Inhibition of Soluble Epoxide Hydrolase by Cembranoid Diterpenes from Soft Coral Sinularia maxima: Enzyme Kinetics, Molecular Docking, and Molecular Dynamics
Abstract
1. Introduction
2. Results
2.1. sEH Inhibitory Effects of the Isolated Cembranoid Diterpenes 1–10
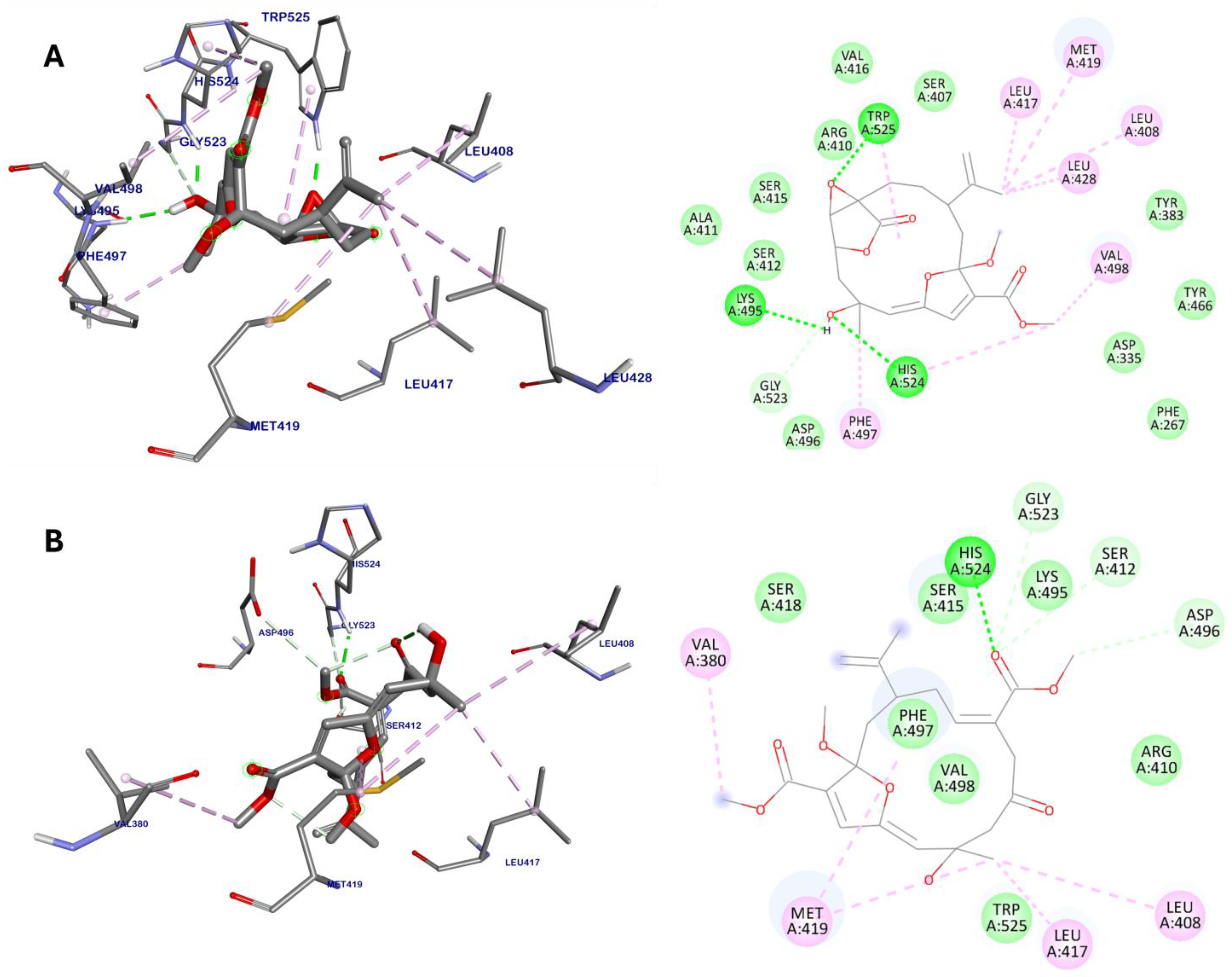
2.2. Molecular Docking
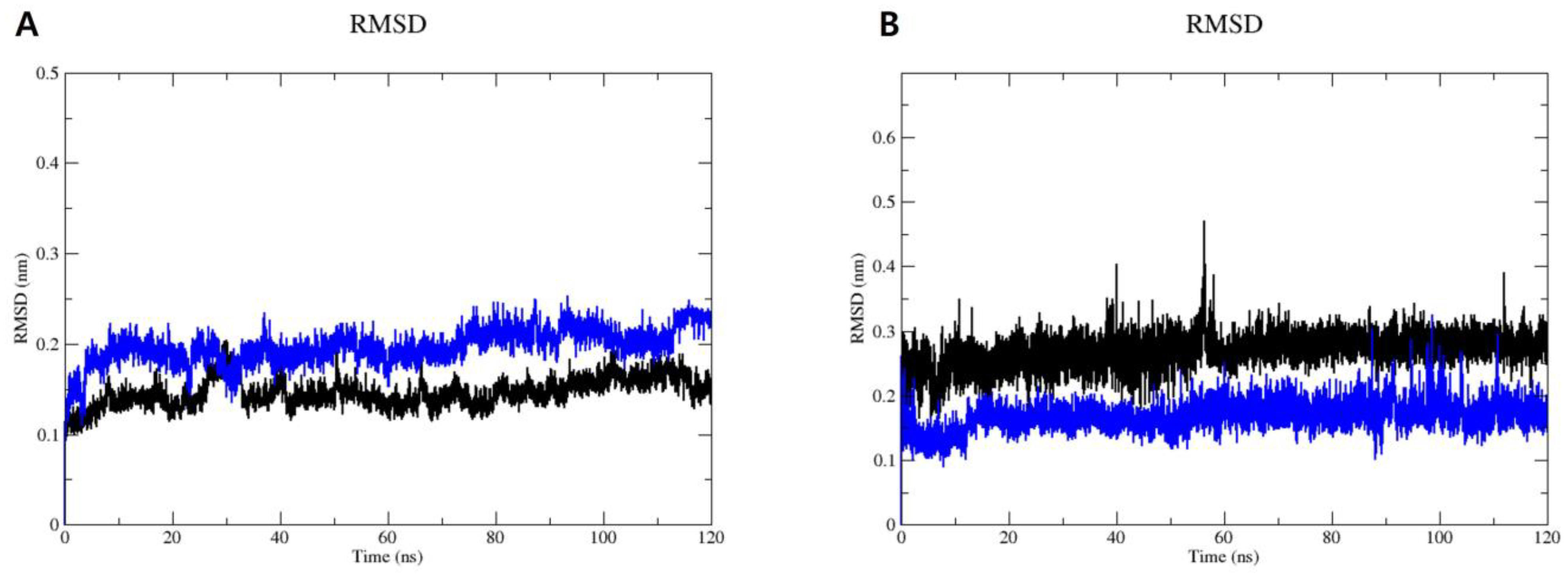
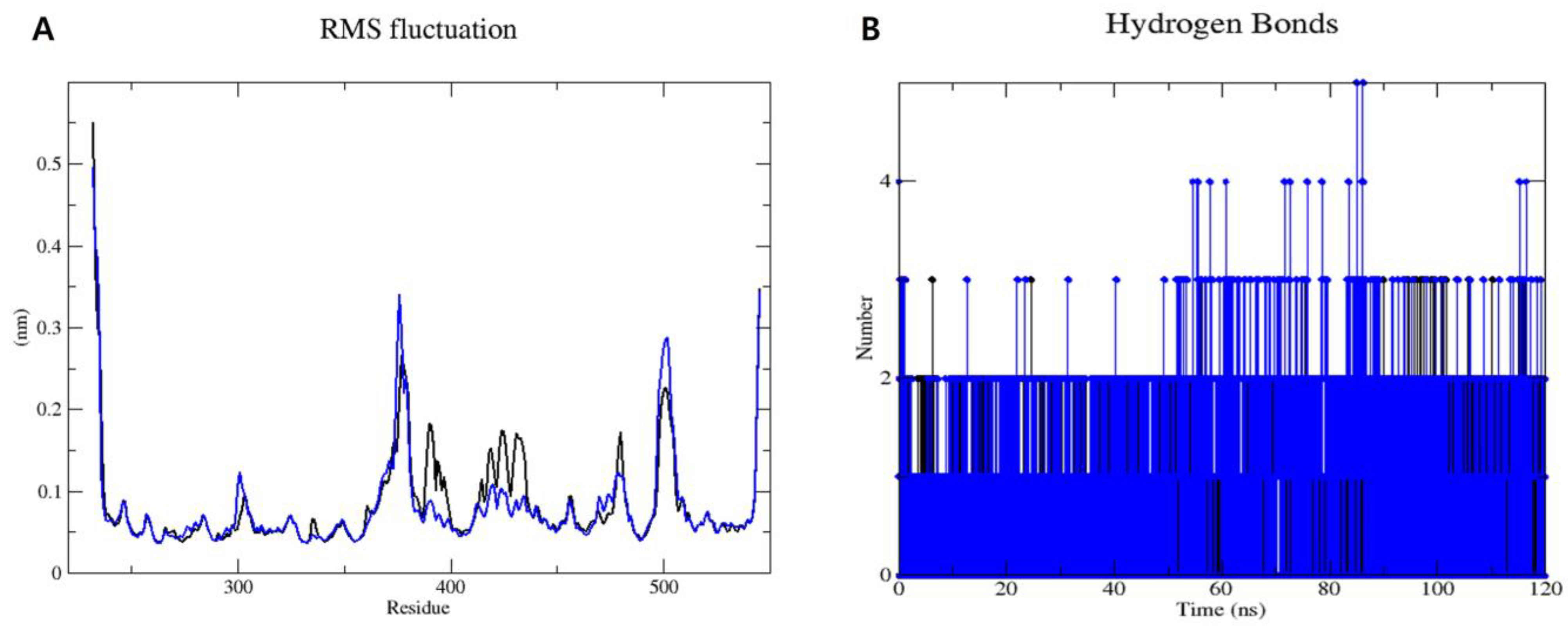
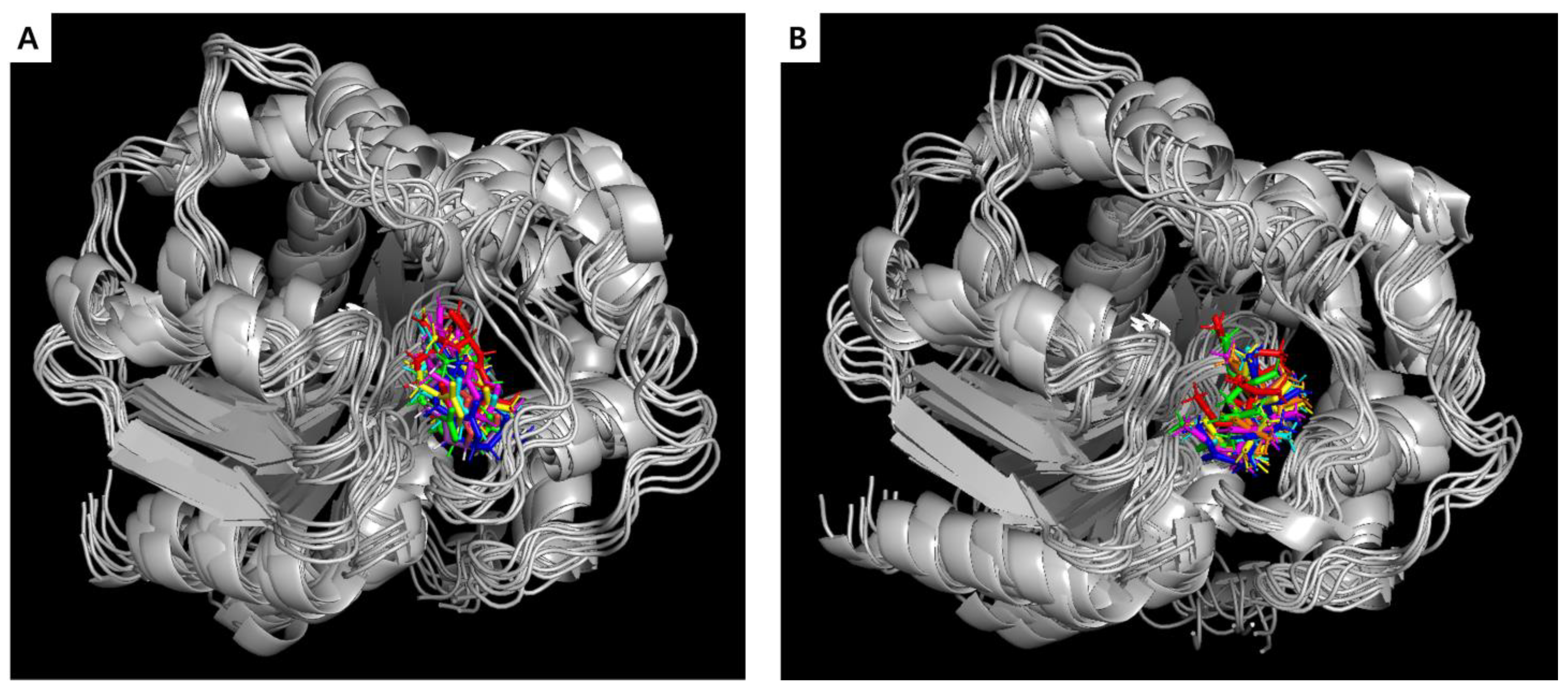
2.3. Molecular Dynamics
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Chemicals and Reagents
4.3. sEH Inhibition Assay
4.4. Molecular Docking Simulation
4.5. Molecular Dynamics Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kitamura, S.; Morisseau, C.; Inceoglu, B.; Kamita, S.G.; De Nicola, G.R.; Nyegue, M.; Hammock, B.D. Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana Baillon: Synthesis, quantification, and measurement of biological activities in vitro and in vivo. PLoS ONE 2015, 10, e0117438. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Hashimoto, K. Soluble epoxide hydrolase as a therapeutic target for neuropsychiatric disorders. Int. J. Mol. Sci. 2022, 23, 4951. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.-Y.; Qiu, H.; Harris, T.R.; Sirish, P.; Hammock, B.D.; Chiamvimonvat, N. Use of metabolomic profiling in the study of arachidonic acid metabolism in cardiovascular disease. Congest. Heart Fail. 2011, 17, 42–46. [Google Scholar] [CrossRef]
- Zarriello, S.; Tuazon, J.P.; Corey, S.; Schimmel, S.; Rajani, M.; Gorsky, A.; Incontri, D.; Hammock, B.D.; Borlongan, C.V. Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders. Prog. Neurobiol. 2019, 172, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Sura, P.; Sura, R.; EnayetAllah, A.E.; Grant, D.F. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 2008, 56, 551–559. [Google Scholar] [CrossRef]
- Lee, G.H.; Oh, S.J.; Lee, S.Y.; Lee, J.-Y.; Ma, J.Y.; Kim, Y.H.; Kim, S.K. Discovery of soluble epoxide hydrolase inhibitors from natural products. Food Chem. Toxicol. 2014, 64, 225–230. [Google Scholar] [CrossRef]
- Buscató, E.l.; Büttner, D.; Brüggerhoff, A.; Klingler, F.-M.; Weber, J.; Scholz, B.; Živković, A.; Marschalek, R.; Stark, H.; Steinhilber, D.; et al. From a multipotent stilbene to soluble epoxide hydrolase inhibitors with antiproliferative properties. ChemMedChem 2013, 8, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Obeme-Nmom, J.I.; Udenigwe, C.C. Soluble epoxide hydrolase: An emerging target for nutraceuticals against inflammation and oxidative stress. Curr. Opin. Food Sci. 2024, 57, 101174. [Google Scholar] [CrossRef]
- Barzkar, N.; Sukhikh, S.; Babich, O. Study of marine microorganism metabolites: New resources for bioactive natural products. Front. Microbiol. 2024, 14, 1285902. [Google Scholar] [CrossRef]
- Mavrommatis, Y.; Ross, K.; Rucklidge, G.; Reid, M.; Duncan, G.; Gordon, M.-J.; Thies, F.; Sneddon, A.; de Roos, B. Intervention with fish oil, but not with docosahexaenoic acid, results in lower levels of hepatic soluble epoxide hydrolase with time in apoE knockout mice. Br. J. Nutr. 2010, 103, 16–24. [Google Scholar] [CrossRef]
- Harris, T.R.; Hammock, B.D. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene 2013, 526, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Jo, A.R.; Kim, Y.H.; Na, M. Epi-leptosphaerin: A new L-isoascorbic acid derivative from marine sponges. Nat. Prod. Sci. 2015, 21, 293–296. [Google Scholar] [CrossRef][Green Version]
- Cho, I.S.; Kim, J.H.; Lin, Y.; Su, X.D.; Kang, J.S.; Yang, S.Y.; Kim, Y.H. Inhibitory activity of quercetin 3-O-arabinofuranoside and 2-oxopomolic acid derived from Malus domestica on soluble epoxide hydrolase. Molecules 2020, 25, 4352. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Zhang, X.-Y.; Zhou, M.-R.; Tian, X.-G.; Lv, X.; Zhang, H.-L.; Deng, S.; Zhang, B.-J.; Sun, C.-P.; Ma, X.-C. Natural soluble epoxide hydrolase inhibitors from Alisma orientale and their potential mechanism with soluble epoxide hydrolase. Int. J. Biol. Macromol. 2021, 183, 811–817. [Google Scholar] [CrossRef]
- Sun, C.-P.; Zhang, J.; Zhao, W.-Y.; Yi, J.; Yan, J.-K.; Wang, Y.-L.; Morisseau, C.; Liu, Z.-B.; Hammock, B.D.; Ma, X.-C. Protostane-type triterpenoids as natural soluble epoxide hydrolase inhibitors: Inhibition potentials and molecular dynamics. Bioorg. Chem. 2020, 96, 103637. [Google Scholar] [CrossRef]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Tung, P.T.; Tai, B.H.; Luyen, B.T.T.; Chae, D.; Kim, S.; Koh, Y.-S.; et al. Diterpenoids from the soft coral Sinularia maxima and their inhibitory effects on lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Chem. Pharm. Bull. 2012, 60, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, J.; Leng, X.; Ouyang, H. Chemical diversity and biological activity of secondary metabolites from soft coral genus Sinularia since 2013. Mar. Drugs 2021, 19, 335. [Google Scholar] [CrossRef]
- Tran, H.H.T.; Nguyen Viet, P.; Nguyen Van, T.; Tran, H.T.; Nguyen Xuan, C.; Nguyen Hoai, N.; Do Cong, T.; Phan Van, K.; Chau Van, M. Cytotoxic steroid derivatives from the Vietnamese soft coral Sinularia brassica. J. Asian Nat. Prod. Res. 2017, 19, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, M.K.; Beeftink, H.H.; Wijffels, R.H. Flow-dependent growth in the zooxanthellate soft coral Sinularia flexibilis. J. Exp. Mar. Biol. Ecol. 2007, 351, 106–113. [Google Scholar] [CrossRef]
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar] [CrossRef]
- Elkhouly, B.H.; Attia, Z.E.; Khedr, I.M.A.; Samy, N.M.; Fouad, A.M. Recent updates on Sinularia soft coral. Mini-Rev. Med. Chem. 2022, 22, 1152–1196. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.; Starmer, J.; Paul, V. Temporal and spatial variation in defensive metabolites of the tropical Pacific soft corals Sinularia maxima and S. polydactyla. Mar. Biol. 2001, 138, 1183–1193. [Google Scholar]
- Slattery, M.; Paul, V.J. Indirect effects of bleaching on predator deterrence in the tropical Pacific soft coral Sinularia maxima. Mar. Ecol. Prog. Ser. 2008, 354, 169–179. [Google Scholar] [CrossRef]
- Wylie, C.R.; Paul, V.J. Chemical defenses in three species of Sinularia (Coelenterata, Alcyonacea): Effects against generalist predators and the butterflyfish Chaetodon unimaculatus Bloch. J. Exp. Mar. Biol. Ecol. 1989, 129, 141–160. [Google Scholar] [CrossRef]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Tung, P.T.; Dat, L.D.; Chae, D.; Kim, S.; Koh, Y.-S.; Kiem, P.V.; et al. Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorg. Med. Chem. Lett. 2013, 23, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Morisseau, C.; Goodrow, M.H.; Newman, J.W.; Wheelock, C.E.; Dowdy, D.L.; Hammock, B.D. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem. Pharmacol. 2002, 63, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, K.-S.; Lee, E.-S.; Kim, Y.-G.; Kim, Y.-I.; Cho, B.O.; Lee, I.S. The inhibition activity of natural methoxyflavonoid from Inula britannica on soluble epoxide hydrolase and NO production in RAW264.7 cells. Int. J. Mol. Sci. 2024, 25, 4357. [Google Scholar] [CrossRef]
- Shi, D.-H.; Xu, C.; Guo, B.-X.; Wang, X.-T.; Chen, Y.-X.; Tan, R.-X. Inhibition of soluble epoxide hydrolase by extracts derived from inflammation-treating Chinese medicinal herbs. Phytother. Res. 2008, 22, 1264–1268. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Butterworth, P.J. The use of dixon plots to study enzyme inhibition. Biochim. Biophys. Acta Enzymol. 1972, 289, 251–253. [Google Scholar] [CrossRef]
- Burlingham, B.T.; Widlanski, T.S. An intuitive look at the relationship of Ki and IC50: A more general use for the Dixon plot. J. Chem. Educ. 2003, 80, 214. [Google Scholar] [CrossRef]
- Palmer, T.; Bonner, P.L. 8—Enzyme Inhibition. In Enzymes, 2nd ed.; Palmer, T., Bonner, P.L., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 126–152. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Tanaka, D.; Tsuda, Y.; Shiyama, T.; Nishimura, T.; Chiyo, N.; Tominaga, Y.; Sawada, N.; Mimoto, T.; Kusunose, N. A practical use of ligand efficiency indices out of the fragment-based approach: Ligand efficiency-guided lead identification of soluble epoxide hydrolase inhibitors. J. Med. Chem. 2011, 54, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-H.; Liao, Y.-J.; Hsiao, S.-H.; Shyue, S.-K.; Lee, T.-S. Role of phosphatase activity of soluble epoxide hydrolase in regulating simvastatin-activated endothelial nitric oxide synthase. Sci. Rep. 2015, 5, 13524. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.V.; Van Cong, P.; Min, B.S.; Yang, S.Y.; Kim, J.A. Inhibitory effects of phenylpropanoids and lignans isolated from the bark of Cinnamomum cassia L. on soluble epoxide hydrolase: Spectroscopic, kinetic, molecular docking, and molecular dynamics studies. J. Mol. Struct. 2024, 1301, 137376. [Google Scholar] [CrossRef]
- Kramer, J.; Proschak, E. Phosphatase activity of soluble epoxide hydrolase. Prostaglandins Other Lipid Mediat. 2017, 133, 88–92. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Sun, C.-P.; Xu, J.-X.; Morisseau, C.; Hammock, B.D.; Qiu, F. Phytochemical constituents from Scutellaria baicalensis in soluble epoxide hydrolase inhibition: Kinetics and interaction mechanism merged with simulations. Int. J. Biol. Macromol. 2019, 133, 1187–1193. [Google Scholar] [CrossRef]
- Gurung, A.B.; Mayengbam, B.; Bhattacharjee, A. Discovery of novel drug candidates for inhibition of soluble epoxide hydrolase of arachidonic acid cascade pathway implicated in atherosclerosis. Comput. Biol. Chem. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Barbosa-Sicard, E.; Frömel, T.; Keserü, B.; Brandes, R.P.; Morisseau, C.; Hammock, B.D.; Braun, T.; Krüger, M.; Fleming, I. Inhibition of the soluble epoxide hydrolase by tyrosine nitration. J. Biol. Chem. 2009, 284, 28156–28163. [Google Scholar] [CrossRef]
- Dötsch, L.; Davies, C.; Hennes, E.; Schönfeld, J.; Kumar, A.; Guita, C.D.C.L.; Ehrler, J.H.M.; Hiesinger, K.; Thavam, S.; Janning, P.; et al. Discovery of the sEH inhibitor epoxykynin as a potent kynurenine pathway modulator. J. Med. Chem. 2024, 67, 4691–4706. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Abis, G.; Mattingly-Peck, F.; Lynham, S.; Fraternali, F.; Conte, M.R. Allosteric regulation of the soluble epoxide hydrolase by nitro fatty acids: A combined experimental and computational approach. J. Mol. Biol. 2022, 434, 167600. [Google Scholar] [CrossRef]
- Oanh, V.T.; Phong, N.V.; Min, B.S.; Yang, S.Y.; Kim, J.A. Insights into the inhibitory activities of neolignans and diarylnonanoid derivatives from nutmeg (Myristica fragrans Houtt.) seeds on soluble epoxide hydrolase using in vitro and in silico approaches. J. Enzym. Inhib. Med. Chem. 2023, 38, 2251099. [Google Scholar] [CrossRef] [PubMed]
- Das Mahapatra, A.; Choubey, R.; Datta, B. Small molecule soluble epoxide hydrolase inhibitors in multitarget and combination therapies for inflammation and cancer. Molecules 2020, 25, 5488. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Paliwal, S.; Sharma, S.; Verma, K.; Gururani, R.; Tiwari, A.; Verma, A.; Chauhan, M.; Singh, A.; Kumar, D.; et al. Discovery of novel soluble epoxide hydrolase inhibitors as potent vasodilators. Sci. Rep. 2018, 8, 14604. [Google Scholar] [CrossRef]
- Han, Y.K.; Lee, J.S.; Yang, S.Y.; Lee, K.Y.; Kim, Y.H. In vitro and in silico studies of soluble epoxide hydrolase inhibitors from the roots of Lycopus lucidus. Plants 2021, 10, 356. [Google Scholar] [CrossRef]
- Ahamad, S.; Gupta, D.; Kumar, V. Targeting SARS-CoV-2 nucleocapsid oligomerization: Insights from molecular docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2022, 40, 2430–2443. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.G.; Weiskirchen, R.; Al-Musharafi, S.K. The use of marine-derived bioactive compounds as potential hepatoprotective agents. Acta Pharmacol. Sin. 2015, 36, 158–170. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y. A review on bioactive compounds from marine-derived Chaetomium species. J. Microbiol. Biotechnol. 2022, 32, 541–550. [Google Scholar] [CrossRef]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [PubMed]
- Congreve, M.; Oswald, C.; Marshall, F.H. Applying structure-based drug design approaches to allosteric modulators of GPCRs. Trends Pharmacol. Sci. 2017, 38, 837–847. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, X.; Xiang, Y.; Xie, L.; Sun, M.; Shi, J. Advances in CD73 inhibitors for immunotherapy: Antibodies, synthetic small molecule compounds, and natural compounds. Eur. J. Med. Chem. 2023, 258, 115546. [Google Scholar] [CrossRef]
- Waltenberger, B.; Garscha, U.; Temml, V.; Liers, J.; Werz, O.; Schuster, D.; Stuppner, H. Discovery of potent soluble epoxide hydrolase (sEH) inhibitors by pharmacophore-based virtual screening. J. Chem. Inf. Model. 2016, 56, 747–762. [Google Scholar] [CrossRef]
- Trang, N.M.; Kim, E.-N.; Pham, T.H.; Jeong, G.-S. Citropten ameliorates osteoclastogenesis related to MAPK and PLCγ/Ca2+ signaling pathways through the regulation of amyloid beta. J. Agric. Food Chem. 2023, 71, 10037–10049. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Yan, J.-J.; Zhang, M.; Wang, C.; Feng, L.; Lv, X.; Huo, X.-K.; Sun, C.-P.; Chen, L.-X.; Ma, X.-C. Natural soluble epoxide hydrolase inhibitors from Inula britanica and their potential interactions with soluble epoxide hydrolase: Insight from inhibition kinetics and molecular dynamics. Chem. Biol. Interact. 2021, 345, 109571. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-P.; Zhang, X.-Y.; Morisseau, C.; Hwang, S.H.; Zhang, Z.-J.; Hammock, B.D.; Ma, X.-C. Discovery of soluble epoxide hydrolase inhibitors from chemical synthesis and natural products. J. Med. Chem. 2020, 64, 184–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jo, Y.D.; Kim, H.-Y.; Kim, B.-R.; Nam, B. In vitro and in silico insights into sEH inhibitors with amide-scaffold from the leaves of Capsicum chinense Jacq. Comput. Struct. Biotechnol. J. 2018, 16, 404–411. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell Jr, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.V.; Heo, M.-S.; Vinh, L.B.; Kim, Y.H.; Yang, S.Y. Investigation of the inhibitory activity of triterpenoids isolated from Actinidia polygama stems against β-glucuronidase via enzyme kinetics, molecular docking, and molecular dynamics analyses. J. Mol. Struct. 2024, 1317, 139135. [Google Scholar] [CrossRef]
- Yang, S.Y.; Phong, N.V. Phenolic compounds from the stems of Zanthoxylum piperitum and their inhibition of β-glucuronidase: Enzyme kinetics, molecular docking, and molecular dynamics analysis. J. Mol. Struct. 2024, 1317, 139117. [Google Scholar] [CrossRef]
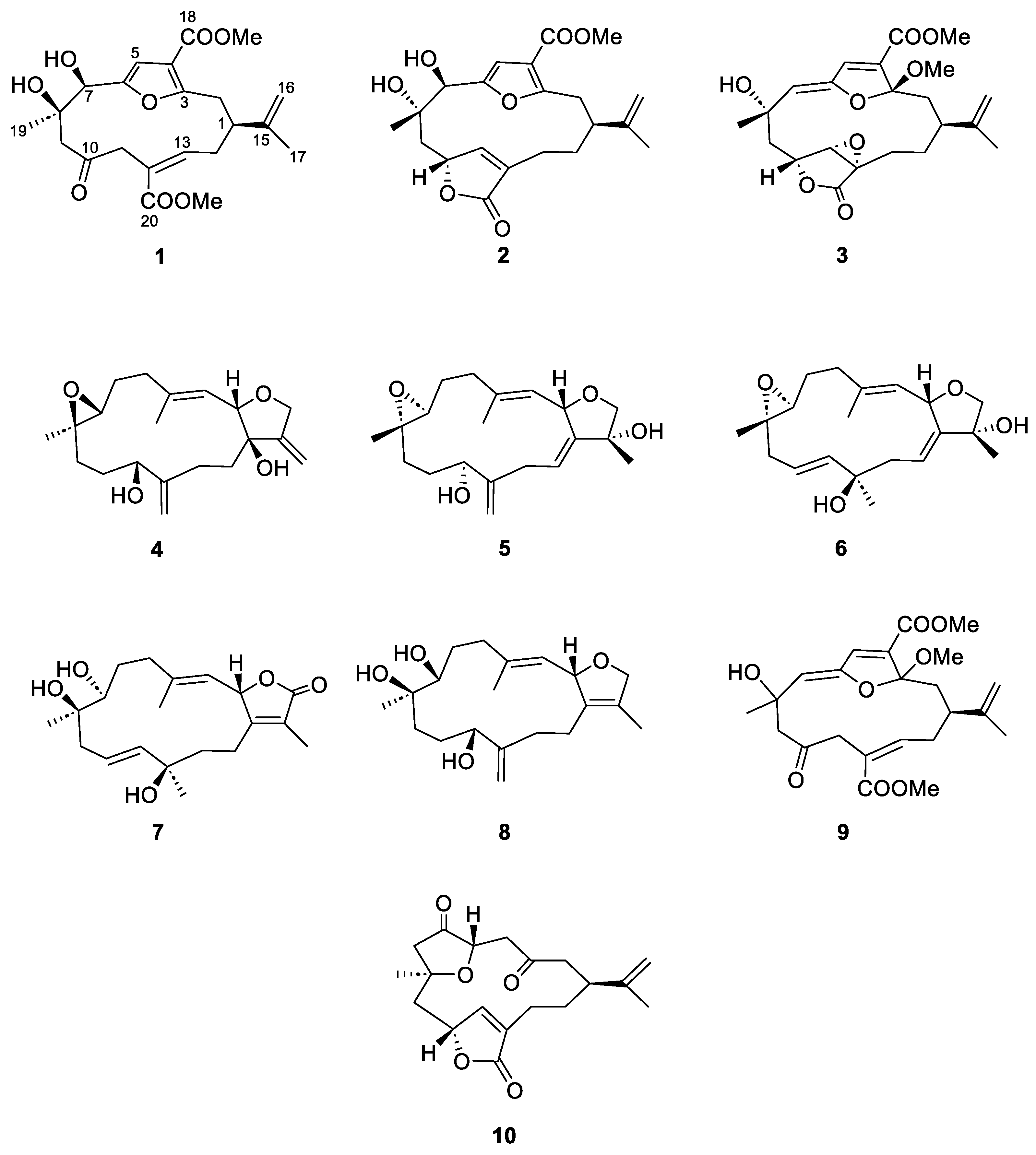
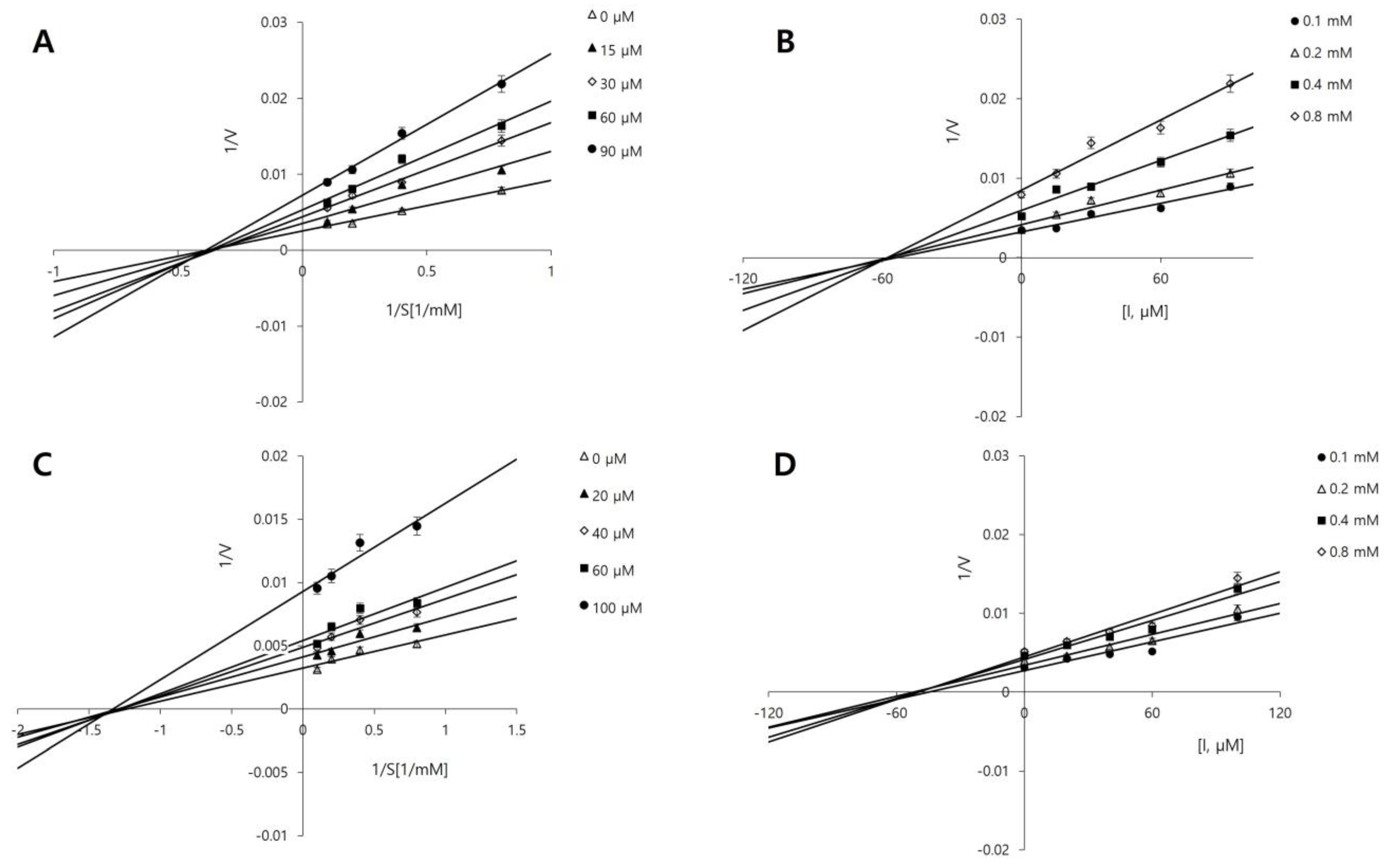
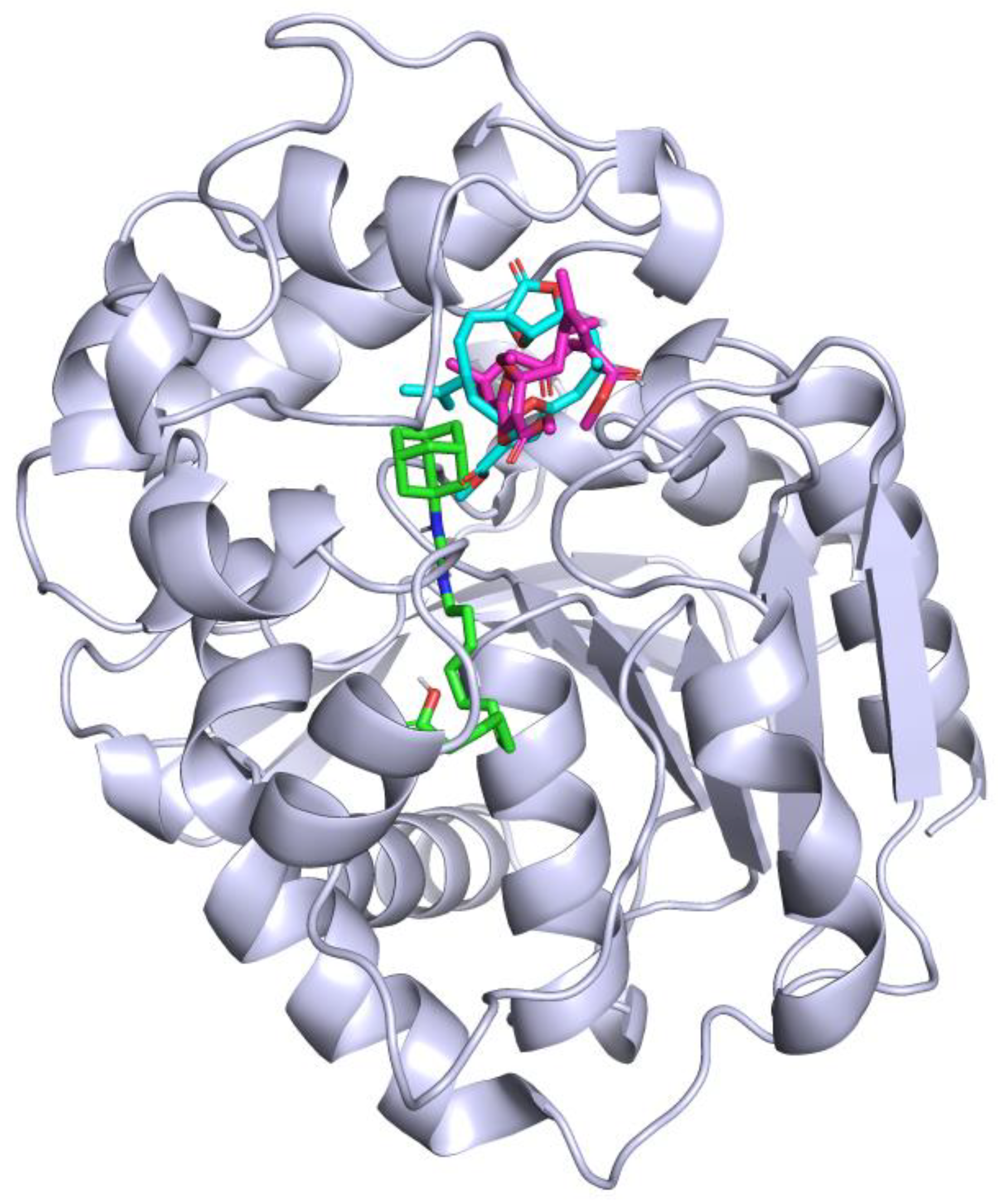
| Compounds | IC50 (μM) 1 | Inhibition Type 2 | Ki (μM) 3 |
|---|---|---|---|
| 1 | >100 | – | – |
| 2 | >100 | – | – |
| 3 | 70.68 ± 1.44 | Non-competitive | 59.44 |
| 4 | >100 | – | – |
| 5 | >100 | – | – |
| 6 | >100 | – | – |
| 7 | >100 | – | – |
| 8 | >100 | – | – |
| 9 | 78.83 ± 2.26 | Non-competitive | 55.03 |
| 10 | >100 | – | – |
| AUDA 4 | 14.69 ± 0.07 nM | – | – |
| Compound | Binding Energy (kcal/mol) | Hydrogen Bonds | van der Waals Interactions | Hydrophobic Interactions |
|---|---|---|---|---|
| 3 | −9.18 | Lys495 Gly523 His524 Trp525 | Phe267 Asp335 Tyr383 Ser407 Arg410 Ala411 Ser412 Ser415 Val416 Tyr466 Asp496 | Leu408 (alkyl) Leu417 (alkyl) Met419 (alkyl) Leu428 (alkyl) Phe497 (π−alkyl) Val498 (alkyl) His524 (π−alkyl) Trp525 (π−alkyl) |
| 9 | −8.94 | Ser412 Asp496 Gly523 His524 | Arg410 Ser415 Ser418 Lys495 Phe497 Val498 Trp525 | Val380 (alkyl) Leu408 (alkyl) Leu417 (alkyl) Met419 (alkyl) |
| AUDA | −8.53 | Asp335 Gln384 Tyr466 | Phe267 Pro268 Thr360 Phe387 Leu428 Met469 Val498 Met503 | Trp336 (π−alkyl) Met339 (alkyl) Phe381 (π−σ) Tyr383 (π−alkyl) Leu408 (alkyl) Met419 (alkyl) Leu499 (alkyl) His524 (π−alkyl) Trp525 (π−alkyl) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phong, N.V.; Thao, N.P.; Vinh, L.B.; Luyen, B.T.T.; Minh, C.V.; Yang, S.Y. Inhibition of Soluble Epoxide Hydrolase by Cembranoid Diterpenes from Soft Coral Sinularia maxima: Enzyme Kinetics, Molecular Docking, and Molecular Dynamics. Mar. Drugs 2024, 22, 373. https://doi.org/10.3390/md22080373
Phong NV, Thao NP, Vinh LB, Luyen BTT, Minh CV, Yang SY. Inhibition of Soluble Epoxide Hydrolase by Cembranoid Diterpenes from Soft Coral Sinularia maxima: Enzyme Kinetics, Molecular Docking, and Molecular Dynamics. Marine Drugs. 2024; 22(8):373. https://doi.org/10.3390/md22080373
Chicago/Turabian StylePhong, Nguyen Viet, Nguyen Phuong Thao, Le Ba Vinh, Bui Thi Thuy Luyen, Chau Van Minh, and Seo Young Yang. 2024. "Inhibition of Soluble Epoxide Hydrolase by Cembranoid Diterpenes from Soft Coral Sinularia maxima: Enzyme Kinetics, Molecular Docking, and Molecular Dynamics" Marine Drugs 22, no. 8: 373. https://doi.org/10.3390/md22080373
APA StylePhong, N. V., Thao, N. P., Vinh, L. B., Luyen, B. T. T., Minh, C. V., & Yang, S. Y. (2024). Inhibition of Soluble Epoxide Hydrolase by Cembranoid Diterpenes from Soft Coral Sinularia maxima: Enzyme Kinetics, Molecular Docking, and Molecular Dynamics. Marine Drugs, 22(8), 373. https://doi.org/10.3390/md22080373






