Jellyfish Venom-Induced Cardiotoxicity and Immune Responses: Mechanisms and Potential Therapeutic Strategies
Abstract
1. Introduction
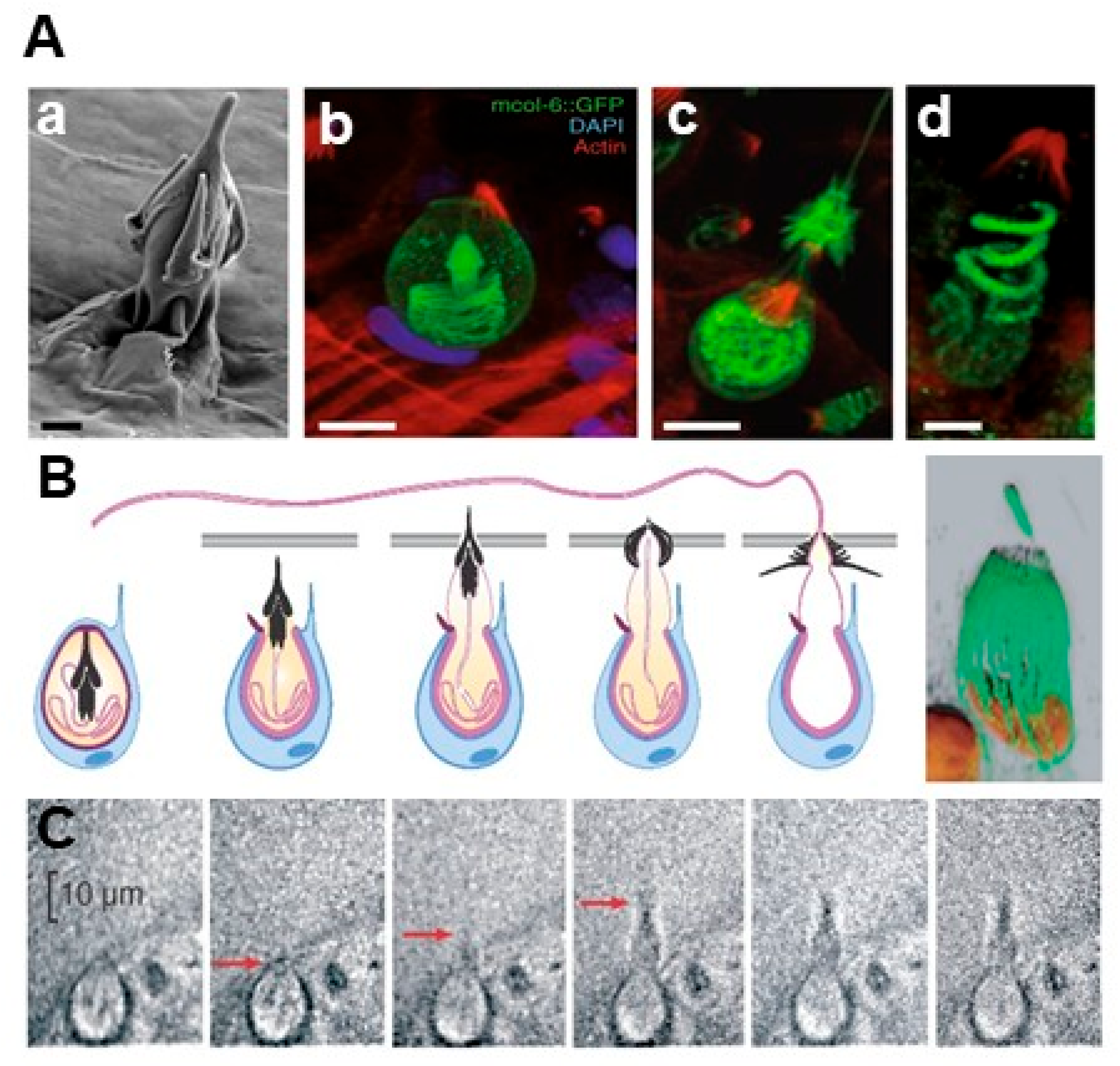
2. Jellyfish Morphology and Nematocyst Ultrastructure
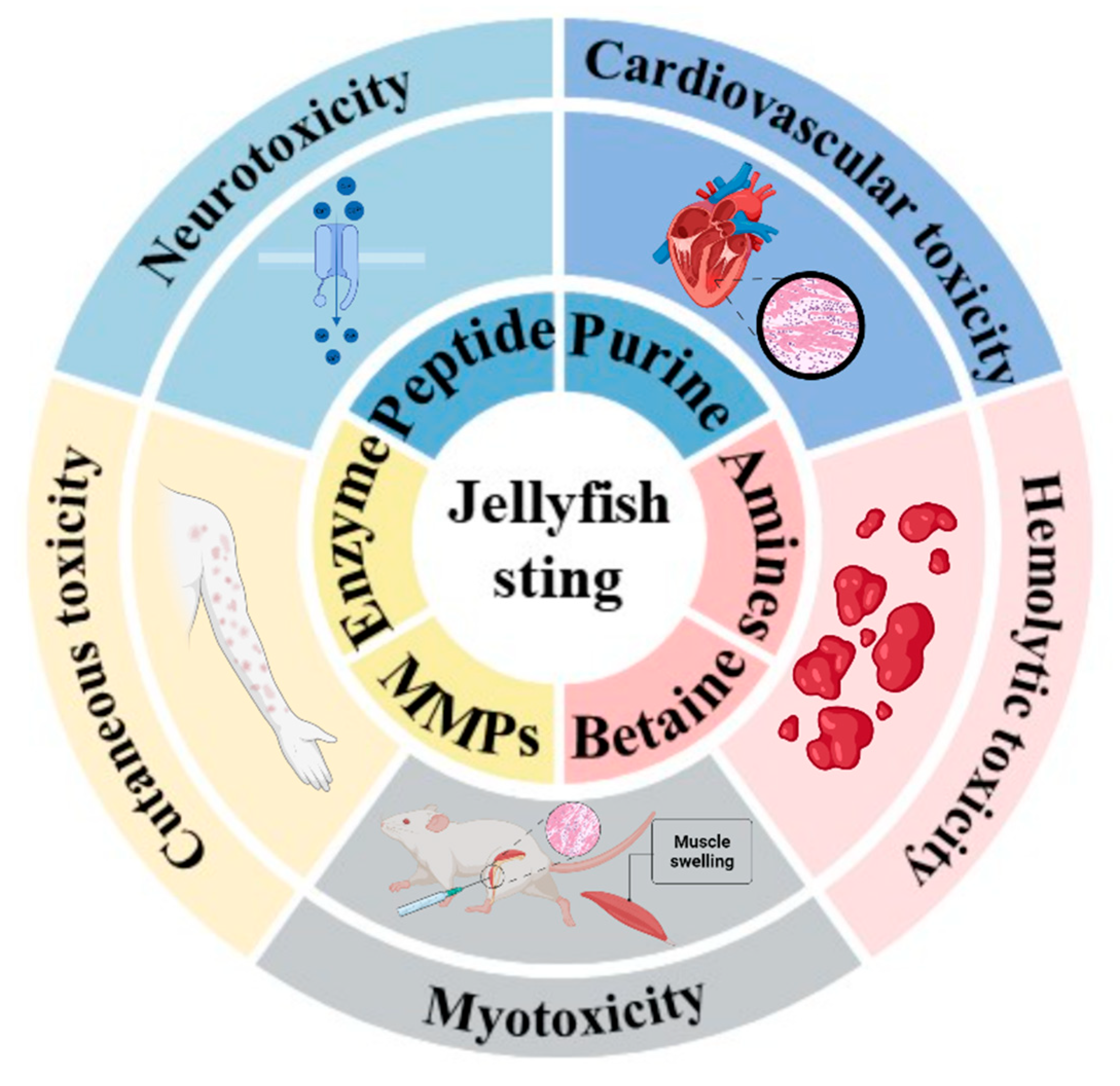
3. Jellyfish Venom Composition
3.1. Matrix Metalloproteinases
3.2. Phospholipase A2, PLA2
3.3. Serine Protease
3.4. Pore-Forming Toxins, PFTs
3.5. Others
| Class | Species | Source | Protein Component | Molecular Weight (kDa) | Bioactivity/Toxicological manifestaion | Major Potential Toxin Families | Ref. |
|---|---|---|---|---|---|---|---|
| Cardiotoxicity-dominant | Carukia barnesi | Double Island (northern Australia) | Crude venom | 25–250 | pulmonary hypertension; tachycardia | Neural sodium channel activator | [68] |
| Chrysaora quinquecirrha | Bay of Bengal (India) | Frc-1 | 105 | antioxidant potential | U | [69,70] | |
| Frc-2 | 65 | ||||||
| Frc-3 | 9 | ||||||
| Meredith Creek (Maryland) | SNLF | 100 | cardiotoxic; neurotoxic | ||||
| 190 | |||||||
| Physalia physalis | Miami (Florida) | Physalitoxin | 240 | haemolytic activity | U | [71] | |
| Immunotoxicity-dominant | Stomolophus meleagris | Qingdao (China) | SmTX | ~45 | haemolytic activity | U | [72] |
| 52 | |||||||
| Pelagia noctiluca | Sicilian coasts (Strait of Messina) | Crude venom | 20–100 | haemolytic activity | U | [73] | |
| Dual-mechanism synergistic toxicity | Chironex fleckeri | Cairns, Townsville, and Weipa (Queensland, Australia) | CfTX-1 | 43 | pore-forming toxins; haemolytic activity | U | [61,74] |
| CfTX-2 | 45 | ||||||
| Weipa (Queensland, Australia) | CfTX-A | ~40 | |||||
| CfTX-B | ~42 | ||||||
| Nemopilema nomurai | Yellow Sea (South Korea) | Crude venom | 15–250 | cardiotoxic, hepatotoxic, hemolytic and cytotoxic biological activities | Metalloproteinase, PLA2 activity | [75] | |
| ROK Coast | JVMP17-1 | ~25 | dermotoxicity, cytotoxicity, and lethality | Metalloproteinase | |||
| JVMP17-2 | 50–70 | ||||||
| Cyanea capillata | Isle of Lewis (Western Isles, Scotland) | CcTX-1 | 31.17 | haemolytic activity | Haemolytic proteins | [76,77] | |
| Isle of Lewis, Rousay (Scotland) | CcNT | 8.22 | neurotoxin | Neurotoxic peptide | |||
| Unkown | Cyanea nozakii | Qingdao (China) | Letoxcn | ~50 | metalloproteinase activity | U | [42] |
4. Cardiotoxicity-Dominant Jellyfish Species
4.1. Carukia barnesi
4.2. Chrysaora quinquecirrha
4.3. Physalia physalis
5. Immunotoxicity-Dominant Jellyfish Species
5.1. Stomolophus meleagris
5.2. Pelagia noctiluca
6. Dual-Mechanism Synergistic Toxicity
6.1. Chironex fleckeri
6.2. Nemopilema nomurai
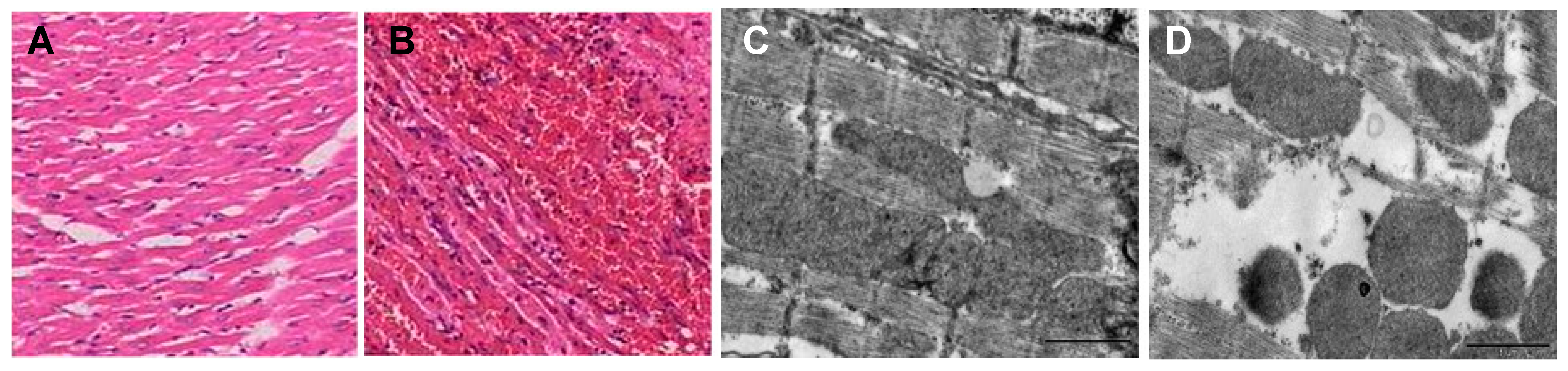
6.3. Cyanea capillata
7. Treatment Strategy
7.1. Comprehensive Treatment of Jellyfish Sting
7.2. Therapeutic Strategies for Cardiotoxicity
7.3. Therapeutic Strategies for Immunotoxicity
8. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish stings and their management: A review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W. Clinical manifestations of jellyfish envenomation. Hydrobiologia 1991, 216, 629–635. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Canepa, A.; Fuentes, V.; Tamburello, L.; Purcell, J.E.; Piraino, S.; Roberts, J.; Boero, F.; Halpin, P. Deterministic Factors Overwhelm Stochastic Environmental Fluctuations as Drivers of Jellyfish Outbreaks. PLoS ONE 2015, 10, e0141060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lyu, Z.; Wu, J.; Cheng, C.; Wang, Y.; Liu, Z.; Du, B.; Yang, Y.; Li, F.; Chen, Q. Epidemiological analysis of jellyfish stings in coastal bathing beaches in Qinhuangdao City from 2017 to 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.Y.; Fonfría, E.S.; Flores-García, A.; Bordehore, C. Epidemiology of jellyfish stings using the Sting Index to identify trends and support proactive management. Ocean Coast. Manag. 2024, 256, 107308. [Google Scholar] [CrossRef]
- Boulware, D.R. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J. Travel Med. 2006, 13, 166–171. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Li, A.; Yu, C.; Li, P. Preparation and Neutralization Efficacy of Novel Jellyfish Antivenoms against Cyanea nozakii Toxins. Toxins 2021, 13, 165. [Google Scholar] [CrossRef]
- Curatolo, R.; Madanchi, M.; Juratli, H.A. Delayed-type hypersensitivity reaction to jellyfish. Int. J. Dermatol. 2024, 63, 818–819. [Google Scholar] [CrossRef]
- Amato, G.; Vita, F.; Gemelli, F.; Tigano, V.; Minciullo, P.L.; Gangemi, S. Jellyfish anaphylaxis: A wide spectrum of sensitization routes. Allergy Asthma Proc. 2020, 41, 158–166. [Google Scholar] [CrossRef]
- Peng, X.; Liu, K.T.; Chen, J.B.; Yan, Z.H.; Danso, B.; Wang, M.K.; Peng, Z.Y.; Xiao, L. Jellyfish Stings: A Review of Skin Symptoms, Pathophysiology, and Management. Med. Sci. Monit. 2024, 30, e944265. [Google Scholar] [CrossRef]
- Srinivasan, M.T.; Varadharajan, A.; Logamoorthy, R.; Karthikeyan, K. The touch of the tentacles—Dermoscopy of jellyfish dermatitis. QJM Int. J. Med. 2024, 118, 355–356. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Yue, Y.; Li, P. Combined Proteome and Toxicology Approach Reveals the Lethality of Venom Toxins from Jellyfish Cyanea nozakii. J. Proteome Res. 2018, 17, 3904–3913. [Google Scholar] [CrossRef]
- Beilei, W.; Lin, Z.; Qian, H.; Qianqian, W.; Tao, W.; Jia, L.; Xiaojuan, W.; Xuting, Y.; Liang, X.; Liming, Z. Direct cardiac toxicity of the tentacle-only extract from the jellyfish Cyanea capillata demonstrated in isolated rat heart. J. Cardiovasc. Pharmacol. 2012, 59, 331–338. [Google Scholar] [CrossRef]
- Brinkman, D.; Burnell, J. Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2007, 50, 850–860. [Google Scholar] [CrossRef]
- Choudhary, I.; Hwang, D.H.; Lee, H.; Yoon, W.D.; Chae, J.; Han, C.H.; Yum, S.; Kang, C.; Kim, E. Proteomic analysis of novel components of Nemopilema nomurai jellyfish venom: Deciphering the mode of action. Toxins 2019, 11, 153. [Google Scholar] [CrossRef]
- Li, A.; Yue, Y.; Li, R.; Yu, C.; Wang, X.; Liu, S.; Xing, R.; Li, P.; Zhang, Q.; Yu, H. Fucoidan may treat jellyfish dermatitis by inhibiting the inflammatory effect of jellyfish venom. Int. J. Biol. Macromol. 2023, 253, 127449. [Google Scholar] [CrossRef] [PubMed]
- Thaikruea, L. The dermatological effects of box jellyfish envenomation in stinging victims in Thailand: Underestimated severity. Wilderness Environ. Med. 2023, 34, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Yang, F.; Chen, J.; Geng, X.; Sun, Q.; Zhang, J.; Liu, C.; Lv, J.; Hou, X. Cytokine Storm Induction Linked to Multi-Organ Failure in Fatal Jellyfish Stings. Adv. Sci. 2025, e01104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Huang, H.; Zhu, J. Bio-inspired transparent soft jellyfish robot. Soft Robot. 2023, 10, 590–600. [Google Scholar] [CrossRef]
- Miles, J.G.; Battista, N.A. Naut your everyday jellyfish model: Exploring how tentacles and oral arms impact locomotion. Fluids 2019, 4, 169. [Google Scholar] [CrossRef]
- Askew, G.; Neil, T.R. Jet-paddling jellies: Swimming performance in the Rhizostomeae jellyfish Catostylus mosaicus. J. Exp. Biol. 2018, 221, jeb191148. [Google Scholar] [CrossRef]
- Beckmann, A.; Özbek, S. The nematocyst: A molecular map of the cnidarian stinging organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef]
- Anderson, P.A.; Bouchard, C. The regulation of cnidocyte discharge. Toxicon 2009, 54, 1046–1053. [Google Scholar] [CrossRef]
- Garm, A.; Lebouvier, M.; Tolunay, D. Mating in the box jellyfish Copula sivickisi--Novel function of cnidocytes. J. Morphol. 2015, 276, 1055–1064. [Google Scholar] [CrossRef]
- Slautterback, D.B.; Fawcett, D.W. The development of the cnidoblasts of Hydra; an electron microscope study of cell differentiation. J. Cell Biol. 1959, 5, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Holstein, T. The morphogenesis of nematocytes in Hydra and Forskålia: An ultrastructural study. J. Ultrastruct. Res. 1981, 75, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J.; Yanagihara, A.A.; Turner, H.C.; Winkel, K. Immunological and toxinological responses to jellyfish stings. Inflamm. Allergy Drug Targets 2011, 10, 438–446. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Giacco, E.; Pane, L. The Mauve Stinger Pelagia noctiluca (Forsskål, 1775). Distribution, Ecology, Toxicity and Epidemiology of Stings. Mar. Drugs 2008, 6, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Pantin, C. The excitation of nematocysts. J. Exp. Biol. 1942, 19, 294–310. [Google Scholar] [CrossRef]
- Holstein, T.; Tardent, P. An ultrahigh-speed analysis of exocytosis: Nematocyst discharge. Science 1984, 223, 830–833. [Google Scholar] [CrossRef]
- Ozbek, S.; Balasubramanian, P.G.; Holstein, T.W. Cnidocyst structure and the biomechanics of discharge. Toxicon 2009, 54, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Khalturin, K.; Shinzato, C.; Khalturina, M.; Hamada, M.; Fujie, M.; Koyanagi, R.; Kanda, M.; Goto, H.; Anton-Erxleben, F.; Toyokawa, M. Medusozoan genomes inform the evolution of the jellyfish body plan. Nat. Ecol. Evol. 2019, 3, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Nüchter, T.; Benoit, M.; Engel, U.; Özbek, S.; Holstein, T.W. Nanosecond-scale kinetics of nematocyst discharge. Curr. Biol. 2006, 16, R316–R318. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Lane, A.N.; Nash, P.D.; Ellsworth, S.A.; Nystrom, G.S.; Rokyta, D.R. The arylsulfatase- and phospholipase-rich venom of the plutoniumid centipede Theatops posticus. Toxicon 2023, 233, 107231. [Google Scholar] [CrossRef]
- Takeda, S. ADAM and ADAMTS Family Proteins and Snake Venom Metalloproteinases: A Structural Overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, S.; Girish, K.S.; Fox, J.W.; Kemparaju, K. ‘Partitagin’ a hemorrhagic metalloprotease from Hippasa partita spider venom: Role in tissue necrosis. Biochimie 2007, 89, 1322–1331. [Google Scholar] [CrossRef]
- Sharma, A.; Balde, A.; Nazeer, R.A. A review on animal venom-based matrix metalloproteinase modulators and their therapeutic implications. Int. Immunopharmacol. 2025, 157, 114703. [Google Scholar] [CrossRef]
- Kang, C.; Han, D.Y.; Park, K.I.; Pyo, M.J.; Heo, Y.; Lee, H.; Kim, G.S.; Kim, E. Characterization and neutralization of Nemopilema nomurai (Scyphozoa: Rhizostomeae) jellyfish venom using polyclonal antibody. Toxicon 2014, 86, 116–125. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef]
- Leung, T.C.N.; Qu, Z.; Nong, W.; Hui, J.H.L.; Ngai, S.M. Proteomic Analysis of the Venom of Jellyfishes Rhopilema esculentum and Sanderia malayensis. Mar. Drugs 2020, 18, 655. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Li, A.; Yu, C.; Li, P. Identification and characterization of the key lethal toxin from jellyfish Cyanea nozakii. Int. J. Biol. Macromol. 2023, 230, 123176. [Google Scholar] [CrossRef]
- Mohan Prakash, R.L.; Hwang, D.H.; Asirvatham, R.D.; Hong, I.H.; Kang, C.; Kim, E. Identification of cardiorespiratory toxic components of Nemopilema nomurai jellyfish venom using sequential chromatography methods. Toxicon 2023, 229, 107126. [Google Scholar] [CrossRef]
- Hernández, R.; Cabalceta, C.; Saravia-Otten, P.; Chaves, A.; Gutiérrez, J.M.; Rucavado, A. Poor regenerative outcome after skeletal muscle necrosis induced by Bothrops asper venom: Alterations in microvasculature and nerves. PLoS ONE 2011, 6, e19834. [Google Scholar] [CrossRef]
- Yu, C.; Yin, X.; Li, A.; Li, R.; Yu, H.; Xing, R.; Liu, S.; Li, P. Toxin metalloproteinases exert a dominant influence on pro-inflammatory response and anti-inflammatory regulation in jellyfish sting dermatitis. J. Proteom. 2024, 292, 105048. [Google Scholar] [CrossRef]
- Helmholz, H.; Ruhnau, C.; Schütt, C.; Prange, A. Comparative study on the cell toxicity and enzymatic activity of two northern scyphozoan species Cyanea capillata (L.) and Cyanea lamarckii (Péron & Léslieur). Toxicon 2007, 50, 53–64. [Google Scholar] [CrossRef]
- Feng, J.; Yu, H.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Partial characterization of the hemolytic activity of the nematocyst venom from the jellyfish Cyanea nozakii Kishinouye. Toxicol 2010, 24, 1750–1756. [Google Scholar] [CrossRef]
- Heo, Y.; Kwon, Y.C.; Shin, K.; Yoon, W.D.; Han, C.H.; Yum, S.; Kim, E. cDNA and gene structures of two phospholipase A2 isoforms, acidic PLA2 PA4 and PLA2 PA3A/PA3B/PA5, in Nemopilema nomurai jellyfish venom. Toxicon 2016, 122, 160–166. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Peuravuori, H.J.; Quinn, R.J.; Llewellyn, L.E.; Benzie, J.A.; Fenner, P.J.; Winkel, K.D. Phospholipase A2 in cnidaria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Nampoothiri, M.; Khandibharad, S.; Singh, S.; Nayak, A.G.; Hariharapura, R.C. Proteomic diversity of Russell’s viper venom: Exploring PLA2 isoforms, pharmacological effects, and inhibitory approaches. Arch. Toxicol. 2024, 98, 3569–3584. [Google Scholar] [CrossRef]
- Urs, N.A.; Yariswamy, M.; Joshi, V.; Nataraju, A.; Gowda, T.; Vishwanath, B. Implications of phytochemicals in snakebite management: Present status and future prospective. Toxin Rev. 2014, 33, 60–83. [Google Scholar]
- Hessinger, D.A.; Lenhoff, H.M. Membrane structure and function. Mechanism of hemolysis induced by nematocyst venom: Roles of phospholipase A and direct lytic factor. Arch. Biochem. Biophys. 1976, 173, 603–613. [Google Scholar] [CrossRef]
- Swenson, S.; Markland, F.S., Jr. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef]
- Heo, Y.; Kwon, Y.C.; Bae, S.K.; Hwang, D.; Yang, H.R.; Choudhary, I.; Lee, H.; Yum, S.; Shin, K.; Yoon, W.D.; et al. Cloning a Chymotrypsin-Like 1 (CTRL-1) Protease cDNA from the Jellyfish Nemopilema nomurai. Toxins 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Lee, H.; Heo, Y.; Pyo, M.J.; Choudhary, I.; Han, C.H.; Yoon, W.D.; Kang, C.; Kim, E. In vitro characterization of jellyfish venom fibrin(ogen)olytic enzymes from Nemopilema nomurai. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 35. [Google Scholar] [CrossRef]
- Anderluh, G.; Macek, P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Ito, E.; Miyake, M.; Noda, M.; Nakajima, T. Novel proteinaceous toxins from the box jellyfish (sea wasp) Carybdea rastoni. Biochem. Biophys. Res. Commun. 2000, 275, 582–588. [Google Scholar] [CrossRef]
- Nagai, H.; Takuwa, K.; Nakao, M.; Sakamoto, B.; Crow, G.L.; Nakajima, T. Isolation and characterization of a novel protein toxin from the Hawaiian box jellyfish (sea wasp) Carybdea alata. Biochem. Biophys. Res. Commun. 2000, 275, 589–594. [Google Scholar] [CrossRef]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A novel protein toxin from the deadly box jellyfish (Sea Wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, B.; Wang, B.; Wang, Q.; Liu, G.; Wang, T.; He, Q.; Zhang, L. Unique Diversity of Sting-Related Toxins Based on Transcriptomic and Proteomic Analysis of the Jellyfish Cyanea capillata and Nemopilema nomurai (Cnidaria: Scyphozoa). J. Proteome Res. 2019, 18, 436–448. [Google Scholar] [CrossRef]
- Yap, W.Y.; Hwang, J.S. Response of Cellular Innate Immunity to Cnidarian Pore-Forming Toxins. Molecules 2018, 23, 2537. [Google Scholar] [CrossRef]
- Gold, D.A.; Katsuki, T.; Li, Y.; Yan, X.; Regulski, M.; Ibberson, D.; Holstein, T.; Steele, R.E.; Jacobs, D.K.; Greenspan, R.J. The genome of the jellyfish Aurelia and the evolution of animal complexity. Nat. Ecol. Evol. 2019, 3, 96–104. [Google Scholar] [CrossRef]
- Klompen, A.M.L.; Macrander, J.; Reitzel, A.M.; Stampar, S.N. Transcriptomic Analysis of Four Cerianthid (Cnidaria, Ceriantharia) Venoms. Mar. Drugs 2020, 18, 413. [Google Scholar] [CrossRef]
- Rachamim, T.; Morgenstern, D.; Aharonovich, D.; Brekhman, V.; Lotan, T.; Sher, D. The dynamically evolving nematocyst content of an anthozoan, a scyphozoan, and a hydrozoan. Mol. Biol. Evol. 2015, 32, 740–753. [Google Scholar] [CrossRef]
- Hodgson, W.C. Pharmacological action of Australian animal venoms. Clin. Exp. Pharmacol. Physiol. 1997, 24, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Weinrich, D.; Williamson, J.A.; Fenner, P.J.; Lutz, L.L.; Bloom, D.A. Autonomic neurotoxicity of jellyfish and marine animal venoms. Clin. Auton. Res. 1998, 8, 125–130. [Google Scholar] [CrossRef]
- Winkel, K.D.; Tibballs, J.; Molenaar, P.; Lambert, G.; Coles, P.; Ross-Smith, M.; Wiltshire, C.; Fenner, P.J.; Gershwin, L.A.; Hawdon, G.M.; et al. Cardiovascular actions of the venom from the Irukandji (Carukia barnesi) jellyfish: Effects in human, rat and guinea-pig tissues in vitro and in pigs in vitro. Clin. Exp. Pharmacol. Physiol. 2005, 32, 777–788. [Google Scholar] [CrossRef]
- Balamurugan, E.; Menon, V.P. In vitro radical scavanging activities of Chrysaora quinquecirrha nematocyst venom. Drug Discov. Ther. 2009, 3, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cobbs, C.S.; Gaur, P.K.; Russo, A.J.; Warnick, J.E.; Calton, G.J.; Burnett, J.W. Immunosorbent chromatography of sea nettle (Chrysaora quinquecirrha) venom and characterization of toxins. Toxicon 1983, 21, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tamkun, M.M.; Hessinger, D.A. Isolation and partial characterization of a hemolytic and toxic protein from the nematocyst venom of the Portuguese Man-of-War, Physalia physalis. Biochim. Biophys. Acta Protein Struct. 1981, 667, 87–98. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation and in vitro partial characterization of hemolytic proteins from the nematocyst venom of the jellyfish Stomolophus meleagris. Toxicol. Vitr. 2013, 27, 1620–1625. [Google Scholar] [CrossRef]
- Maisano, M.; Trapani, M.; Parrino, V.; Parisi, M.; Cappello, T.; D’Agata, A.; Benenati, G.; Natalotto, A.; Mauceri, A.; Cammarata, M. Haemolytic activity and characterization of nematocyst venom from Pelagia noctiluca (Cnidaria: Scyphozoa). Ital. J. Zool. 2013, 80, 168–176. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins: Expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Rowley, O.C.; Courtney, R.; Northfield, T.; Seymour, J. Environmental drivers of the occurrence and abundance of the Irukandji jellyfish (Carukia barnesi). PLoS ONE 2022, 17, e0272359. [Google Scholar] [CrossRef] [PubMed]
- Lassen, S.; Helmholz, H.; Ruhnau, C.; Prange, A. A novel proteinaceous cytotoxin from the northern Scyphozoa Cyanea capillata (L.) with structural homology to cubozoan haemolysins. Toxicon 2011, 57, 721–729. [Google Scholar] [CrossRef]
- Lassen, S.; Wiebring, A.; Helmholz, H.; Ruhnau, C.; Prange, A. Isolation of a Nav channel blocking polypeptide from Cyanea capillata medusae—A neurotoxin contained in fishing tentacle isorhizas. Toxicon 2012, 59, 610–616. [Google Scholar] [CrossRef]
- Tibballs, J.; Li, R.; Tibballs, H.A.; Gershwin, L.A.; Winkel, K.D. Australian carybdeid jellyfish causing “Irukandji syndrome”. Toxicon 2012, 59, 617–625. [Google Scholar] [CrossRef]
- Barnes, J.H. Cause and Effect in Irukandji Stingings. Med. J. Aust. 1964, 1, 897–904. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Granados, J.; Agudelo, R. Tako-tsubo cardiomyopathy in clinical toxinology: A systematic review. Toxicon 2022, 219, 106929. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.H.; Seymour, J.E. Venom ontogeny, diet and morphology in Carukia barnesi, a species of Australian box jellyfish that causes Irukandji syndrome. Toxicon 2007, 49, 1073–1082. [Google Scholar] [CrossRef]
- Houck, H.E.; Lipsky, M.M.; Marzella, L.; Burnett, J.V. Toxicity of sea nettle (Chrysaora quinquecirrha) fishing tentacle nematocyst venom in cultured rat hepatocytes. Toxicon 1996, 34, 771–778. [Google Scholar] [CrossRef]
- Kelman, S.N.; Calton, G.J.; Burnett, J.W. Isolation and partial characterization of a lethal sea nettle (Chrysaora quinquecirrha) mesenteric toxin. Toxicon 1984, 22, 139–144. [Google Scholar] [CrossRef]
- Lin, W.W.; Lee, C.Y.; Burnett, J.W. Effect of sea nettle (Chrysaora quinquecirrha) venom on isolated rat aorta. Toxicon 1988, 26, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.; Vue, Z.; Behringer, R.R.; Dunn, C.W. Morphology and development of the Portuguese man of war, Physalia physalis. Sci. Rep. 2019, 9, 15522. [Google Scholar] [CrossRef] [PubMed]
- Morishige, H.; Sugahara, T.; Nishimoto, S.; Muranaka, A.; Ohno, F.; Shiraishi, R.; Doi, M. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology 2011, 63, 481–492. [Google Scholar] [CrossRef]
- Olson, C.E.; Cargo, D.G.; Calton, G.J.; Burnett, J.W. Immunochromatography and cardiotoxicity of sea nettle (Chrysaora quinquecirrha) polyps and cysts. Toxicon 1985, 23, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.; Hessinger, D.A. Portuguese Man-of-war (Physalia physalis) venom induces calcium influx into cells by permeabilizing plasma membranes. Toxicon 2000, 38, 1015–1028. [Google Scholar] [CrossRef]
- Tomkielska, Z.; Frias, J.; Simões, N.; de Bastos, B.P.; Fidalgo, J.; Casas, A.; Almeida, H.; Toubarro, D. Revealing the Bioactivities of Physalia physalis Venom Using Drosophila as a Model. Toxins 2024, 16, 491. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Kuai, W.; Li, W.; Wang, Z.; Xiao, L.; Wu, J. Troxerutin suppress inflammation response and oxidative stress in jellyfish dermatitis by activating Nrf2/HO-1 signaling pathway. Front. Immunol. 2024, 15, 1369849. [Google Scholar] [CrossRef]
- Frazão, B.; Campos, A.; Osório, H.; Thomas, B.; Leandro, S.; Teixeira, A.; Vasconcelos, V.; Antunes, A. Analysis of Pelagia noctiluca proteome Reveals a Red Fluorescent Protein, a Zinc Metalloproteinase and a Peroxiredoxin. Protein J. 2017, 36, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, G.L.; Pane, L. Mediterranean jellyfish venoms: A review on scyphomedusae. Mar. Drugs 2010, 8, 1122–1152. [Google Scholar] [CrossRef]
- Del Negro, P.; Sciancalepore, M.; Mulas, G. Studi preliminari sulla dermotossicità da pelagia noctiluca nell’animale da esperimento. Atti Dell’Accademia Delle Scienze Dell’Istituto di Bologna. Classe di Scienze Fisiche. Rendi-Conti. 1987, 86, 147. [Google Scholar]
- Bruschetta, G.; Impellizzeri, D.; Morabito, R.; Marino, A.; Ahmad, A.; Spanò, N.; Spada, G.L.; Cuzzocrea, S.; Esposito, E. Pelagia noctiluca (Scyphozoa) crude venom injection elicits oxidative stress and inflammatory response in rats. Mar. Drugs 2014, 12, 2182–2204. [Google Scholar] [CrossRef]
- Freeman, S.E.; Turner, R.J. A pharmacological study of the toxin in a Cnidarian, Chironex fleckeri Southcott. Br. J. Pharmacol. 1969, 35, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Andreosso, A.; Bansal, P.S.; Smout, M.J.; Wilson, D.; Seymour, J.E.; Daly, N.L. Structural Characterisation of Predicted Helical Regions in the Chironex fleckeri CfTX-1 Toxin. Mar. Drugs 2018, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Tamadoni Jahromi, S.; Zargan, J.; Zamani, E.; Ranjbar, R.; Honari, H. Cloning and Expression of N-CFTX-1 Antigen from Chironex fleckeri in Escherichia coli and Determination of Immunogenicity in Mice. Iran. J. Public Health. 2021, 50, 376–383. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Li, T.; Li, P. Comprehensive Proteome Reveals the Key Lethal Toxins in the Venom of Jellyfish Nemopilema nomurai. J. Proteome Res. 2020, 19, 2491–2500. [Google Scholar] [CrossRef]
- Li, A.; Yu, H.; Li, R.; Liu, S.; Xing, R.; Li, P. Inhibitory Effect of Metalloproteinase Inhibitors on Skin Cell Inflammation Induced by Jellyfish Nemopilema nomurai Nematocyst Venom. Toxins 2019, 11, 156. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Kim, J.S.; Yoon, W.D.; Lim, D.; Hart, A.J.; Hodgson, W.C. Cardiovascular effects of Nemopilema nomurai (Scyphozoa: Rhizostomeae) jellyfish venom in rats. Toxicol. Lett. 2006, 167, 205–211. [Google Scholar] [CrossRef]
- Choudhary, I.; Lee, H.; Pyo, M.J.; Heo, Y.; Bae, S.K.; Kwon, Y.C.; Yoon, W.D.; Kang, C.; Kim, E. Proteomics approach to examine the cardiotoxic effects of Nemopilema nomurai Jellyfish venom. J. Proteom. 2015, 128, 123–131. [Google Scholar] [CrossRef]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.T.; Lee, H.; Kim, J.S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.D.; Åtland, Å.; Dale, T. Acute lion’s mane jellyfish, Cyanea capillata (Cnideria: Scyphozoa), exposure to Atlantic salmon (Salmo salar L.). J. Fish Dis. 2018, 41, 751–759. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.S.; Wang, Q.Q.; He, Q.; Liu, S.H.; Li, Y.; Zhang, J.; Zhang, L.M. The lethality of tentacle-only extract from jellyfish Cyanea capillata is primarily attributed to cardiotoxicity in anaesthetized SD rats. Toxicon 2010, 55, 838–845. [Google Scholar] [CrossRef]
- Wilcox, C.L.; Yanagihara, A.A. Heated Debates: Hot-Water Immersion or Ice Packs as First Aid for Cnidarian Envenomations? Toxins 2016, 8, 97. [Google Scholar] [CrossRef]
- Remigante, A.; Costa, R.; Morabito, R.; La Spada, G.; Marino, A.; Dossena, S. Impact of Scyphozoan Venoms on Human Health and Current First Aid Options for Stings. Toxins 2018, 10, 133. [Google Scholar] [CrossRef]
- Morabito, R.; Marino, A.; Dossena, S.; La Spada, G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon 2014, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W. Treatment of Atlantic cnidarian envenomations. Toxicon 2009, 54, 1201–1205. [Google Scholar] [CrossRef]
- Exton, D.R.; Fenner, P.J.; Williamson, J.A. Cold packs: Effective topical analgesia in the treatment of painful stings by Physalia and other jellyfish. Med. J. Aust. 1989, 151, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Vucenik, I.; Shamsuddin, A.; Niculescu, F.; Burnett, J.W. Two new actions of sea nettle (Chrysaora quinquecirrha) nematocyst venom: Studies on the mechanism of actions on complement activation and on the central nervous system. Toxicon 2004, 44, 895–899. [Google Scholar] [CrossRef]
- Ward, N.T.; Darracq, M.A.; Tomaszewski, C.; Clark, R.F. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann. Emerg. Med. 2012, 60, 399–414. [Google Scholar] [CrossRef]
- Pyo, M.J.; Lee, H.; Bae, S.K.; Heo, Y.; Choudhary, I.; Yoon, W.D.; Kang, C.; Kim, E. Modulation of jellyfish nematocyst discharges and management of human skin stings in Nemopilema nomurai and Carybdea mora. Toxicon 2016, 109, 26–32. [Google Scholar] [CrossRef]
- Montgomery, L.; Seys, J.; Mees, J. To Pee, or Not to Pee: A Review on Envenomation and Treatment in European Jellyfish Species. Mar. Drugs 2016, 14, 127. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Wang, B.; Wang, C.; Xiao, L.; Zhang, L. β adrenergic receptor/cAMP/PKA signaling contributes to the intracellular Ca2+ release by tentacle extract from the jellyfish Cyanea capillata. BMC Pharmacol. Toxicol. 2017, 18, 60. [Google Scholar] [CrossRef]
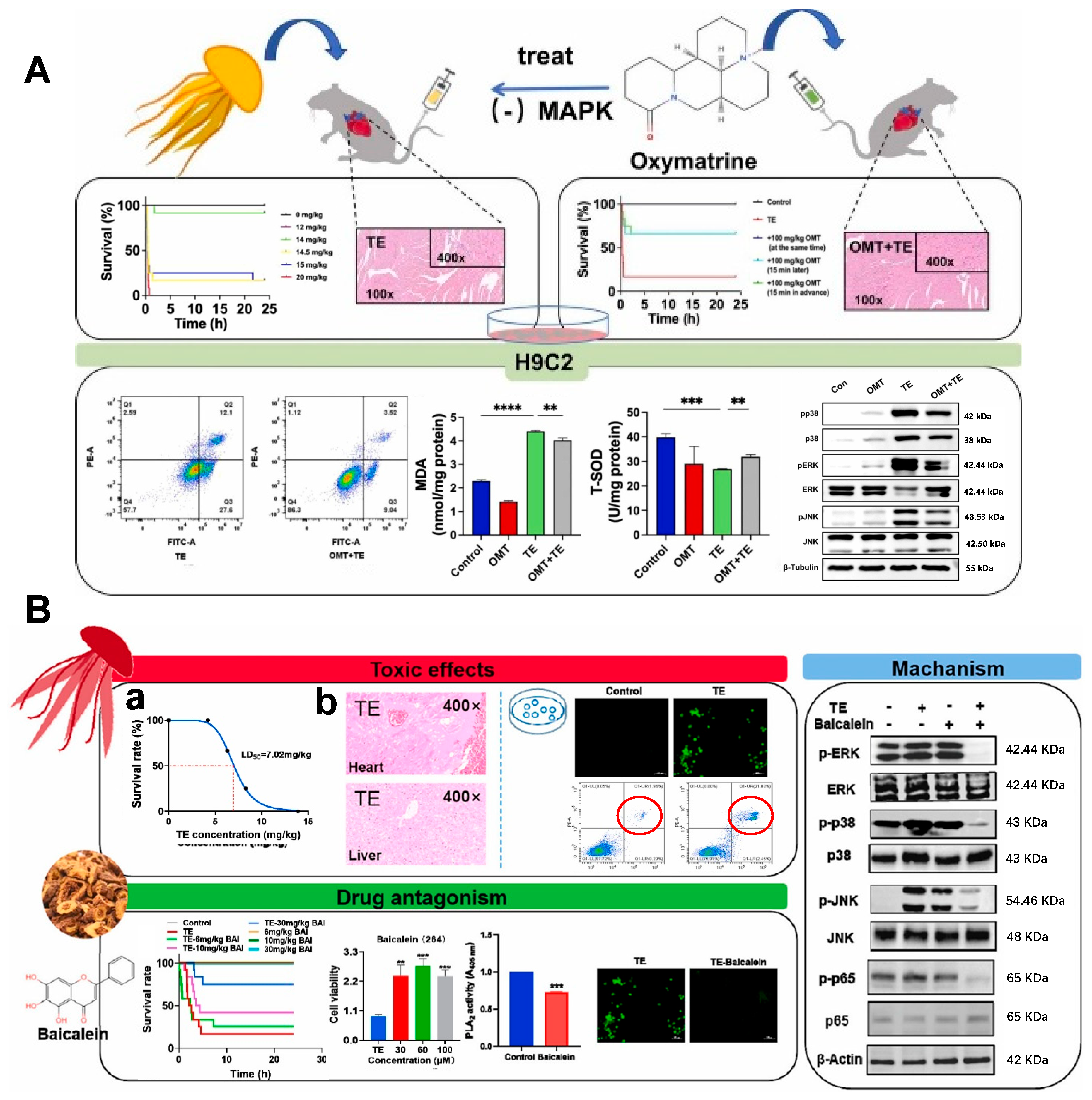
- Wang, X.; Wang, Y.; Geng, X.; Wang, Z.; Zhang, J.; Liu, T.; Chen, W.; Yang, J.; Xiao, L.; Dong, W. Oxymatrine antagonises oxidative stress and apoptosis in Nemopilema nomurai toxin-induced cardiotoxicity by inhibiting mitogen-activated protein kinase. Toxicol. Lett. 2025, 403, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, F.; Wang, Y.; Geng, X.; Zhang, J.; Wang, X.; Liu, C.; Danso, B.; Chen, J.; Pozzolini, M. Baicalein antagonises Rhopilema esculentum toxin-induced oxidative stress and apoptosis by modulating ROS-MAPK-NF-κB and inhibiting PLA2 activity. Toxicon 2025, 256, 108266. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Rimessi, A.; Patergnani, S.; Vitto, V.A.; Danese, A.; Kahsay, A.; Palumbo, L.; Bonora, M.; Wieckowski, M.R.; Giorgi, C. Calcium dysregulation in heart diseases: Targeting calcium channels to achieve a correct calcium homeostasis. Pharmacol. Res. 2022, 177, 106119. [Google Scholar] [CrossRef] [PubMed]
- Endean, R.; Sizemore, D.J. The effectiveness of antivenom in countering the actions of box-jellyfish (Chironex fleckeri) nematocyst toxins in mice. Toxicon 1988, 26, 425–431. [Google Scholar] [CrossRef]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vivo cardiovascular effects of box jellyfish Chironex fleckeri venom in rats: Efficacy of pre-treatment with antivenom, verapamil and magnesium sulphate. Toxicon 2004, 43, 685–690. [Google Scholar] [CrossRef]
- Winter, K.L.; Isbister, G.K.; Jacoby, T.; Seymour, J.E.; Hodgson, W.C. An in vivo comparison of the efficacy of CSL box jellyfish antivenom with antibodies raised against nematocyst-derived Chironex fleckeri venom. Toxicol. Lett. 2009, 187, 94–98. [Google Scholar] [CrossRef]
- Andreosso, A.; Smout, M.J.; Seymour, J.E. Dose and time dependence of box jellyfish antivenom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 34. [Google Scholar] [CrossRef]
- Bloom, D.A.; Burnett, J.W.; Hebel, J.R.; Alderslade, P. Effects of verapamil and CSL antivenom on Chironex fleckeri (box-jellyfish) induced mortality. Toxicon 1999, 37, 1621–1626. [Google Scholar] [CrossRef]
- Masoud, W.G.; Ussher, J.R.; Wang, W.; Jaswal, J.S.; Wagg, C.S.; Dyck, J.R.; Lygate, C.A.; Neubauer, S.; Clanachan, A.S.; Lopaschuk, G.D. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc. Res. 2014, 101, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chen, J.; Liu, F.; Li, B.; Zhang, C.; Wang, X.; Liu, L.; Wang, M.; Wang, T.; Wang, S.; et al. Jellyfish stings-induced cardiac failure was ameliorated through AAG-mediated glycogen-driven ATP production. Exploration 2025, 5, 20230089. [Google Scholar] [CrossRef] [PubMed]
- Geetha, R.; Sathiya Priya, C.; Anuradha, C.V. Troxerutin abrogates mitochondrial oxidative stress and myocardial apoptosis in mice fed calorie-rich diet. Chem. Biol. Interact. 2017, 278, 74–83. [Google Scholar] [CrossRef] [PubMed]
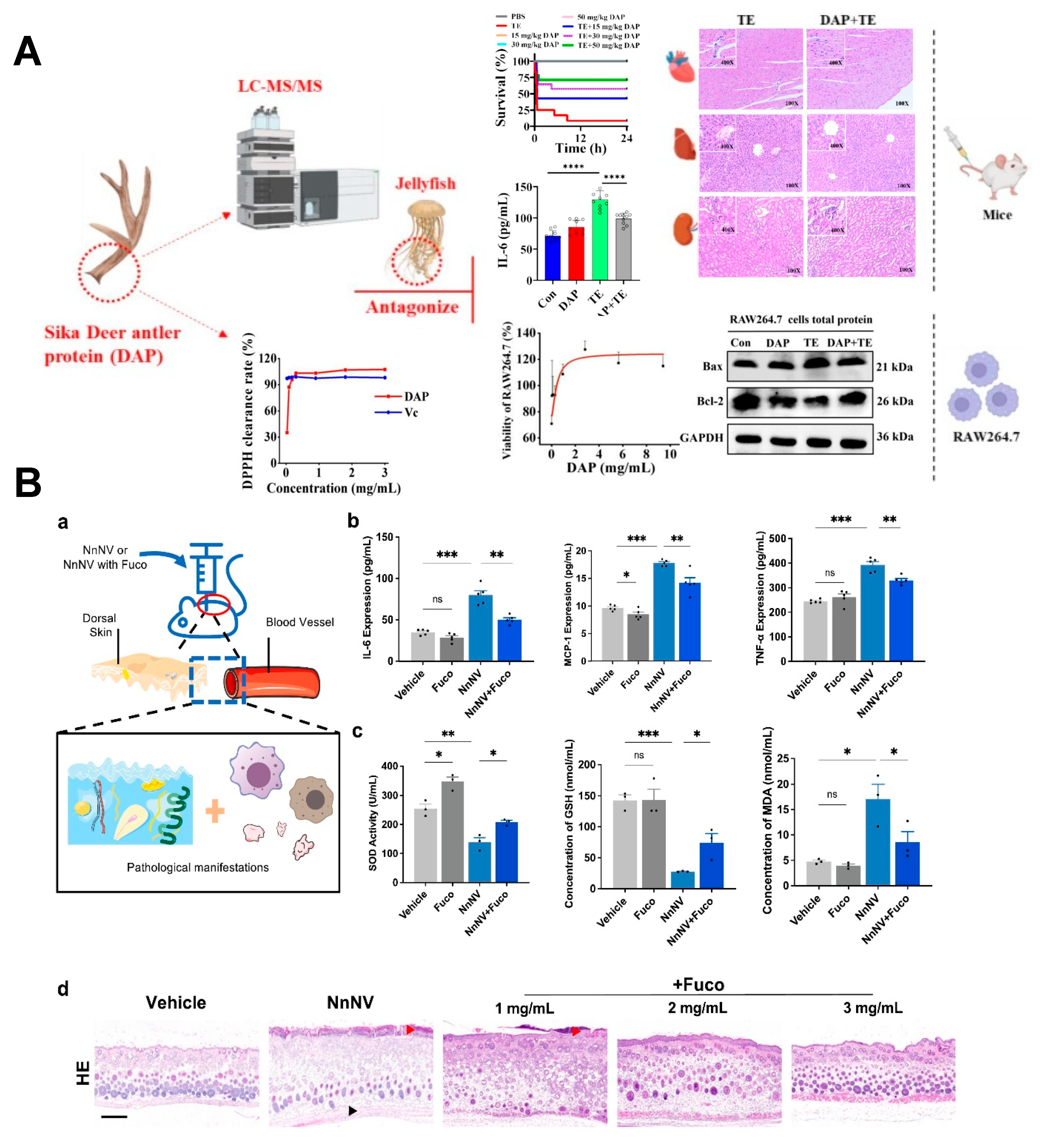
- Zhang, J.; Yang, F.; Tang, D.; Wang, Z.; He, K.; Chen, J.; Danso, B.; Wei, D.; Höfer, J.; Sun, Y.; et al. Sika Deer antler protein antagonizes the inflammatory response and oxidative damage induced by jellyfish venom. Int. Immunopharmacol. 2024, 143, 113343. [Google Scholar] [CrossRef]
- Loredana Asztalos, M.; Rubin, A.I.; Elenitsas, R.; Groft MacFarlane, C.; Castelo-Soccio, L. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: A case report and review of literature. Pediatr. Dermatol. 2014, 31, 217–219. [Google Scholar] [CrossRef]
- Xie, B.; Dashevsky, D.; Rokyta, D.; Ghezellou, P.; Fathinia, B.; Shi, Q.; Richardson, M.K.; Fry, B.G. Dynamic genetic differentiation drives the widespread structural and functional convergent evolution of snake venom proteinaceous toxins. BMC Biol. 2022, 20, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qiu, Z.; Li, B.; Geng, X.; Yu, X.; Li, Y.; Li, W.; Yang, J. Jellyfish Venom-Induced Cardiotoxicity and Immune Responses: Mechanisms and Potential Therapeutic Strategies. Mar. Drugs 2025, 23, 369. https://doi.org/10.3390/md23100369
Li Y, Qiu Z, Li B, Geng X, Yu X, Li Y, Li W, Yang J. Jellyfish Venom-Induced Cardiotoxicity and Immune Responses: Mechanisms and Potential Therapeutic Strategies. Marine Drugs. 2025; 23(10):369. https://doi.org/10.3390/md23100369
Chicago/Turabian StyleLi, Yueyue, Zhiwen Qiu, Bingbing Li, Xiaoyu Geng, Xuelu Yu, Yue Li, Wei Li, and Jishun Yang. 2025. "Jellyfish Venom-Induced Cardiotoxicity and Immune Responses: Mechanisms and Potential Therapeutic Strategies" Marine Drugs 23, no. 10: 369. https://doi.org/10.3390/md23100369
APA StyleLi, Y., Qiu, Z., Li, B., Geng, X., Yu, X., Li, Y., Li, W., & Yang, J. (2025). Jellyfish Venom-Induced Cardiotoxicity and Immune Responses: Mechanisms and Potential Therapeutic Strategies. Marine Drugs, 23(10), 369. https://doi.org/10.3390/md23100369






