Progress in the Application of Marine Polysaccharide Drug Delivery Systems in Tumor Immunotherapy: Multiple Mechanisms and Material Forms
Abstract
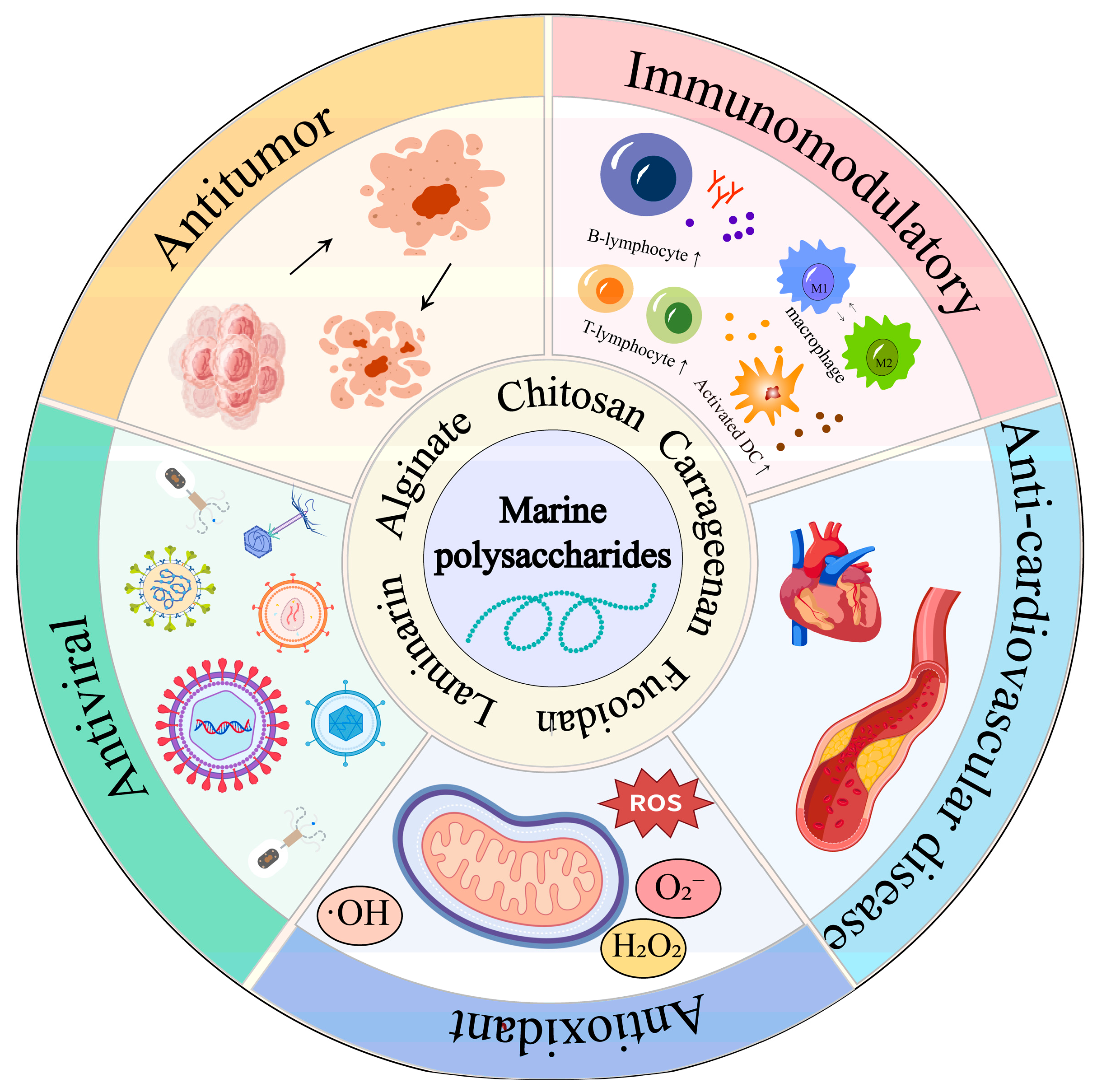
1. Introduction
2. Potential Advantages of Marine Polysaccharides for Tumor Immunotherapy
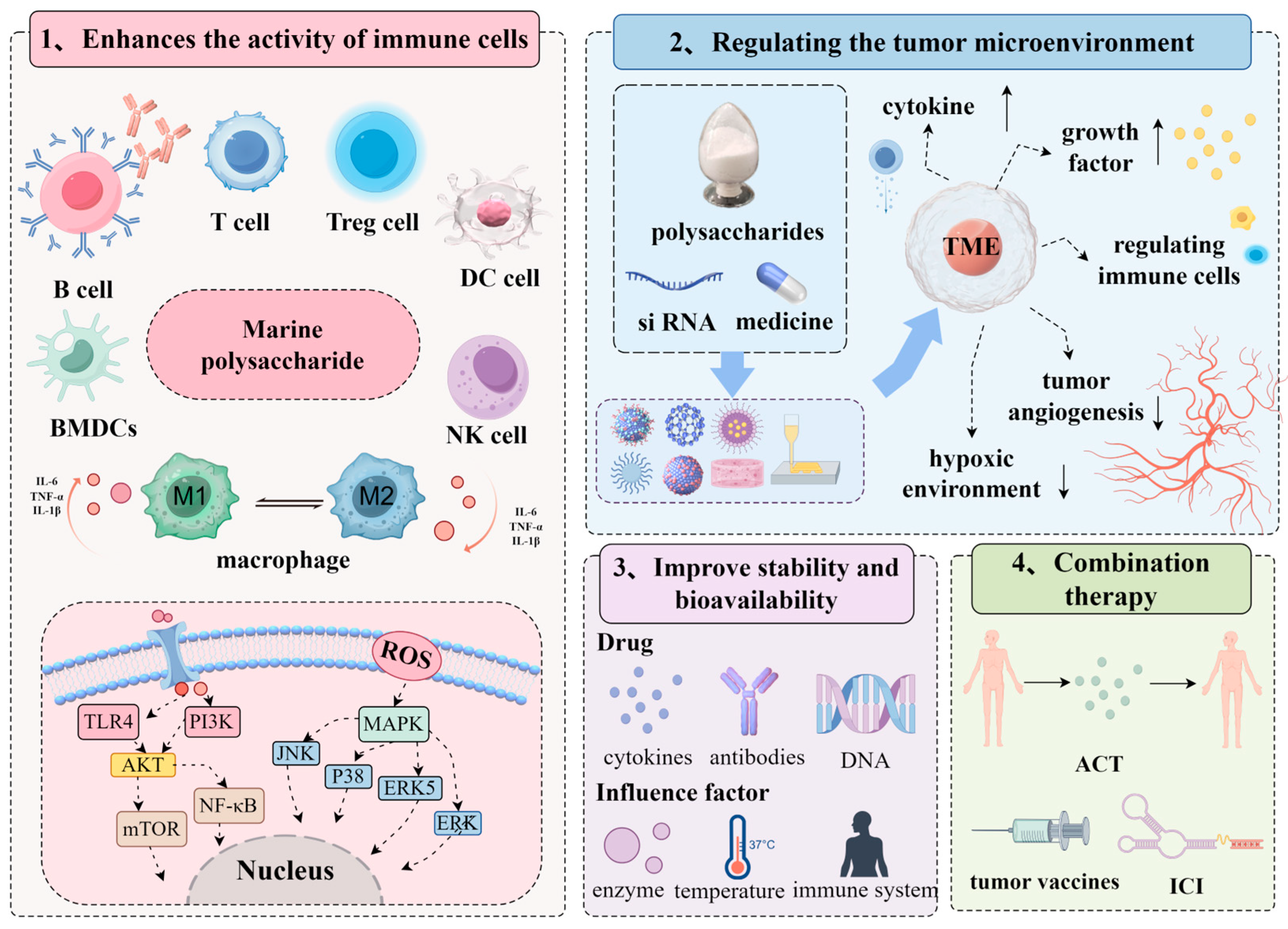
2.1. Enhances the Activity of Immune Cells
2.2. Regulating the Tumor Microenvironment
2.3. Improve Drug Stability and Bioavailability
2.4. Combination Therapy
3. Application of Marine Polysaccharide Drug Delivery System in Tumor Immunotherapy
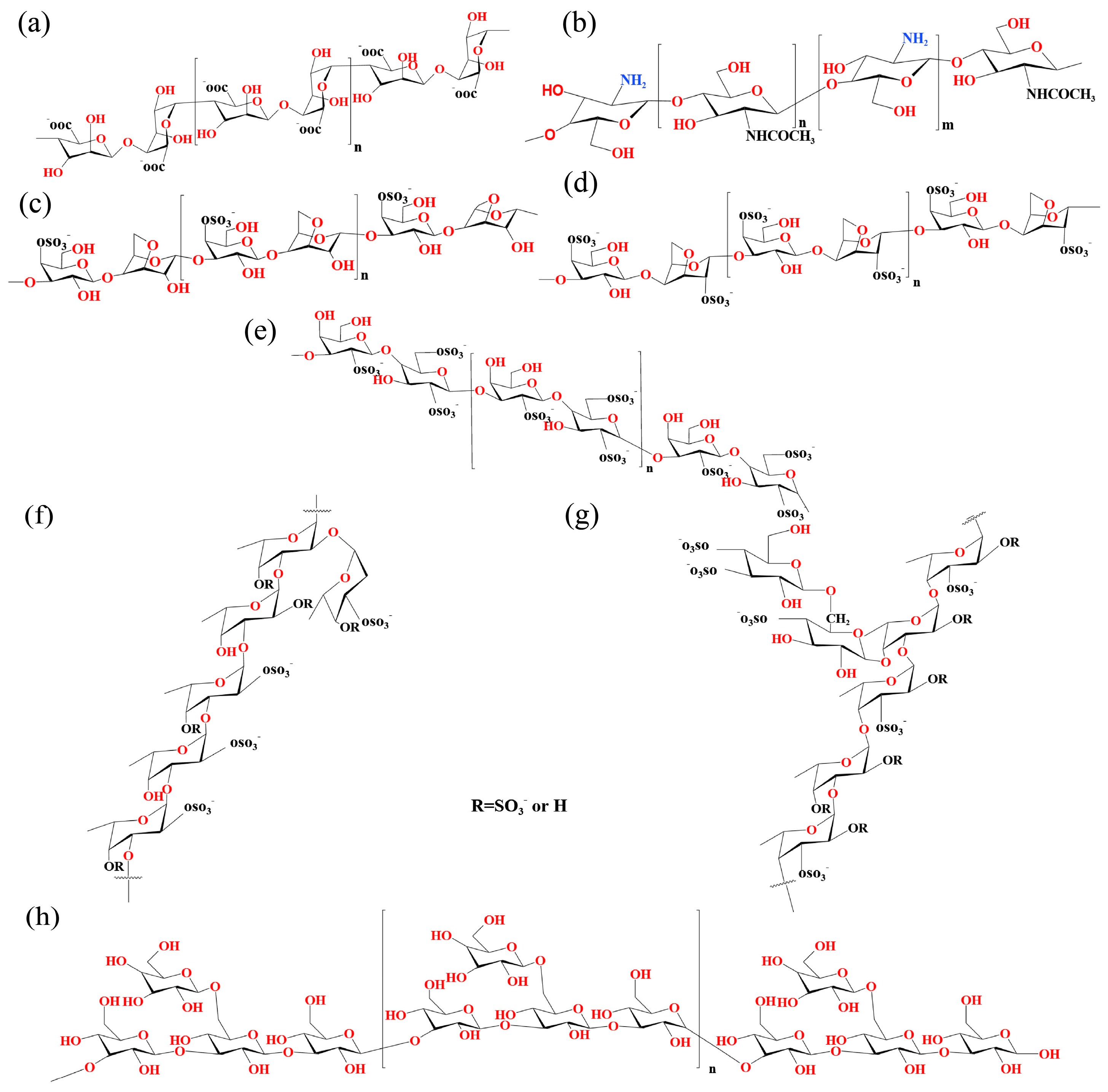
3.1. Alginate
3.1.1. Alginate Hydrogel Delivery Systems
3.1.2. Alginate Beads Delivery Systems
3.1.3. Alginate Microsphere Delivery Systems
3.1.4. Alginate Nanoparticle Delivery Systems
3.1.5. Others
3.2. Chitosan
3.2.1. Chitosan Hydrogel Delivery System
3.2.2. Chitosan Microsphere Delivery Systems
3.2.3. Chitosan Nanoparticle Delivery Systems
3.2.4. Others
3.3. Carrageenan
3.4. Fucoidan
3.5. Laminarin
4. Discussion
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, S.Y.; Zhang, Y.B.; Wang, H.B.; Liang, W.H.; Xie, L.; Li, N.; Fang, Y.; Wang, Y.T.; Liu, J.Y.; Chi, H.; et al. ITPRIPL1 binds CD3 8 to impede T cell activation and enable tumor immune evasion. Cell 2024, 187, 2305–2323.e33. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, A.; Huang, Y.; Li, H.; Cui, J.; Yang, H.; Si, L.; Jiao, T.; Ren, Z.; Zhang, Z.; et al. Ligand-induced ubiquitination unleashes LAG3 immune checkpoint function by hindering membrane sequestration of signaling motifs. Cell 2025, 188, 2354–2371.e18. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.H.; Liu, S.S.; Liu, J.Y.; Hu, H.W.; Yang, L.; Zhao, Q.T.; Li, C.C.; Zhang, B.; Zhang, Y. Overcoming resistance to immunotherapy by targeting GPR84 in myeloid-derived suppressor cells. Signal Transduct. Target. Ther. 2023, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Li, K.M.; Li, X.L.; Wu, J.; Wu, H.S.; Wu, M.; Zhou, Y.P.; Lin, Y.; Zou, Y.F.; Jiang, X.Q.; Xu, H.E. A Dual Enhancing Strategy of Novel Nanovaccine Based on TIM3 Silencing Nanoadjuvants and Desialylated Cancer Cell Membrane Antigens for Personalized Vaccination Immunotherapy of Cancer. Adv. Funct. Mater. 2024, 34, 2404956. [Google Scholar] [CrossRef]
- Mao, L.Z.; Ma, P.Q.; Luo, X.; Cheng, H.W.; Wang, Z.X.; Ye, E.Y.; Loh, X.J.; Wu, Y.L.; Li, Z.B. Stimuli-Responsive Polymeric Nanovaccines Toward Next-Generation Immunotherapy. ACS Nano 2023, 17, 9826–9849. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yan, Y.; Guo, X.R.; Lu, A.; Jiang, L.X.; Zhu, Y.J.; Shi, Y.J.; Liu, X.Y.; Wang, J.C. Enhanced Tumor Immunotherapy by Triple Amplification Effects of Nanomedicine on the STING Signaling Pathway in Dendritic Cells. Adv. Healthc. Mater. 2025, 14, 2403143. [Google Scholar] [CrossRef]
- Yan, J.X.; Zhang, C.; Xu, Y.L.; Huang, Z.H.; Ye, Q.Y.; Qian, X.J.; Zhu, L.; Huang, G.M.; Wang, X.Q.; Jiang, W.; et al. GPR34 is a metabolic immune checkpoint for ILC1-mediated antitumor immunity. Nat. Immunol. 2024, 25, 2057–2067. [Google Scholar] [CrossRef]
- Zou, J.L.; Jiang, C.; Hu, Q.S.; Jia, X.L.; Wang, S.Q.; Wan, S.Y.; Mao, Y.Q.; Zhang, D.P.; Zhang, P.; Dai, B.; et al. Tumor microenvironment-responsive engineered hybrid nanomedicine for photodynamic-immunotherapy via multi-pronged amplification of reactive oxygen species. Nat. Commun. 2025, 16, 424. [Google Scholar] [CrossRef]
- Xu, J.C.; Wan, R.; Cai, Y.R.; Cai, S.L.; Wu, L.; Li, B.L.; Duan, J.C.; Cheng, Y.; Li, X.L.; Wang, X.C.; et al. Circulating tumor DNA-based stratification strategy for chemotherapy plus PD-1 inhibitor in advanced non-small-cell lung cancer. Cancer Cell 2024, 42, 1598–1613. [Google Scholar] [CrossRef]
- Piper, M.; Hoen, M.; Darragh, L.B.; Knitz, M.W.; Nguyen, D.; Gadwa, J.; Durini, G.; Karakoc, I.; Grier, A.; Neupert, B.; et al. Simultaneous targeting of PD-1 and IL-2Rbg with radiation therapy inhibits pancreatic cancer growth and metastasis. Cancer Cell 2023, 41, 950–969.e6. [Google Scholar] [CrossRef]
- Guan, X.; Wang, F.; Zhou, B.; Sang, X.; Zhao, Q. The nutritional function of active polysaccharides from marine animals: A review. Food Biosci. 2024, 58, 103693. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, F.; Zhang, J.; Wang, W.; Li, L.; Yan, J. Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Gurpilhares, D.d.B.; Cinelli, L.P.; Simas, N.K.; Pessoa Jr, A.; Sette, L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Kearney, R. Specific tumor immunity induced with mitomycin C-treated syngeneic tumor cells (MCT). Effects of carrageenan and trypan blue on MCT-induced immunity in mice. J. Natl. Cancer Inst. 1980, 64, 81–87. [Google Scholar]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef]
- Cheong, K.L.; Yu, B.; Chen, J.; Zhong, S. A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Rajasekar, P.; Palanisamy, S.; Anjali, R.; Vinosha, M.; Elakkiya, M.; Marudhupandi, T.; Tabarsa, M.; You, S.; Prabhu, N.M. Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int. J. Biol. Macromol. 2019, 141, 809–821. [Google Scholar] [CrossRef]
- Tang, Y.P.; Pu, Q.Y.; Zhao, Q.L.; Zhou, Y.F.; Jiang, X.X.; Han, T. Effects of Fucoidan Isolated From Laminaria japonica on Immune Response and Gut Microbiota in Cyclophosphamide-Treated Mice. Front. Immunol. 2022, 13, 916618. [Google Scholar] [CrossRef]
- Hwang, P.A.; Lin, H.T.V.; Lin, H.Y.; Lo, S.K. Dietary Supplementation with Low-Molecular-Weight Fucoidan Enhances Innate and Adaptive Immune Responses and Protects against Mycoplasma pneumoniae Antigen Stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hunter, R.; Zhang, Q.; Yu, H.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. The application of marine polysaccharides to antitumor nanocarriers. Carbohydr. Polym. 2024, 342, 122407. [Google Scholar] [CrossRef]
- Ju, H.; Yu, C.; Liu, W.; Li, H.-H.; Fu, Z.; Wu, Y.-C.; Gong, P.-X.; Li, H.-J. Polysaccharides from marine resources exhibit great potential in the treatment of tumor: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100308. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Deng, Z.Z.; Qishan, S.; Zhang, Q.B.; Wang, J.; Yue, Y.; Geng, L.H.; Wu, N. Low molecular weight fucoidan LF2 improves the immunosuppressive tumor microenvironment and enhances the anti-pancreatic cancer activity of oxaliplatin. Biomed. Pharmacother. 2024, 173, 116360. [Google Scholar] [CrossRef]
- Lu, S.Y.; Zhou, T.; Shabbir, I.; Choi, J.; Kim, Y.H.; Park, M.; Aweya, J.J.; Tan, K.; Zhong, S.Y.; Cheong, K.L. Marine algal polysaccharides: Multifunctional bioactive ingredients for cosmetic formulations. Carbohydr. Polym. 2025, 353, 123276. [Google Scholar] [CrossRef]
- Wu, X.Y.; Liu, Z.C.; Liu, Y.; Yang, Y.; Shi, F.L.; Cheong, K.L.; Teng, B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef]
- Yu, P.; Gu, T.W.; Rao, Y.Y.; Liang, W.M.; Zhang, X.; Jiang, H.G.; Lu, J.D.; She, J.L.; Guo, J.M.; Yang, W.; et al. A novel marine-derived anti-acute kidney injury agent targeting peroxiredoxin 1 and its nanodelivery strategy based on ADME optimization. Acta Pharm. Sin. B 2024, 14, 3232–3250. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.L.; Hu, H. Marine Polysaccharides as a Versatile Biomass for the Construction of Nano Drug Delivery Systems. Mar. Drugs 2021, 19, 345. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Shabana, S.; Hamouda, H.I.; Abdalla, M.; Sharaf, M.; Chi, Z.; Liu, C. Multifunctional nanoparticles based on marine polysaccharides for apremilast delivery to inflammatory macrophages: Preparation, targeting ability, and uptake mechanism. Int. J. Biol. Macromol. 2022, 222, 1709–1722. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Allela, O.Q.B.; Hussein, A.M.; Mustafa, M.A.; Kaur, M.; Alaraj, M.; Al-Hussainy, A.F.; Radi, U.K.; Ubaid, M.; Idan, A.H.; et al. Recent advances in polysaccharide-based drug delivery systems for cancer therapy: A comprehensive review. Artif. Cells Nanomed. Biotechnol. 2024, 52, 564–586. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Zheng, Y.; Wang, H.; Zhao, F.; Yang, X.; Zhao, Q.; Jiang, Y.; Man, C. Recent advances in fucoidan-based improved delivery systems: Structure, carrier types and biomedical applications. Carbohydr. Polym. 2025, 352, 123183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Na, K.; Wei, J.; Zhang, L.; Guo, X. Alginate oligosaccharides: The structure-function relationships and the directional preparation for application. Carbohydr. Polym. 2022, 284, 119225. [Google Scholar] [CrossRef]
- Zhang, B.; Lan, W.; Xie, J. Chemical modifications in the structure of marine polysaccharide as serviceable food processing and preservation assistant: A review. Int. J. Biol. Macromol. 2022, 223, 1539–1555. [Google Scholar] [CrossRef]
- Pramanik, S.; Singh, A.; Abualsoud, B.M.; Deepak, A.; Nainwal, P.; Sargsyan, A.S.; Bellucci, S. From algae to advancements: Laminarin in biomedicine. RSC Adv. 2024, 14, 3209–3231. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, N.; Suo, Q.; Wang, J.; Yue, Y.; Geng, L.; Zhang, Q. Fucoidan, as an immunostimulator promotes M1 macrophage differentiation and enhances the chemotherapeutic sensitivity of capecitabine in colon cancer. Int. J. Biol. Macromol. 2022, 222, 562–572. [Google Scholar] [CrossRef]
- Dai, X.; Liu, X.; Li, Y.; Xu, Q.; Yang, L.; Gao, F. Nitrogen-phosphorous co-doped carbonized chitosan nanoparticles for chemotherapy and ROS-mediated immunotherapy of intracellular Staphylococcus aureus infection. Carbohydr. Polym. 2023, 315, 121013. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, L.; Yang, F.; Cheung, P.C.K. Beta-d-glucan-based drug delivery system and its potential application in targeting tumor associated macrophages. Carbohydr. Polym. 2021, 253, 117258. [Google Scholar] [CrossRef]
- Christensen, M.D.; Allahgholi, L.; Dobruchowska, J.M.; Moenaert, A.; Guðmundsson, H.; Friðjónsson, Ó.; Karlsson, E.N.; Hreggviðsson, G.; Freysdottir, J. Laminarins and their derivatives affect dendritic cell activation and their crosstalk with T cells. Int. J. Biol. Macromol. 2025, 306, 141287. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.O. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017, 8, 38554–38567. [Google Scholar] [CrossRef]
- Han, M.; Sun, P.; Li, Y.; Wu, G.; Nie, J. Structural characterization of a polysaccharide from Sargassum henslowianum, and its immunomodulatory effect on gastric cancer rat. Int. J. Biol. Macromol. 2018, 108, 1120–1127. [Google Scholar] [CrossRef]
- Zhao, X.; Jiao, G.; Yang, Y.; Li, M.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Hao, J.; Yu, G. Structure and immunomodulatory activity of a sulfated agarose with pyruvate and xylose substitutes from Polysiphonia senticulosa Harvey. Carbohydr. Polym. 2017, 176, 29–37. [Google Scholar] [CrossRef]
- Park, H.B.; Hwang, J.; Zhang, W.; Go, S.; Kim, J.; Choi, I.; You, S.; Jin, J.O. Polysaccharide from Codium fragile Induces Anti-Cancer Immunity by Activating Natural Killer Cells. Mar. Drugs 2020, 18, 626. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Zhang, J.; Ding, X.; Cui, J.; Wang, G.; Wang, Z.; Wang, L. Alginate Enhances Memory Properties of Antitumor CD8(+) T Cells by Promoting Cellular Antioxidation. ACS Biomater. Sci. Eng. 2019, 5, 4717–4725. [Google Scholar] [CrossRef]
- Park, H.B.; Hwang, J.; Lim, S.M.; Zhang, W.; Jin, J.O. Dendritic cell-mediated cancer immunotherapy with Ecklonia cava fucoidan. Int. J. Biol. Macromol. 2020, 159, 941–947. [Google Scholar] [CrossRef]
- Geng, H.; Chen, M.; Guo, C.; Wang, W.; Chen, D. Marine polysaccharides: Biological activities and applications in drug delivery systems. Carbohydr. Res. 2024, 538, 109071. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yin, H.; Li, R.; Shi, W.; Mou, J.; Yang, J. The activation effects of fucoidan from sea cucumber Stichopus chloronotus on RAW264.7 cells via TLR2/4-NF-κB pathway and its structure-activity relationship. Carbohydr. Polym. 2021, 270, 118353. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Z.; Zheng, C.; Liu, Y.; Hao, R.; Ji, X.; Xi, Q.; Shen, J.; Li, Z. Chitosan oligosaccharide regulates AMPK and STAT1 pathways synergistically to mediate PD-L1 expression for cancer chemoimmunotherapy. Carbohydr. Polym. 2022, 277, 118869. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, C.W.; Lee, S.J.; Park, H.-R.; Seo, D.B.; Park, J.Y.; Park, J.; Shin, K.-S. Immunostimulating and Antimetastatic Effects of Polysaccharides Purified from Ginseng Berry. Am. J. Chin. Med. 2019, 47, 823–839. [Google Scholar] [CrossRef]
- Zong, S.; Li, J.; Ye, Z.; Zhang, X.; Yang, L.; Chen, X.; Ye, M. Lachnum polysaccharide suppresses S180 sarcoma by boosting anti-tumor immune responses and skewing tumor-associated macrophages toward M1 phenotype. Int. J. Biol. Macromol. 2020, 144, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, J.; Li, Y.; Lu, B.; Du, J.; Xu, J.; Qin, Z.; Ning, T.; Dong, C. A polysaccharide from native Curcuma kwangsiensis and its mechanism of reversing MDSC-induced suppressive function. Carbohydr. Polym. 2022, 297, 120020. [Google Scholar] [CrossRef] [PubMed]
- Muliawan, G.K.; Lee, T.K. The roles of cancer stem cell-derived secretory factors in shaping the immunosuppressive tumor microenvironment in hepatocellular carcinoma. Front. Immunol. 2024, 15, 1400112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dang, D.; Cong, L.; Sun, H.; Cong, X. Pivotal factors associated with the immunosuppressive tumor microenvironment and melanoma metastasis. Cancer Med. 2021, 10, 4710–4720. [Google Scholar] [CrossRef]
- Tian, L.; Li, C.M.; Li, Y.F.; Huang, T.M.; Chao, N.X.; Luo, G.R.; Mo, F.R. Laminarin from Seaweed (Laminaria japonica) Inhibits Hepatocellular Carcinoma Through Upregulating Senescence Marker Protein-30. Cancer Biother. Radiopharm. 2020, 35, 277–283. [Google Scholar] [CrossRef]
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431. [Google Scholar] [CrossRef]
- Park, J.; Gerber, M.H.; Babensee, J.E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. A 2015, 103, 170–184. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Tian, X.; Wang, X.; Yang, J.; Leng, X.; Zhang, H. Synergy of Polydopamine Nanovaccine and Endostar Alginate Hydrogel for Improving Antitumor Immune Responses Against Colon Tumor. Int. J. Nanomed. 2022, 17, 4791–4805. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, H.; Wu, Y.; Yu, H.; Yang, H.; Li, J. Local nasal immunotherapy: Efficacy of Dermatophagoides farinae-chitosan vaccine in murine asthma. Int. Arch. Allergy Immunol. 2009, 150, 221–228. [Google Scholar] [CrossRef]
- Sun, J.; Sun, J.; Song, B.; Zhang, L.; Shao, Q.; Liu, Y.; Yuan, D.; Zhang, Y.; Qu, X. Fucoidan inhibits CCL22 production through NF-kappaB pathway in M2 macrophages: A potential therapeutic strategy for cancer. Sci. Rep. 2016, 6, 35855. [Google Scholar] [CrossRef]
- Karuppan Perumal, M.K.; Gandhi, D.; Rajasekaran, M.B.S.; Kudiyarasu, S.; Renuka, R.R.; Julius, A.; Samrot, A.V.; Lakshmi Narayanan, A. Inhibition of angiogenesis using laminarin a natural polysaccharide from brown seaweeds—A review. Biocatal. Agric. Biotechnol. 2023, 54, 102947. [Google Scholar] [CrossRef]
- Zhao, Z.; Yao, Y.; Ding, Z.; Chen, X.; Xie, K.; Luo, Y.; Zhang, J.; Chen, X.; Wu, X.; Xu, J.; et al. Antitumour immunity mediated by mannan-modified adenovirus vectors expressing VE-cadherin. Vaccine 2011, 29, 4218–4224. [Google Scholar] [CrossRef]
- Salim, S.A.; Salaheldin, T.A.; Elmazar, M.M.; Abdel-Aziz, A.F.; Kamoun, E.A. Smart biomaterials for enhancing cancer therapy by overcoming tumor hypoxia: A review. RSC Adv. 2022, 12, 33835–33851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Q.; Chen, X.; Nie, X.; Xue, F.; Xu, W.; Luan, Y. An Injectable Hydrogel to Modulate T Cells for Cancer Immunotherapy. Small 2022, 18, e2202663. [Google Scholar] [CrossRef]
- Chiang, C.S.; Lin, Y.J.; Lee, R.; Lai, Y.H.; Cheng, H.W.; Hsieh, C.H.; Shyu, W.C.; Chen, S.Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, M.; Cao, Y.; Zhu, B.; Chen, J.; Li, Y.; Shao, J.; Zhou, S.; Shan, P.; Zheng, C.; et al. Combination of a STING Agonist and Photothermal Therapy Using Chitosan Hydrogels for Cancer Immunotherapy. Biomacromolecules 2023, 24, 2790–2803. [Google Scholar] [CrossRef] [PubMed]
- Adibfar, S.; Masjedi, A.; Nazer, A.; Rashidi, B.; Karpisheh, V.; Izadi, S.; Hassannia, H.; Gholizadeh Navashenaq, J.; Mohammadi, H.; Hojjat-Farsangi, M.; et al. Combined inhibition of EZH2 and CD73 molecules by folic acid-conjugated SPION-TMC nanocarriers loaded with siRNA molecules prevents TNBC progression and restores anti-tumor responses. Life Sci. 2022, 309, 121008. [Google Scholar] [CrossRef]
- Lima, B.V.; Oliveira, M.J.; Barbosa, M.A.; Goncalves, R.M.; Castro, F. Immunomodulatory potential of chitosan-based materials for cancer therapy: A systematic review of in vitro, in vivo and clinical studies. Biomater. Sci. 2021, 9, 3209–3227. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Wolkers, W.F.; Oliver, A.E.; Ma, X.; Auh, J.H.; Tang, M.; Zhu, S.; Norris, J.; Tablin, F. Stabilization of dry Mammalian cells: Lessons from nature. Integr. Comp. Biol. 2005, 45, 810–820. [Google Scholar] [CrossRef]
- Argenziano, M.; Occhipinti, S.; Scomparin, A.; Angelini, C.; Novelli, F.; Soster, M.; Giovarelli, M.; Cavalli, R. Exploring chitosan-shelled nanobubbles to improve HER2 + immunotherapy via dendritic cell targeting. Drug Deliv. Transl. Res. 2022, 12, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Liu, B.Y.; Wu, J.L.; Ai, S.L.; Zhuo, R.X.; Cheng, S.X. A Dual Macrophage Targeting Nanovector for Delivery of Oligodeoxynucleotides To Overcome Cancer-Associated Immunosuppression. ACS Appl. Mater. Interfaces 2017, 9, 42566–42576. [Google Scholar] [CrossRef]
- Yang, R.; Xu, K.; Li, H.; Feng, Y.; Xiang, G.; Zhou, X.; Zhang, C. Laminarin-mediated oral delivery of miRNA-223 for targeted macrophage polarization in inflammatory bowel disease. Int. J. Biol. Macromol. 2025, 311, 143052. [Google Scholar] [CrossRef]
- Liu, X.; Ren, X.; Zhou, L.; Liu, K.; Deng, L.; Qing, Q.; Li, J.; Zhi, F.; Li, M. Tollip Orchestrates Macrophage Polarization to Alleviate Intestinal Mucosal Inflammation. J. Crohns Colitis 2022, 16, 1151–1167. [Google Scholar] [CrossRef]
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef]
- Kim, D.; Jo, S.; Lee, D.; Kim, S.M.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Lee, J.J.; Lee, K.; Kim, T.D.; et al. NK cells encapsulated in micro/macropore-forming hydrogels via 3D bioprinting for tumor immunotherapy. Biomater. Res. 2023, 27, 60. [Google Scholar] [CrossRef]
- Chen, S.; Ding, R.; Zhou, Y.; Zhang, X.; Zhu, R.; Gao, X.D. Immunomodulatory effects of polysaccharide from marine fungus Phoma herbarum YS4108 on T cells and dendritic cells. Mediat. Inflamm. 2014, 2014, 738631. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Chaleshtari, M.; Kiaie, S.H.; Irandoust, M.; Karami, H.; Nabi Afjadi, M.; Ghani, S.; Aghaei Vanda, N.; Ghaderi Sede, M.J.; Ahmadi, A.; Masjedi, A.; et al. Concomitant blockade of A2AR and CTLA-4 by siRNA-loaded polyethylene glycol-chitosan-alginate nanoparticles synergistically enhances antitumor T-cell responses. J. Cell Physiol. 2020, 235, 10068–10080. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Masjedi, A.; Hallaj, S.; Nabi Afjadi, M.; Malakotikhah, F.; Ghani, S.; Ahmadi, A.; Sojoodi, M.; Hassannia, H.; Atyabi, F.; et al. Blockade of CTLA-4 increases anti-tumor response inducing potential of dendritic cell vaccine. J. Control Release 2020, 326, 63–74. [Google Scholar] [CrossRef]
- Fathi, M.; Bahmanpour, S.; Barshidi, A.; Rasouli, H.; Karoon Kiani, F.; Mahmoud Salehi Khesht, A.; Izadi, S.; Rashidi, B.; Kermanpour, S.; Mokhtarian, R.; et al. Simultaneous blockade of TIGIT and HIF-1alpha induces synergistic anti-tumor effect and decreases the growth and development of cancer cells. Int. Immunopharmacol. 2021, 101, 108288. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, W.; Zhu, J.; Zhu, J.; Shen, J.; Liu, Z.; Yang, Y.; Chen, Q. Nanoparticle-Mediated Delivery of Inhaled Immunotherapeutics for Treating Lung Metastasis. Adv. Mater. 2021, 33, e2007557. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Tan, L.; Li, X.; Dai, Z.; Cheng, Q.; Liu, J.; Wang, Y.; Huang, L.; Wang, L.; et al. LncRNA-edited biomimetic nanovaccines combined with anti-TIM-3 for augmented immune checkpoint blockade immunotherapy. J. Control Release 2023, 361, 671–680. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, T.; Wang, X.; Yang, L.; Wang, Z.; Qin, Y.; Hu, Y.; Zhang, T.; Wang, D.; Liu, A.; et al. Co-delivery of immunochemotherapeutic by classified targeting based on chitosan and cyclodextrin derivatives. Int. J. Biol. Macromol. 2023, 226, 1396–1410. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-Responsive Smart Hydrogel Releasing Immune Adjuvant Synchronized with Repeated Chemotherapy or Radiotherapy to Boost Antitumor Immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Farmanbordar, H.; Mohammadi, R. Synthesis of magnetic bio-nanocomposite hydrogel beads based on sodium alginate and β-cyclodextrin: Potential pH-responsive oral delivery anticancer systems for colorectal cancer. Int. J. Biol. Macromol. 2025, 305, 140748. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadzadeh, A.; Javanbakht, S.; Mohammadi, R.; Ghorbani, M. Alginate/magnetic hydroxyapatite bio-nanocomposite hydrogel bead as a pH-responsive oral drug carrier for potential colon cancer therapy. Results Chem. 2025, 15, 102177. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Mohammadi, R. Green synthesis of pH-sensitive magnetic bio-nanocomposite hydrogel based on galactomannan and sodium alginate for targeted colorectal cancer drug delivery. J. Sci. Adv. Mater. Devices 2025, 10, 100892. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Mohammadi, R. Synthesizing of pH-sensitive bio-nanocomposite hydrogels based on okra polysaccharide and sodium alginate for targeted colorectal cancer drug delivery with antibacterial and antioxidant properties. Carbohydr. Polym. Technol. Appl. 2025, 11, 100890. [Google Scholar] [CrossRef]
- Patel, T.; Himaja, A.; Biswas, S.; Ghosh, B. Dual Stimuli-Responsive Gemcitabine-Conjugated Alginate-Chitosan Nanoparticles for Triple-Negative Breast Cancer Therapy: A Smart Approach. Bioconjugate Chem. 2025, 36, 2037–2053. [Google Scholar] [CrossRef]
- Tripathi, A.; Pandey, V.K.; Rustagi, S.; Lai, W.-F.; Samrot, A.V. Alginate-based NPs for targeted ovarian cancer therapy: Navigating current progress and biomedical applications. Int. J. Biol. Macromol. 2025, 319, 145365. [Google Scholar] [CrossRef]
- Li, B.Y.; Lin, T.Y.; Lai, Y.J.; Chiu, T.H.; Yeh, Y.C. Engineering Multiresponsive Alginate/PNIPAM/Carbon Nanotube Nanocomposite Hydrogels as On-Demand Drug Delivery Platforms. Small 2025, 21, e2407420. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; Gurikov, P.; Schroeter, B.; Fini, E.; deClaville Christiansen, J. Multivalent ion-crosslinked alginate–montmorillonite nanocomposite hydrogels for hydrophilic drug release. J. Drug Deliv. Sci. Technol. 2025, 112, 107275. [Google Scholar] [CrossRef]
- Rajwar, T.K.; Sahoo, R.K.; Halder, J.; Mishra, A.; Satapathy, B.; Saha, I.; Sahoo, G.P.; Mahanty, R.; Rai, V.K.; Pradhan, D.; et al. Doxorubicin loaded salicylic acid crosslinked chitosan nanoparticles as postsurgical implants for breast cancer. Int. J. Biol. Macromol. 2025, 320, 145874. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Pareek, A.; Patel, S.; Fareed, M.; Alsaidan, O.A.; Prajapati, B.G. Advances in cancer therapy using fluorinated chitosan: A promising nanoplatform for drug delivery. Med. Oncol. 2025, 42, 452. [Google Scholar] [CrossRef] [PubMed]
- Amparo, T.R.; Anunciação, K.d.F.d.; Almeida, T.C.; Sousa, L.R.D.; Xavier, V.F.; Seibert, J.B.; Barboza, A.P.M.; Vieira, P.M.d.A.; dos Santos, O.D.H.; da Silva, G.N.; et al. Chitosan Nanoparticles Enhance the Antiproliferative Effect of Lapachol in Urothelial Carcinoma Cell Lines. Pharmaceutics 2025, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Blebea, N.-M.; Pușcașu, C.; Vlad, R.-A.; Hancu, G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels 2025, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.; Liszbinski, R.B.; Carvalho, S.G.; Junior, A.G.T.; Chorilli, M.; de Jesus, M.B.; Gremião, M.P.D. 5-Fluorouracil-loaded chitosan nanoparticles conjugated with methotrexate for targeted therapy of colorectal cancer. Int. J. Biol. Macromol. 2025, 287, 138342. [Google Scholar] [CrossRef]
- Alemi, P.S.; Mohamadali, M.; Arabahmadi, S.; Irani, S.; Sharifi, F. Carboxymethyl Chitosan and Chitosan as a Bioactive Delivery System: A Review. Biotechnol. Appl. Biochem. 2025, e2758. [Google Scholar] [CrossRef]
- Nabipour, H.; Aliakbari, F.; Volkening, K.; Strong, M.J.; Rohani, S. Novel metal-organic framework coated with chitosan-κ-carrageenan as a platform for curcumin delivery to cancer cells. Int. J. Biol. Macromol. 2025, 301, 140027. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, M.W.; Mohamed, R.R.; Hanna, D.H. Preparation of carrageenan/ chitosan-based (N,N,N-trimeth(yl chitosan chloride) silver nanocomposites as pH sensitive carrier for effective controlled curcumin delivery in cancer cells. OpenNano 2022, 7, 100050. [Google Scholar] [CrossRef]
- Hanna, D.H.; El-Mazaly, M.H.; Mohamed, R.R. Synthesis of biodegradable antimicrobial pH-sensitive silver nanocomposites reliant on chitosan and carrageenan derivatives for 5-fluorouracil drug delivery toward HCT116 cancer cells. Int. J. Biol. Macromol. 2023, 231, 123364. [Google Scholar] [CrossRef]
- Jafari, H.; Namazi, H. κ-carrageenan coated magnetic hydroxypropyl methylcellulose/chitosan nanoparticles as a pH-sensitive nanocarrier for efficient methotrexate release. Int. J. Biol. Macromol. 2025, 322, 146750. [Google Scholar] [CrossRef]
- Fathi, R.; Mohammadi, R. Preparation of pH-responsive magnetic nanocomposite hydrogels based on k-carrageenan/chitosan/silver nanoparticles: Antibacterial carrier for potential targeted anticancer drug delivery. Int. J. Biol. Macromol. 2023, 246, 125546. [Google Scholar] [CrossRef]
- Karimi, M.H.; Mahdavinia, G.R.; Massoumi, B. pH-controlled sunitinib anticancer release from magnetic chitosan nanoparticles crosslinked with κ-carrageenan. Mater. Sci. Eng. C 2018, 91, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, K. Polyphenol modified CuO nanorods capped by kappa-carrageenan for controlled paclitaxel release in furnishing targeted chemotherapy in breast carcinoma cells. Int. J. Biol. Macromol. 2024, 255, 127893. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Meylina, L.; Rusdin, A.; Mohammed, A.F.A.; Tirtamie, D.; Herdiana, Y.; Motoyama, K.; Panatarani, C.; Joni, I.M.; Lesmana, R.; et al. The Potential Cytotoxic Activity Enhancement of α-Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against MCF-7 Cell Line. Polymers 2021, 13, 1681. [Google Scholar] [CrossRef]
- Karabatak, A.; Danışman-Kalındemirtaş, F.; Tan, E.; Erdem-Kuruca, S.; Karakuş, S. Kappa carrageenan/PEG-CuO nanoparticles as a multifunctional nanoplatform: Digital colorimetric biosensor and anticancer drug nanocarrier. Appl. Phys. A 2022, 128, 661. [Google Scholar] [CrossRef]
- Ma, M.; Yu, C.; Liu, H.; Lv, X.; Zhu, H.; Tang, W.; Teng, G.; Xiong, F. Methacrylated Carrageenan/Gelatin Interpenetrating Network Microspheres Loaded with Targeted Drugs and combined with PD-L1 Inhibitors for Hepatocellular Carcinoma Treatment. ACS Appl. Mater. Interfaces 2025, 17, 42893–42914. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, L.; Long, J.; Luo, J.; Yang, L.; Lin, F.; Liang, R.; Zhang, X.; Liu, J.; Cao, J.; et al. Spatially Targeted PD-L1 Blockade for Restoring Exhausted Cytotoxic T Lymphocyte Rejuvenation to Potentiate Multimodal-Immune Synergistic Therapies for Breast Cancer Treatment. Small 2025, 21, e2410953. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Anees, M.; Zofair, S.F.F.; Rasool, F.; Khan, M.A.; Moin, S.; Younus, H. Fucoidan based polymeric nanoparticles encapsulating epirubicin: A novel and effective chemotherapeutic formulation against colorectal cancer. Int. J. Pharm. 2024, 664, 124622. [Google Scholar] [CrossRef] [PubMed]
- Pai, F.-T.; Lin, W.J. Synergistic cytotoxicity of irinotecan combined with polysaccharide-based nanoparticles for colorectal carcinoma. Biomater. Adv. 2023, 153, 213577. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Diao, N.; Song, S.; Wang, W.; Cao, M.; Yang, W.; Guo, C.; Chen, D. Inflammatory macrophage reprogramming strategy of fucoidan microneedles-mediated ROS-responsive polymers for rheumatoid arthritis. Int. J. Biol. Macromol. 2024, 271, 132442. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Lee, M.-F.; Chen, J.-H.; Tsou, M.-H.; Wu, Z.-Y.; Lee, C.-Z.; Huang, Y.-Y.; Lin, S.-M.; Lin, H.-M. Fucoidan with three functions extracted from Sargassum aquifolium integrated rice-husk synthesis dual-imaging mesoporous silica nanoparticle. J. Nanobiotechnology 2022, 20, 298. [Google Scholar] [CrossRef]
- DuRoss, A.N.; Landry, M.R.; Thomas, C.R., Jr.; Neufeld, M.J.; Sun, C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021, 500, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Du, H.; Aslam, M.; Wang, W.; Chen, W.; Li, T.; Liu, Z.; Liu, X. Laminarin, a Major Polysaccharide in Stramenopiles. Mar. Drugs 2021, 19, 576. [Google Scholar] [CrossRef]
- Yao, W.; Lin, Y.; Xu, N.; Xi, Q.; Liu, Y.; Li, L. Laminarin-coated Genexol-PM pH sensitive nanomicelles targeting miR-620/IRF2BP2 axis for inhibition of cell proliferation and induction of apoptosis in Invitro thyroid carcinoma. Int. J. Biol. Macromol. 2025, 310, 143198. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhuang, Y.; He, T.; Wu, X.; Su, L.; Kang, J.; Chang, J.; Wang, H. Sodium Alginate Hydrogel-Mediated Cancer Immunotherapy for Postoperative In Situ Recurrence and Metastasis. ACS Biomater. Sci. Eng. 2021, 7, 5717–5726. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, S.; Ma, Y.; Ma, L.; Zu, M.; Sun, J.; Dai, F.; Duan, L.; Xiao, B. Oral Nanomotor-Enabled Mucus Traverse and Tumor Penetration for Targeted Chemo-Sono-Immunotherapy against Colon Cancer. Small 2022, 18, e2203466. [Google Scholar] [CrossRef]
- Li, Y.; Fang, M.; Zhang, J.; Wang, J.; Song, Y.; Shi, J.; Li, W.; Wu, G.; Ren, J.; Wang, Z.; et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2016, 5, e1074374. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Gordo, S.; Schubert, D.A.; Lewin, S.A.; Desai, R.M.; Dobbins, J.; Wucherpfennig, K.W.; Mooney, D.J. Multicomponent Injectable Hydrogels for Antigen-Specific Tolerogenic Immune Modulation. Adv. Heal. Mater. 2017, 6, 1600773. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Y.; Liu, J.; Wang, J.; Mo, F.; Wang, Y.; Chen-Mayfield, T.J.; Sondel, P.M.; Hong, S.; Hu, Q. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat. Commun. 2022, 13, 1845. [Google Scholar] [CrossRef]
- Han, J.; Bhatta, R.; Liu, Y.; Bo, Y.; Wang, H. In Situ Dendritic Cell Recruitment and T Cell Activation for Cancer Immunotherapy. Front. Pharmacol. 2022, 13, 954955. [Google Scholar] [CrossRef]
- Cheng, Z.; Xue, C.; Liu, M.; Cheng, Z.; Tian, G.; Li, M.; Xue, R.; Yao, X.; Zhang, Y.; Luo, Z. Injectable microenvironment-responsive hydrogels with redox-activatable supramolecular prodrugs mediate ferroptosis-immunotherapy for postoperative tumor treatment. Acta Biomater. 2023, 169, 289–305. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Ding, M.; Yu, N.; Zhang, Y.; Wu, H.; Zhang, Q.; Liu, J.; Li, J. Prodrug-loaded semiconducting polymer hydrogels for deep-tissue sono-immunotherapy of orthotopic glioblastoma. Biomater. Sci. 2023, 11, 6823–6833. [Google Scholar] [CrossRef]
- Ding, M.; Fan, Y.; Lv, Y.; Liu, J.; Yu, N.; Kong, D.; Sun, H.; Li, J. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022, 149, 334–346. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; Sun, G.; Yu, M.; Qin, J.; Zhang, Y.; Ding, D. Advances in cancer diagnosis and therapy by alginate-based multifunctional hydrogels: A review. Int. J. Biol. Macromol. 2024, 283, 137707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, J.; Shi, S.; He, J.; Liu, W.; Li, Y.; Zeng, X.; Pang, J.; Wu, C. Preparation and characterization of pH-sensitive calcium alginate hydrogel beads as delivery carriers for the controlled release of fucoxanthin. Food Hydrocoll. 2025, 163, 111106. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Janchai, P.; Aksornsri, T.; Vaithanomsat, P. Design and evaluation of bromelain-encapsulated alginate beads reinforced with gum Arabic: Formulation, characterization, and stability in simulated gastrointestinal conditions. J. Agric. Food Res. 2025, 19, 101698. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, X.; Ma, Y.; Nan, W.; Wu, W.; Chu, Z.; Sun, X.; Huang, J.; Muratkhan, M.; Yue, F.; et al. Sodium alginate/low methoxyl pectin composite hydrogel beads prepared via gas-shearing technology for enhancing the colon-targeted delivery of probiotics and modulating gut microbiota. Int. J. Biol. Macromol. 2025, 300, 140375. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.S.; Sobh, R.A.; Fahim, A.M.; Elsayed, G.H. Alginate sulfonamide hydrogel beads for 5-fluorouracil delivery: Antitumor activity, cytotoxicity assessment, and theoretical investigation. Int. J. Biol. Macromol. 2024, 282, 136573. [Google Scholar] [CrossRef]
- Gomathi, T.; Suganya, R.; Joseph, J.J.; Pandiaraj, S.; Alibrahim, K.A.; Alodhayb, A.N.; Rajakumar, G.; Viswanathan, D.; Thiruvengadam, M.; Shobha, K.; et al. Development and evaluation of biodegradable alginate beads loaded with sorafenib for cancer treatment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135083. [Google Scholar] [CrossRef]
- Chernykh, I.N.; Gopin, A.V.; Evdokimov, A.A.; Kharlanov, A.N.; Šandalová, S.; Nikolaev, A.L. Novel yttrium-90 carriers based on enzymatically mineralized calcium and yttrium alginate beads. Surf. Interfaces 2025, 69, 106691. [Google Scholar] [CrossRef]
- Pan, C.T.; Yu, R.S.; Yang, C.J.; Chen, L.R.; Wen, Z.H.; Chen, N.Y.; Ou, H.Y.; Yu, C.Y.; Shiue, Y.L. Sustained-Release and pH-Adjusted Alginate Microspheres-Encapsulated Doxorubicin Inhibit the Viabilities in Hepatocellular Carcinoma-Derived Cells. Pharmaceutics 2021, 13, 1417. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Yang, S.; Mu, C.; Liu, T.; Pei, P.; Shen, W.; Zhang, Y.; Wang, G.; Chen, L.; Yang, K. Radionuclide-Labeled Microspheres for Radio-Immunotherapy of Hepatocellular Carcinoma. Adv. Heal. Mater. 2023, 12, e2300944. [Google Scholar] [CrossRef]
- de las Heras, A.I.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.I. Immunogenic and protective effects of an oral DNA vaccine against infectious pancreatic necrosis virus in fish. Fish. Shellfish. Immunol. 2010, 28, 562–570. [Google Scholar] [CrossRef]
- Stewart, S.; Arminan, A.; He, X. Nanoparticle-Mediated Delivery of Cryoprotectants for Cryopreservation. Cryo Lett. 2020, 41, 308–316. [Google Scholar]
- Chen, G.; Lv, Y. Nanotechnology-Based Cryopreservation of Cellscaffold Constructs: A New Breakthrough to Clinical Application. CryoLetters 2016, 37, 381–387. [Google Scholar] [PubMed]
- Borges, O.; Borchard, G.; Verhoef, J.C.; de Sousa, A.; Junginger, H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005, 299, 155–166. [Google Scholar] [CrossRef]
- Marrella, A.; Dondero, A.; Aiello, M.; Casu, B.; Olive, D.; Regis, S.; Bottino, C.; Pende, D.; Meazza, R.; Caluori, G.; et al. Cell-Laden Hydrogel as a Clinical-Relevant 3D Model for Analyzing Neuroblastoma Growth, Immunophenotype, and Susceptibility to Therapies. Front. Immunol. 2019, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- De Dios-Figueroa, G.T.; Aguilera-Marquez, J.D.R.; Garcia-Uriostegui, L.; Hernandez-Gutierrez, R.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H. Embedded Living HER2+ Cells in a 3D Gelatin-Alginate Hydrogel as an In Vitro Model for Immunotherapy Delivery for Breast Cancer. Polymers 2023, 15, 3726. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Espinosa-Andrews, H.; Cervantes-Uc, J.M.; Limón-Rocha, I.; Luna-Bárcenas, G.; Vázquez-Lepe, M.; Morales-Hernández, N.; Jiménez-Ávalos, J.A.; Mejía-Torres, D.G.; Ramos-Martínez, P.; et al. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers 2023, 15, 2203. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Alizadeh, A.H. Biofilm hydrogel derived from physical crosslinking (self-assembly) of xanthan gum and chitosan for removing Cd(2+), Ni(2+), and Cu(2+) from aqueous solution. Int. J. Biol. Macromol. 2024, 266, 131394. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Deng, L.; Hu, R.; Dong, A. Performance optimization of injectable chitosan hydrogel by combining physical and chemical triple crosslinking structure. J. Biomed. Mater. Res. A 2013, 101, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, S.; Liu, Y.; Guo, F.; Miao, Q.; Huang, H. A composite hydrogel scaffold based on collagen and carboxymethyl chitosan for cartilage regeneration through one-step chemical crosslinking. Int. J. Biol. Macromol. 2023, 226, 706–715. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, S.; Xie, Y.; Lv, X.; Sun, S. High Strength, Conductivity, and Bacteriostasis of the P(AM-co-AA)/Chitosan Quaternary Ammonium Salt Composite Hydrogel through Ionic Crosslinking and Hydrogen Bonding. Langmuir 2023, 39, 8698–8709. [Google Scholar] [CrossRef]
- Yan, K.; Wan, Y.; Xu, F.; Lu, J.; Yang, C.; Li, X.; Lu, Z.; Wang, X.; Wang, D. Ionic crosslinking of alginate/carboxymethyl chitosan fluorescent hydrogel for bacterial detection and sterilization. Carbohydr. Polym. 2023, 302, 120427. [Google Scholar] [CrossRef]
- Yang, J.; Liang, G.; Xiang, T.; Situ, W. Effect of crosslinking processing on the chemical structure and biocompatibility of a chitosan-based hydrogel. Food Chem. 2021, 354, 129476. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Li, S.; Yang, Y.; Chen, A.; Xu, M.; Zhai, H.; Cai, T.; Peng, Y. Self-Cross-Linked Chitosan/Albumin-Bound Nanoparticle Hydrogel for Inhibition of Postsurgery Malignant Glioma Recurrence. ACS Appl. Mater. Interfaces 2023, 15, 56774–56785. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, G.; Yu, J.; Xu, P.; Yan, J.; Jin, Z.; Chen, S.; Wang, Y.; Zhang, L.W.; Wang, Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnology 2022, 20, 372. [Google Scholar] [CrossRef]
- Wu, H.; Wei, G.; Luo, L.; Li, L.; Gao, Y.; Tan, X.; Wang, S.; Chang, H.; Liu, Y.; Wei, Y.; et al. Ginsenoside Rg3 nanoparticles with permeation enhancing based chitosan derivatives were encapsulated with doxorubicin by thermosensitive hydrogel and anti-cancer evaluation of peritumoral hydrogel injection combined with PD-L1 antibody. Biomater. Res. 2022, 26, 77. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Cheng, K.; Li, Y.; Gong, R.; Zhao, X.; Nie, G.; Ren, H. Injectable Immunotherapeutic Hydrogel Containing RNA-Loaded Lipid Nanoparticles Reshapes Tumor Microenvironment for Pancreatic Cancer Therapy. Nano Lett. 2022, 22, 8801–8809. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Liu, H.; Nordquist, J.A.; Nordquist, R.E. Tumour cell damage and leucocyte infiltration after laser immunotherapy treatment. Lasers Med. Sci. 2000, 15, 43–48. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Chen, Y.; Wang, F.; Jiang, W. Preparation and characterization of curdlan-chitosan conjugate nanoparticles as mucosal adjuvants for intranasal influenza H1N1 subunit vaccine. Int. J. Biol. Macromol. 2024, 266, 131289. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, W.; Li, S.; Zhang, Y.; Hu, X.; Liu, S.; Li, Y. Fabrication of chitosan-based emulsion as an adjuvant to enhance nasal mucosal immune responses. Int. J. Biol. Macromol. 2024, 272, 132913. [Google Scholar] [CrossRef]
- Liu, Y.; Long, M.; Wang, Y.; Liang, Z.; Dong, Y.; Qu, M.; Ge, X.; Nan, Y.; Chen, Y.; Zhou, X. Chitosan-alginate/R8 ternary polyelectrolyte complex as an oral protein-based vaccine candidate induce effective mucosal immune responses. Int. J. Biol. Macromol. 2024, 275, 133671. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiao, S.; Jin, Z.; Li, H.; Yu, H.; Zhang, C.; Yin, T.H.; Zhao, K. Acidified sucralfate encapsulated chitosan derivative nanoparticles as oral vaccine adjuvant delivery enhancing mucosal and systemic immunity. Int. J. Biol. Macromol. 2024, 279, 135424. [Google Scholar] [CrossRef]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R. Chitin, chitosan, and glycated chitosan regulate immune responses: The novel adjuvants for cancer vaccine. Clin. Dev. Immunol. 2013, 2013, 387023. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Li, X.; He, T.; Gong, S.; Zhu, S.; Zhang, M.; Wu, Q.; Gong, C. A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response. Theranostics 2021, 11, 6936–6949. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xue, W.; Yu, X.; Qiu, X.; Liu, Z. pH Sensitive phosphorylated chitosan hydrogel as vaccine delivery system for intramuscular immunization. J. Biomater. Appl. 2017, 31, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Zhou, S.; Chen, D.; Zhou, M.; Chen, Q.; Lu, Y.; Cai, N.; Liu, C.; Guo, Y.; et al. Sequentially sustained release of anticarcinogens for postsurgical chemoimmunotherapy. J. Control Release 2022, 350, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tang, K.Y.; Wang, Y.Y. Preparation of drug-loaded chitosan microspheres repair materials. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6489–6495. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, M.; Ge, Y.; Fan, J. Novel Alginate-Chitosan Composite Microspheres for Implant Delivery of Vancomycin and In Vivo Evaluation. Chem. Biol. Drug Des. 2016, 88, 434–440. [Google Scholar] [CrossRef]
- Pahuja, S.; Aggarwal, S.; Sarup, P. Formulation and Characterization of Losartan Loaded Chitosan Microspheres: Effect of Crosslinking Agents. Drug Res. 2021, 71, 204–212. [Google Scholar] [CrossRef]
- Meskelis, L.; Agondi, R.F.; Duarte, L.G.R.; de Carvalho, M.D.; Sato, A.C.K.; Picone, C.S.F. New approaches for modulation of alginate-chitosan delivery properties. Food Res. Int. 2024, 175, 113737. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, L.; Rajoka, M.S.R.; Mai, Z.; Bahadur, A.; Mehwish, H.M.; Umair, M.; Zhao, L.; Wu, Y.; Song, X. Jawbones Scaffold Constructed by TGF-β1 and BMP-2 Loaded Chitosan Microsphere Combining with Alg/HA/ICol for Osteogenic-Induced Differentiation. Polymers 2021, 13, 3079. [Google Scholar] [CrossRef]
- Katsarov, P.D.; Pilicheva, B.A.; Manev, H.M.; Lukova, P.K.; Kassarova, M.I. Optimization of Chitosan Microspheres Spray Drying via 32 Full Factorial Design. Folia Med. 2017, 59, 310–317. [Google Scholar] [CrossRef]
- Ogunjimi, A.T.; Fiegel, J.; Brogden, N.K. Design and Characterization of Spray-Dried Chitosan-Naltrexone Microspheres for Microneedle-Assisted Transdermal Delivery. Pharmaceutics 2020, 12, 496. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, L.J.; Sun, D.; Wang, M.; Shi, J.R.; Yang, D.; Wang, L.H.; Zou, S.C. Preparation and properties of chitosan-based microspheres by spray drying. Food Sci. Nutr. 2020, 8, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Y.; Luo, M.; Deng, F.; Lin, S.; Wu, W.; Li, G.; Nan, K. Dual cross-linked chitosan microspheres formulated with spray-drying technique for the sustained release of levofloxacin. Drug Dev. Ind. Pharm. 2019, 45, 568–576. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Wang, Y.; Liu, S.; Xu, Y.; Zhang, C.; Li, L.; Si, S.; Yao, B.; Dai, W.; et al. Re-exposure of chitosan by an inhalable microsphere providing the re-education of TAMs for lung cancer treatment with assistant from sustained H(2)S generation. Int. J. Pharm. 2023, 642, 123142. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Yao, X.; Jovevski, J.J.; Todd, M.F.; Xu, R.; Li, Y.; Wang, J.; Matosevic, S. Nanoparticle-Mediated Intracellular Protection of Natural Killer Cells Avoids Cryoinjury and Retains Potent Antitumor Functions. Adv. Sci. 2020, 7, 1902938. [Google Scholar] [CrossRef]
- Lin, L.; He, J.; Li, J.; Xu, Y.; Li, J.; Wu, Y. Chitosan Nanoparticles Strengthen Vgamma9Vdelta2 T-Cell Cytotoxicity Through Upregulation Of Killing Molecules And Cytoskeleton Polarization. Int. J. Nanomed. 2019, 14, 9325–9336. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Cancer, chitosan nanoparticles and catalytic nucleic acids. J. Pharm. Pharmacol. 2009, 61, 3–12. [Google Scholar] [CrossRef]
- Chakraborty, A.; Roy, G.; Swami, B.; Bhaskar, S. Tumor targeted delivery of mycobacterial adjuvant encapsulated chitosan nanoparticles showed potential anti-cancer activity and immune cell activation in tumor microenvironment. Int. Immunopharmacol. 2023, 114, 109463. [Google Scholar] [CrossRef]
- Bastaki, S.; Aravindhan, S.; Ahmadpour Saheb, N.; Afsari Kashani, M.; Evgenievich Dorofeev, A.; Karoon Kiani, F.; Jahandideh, H.; Beigi Dargani, F.; Aksoun, M.; Nikkhoo, A.; et al. Codelivery of STAT3 and PD-L1 siRNA by hyaluronate-TAT trimethyl/thiolated chitosan nanoparticles suppresses cancer progression in tumor-bearing mice. Life Sci. 2021, 266, 118847. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, H.; Huang, D.; Feng, S.; Fujita, M.; Gao, X.D. Chitosan-Functionalized Graphene Oxide as a Potential Immunoadjuvant. Nanomaterials 2017, 7, 59. [Google Scholar] [CrossRef]
- Yang, X.; Yu, T.; Zeng, Y.; Lian, K.; Zhou, X.; Li, S.; Qiu, G.; Jin, X.; Yuan, H.; Hu, F. Tumor-draining lymph node targeting chitosan micelles as antigen-capturing adjuvants for personalized immunotherapy. Carbohydr. Polym. 2020, 240, 116270. [Google Scholar] [CrossRef]
- Korbelik, M.; Banath, J.; Zhang, W.; Gallagher, P.; Hode, T.; Lam, S.S.K.; Chen, W.R. N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects of photodynamic therapy and photodynamic therapy-generated vaccines. Int. Immunopharmacol. 2019, 75, 105764. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhou, F.; Nordquist, R.E.; Carubelli, R.; Liu, H.; Chen, W.R. Glycated chitosan as a new non-toxic immunological stimulant. Immunopharmacol. Immunotoxicol. 2009, 31, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Quan, G.; Wen, T.; Yang, P.; Qin, W.; Mai, H.; Sun, Y.; Lu, C.; Pan, X.; Wu, C. Cold to Hot: Binary Cooperative Microneedle Array-Amplified Photoimmunotherapy for Eliciting Antitumor Immunity and the Abscopal Effect. ACS Appl. Mater. Interfaces 2020, 12, 32259–32269. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Thakur, N.; Singh, B.; Sharma, S.; Kanwar, S.S. Designing carrageenan-based hydrogels for drug delivery applications: Evaluation of physiochemical and biomedical properties. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100439. [Google Scholar] [CrossRef]
- Vaid, V.; Jindal, R. RSM-CCD optimized in air synthesis of novel kappa-carrageenan/tamarind kernel powder hybrid polymer network incorporated with inclusion complex of (2-hydroxypropyl)-β-cyclodextrin and adenosine for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2022, 67, 102976. [Google Scholar] [CrossRef]
- Mozaffari, E.; Tanhaei, B.; Khajenoori, M.; Movaghar Khoshkho, S. Unveiling the swelling behavior of κ-carrageenan hydrogels: Influence of composition and physiological environment on drug delivery potential. J. Ind. Eng. Chem. 2024, 141, 217–227. [Google Scholar] [CrossRef]
- Volod’ko, A.V.; Son, E.Y.; Glazunov, V.P.; Davydova, V.N.; Alexander-Sinkler, E.I.; Aleksandrova, S.A.; Blinova, M.I.; Yermak, I.M. Carrageenan films as promising mucoadhesive ocular drug delivery systems. Colloids Surf. B Biointerfaces 2024, 237, 113854. [Google Scholar] [CrossRef]
- Lim, H.-P.; Ooi, C.-W.; Tey, B.-T.; Chan, E.-S. Controlled delivery of oral insulin aspart using pH-responsive alginate/κ-carrageenan composite hydrogel beads. React. Funct. Polym. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Gu, L.; Mcclements, D.J.; Li, J.; Su, Y.; Li, J. Formulation of alginate/carrageenan microgels to encapsulate, protect and release immunoglobulins: Egg Yolk IgY. Food Hydrocoll. 2021, 112, 106349. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.S.; Negreanu-Pirjol, T.; Popoviciu, D.R.; Anton, R.E.; Prelipcean, A.M. Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1781. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Yang, C.; Li, J.; Sun, T.; Yu, G. Recent Advances in Pharmaceutical Potential of Brown Algal Polysaccharides and their Derivatives. Curr. Pharm. Des. 2019, 25, 1290–1311. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pavuluri, S.; Bruggeman, K.; Long, B.M.; Parnell, A.J.; Martel, A.; Parnell, S.R.; Pfeffer, F.M.; Dennison, A.J.; Nicholas, K.R.; et al. Coassembled nanostructured bioscaffold reduces the expression of proinflammatory cytokines to induce apoptosis in epithelial cancer cells. Nanomedicine 2016, 12, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Lin, X.Z.; Kuo, K.L.; Hsu, F.Y. Fabrication and Cytotoxicity of Fucoidan-Cisplatin Nanoparticles for Macrophage and Tumor Cells. Materials 2017, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Choi, D.S.; Choi, S.; Won, J.Y.; Jo, Y.; Kim, H.B.; Jung, Y.; Shin, S.C.; Min, H.; Choi, H.W.; et al. Enhancing adoptive T-cell therapy with fucoidan-based IL-2 delivery microcapsules. Bioeng. Transl. Med. 2023, 8, e10362. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, B.; Wang, T.; Zhang, L.; Chen, X.; Zhao, X. TAM-Hijacked Immunoreaction Rescued by Hypoxia-Pathway-Intervened Strategy for Enhanced Metastatic Cancer Immunotherapy. Small 2023, 20, e2305728. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Y.; Gong, X.; Nie, F.; Xu, J.; Guo, Y. Construction of quercetin-fucoidan nanoparticles and their application in cancer chemo-immunotherapy treatment. Int. J. Biol. Macromol. 2023, 256, 128057. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Yadav, D.; An, E.K.; Kwak, M.; Lee, P.C.; Jin, J.O. Enhancement of Immune Checkpoint Inhibitor-Mediated Anti-Cancer Immunity by Intranasal Treatment of Ecklonia cava Fucoidan against Metastatic Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9125. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, J.; Song, R.; Dong, X.; Wei, Z.; Li, X.; Zeng, X.; Yao, J. Laminarin Alleviates Acute Lung Injury Induced by LPS Through Inhibition of M1 Macrophage Polarisation. J. Cell Mol. Med. 2025, 29, e70440. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.-F.; Ji, Y.-B.; Meng, D.-Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef]
- Zhou, S.; Qin, H.; Long, Z.; Kong, L.; Ma, J.; Lin, Y.; Lin, H.; Huang, Z.; Li, Z. Effects of laminarin on antioxidant capacity and non-specific immunity of spotted sea bass (Lateolabrax maculatus). Aquac. Rep. 2025, 40, 102549. [Google Scholar] [CrossRef]
- Li, X.-Y.; Wang, Z.-X.; Li, L.-Z.; Huang, L.; Wu, Y.-C.; Li, H.-J. A review oriented by structure-activity relationship: Preparation, bioactivities, and applications of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2025, 328, 147675. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Sabir, A.; Wang, M.; Zhong, S.; Tan, K. Advancements in the Extraction, Characterization, and Bioactive Potential of Laminaran: A Review. Foods 2025, 14, 1683. [Google Scholar] [CrossRef]
- Custódio, C.A.; Reis, R.L.; Mano, J.F. Photo-Cross-Linked Laminarin-Based Hydrogels for Biomedical Applications. Biomacromolecules 2016, 17, 1602–1609. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.-S. Seaweed-derived laminarin and alginate as potential chemotherapeutical agents: An updated comprehensive review considering cancer treatment. Int. J. Biol. Macromol. 2025, 293, 136593. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Song, Z.; Jin, L.; Jiao, L.; Liu, H.; Zhang, S.; Hu, Y.; Sun, Y.; Li, E.; Zhao, G.; et al. Polyethyleneimine-modified Laminarin nanoparticles as a novel vaccine adjuvant for ovalbumin to enhance the immune responses. Int. J. Biol. Macromol. 2025, 292, 139157. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, Q.; Gao, W.; Xiao, Y.; Shi, L.; Lin, F.; Xiong, Y.; Zhang, Y.; Xu, Q.; Wang, L.; et al. Synergistic microglial modulation by laminarin-based platinum nanozymes for potential intracerebral hemorrhage therapy. Biomaterials 2025, 319, 123212. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, N.E.; Youssef, F.R.; Alshishtawy, A.A.K.; Elshikh, F.M.; Newir, O.; Abdelazeem, S.H.; Ma’ruf, N.K.; Shouman, H.; Ali, S.S.; El-Sheekh, M.M. Marine algal polysaccharides for drug delivery applications: A review. Int. J. Biol. Macromol. 2025, 295, 139551. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, J.; Feng, X.; Gao, X.; Cheng, W.; Kong, L.; Liang, F. Cell membrane-inspired chitosan nanoparticles for prolonged circulation and tumor-targeted drug delivery. Int. J. Biol. Macromol. 2025, 304, 140934. [Google Scholar] [CrossRef]
- Li, Q.; Han, J.; Yang, Y.; Chen, Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front. Immunol. 2022, 13, 1070961. [Google Scholar] [CrossRef]
- Li, J.; Jia, J.; Teng, Y.; Wang, X.; Xia, X.; Song, S.; Zhu, B.; Xia, X. Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome. Foods 2025, 14, 387. [Google Scholar] [CrossRef]
- Su, W.; Qiu, W.; Li, S.-J.; Wang, S.; Xie, J.; Yang, Q.-C.; Xu, J.; Zhang, J.; Xu, Z.; Sun, Z.-J. A Dual-Responsive STAT3 Inhibitor Nanoprodrug Combined with Oncolytic Virus Elicits Synergistic Antitumor Immune Responses by Igniting Pyroptosis. Adv. Mater. 2023, 35, 2209379. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Li, Z.; Zhang, X.; Zhang, H.; Zhang, T.; Meng, X.; Sheng, F.; Hou, Y. Amelioration of systemic antitumor immune responses in cocktail therapy by immunomodulatory nanozymes. Sci. Adv. 2022, 8, eabn3883. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, W.; Su, F.; Wang, J.; Li, H.; Sun, M.; Ma, Y.; Xu, Y.; Li, D.; Wang, Y.; et al. Hybrid Membrane-Camouflaged Biomimetic Immunomodulatory Nanoturrets with Sequential Multidrug Release for Potentiating T Cell-Mediated Anticancer Immunity. J. Am. Chem. Soc. 2024, 146, 18592–18605. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Zhang, Y.; Yang, Y.; Cao, J.; Huang, J.; Chen, J.; Chen, H.; Zhang, J.; Wang, L.; et al. Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy. Leukemia 2024, 38, 2517–2543. [Google Scholar] [CrossRef]
- Huang, G.; He, Y.; Chen, X.; Yin, T.; Ma, A.; Zhu, L.; Chen, L.; Liang, R.; Zhang, P.; Pan, H.; et al. Bioorthogonal oncolytic-virus nanovesicles combined bio-immunotherapy with CAR-T cells for solid tumors. Biomater. Sci. 2025, 13, 457–465. [Google Scholar] [CrossRef] [PubMed]
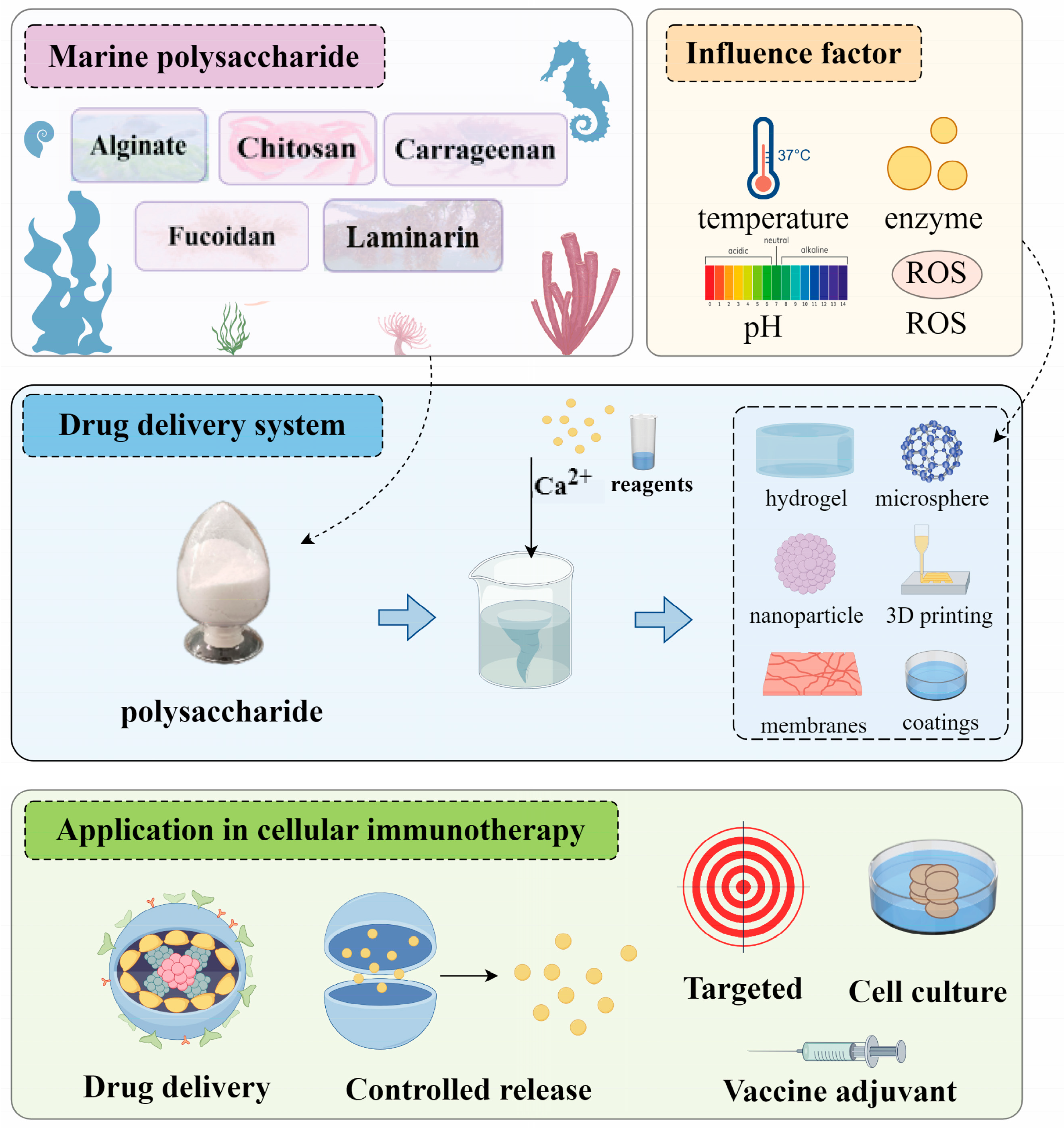
| Polysaccharide | Polysaccharide Source | Type of Nanoparticle Delivery System | Relevant Experimental Studies | References |
|---|---|---|---|---|
| Alginate | Brown algae (e.g., Macrocystis, Laminaria, Ecklonia, and Sargassum) and certain bacteria (Azotobacter and Pseudomonas) | Magnetic bio-nanocomposite hydrogel beads | Controlled Release: pH-sensitive release, drug release | [85,86,87,88] |
| Alginate-based nanoparticles (NPs) | Targeting: Effective targeting of ovarian cancer with minimal off-target effects | [89,90] | ||
| Alginate Nanocomposite Hydrogels | Stimuli-Responsive: responsive drug release demonstrated by hydrogels | [87,91,92] | ||
| Chitosan | Chitin, sourced from crustacean shells (e.g., shrimp, crab, lobster) and the cell walls of mushrooms, coral, algae, and nematodes | Salicylic acid chitosan nanoparticle | Therapeutic Effects: Salicylic acid-chitosan nanoparticles inhibit tumor growth and promote tissue regeneration | [93,94,95,96] |
| Tripolyphosphate cross-linked chitosan nanoparticles | Cytotoxicity: Cytotoxicity was assessed against the cancer cell line | [97,98] | ||
| Carrageenan | Primarily from aquaculture-based seaweed farming, with Eucheuma and Kappaphycus species accounting for >90% of global production | Chitosan-Kappa-Carrageenan composite | Controlled Release | [99,100,101] |
| Kappa-carrageenan-coated magnetic hydroxypropyl methylcellulose/chitosan nanoparticles | Cytotoxicity and Targeting | [102,103,104] | ||
| Kappa-carrageenan-coated nanoparticles | Carrageenan nanosystems exhibit targeted chemotherapeutic effects and cytotoxicity against breast cancer cell lines (e.g., MCF-7) | [105,106,107] | ||
| Methacrylated carrageenan/gelatin hydrogel microspheres (MCGs) | Immunomodulation: MCGs reshape the TME to enhance response to PD-L1 inhibitors | [108,109] | ||
| Fucoidan | Brown algae (Laminariaceae, Fucaceae, Chordariaceae, Alariaceae), sea cucumbers (Stichopodidae, Holothuriidae), sea urchin eggs (Strongylocentrotidae, Arbaciidae), and seagrasses (Cymodoceaceae) | Fucoidan-based polymeric nanoparticles (NPs) | Anticancer Activity: Assessed in HCT116 colorectal cancer cells | [110,111] |
| Fucoidan-based nanoparticles (NPs) | Improved Efficacy and Safety: Enhanced tumor regression, improved survival, and reduced off-target cardiotoxicity | [112,113,114] | ||
| Laminarin | Brown seaweeds such as Saccharina, Laminaria, and Fucus, with particularly high levels in Laminaria and Fucus species | Laminarin–peptide dendrimer-based composite nanoparticles | Influence on TAM polarization, cytokine secretion, and the proliferation and apoptosis of tumor cells | [37,73,115] |
| Laminarin-coated pH-sensitive Genexol-PM nanomicelles | Targeted delivery and pH-sensitive release of nanoparticles for selective tumor cell killing, reduced normal tissue injury, and alleviation of the immunosuppressive microenvironment to enhance antitumor immunity | [116,117,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, M.; Yan, S.; Zhang, Y.; Wang, P. Progress in the Application of Marine Polysaccharide Drug Delivery Systems in Tumor Immunotherapy: Multiple Mechanisms and Material Forms. Mar. Drugs 2025, 23, 384. https://doi.org/10.3390/md23100384
Cha M, Yan S, Zhang Y, Wang P. Progress in the Application of Marine Polysaccharide Drug Delivery Systems in Tumor Immunotherapy: Multiple Mechanisms and Material Forms. Marine Drugs. 2025; 23(10):384. https://doi.org/10.3390/md23100384
Chicago/Turabian StyleCha, Mingxue, Shuqiang Yan, Yiping Zhang, and Peipei Wang. 2025. "Progress in the Application of Marine Polysaccharide Drug Delivery Systems in Tumor Immunotherapy: Multiple Mechanisms and Material Forms" Marine Drugs 23, no. 10: 384. https://doi.org/10.3390/md23100384
APA StyleCha, M., Yan, S., Zhang, Y., & Wang, P. (2025). Progress in the Application of Marine Polysaccharide Drug Delivery Systems in Tumor Immunotherapy: Multiple Mechanisms and Material Forms. Marine Drugs, 23(10), 384. https://doi.org/10.3390/md23100384







