Structure-Activity Relationship Study of Majusculamide D: Overcoming Metabolic Instability and Severe Toxicity with a Fluoro Analogue
Abstract
1. Introduction
2. Results and Discussion
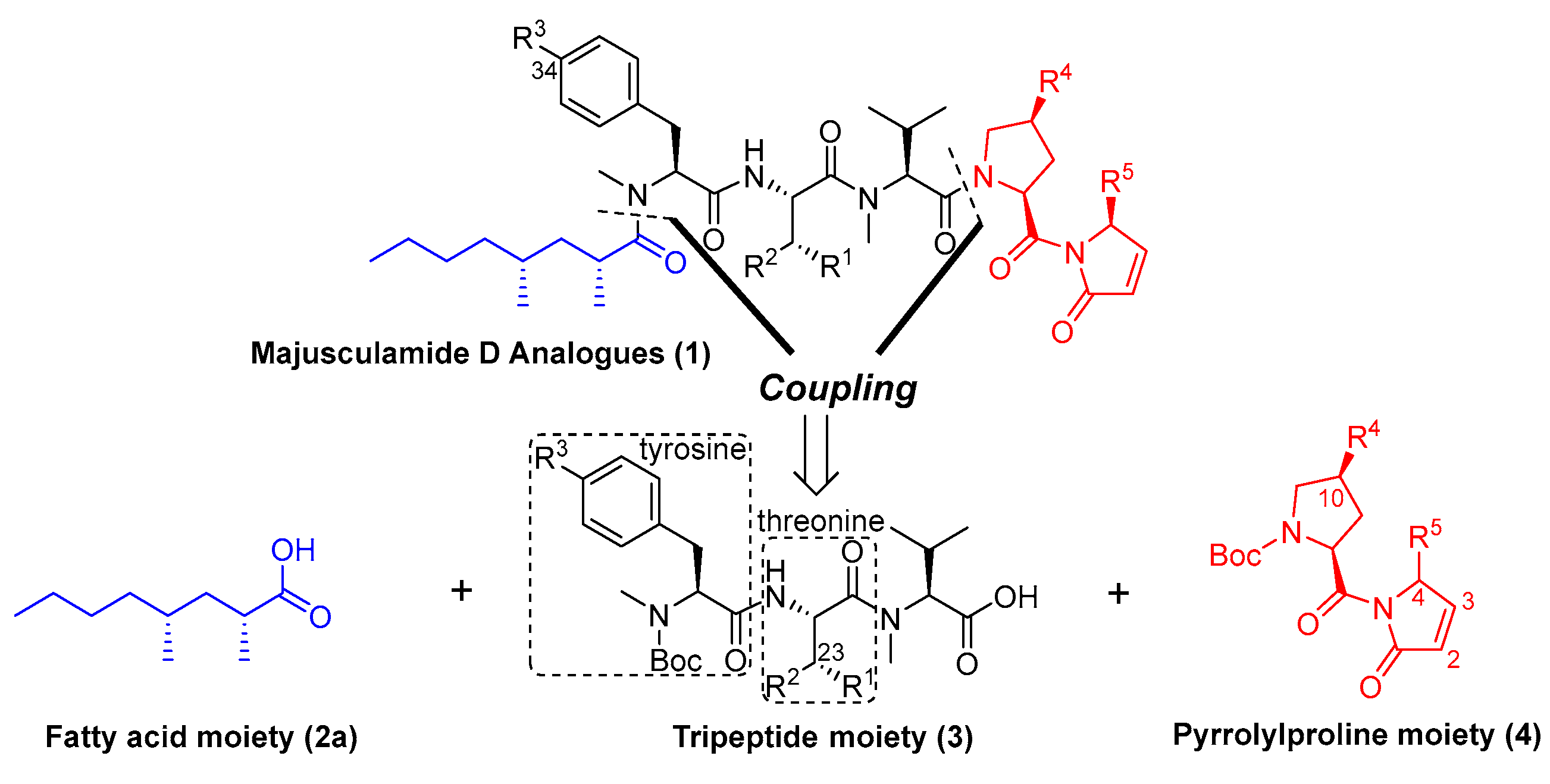
2.1. Syntheses and Inhibitory Activities of Majusculamide D Analogues
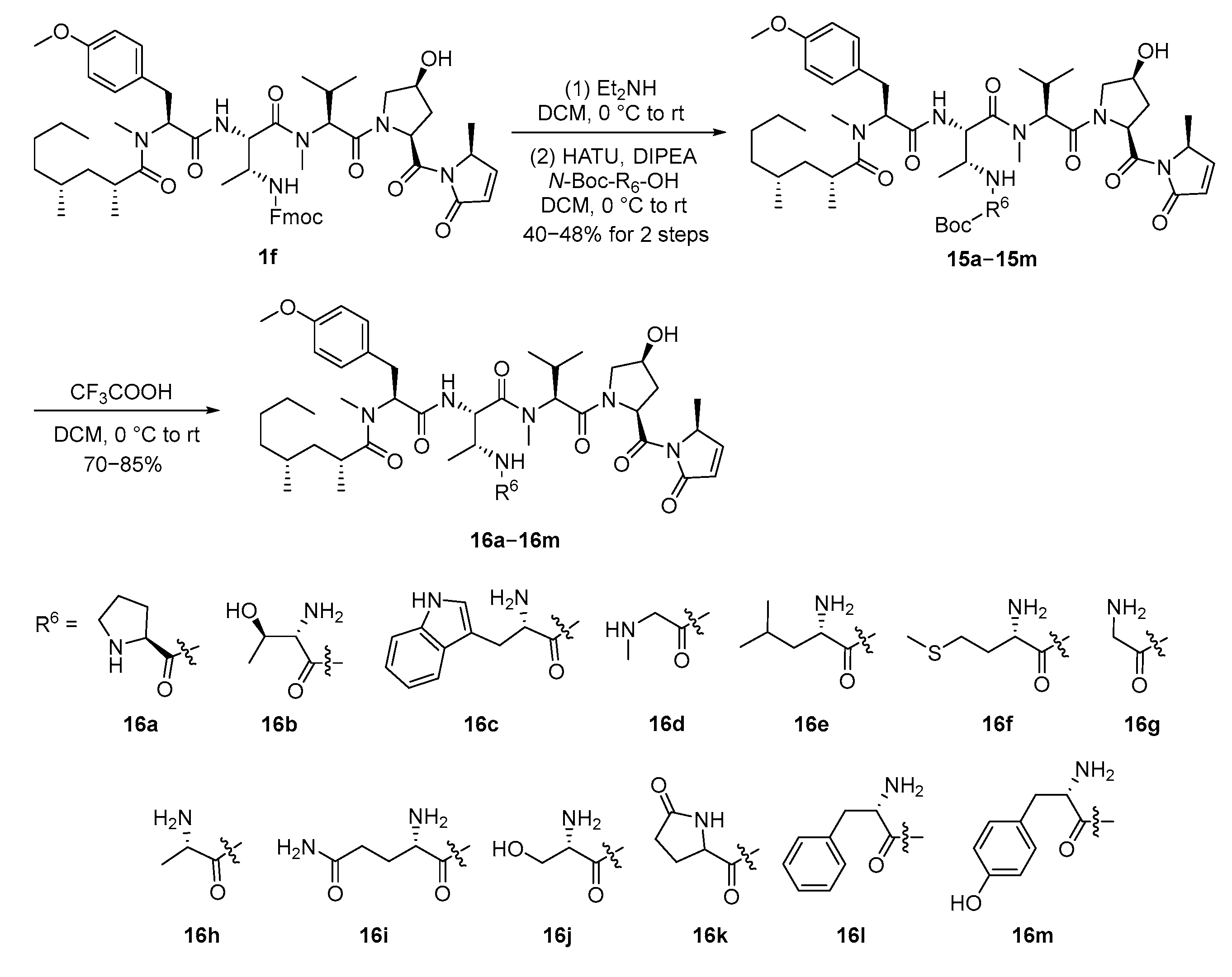
2.2. Syntheses and Inhibitory Activities of Majusculamide D Analogues Conjugated with One Extra Amino Acid
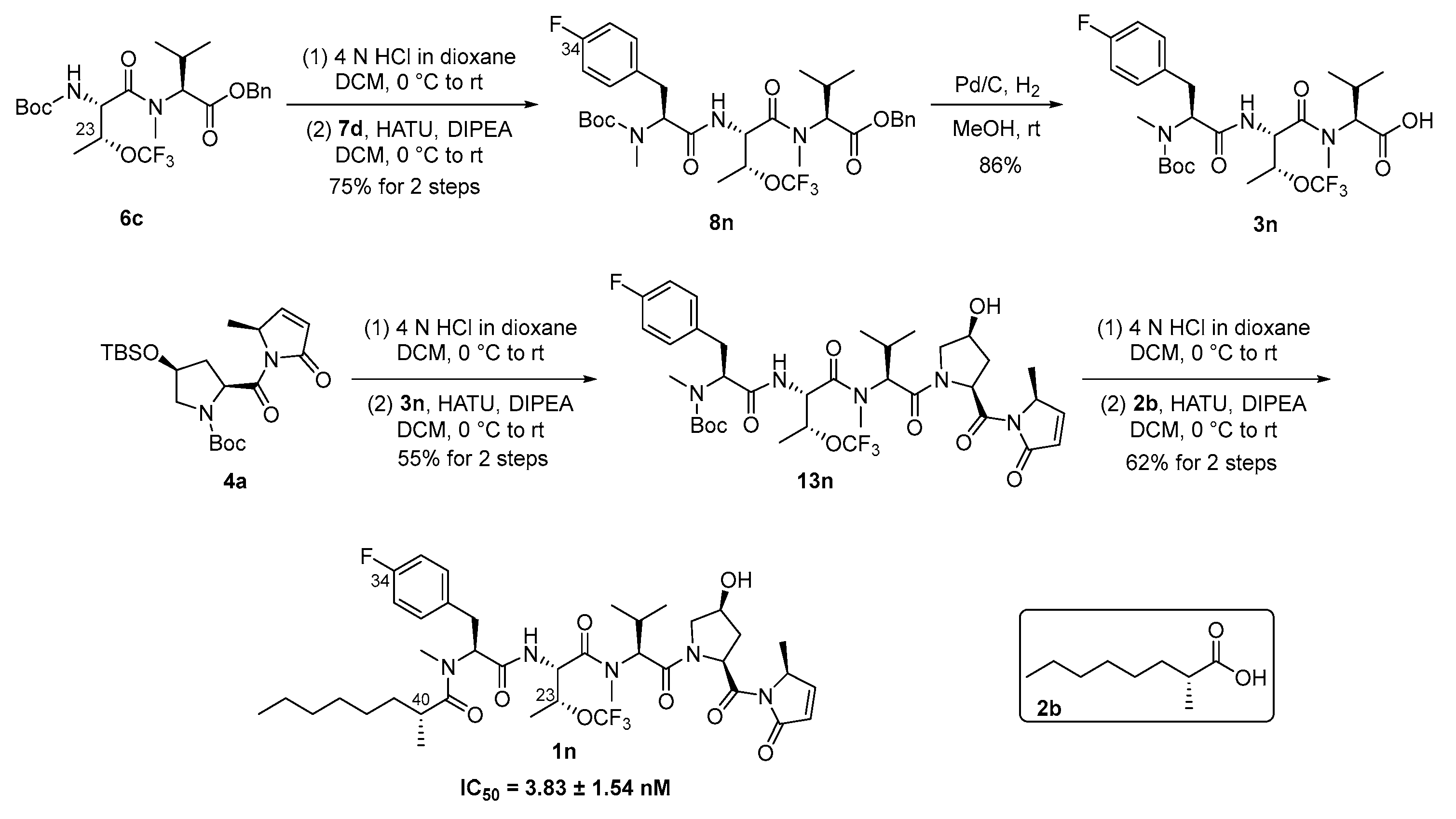
2.3. Synthesis, Inhibitory Activity, Metabolic Stability Assay, and In Vivo Antitumor Activity of Majusculamide D Analogue 1n
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of 8b–8j and 8n (Take 8n for Example)
3.1.2. General Procedure for the Synthesis of 3b–3j and 3n (Take 3n for Example)
3.1.3. General Procedure for the Synthesis of 13b–13n (Take 13n for Example)
3.1.4. General Procedure for the Synthesis of 1b–1n (Take 1n for Example)
3.2. Experimental Cells and Animals
3.3. MTT Cell Activity Assay
3.4. Stability in Mouse Plasma Assay
3.5. In Vivo Antitumor Activity
3.6. H&E Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 8 August 2024).
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology 2022, 163, 386–402. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tian, L.; Zhang, Y.; Wu, Y.; Li, B.; Liu, J. Recent advances in molecular and nanoparticle probes for fluorescent bioanalysis. Nano Res. 2024, 17, 6443–6474. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xie, X.; Chen, B.; Liu, L.; Jiang, C.; Qian, Q. Marine natural products: A potential source of anti-hepatocellular carcinoma drugs. J. Med. Chem. 2021, 64, 7879–7899. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Longley, R.E.; Reed, J.K. Microcolins A and B, new immunosuppressive peptides from the blue-green alga Lyngbya majuscula. J. Nat. Prod. 1992, 55, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Entzeroth, M. Majusculamide D and deoxymajusculamide D, two cytotoxins from Lyngbya majuscula. Phytochemistry 1988, 27, 3101–3103. [Google Scholar] [CrossRef]
- Takamatsu, S.; Nagle, D.G.; Gerwick, W.H. Secondary metabolites from marine cyanobacteria and algae inhibit LFA-1/ICAM-1 mediated cell adhesion. Planta Med. 2004, 70, 127–131. [Google Scholar] [PubMed]
- Meickle, T.; Matthew, S.; Ross, C.; Luesch, H.; Paul, V. Bioassay-guided isolation and identification of Desacetylmicrocolin B from Lyngbya cf. polychroa. Planta Med. 2009, 75, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.A.; Harmody, D.; Pitts, T.P.; Vera-Diaz, B.; Winder, P.L.; Yu, Y.; Wright, A.E. Inhibition of IL-8 secretion on BxPC-3 and MIA PaCa-2 cells and induction of cytotoxicity in pancreatic cancer cells with marine natural products. Anti-Cancer Drugs 2017, 28, 153–160. [Google Scholar] [CrossRef]
- Yu, H.-B.; Glukhov, E.; Li, Y.; Iwasaki, A.; Gerwick, L.; Dorrestein, P.C.; Jiao, B.-H.; Gerwick, W.H. Cytotoxic Microcolin lipopeptides from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2019, 82, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Caro-Diaz, E.J.E.; Valeriote, F.A.; Gerwick, W.H. Highly convergent total synthesis and assignment of absolute configuration of Majusculamide D, a potent and selective cytotoxic metabolite from Moorea sp. Org. Lett. 2019, 21, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Wang, C.; Zhang, H.; Yi, J.; Wang, K.; Hou, Y.; Ji, P.; Jin, X.; Li, C.; et al. Microcolin H, a novel autophagy inducer, exerts potent antitumour activity by targeting PITPα/β. Signal Transduct. Target. Ther. 2023, 8, 428. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, M.; Xi, X.; Lu, Y.; Wang, L.; Chen, Y. Synthesis of anti-pancreatic cancer natural product Majusculamide D and analogues reveals a preliminary structure-activity relationships. Chin. J. Chem. 2024, 42, 605–610. [Google Scholar] [CrossRef]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Wang, S.; Dong, G.; Sheng, C. Structural simplification of natural products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Qing, F. Silver-mediated oxidative trifluoromethylation of alcohols to alkyl trifluoromethyl ethers. Org. Lett. 2015, 17, 5048–5051. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bi, K.; Wu, S.; Li, Y.; Huang, Y.; Sheng, C.; Dong, G. Water-soluble derivatives of evodiamine: Discovery of evodiamine-10-phosphate as an orally active antitumor lead compound. Eur. J. Med. Chem. 2021, 220, 113544. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ji, H. Direct targeting of β-catenin in the Wnt signaling pathway: Current progress and perspectives. Med. Res. Rev. 2021, 41, 2109–2129. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Zhang, Y.; Zhou, Z.; Cui, X.; Zhang, L.; Fung, K.-M.; Zheng, W.; Allard, F.D.; Yee, E.U.; et al. ZIP4 promotes pancreatic cancer progression by repressing ZO-1 and Claudin-1 through a ZEB1-dependent transcriptional mechanism. Clin. Cancer Res. 2018, 24, 3186–3196. [Google Scholar] [CrossRef]
- Singh, A.B.; Sharma, A.; Smith, J.J.; Krishnan, M.; Chen, X.; Escheich, S.; Washington, M.K.; Yeatman, T.J.; Beauchamp, R.D.; Dhawan, P. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-Cadherin expression in colon cancer cells. Gastroenterology 2011, 141, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.J.; Kahata, K.; Idås, O.; Thuault, S.; Heldin, C.H.; Moustakas, A. The high mobility group A2 protein epigenetically silences the Cdh1 gene during epithelial-to-mesenchymal transition. Nucleic Acids Res. 2015, 43, 162–178. [Google Scholar] [CrossRef]
- Zhou, Z.; Qutaish, M.; Han, Z.; Schur, R.M.; Liu, Y.; Wilson, D.L.; Lu, Z.-R. LMRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat. Commun. 2015, 6, 7984. [Google Scholar] [CrossRef] [PubMed]
- Sara, M.R.; Vera, M.G.; Catarina, G.T.; Cláudia, M.L.; João, L.; Diana, M.; Paula, C.D.; Helene, N.K.; Isabelle, B.P.; Rui, H.; et al. Vimentin epigenetic deregulation in bladder cancer associates with acquisition of invasive and metastatic phenotype through epithelial-to-mesenchymal transition. Int. J. Biol. Sci. 2023, 19, 1–12. [Google Scholar]




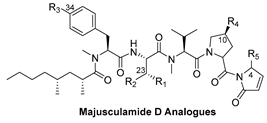 | ||||||
| Compound | R1 | R2 | R3 | R4 | R5 | IC50 (nM) 1 |
| 1a (Majusculamide D) | OAc | CH3 | OMe | OH | CH3 (R) | 1.88 ± 0.82 |
| 14a | OH | CH3 | OMe | OH | CH3 (R) | 1162 ± 194.9 |
| 1c | OCF3 | CH3 | OMe | OH | CH3 (R) | 2.07 ± 0.67 |
| 1d | OAc | H | OMe | OH | CH3 (R) | 2.15 ± 0.37 |
| 1e | 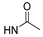 | CH3 | OMe | OH | CH3 (R) | 2.94 ± 0.93 |
| 14b | 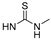 | CH3 | OMe | OH | CH3 (R) | 1.06 ± 0.35 |
| 14c | OAc | CH3 | OH | OH | CH3 (R) | 5.31 ± 1.46 |
| 1h | OAc | CH3 | H | OH | CH3 (R) | 72.02 ± 21.88 |
| 1i | OAc | CH3 | F | OH | CH3 (R) | 0.41 ± 0.08 |
| 1j | OAc | CH3 | Ph | OH | CH3 (R) | 0.70 ± 0.07 |
| 1k | OAc | CH3 | OMe | F | CH3 (R) | 2.55 ± 0.55 |
| 1l | OAc | CH3 | OMe | OH | H | 134.20 ± 78.18 |
| 1m | OAc | CH3 | OMe | OH | CH3 (S) | 4.68 ± 0.59 |
| Compound | IC50 (nM) 1 | Solubility (mg/mL) 2 |
|---|---|---|
| 1a (Majusculamide D) | 1.99 ± 0.68 | Insoluble |
| 16a | 40.76 ± 14.06 | 0.21 |
| 16b | 116.18 ± 53.97 | 0.41 |
| 16c | 34.36 ± 4.50 | 0.20 |
| 16d | 16.16 ± 6.72 | 0.81 |
| 16e | 34.21 ± 6.36 | 0.23 |
| 16f | 13.07 ± 6.30 | 0.17 |
| 16g | 43.14 ± 29.8 | 0.16 |
| 16h | 79.93 ± 45.89 | 0.36 |
| 16i | 739.80 ± 712.91 | 0.53 |
| 16j | 184.15 ± 33.25 | 0.36 |
| 16k | 471.58 ± 69.72 | 0.33 |
| 16l | 55.41 ± 14.73 | 0.13 |
| 16m | 278.4 ± 14.95 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Xi, X.; Zhang, M.; Lv, M.; Zhang, X.; Lu, Y.; Wang, L.; Chen, Y. Structure-Activity Relationship Study of Majusculamide D: Overcoming Metabolic Instability and Severe Toxicity with a Fluoro Analogue. Mar. Drugs 2024, 22, 537. https://doi.org/10.3390/md22120537
Zhao X, Xi X, Zhang M, Lv M, Zhang X, Lu Y, Wang L, Chen Y. Structure-Activity Relationship Study of Majusculamide D: Overcoming Metabolic Instability and Severe Toxicity with a Fluoro Analogue. Marine Drugs. 2024; 22(12):537. https://doi.org/10.3390/md22120537
Chicago/Turabian StyleZhao, Xiuhe, Xiaonan Xi, Mingxiao Zhang, Mengxue Lv, Xiang Zhang, Yaxin Lu, Liang Wang, and Yue Chen. 2024. "Structure-Activity Relationship Study of Majusculamide D: Overcoming Metabolic Instability and Severe Toxicity with a Fluoro Analogue" Marine Drugs 22, no. 12: 537. https://doi.org/10.3390/md22120537
APA StyleZhao, X., Xi, X., Zhang, M., Lv, M., Zhang, X., Lu, Y., Wang, L., & Chen, Y. (2024). Structure-Activity Relationship Study of Majusculamide D: Overcoming Metabolic Instability and Severe Toxicity with a Fluoro Analogue. Marine Drugs, 22(12), 537. https://doi.org/10.3390/md22120537






