of isomeric cyclo(leu-pro) produced by Pseudomonas sesami BC42 and its differential antifungal activities against Colletotrichum orbiculare. Front. Microbiol. 2023, 14, 1230345. [Google Scholar] [CrossRef]
- Saadouli, I.; Zendah El Euch, I.; Trabelsi, E.; Mosbah, A.; Redissi, A.; Ferjani, R.; Fhoula, I.; Cherif, A.; Sabatier, J.M.; Sewald, N.; et al. Isolation, Characterization and Chemical Synthesis of Large Spectrum Antimicrobial Cyclic Dipeptide (l-leu-l-pro) from Streptomyces misionensis V16R3Y1 Bacteria Extracts. A Novel 1H NMR Metabolomic Approach. Antibiotics 2020, 9, 270. [Google Scholar] [CrossRef]
- Salman, M.; Tariq, A.; Mustafa, G.; Javed, M.R.; Naheed, S.; Qamar, S.A. Cyclo(L-Leucyl-L-Prolyl) from Lactobacillus coryniformis BCH-4 inhibits the proliferation of Aspergillus flavus: An in vitro to in silico approach. Arch. Microbiol. 2022, 204, 267. [Google Scholar] [CrossRef]
- Ivanov, I.; Petrov, K.; Lozanov, V.; Hristov, I.; Wu, Z.J.; Liu, Z.M.; Petrova, P. Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt. Processes 2021, 9, 114. [Google Scholar] [CrossRef]
- Liu, R.; Kim, A.H.; Kwak, M.K.; Kang, S.O. Proline-Based Cyclic Dipeptides from Korean Fermented Vegetable Kimchi and from Leuconostoc mesenteroides LBP-K06 Have Activities against Multidrug-Resistant Bacteria. Front. Microbiol. 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.S.; Song, Y.; Sakuno, E.; Nakajima, H.; Nakagawa, H.; Yabe, K. Cyclo(L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 2004, 70, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, F.; Jin, X.; Jiang, J.; Hu, S.; Cheng, L.; Zhang, G. A new diketopiperazine from an endophytic fungus Aspergillus aculeatus F027. Nat. Prod. Res. 2021, 35, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Poornima, B.; Pandian, S.K. Inhibitory efficacy of cyclo(L-leucyl-L-prolyl) from mangrove rhizosphere bacterium-Bacillus amyloliquefaciens (MMS-50) toward cariogenic properties of Streptococcus mutans. Res. Microbiol. 2014, 165, 278–289. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; Abd-Elnaby, H.M.; Abou-Shousha, S.A.; Abdul-Raouf, U.M.; Abouelwafa, A.E. Production of Anti-Inflammatory Pyrrol Compound from Marine Bacillus baekryungensis AMHSU. World J. Fish. Marine Sci. 2016, 8, 74–84. [Google Scholar]
- Martínez-Luis, S.; Gómez, J.F.; Spadafora, C.; Guzmán, H.M.; Gutiérrez, M. Antitrypanosomal alkaloids from the marine bacterium Bacillus pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef]
- Nishanth Kumar, S.; Mohandas, C.; Siji, J.V.; Rajasekharan, K.N.; Nambisan, B. Identification of antimicrobial compound, diketopiperazines, from a Bacillus sp. N strain associated with a rhabditid entomopathogenic nematode against major plant pathogenic fungi. J. Appl. Microbiol. 2012, 113, 914–924. [Google Scholar] [CrossRef]
- Noh, S.W.; Seo, R.; Park, J.K.; Manir, M.M.; Park, K.; Sang, M.K.; Moon, S.S.; Jung, H.W. Cyclic Dipeptides from Bacillus vallismortis BS07 Require Key Components of Plant Immunity to Induce Disease Resistance in Arabidopsis against Pseudomonas Infection. Plant Pathol. J. 2017, 33, 402–409. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, L.; Okyere, S.K.; Wen, J.; Ran, Y.; Nong, X.; Hu, Y. Antibacterial Activity of Two Metabolites Isolated From Endophytic Bacteria Bacillus velezensis Ea73 in Ageratina adenophora. Front. Microbiol. 2022, 13, 860009. [Google Scholar] [CrossRef]
- Khaled, J.M.; Al-Mekhlafi, F.A.; Mothana, R.A.; Alharbi, N.S.; Alzaharni, K.E.; Sharafaddin, A.H.; Kadaikunnan, S.; Alobaidi, A.S.; Bayaqoob, N.I.; Govindarajan, M.; et al. Brevibacillus laterosporus isolated from the digestive tract of honeybees has high antimicrobial activity and promotes growth and productivity of honeybee’s colonies. Environ. Sci. Pollut. Res. Int. 2018, 25, 10447–10455, Erratum in Environ. Sci. Pollut. Res. Int. 2018, 25, 24516. [Google Scholar] [CrossRef]
- Bofinger, M.R.; de Sousa, L.S.; Fontes, J.E.N.; Marsaioli, A.J. Diketopiperazines as Cross-Communication Quorum-Sensing Signals between Cronobacter sakazakii and Bacillus cereus. ACS Omega 2017, 2, 1003–1008. [Google Scholar] [CrossRef]
- Jinendiran, S.; Teng, W.; Dahms, H.U.; Liu, W.; Ponnusamy, V.K.; Chiu, C.C.; Kumar, B.S.D.; Sivakumar, N. Induction of mitochondria-mediated apoptosis and suppression of tumor growth in zebrafish xenograft model by cyclic dipeptides identified from Exiguobacterium acetylicum. Sci. Rep. 2020, 10, 13721. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Xu, H.; Liu, W.; Yu, J.; Wang, Q.; Yu, H.; Wei, J.W.; Dai, R.; Zhou, J.; et al. Root microbiota regulates tiller number in rice. Cell 2025, 188, 3152–3166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, F.; Chen, T. Secondary metabolites of Galactomyces geotrichum from Laminaria japonica ameliorate cognitive deficits and brain oxidative stress in D-galactose induced Alzheimer’s disease mouse model. Nat. Prod. Res. 2021, 35, 5323–5328. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, M.; Ares-Arroyo, M.; Wedel, E.; Montero, N.; Barbas, C.; Rey-Stolle, M.F.; González-Zorn, B.; García, A. Multiplatform Metabolomics Characterization Reveals Novel Metabolites and Phospholipid Compositional Rules of Haemophilus influenzae Rd KW20. Int. J. Mol. Sci. 2023, 24, 11150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Zhao, J.; Zhang, H.; Chen, W. Streptococcus mutans and Candida albicans Biofilm Inhibitors Produced by Lactiplantibacillus plantarum CCFM8724. Curr. Microbiol. 2022, 79, 143. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, S.; Cui, W.; Lv, J. Identification of antifungal compounds produced by Lactobacillus casei AST18. Curr. Microbiol. 2012, 65, 156–161. [Google Scholar] [CrossRef]
- Kang, S.O.; Kwak, M.K. Antimicrobial Cyclic Dipeptides from Japanese Quail (Coturnix japonica) Eggs Supplemented with Probiotic Lactobacillus plantarum. J. Microbiol. Biotechnol. 2024, 34, 314–329. [Google Scholar] [CrossRef]
- Niranjan, R.; Patil, S.; Dubey, A.; Lochab, B.; Priyadarshini, R. Small cyclic dipeptide produced by Lactobacillus rhamnosus with anti-biofilm properties against Streptococcus mutans biofilm. Biofilm 2024, 8, 100237. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kapadnis, B. Lactococcus lactis subsp. cremoris of Plant Origin Produces Antifungal Cyclo-(Leu-Pro) and Tetradecanoic Acid. Indian J. Microbiol. 2021, 61, 74–80. [Google Scholar] [CrossRef]
- Delgado Gómez, L.M.; Torres-Mendoza, D.; Hernández-Torres, K.; Ortega, H.E.; Cubilla-Rios, L. Identification of Secondary Metabolites from the Mangrove-Endophyte Lasiodiplodia iranensis F0619 by UPLC-ESI-MS/MS. Metabolites 2023, 13, 912. [Google Scholar] [CrossRef]
- Liu, R.; Shin, G.; No, Y.; Shin, J.; Kang, S.; Park, P. Optimization of the Culture Conditions of Lactic Acid Bacteria for Antimicrobial Activity and Mass Production of Cyclic Dipeptides. J. Microbiol. Biotechnol. 2025, 35, e2408007. [Google Scholar] [CrossRef]
- Hirozawa, M.T.; Ono, M.A.; de Souza Suguiura, I.M.; Garcia, S.; Bordini, J.G.; Amador, I.R.; Hirooka, E.Y.; Ono, E.Y.S. Limosilactobacillus reuteri as sustainable biological control agent against toxigenic Fusarium verticillioides. Braz. J. Microbiol. 2023, 54, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Bejarano, A.; Masi, M.; Puopolo, G.; Evidente, A. Isolation of 2,5-diketopiperazines from Lysobacter capsici AZ78 with activity against Rhodococcus fascians. Nat. Prod. Res. 2021, 35, 4969–4977. [Google Scholar] [CrossRef] [PubMed]
- Stelmasiewicz, M.; Świątek, Ł.; Ludwiczuk, A. Chemical and Biological Studies of Endophytes Isolated from Marchantia polymorpha. Molecules 2023, 28, 2202. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Ferron, S.; Rouaud, I.; Gouault, N.; Hurvois, J.P.; Tomasi, S. Isolation and Structure Identification of Novel Brominated Diketopiperazines from Nocardia ignorata—A Lichen-Associated Actinobacterium. Molecules 2017, 22, 371. [Google Scholar] [CrossRef]
- Xiang, W.X.; Liu, Q.; Li, X.M.; Lu, C.H.; Shen, Y.M. Four pairs of proline-containing cyclic dipeptides from Nocardiopsis sp. HT88, an endophytic bacterium of Mallotus nudiflorus L. Nat. Prod. Res. 2020, 34, 2219–2224. [Google Scholar]
- Wang, N.; Cui, C.B.; Li, C.W. A new cyclic dipeptide penicimutide: The activated production of cyclic dipeptides by introduction of neomycin-resistance in the marine-derived fungus Penicillium purpurogenum G59. Arch. Pharm. Res. 2016, 39, 762–770. [Google Scholar] [CrossRef]
- Parasuraman, P.; Devadatha, B.; Sarma, V.V.; Ranganathan, S.; Ampasala, D.R.; Reddy, D.; Kumavath, R.; Kim, I.W.; Patel, S.K.S.; Kalia, V.C.; et al. Inhibition of Microbial Quorum Sensing Mediated Virulence Factors by Pestalotiopsis sydowiana. J. Microbiol. Biotechnol. 2020, 30, 571–582. [Google Scholar] [CrossRef]
- Xiang, S.L.; Xu, K.Z.; Yin, L.J.; Jia, A.Q. An Investigation of Quorum Sensing Inhibitors against Bacillus cereus in The Endophytic Fungus Pithomyces sacchari of the Laurencia sp. Mar. Drugs. 2024, 22, 161. [Google Scholar]
- Alves, B.V.B.; Borges, L.J.; Hanna, S.A.; Soares, M.B.P.; Bezerra, D.P.; Moreira, L.L.P.F.; Borges, W.S.; Portela, R.W.D.; Fernandez, C.C.; Umsza-Guez, M.A. Pigment Production by Pseudofusicoccum sp.: Extract Production, Cytotoxicity Activity, and Diketopiperazines Identified. Microorganisms 2025, 13, 277. [Google Scholar] [CrossRef]
- Santos, O.C.S.; Soares, A.R.; Machado, F.L.S.; Romanos, M.T.V.; Muricy, G.; Giambiagi-deMarval, M.; Laport, M.S. Investigation of biotechnological potential of sponge-associated bacteria collected in Brazilian coast. Lett. Appl. Microbiol. 2015, 60, 140–147. [Google Scholar] [CrossRef]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Yu, Z.; Zhang, J. Cyclo(l-Pro-l-Leu) of Pseudomonas putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne incognita. Molecules 2019, 24, 768. [Google Scholar] [CrossRef]
- Degrassi, G.; Aguilar, C.; Bosco, M.; Zahariev, S.; Pongor, S.; Venturi, V. Plant growth-promoting Pseudomonas putida WCS358 produces and secretes four cyclic dipeptides: Cross-talk with quorum sensing bacterial sensors. Curr. Microbiol. 2002, 45, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ju, H.J.; Sang, M.K. Bioactive extract of Pseudomonas sp. BC42 suppresses the infection stages of Colletotrichum orbiculare. J. Plant Pathol. 2022, 104, 1443–1455. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, R.; Ding, M.; Liu, Y.; Li, L. Biocontrol of the root-knot nematode Meloidogyne incognita by a nematicidal bacterium Pseudomonas simiae MB751 with cyclic dipeptide. Pest. Manag. Sci. 2021, 77, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Prastya, M.E.; Astuti, R.I.; Batubara, I.; Takagi, H.; Wahyudi, A.T. Chemical screening identifies an extract from marine Pseudomonas sp.-PTR-08 as an anti-aging agent that promotes fission yeast longevity by modulating the Pap1-ctt1+ pathway and the cell cycle. Mol. Biol. Rep. 2020, 47, 33–43. [Google Scholar] [CrossRef]
- Kalinovskaya, N.I.; Romanenko, L.A.; Kalinovsky, A.I. Antibacterial low-molecular-weight compounds produced by the marine bacterium Rheinheimera japonica KMM 9513T. Antonie Van Leeuwenhoek 2017, 110, 719–726. [Google Scholar] [CrossRef]
- Chen, Y.S. Studies on the Metabolic Products of Rosellinia necatrix Berlese: Part I. Isolation and Characterization of Several Physiologically Active Neutral Substances. Bull. Agric. Chem. Soc. Japan 1960, 24, 372–381. [Google Scholar]
- Kristiana, R.; Cahyani, N.K.D.; Jin, Y.; Mudianta, I.W.; Putri, F.R.; Halisah, K.A.Z.; Wang, M.X.; Guo, Y.W.; Li, X.W.; Radjasa, O.K. Antibacterial metabolites from a heterobranchia-associated bacteria and their prey from Bali, Indonesia. Curr. Res. Microb. Sci. 2025, 9, 100448. [Google Scholar] [CrossRef]
- Romero-Diaz, C.; Campos, S.M.; Herrmann, M.A.; Lewis, K.N.; Williams, D.R.; Soini, H.A.; Novotny, M.V.; Hews, D.K.; Martins, E.P. Structural Identification, Synthesis and Biological Activity of Two Volatile Cyclic Dipeptides in a Terrestrial Vertebrate. Sci. Rep. 2020, 10, 4303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, H.; Zeng, M.; Liu, Z.; Wang, Y. The involvement of bacterial quorum sensing in the spoilage of refrigerated Litopenaeus vannamei. Int. J. Food Microbiol. 2015, 192, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Taechowisan, T.; Chuen-Im, T.; Phutdhawong, W.S. Antibacterial and Anticancer Properties of Diketopiperazines from Streptomyces antimicrobicus BN122, an Endophyte in Oryza sativa var. glutinosa. Pak. J. Biol. Sci. 2025, 28, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, J.H.; Rho, J.Y. Antifungal Activities of Streptomyces blastmyceticus Strain 12-6 Against Plant Pathogenic Fungi. Mycobiology 2019, 47, 329–334. [Google Scholar] [CrossRef]
- Kaaniche, F.; Hamed, A.; Elleuch, L.; Chakchouk-Mtibaa, A.; Smaoui, S.; Karray-Rebai, I.; Koubaa, I.; Arcile, G.; Allouche, N.; Mellouli, L. Purification and characterization of seven bioactive compounds from the newly isolated Streptomyces cavourensis TN638 strain via solid-state fermentation. Microb. Pathog. 2020, 142, 104106. [Google Scholar] [CrossRef]
- Li, X.; Dobretsov, S.; Xu, Y.; Xiao, X.; Hung, O.S.; Qian, P.Y. Antifouling diketopiperazines produced by a deep-sea bacterium, Streptomyces fungicidicus. Biofouling 2006, 22, 201–208. [Google Scholar] [CrossRef]
- Çetinkaya, S.; Yenidünya, A.F.; Arslan, K.; Arslan, D.; Doğan, Ö.; Daştan, T. Secondary Metabolites of an of Streptomyces griseorubens Isolate Are Predominantly Pyrrole- and Linoleic-acid like Compounds. J. Oleo Sci. 2020, 69, 1273–1280. [Google Scholar] [CrossRef]
- Kubo, A.; Takahashi, K.; Arai, T. Diketopiperazines containing L-proline from Streptomyces lavendulae and their stereochemistry in solution. Experientia 1977, 33, 12–13. [Google Scholar] [CrossRef]
- Rhee, K.H. Purification and identification of an antifungal agent from Streptomyces sp. KH-614 antagonistic to rice blast fungus, Pyricularia oryzae. J. Microbiol. Biotechnol. 2003, 13, 984–988. [Google Scholar]
- Rhee, K.H. Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti-mutagenic properties. Int. J. Antimicrob. Agents 2004, 24, 423–427. [Google Scholar] [CrossRef]
- Siddharth, S.; Vittal, R.R. Evaluation of antimicrobial, enzyme inhibitory, antioxidant and cytotoxic activities of partially purified volatile metabolites of marine Streptomyces sp. S2A. Microorganisms 2018, 6, 72. [Google Scholar] [CrossRef]
- Koaze, Y. On the Mechanism of L-Prolyl Diketopiperazine Formation by Streptomyces. Bull. Agric. Chem. Soc. Japan 1960, 24, 449–458. [Google Scholar]
- Tamura, S.; Suzuki, A.; Aoki, Y.; Otake, N. Isolation of Several Diketopiperazines from Peptone. Agric. Biol. Chem. 1964, 28, 650–652. [Google Scholar] [CrossRef]
- Bhandari, S.; Bhattarai, B.R.; Adhikari, A.; Aryal, B.; Shrestha, A.; Aryal, N.; Lamichhane, U.; Thapa, R.; Thapa, B.B.; Yadav, R.P.; et al. Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking. Processes 2022, 10, 2149. [Google Scholar] [CrossRef]
- Alshaibani, M.; Zin, N.M.; Jalil, J.; Sidik, N.; Ahmad, S.J.; Kamal, N.; Edrada-Ebel, R. Isolation, Purification, and Characterization of Five Active Diketopiperazine Derivatives from Endophytic Streptomyces SUK 25 with Antimicrobial and Cytotoxic Activities. J. Microbiol. Biotechnol. 2017, 27, 1249–1256, Erratum in J. Microbiol. Biotechnol. 2017, 27, 2074. [Google Scholar] [CrossRef]
- Buedenbender, L.; Robertson, L.P.; Lucantoni, L.; Avery, V.M.; Kurtböke, D.İ.; Carroll, A.R. HSQC-TOCSY Fingerprinting-Directed Discovery of Antiplasmodial Polyketides from the Marine Ascidian-Derived Streptomyces sp. (USC-16018). Mar. Drugs 2018, 16, 189. [Google Scholar]
- Manimaran, M.; Kannabiran, K. Marine sp. VITMK1 Derived Pyrrolo [1, 2-A] Pyrazine-1, 4-Dione, Hexahydro-3-(2-Methylpropyl) and Its Free Radical Scavenging Activity. Open Bioact. Compd. J. 2017, 5, 23–30. [Google Scholar]
- Guo, Z.K.; Wang, R.; Chen, F.X.; Liu, T.M.; Yang, M.Q. Bioactive aromatic metabolites from the sea urchin-derived actinomycete Streptomyces spectabilis strain HDa1. Phytochem. Lett. 2018, 25, 132–135. [Google Scholar] [CrossRef]
- Mashima, I.; Miyakawa, H.; Scannapieco, F.A.; Nakazawa, F. Identification of an early stage biofilm inhibitor from Veillonella tobetsuensis. Anaerobe 2018, 52, 86–91. [Google Scholar] [CrossRef]
- Chen, M.Z.; Dewis, M.L.; Kraut, K.; Merritt, D.; Reiber, L.; Trinnaman, L.; Da Costa, N.C. 2,5-diketopiperazines (cyclic dipeptides) in beef: Identification, synthesis, and sensory evaluation. J. Food Sci. 2009, 74, 100–105. [Google Scholar] [CrossRef]
- Tabassum, T.; Rahman, H.; Tawab, A.; Murad, W.; Hameed, H.; Shah, S.A.R.; Alzahrani, K.J.; Banjer, H.J.; Alshiekheid, M.A. Fagonia cretica: Identification of compounds in bioactive gradient high performance liquid chromatography fractions against multidrug resistant human gut pathogens. Trop. Biomed. 2022, 39, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Zi, J.; Zhu, C.; Lin, S.; Yang, Y.; Shi, J. Constituents of Gymnadenia conopsea. Zhongguo Zhong Yao Za Zhi 2010, 35, 2852–2861. [Google Scholar] [PubMed]
- Gautschi, M.; Schmid, J.P.; Peppard, T.L.; Ryan, T.P.; Tuorto, R.M.; Yang, X. Chemical Characterization of Diketopiperazines in Beer. J. Agric. Food Chem. 1997, 45, 3183–3189. [Google Scholar] [CrossRef]
- Ginz, M.; Engelhardt, U.H. Identification of Proline-Based Diketopiperazines in Roasted Coffee. J. Agric. Food Chem. 2000, 48, 3528–3532. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, T.; Elfrink, H.; Azadi-Chegeni, F.; Dubbelman, A.C.; Harms, A.C.; Jacobs, D.M.; Braumann, U.; Velders, A.H.; van Duynhoven, J.; Hankemeier, T. Fractionation platform for target identification using off-line directed two-dimensional chromatography, mass spectrometry and nuclear magnetic resonance. Anal. Chim. Acta. 2021, 1142, 28–37. [Google Scholar] [CrossRef]
- Majumder, S.; Ghosh, A.; Chakraborty, S.; Bhattacharya, M. The Himalayan ethnic beverage tongba with therapeutic properties in high-altitude illnesses and metabolomic similarities to Japanese sake. Acta Univ. Sapientiae Aliment. 2022, 15, 67–83. [Google Scholar] [CrossRef]
- Majumder, S.; Chakraborty, S.; Ghoshi, A.; Bhattacharya, M. In silico insights into the efficacy of Djareeling Himalaya’s traditional fermented beverages to combat various high-altitude sicknesses. Acta Univ. Cibiniensis Ser. E 2023, 27, 261–292. [Google Scholar]
- Ryan, L.A.; Dal Bello, F.; Arendt, E.K.; Koehler, P. Detection and quantitation of 2,5-diketopiperazines in wheat sourdough and bread. J. Agric. Food Chem. 2009, 57, 9563–9568. [Google Scholar] [CrossRef]
- Agarwal, P.; Fischer, H.D.; Camalle, M.D.; Skirycz, A. Not to be overlooked: Dipeptides and their role in plant stress resilience. J. Exp. Bot. 2025, eraf311, online ahead of print. [Google Scholar] [CrossRef]
- Wei, B.; Ying, T.T.; Lv, H.W.; Zhou, Z.Y.; Cai, H.; Hu, G.A.; Liang, H.M.; Yu, W.C.; Yu, Y.L.; Fan, A.L.; et al. Global analysis of fungal biosynthetic gene clusters reveals the diversification of diketopiperazine biosynthesis. Bioresour. Technol. 2025, 422, 132218. [Google Scholar] [CrossRef]
- González, O.; Ortíz-Castro, R.; Díaz-Pérez, C.; Díaz-Pérez, A.L.; Magaña-Dueñas, V.; López-Bucio, J.; Campos-García, J. Non-ribosomal Peptide Synthases from Pseudomonas aeruginosa Play a Role in Cyclodipeptide Biosynthesis, Quorum-Sensing Regulation, and Root Development in a Plant Host. Microb. Ecol. 2017, 73, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Campbell, J.; Lin, Q.; Geske, G.D.; Blackwell, H.E. New and unexpected insights into the modulation of LuxR-type quorum sensing by cyclic dipeptides. ACS Chem. Biol. 2009, 4, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Domzalski, A.; Margent, L.; Vigo, V.; Dewan, F.; Pilarsetty, N.V.K.; Xu, Y.; Kawamura, A. Unambiguous Stereochemical Assignment of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro) by Electronic Circular Dichroic Spectroscopy. Molecules 2021, 26, 5981. [Google Scholar]
- Beneš, R.; Koval, D.; Švec, I.; Macůrková, A.; Vrchotová, B.; Honzíková, T.; Kalenchak, K.; Bárta, J.; Bártová, V.; Bedrníček, J.; et al. Antimicrobial and Antifungal Activities of Proline-Based 2,5-Diketopiperazines Occurring in Food and Beverages and Their Synergism with Lactic Acid. ACS Agric. Sci. Technol. 2025, 5, 1681–1692. [Google Scholar] [CrossRef]
- Eguchi, C.; Kakuta, A. Studies on cyclic dipeptides. I. Thermodynamics of the cis-trans isomerization of the side chains in cyclic dipeptides. J. Am. Chem. Soc. 1974, 96, 3985–3989. [Google Scholar] [CrossRef]
- Ying, J.; Lin, R.; Xu, P.; Wu, Y.; Liu, Y.; Zhao, Y. Prebiotic formation of cyclic dipeptides under potentially early Earth conditions. Sci. Rep. 2018, 8, 936. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Ying, J.; Liu, Y.; Zhang, G.; Zhao, Y. Selection of Amino Acid Chirality Induced by Cyclic Dipeptide Synthesis in Plausible Prebiotic Conditions. Front. Astron. Space Sci. 2022, 9, 794932. [Google Scholar] [CrossRef]
- Chi, Y.; Li, X.; Chen, Y.; Zhang, Y.; Liu, Y.; Gao, X.; Zhao, Y. Prebiotic Formation of Catalytically Active Dipeptides via Trimetaphosphate Activation. Chem. Asian J. 2022, 17, e202200926. [Google Scholar] [CrossRef]
- Otsuka, Y.; Arita, H.; Sakaji, M.; Yamamoto, K.; Kashiwagi, T.; Shimamura, T.; Ukeda, H. Investigation of the formation mechanism of proline-containing cyclic dipeptide from the linear peptide. Biosci. Biotechnol. Biochem. 2019, 83, 2355–2363. [Google Scholar] [CrossRef]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules. 2021, 11, 1515. [Google Scholar]
- Nsengiyumva, O.; Hamedzadeh, S.; McDaniel, J.; Macho, J.; Simpson, G.; Panda, S.S.; Ha, K.; Lebedyeva, I.; Faidallah, H.M.; Al-Mohammadi, M.M.; et al. A benzotriazole-mediated route to protected marine-derived hetero-2,5-diketopiperazines containing proline. Org. Biomol. Chem. 2015, 13, 4399–4403. [Google Scholar] [CrossRef]
- Karakama, S.; Suzuki, S.; Kino, K. One-pot synthesis of 2,5-diketopiperazine with high titer and versatility using adenylation enzyme. Appl. Microbiol. Biotechnol. 2022, 106, 4469–4479. [Google Scholar] [CrossRef]
- Kano, S.; Suzuki, S.; Hara, R.; Kino, K. Synthesis of d-Amino Acid-Containing Dipeptides Using the Adenylation Domains of Nonribosomal Peptide Synthetase. Appl. Environ. Microbiol. 2019, 85, e00120-19. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, I.; Boateng, A.; Hattori, T.; Nakagai, K.; Kawase, M.; Ogata, S.; Yamamoto, H. Synthesis of Mono-Boc-2,5-Diketopiperazine: A Key Building Block for Amide and Peptide Synthesis. J. Org. Chem. 2025, 90, 4357–4364. [Google Scholar]
- Farran, D.; Echalier, D.; Martinez, J.; Dewynter, G. Regioselective and sequential reactivity of activated 2,5-diketopiperazines. J. Pept. Sci. 2009, 15, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Tullberg, M.; Luthman, K.; Grøtli, M. Microwave-assisted solid-phase synthesis of 2,5-diketopiperazines: Solvent and resin dependence. J. Comb. Chem. 2006, 8, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Tullberg, M.; Grøtli, M.; Luthman, K. Synthesis of functionalized, unsymmetrical 1,3,4,6-tetrasubstituted 2,5-diketopiperazines. J. Org. Chem. 2007, 72, 195–199. [Google Scholar] [CrossRef]
- Fischer, P.M. Diketopiperazines in peptide and combinatorial chemistry. J. Pept. Sci. 2003, 9, 9–35. [Google Scholar] [CrossRef]
- Takaya, Y.; Furukawa, T.; Miura, S.; Akutagawa, T.; Hotta, Y.; Ishikawa, N.; Niwa, M. Antioxidant constituents in distillation residue of Awamori spirits. J. Agric. Food Chem. 2007, 55, 75–79. [Google Scholar] [CrossRef]
- Furukawa, T.; Akutagawa, T.; Funatani, H.; Uchida, T.; Hotta, Y.; Niwa, M.; Takaya, Y. Cyclic dipeptides exhibit potency for scavenging radicals. Bioorg Med. Chem. 2012, 20, 2002–2009. [Google Scholar] [CrossRef]
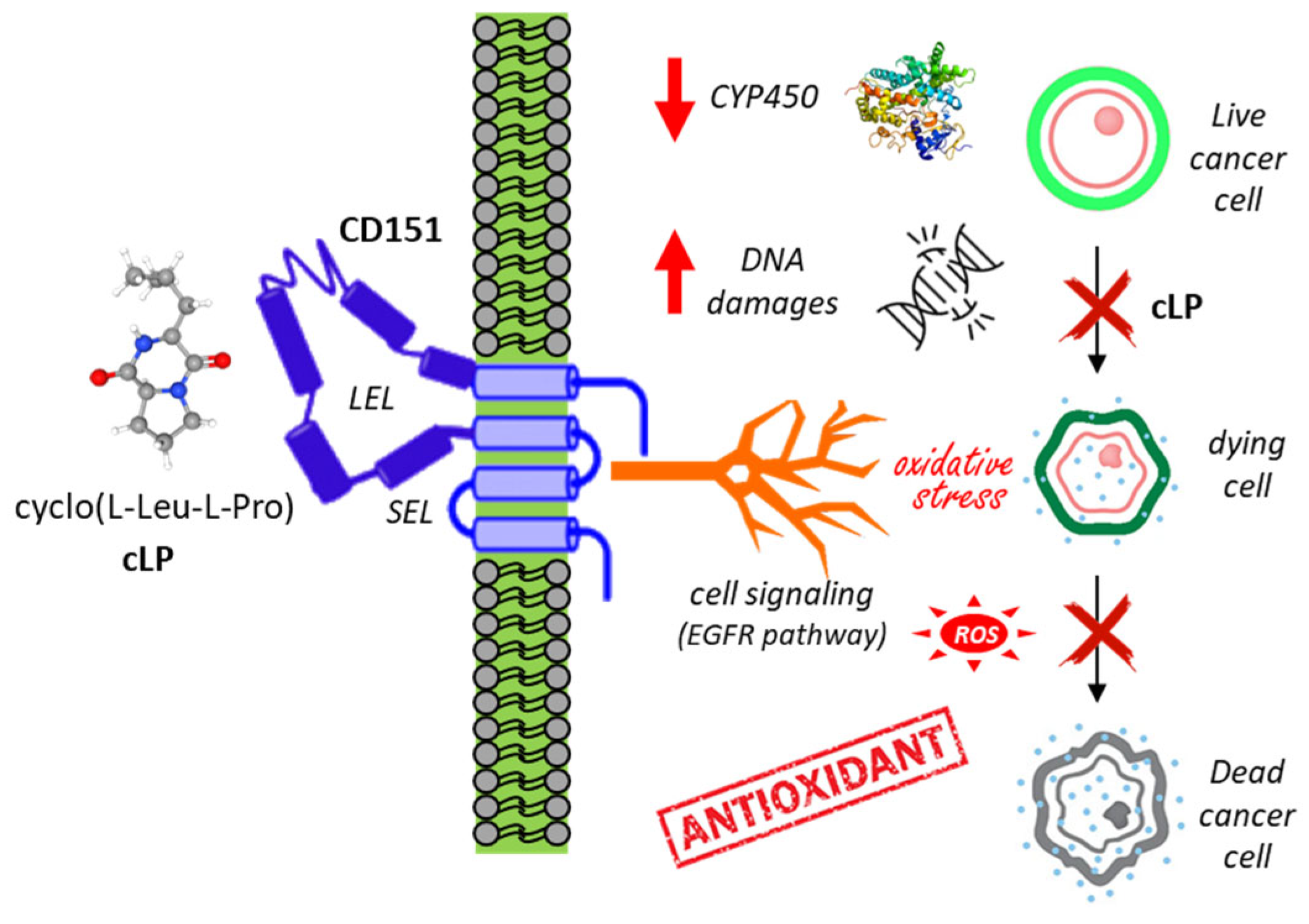
- Deepak, K.G.K.; Kumari, S.; Malla, R.R. Marine Cyclic Dipeptide Cyclo (L-Leu-L-Pro) Protects Normal Breast Epithelial Cells from tBHP-induced Oxidative Damage by Targeting CD151. Arch. Breast Cancer 2021, 8, 162–173. [Google Scholar]
- Gowrishankar, S.; Pandian, S.K. Modulation of Staphylococcus epidermidis (RP62A) extracellular polymeric layer by marine cyclic dipeptide-cyclo(l-leucyl-l-prolyl) thwarts biofilm formation. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1254–1262. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Pandian, S.K.; Balasubramaniam, B.; Balamurugan, K. Quorum quelling efficacy of marine cyclic dipeptide -cyclo(L-leucyl-L-prolyl) against the uropathogen Serratia marcescens. Food Chem. Toxicol. 2019, 123, 326–336. [Google Scholar]
- Li, L.; Xu, Z.; Cao, R.; Li, J.; Wu, C.J.; Wang, Y.; Zhu, H. Effects of hydroxyl group in cyclo(Pro-Tyr)-like cyclic dipeptides on their anti-QS activity and self-assembly. iScience 2023, 26, 107048. [Google Scholar]
- Jothi, R.; Hari Prasath, N.; Gowrishankar, S.; Pandian, S.K. Bacterial Quorum-Sensing Molecules as Promising Natural Inhibitors of Candida albicans Virulence Dimorphism: An In Silico and In Vitro Study. Front. Cell Infect. Microbiol. 2021, 11, 781790. [Google Scholar]
- Zhang, C.; Wang, C.; Jatt, A.N.; Liu, H.; Liu, Y. Role of RpoS in stress resistance, biofilm formation and quorum sensing of Shewanella baltica. Lett. Appl. Microbiol. 2021, 72, 307–315. [Google Scholar] [PubMed]
- Paopradit, P.; Tansila, N.; Surachat, K.; Mittraparp-Arthorn, P. Vibrio alginolyticus influences quorum sensing-controlled phenotypes of acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus. PeerJ 2021, 9, e11567. [Google Scholar]
- Kachhadia, R.; Kapadia, C.; Singh, S.; Gandhi, K.; Jajda, H.; Alfarraj, S.; Ansari, M.J.; Danish, S.; Datta, R. Quorum Sensing Inhibitory and Quenching Activity of Bacillus cereus RC1 Extracts on Soft Rot-Causing Bacteria Lelliottia amnigena. ACS Omega 2022, 7, 25291–25308. [Google Scholar] [CrossRef]
- Simon, G.; Bérubé, C.; Voyer, N.; Grenier, D. Anti-biofilm and anti-adherence properties of novel cyclic dipeptides against oral pathogens. Bioorganic Med. Chem. 2019, 27, 2323–2331. [Google Scholar]
- Marchesan, J.T.; Morelli, T.; Moss, K.; Barros, S.P.; Ward, M.; Jenkins, W.; Aspiras, M.B.; Offenbacher, S. Association of Synergistetes and Cyclodipeptides with Periodontitis. J. Dent. Res. 2015, 94, 1425–1431. [Google Scholar] [CrossRef]
- Wu, M.; Huang, S.; Du, J.; Li, Y.; Jiang, S.; Zhan, L.; Huang, X. D-alanylation of lipoteichoic acid contributes to biofilm formation and acidogenesis capacity of Streptococcus mutans. Microb. Pathog. 2022, 169, 105666. [Google Scholar] [CrossRef]
- Sangavi, R.; Malligarjunan, N.; Pandian, S.K.; Gowrishankar, S. Marine-Derived Cyclo(l-Leucyl-l-Prolyl) Targets d-Alanylation of Lipoteichoic Acid to Combat Streptococcus mutans UA159 Mediated Dental Cariogenesis. Mol. Oral. Microbiol. 2025, 40, 202–222. [Google Scholar] [CrossRef]
- Yabe, K.; Yan, P.S.; Song, Y.; Ichinomiya, M.; Nakagawa, H.; Shima, Y.; Ando, Y.; Sakuno, E.; Nakajima, H. Isolation of microorganisms and substances inhibitory to aflatoxin production. Food Addit. Contam. Part A 2008, 25, 1111–1117. [Google Scholar] [CrossRef]
- Deslauriers, R.; Grzonka, Z.; Walter, R. Influence of D and L amino-acid residues on the conformation of peptides in solution: A carbon-13 nuclear magnetic resonance study of cyclo(prolyl-leucyl). Biopolymers 1976, 15, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Okuda-Shimazaki, J.; Yoshida, H.; Sode, K. AD dependent glucose dehydrogenases—Discovery and engineering of representative glucose sensing enzymes. Bioelectrochemistry 2020, 132, 107414. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Sakai, G.; Mori, K.; Kojima, K.; Kamitori, S.; Sode, K. Structural analysis of fungus-derived FAD glucose dehydrogenase. Sci. Rep. 2015, 5, 13498. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Tariq, A.; Ijaz, A.; Naheed, S.; Hashem, A.; Abd Allah, E.F.; Soliman, M.H.; Javed, M.R. In Vitro Antimicrobial and Antioxidant Activities of Lactobacillus coryniformis BCH-4 Bioactive Compounds and Determination of their Bioprotective Effects on Nutritional Components of Maize (Zea mays L.). Molecules 2020, 25, 4685. [Google Scholar] [CrossRef]
- Iimura, K.; Furukawa, T.; Yamamoto, T.; Negishi, L.; Suzuki, M.; Sakuda, S. The Mode of Action of Cyclo(l-Ala-l-Pro) in Inhibiting Aflatoxin Production of Aspergillus flavus. Toxins 2017, 9, 219. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Salman, M.; Numan, M.; Javed, M.R.; Zubair, M.; Mustafa, G. Characterization of antifungal metabolites produced by Lactobacillus plantarum and Lactobacillus coryniformis isolated from rice rinsed water. Mol. Biol. Rep. 2020, 47, 1871–1881. [Google Scholar] [CrossRef]
- Ahmad, S.J.; Abdul Rahim, M.B.H.; Baharum, S.N.; Baba, M.S.; Zin, N.M. Discovery of Antimalarial Drugs from Streptomycetes Metabolites Using a Metabolomic Approach. J. Trop. Med. 2017, 2017, 2189814. [Google Scholar] [CrossRef]
- Ceravolo, I.P.; Leoni, L.F.; Krettli, A.U.; Murta, S.M.F.; Resende, D.M.; Cruz, M.G.F.M.L.; Varejão, J.O.S.; Mendes, L.L.; Varejão, E.V.V.; Kohlhoff, M. Novel 2,5-Diketopiperazines with In Vitro Activities against Protozoan Parasites of Tropical Diseases. Pharmaceuticals 2024, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.; Marni, R.; Chakraborty, A. Exploring the role of CD151 in the tumor immune microenvironment: Therapeutic and clinical perspectives. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188898. [Google Scholar]
- Erfani, S.; Hua, H.; Pan, Y.; Zhou, B.P.; Yang, X.H. The Context-Dependent Impact of Integrin-Associated CD151 and Other Tetraspanins on Cancer Development and Progression: A Class of Versatile Mediators of Cellular Function and Signaling, Tumorigenesis and Metastasis. Cancers 2021, 13, 2005. [Google Scholar]
- Gao, X.; Liu, S.; Cao, Y.; Shi, L.; Yin, Y. The controversial role of CD151 in different solid tumors: Promoter or suppressor? Cancer Cell Int. 2025, 25, 110. [Google Scholar] [CrossRef]
- Shanmukhappa, K.; Kim, J.K.; Kapil, S. Role of CD151, A tetraspanin, in porcine reproductive and respiratory syndrome virus infection. Virol. J. 2007, 4, 62. [Google Scholar] [CrossRef]
- Kawashima, K.; Saigo, C.; Kito, Y.; Hanamatsu, Y.; Egawa, Y.; Takeuchi, T. CD151 confers metastatic potential to clear cell sarcoma of the soft tissue in animal model. Oncol. Lett. 2019, 17, 4811–4818. [Google Scholar] [CrossRef]
- Haeuw, J.F.; Goetsch, L.; Bailly, C.; Corvaia, N. Tetraspanin CD151 as a target for antibody-based cancer immunotherapy. Biochem. Soc. Trans. 2011, 39, 553–558. [Google Scholar] [CrossRef]
- Marni, R.; Kundrapu, D.B.; Chakraborti, A.; Malla, R. Insight into drug sensitizing effect of diallyl disulfide and diallyl trisulfide from Allium sativum L. on paclitaxel-resistant triple-negative breast cancer cells. J. Ethnopharmacol. 2022, 296, 115452. [Google Scholar] [CrossRef]
- Akella, M.; Malla, R. Molecular modeling and in vitro study on pyrocatechol as potential pharmacophore of CD151 inhibitor. J. Mol. Graph. Model. 2020, 100, 107681. [Google Scholar] [CrossRef]
- Akella, M.; Amajala, K.C.; Malla, R.R. Bioinformatics analysis of regulatory elements of the CD151 gene and insilico docking of CD151 with diallyl sulfide. Gene Rep. 2019, 17, 100551. [Google Scholar] [CrossRef]
- Gavara, M.M.; Zaveri, K.; Badana, A.K.; Gugalavath, S.; Amajala, K.C.; Patnala, K.; Malla, R.R. A novel small molecule inhibitor of CD151 inhibits proliferation of metastatic triple negative breast cancer cell lines. Process Biochem. 2018, 66, 254–262. [Google Scholar] [CrossRef]
- Kgk, D.; Kumari, S.; G, S.; Malla, R.R. Marine natural compound cyclo(L-leucyl-L-prolyl) peptide inhibits migration of triple negative breast cancer cells by disrupting interaction of CD151 and EGFR signaling. Chem. Biol. Interact. 2020, 315, 108872. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.; Nga, M.E.; Chin, C.Y.; Tai, Y.K.; Wong, H.C.; Soo, R.; An, O.; Yang, H.; Seet, J.E.; Lim, Y.C.; et al. Impact of CD151 overexpression on prognosis and therapy in non-small cell lung cancer patients lacking EGFR mutations. Cell Prolif. 2024, 57, e13708. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, T.; Zhou, J.; Du, W.; Zeng, Y.; Liu, T.; Fu, Y.; Li, Y.; Qian, Q.; Yang, X.H.; et al. CD151 drives cancer progression depending on integrin α3β1 through EGFR signaling in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 192. [Google Scholar] [CrossRef]
- Jia, J.; Yao, J.; Kong, J.; Yu, A.; Wei, J.; Dong, Y.; Song, R.; Shan, D.; Zhong, X.; Lv, F.; et al. 2,5-Diketopiperazines: A Review of Source, Synthesis, Bioactivity, Structure, and MS Fragmentation. Curr. Med. Chem. 2023, 30, 1060–1085. [Google Scholar] [CrossRef]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and Biological Activities of Diketopiperazines from Marine Organisms: A Review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef]
- de Carvalho, M.P.; Abraham, W.R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef]
- Ding, L.; Xu, P.; Zhang, W.; Yuan, Y.; He, X.; Su, D.; Shi, Y.; Naman, C.B.; Yan, X.; Wu, B.; et al. Three New Diketopiperazines from the Previously Uncultivable Marine Bacterium Gallaecimonas mangrovi HK-28 Cultivated by iChip. Chem. Biodivers. 2020, 17, e2000221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Insights into the Bioactivities and Mechanism of Action of the Microbial Diketopiperazine Cyclic Dipeptide Cyclo(L-leucyl-L-prolyl). Mar. Drugs 2025, 23, 397. https://doi.org/10.3390/md23100397
Bailly C. Insights into the Bioactivities and Mechanism of Action of the Microbial Diketopiperazine Cyclic Dipeptide Cyclo(L-leucyl-L-prolyl). Marine Drugs. 2025; 23(10):397. https://doi.org/10.3390/md23100397
Chicago/Turabian StyleBailly, Christian. 2025. "Insights into the Bioactivities and Mechanism of Action of the Microbial Diketopiperazine Cyclic Dipeptide Cyclo(L-leucyl-L-prolyl)" Marine Drugs 23, no. 10: 397. https://doi.org/10.3390/md23100397
APA StyleBailly, C. (2025). Insights into the Bioactivities and Mechanism of Action of the Microbial Diketopiperazine Cyclic Dipeptide Cyclo(L-leucyl-L-prolyl). Marine Drugs, 23(10), 397. https://doi.org/10.3390/md23100397







