Syntheses of Marine Natural Products via Matteson Homologations and Related Processes
Abstract
1. Introduction
2. Homologations of Boronic Esters
2.1. Matteson Homologations
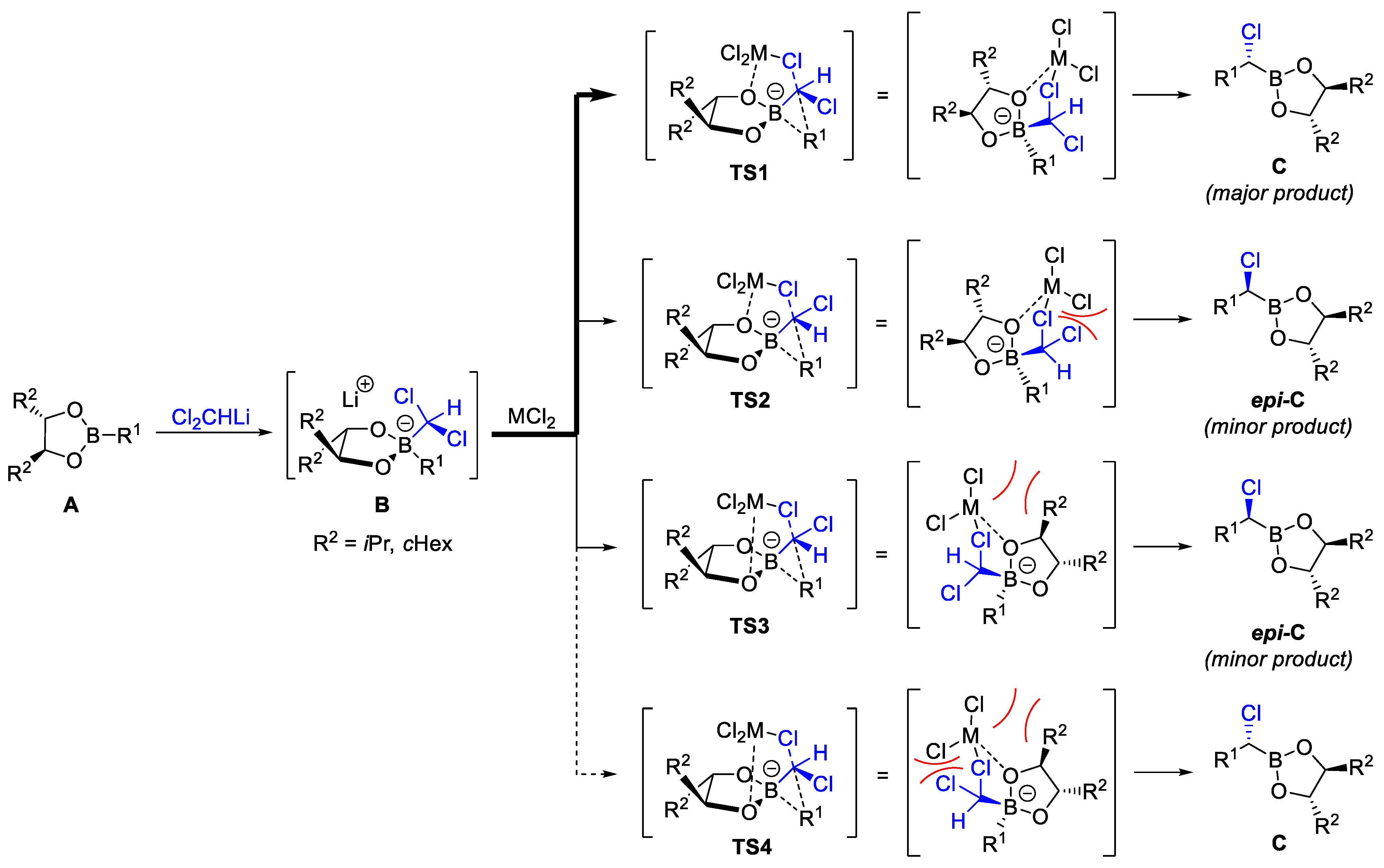

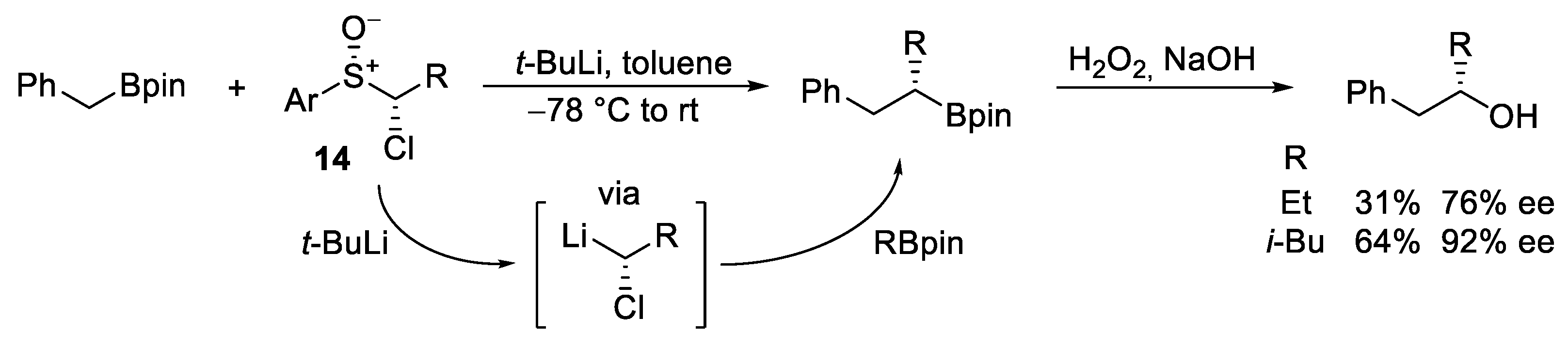
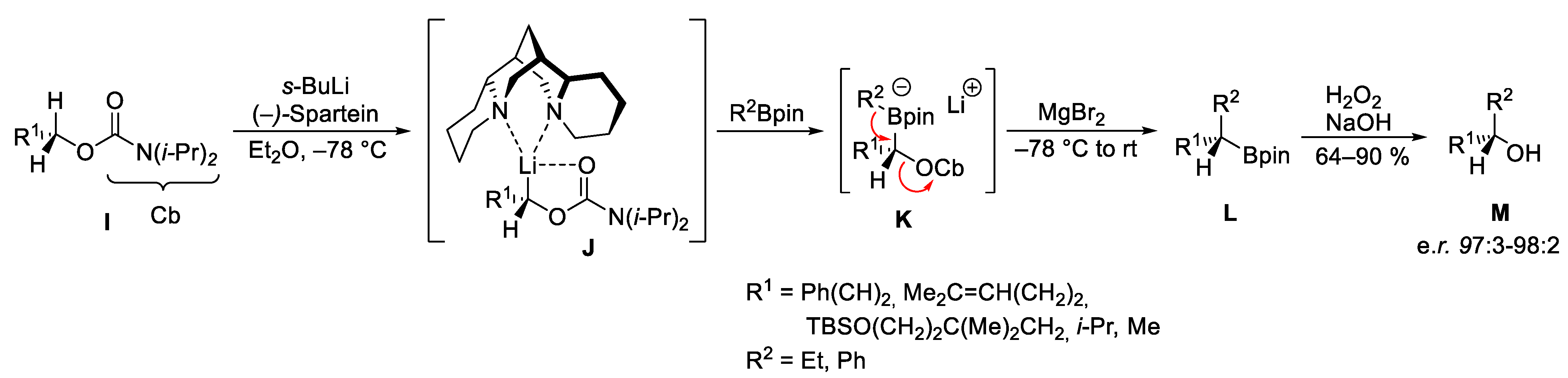
2.2. Reagent-Controlled Homologations
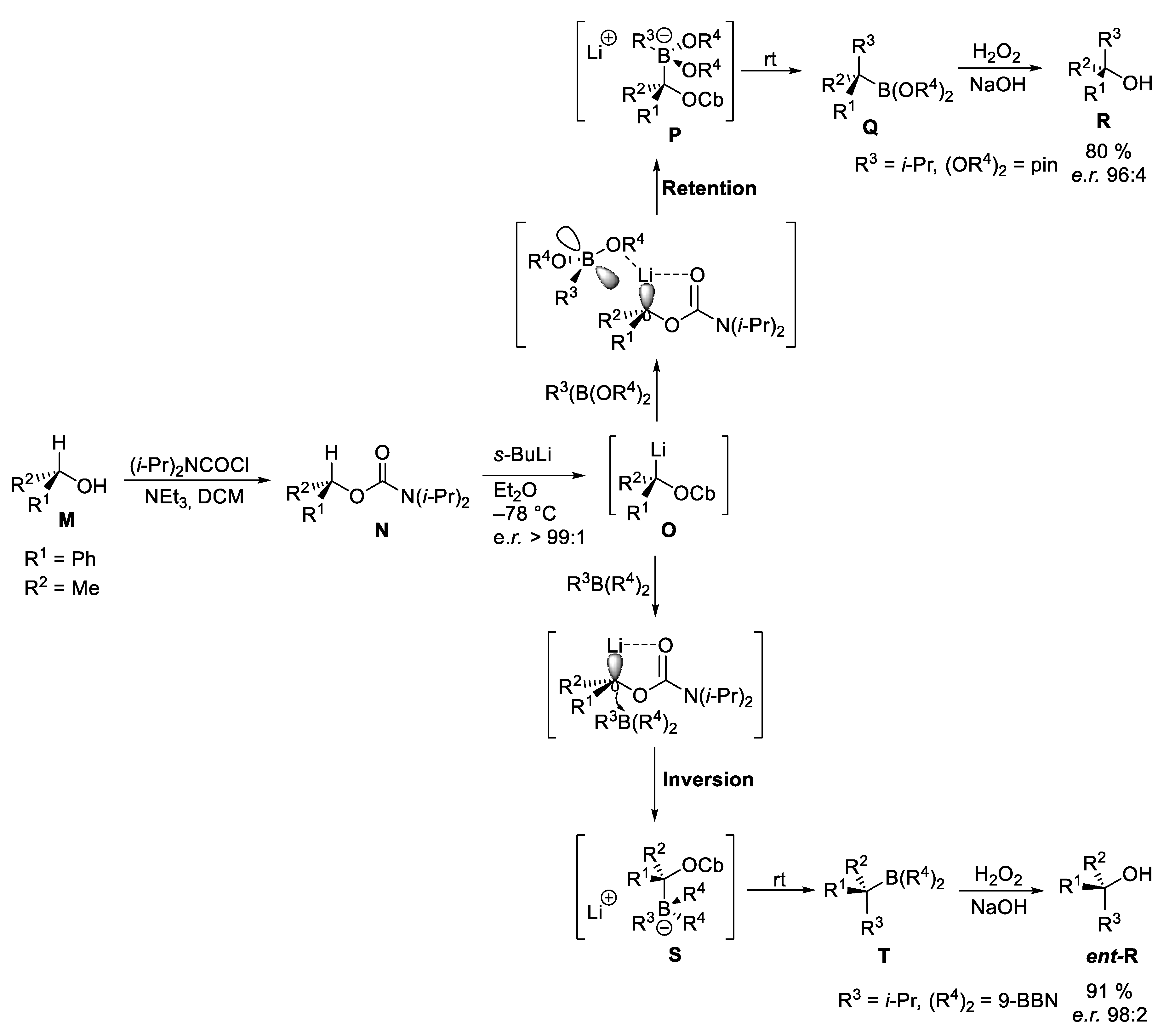
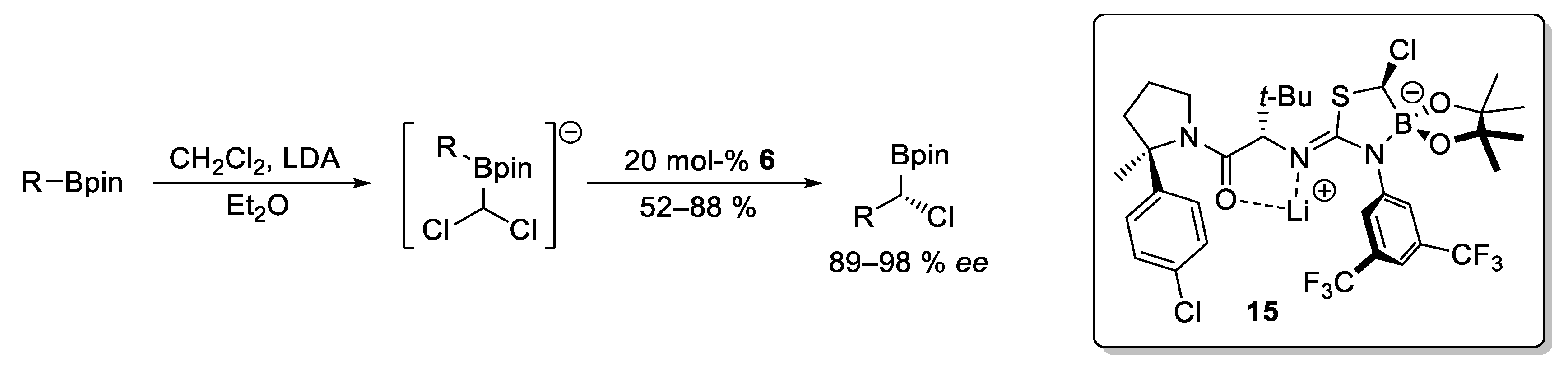
2.3. Catalytic Homologations
3. Syntheses of Marine Natural Products via Matteson Homologation
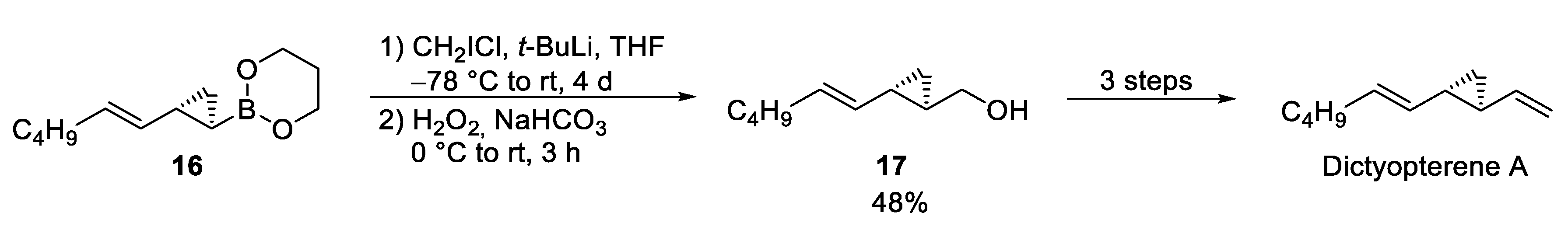
3.1. Dictyopterene A
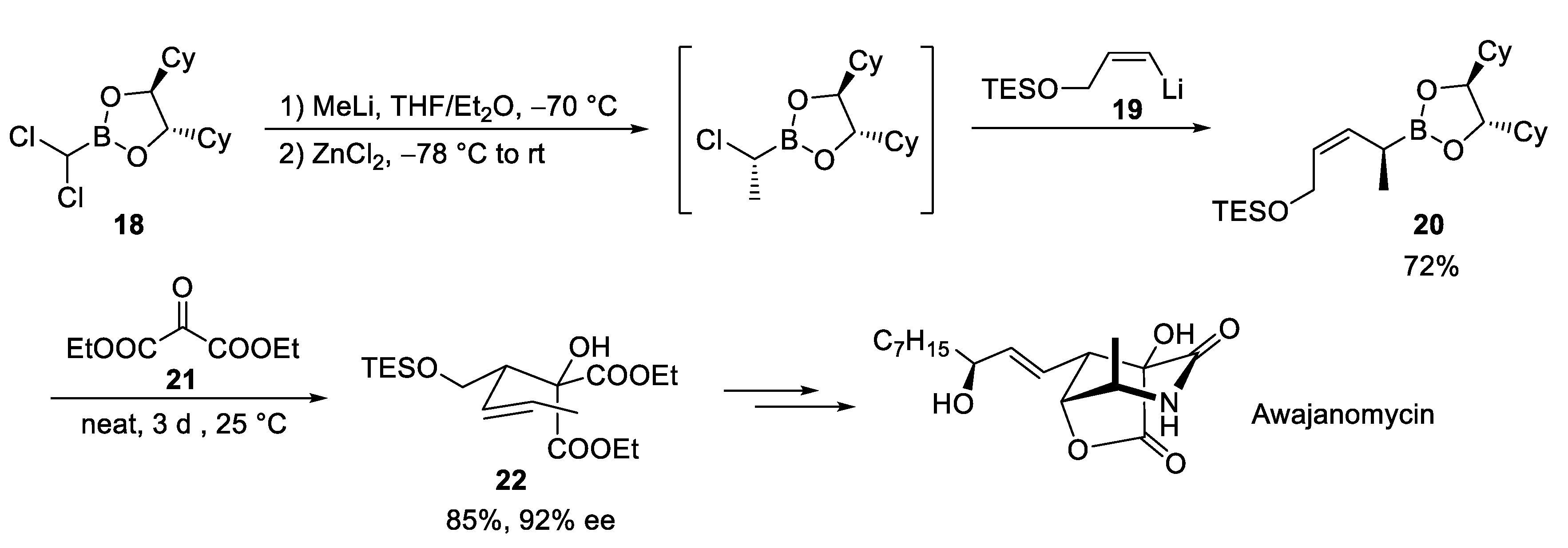
3.2. Awajanomycin
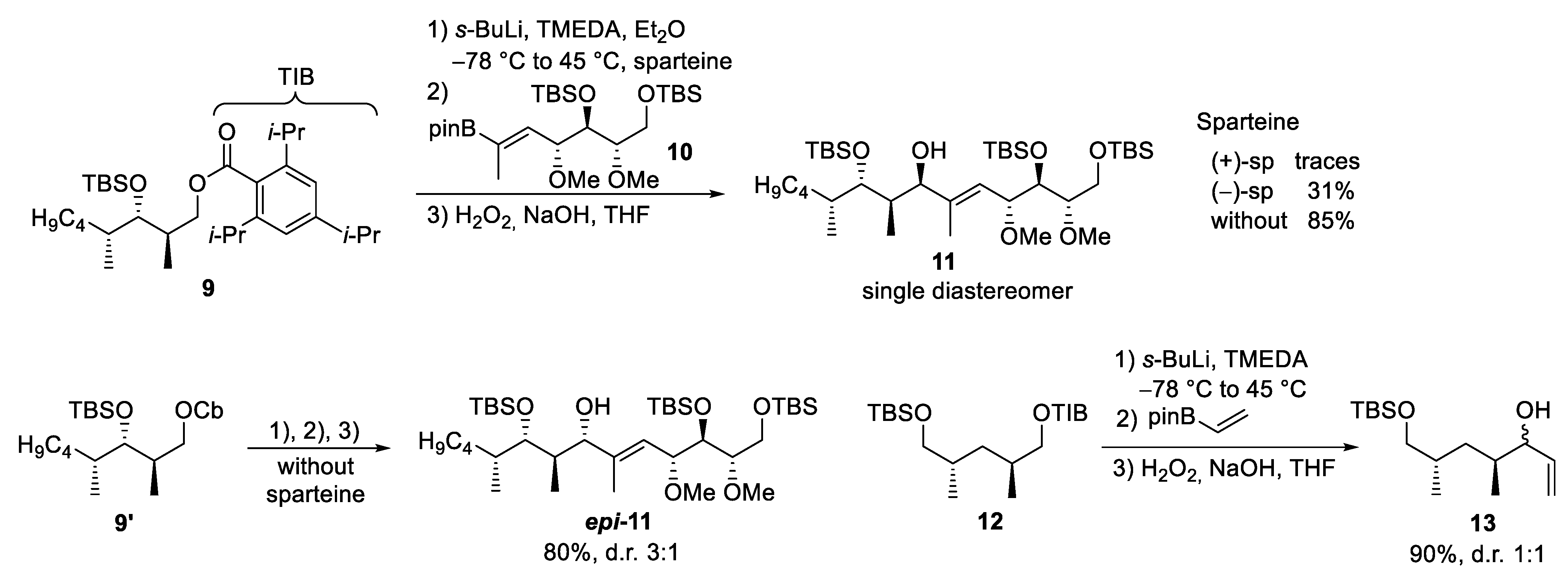
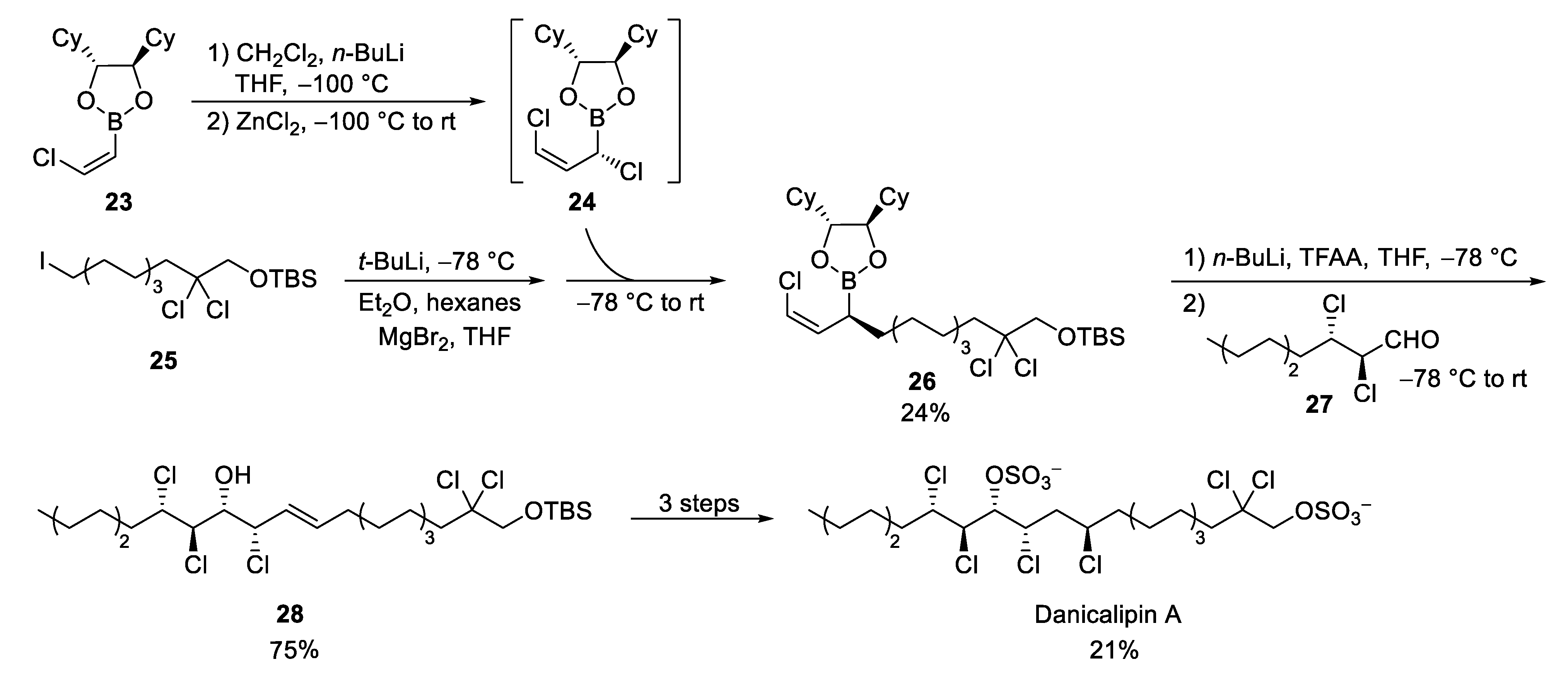
3.3. Danicalipin A
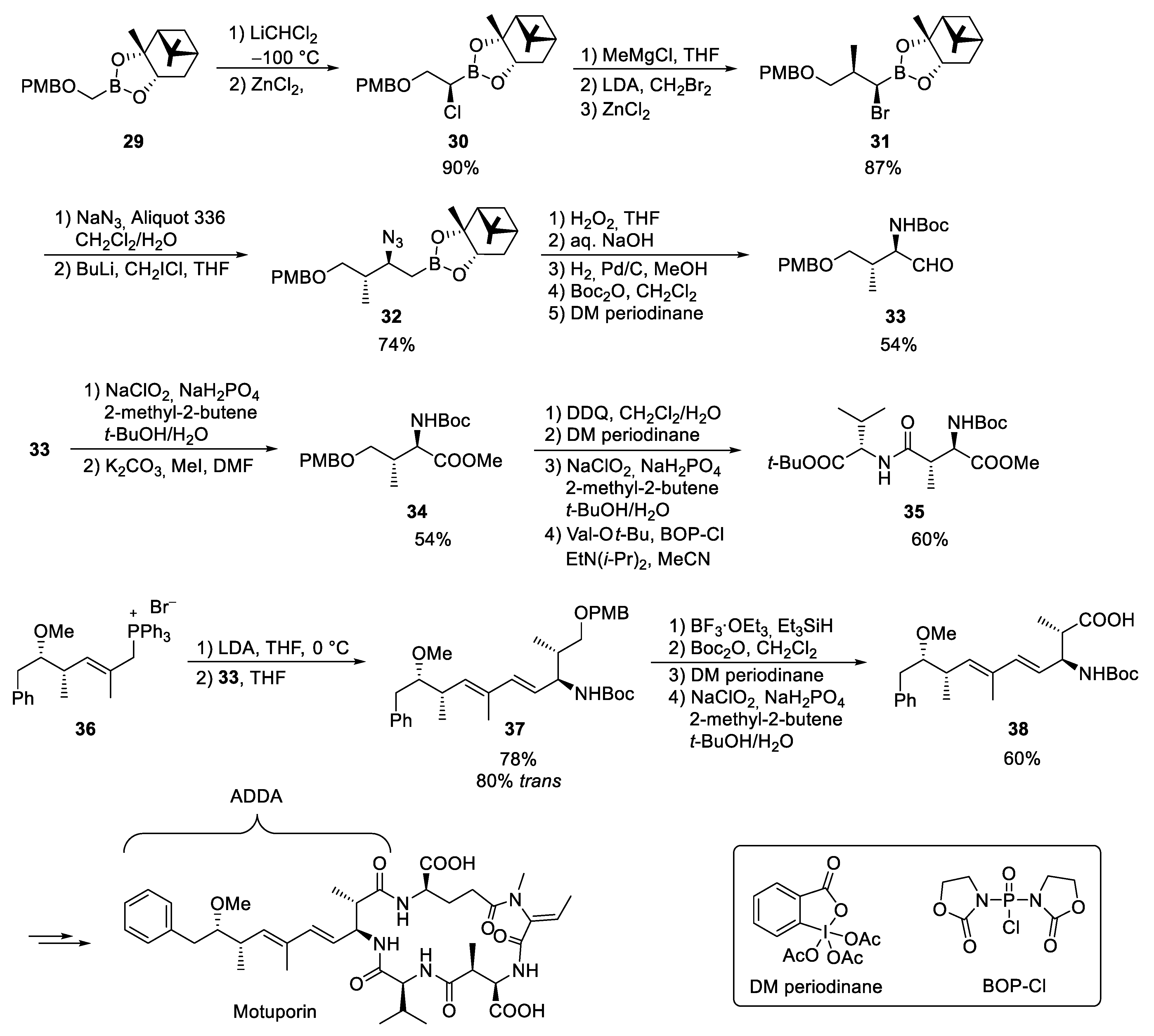
3.4. Motuporin
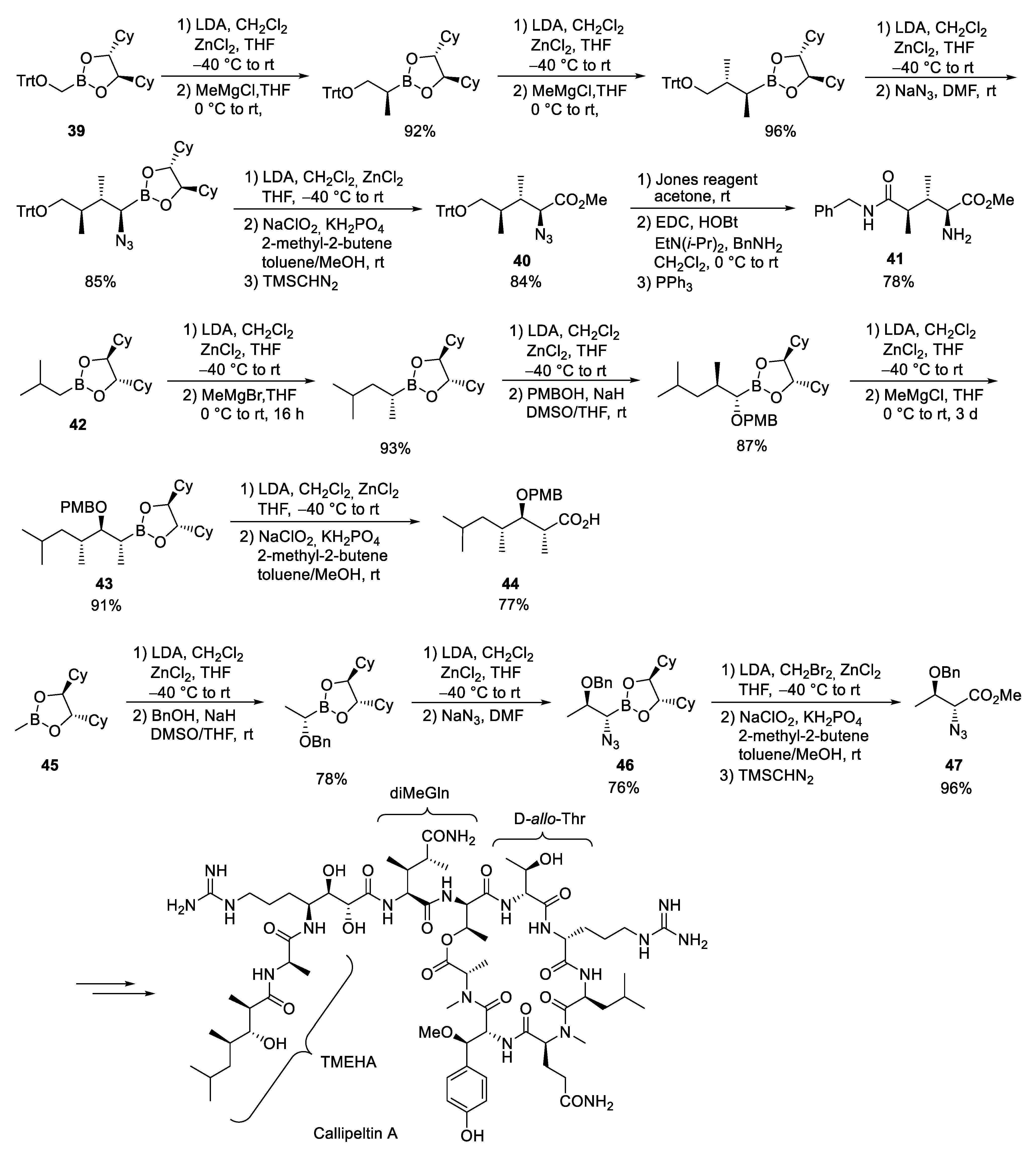
3.5. Callipeltin A
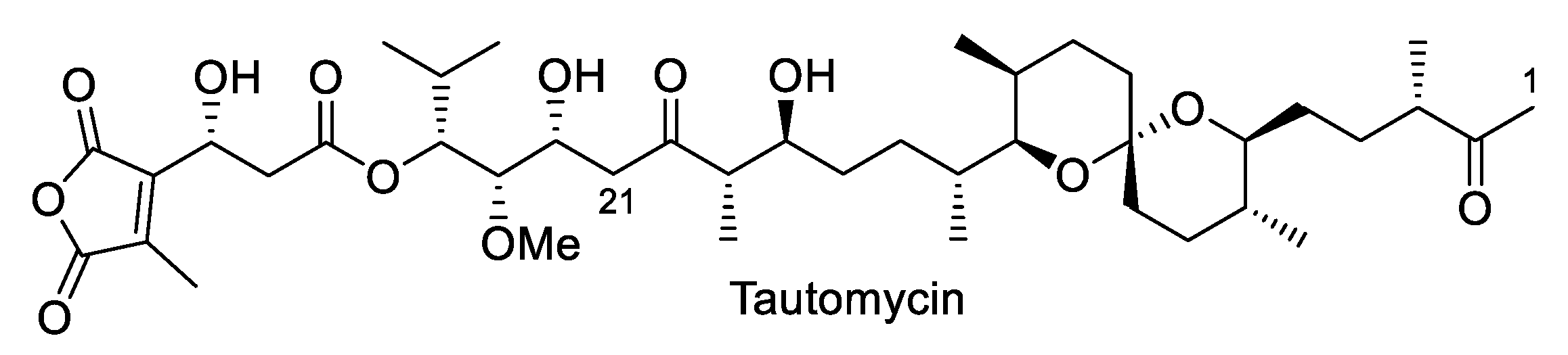
3.6. Tautomycin
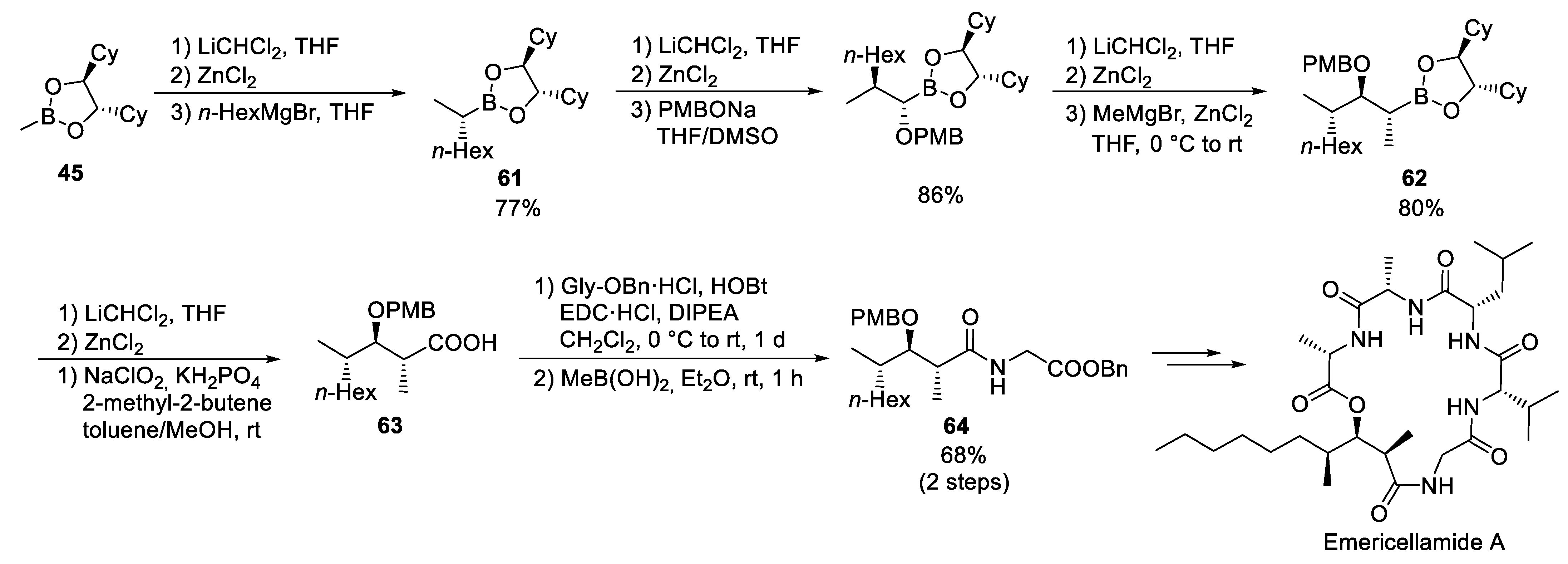
3.7. Emericellamide A
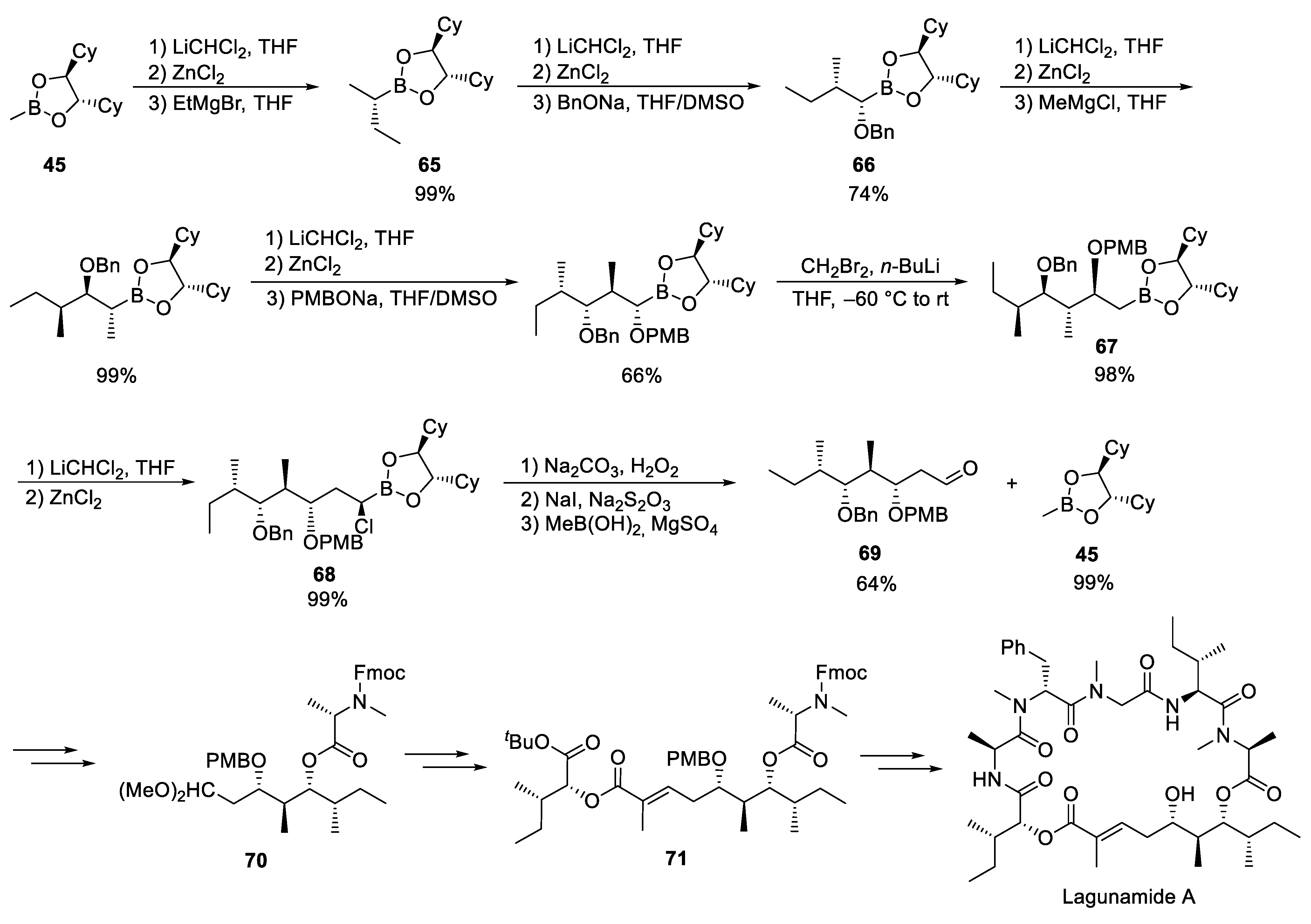
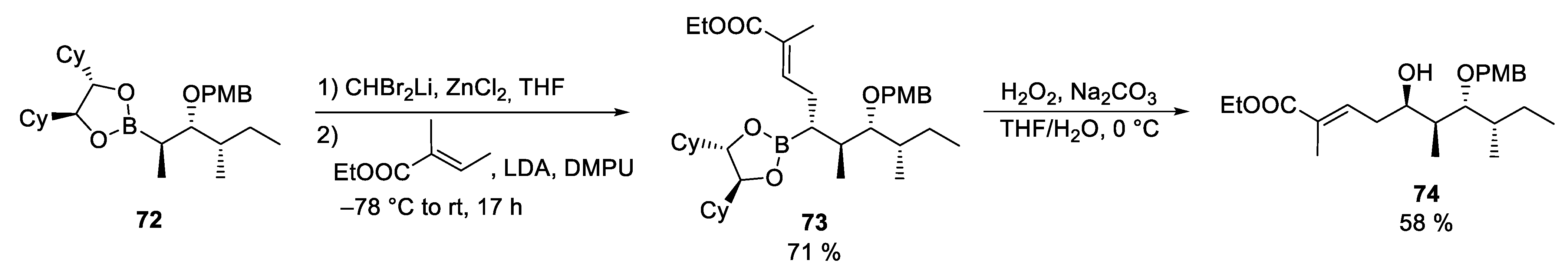
3.8. Lagunamides A
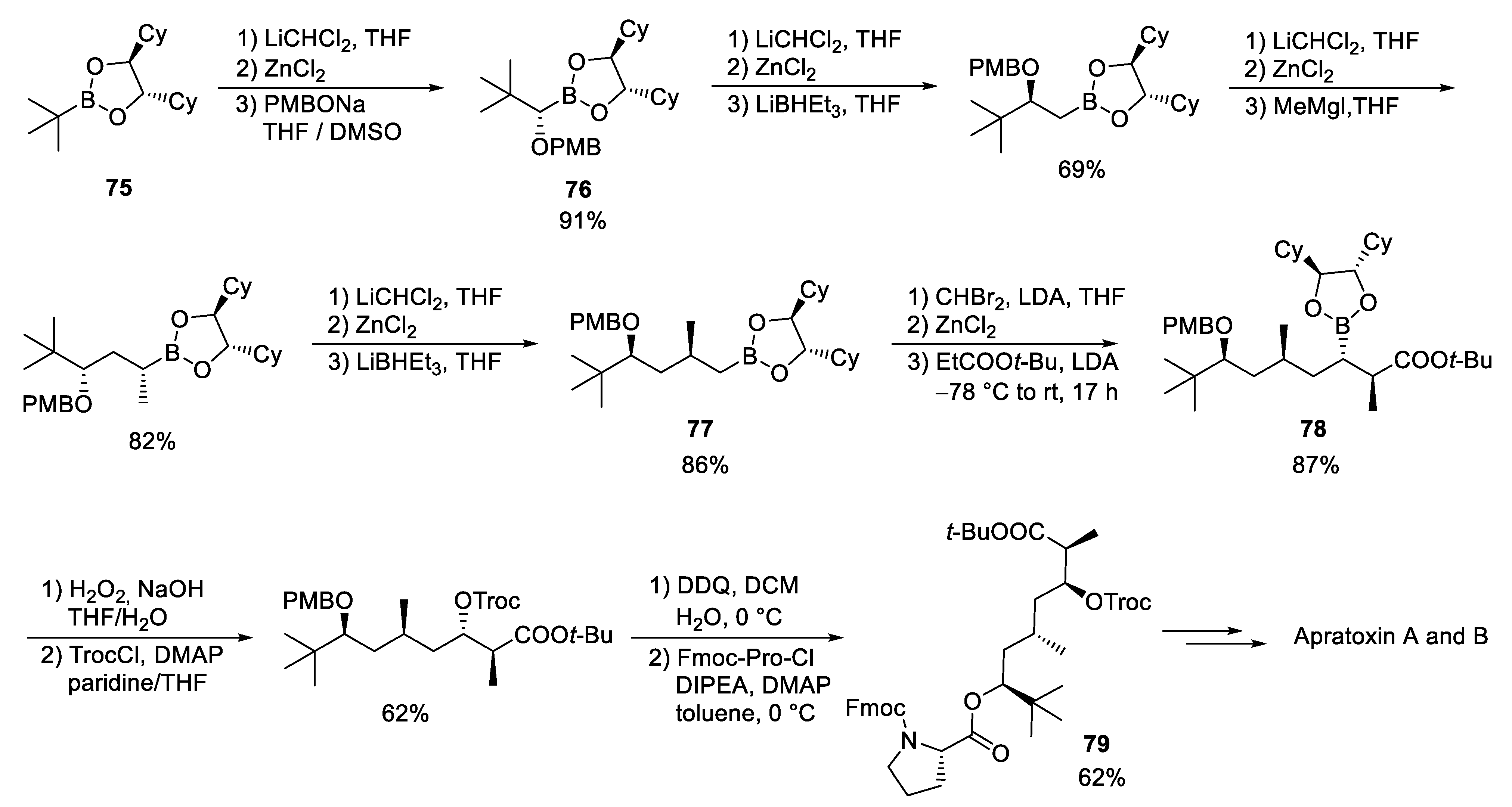
3.9. Apratoxin A and B
3.10. Doliculide
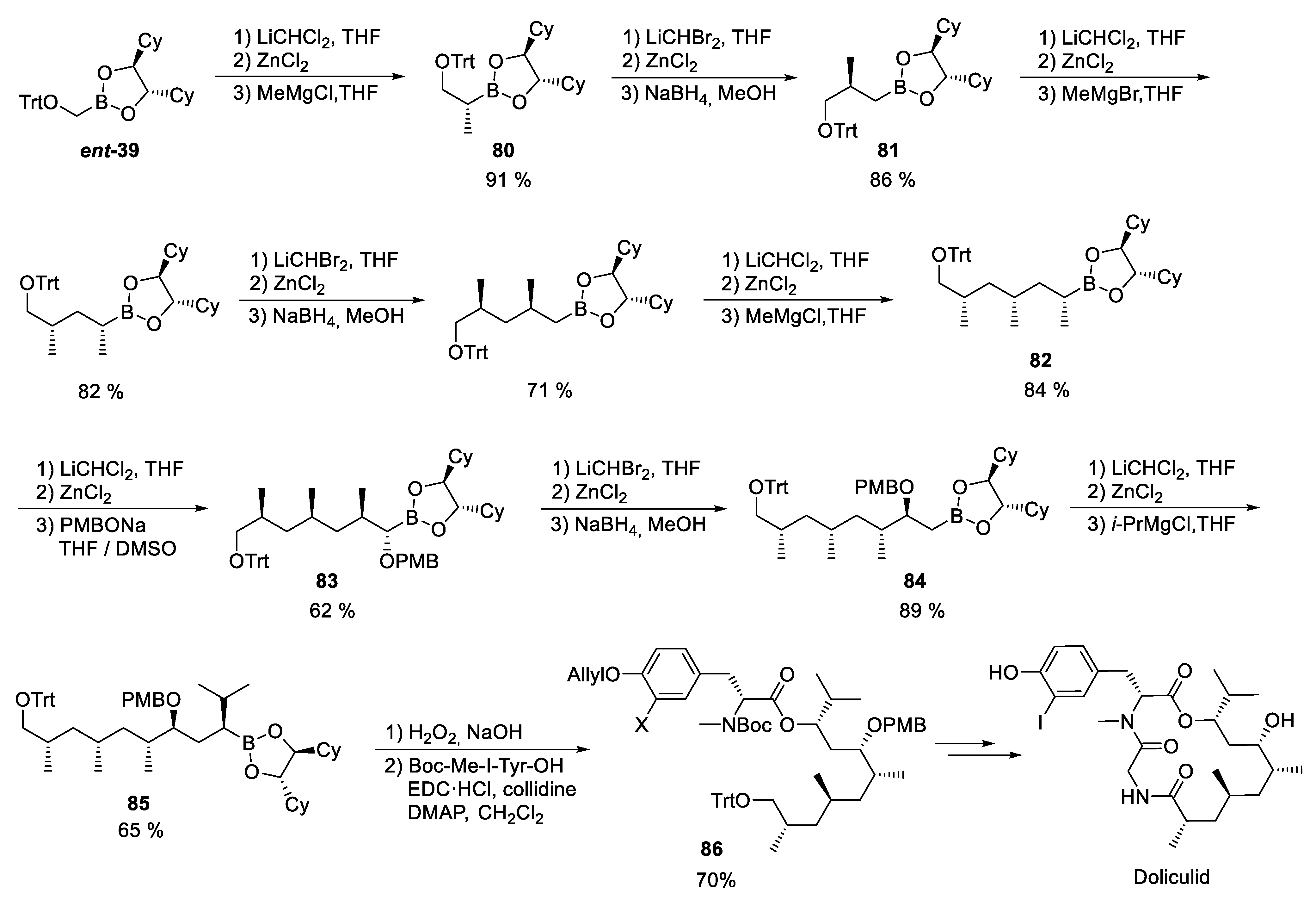
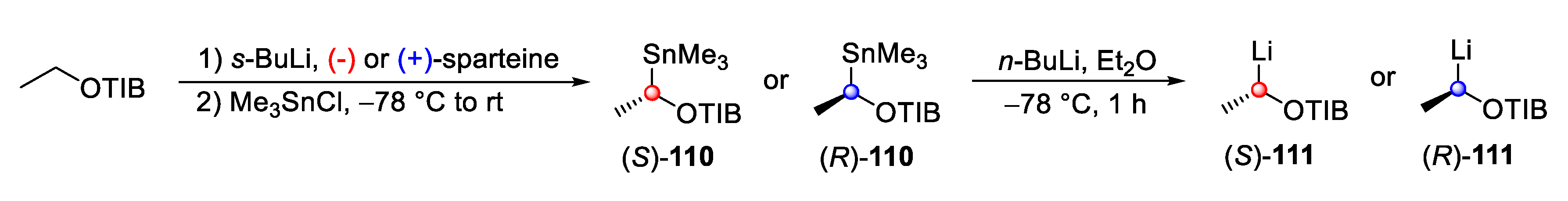
4. Syntheses of Marine Natural Products Using the Lithiation–Borylation Protocol
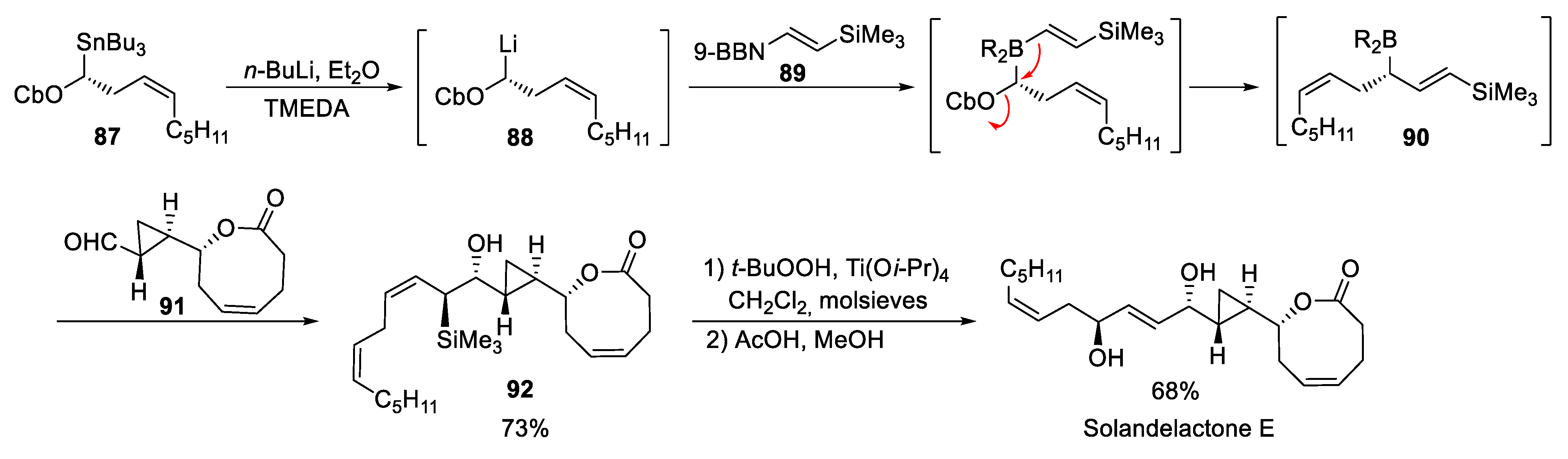
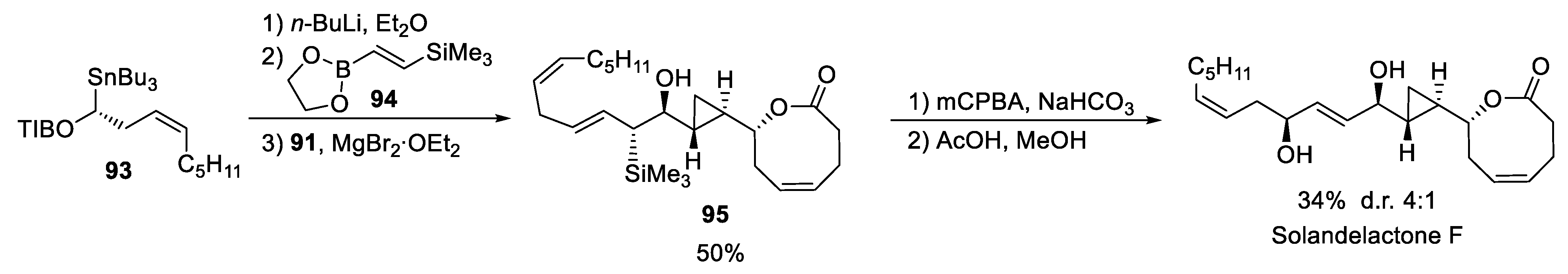
4.1. Solandelactone E and F
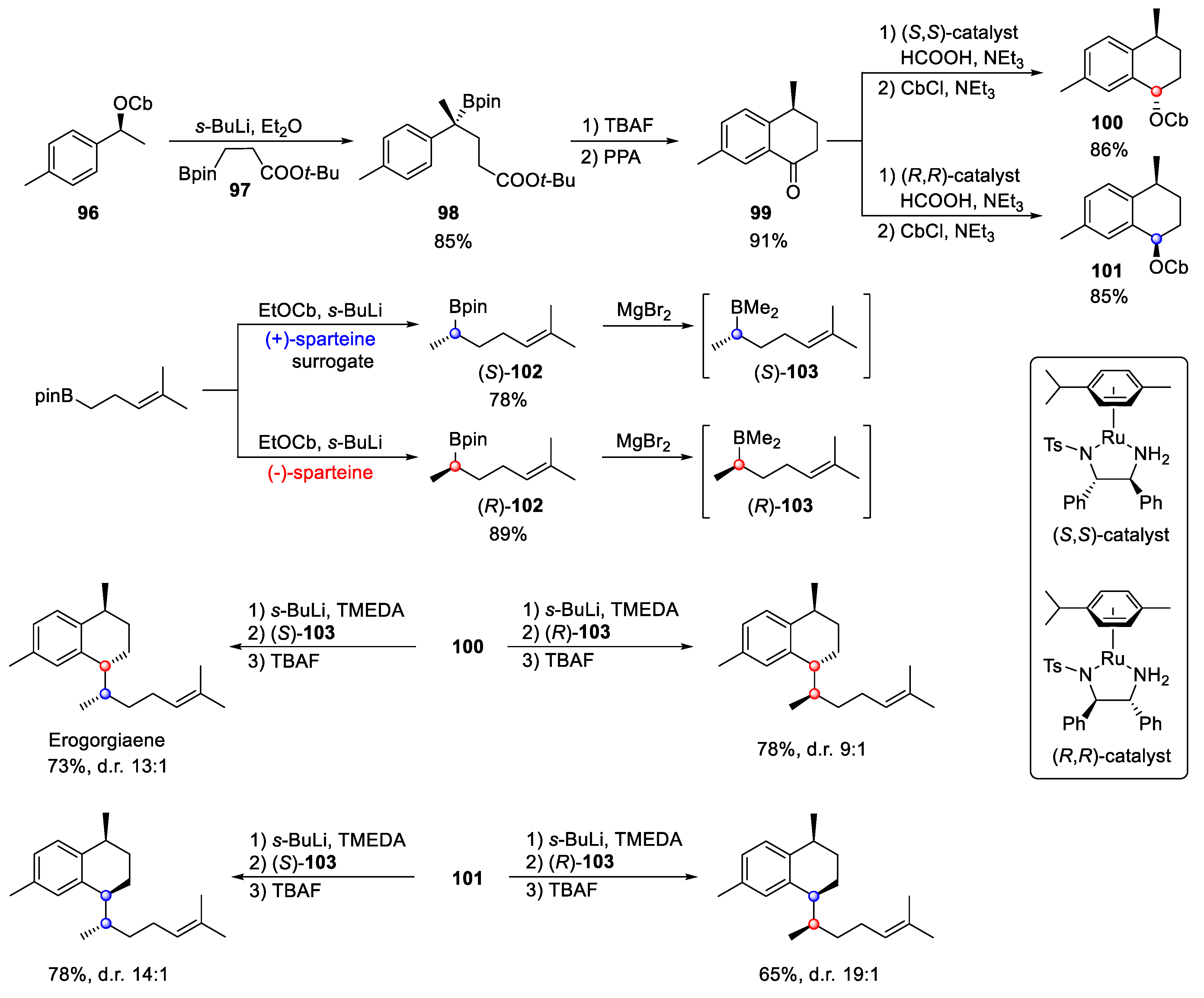
4.2. Erogorgiaene
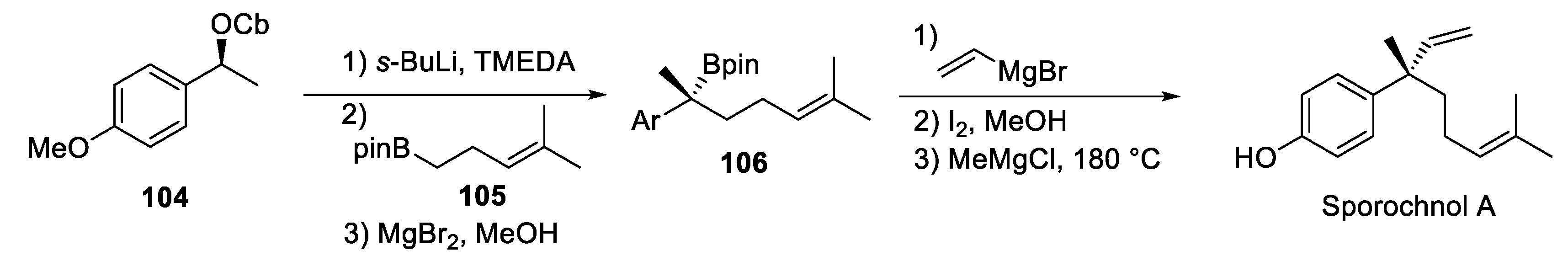
4.3. Sporochnol A
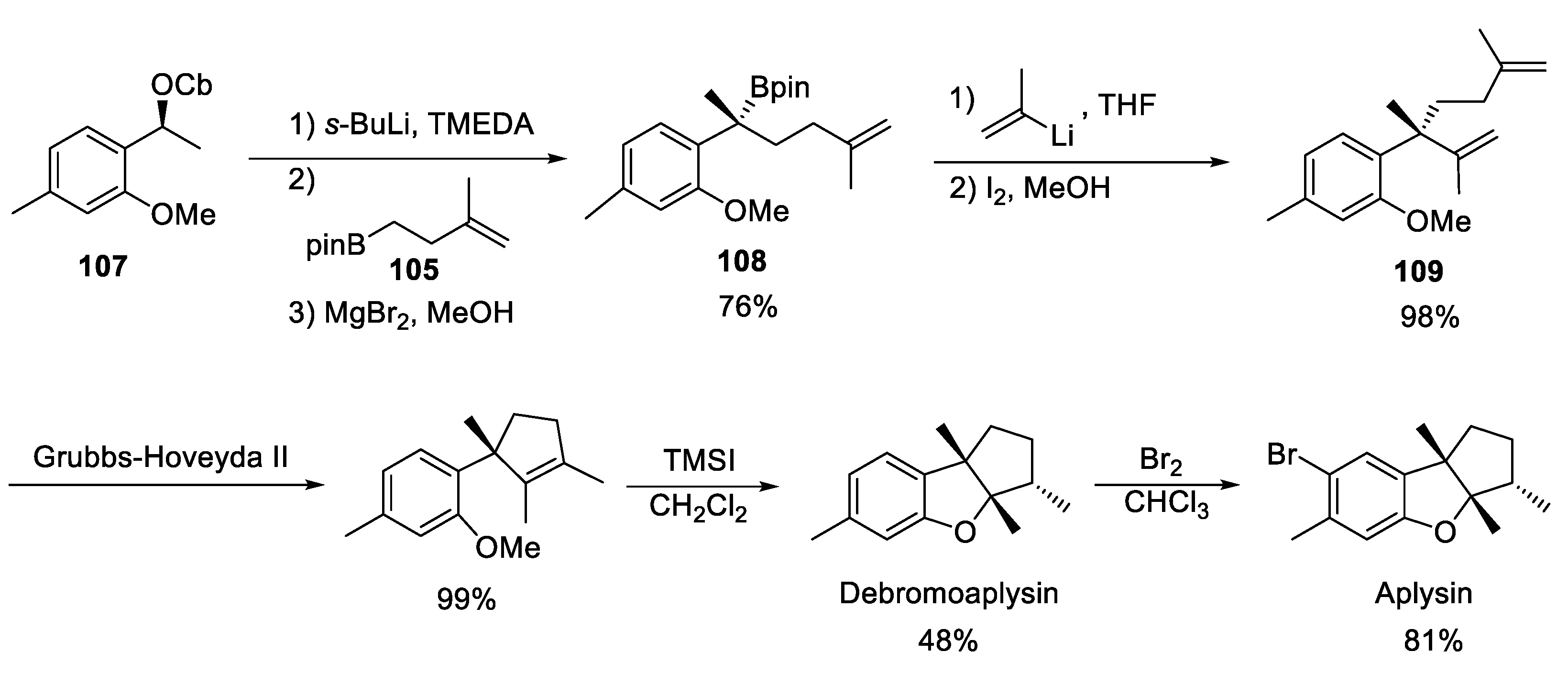
4.4. Aplysin
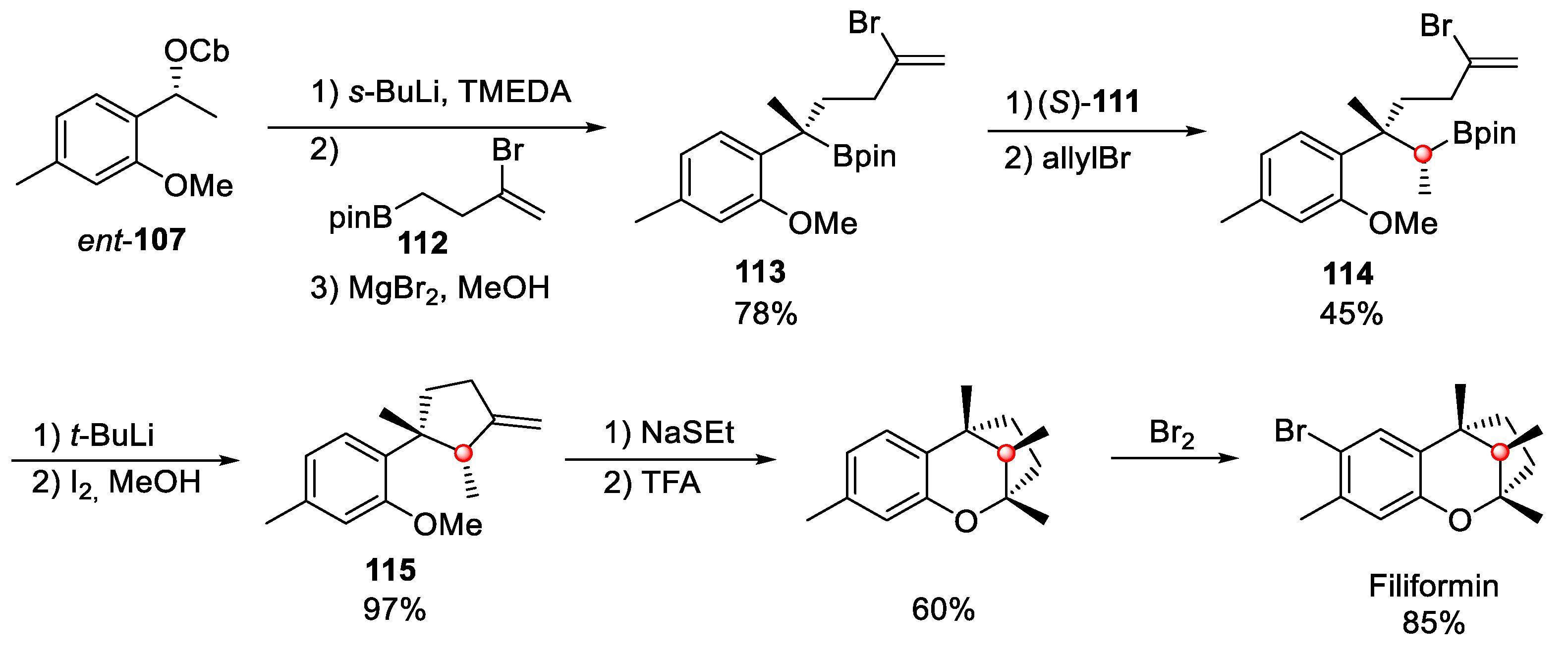
4.5. Filiformin
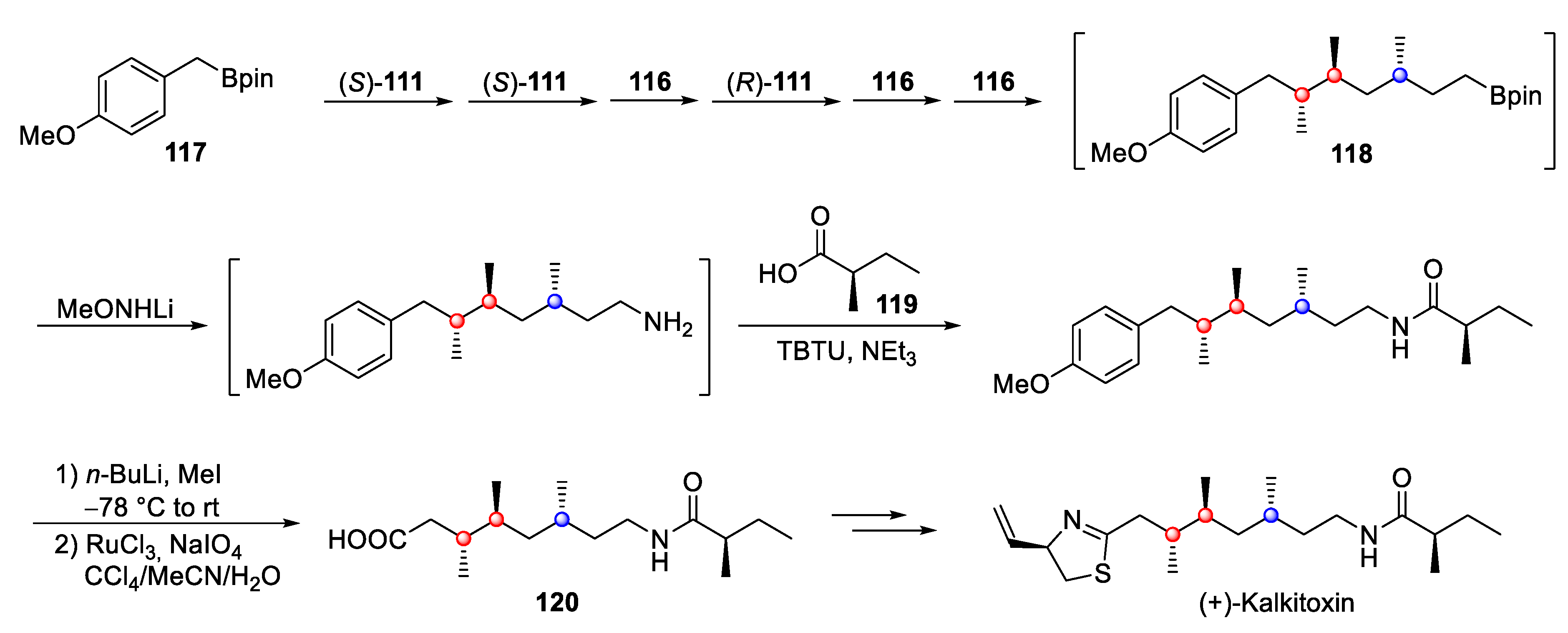
4.6. Kalkitoxin
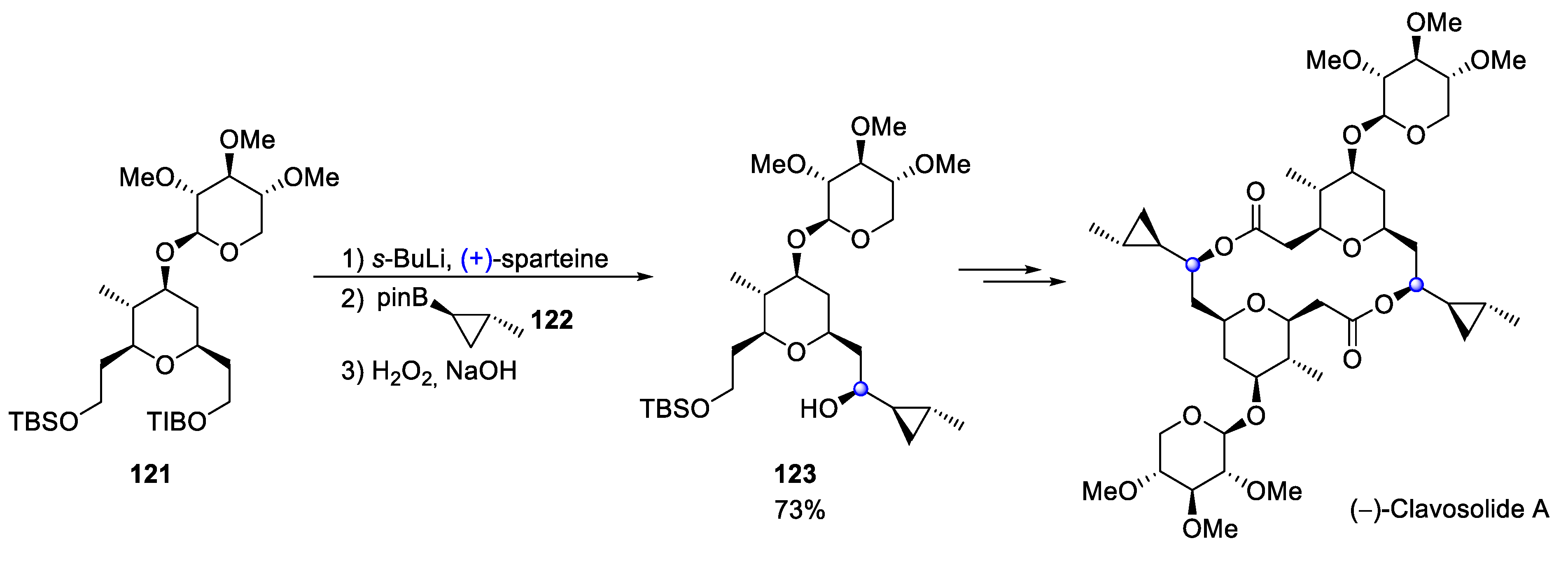
4.7. Clavosolide A
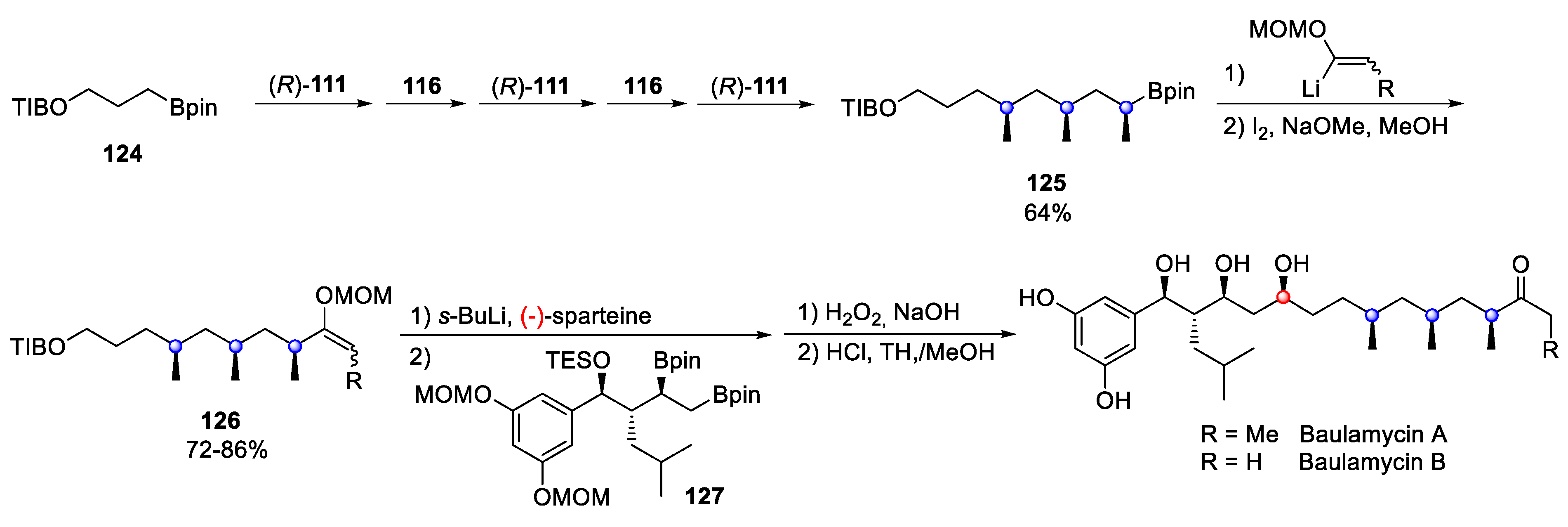
4.8. Baulamycin A and B
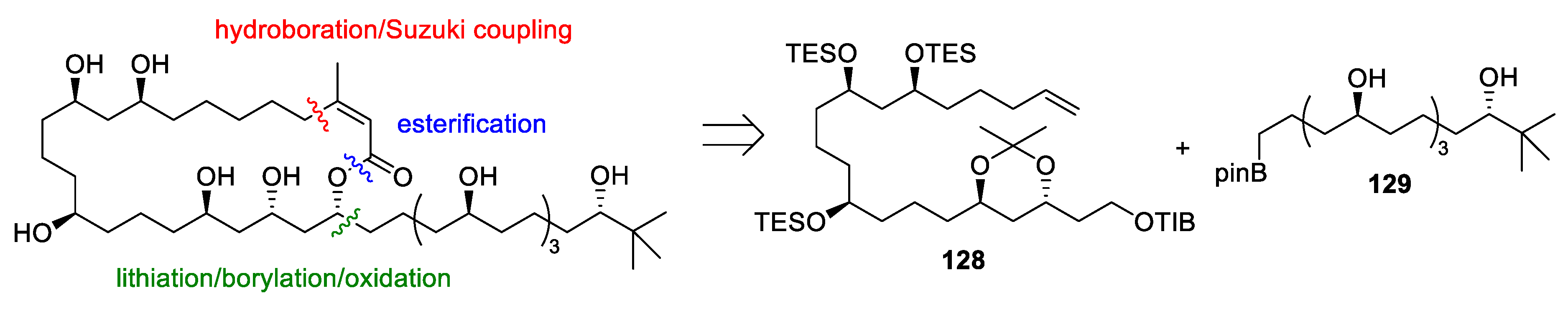
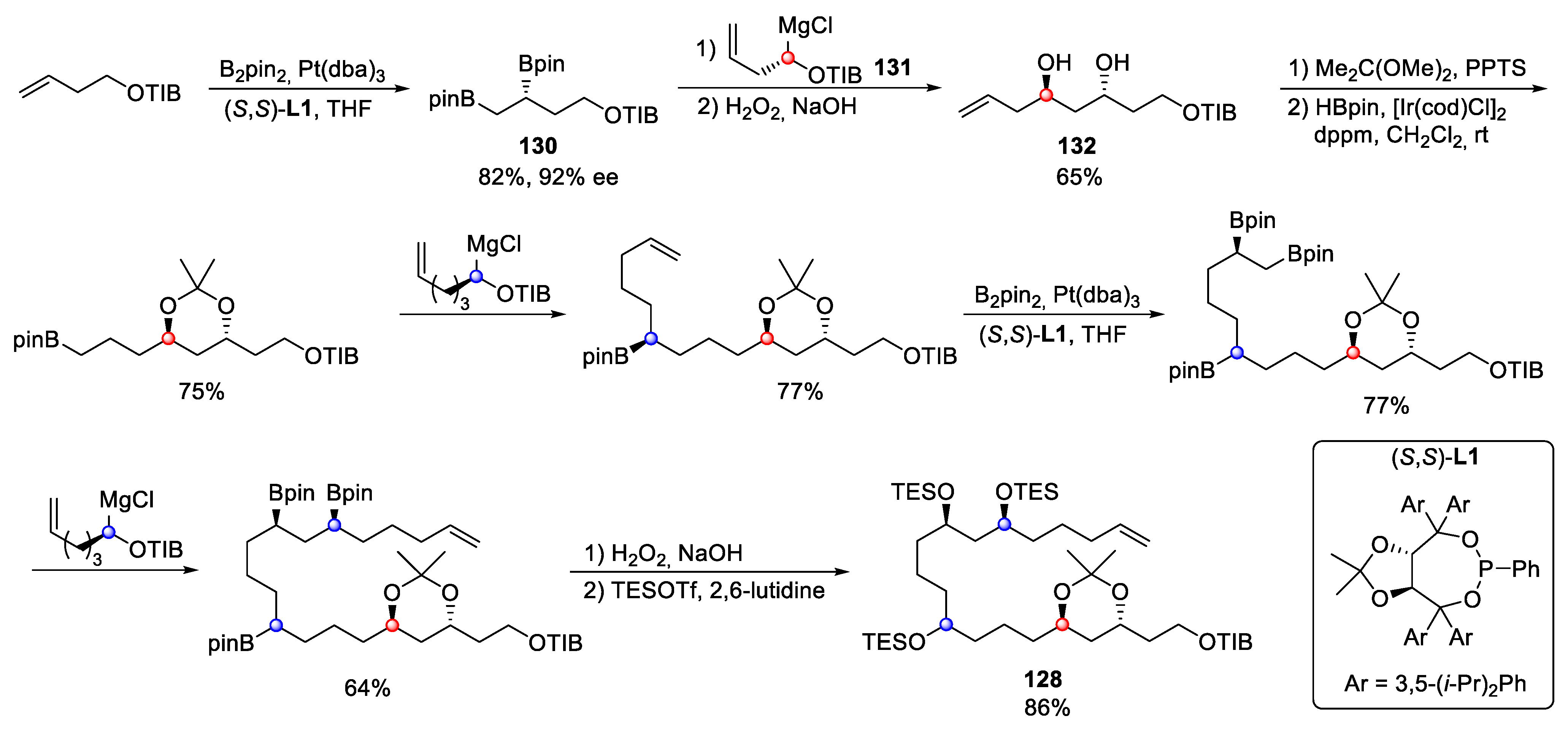
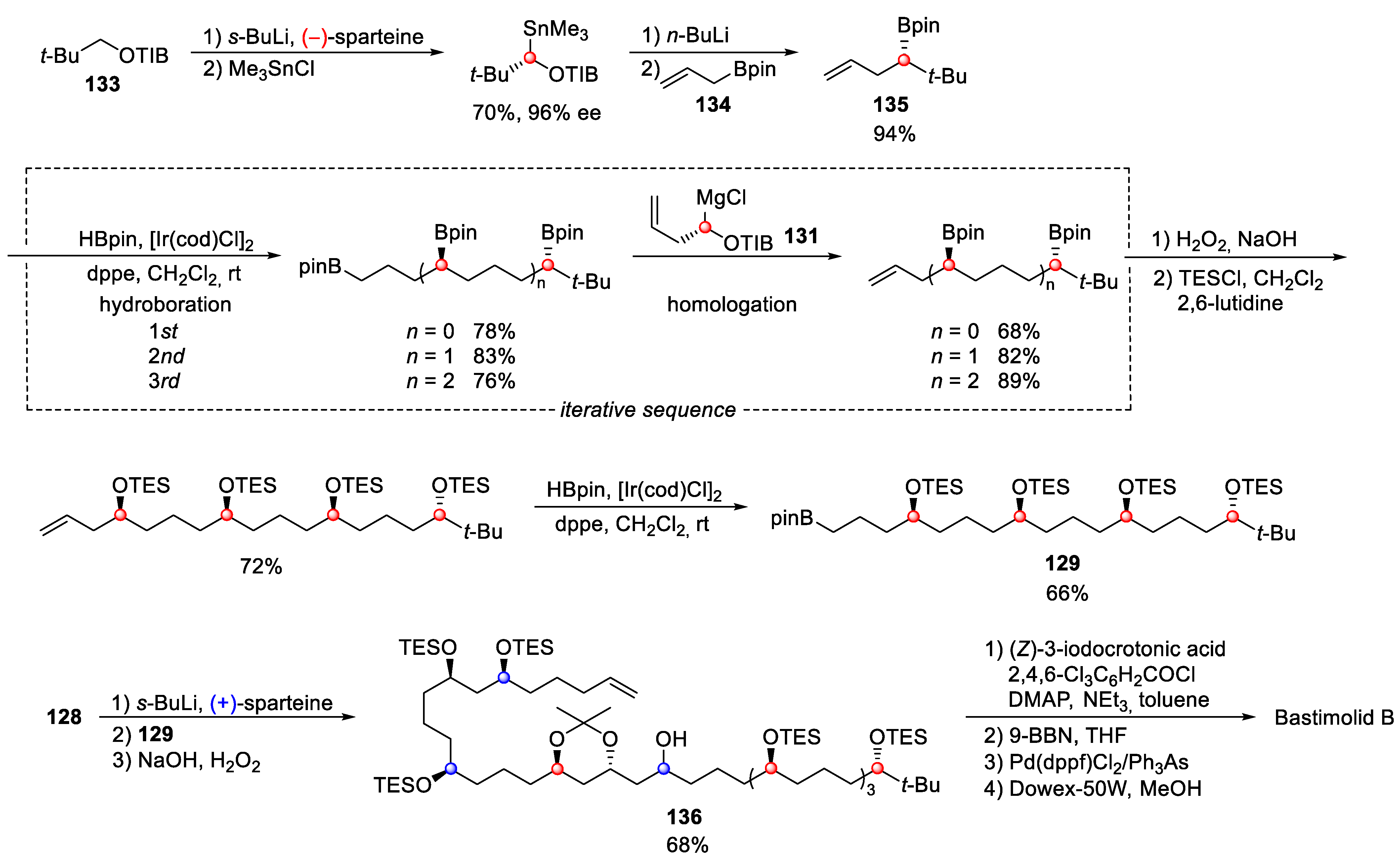
4.9. Bastimolide B
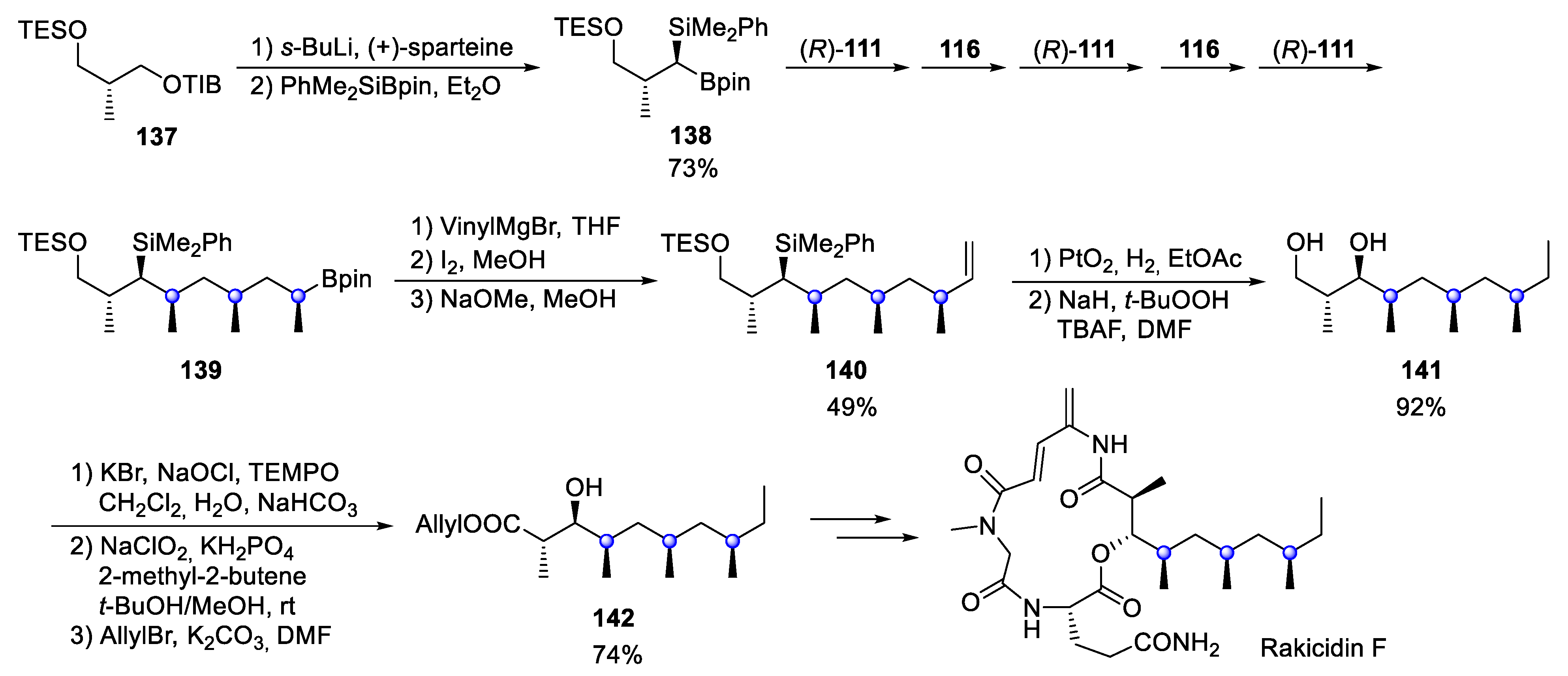
4.10. Rakicidin F
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chintoju, N.; Konduru, P.; Kathula, R.L.; Remella, R. Importance of Natural Products in the Modern History. Res. Rev. J. Hosp. Clin. Pharm. 2015, 1, 5–10. [Google Scholar]
- Hamburger, M.; Hostettmann, K. Bioactivity in Plants: The link between Phytochemistry and Medicine. Phytochemistry 1991, 30, 3864–3874. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; Mcintosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The Odyssey of Marine Pharmaceuticals: A Current Pipeline Perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Mayer, V.A.; Swanson-Mungerson, M.; Pierce, M.L.; Rodríguez, A.D.; Nakamura, F.; Taglialatela-Scafati, O. Marine Pharmacology in 2019–2021: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2024, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Lagunamides A and B: Cytotoxic and Antimalarial Cyclodepsipeptides from the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total Structure Determination of Apratoxin A, a Potent Novel Cytotoxin from the Marine Cyanobacterium Lyngbya majuscule. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef]
- Zampella, A.; D’Auria, M.V.; Gomez Paloma, L.; Casapullo, A.; Minale, L.; Debitus, C.; Henin, Y. Callipeltin A, an Anti-HIV Cyclic Depsipeptide from the New Caledonian Lithistida Sponge Callipelta sp. J. Am. Chem. Soc. 1996, 118, 6202–6209. [Google Scholar] [CrossRef]
- Fuwa, H. Structure Determination, Correction, and Disproof of Marine Macrolide Natural Products by Chemical Synthesis. Org. Chem. Front. 2021, 8, 3990–4023. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M. Catalytic Asymmetric Synthesis of α-Amino Acids. Chem. Rev. 2007, 107, 4584–4671. [Google Scholar] [CrossRef]
- Perdih, A.; Sollner Dolenc, M. Recent Advances in the Synthesis of Unnatural α-Amino Acids. Curr. Org. Chem. 2007, 11, 801–832. [Google Scholar] [CrossRef]
- Troyano, F.J.A.; Merkens, K.; Anwar, K.; Gómez-Suárez, A. Radical-based Synthesis and Modification of Amino Acids. Angew. Chem. Int. Ed. 2021, 60, 1098–1115. [Google Scholar] [CrossRef] [PubMed]
- Paterson, I.; Lam, N.Y.S. Challenges and Discoveries in the Total Synthesis of Complex Polyketide Natural Products. J. Antibiot. 2018, 71, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, T.P.; Lam, N.Y.S.; Anketell, M.J.; Paterson, I. The Stereocontrolled Total Synthesis of Polyketide Natural Products: A Thirty-Year Journey. Bull. Chem. Soc. Jpn. 2021, 94, 713–731. [Google Scholar] [CrossRef]
- Matteson, D.S.; Ray, R. Directed Chiral Synthesis with Pinanediol Boronic Esters. J. Am. Chem. Soc. 1980, 102, 7590–7591. [Google Scholar] [CrossRef]
- Matteson, D.S.; Collins, D.S.L.; Aggarwal, V.K.; Ciganek, E. in The Matteson Reaction. Org. React. 2021, 105, 427–860. [Google Scholar] [CrossRef]
- Matteson, D.S.; Mah, R.W.H. Neighboring Boron in Nucleophilic Displacement. J. Am. Chem. Soc. 1963, 85, 2599–2603. [Google Scholar] [CrossRef]
- Matteson, D.S.; Majumdar, D. α-Chloro boronic esters from homologation of boronic esters. J. Am. Chem. Soc. 1980, 102, 7588–7590. [Google Scholar] [CrossRef]
- Matteson, D.S.; Majumdar, D. Homologation of Boronic Esters to α-Chloro Boronic Esters. Organometallics 1983, 2, 1529–1535. [Google Scholar] [CrossRef]
- Matteson, D.S.; Peterson, M.L. Synthesis of L-(+)-Ribose via (S)-Pinanediol (αS)-α-Bromo Boronic Esters. J. Org. Chem. 1987, 52, 5116–5121. [Google Scholar] [CrossRef]
- Matteson, D.S.; Sadhu, K.M.; Peterson, M.L. 99% Chirally Selective Syntheses via Pinanediol Boronic Esters: Insect Pheromones, Diols, and an Amino Alcohol. J. Am. Chem. Soc. 1986, 108, 810–819. [Google Scholar] [CrossRef]
- Matteson, D.S.; Michnick, T.J. Stereoselective Reaction of an Enolate with Chiral α-Halo Boronic Acid Esters. Organometallics 1990, 9, 3171–3177. [Google Scholar] [CrossRef]
- Matteson, D.S. Boronic Esters in Asymmetric Synthesis. J. Org. Chem. 2013, 78, 10009–10023. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Matteson, D.S. Osmium tetroxide catalyzed hydroxylation of hindered olefins. Tetrahedron Lett. 1980, 21, 449–450. [Google Scholar] [CrossRef]
- Matteson, D.S.; Kandil, A.A. (S,S)-Diisopropylethanediol (diped)—A new chiral director for the α-chloro boronic ester synthesis. Tetrahedron Lett. 1986, 27, 3831–3834. [Google Scholar] [CrossRef]
- Matteson, D.S.; Beedle, E.C.; Kandil, A.A. Preparation of (3S,4S)-2,5-dimethyl-3,4-hexanediol [(S)-DIPED] from (R,R)-tartaric acid via trimethylsilyl chloride catalyzed acetylation of a hindered 1,4-diol. J. Org. Chem. 1987, 52, 5034–5036. [Google Scholar] [CrossRef]
- Hoffmann, R.W.; Ditrich, K.; Köster, G.; Stürmer, R. Stereoselective synthesis of alcohols, XXXI: Stereoselective C–C bond formation using chiral (Z)-pentenylboronates. Chem. Ber. 1989, 122, 1783–1789. [Google Scholar] [CrossRef]
- Hiscox, W.C.; Matteson, D.S. An Efficient Preparation of (R*,R*)-1,2-Dicyclohexylethane-1,2-diol, a Superior Chiral Director for Synthesis with Boronic Esters. J. Org. Chem. 1996, 61, 8315–8316. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Barnes-Seeman, D.; Lee, T.W. The mechanistic basis for diastereoselectivity in the Matteson rearrangement. Tetrahedron Asymmetry 1997, 8, 3711–3713. [Google Scholar] [CrossRef]
- Midland, M.M. Ab Initio Investigation of the Transition State for Asymmetric Synthesis with Boronic Esters. J. Org. Chem. 1998, 63, 914–915. [Google Scholar] [CrossRef]
- Tripathy, P.B.; Matteson, D.S. Asymmetric Synthesis of the Four Stereoisomers of 4-Methyl-3-heptanol via Boronic Esters: Sequential Double Stereodifferentiation Leads to Very High Purity. Synthesis 1990, 1990, 200–206. [Google Scholar] [CrossRef]
- Rangaishenvi, M.V.; Singaram, B.; Brown, H.C. Chiral Synthesis via Organoboranes. 30. Facile Synthesis, by the Matteson Asymmetric Homologation Procedure, of α-Methyl Boronic Acids Not Available from Asymmetric Hydroboration and Their Conversion into the Corresponding Aldehydes, Ketones, Carboxylic Acids, and Amines of High Enantiomeric Purity. J. Org. Chem. 1991, 56, 3286–3294. [Google Scholar] [CrossRef]
- Matteson, D.S.; Man, H.-W.; Ho, O.C. Asymmetric Synthesis of Stegobinone via Boronic Ester Chemistry. J. Am. Chem. Soc. 1996, 118, 4560–4566. [Google Scholar] [CrossRef]
- Villieras, J.; Bacquet, C.; Masure, D.; Normant, J.F. Les carbénoïdes lithiens dibromés et leur intérêt synthétique. J. Organomet. Chem. 1973, 50, C7–C11. [Google Scholar] [CrossRef]
- Matteson, D.S.; Man, H.W. Enantioselective capture and retroracemization of (1-bromoalkyl)boronic esters by an n-propanoyloxazolidinone enolate and iodide-ion. J. Org. Chem. 1994, 59, 5734–5741. [Google Scholar] [CrossRef]
- Sadhu, K.M.; Matteson, D.S. (Chloromethyl) lithium: Efficient generation and capture by boronic esters and a simple preparation of diisopropyl (chloromethyl) boronate. Organometallics 1985, 4, 1687–1689. [Google Scholar] [CrossRef]
- Brown, H.C.; Singh, S.M.; Rangaishenvi, M.V. Organoboranes. 46. New Procedures for the Homologation of Boronic Esters: A Critical Examination of the Available Procedures to Achieve Convenient Homologation of Boronic Esters. J. Org. Chem. 1986, 51, 3150–3155. [Google Scholar] [CrossRef]
- Michnick, T.J.; Matteson, D.S. (Bromomethyl) lithium: Efficient in situ reactions. Synlett 1991, 1991, 631–632. [Google Scholar] [CrossRef]
- Gorovoy, A.S.; Gozhina, O.; Svendsen, J.-S.; Tetz, G.V.; Domorad, A.; Tetz, V.V.; Lejon, T. Syntheses and anti-tubercular activity of β-substituted and α,β-disubstituted peptidyl β-aminoboronates and boronic acids. J. Pept. Sci. 2013, 19, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Gorovoy, A.S.; Gozhina, O.; Svendsen, J.-S.; Domorad, A.A.; Tetz, G.V.; Tetz, V.V.; Lejon, T. Boron-Containing Peptidomimetics—A Novel Class of Selective Anti-tubercular Drugs. Chem. Biol. Drug Des. 2013, 81, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Matteson, D.S.; Soundararajan, R.; Ho, O.C.; Gatzweiler, W. (Alkoxyalkyl)boronic Ester Intermediates for Asymmetric Synthesis. Organometallics 1996, 15, 152–163. [Google Scholar] [CrossRef]
- Tost, M.; Andler, O.; Kazmaier, U. A Matteson Homologation-Based Synthesis of Doliculide and Derivatives. Eur. J. Org. Chem. 2021, 2021, 6459–6471. [Google Scholar] [CrossRef]
- Matteson, D.S.; Kandil, A.A.; Soundararajan, R. Synthesis of Asymmetrically Deuterated Glycerol and Dibenzylglyceraldehyde via Boronic Esters. J. Am. Chem. Soc. 1990, 112, 3964–3969. [Google Scholar] [CrossRef]
- Matteson, D.S. Asymmetric synthesis with boronic esters. Acc. Chem. Res. 1988, 21, 294–300. [Google Scholar] [CrossRef]
- Matteson, D.S. Boronic Esters in Stereodirected Synthesis. Tetrahedron 1989, 45, 1859–1885. [Google Scholar] [CrossRef]
- Matteson, D.S.; Wan, H.-W. Hydrolysis of Substituted 1,3,2-Dioxaborolanes and an Asymmetric Synthesis of a Differently Protected Syn,syn-3-Methyl-2,4-hexanediol. J. Org. Chem. 1996, 61, 6047–6051. [Google Scholar] [CrossRef]
- Matteson, D.S.; Kandil, A.A. Conversion of α-halo boronic esters to inverted α-(methylsulfonyl)oxy boronic esters. J. Org. Chem. 1987, 52, 5121–5124. [Google Scholar] [CrossRef]
- Stymiest, J.L.; Dutheuil, G.; Mahmood, A.; Aggarwal, V.K. Lithiated Carbamates: Chiral Carbenoids for Iterative Homologation of Boranes and Boronic Esters. Angew. Chem. Int. Ed. 2007, 46, 7491–7494. [Google Scholar] [CrossRef]
- Hoppe, D.; Hintze, F.; Tebben, P.; Paetow, M.; Ahrens, H.; Schwerdtfeger, J.; Sommerfeld, P.; Haller, J.; Guarnieri, W.; Kolczewski, S.; et al. Enantioselective synthesis via sparteine-induced asymmetric deprotonation. Pure App. Chem. 1994, 66, 1479–1486. [Google Scholar] [CrossRef]
- Hoppe, D.; Hense, T. Enantioselective Synthesis with Lithium/(−)-Sparteine Carbanion Pairs. Angew. Chem. Int. Ed. Engl. 1997, 36, 2282–2316. [Google Scholar] [CrossRef]
- Hoppe, D.; Hintze, F.; Tebben, P. Chiral Lithium-1-oxyalkanides by Asymmetric Deprotonation; Enantioselective Synthesis of 2-Hydroxyalkanoic Acids and Secondary Alkanols. Angew. Chem. Int. Ed. 1990, 29, 1422–1424. [Google Scholar] [CrossRef]
- Beckmann, E.; Desai, V.; Hoppe, D. Stereospecific Reaction of α-Carbamoyloxy-2-alkenylboronates and α-Carbamoyloxy-alkylboronates with Grignard Reagents—Synthesis of Highly Enantioenriched Secondary Alcohols. Synlett 2004, 2275–2280. [Google Scholar] [CrossRef]
- Beckmann, E.; Hoppe, D. Synthesis of an Enantioenriched α-Carbamoyloxy-crotylboronate and Its Homoaldol Reaction with Aldehydes. Synthesis 2005, 2005, 217–222. [Google Scholar] [CrossRef]
- Beak, P.; Baillargeon, M.; Carter, L.G. Lithiation of Ethyl 2,4,6-Triisopropylbenzoate Adjacent to Oxygen: The a-Lithioalkyl Alcohol Synthon. J. Org. Chem. 1978, 43, 4255–4256. [Google Scholar] [CrossRef]
- Dearden, M.J.; Firkin, C.R.; Hermet, J.-P.R.; O’Brien, P. A Readily-Accessible (+)-Sparteine Surrogate. J. Am. Chem. Soc. 2002, 124, 11870–11871. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.D.; Canipa, S.J.; Ferris, L.; O’Brien, P. Gram-Scale Synthesis of the (−)-Sparteine Surrogate and (−)-Sparteine. Angew. Chem. Int. Ed. 2018, 57, 223–226. [Google Scholar] [CrossRef]
- Leonori, D.; Aggarwal, V.K. Lithiation−Borylation Methodology and Its Application in Synthesis. Acc. Chem. Res. 2014, 47, 3174–3183. [Google Scholar] [CrossRef]
- Linne, Y.; Schönwald, A.; Weißbach, S.; Kalesse, M. Desymmetrization of C2-Symmetric Bis(Boronic Esters) by Zweifel Olefinations. Chem. Eur. J. 2020, 26, 7998–8002. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, D.; Carstens, A.; Krämer, T. Generation of a Configurationally Stable Chiral Benzyllithium Derivative, and the Capricious Stereochemistry of Its Electrophilic Substitution. Angew. Chem. Int. Ed. 1990, 29, 1424–1425. [Google Scholar] [CrossRef]
- Derwing, C.; Hoppe, D. Synthesis of Enantioenriched Tertiary Benzylic Alcohols via Stereospecific Lithiation of Secondary Benzyl Carbamates—Design of Dialkylcarbamates, Cleavable under Basic, Mild Conditions. Synthesis 1996, 1996, 149–154. [Google Scholar] [CrossRef]
- Scott, H.K.; Aggarwal, V.K. Highly Enantioselective Synthesis of Tertiary Boronic Esters and their Stereospecific Conversion to other Functional Groups and Quaternary Stereocentres. Chem. Eur. J. 2011, 17, 13124–13132. [Google Scholar] [CrossRef]
- Stymiest, J.L.; Bagutski, V.; French, R.M.; Aggarwal, V.K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 2008, 456, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Bagutski, V.; French, R.M.; Aggarwal, V.K. Full Chirality Transfer in the Conversion of Secondary Alcohols into Tertiary Boronic Esters and Alcohols Using Lithiation–Borylation Reactions. Angew. Chem. Int. Ed. 2010, 49, 5142–5145. [Google Scholar] [CrossRef] [PubMed]
- Linne, Y.; Bonandi, E.; Tabet, C.; Geldsetzer, J.; Kalesse, M. The Total Synthesis of Chondrochloren A. Angew. Chem. Int. Ed. 2021, 60, 6938–6942. [Google Scholar] [CrossRef]
- Linne, E.; Kalesse, M. Stereoselective Construction of β-chiral Homoallyl Functionalities by Substrate- and Reagent-Controlled Iterative 1,2-Metallate Rearrangements. Org. Lett. 2023, 25, 8210–8214. [Google Scholar] [CrossRef]
- Linne, Y.; Birkner, M.; Flormann, J.; Lücke, D.; Becker, J.A.; Kalesse, M. Sparteine-Free, Highly Stereoselective Construction of Complex Allylic Alcohols Using 1,2-Metallate Rearrangements. J. Am. Chem. Soc. Au 2023, 3, 1695–1710. [Google Scholar] [CrossRef]
- Blakemore, P.R.; Burge, M.S. Iterative Stereospecific Reagent-Controlled Homologation of Pinacol Boronates by Enantioenrichedalpha-Chloroalkyllithium Reagents. J. Am. Chem. Soc. 2007, 129, 3068–3069. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, P.R.; Burge, M.S.; Stephton, M.A. Competing reaction pathways from α-halo-α-protioalkyl aryl sulfoxides initiated by organometallic reagents. Tetrahedron Lett. 2007, 48, 3999–4002. [Google Scholar] [CrossRef]
- Drago, C.; Caggiano, L.; Jackson, R.F.W. Vanadium-Catalyzed Sulfur Oxidation/Kinetic Resolution in the Synthesis of Enantiomerically Pure Alkyl Aryl Sulfoxides. Angew. Chem. Int. Ed. 2005, 44, 7221–7223. [Google Scholar] [CrossRef] [PubMed]
- Cogan, D.A.; Liu, G.; Kim, K.; Backes, B.J.; Ellman, J.A. Catalytic Asymmetric Oxidation of tert-Butyl Disulfide. Synthesis of tert-Butanesulfinamides, tert-Butyl Sulfoxides, and tert-Butanesulfinimines. J. Am. Chem. Soc. 1998, 120, 8011–8019. [Google Scholar] [CrossRef]
- Bolm, C.; Bienewald, F. Asymmetric Sulfide Oxidation with Vanadium Catalysts and H2O2. Angew. Chem. Int. Ed. Engl. 1995, 34, 2640–2642. [Google Scholar] [CrossRef]
- Satoh, T.; Oohara, T.; Ueda, Y.; Yamakawa, K. The practical procedure for a preparation of 1-chloroalkyl p-tolyl sulfoxides in high optically active form: A very short synthesis of optically active disparlure. Tetrahedron Lett. 1988, 29, 313–316. [Google Scholar] [CrossRef]
- Hoyt, A.L.; Blakemore, P.R. On the nature of the chainextending species in organolithium initiated stereospecific reagentcontrolled homologation reactions using α-chloroalkyl aryl sulfoxides. Tetrahedron Lett. 2015, 56, 2980–2982. [Google Scholar] [CrossRef]
- Emerson, C.R.; Zakharov, L.N.; Blakemore, P.R. Investigation of Functionalized α-Chloroalkyllithiums for a Stereospecific Reagent-Controlled Homologation Approach to the Analgesic Alkaloid (−)-Epibatidine. Chem. Eur. J. 2013, 19, 16342–16356. [Google Scholar] [CrossRef]
- Sun, X.; Blakemore, P.R. Programmed Synthesis of a Contiguous Stereotriad Motif by Triple Stereospecific Reagent-Controlled Homologation. Org. Lett. 2013, 15, 4500–4503. [Google Scholar] [CrossRef] [PubMed]
- Emerson, C.R.; Zakharov, L.N.; Blakemore, P.R. Iterative Stereospecific Reagent-Controlled Homologation Using a Functionalized α-Chloroalkyllithium: Synthesis of Cyclic Targets Related to Epibatidine. Org. Lett. 2011, 13, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, P.R.; Marsden, S.P.; Vater, H.D. Reagent-Controlled Asymmetric Homologation of Boronic Esters by Enantioenriched Main-Group Chiral Carbenoids. Org. Lett. 2006, 8, 773–776. [Google Scholar] [CrossRef]
- Jadhav, P.K.; Man, H.-W. Enantiotopic Differentiation of pro-R or pro-S Chlorides in (Dichloromethyl)borates by Chiral Lewis Acids: Enantioselective Synthesis of (α-Chloroalkyl)boronates. J. Am. Chem. Soc. 1997, 119, 846–847. [Google Scholar] [CrossRef]
- Smith, K.; Saleh, B.A.; Alshammari, M.B.; El-Hiti, G.A.; Elliott, M.C. Studies on a catalytic version of the Matteson asymmetric homologation reaction. Org. Biomol. Chem. 2021, 19, 4279–4284. [Google Scholar] [CrossRef]
- Sharma, H.A.; Essman, J.Z.; Jacobsen, E.N. Enantioselective Catalytic 1,2-Boronate Rearrangements. Science 2021, 374, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Essman, J.Z.; Sharma, H.A.; Jacobsen, E.N. Development of Enantioselective Lithium-Isothiourea-Boronate–Catalyzed Matteson Homologations. Synlett 2023, 34, 2061. [Google Scholar] [CrossRef] [PubMed]
- Angle, S.R.; Sharma, H.A.; Choi, C.K.; Carlson, K.E.; Hou, Y.; Nwachukwu, J.C.; Kim, S.H.; Katzenellenbogen, B.S.; Nettles, K.W.; Katzenellenbogen, J.A.; et al. Iterative Catalyst-Controlled Diastereoselective Matteson Homologations Enable the Selective Synthesis of Benzestrol Isomers. J. Am. Chem. Soc. 2024, 146, 30771–30777. [Google Scholar] [CrossRef] [PubMed]
- Janetzko, J.; Batey, R.A. Organoboron-Based Allylation Approach to the Total Synthesis of the Medium-Ring Dilactone (+)-Antimycin A1b. J. Org. Chem. 2014, 79, 7415–7424. [Google Scholar] [CrossRef] [PubMed]
- Lebarbier, C.; Carreaux, F.; Carboni, B. Synthesis of as boronic acid Analoque of L-ornithine. Synthesis 1996, 1371–1374. [Google Scholar] [CrossRef]
- Andler, O.; Kazmaier, U. Stereoselective Synthesis of the Side Chain of Meliponamycin A. Org. Lett. 2022, 24, 2541–2545. [Google Scholar] [CrossRef]
- Kohr, M.; Papenkordt, N.; Jung, M.; Kazmaier, U. Total synthesis and biological evaluation of the histone deacetylase inhibitor WF-3161. Org. Biomol. Chem. 2023, 21, 4382–4387. [Google Scholar] [CrossRef]
- Kempf, M.; Andler, O.; Kazmaier, U. Total synthesis of salviaquinesin A using the homologation approach of Matteson. Helv. Chim. Acta 2023, e202300136. [Google Scholar] [CrossRef]
- Andler, O.; Kazmaier, U. Matteson Homologation-based total synthesis of meliponamycin A. Org. Lett. 2024, 26, 148–152. [Google Scholar] [CrossRef]
- Yue, G.; Yang, L.; Yuan, C.; Du, B.; Liu, B. Total syntheses of lindenane-type sesquiterpenoids: (±)-chloranthalactones A, B, F, (±)-9-hydroxy heterogorgiolide, and (±)-shizukanolide E. Tetrahedron 2012, 68, 9624–9637. [Google Scholar] [CrossRef]
- Struth, F.R.; Hirschhäuser, C. A Modular Approach to the Asymmetric Synthesis of Cytisine. Eur. J. Org. Chem. 2016, 2016, 958–964. [Google Scholar] [CrossRef]
- Matteson, D.S.; Singh, R.P.; Schafman, B.; Yang, J.-J. Asymmetric Synthesis of Serricornin via Boronic Esters. J. Org. Chem. 1998, 63, 4466–4469. [Google Scholar] [CrossRef]
- Matteson, D.S.; Sadhu, K.M. Boronic ester homologation with 99% chiral selectivity and its use in syntheses of the insect pheromones (3S,4S)-4-methyl-3-heptanol and exo-brevicomin. J. Am. Chem. Soc. 1983, 105, 2077–2078. [Google Scholar] [CrossRef]
- Matteson, D.S.; Man, H.-W. High-Precision Asymmetric Synthesis of Stegobiol and Stegobinone over Boronic Acid Esters. J. Org. Chem. 1993, 58, 6545–6547. [Google Scholar] [CrossRef]
- Anderbrant, O.; Matteson, D.S.; Unelius, C.R.; Pharazyn, P.S.; Santangelo, E.M.; Schlyter, F.; Birgersson, G. Pheromone of the elm bark beetle Scolytus laevis (Coleoptera: Scolytidae): Stereoisomers of 4-methyl-3-heptanol reduce interspecific competition. Chemoecology 2010, 20, 179–187. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr.; Doty, M.S. Dictyopterene A. An odoriferous constituent from algae of the genus dictyopteris. Tetrahedron Lett. 1968, 9, 4787–4790. [Google Scholar] [CrossRef]
- Hohn, E.; Paleček, J.; Pietruszka, J. Synthesis of Dictyopterene A. Synlett 2008, 971–974. [Google Scholar] [CrossRef]
- Jang, J.-H.; Kanoh, K.; Adachi, K.; Shizuri, Y. Awajanomycin, a Cytotoxic γ-Lactone-δ-lactam Metabolite from Marine-Derived Acremonium sp. AWA16-1, J. Nat. Prod. 2006, 69, 1358–1360. [Google Scholar] [CrossRef]
- Wohlfahrt, M.; Harms, K.; Koert, U. Total Synthesis of (+)-Awajanomycin. Eur. J. Org. Chem. 2012, 2260–2265. [Google Scholar] [CrossRef]
- Chen, L.L.; Pousada, M.; Haines, T.H.J. The flagellar membrane of Ochromonas danica. Lipid composition. J. Biol. Chem. 1976, 251, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.L.; Hu, D.X.; McKenna, G.M.; Burns, N.Z. Catalytic Enantioselective Dihalogenation and the Selective Synthesis of (−)-Deschloromytilipin A and (−)-Danicalipin A. J. Am. Chem. Soc. 2016, 138, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.-Y.; Scott, H.K.; Hesse, M.J.; Willis, C.L.; Aggarwal, V.K. Highly diastereo- and enantioselective allylboration of aldehydes using α-substituted allyl/crotyl pinacol boronic esters via in situ generated borinic esters. J. Am. Chem. Soc. 2013, 135, 5316–5319. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.-Y.; Aggarwal, V.K. Highly Diastereoselective and Enantiospecific Allylation of Ketones and Imines Using Borinic Esters: Contiguous Quaternary Stereogenic Centers. Angew. Chem. Int. Ed. 2014, 53, 10992–10996. [Google Scholar] [CrossRef]
- De Silva, E.D.; Williams, D.E.; Andersen, R.J.; Klix, H.; Holmes, C.F.B.; Allen, T.M. Motuporin, a potent protein phosphatase inhibitor isolated from the Papua New Guinea sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992, 33, 1561–1564. [Google Scholar] [CrossRef]
- Honkanen, R.A.; Dukelow, M.; Zwiller, J.; Moore, R.A.; Khatra, B.S.; Boynton, A. L Cyanobacterial nodularin is a potent inhibitor of type 1 and type 2A protein phosphatases. Mol. Pharmacol. 1991, 40, 577–583. [Google Scholar] [PubMed]
- Honkanen, R.A.; Zwiller, J.; Moore, R.A.; Dailey, S.L.; Khatra, B.S.; Dukelow, M.; Boynton, A.L. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J. Biol. Chem. 1990, 265, 19401–19404. [Google Scholar] [CrossRef] [PubMed]
- Matteson, D.S.; Sadhu, K.M. Synthesis of 1-Amino-2-phenylethane-I-boronic Acid Derivatives. Organometallics 1984, 3, 614–618. [Google Scholar] [CrossRef]
- Matteson, D.S.; Jesthi, P.K.; Sadhu, K.M. Synthesis and Properties of Pinanediol a-Amido Boronic Esters. Organometallics 1984, 3, 1284–1288. [Google Scholar] [CrossRef]
- Matteson, D.S.; Beedle, E.C. A direct chiral synthesis of amino acids from boronic esters. Tetrahedron Lett. 1987, 28, 4499–4502. [Google Scholar] [CrossRef]
- Matteson, D.S.; Majumdar, D. Iodomethaneboronic esters and aminomethaneboronic esters. J. Organomet. Chem. 1979, 170, 259–264. [Google Scholar] [CrossRef]
- Matteson, D.S.; Lu, J. Asymmetric synthesis of 1-acyl-3,4-disubstituted pyrrolidine-2-boronic acid derivatives. Tetrahedron Asymmetry 1998, 9, 2423–2436. [Google Scholar] [CrossRef]
- Matteson, D.S.; Singh, R.P.; Sutton, C.H.; Verheyden, J.D.; Lu, J. An Exploratory Study of Silylated Amino Boronic Ester Chemistry. Heteroatom Chem. 1997, 8, 487–494. [Google Scholar] [CrossRef]
- Bauer, S.M.; Armstrong, R.W. Total Synthesis of Motuporin (Nodularin-V). J. Am. Chem. Soc. 1999, 121, 6355–6366. [Google Scholar] [CrossRef]
- Matteson, D.S.; Beedle, E.C. A chiral synthesis of (2S, 3S)-phenylalanine-3-2H via boronic esters. J. Label Compd. Radiopharm. 1988, 25, 675–683. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily Accessible 12-I-5 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Tanaka, T.; Oikawa, Y.; Hamada, T.; Yonemitsu, O. Total synthesis of tylonolide, the aglycone of the 16-membered ring macrolide tylosin, from D-glucose. Selective application of MPM and DMPM protecting groups for hydroxy functions. Tetrahedron Lett. 1986, 27, 3651–3654. [Google Scholar] [CrossRef]
- Bal, B.S.; Childers, W.E.; Pinnick, H.W. Oxidation of α, β-unsaturated aldehydes. Tetrahedron 1981, 37, 2091–2096. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Zampella, A.; Paloma, L.G.; Minale, L.; Debitus, C.; Roussakis, C.; Le Bert, V. Callipeltins B and C.; Bioactive Peptides from a Marine Lithistida Sponge Callipelta sp. Tetrahedron 1996, 52, 9589–9596. [Google Scholar] [CrossRef]
- Zampella, A.; Randazzo, A.; Borbone, N.; Luciani, S.; Trevisi, L.; Debitus, C.; D’Auria, M.V. Isolation of callipeltins A–C and of two new open-chain derivatives of callipeltin A from the marine sponge Latrunculia sp. A revision of the stereostructure of callipeltins. Tetrahedron Lett. 2002, 43, 6163–6166. [Google Scholar] [CrossRef]
- Sepe, V.; D’Orsi, R.; Borbone, N.; D’Auria, M.V.; Bifulco, G.; Monti, M.C.; Catania, A.; Zampella, A. Callipeltins F–I: New antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron 2006, 62, 833–840. [Google Scholar] [CrossRef]
- Stierhof, M.; Hansen, K.Ø.; Sharma, M.; Feussner, K.; Subko, K.; Díaz-Rullo, F.F.; Isaksson, J.; Pérez-Victoria, I.; Clarke, D.; Hansen, E.; et al. New cytotoxic callipeltins from the Solomon Island marine sponge Asteropus sp. Tetrahedron 2016, 72, 6929–6934. [Google Scholar] [CrossRef]
- Horn, A.; Kazmaier, U. Synthesis of the cyclic heptapeptide nucleus of Callipeltin A. Org. Chem. Front. 2022, 9, 5213–5218. [Google Scholar] [CrossRef]
- Horn, A.; Kazmaier, U. Stereoselective synthesis of the side chain of Callipeltin A. Org. Lett. 2022, 24, 7072–7076. [Google Scholar] [CrossRef]
- Horn, A.; Papadopoulos, E.; Kinsinger, T.; Greve, J.; Bickel, E.; Pachoula, S.; Kazmaier, U. Stereoselective synthesis of α-azido esters and α-amino acid derivatives via Matteson homologation of boron esters. Z. Anorg. Allg. Chem. 2024, 650, e202400113. [Google Scholar] [CrossRef]
- Bickel, E.; Kazmaier, U. Simple syntheses of bottromycin derivatives via Ugi reactions and Matteson homologations. Org. Biomol. Chem. 2024, 22, 8811–8816. [Google Scholar] [CrossRef]
- Cheng, X.C.; Kihara, T.; Kusakbe, H.; Magea, J.; Kobayashi, Y.; Fang, R.P.; Ni, Z.F.; Shen, Y.C.; Ko, K.; Yamaguchi, I.; et al. A new antibiotic, tautomycin. J. Antibiot. 1987, 40, 907–909. [Google Scholar] [CrossRef][Green Version]
- Maurer, K.W.; Armstrong, R.W. Synthesis of the C1-C21 Fragment of the Serine/Threonine Phosphatase Inhibitor Tautomycin. J. Org. Chem. 1996, 61, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Niit, K.; Utimoton, K. Simple and Selective Method for RCHO to (E)-RCH=CHX Conversion by Means of a CHX3–CrCl2 System. J. Am. Chem. Soc. 1986, 108, 7408–7410. [Google Scholar] [CrossRef]
- Kishi, Y. Applications of Ni (II)/Cr (II)-mediated coupling reactions to natural products syntheses. Pure Appl. Chem. 1992, 64, 343–350. [Google Scholar] [CrossRef]
- Takai, K.; Tagashira, M.; Kuroda, T.; Oshima, K.; Utimoto, K.; Nozaki, H. Reactions of alkenylchromium reagents prepared from alkenyl trifluoromethanesulfonates (triflates) with chromium(II) chloride under nickel catalysis. J. Am. Chem. Soc. 1986, 108, 6048–6050. [Google Scholar] [CrossRef]
- Priester, R.; Kazmaier, U. A Straightforward Synthesis of Emericellamide A using Matteson’s Homologation Approach. Synlett 2023, 34, 2159–2164. [Google Scholar] [CrossRef]
- Nakao, Y.; Age, W.Y.; Takada, Y.; Kimura, J.; Yang, L.; Mooberry, S.L.; Scheuer, P.J. Kulokekahilide-2, a Cytotoxic Depsipeptide from a Cephalaspidean Mollusk Philinopsis speciosa. J. Nat. Prod. 2004, 67, 1332–1340. [Google Scholar] [CrossRef]
- Takada, Y.; Mori, E.; Umehara, M.; Nakao, Y.; Kimura, J. Reinvestigation of the stereochemistry of kulokekahilide-2. Tetrahedron Lett. 2007, 48, 7653–7656. [Google Scholar] [CrossRef]
- Takada, Y.; Umehara, M.; Nakao, Y.; Kimura, J. Revised absolute stereochemistry of natural kulokekahilide-2. Tetrahedron Lett. 2008, 49, 1163–1165. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Kaneda, M.; Sumimoto, S.; Oishi, S.; Fujii, N.; Suenaga, K.; Teruya, T. Odoamide, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Okeania sp. Tetrahedron 2016, 72, 5472–5478. [Google Scholar] [CrossRef]
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Kigoshi, H.; Yamada, K. Isolation and stereostructure of aurilide, a novel cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron Lett. 1996, 37, 6771–6774. [Google Scholar] [CrossRef]
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Fujita, T.; Takada, N.; Hayamizu, K.; Takagi, M.; Irifune, T.; Kigoshi, H.; et al. Aurilide, a cytotoxic depsipeptide from the sea hare Dolabella auricularia: Isolation, structure determination, synthesis, and biological activity. Tetrahedron 2004, 60, 8509–8527. [Google Scholar] [CrossRef]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, Cancer Cell Toxins from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya majuscule. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, W.; Li, L.; Sun, X.; Song, S.; Xu, Q.; Zhang, L.; Wei, B.; Deng, X. Structure Determinants of Lagunamide A for Anticancer Activity and Its Molecular Mechanism of Mitochondrial Apoptosis. Mol. Pharm. 2016, 13, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mostert, D.; Orgler, C.; Andler, O.; Zischka, H.; Kazmaier, U.; Vollmar, A.M.; Braig, S.; Sieber, S.A.; Zahler, S. Lagunamide A causes potent antiproliferative and pro-apoptotic effects in cancer cells by targeting EYA3. ChemBioChem 2024, e202400024. [Google Scholar] [CrossRef]
- Suenaga, K.; Kajiwara, S.; Kuribayashi, S.; Handa, T.; Kigoshi, H. Synthesis and cytotoxicity of aurilide analogs. Bioorg. Med. Chem. Lett. 2008, 18, 3902–3905. [Google Scholar] [CrossRef]
- Andler, O.; Kazmaier, U. A Straightforward Synthesis of Polyketides via Ester Dienolate Matteson Homologation. Chem. Eur. J. 2021, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a Potent Cytotoxic Cyclodepsipeptide from Papua New Guinea Collections of the Marine Cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a Cytotoxic Peptolide from a Guamanian Collection of the Marine Cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116, Erratum in J. Nat. Prod. 2018, 81, 217–217. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Cowley, E.S.; Sikorska, J.; Shaala, L.A.; Ishmael, J.E.; Youssef, D.T.A.; McPhail, K.L. Apratoxin H and Apratoxin A Sulfoxide from the Red Sea Cyanobacterium Moorea producens. J. Nat. Prod. 2013, 76, 1781–1788. [Google Scholar] [CrossRef]
- Tarsis, E.M.; Rastelli, E.J.; Wengryniuk, S.E.; Coltart, D.M. The apratoxin marine natural products: Isolation, structure determination, and asymmetric total synthesis. Tetrahedron 2015, 71, 5029–5044. [Google Scholar] [CrossRef]
- Liu, Y.; Law, B.K.; Luesch, H. Apratoxin A Reversibly Inhibits the Secretory Pathway by Preventing Cotranslational Translocation. Mol. Pharmacol. 2009, 76, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Chanda, S.K.; Raya, R.M.; DeJesus, P.D.; Orth, A.P.; Walker, J.R.; Izpisúa Belmonte, J.C.; Schultz, P.G. A functional genomics approach to the mode of action of apratoxin A. Nat. Chem. Biol. 2006, 2, 158–167. [Google Scholar] [CrossRef]
- Wan, X.; Serrill, J.D.; Humphreys, I.R.; Tan, M.; McPhail, K.L.; Ganley, I.G.; Ishmael, J.E. ATG5 Promotes Death Signaling in Response to the Cyclic Depsipeptides Coibamide A and Apratoxin A. Mar. Drugs 2018, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Luesch, H. Systematic Chemical Mutagenesis Identifies a Potent Novel Apratoxin A/E Hybrid with Improved in Vivo Antitumor Activity. ACS Med. Chem. Lett. 2011, 2, 861–865. [Google Scholar] [CrossRef]
- Andler, O.; Kazmaier, U. Total synthesis of apratoxin A and B using Matteson’s homologation approach. Org. Biomol. Chem. 2021, 19, 4866–4870. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, H.; Nemoto, T.; Ojika, M.; Yamada, K. Isolation and Stereostructure of Doliculide, a Cytotoxic Cyclodepsipeptide from the Japanese Sea Hare Dolabella auricularia. J. Org. Chem. 1994, 59, 4710–4711. [Google Scholar] [CrossRef]
- Harrigan, G.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Nagle, D.G.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H.; Valeriote, F.A. Symplostatin 1: A Dolastatin 10 Analogue from the Marine Cyanobacterium Symploca hydnoides. J. Nat. Prod. 1998, 61, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Covell, D.G.; Liu, C.; Ghosh, A.K.; Hamel, E. (−)-Doliculide, a New Macrocyclic Depsipeptide Enhancer of Actin Assembly. J. Biol. Chem. 2002, 277, 32165–32171. [Google Scholar] [CrossRef] [PubMed]
- Franklin-Tong, V.E.; Gourlay, C.W. A Role for Actin in Regulating Apoptosis/Programmed Cell Death: Evidence Spanning Yeast, Plants and Animals. Biochem. J. 2008, 413, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef]
- Matcha, K.; Madduri, A.V.R.; Roy, S.; Ziegler, S.; Waldmann, H.; Hirsch, A.K.H.; Minnaard, A.J. Total Synthesis of (−)-Doliculide, Structure–Activity Relationship Studies and Its Binding to F-Actin. ChemBioChem 2012, 13, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Braig, S.; Chen, T.; Altmann, K.-H.; Vollmar, A.M. Pharmacological Characterization of Actin-Binding (−)-Doliculide. Bioorg. Med. Chem. 2014, 22, 5117–5122. [Google Scholar] [CrossRef]
- Sumiya, E.; Shimogawa, H.; Sasaki, H.; Tsutsumi, M.; Yoshita, K.; Ojika, M.; Suenaga, K.; Uesugi, M. Cell-Morphology Profiling of a Natural Product Library Identifies Bisebromoamide and Miuraenamide A as Actin Filament Stabilizers. ACS Chem. Biol. 2011, 6, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Rüdiger, D.; Förster, F.; von Blume, J.; Yu, P.; Küster, B.; Kazmaier, U.; Vollmar, A.M.; Zahler, S. Persistent inhibition of pore-based cell migration by sub-toxic doses of miuraenamide, an actin filament stabilizer. Sci. Rep. 2017, 7, 16407. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meixner, M.; Yu, L.; Karmann, L.; Kazmaier, U.; Vollmar, A.M.; Antes, I.; Zahler, S. Turning the Actin Nucleating Compound Miuraenamide into Nucleation Inhibitors. ACS Omega 2021, 6, 22165–22172. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Chen, T.; Altmann, K.-H.; Vollmar, A.M. Actin-Binding Doliculide Causes Premature Senescence in P53 Wild Type Cells. Bioorg. Med. Chem. 2016, 24, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ho, O.C.; Soundararajan, R.; Lu, J.; Matteson, D.S.; Wang, Z.; Chen, X.; Wei, M.; Willett, R.D. ((Trityloxy)methyl)boronic Esters. Organometallics 1995, 14, 2855–2860. [Google Scholar] [CrossRef]
- Tost, M.; Kazmaier, U. Synthesis and Late-Stage Modification of (–)-Doliculide Derivatives Using Matteson’s Homologation Approach. Mar. Drugs 2024, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Millán, A.; Martinez, P.D.G.; Aggarwal, V.K. Stereocontrolled Synthesis of Polypropionate Fragments based on a Building Block Assembly Strategy using Lithiation-Borylation Methodologies. Chem. Eur. J. 2018, 24, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Cho, K.W.; Rho, J.-R.; Shin, J. Solandelactones A-I, Lactonized Cyclopropyl Oxylipins Isolated from the Hydroid Solanderia secunda. Tetrahedron 1996, 52, 10583–10596. [Google Scholar] [CrossRef]
- Binanzer, M.; Fang, G.Y.; Aggarwal, V.K. Asymmetric Synthesis of Allylsilanes by the Borylation of Lithiated Carbamates: Formal Total Synthesis of (–)-Decarestrictine D. Angew. Chem. Int. Ed. 2010, 49, 4264–4268. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Aggarwal, V.K. Asymmetric Total Synthesis of Solandelactone E: Stereocontrolled Synthesis of the 2-ene-1,4-diol Core Through a Lithiation–Borylation—Allylation Sequence. Angew. Chem. Int. Ed. 2010, 49, 6673–6675. [Google Scholar] [CrossRef]
- Landais, Y.; Parra-Rapado, L. Epoxidation and cyclopropanation of 2-silyl-3-alkenols. A study of 1,2-asymmetric induction. Tetrahedron Lett. 1996, 37, 1205–1208. [Google Scholar] [CrossRef]
- Robinson, A.; Aggarwal, V.K. Stereocontrolled asymmetric synthesis of syn-E-1,4-diol-2-enes using allyl boronates and its application in the total synthesis of solandelactone F. Org. Biomol. Chem. 2012, 10, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.D.; Ramirez, C. Serrulatane Diterpenes with Antimycobacterial Activity Isolated from the West Indian Sea Whip Pseudopterogorgia elisabethae. J. Nat. Prod. 2001, 64, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Hashiguchi, S.; Uematsu, N.; Ikariya, T.; Noyori, R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid−Triethylamine Mixture. J. Am. Chem. Soc. 1996, 118, 2521–2522. [Google Scholar] [CrossRef]
- Nave, S.; Sonawane, R.P.; Elford, T.G.; Aggarwal, V.K.J. Protodeboronation of Tertiary Boronic Esters: Asymmetric Synthesis of Tertiary Alkyl Stereogenic Centers. J. Am. Chem. Soc. 2010, 132, 17096–17098. [Google Scholar] [CrossRef]
- Elford, T.G.; Nave, S.; Sonawane, R.P.; Aggarwal, V.K. Total Synthesis of (+)-Erogorgiaene Using Lithiation–Borylation Methodology, and Stereoselective Synthesis of Each of Its Diastereoisomers. J. Am. Chem. Soc. 2011, 133, 16798–16801. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Tsai, P.I.; Fenical, W.; Hay, M.E. Secondary metabolite chemistry of the caribbean marine alga Sporochnus bolleanus: A basis for herbivore chemical defence. Phytochemistry 1993, 52, 71. [Google Scholar] [CrossRef]
- Zweifel, G.; Arzoumanian, H.; Whitney, C.C. A Convenient Stereoselective Synthesis of Substituted Alkenes via Hydroboration-Iodination of Alkynes. J. Am. Chem. Soc. 1967, 89, 3652–3653. [Google Scholar] [CrossRef]
- Zweifel, G.; Polston, N.L.; Whitney, C.C. A stereoselective synthesis of conjugated dienes from alkynes via the hydroboration-iodination reaction. J. Am. Chem. Soc. 1968, 90, 6243–6245. [Google Scholar] [CrossRef]
- Evans, D.A.; Crawford, T.C.; Thomas, R.C.; Walker, J.A. Studies directed toward the synthesis of prostaglandins. Useful boron-mediated olefin syntheses. J. Org. Chem. 1976, 41, 3947–3953. [Google Scholar] [CrossRef]
- Sonawane, R.P.; Jheengut, V.; Rabalakos, C.; Larouche-Gauthier, R.; Scott, H.K.; Aggarwal, V.K. Enantioselective Construction of Quaternary Stereogenic Centers from Tertiary Boronic Esters: Methodology and Applications. Angew. Chem. Int. Ed. 2011, 50, 3760–3763. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Ball, L.T.; Carobene, S.; Connelly, R.L.; Hesse, M.J.; Partridge, B.M.; Roth, P.; Thomas, S.P.; Webster, M.P. Application of the lithiation–borylation reaction to the rapid and enantioselective synthesis of the bisabolane family of sesquiterpenes. Chem. Commun. 2012, 48, 9230–9232. [Google Scholar] [CrossRef] [PubMed]
- Jongaramruong, J.; Blackman, A.J.; Skelton, B.W.; White, A.H. Chemical relationships between the sea hare Aplysia parvula and the red seaweed Laurencia filiformis from Tasmania. Aust. J. Chem. 2002, 55, 275–280. [Google Scholar] [CrossRef]
- Yamamura, S.; Hirata, Y. Structures of aplysin and aplysinol, naturally occurring bromo compounds. Tetrahedron 1963, 19, 1485–1496. [Google Scholar] [CrossRef]
- Fletcher, C.J.; Blair, D.J.; Wheelhouse, K.M.P.; Aggarwal, V.K. The total synthesis of (–)-aplysin via a lithiation–borylation–propenylation sequence. Tetrahedron 2012, 68, 7598–7604. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. New laurene derivatives from Laurencia filiformis. Aust. J. Chem. 1976, 29, 2533–2539. [Google Scholar] [CrossRef]
- Blair, D.J.; Fletcher, C.J.; Wheelhouse, K.M.P.; Aggarwal, V.K. Stereocontrolled Synthesis of Adjacent Acyclic Quaternary-Tertiary Motifs: Application to a Concise Total Synthesis of (–)-Filiformin. Angew. Chem. Int. Ed. 2014, 53, 5552–5555. [Google Scholar] [CrossRef]
- Wu, M.; Okino, T.; Nogle, L.M.; Marquez, B.L.; Williamson, R.T.; Sitachitta, N.; Berman, F.W.; Murray, T.F.; McGough, K.; Jacobs, R.; et al. Structure, Synthesis, and Biological Properties of Kalkitoxin, a Novel Neurotoxin from the Marine Cyanobacterium Lyngbya majuscule. J. Am. Chem. Soc. 2000, 122, 12041–12042. [Google Scholar] [CrossRef]
- Berman, F.W.; Gerwick, W.H.; Murray, T.F. Antillatoxin and kalkitoxin, ichthyotoxins from the tropical cyanobacterium Lyngbya majuscula, induce distinct temporal patterns of NMDA receptor-mediated neurotoxicity. Toxicon 1999, 37, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Hokama, Y.; Dickey, R.W.; Granade, H.R.; Lewis, R.; Yasumoto, T.; Wekell, M.M. Detection of sodium channel toxins: Directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J. AOAC Int. 1995, 78, 521–527. [Google Scholar] [CrossRef]
- Burns, M.; Essafi, S.; Bame, J.R.; Bull, S.P.; Webster, M.P.; Balieu, S.; Dale, J.W.; Butts, C.P.; Harvey, J.N.; Aggarwal, V.K. Assembly-line synthesis of organic molecules with tailored shapes. Nature 2014, 513, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Balieu, S.; Hallett, G.E.; Burns, M.; Bootwicha, T.; Studley, J.; Aggarwal, V.K. Toward Ideality: The Synthesis of (+)-Kalkitoxin and (+)-Hydroxyphthioceranic Acid by Assembly-Line Synthesis. J. Am. Chem. Soc. 2015, 137, 4398–4403. [Google Scholar] [CrossRef] [PubMed]
- Bootwicha, T.; Feilner, J.M.; Myers, E.L.; Aggarwal, V.K. Iterative assembly line synthesis of polypropionates with full stereocontrol. Nat. Chem. 2017, 9, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.; Mykura, R.C.; Aggarwal, V.K. Lithiation–borylation methodology in the total synthesis of natural products. Nat. Synth. 2022, 1, 117–126. [Google Scholar] [CrossRef]
- Mlynarski, S.N.; Karns, A.S.; Morken, J.P. Direct Stereospecific Amination of Alkyl and Aryl Pinacol Boronates. J. Am. Chem. Soc. 2012, 134, 16449–16451. [Google Scholar] [CrossRef]
- Rao, R.M.; Faulkner, D.J. Clavosolides A and B, Dimeric Macrolides from the Philippines Sponge Myriastra clavosa. J. Nat. Prod. 2002, 65, 386–388. [Google Scholar] [CrossRef]
- Erickson, K.L.; Gustafson, K.R.; Pannell, L.K.; Beutler, J.A.; Boyd, M. New Dimeric Macrolide Glycosides from the Marine Sponge Myriastra clavosa. J. Nat. Prod. 2002, 65, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Millán, A.; Smith, J.R.; Chen, J.L.-Y.; Aggarwal, V.K. Tandem Allylboration–Prins Reaction for the Rapid Construction of Substituted Tetrahydropyrans: Application to the Total Synthesis of (−)-Clavosolide A. Angew. Chem. Int. Ed. 2016, 55, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Schofield, M.M.; Chlipala, G.E.; Schultz, P.J.; Yim, I.; Newmister, S.A.; Nusca, T.D.; Scaglione, J.B.; Hanna, P.C.; Tamayo-Castillo, G.; et al. Baulamycins A and B, broad-spectrum antibiotics identified as inhibitors of siderophore biosynthesis in Staphylococcus aureus and Bacillus anthracis. J. Am. Chem. Soc. 2014, 136, 1579–1586. [Google Scholar] [CrossRef]
- Wu, J.; Lorenzo, P.; Zhong, S.; Ali, M.; Butts, C.P.; Myers, E.L.; Aggarwal, V.K. Synergy of synthesis, computation and NMR reveals correct baulamycin structures. Nature 2017, 547, 436–440. [Google Scholar] [CrossRef]
- Shao, C.-L.; Mou, X.-F.; Cao, F.; Spadafora, C.; Glukhov, E.; Gerwick, L.; Wang, C.-Y.; Gerwick, W.H. Bastimolide B, an Antimalarial 24-Membered Marine Macrolide Possessing a tert-Butyl Group. J. Nat. Prod. 2018, 81, 211–215. [Google Scholar] [CrossRef]
- Shao, C.-L.; Linington, R.G.; Balunas, M.J.; Centeno, A.; Boudreau, P.; Zhang, C.; Engene, N.; Spadafora, C.; Mutka, T.S.; Kyle, D.E.; et al. Bastimolide A, a Potent Antimalarial Polyhydroxy Macrolide from the Marine Cyanobacterium Okeania hirsute. J. Org. Chem. 2015, 80, 7849–7855. [Google Scholar] [CrossRef] [PubMed]
- Friestad, G.K.; Sreenilayam, G. 1,5-Polyols: Challenging Motifs for Configurational Assignment and Synthesis. Pure Appl. Chem. 2011, 83, 461–478. [Google Scholar] [CrossRef]
- Fiorito, D.; Keskin, S.; Bateman, J.M.; George, M.; Noble, A.; Aggarwal, V.K. Stereocontrolled Total Synthesis of Bastimolide B Using Iterative Homologation of Boronic Esters. J. Am. Chem. Soc. 2022, 144, 7995–8001. [Google Scholar] [CrossRef] [PubMed]
- Kliman, L.T.; Mlynarski, S.N.; Ferris, G.E.; Morken, J.P. Catalytic Enantioselective 1,2-Diboration of 1,3-Dienes: Versatile Reagents for Stereoselective Allylation. Angew. Chem. Int. Ed. 2012, 51, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.R.; Haeffner, F.; Kliman, L.T.; Morken, J.P. Scope and Mechanism of the Pt-Catalyzed Enantioselective Diboration of Monosubstituted Alkenes. J. Am. Chem. Soc. 2013, 135, 11222–11231. [Google Scholar] [CrossRef] [PubMed]
- Casoni, G.; Kucukdisli, M.; Fordham, J.M.; Burns, M.; Myers, E.L.; Aggarwal, V.K. α-Sulfinyl Benzoates as Precursors to Li and Mg Carbenoids for the Stereoselective Iterative Homologation of Boronic Esters. J. Am. Chem. Soc. 2017, 139, 11877–11886. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.J.; Chitti, S.; Trobe, M.; Kostyra, D.M.; Haley, H.M.S.; Hansen, R.L.; Ballmer, S.G.; Woods, T.J.; Wang, W.; Mubayi, V.; et al. Automated Iterative Csp3–C Bond Formation. Nature 2022, 604, 92–97. [Google Scholar] [CrossRef]
- Crudden, M.C.; Hleba, Y.B.; Chen, A.C. Regio- and Enantiocontrol in the Room-Temperature Hydroboration of Vinyl Arenes with Pinacol Borane. J. Am. Chem. Soc. 2004, 126, 9200–9201. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Fujikawa, R.; Umemoto, T.; Miyaura, N. Iridium-Catalyzed Hydroboration of Alkenes with Pinacolborane. Tetrahedron 2004, 60, 10695–10700. [Google Scholar] [CrossRef]
- Fiorito, D.; Mazet, C. Ir-Catalyzed Selective Hydroboration of 2-Substituted 1,3-Dienes: A General Method to Access Homoallylic Boronates. ACS Catal. 2018, 8, 9382–9387. [Google Scholar] [CrossRef]
- Chemler, S.R.; Trauner, D.; Danishefsky, S.J. The B-Alkyl Suzuki-Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis. Angew. Chem. Int. Ed. 2001, 40, 4544–4568. [Google Scholar] [CrossRef]
- Kallan, N.C.; Halcomb, R.L. Synthesis of the Ring System of Phomactin D Using a Suzuki Macrocyclization. Org. Lett. 2000, 2, 2687–2690. [Google Scholar] [CrossRef] [PubMed]
- Chemler, S.R.; Danishefsky, S.J. Transannular Macrocyclization via Intramolecular B-Alkyl Suzuk.i Reaction. Org. Lett. 2000, 2, 2695–2698. [Google Scholar] [CrossRef]
- Igarashi, Y.; Shimasaki, R.; Miyanaga, S.; Oku, N.; Onaka, H.; Sakurai, H.; Saiki, I.; Kitani, S.; Nihira, T.; Wimonsiravude, W.; et al. Rakicidin D, ein Inhibitor der Tumorzellinvasion aus marinen Streptomyces sp. J. Antibiot. 2010, 63, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Kitani, S.; Ueguchi, T.; Igarashi, Y.; Leetanasaksakul, K.; Thamchaipenet, A.; Nihira, T. Rakicidin F, a new antibacterial cyclic depsipeptide derived from a marine sponge-derived Streptomyces sp. J. Antibiot. 2018, 71, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Bold, C.P.; Yeung, K.; Pape, F.; Kaiser, D.; Aggarwal, V.K. Application of Lithiation–Borylation to the Total Synthesis of (−)-Rakicidin F. Organic Lett. 2022, 24, 9398–9402. [Google Scholar] [CrossRef] [PubMed]
- Smitrovich, J.H.; Woerpel, K.A. Oxidation of Sterically Hindered Alkoxysilanes and Phenylsilanes under Basic Conditions. J. Org. Chem. 1996, 61, 6044–6046. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazmaier, U. Syntheses of Marine Natural Products via Matteson Homologations and Related Processes. Mar. Drugs 2025, 23, 20. https://doi.org/10.3390/md23010020
Kazmaier U. Syntheses of Marine Natural Products via Matteson Homologations and Related Processes. Marine Drugs. 2025; 23(1):20. https://doi.org/10.3390/md23010020
Chicago/Turabian StyleKazmaier, Uli. 2025. "Syntheses of Marine Natural Products via Matteson Homologations and Related Processes" Marine Drugs 23, no. 1: 20. https://doi.org/10.3390/md23010020
APA StyleKazmaier, U. (2025). Syntheses of Marine Natural Products via Matteson Homologations and Related Processes. Marine Drugs, 23(1), 20. https://doi.org/10.3390/md23010020