A Robust Marine Collagen Peptide–Agarose 3D Culture System for In Vitro Modeling of Hepatocellular Carcinoma and Anti-Cancer Therapeutic Development
Abstract
1. Introduction
2. Results
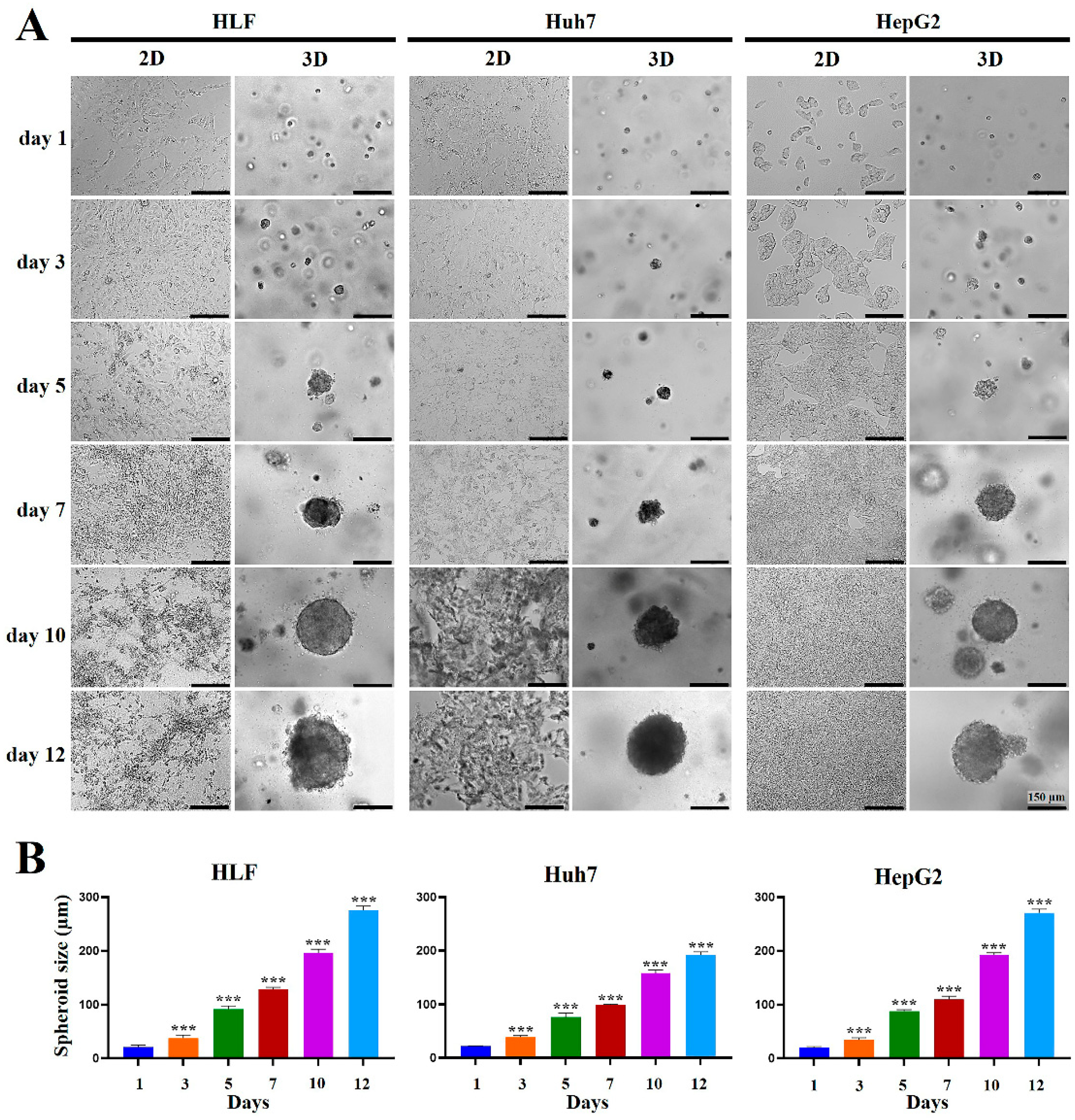
2.1. Formation and Growth of HCC Cell Spheroids Were Promoted in MCP-B Hydrogels
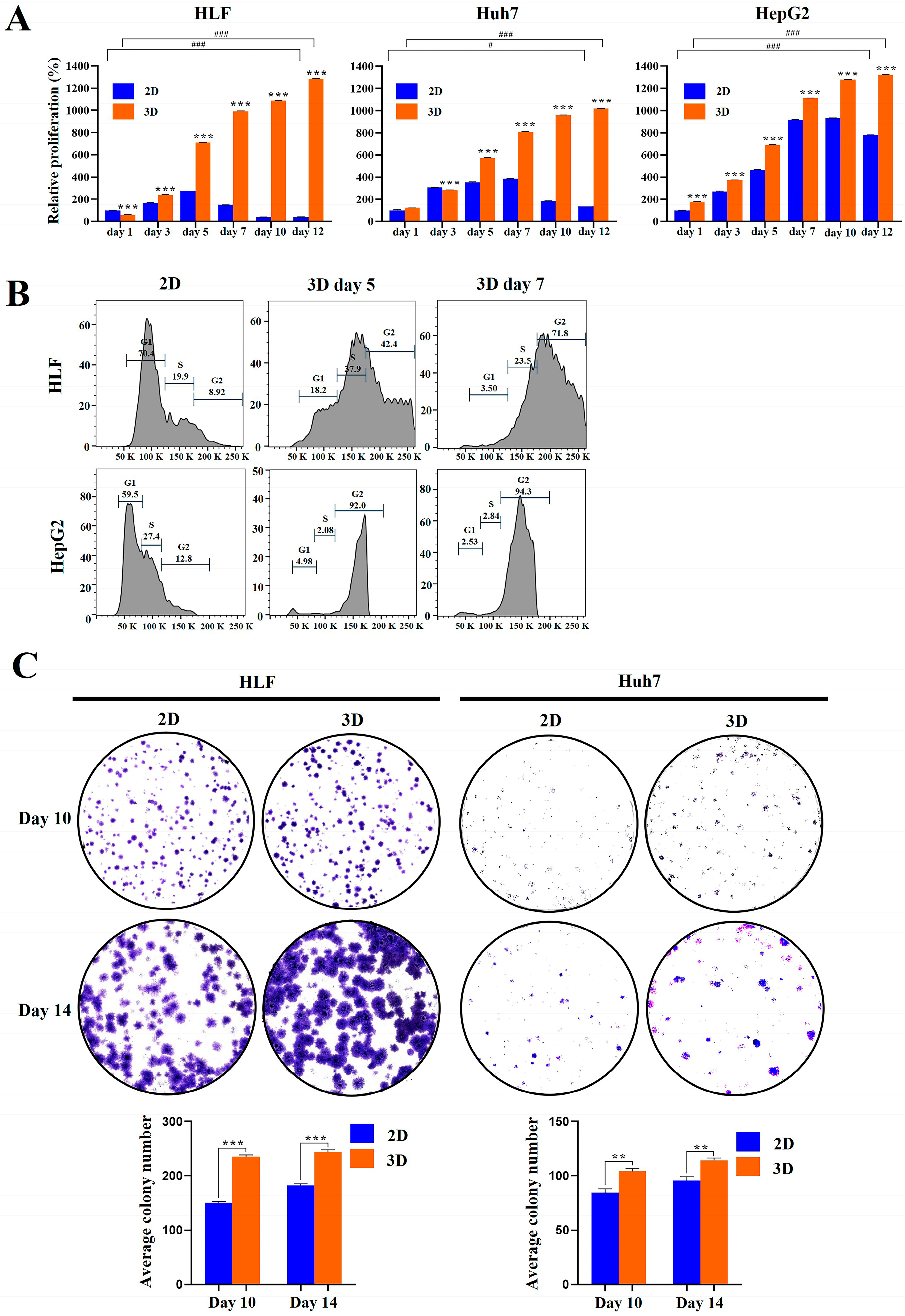
2.2. Proliferation and Clonogenicity of HCC Cells Were Enhanced in MCP-B Hydrogels
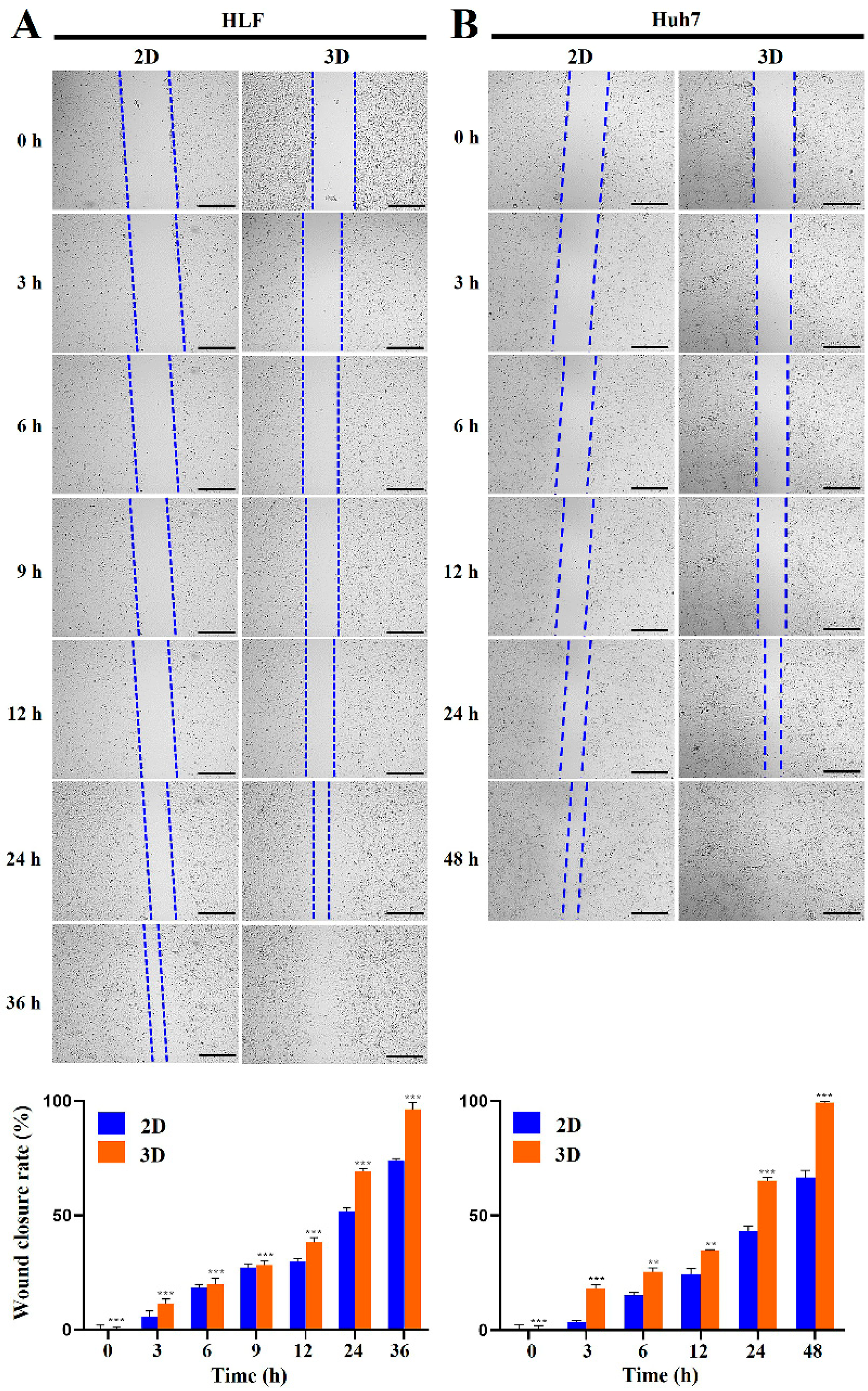
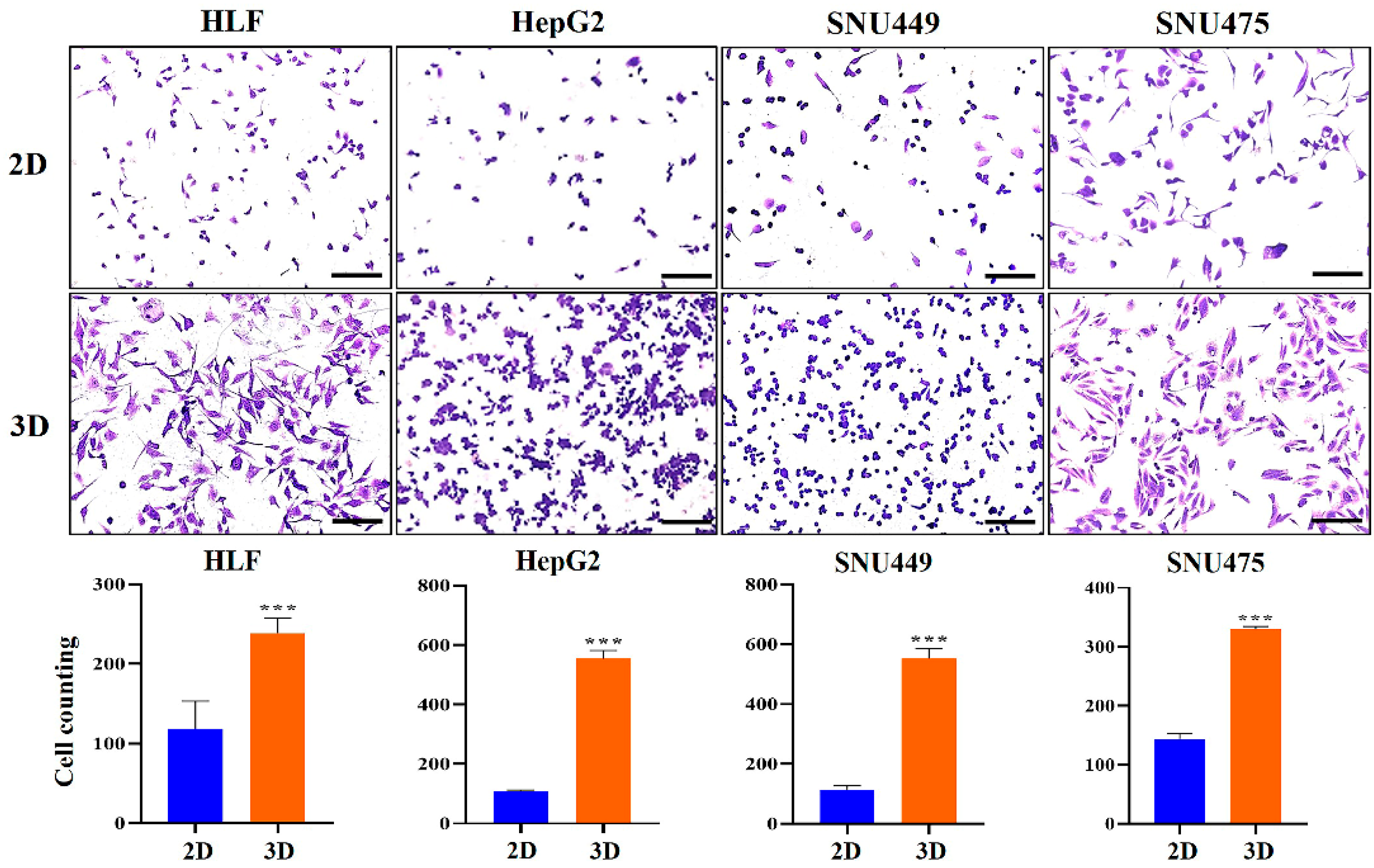
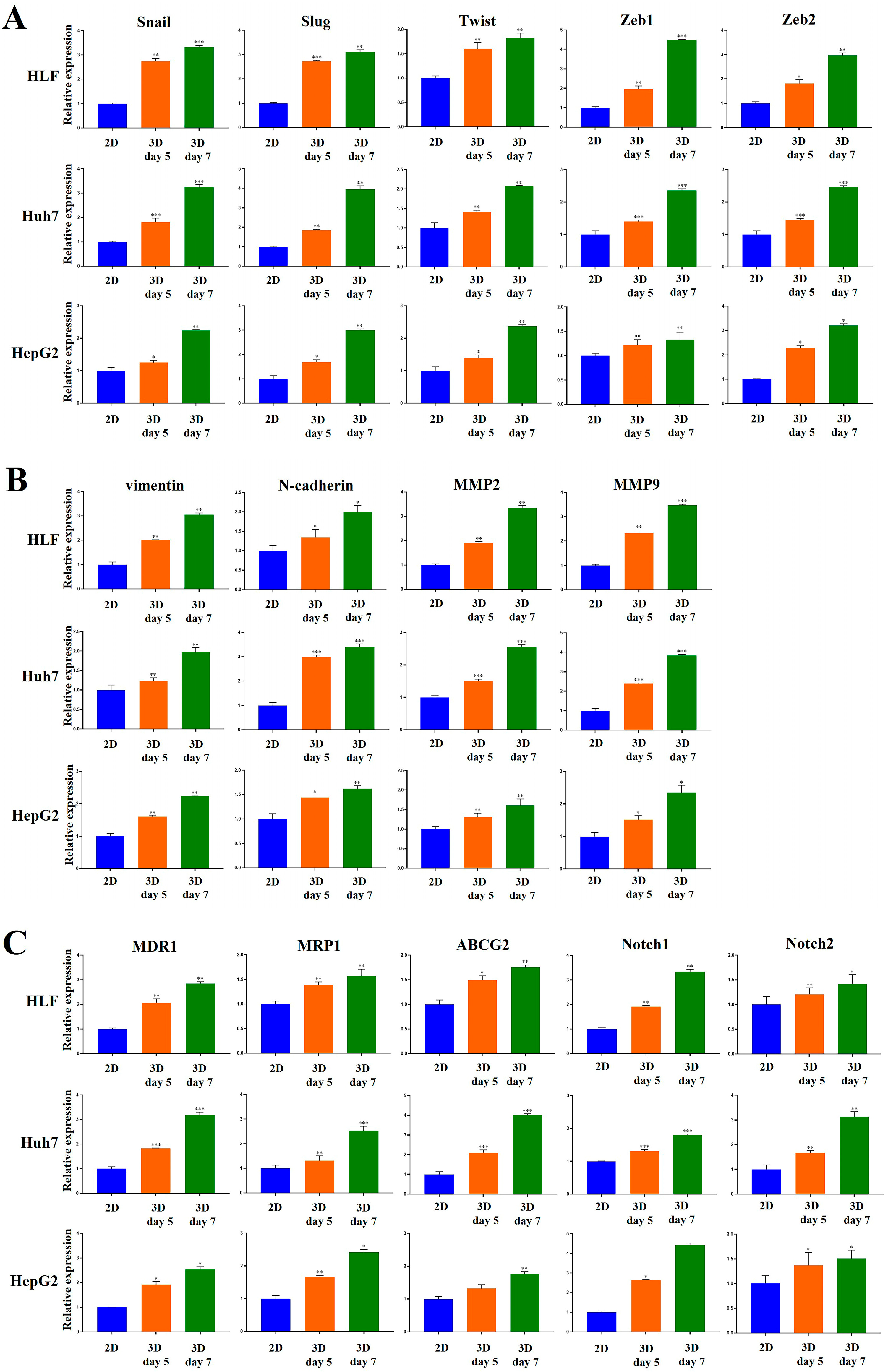
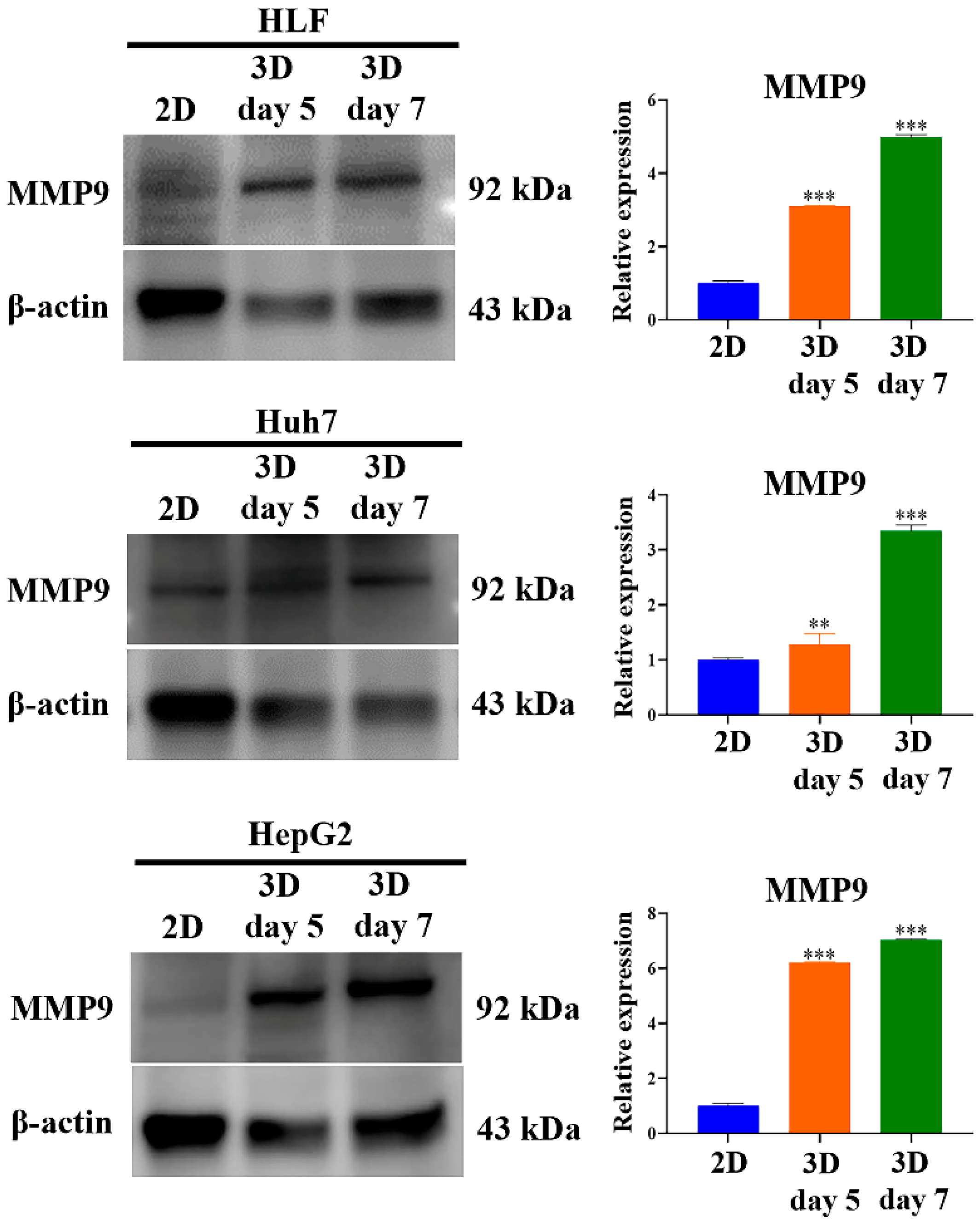
2.3. Metastatic Potential of HCC Cells Was Elevated in MCP-B Hydrogels
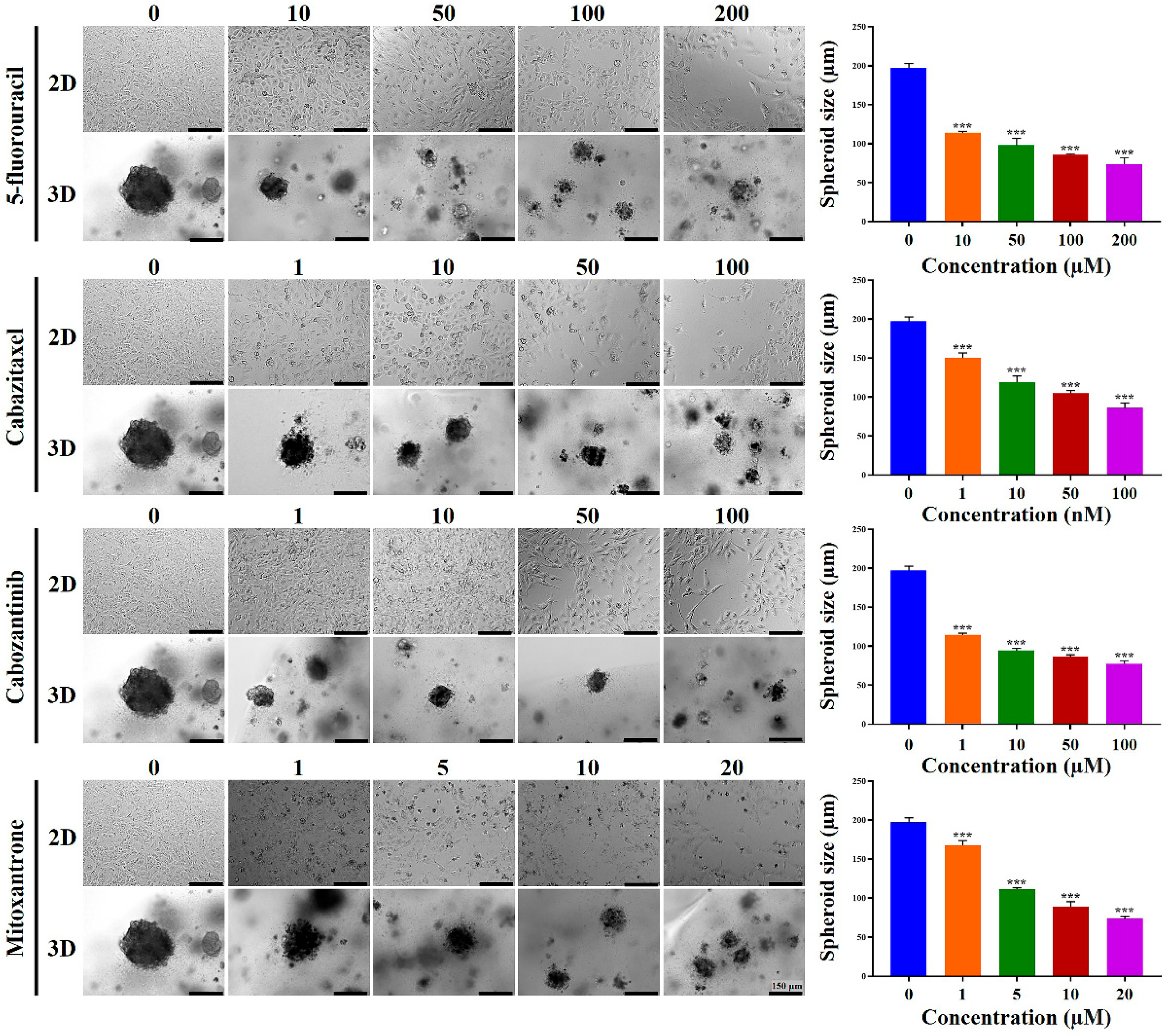
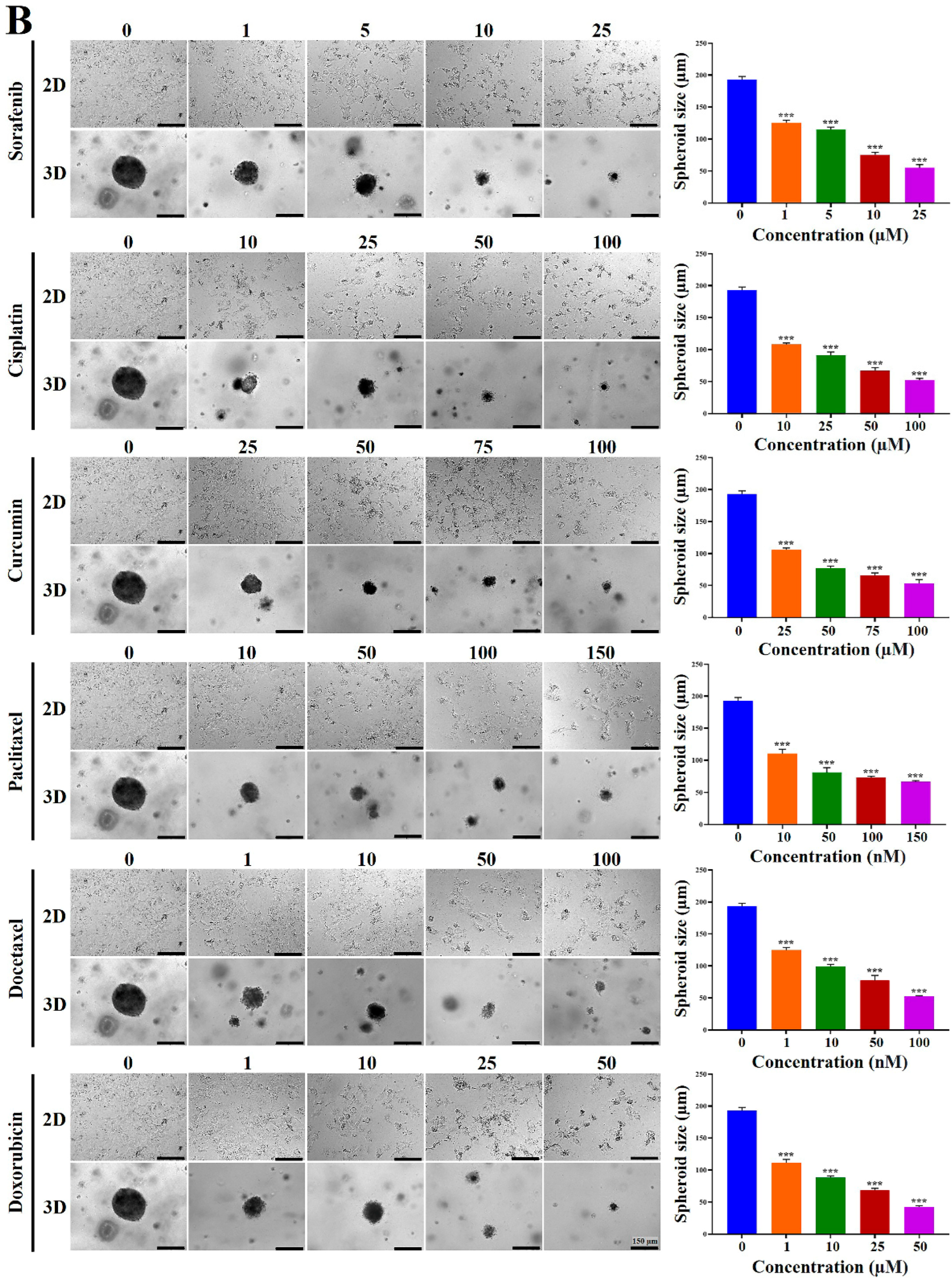
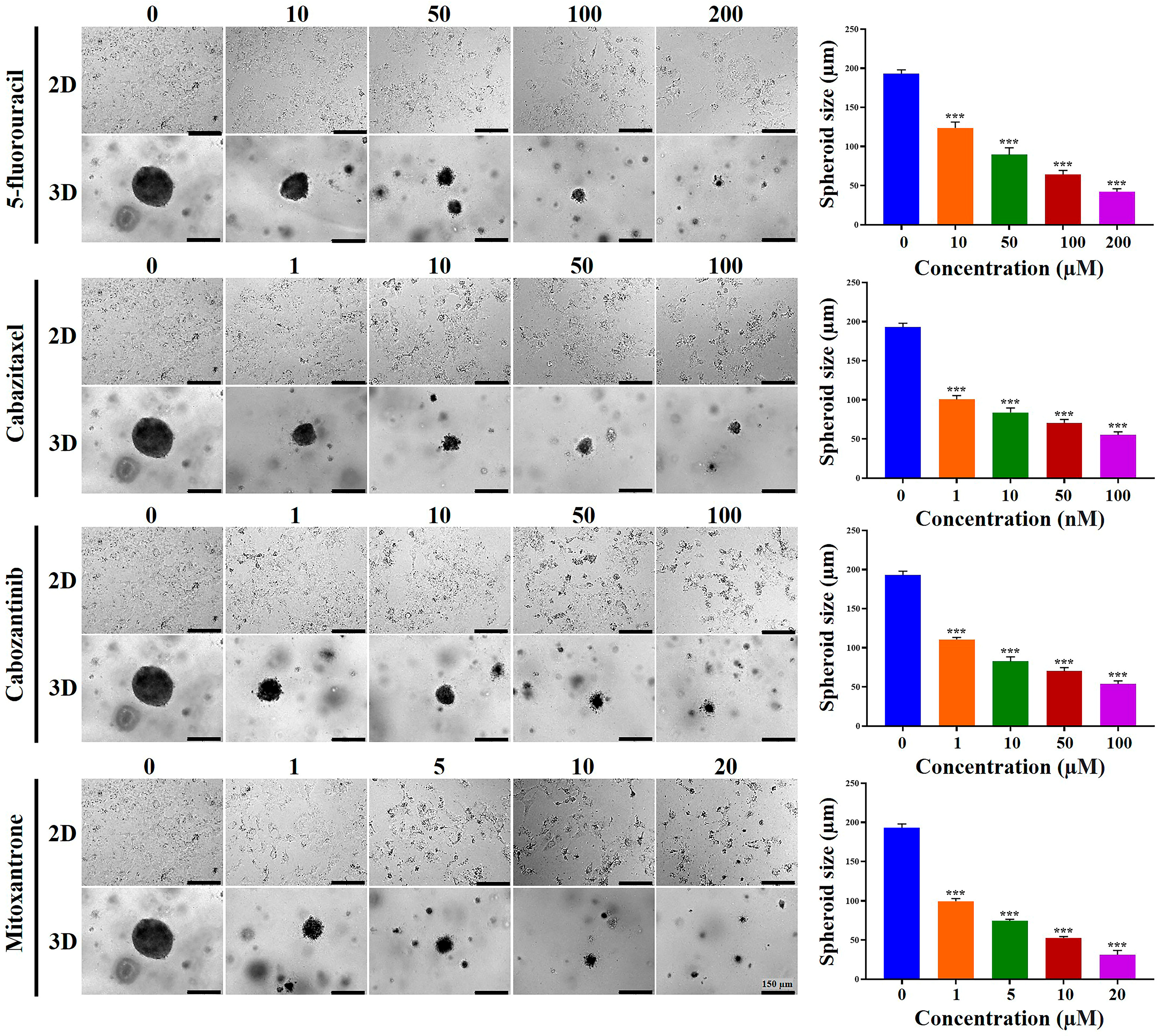
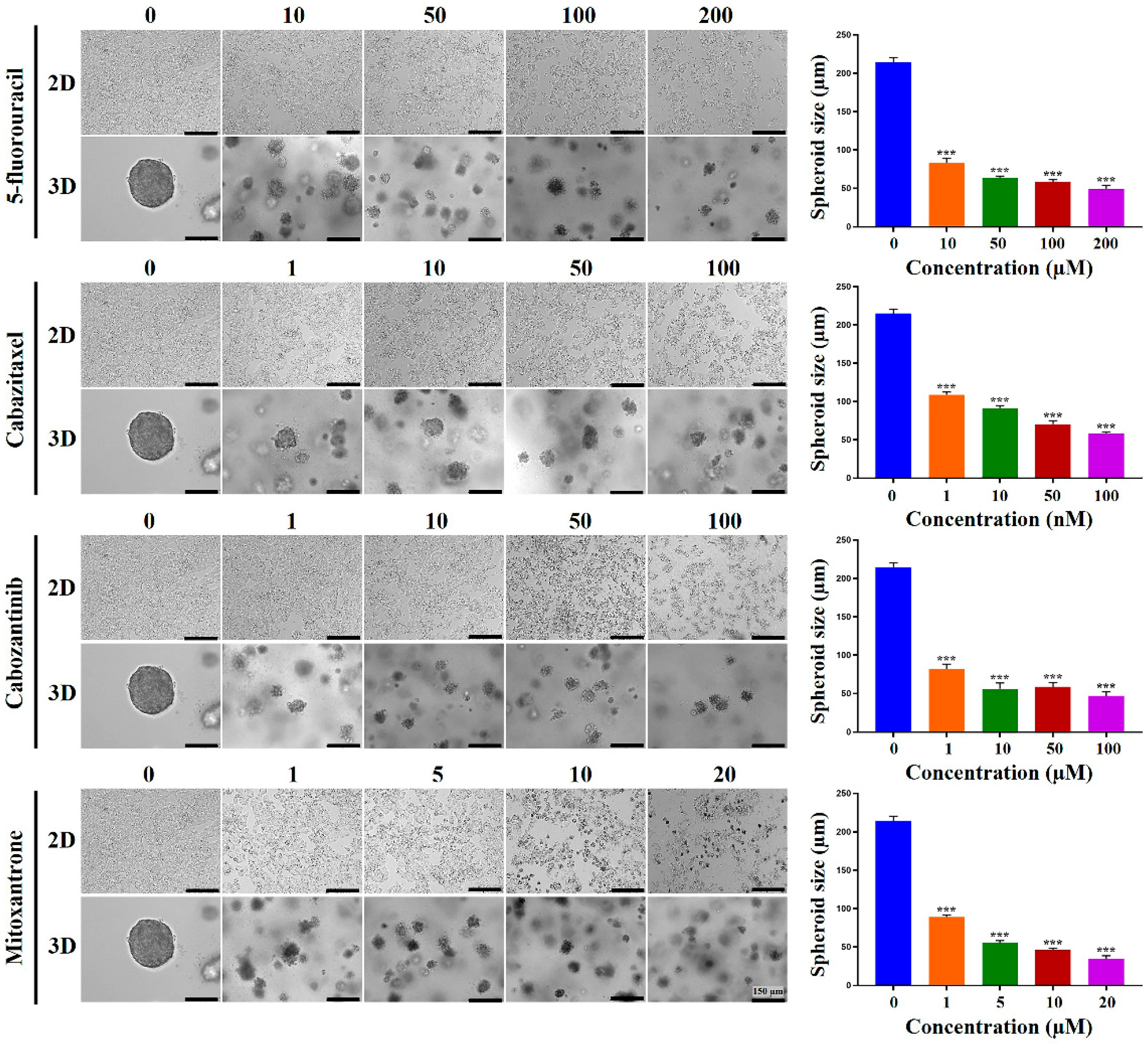
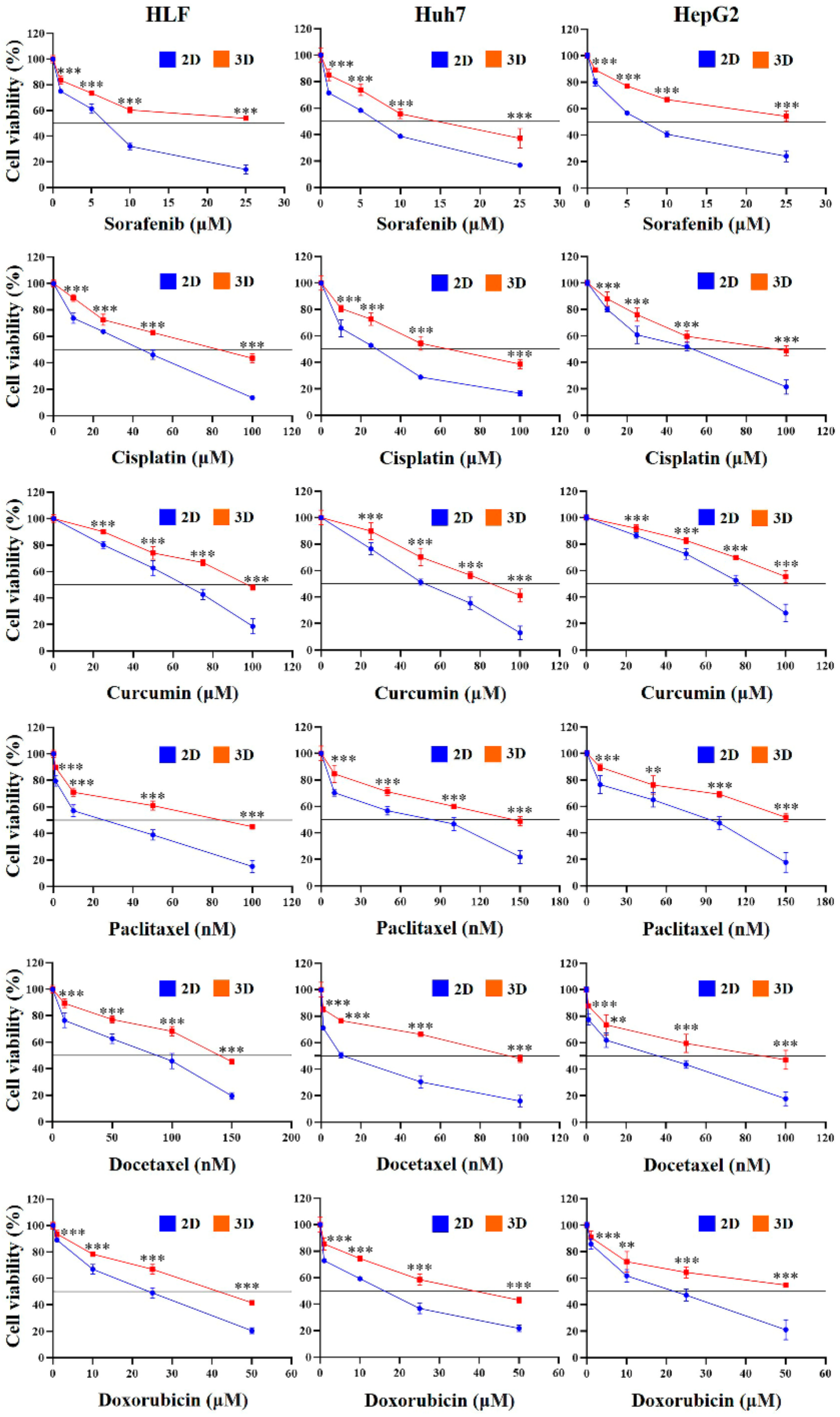
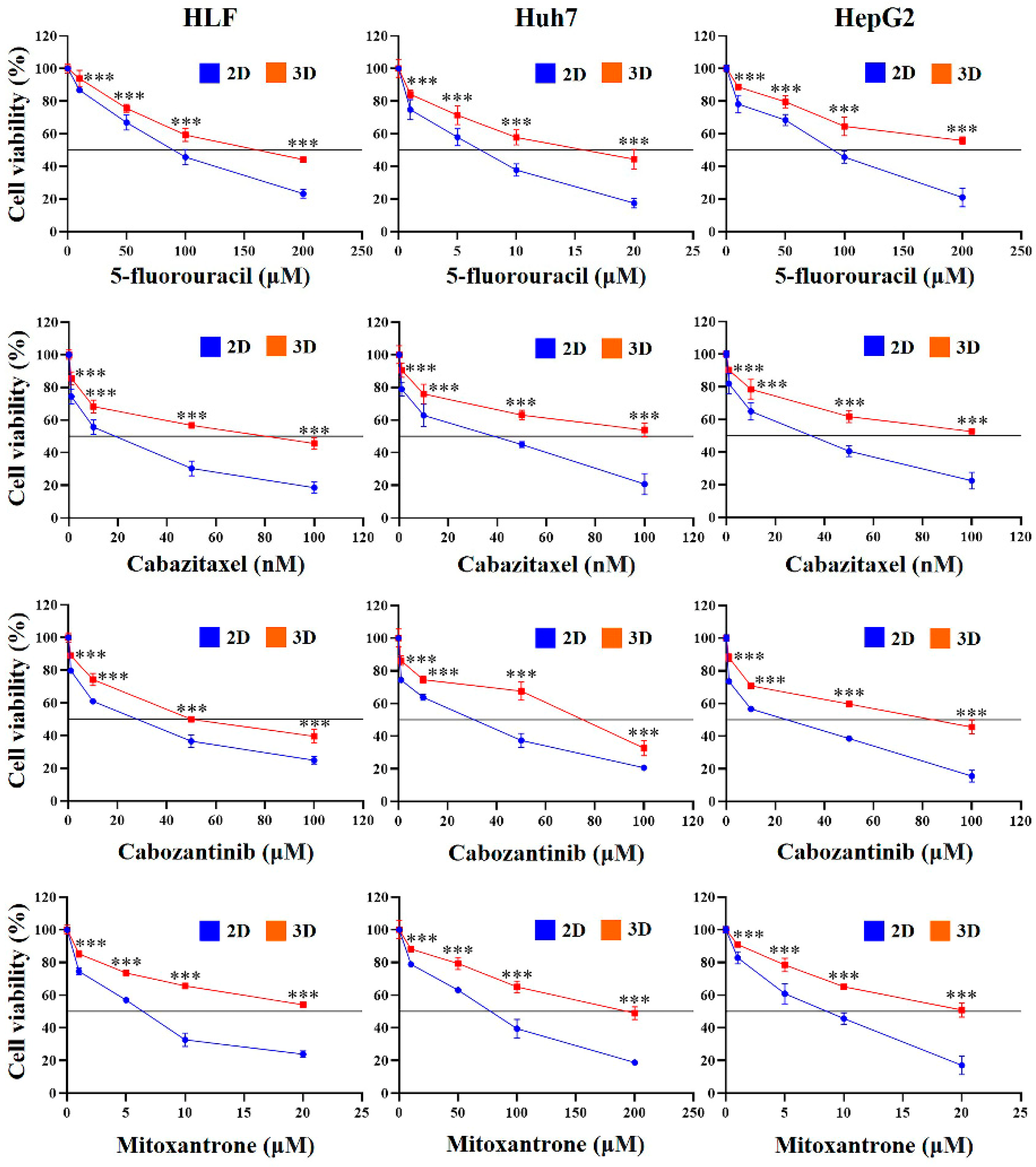
2.4. Chemoresistance of HCC Cells Was Increased in MCP-B Hydrogels
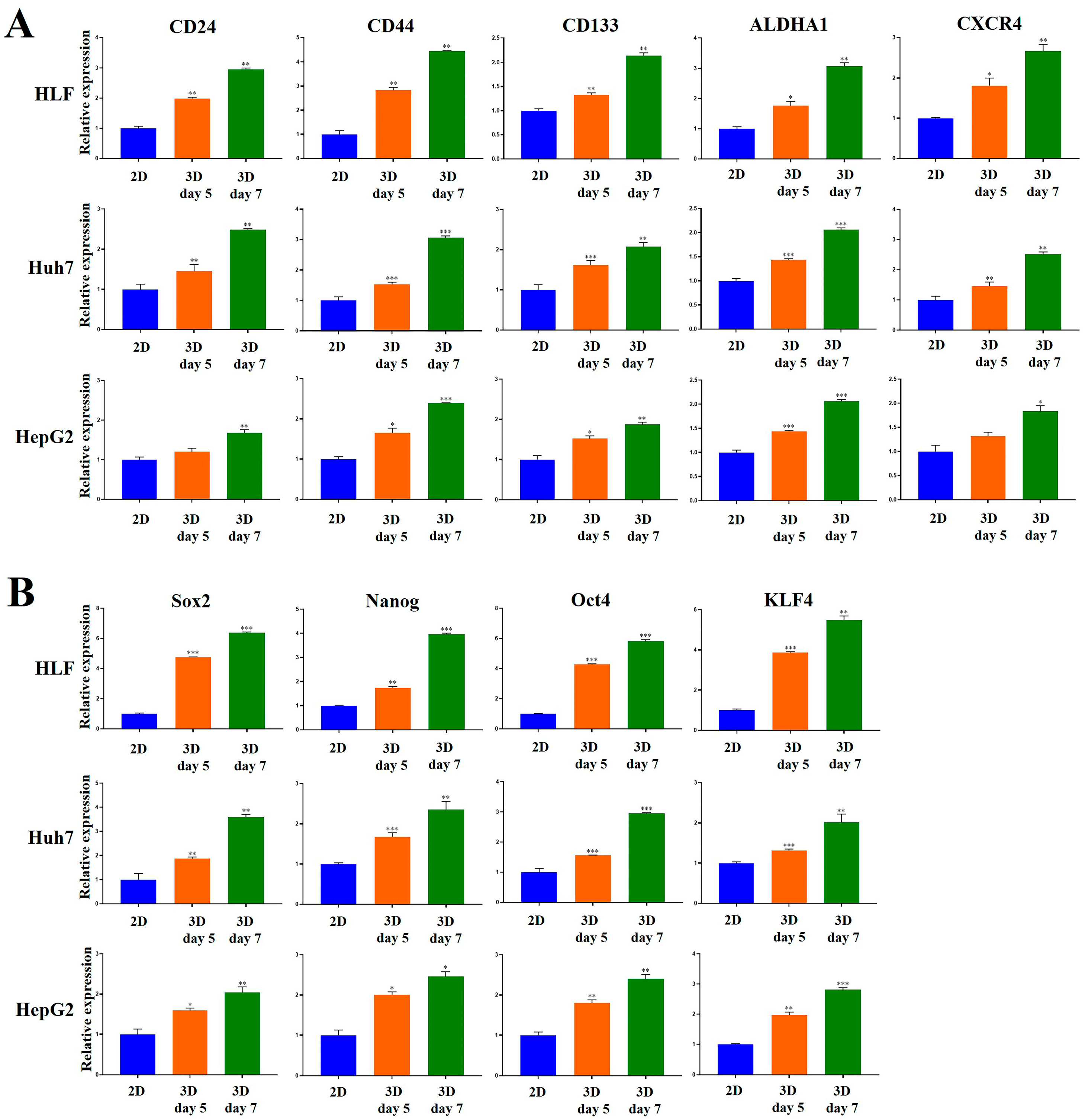
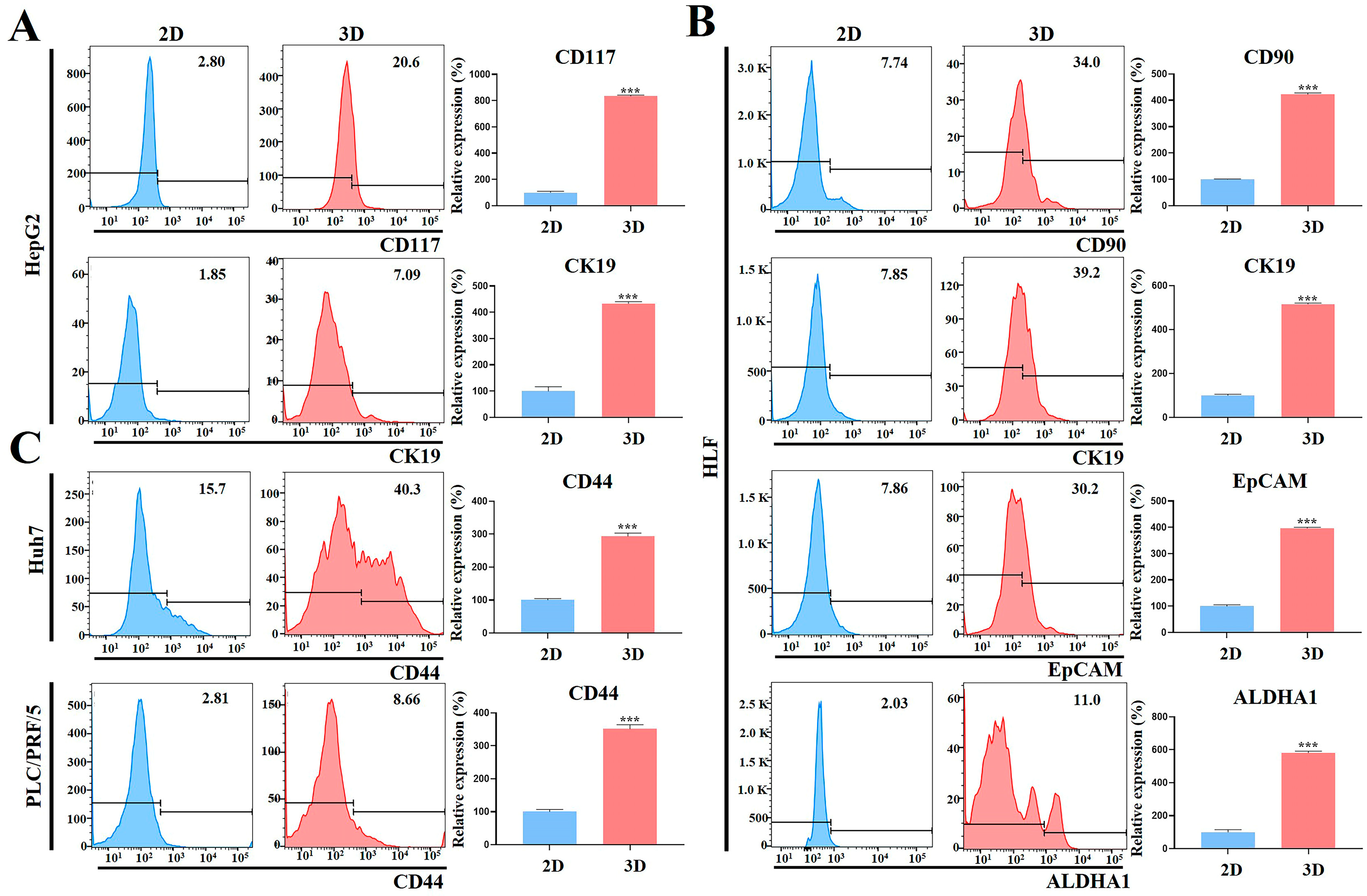
2.5. Stemness-Related Marker Expression of HCC Cells Was Enhanced in MCP-B Hydrogels
2.6. Aggressiveness of HCC Cells Was Reinforced in MCP-B Hydrogels
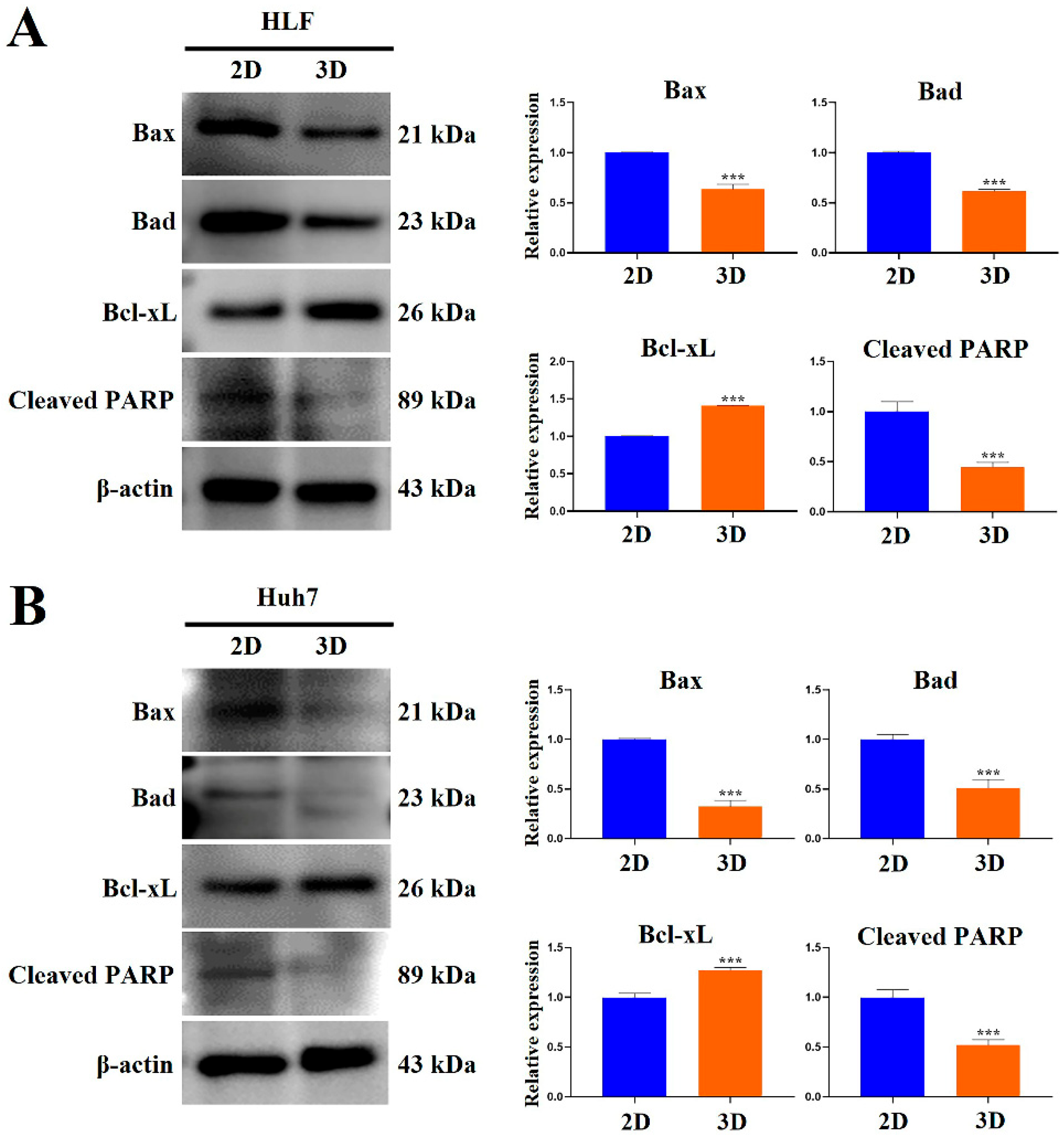
2.7. MCP-B Hydrogels Reduced Human HCC Cell Apoptosis
2.8. HCC Stem Cell Biomarker Expression Was Augmented in MCP-B Hydrogels
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Synthesis of Hydrogels for 3D Cell Culture
4.3. Spheroid Growth Assay
4.4. Cell Proliferation Assay
4.5. Colony-Forming Assay
4.6. Wound-Healing Assay
4.7. Hydrogel Invasion Assay
4.8. RNA Isolation and cDNA Synthesis
4.9. Quantitative Real-Time PCR (qRT-PCR)
4.10. Flow Cytometry
4.11. Cell Cycle Analysis
4.12. Cell Spheroid-Based Anti-Cancer Drug Test
4.13. Western Blot Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| MCP-B | Marine collagen peptides-based |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| CSC | Cancer stem cell |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MDR1 | Multidrug resistance protein 1 |
| MRP1 | Multidrug resistance-associated protein 1 |
| CYP | Cytochrome P450 |
References
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: Signaling pathways and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 35. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.Y.; Danpanichkul, P.; Yong, J.N.; Yu, Z.; Tan, D.J.H.; Lim, W.H.; Koh, B.; Lim, R.Y.Z.; Tham, E.K.J.; Mitra, K.; et al. Liver cancer in 2021: Global Burden of Disease study. J. Hepatol. 2025, 82, 851–860. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Mocci, S.; Perra, A.; Littera, R.; Pes, F.; Melis, M.; Sanna, C.; Mascia, A.; Murgia, M.; Mereu, C.; Lorrai, M.; et al. Human leukocyte antigen-G in hepatocellular carcinoma driven by chronic viral hepatitis or steatotic liver disease. Sci. Rep. 2025, 15, 13331. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Lin, S.; Geller, D.A.; Yan, Y. The microenvironment in the development of MASLD-MASH-HCC and associated therapeutic in MASH-HCC. Front. Immunol. 2025, 16, 1569915. [Google Scholar] [CrossRef]
- Rho, H.; Kim, U.; Song, J. Ubiquitination and ubiquitin-like modifications in metabolic dysfunction-associated steatotic liver disease: Mechanisms and implications. BMB Rep. 2025, 58, 6447. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis virus and hepatocellular carcinoma: Recent advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef]
- Zhang, N.S.; Wong, R.J. Geographical disparities in hepatitis B virus related hepatocellular carcinoma mortality rates worldwide from 1990 to 2019. Medicine 2023, 102, e33666. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Mucinski, J.M.; Salvador, A.F.; Moore, M.P.; Fordham, T.M.; Anderson, J.M.; Shryack, G.; Cunningham, R.P.; Lastra, G.; Gaballah, A.H.; Diaz-Arias, A.; et al. Histological improvements following energy restriction and exercise: The role of insulin resistance in resolution of MASH. J. Hepatol. 2024, 81, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Polpichai, N.; Maneenil, C.; Danpanichkul, P.; Rattananukrom, C.; Choudhury, A.; Wong, Y.J.; Sripongpun, P.; Liangpunsakul, S.; Kaewdech, A. Current and new strategies for hepatocellular carcinoma surveillance. Gastroenterol. Rep. 2025, 13, goaf045. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, H.; Zhang, X.; Wang, S.; Liu, C.; An, K.; Liu, R.; Tian, X. Diversified applications of hepatocellular carcinoma medications: Molecular-targeted, immunotherapeutic, and combined approaches. Front. Pharmacol. 2024, 15, 1422033. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Riaz, I.B.; Naqvi, S.A.A.; Almquist, D.R.; Mina, S.; Almasri, J.; Shah, S.; Almader-Douglas, D.; Uson Junior, P.L.S.; Mahipal, A.; et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: A systematic review and network meta-analysis. JAMA Oncol. 2020, 6, e204930. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Méndez-Blanco, C.; Fondevila, F.; García-Palomo, A.; González-Gallego, J.; Mauriz, J.L. Sorafenib resistance in hepatocarcinoma: Role of hypoxia-inducible factors. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, Y.B.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Yoon, J.H. CKD-5, a novel pan-histone deacetylase inhibitor, synergistically enhances the efficacy of sorafenib for hepatocellular carcinoma. BMC Cancer 2020, 20, 1001. [Google Scholar] [CrossRef]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn. J. Clin. Oncol. 2018, 48, 103–114. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012, 209, 211–215. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Atala, A.; Soker, S. Substrate elasticity controls cell proliferation, surface marker expression and motile phenotype in amniotic fluid-derived stem cells. J. Mech. Behav. Biomed. Mater. 2013, 17, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.; Gil da Costa, R.M.; Coleman, I.M.; Matson, C.K.; Risk, M.C.; Coleman, R.T.; Nelson, P.S. A comparison of prostate cancer cell transcriptomes in 2D monoculture vs 3D xenografts identify consistent gene expression alterations associated with tumor microenvironments. Prostate 2020, 80, 491–499. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Kutle, I.; Polten, R.; Hachenberg, J.; Klapdor, R.; Morgan, M.; Schambach, A. Tumor organoid and spheroid models for cervical cancer. Cancers 2023, 15, 2518. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, J.S.; Kim, S.H.; Park, Y.K.; Yu, E.; Kim, K.H.; Seo, E.J.; Oh, H.B.; Lee, H.C.; Kim, K.M.; et al. Patient-derived multicellular tumor spheroids towards optimized treatment for patients with hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 109. [Google Scholar] [CrossRef]
- Ayvaz, I.; Sunay, D.; Sariyar, E.; Erdal, E.; Karagonlar, Z.F. Three-dimensional cell culture models of hepatocellular carcinoma—A review. J. Gastrointest. Cancer 2021, 52, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cheng, C.; Liu, S.; Yang, L.; Han, P.; Cui, T.; Zhang, Y. Advancements and application prospects of three-dimensional models for primary liver cancer: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1343177. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.; Giachi, A.; Spínola-Lasso, E.; Marra, F.; Raggi, C. Organoids and spheroids: Advanced in vitro models for liver cancer research. Front. Cell Dev. Biol. 2024, 12, 1536854. [Google Scholar] [CrossRef]
- van Tienderen, G.S.; Groot Koerkamp, B.; Ijzermans, J.N.M.; van der Laan, L.J.W.; Verstegen, M.M.A. Recreating tumour complexity in a dish: Organoid models to study liver cancer cells and their extracellular environment. Cancers 2019, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Blidisel, A.; Marcovici, I.; Coricovac, D.; Hut, F.; Dehelean, C.A.; Cretu, O.M. Experimental models of hepatocellular carcinoma—A preclinical perspective. Cancers 2021, 13, 3651. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Silva-Gomez, J.A.; Lucano-Landeros, S.; Santos, A.; Monroy-Ramirez, H.C.; Armendariz-Borunda, J. Liver cancer: Therapeutic challenges and the importance of experimental models. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8837811. [Google Scholar] [CrossRef]
- Romualdo, G.R.; Leroy, K.; Costa, C.J.S.; Prata, G.B.; Vanderborght, B.; da Silva, T.C.; Barbisan, L.F.; Andraus, W.; Devisscher, L.; Câmara, N.O.S.; et al. In vivo and in vitro models of hepatocellular carcinoma: Current strategies for translational modeling. Cancers 2021, 13, 5583. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, J.; Qin, H. Advances in hepatocellular carcinoma drug resistance models. Front. Med. 2024, 11, 1437226. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Liu, Y.; Yu, Y.; Yang, M.; Lu, L.; Chan, L.; Liu, B. Functional hydrogels for hepatocellular carcinoma: Therapy, imaging, and in vitro model. J. Nanobiotechnol. 2024, 22, 381. [Google Scholar] [CrossRef]
- Courtois, A.; Gérard, S.; Esparteiro, D.; Nguyen-Khac, E.; Marcq, I.; Fouquet, G. An overview of in vitro models of alcohol-related hepatocellular carcinoma. Cancer Med. 2025, 14, e70524. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cui, J.; Chen, R.; Guo, K.; Kang, X.; Li, Y.; Gao, D.; Sun, L.; Xu, C.; Chen, J.; et al. A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumour Biol. 2011, 32, 469–479. [Google Scholar] [CrossRef]
- Yip, D.; Cho, C.H. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commun. 2013, 433, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Terashima, J.; Goto, S.; Hattori, H.; Hoshi, S.; Ushirokawa, M.; Kudo, K.; Habano, W.; Ozawa, S. CYP1A1 and CYP1A2 expression levels are differentially regulated in three-dimensional spheroids of liver cancer cells compared to two-dimensional monolayer cultures. Drug Metab. Pharmacokinet. 2015, 30, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of in vitro 3D cell model developed from human hepatocellular carcinoma (HepG2) cell line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Dudas, J.; Ladanyi, A.; Ingruber, J.; Steinbichler, T.B.; Riechelmann, H. Epithelial to mesenchymal transition: A mechanism that fuels cancer radio/chemoresistance. Cells 2020, 9, 428. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-mesenchymal transition in cancer: A historical overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights into the role of matrix metalloproteinases in cancer and its various therapeutic aspects: A review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Pietras, K.; Ostman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- McMillin, D.W.; Negri, J.M.; Mitsiades, C.S. The role of tumour-stromal interactions in modifying drug response: Challenges and opportunities. Nat. Rev. Drug Discov. 2013, 12, 217–228. [Google Scholar] [CrossRef]
- Ho, D.W.H.; Lo, R.C.L.; Chan, L.K.; Ng, I.O.L. Molecular pathogenesis of hepatocellular carcinoma. Liver Cancer 2016, 5, 290–302. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP and TAZ: A signalling hub of the tumour microenvironment. Nat. Rev. Cancer 2019, 19, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Safri, F.; Nguyen, R.; Zerehpooshnesfchi, S.; George, J.; Qiao, L. Heterogeneity of hepatocellular carcinoma: From mechanisms to clinical implications. Cancer Gene Ther. 2024, 31, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Wang, Z.; Zhang, L.; Li, J.; Li, Y. Hepatocellular carcinoma drug resistance models. Cancer Cell Int. 2025, 25, 195. [Google Scholar] [CrossRef] [PubMed]
- Rauner, G.; Gupta, P.B.; Kuperwasser, C. From 2D to 3D and beyond: The evolution and impact of in vitro tumor models in cancer research. Nat. Methods 2025. ahead of print. [Google Scholar] [CrossRef]
- Kim, J.B.; Stein, R.; O’Hare, M.J. Three-dimensional in vitro tissue culture models of breast cancer—A review. Breast Cancer Res. Treat. 2004, 85, 281–291. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Q.; Shang, B.; Zhang, K.; Zhang, W. Organoid co-culture models of the tumor microenvironment promote precision medicine. Cancer Innov. 2024, 3, e101. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, T.; Cheng, H.; Liu, W.; Shi, D.; Yuan, J.; He, Z.; Wang, W.; Chen, B.; Ma, L.; et al. Macrophage-organoid co-culture model for identifying treatment strategies against macrophage-related gemcitabine resistance. J. Exp. Clin. Cancer Res. 2023, 42, 199. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Koutsakis, C.; Piperigkou, Z.; Karamanos, N.K. Recreating the extracellular matrix: Novel 3D cell culture platforms in cancer research. FEBS J. 2023, 290, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Saglam-Metiner, P.; Gulce-Iz, S.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 20, 203–212. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Modeling Neurological Diseases With Human Brain Organoids. Front. Synaptic Neurosci. 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Balak, J.R.A.; Juksar, J.; Carlotti, F.; Lo Nigro, A.; de Koning, E.J.P. Organoids from the Human Fetal and Adult Pancreas. Curr. Diab. Rep. 2019, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, B.; Buono, M.F.; von Boehmer, L.; Hoerstrup, S.P.; Emmert, M.Y. Human Cardiac Organoids for Disease Modeling. Clin. Pharmacol. Ther. 2019, 105, 79–85. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Yousef Yengej, F.A.; Jansen, J.; Rookmaaker, M.B.; Verhaar, M.C.; Clevers, H. Kidney Organoids and Tubuloids. Cells 2020, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Nam, L.; Kannan, S.; Kwon, C. Heart organoids and tissue models for modeling development and disease. Semin. Cell Dev. Biol. 2021, 118, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ogoke, O.; Maloy, M.; Parashurama, N. The science and engineering of stem cell-derived organoids-examples from hepatic, biliary, and pancreatic tissues. Biol. Rev. Camb. Philos. Soc. 2021, 96, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, J.; Knoblich, J.A. Brain organoids: An ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 2021, 28, 52–67. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamagata, K.; Osafune, K. Kidney organoids: Research in developmental biology and emerging applications. Dev. Growth Differ. 2021, 63, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Mateos-Sánchez, C.; González, B.; de Miguel-García, G.; Font-Cugat, A.; Marcote-Corral, I.; Alonso, S. Comparative analysis of 3D-culture techniques for multicellular colorectal tumour spheroids and development of a novel SW48 3D-model. Sci. Rep. 2025, 15, 27687. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hori, Y.; Yamamoto, T.; Urashima, T.; Ohara, Y.; Tanaka, H. 3D spheroid cultures improve the metabolic gene expression profiles of HepaRG cells. Biosci. Rep. 2015, 35, e00208. [Google Scholar] [CrossRef]
- Liao, W.; Wang, J.; Xu, J.; You, F.; Pan, M.; Xu, X.; Weng, J.; Han, X.; Li, S.; Li, Y.; et al. High-throughput three-dimensional spheroid tumor model using a novel stamp-like tool. J. Tissue Eng. 2019, 10, 2041731419889184. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, H.; Wang, Y.; Zhang, X.; Jin, B.; Xie, F.; Jin, Y.; Pang, Y.; Zhao, H.; Lu, X.; et al. Application of a 3D bioprinted hepatocellular carcinoma cell model in antitumor drug research. Front. Oncol. 2020, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.P.; Carey, J.E.; Miah, A.; McMurray, H.F.; Munday, P.W.; James, R.S.; Coleman, R.A.; Brown, A.M. Measurement of cytochrome P450 gene induction in human hepatocytes using quantitative real-time reverse transcriptase-polymerase chain reaction. Drug Metab. Dispos. 2000, 28, 781–788. [Google Scholar] [CrossRef]
- Nishimura, M.; Ueda, N.; Naito, S. Effects of dimethyl sulfoxide on the gene induction of cytochrome P450 isoforms, UGT-dependent glucuronosyl transferase isoforms, and ABCB1 in primary culture of human hepatocytes. Biol. Pharm. Bull. 2003, 26, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Yokoi, T.; Tateno, C.; Kataoka, M.; Takahashi, E.; Horie, T.; Yoshizato, K.; Naito, S. Induction of human CYP1A2 and CYP3A4 in primary culture of hepatocytes from chimeric mice with humanized liver. Drug Metab. Pharmacokinet. 2005, 20, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Koeda, A.; Suganuma, Y.; Suzuki, E.; Shimizu, T.; Nakayama, M.; Satoh, T.; Narimatsu, S.; Naito, S. Comparison of inducibility of CYP1A and CYP3A mRNAs by prototypical inducers in primary cultures of human, cynomolgus monkey, and rat hepatocytes. Drug Metab. Pharmacokinet. 2007, 22, 178–186. [Google Scholar] [CrossRef]
- Khafaga, A.F.; Mousa, S.A.; Aleya, L.; Abdel-Daim, M.M. Three-dimensional (3D) cell culture: A valuable step in advancing treatments for human hepatocellular carcinoma. Cancer Cell Int. 2022, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Shigemoto, K.; Akiyama, H.; Ieda, A.; Ijiri, Y.; Hayashi, T. Human hepatocarcinoma functional liver cell-4 cell line exhibits high expression of drug-metabolizing enzymes in three-dimensional culture. Biol. Pharm. Bull. 2014, 37, 1782–1787. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D cell culture models as recapitulators of the tumor microenvironment for the screening of anti-cancer drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Hsieh, C.H.; Wu, Y.S. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol. Pharm. 2011, 8, 1996–2011. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, N.; Kim, K.; Koo, H.J.; Choi, J.; Kim, H.N. Chemoresistance of cancer cells: Requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 2018, 8, 5259–5275. [Google Scholar] [CrossRef]
- Wind, N.S.; Holen, I. Multidrug resistance in breast cancer: From in vitro models to clinical studies. Int. J. Breast Cancer 2011, 2011, 967419. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Agarwal, S.; Singh, A.; Bajaj, A.; Dasgupta, U. Insights into molecular mechanisms of chemotherapy resistance in cancer. Transl. Oncol. 2024, 42, 101901. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.N.; Chow, E.K.H. Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2013, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Rizzino, A. Concise review: The Sox2-Oct4 connection: Critical players in a much larger interdependent network integrated at multiple levels. Stem Cells 2013, 31, 1033–1039. [Google Scholar] [CrossRef]
- Wang, X.Q.; Ongkeko, W.M.; Chen, L.; Yang, Z.F.; Lu, P.; Chen, K.K.; Lopez, J.P.; Poon, R.T.P.; Fan, S.T. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology 2010, 52, 528–539. [Google Scholar] [CrossRef]
- Cheung, S.T.; Cheung, P.F.Y.; Cheng, C.K.C.; Wong, N.C.L.; Fan, S.T. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology 2011, 140, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.H.; Fan, L.L.; Chen, X.; Bishop, J.M. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology 2012, 56, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Han, J.; Kim, J.B.; Yang, M.G.; Kim, Y.J.; Lim, H.J.; An, S.Y.; Kim, J.H. Interleukin-8 is related to poor chemotherapeutic response and tumourigenicity in hepatocellular carcinoma. Eur. J. Cancer 2014, 50, 341–350. [Google Scholar] [CrossRef]
- Zhou, J.J.; Deng, X.G.; He, X.Y.; Zhou, Y.; Yu, M.; Gao, W.C.; Zeng, B.; Zhou, Q.B.; Li, Z.H.; Chen, R.F. Knockdown of NANOG enhances chemosensitivity of liver cancer cells to doxorubicin by reducing MDR1 expression. Int. J. Oncol. 2014, 44, 2034–2040. [Google Scholar] [CrossRef]
- Wan, X.; Cheng, C.; Shao, Q.; Lin, Z.; Lu, S.; Chen, Y. CD24 promotes HCC progression via triggering Notch-related EMT and modulation of tumor microenvironment. Tumour Biol. 2016, 37, 6073–6084. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yao, Y.; Xu, G.; Zhou, C.; Zhang, Y.; Sun, J.; Jiang, R.; Shao, Q.; Chen, Y. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, X.; Wan, Y.; Zhang, H.; Tang, T.; Zhang, M.; Yu, S.; Zhao, W.; Chen, L. CD44 promotes hepatocellular carcinoma progression via upregulation of YAP. Exp. Hematol. Oncol. 2021, 10, 54. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.J.; Chan, K.W.; Guan, X.Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008, 27, 1749–1758. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Xu, Y.; Lai, Y.; Weng, H.; Tan, L.; Li, Y.; Chen, G.; Luo, X.; Ye, Y. MiR-124 sensitizes cisplatin-induced cytotoxicity against CD133(+) hepatocellular carcinoma cells by targeting SIRT1/ROS/JNK pathway. Aging 2019, 11, 2551–2564. [Google Scholar] [CrossRef]
- Kahraman, D.C.; Kahraman, T.; Cetin-Atalay, R. Targeting PI3K/Akt/mTOR pathway identifies differential expression and functional role of IL8 in liver cancer stem cell enrichment. Mol. Cancer Ther. 2019, 18, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Hemati, H.; Kaur, J.; Sobti, R.C.; Trehanpati, N. Inhibition of NOTCH signaling pathway chemosensitizes HCC CD133+ cells to vincristine and 5-fluorouracil through upregulation of BBC3. Biochem. Biophys. Res. Commun. 2020, 525, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Tanei, T.; Morimoto, K.; Shimazu, K.; Kim, S.J.; Tanji, Y.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin. Cancer Res. 2009, 15, 4234–4241. [Google Scholar] [CrossRef]
- Jackson, B.; Brocker, C.; Thompson, D.C.; Black, W.; Vasiliou, K.; Nebert, D.W.; Vasiliou, V. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum. Genom. 2011, 5, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lingala, S.; Khoobyari, S.; Nolta, J.; Zern, M.A.; Wu, J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J. Hepatol. 2011, 55, 838–845, Erratum in J. Hepatol. 2013, 58, 405. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Konno, M.; Hamabe, A.; Hasegawa, S.; Kano, Y.; Ohta, K.; Fukusumi, T.; Sakai, D.; Kudo, T.; Haraguchi, N.; et al. Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int. J. Oncol. 2013, 42, 1437–1442. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.J.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Na, J.; Ryu, J.; Kim, H.J.; Nam, S.H.; Kang, M.; Jung, J.W.; Lee, M.S.; Song, H.E.; Choi, J.; et al. Interaction of tetraspan(in) TM4SF5 with CD44 promotes self-renewal and circulating capacities of hepatocarcinoma cells. Hepatology 2015, 61, 1978–1997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, M.; Lu, P.; Li, X.; Zhu, S.; Yue, S. NEDD9 may regulate hepatocellular carcinoma cell metastasis by promoting epithelial-mesenchymal-transition and stemness via repressing Smad7. Oncotarget 2017, 8, 1714–1724. [Google Scholar] [CrossRef]
- Kanki, K.; Watanabe, R.; Nguyen Thai, L.; Zhao, C.H.; Naito, K. HDAC9 is preferentially expressed in dedifferentiated hepatocellular carcinoma cells and is involved in an anchorage-independent growth. Cancers 2020, 12, 2734. [Google Scholar] [CrossRef]
- Chaves, L.P.; Melo, C.M.; Saggioro, F.P.; Reis, R.B.D.; Squire, J.A. Epithelial-mesenchymal transition signaling and prostate cancer stem cells: Emerging biomarkers and opportunities for precision therapeutics. Genes 2021, 12, 1900. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, Y.E.; Al-Hetty, H.R.A.K.; Ramaiah, P.; Fazaa, A.H.; Jalil, A.T.; Alsaikhan, F.; Gupta, J.; Ramírez-Coronel, A.A.; Tayyib, N.A.; Peng, H. Harnessing function of EMT in hepatocellular carcinoma: From biological view to nanotechnological standpoint. Environ. Res. 2023, 227, 115683. [Google Scholar] [CrossRef]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Banerjee, S.; Sarkar, F.H. Emerging Role of Notch in Stem Cells and Cancer. Cancer Lett. 2009, 279, 8–12. [Google Scholar] [CrossRef]
- Cho, S.; Lu, M.; He, X.; Ee, P.L.R.; Bhat, U.; Schneider, E.; Miele, L.; Beck, W.T. Notch1 regulates the expression of the multidrug resistance gene ABCC1/MRP1 in cultured cancer cells. Proc. Natl Acad. Sci. USA 2011, 108, 20778–20783. [Google Scholar] [CrossRef] [PubMed]
- Tosello, V.; Ferrando, A.A. The NOTCH signaling pathway: Role in the pathogenesis of T-cell acute lymphoblastic leukemia and implication for therapy. Ther. Adv. Hematol. 2013, 4, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; Li, J.; Zhang, K.; Chen, J.; Chen, D.; Feng, B.; Song, H.; Feng, J.; Wang, R.; et al. Notch-1 confers chemoresistance in lung adenocarcinoma to taxanes through AP-1/microRNA-451 mediated regulation of MDR-1. Mol. Ther. Nucleic Acids 2016, 5, e375. [Google Scholar] [CrossRef]
- Al-Alem, L.F.; Pandya, U.M.; Baker, A.T.; Bellio, C.; Zarrella, B.D.; Clark, J.; DiGloria, C.M.; Rueda, B.R. Ovarian cancer stem cells: What progress have we made? Int. J. Biochem. Cell Biol. 2019, 107, 92–103. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and their inhibitors: Potential for the development of new therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Artacho-Cordón, A.; Artacho-Cordón, F.; Ríos-Arrabal, S.; Calvente, I.; Núñez, M.I. Tumor microenvironment and breast cancer progression: A complex scenario. Cancer Biol. Ther. 2012, 13, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Arvelo, F.; Sojo, F.; Cotte, C. Tumour progression and metastasis. Ecancermedicalscience 2016, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; An, Z.Y.; Jiang, W.; Jin, W.L.; He, X.Y. Collagen code in tumor microenvironment: Functions, molecular mechanisms, and therapeutic implications. Biomed. Pharmacother. 2023, 166, 115390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, X.; Kan, C.; Chen, B.; Qu, N.; Hou, N.; Liu, Y.; Han, F. COL1A1: A novel oncogenic gene and therapeutic target in malignancies. Pathol. Res. Pract. 2022, 236, 154013. [Google Scholar] [CrossRef]
- Ding, D.Y.; Jiang, S.Y.; Zu, Y.X.; Yang, Y.; Gan, X.J.; Yuan, S.X.; Zhou, W.P. Collagen in hepatocellular carcinoma: A novel biomarker and therapeutic target. Hepatol. Commun. 2024, 8, e0489. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.; Iyer, R.; Chakraborty, S. The extracellular matrix in hepatocellular carcinoma: Mechanisms and therapeutic vulnerability. Cell Rep. Med. 2023, 4, 101170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, S.; Chen, J.; Zhou, D.; Gao, H.; Zhou, Z.; Xu, L.; Chen, M. Overexpression of LAMC1 predicts poor prognosis and enhances tumor cell invasion and migration in hepatocellular carcinoma. J. Cancer 2017, 8, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Ikram, M.; Subhan, F.; Kang, H.Y.; Lim, Y.; Lee, R.; Jin, S.; Jeong, Y.H.; Kwak, J.Y.; Na, Y.J.; et al. Alginate–marine collagen–agarose composite hydrogels as matrices for biomimetic 3D cell spheroid formation. RSC Adv. 2016, 6, 46952–46965. [Google Scholar] [CrossRef]
- Moon, S.; Ok, Y.; Hwang, S.; Lim, Y.S.; Kim, H.Y.; Na, Y.J.; Yoon, S. A marine collagen-based biomimetic hydrogel recapitulates cancer stem cell niche and enhances progression and chemoresistance in human ovarian cancer. Mar. Drugs 2020, 18, 498. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| ABCG2 | GTTCTCAGCAGCTCTTCGGCTT | TCCTCCAGACACACCACGGATA |
| Albumin | TTTATGCCCCGGAAGTCCTTT | AGTCTCTGTTTGGCAGACGAA |
| ALDHA1 | CGGGAAAAGCAATCTGAAGAGGG | GATGCGGCTATACAACACTGGC |
| CD24 | CACGCAGATTTATTCCAGTGAAAC | GACCACGAAGAGACTGGCTGTT |
| CD44 | CTGCCGCTTTGCAGGTGTA | CATTGTGGGCAAGGTGCTATT |
| CD133 | CACTACCAAGGACAAGGCGTTC | CAACGCCTCTTTGGTCTCCTTG |
| c-Met | AGGCTTCTGGGCCTTAT | TGCTTCTCTCGCCAGGAATAC |
| COL1A1 | ATTAGTAGGTGTGCTGTGTG | AAGCGTTTGCGTAGTAATTG |
| COL4A1 | ATTAGTAGGTGTGCTGTGTG | AAGCGTTTGCGTAGTAATTG |
| CYP1A1 | TGGATGAGAACGCCAATG | TGGGTTGACCCATAGCTTCT |
| CYP1A2 | AACAAGGGACACAACGCTGAAT | GGAAGAGAAACAAGGGCTGAGT |
| CYP3A4 | TTTTGTCCTACCATAAGGGC | CATAAATCCCACTGGACCAA |
| CXCR4 | CTCCTCTTTGTCATCACGCTTCC | GGATGAGGACACTGCTGTAGAG |
| Fibronectin | ACCTACAACATCATAGTGGAGGCACTG | GTCACAGCGCCAGCCCCGCTGGCCTCC |
| HGF | CTCACACCCGCTGGGAGTAC | TCCTTGACCTTGGATGCATTC |
| HNF4A | GGCCAAGTACATCCCAGCTTT | CAGCACCAGCTCGTCAAGG |
| IGF1 | GCTCTTCAGTTCGTGTGTGG | CGCAATACATCTCCAGCCTC |
| IGF2 | GGGCAAGTTCTTCCAATATGA | TCACTTCCGATTGCTGGC |
| IGF1R | AACCCCAAGACTGAGGTGTG | TGACATCTCTCCGCTTCCTT |
| IGF2R | GGCACAATTACTGCTCCAAAGAC | CAAGGCCCTTTCTCCCCAC |
| KLF4 | CATCTCAAGGCACACCTGCGAA | TCGGTCGCATTTTTGGCACTGG |
| LAMC1 | ACTCCTAATCTTGGACCATAC | ACAACAGCACAACTTGAAC |
| MDR1 | GCTGTCAAGGAAGCCAATGCCT | TGCAATGGCGATCCTCTGCTTC |
| MMP2 | TGACGGTAAGGACGGACTC | ATACTTCACACGGACCACTTG |
| MMP9 | CAGAGATGCGTGGAGAGT | TCTTCCGAGTAGTTTTGG |
| MRP1 | CCGTGTACTCCAACGCTGACAT | ATGCTGTGCGTGACCAAGATCC |
| Nanog | CTCCAACATCCTGAACCTCAGC | CGTCACACCATTGCTATTCTTCG |
| N-cadherin | AGCCAACCTTAACTGAGGAGT | GGCAAGTTGATTGGAGGGATG |
| Notch1 | GGTGAACTGCTCTGAGGAGATC | GGATTGCAGTCGTCCACGTTGA |
| Notch2 | GTGCCTATGTCCATCTGGATGG | AGACACCTGAGTGCTGGCACAA |
| Oct4 | CTTGAATCCCGAATGGAAAGGG | GTGTATATCCCAGGGTGATCCTC |
| Snail | ACTGCAACAAGGAATACCTCAG | GCACTGGTACTTCTTGACATCTG |
| Slug | TGTGACAAGGAATATGTGAGCC | TGAGCCCTCAGATTTGACCTG |
| Sox2 | GCTACAGCATGATGCAGGACCA | TCTGCGAGCTGGTCATGGAGTT |
| Twist | GTCCGCAGTCTTACGAGGAG | GCTTGAGGGTCTGAATCTTGCT |
| VEGFA | TTGCCTTGCTGCTCTACCTCCA | GATGGCAGTAGCTGCGCTGATA |
| Vimentin | CAAAGCAGGAGTCCACTGAG | TAAGGGCATCCACTTCACAG |
| TGFβ | GGGACTATCCACCTGCAAGA | CCTCCTTGGCGTAGTAGTCG |
| TGFβR | GACAACGTCAGGTTCTGGCTCA | CCGCCACTTTCCTCTCCAAACAACT |
| Zeb1 | TTACACCTTTGCATACAGAACCC | TTTACGATTACACCCAGACTGC |
| Zeb2 | GCGATGGTCATGCAGTCAG | CAGGTGGCAGGTCATTTTCTT |
| GAPDH | GGAGAAGGCTGGGGCTCAT | TGATGGCATGGACTGTGGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajbongshi, L.; Kim, J.-E.; Lee, J.-E.; Lee, S.-R.; Hwang, S.-Y.; Kim, Y.; Hong, Y.M.; Oh, S.-O.; Kim, B.S.; Lee, D.; et al. A Robust Marine Collagen Peptide–Agarose 3D Culture System for In Vitro Modeling of Hepatocellular Carcinoma and Anti-Cancer Therapeutic Development. Mar. Drugs 2025, 23, 386. https://doi.org/10.3390/md23100386
Rajbongshi L, Kim J-E, Lee J-E, Lee S-R, Hwang S-Y, Kim Y, Hong YM, Oh S-O, Kim BS, Lee D, et al. A Robust Marine Collagen Peptide–Agarose 3D Culture System for In Vitro Modeling of Hepatocellular Carcinoma and Anti-Cancer Therapeutic Development. Marine Drugs. 2025; 23(10):386. https://doi.org/10.3390/md23100386
Chicago/Turabian StyleRajbongshi, Lata, Ji-Eun Kim, Jin-Eui Lee, Su-Rin Lee, Seon-Yeong Hwang, Yuna Kim, Young Mi Hong, Sae-Ock Oh, Byoung Soo Kim, Dongjun Lee, and et al. 2025. "A Robust Marine Collagen Peptide–Agarose 3D Culture System for In Vitro Modeling of Hepatocellular Carcinoma and Anti-Cancer Therapeutic Development" Marine Drugs 23, no. 10: 386. https://doi.org/10.3390/md23100386
APA StyleRajbongshi, L., Kim, J.-E., Lee, J.-E., Lee, S.-R., Hwang, S.-Y., Kim, Y., Hong, Y. M., Oh, S.-O., Kim, B. S., Lee, D., & Yoon, S. (2025). A Robust Marine Collagen Peptide–Agarose 3D Culture System for In Vitro Modeling of Hepatocellular Carcinoma and Anti-Cancer Therapeutic Development. Marine Drugs, 23(10), 386. https://doi.org/10.3390/md23100386









