Lipids Extracted from Aptocyclus ventricosus Eggs Possess Immunoregulatory Effects on RAW264.7 Cells by Activating the MAPK and NF-κB Signaling Pathways
Abstract
1. Introduction
2. Results
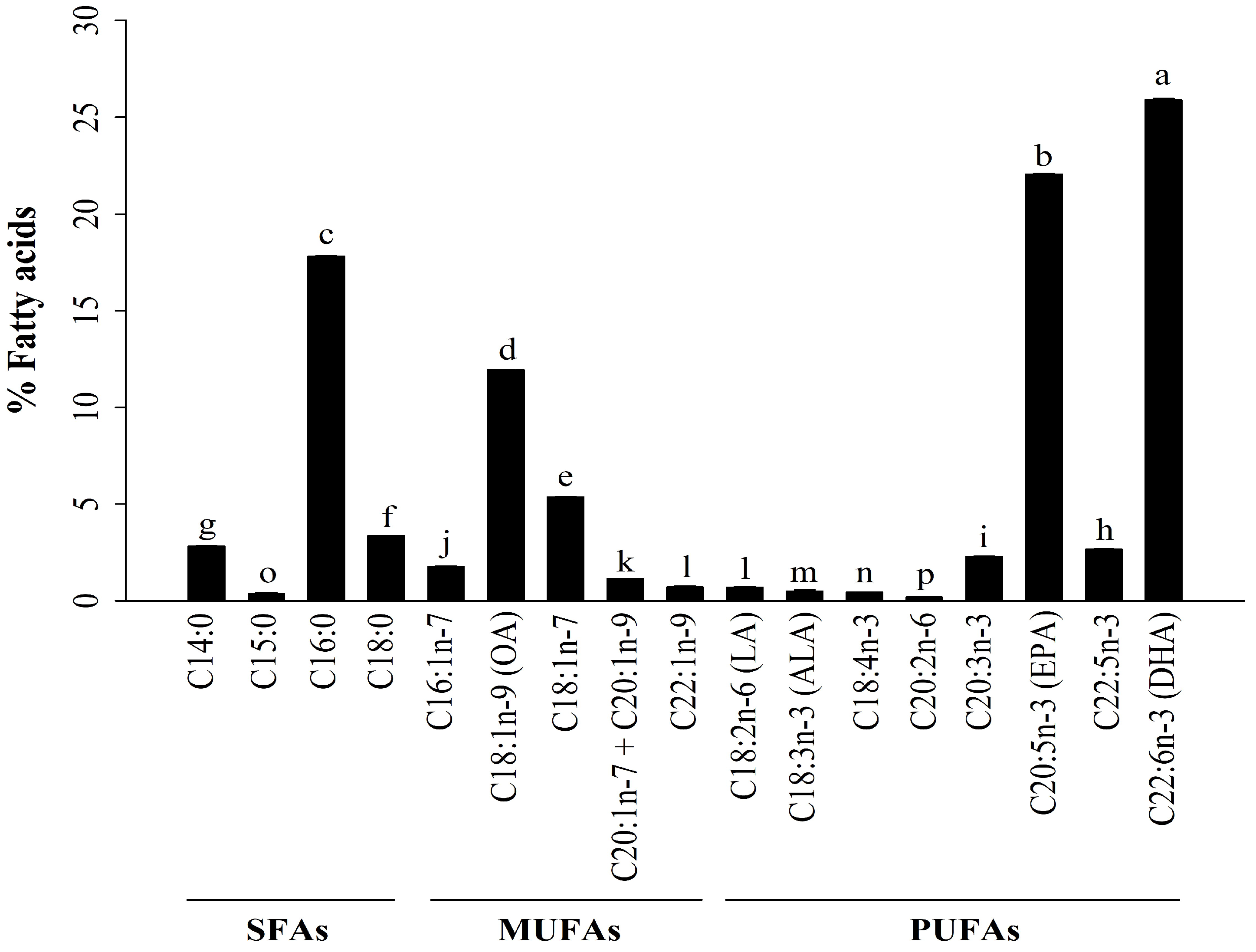
2.1. Analysis of Fatty Acids (FAs) in A. ventricosus Lipids
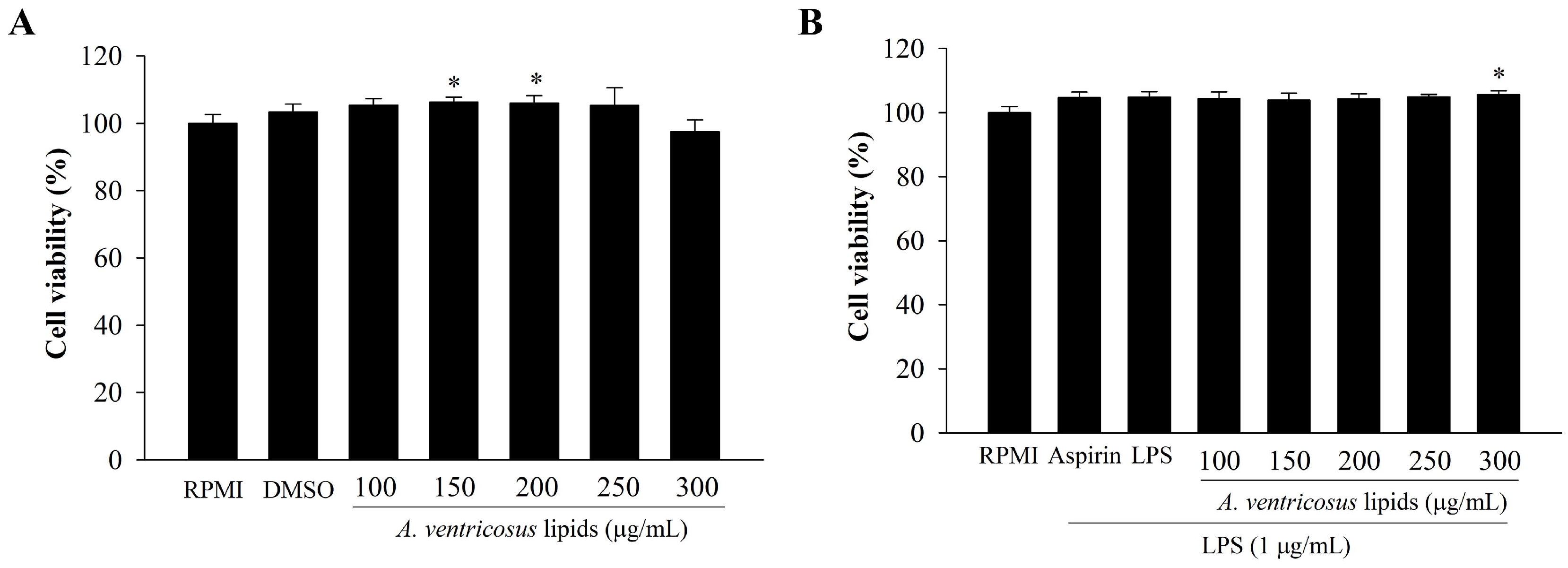
2.2. Cytotoxic Effect of A. ventricosus Lipids on Macrophages
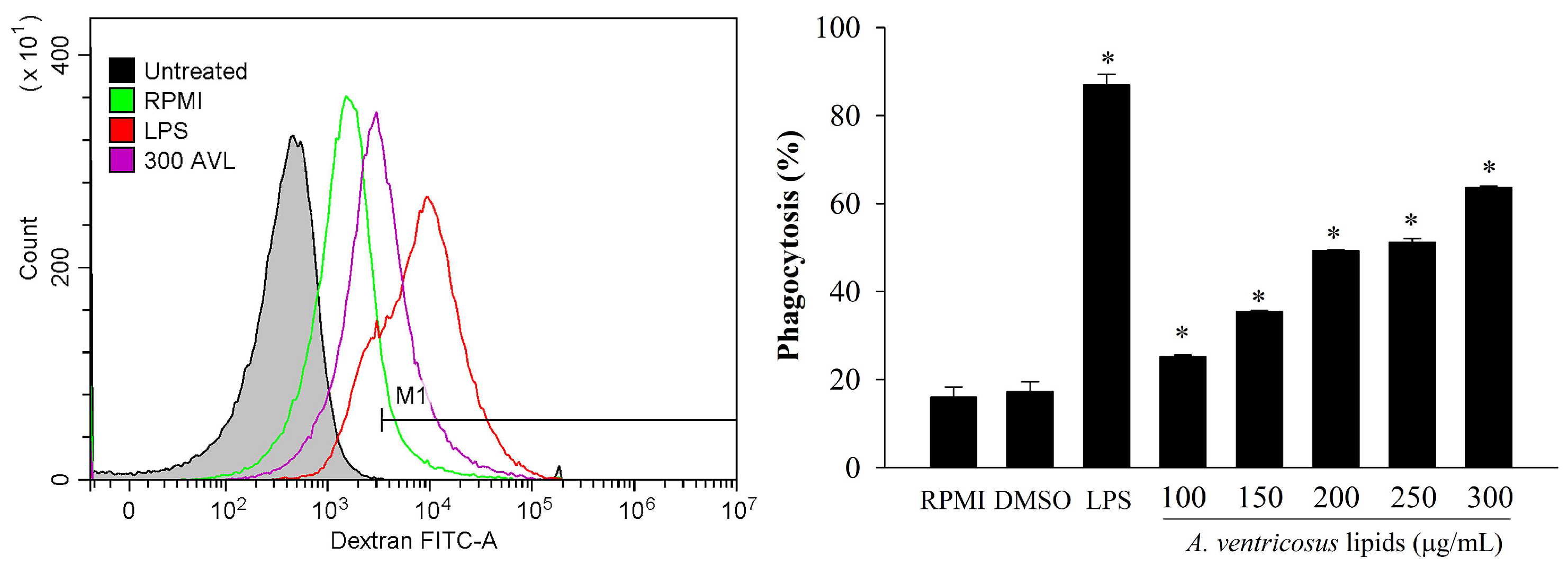
2.3. Effects of A. ventricosus Lipids on Phagocytosis of Macrophages
2.4. Effects of A. ventricosus Lipids on NO Production and iNOS Expression
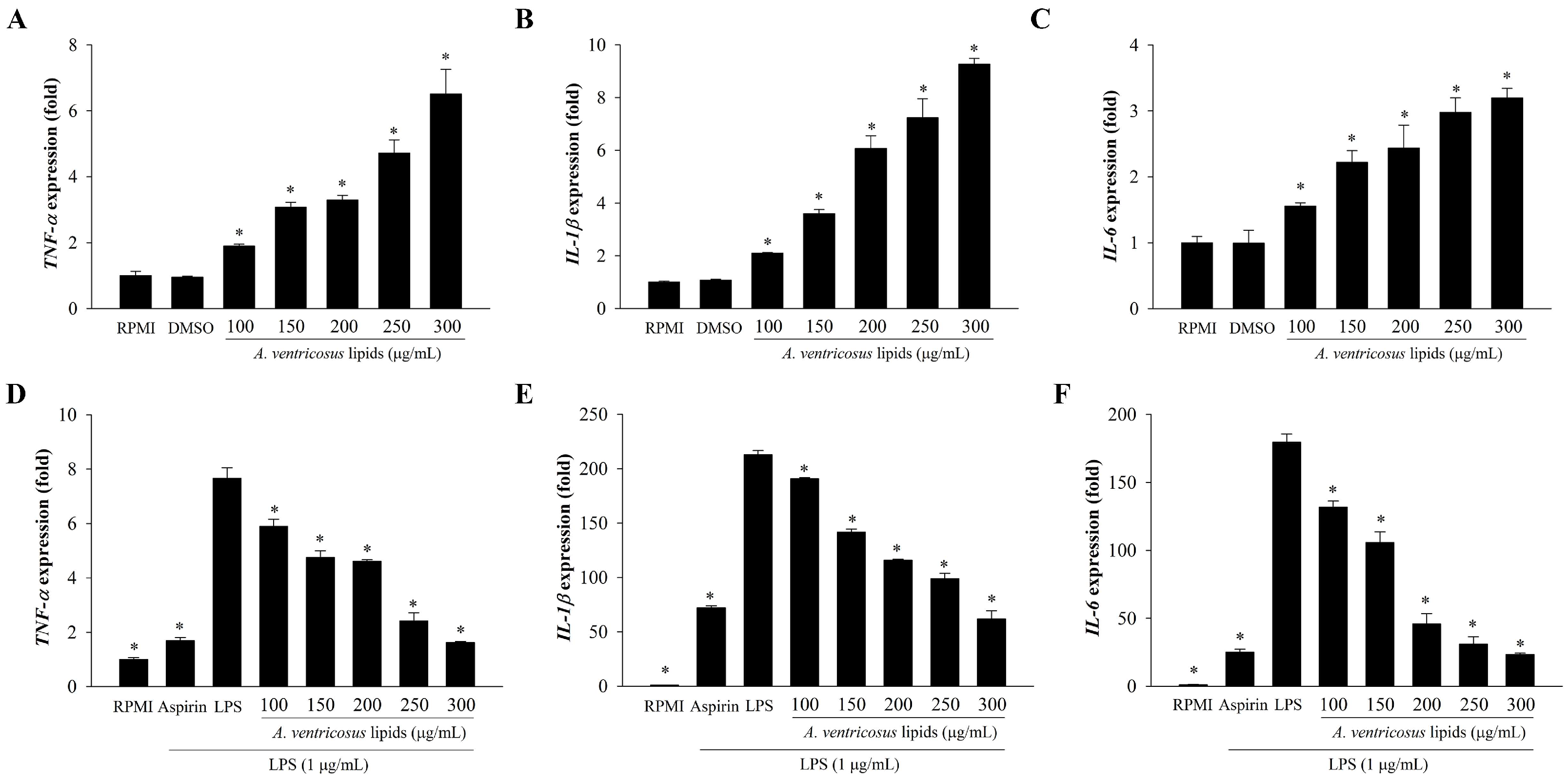
2.5. Effects of A. ventricosus Lipids on Cytokine Expression
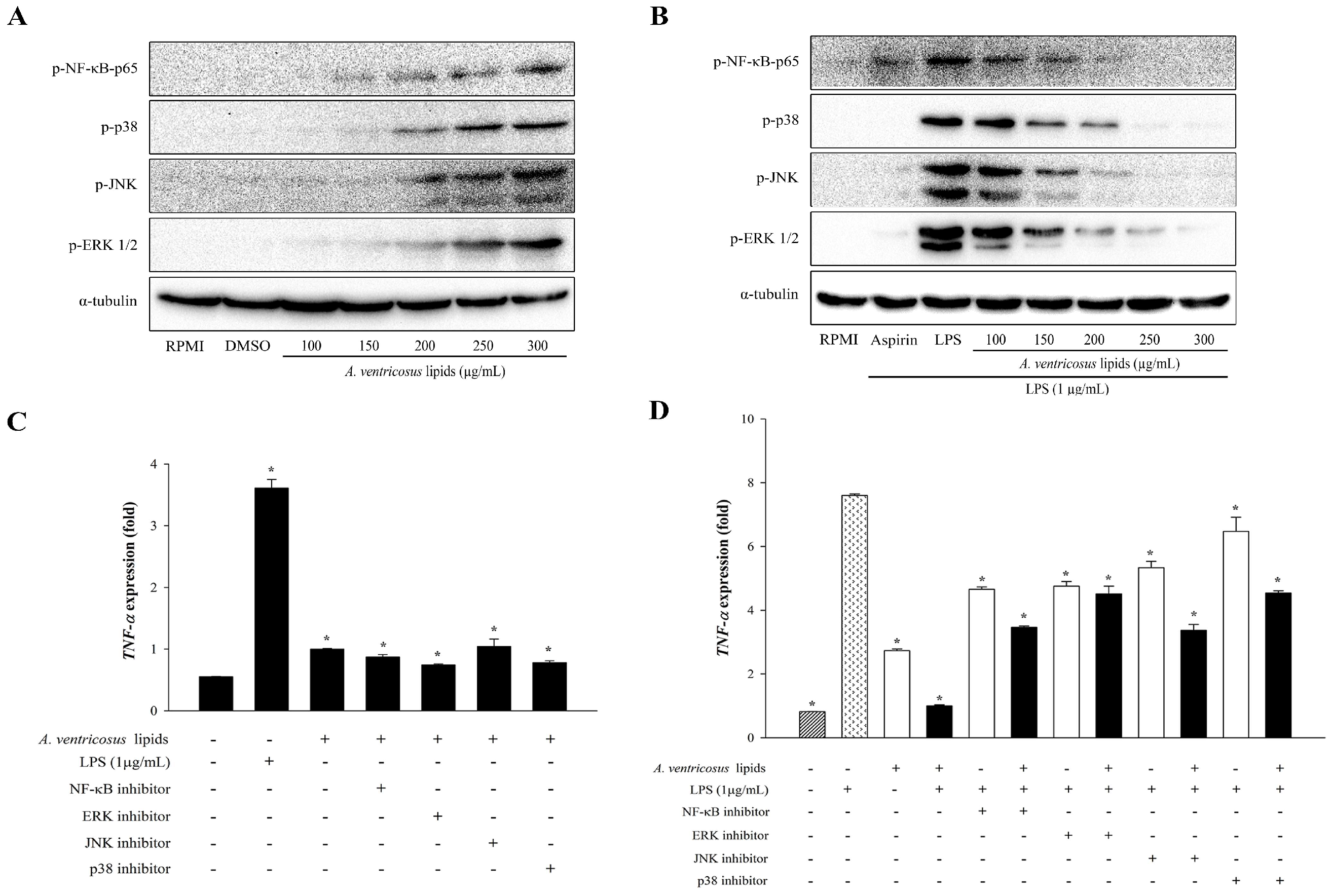
2.6. Effects of A. ventricosus Lipids on NF-κB and MAPK Activation
2.7. Effects of A. ventricosus Lipids on TNF-α Expression after Co-Treatment with Specific Inhibitors via NF-κB and MAPK Activation
2.8. Effects of A. ventricosus Lipids on LPS-Induced Cell Surface Molecule Expression
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Preparation of A. ventricosus Lipids
4.3. Determination of Fatty Acid Compositions
4.4. Cell Culture and Treatments
4.5. Assay of Cell Viability
4.6. Measurement of NO Production
4.7. RNA Isolation and Real-Time qPCR
4.8. Western Blotting Assay
4.9. Inhibition of NF-κB and MAPK Using Specific Inhibitors
4.10. Phagocytic Uptake of Macrophages
4.11. Analysis of Expression of Cell Surface Molecules
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Florean, C.; Dicato, M.; Diederich, M. Immune-modulating and anti-inflammatory marine compounds against cancer. Semin. Cancer Biol. 2022, 80, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, L.; Sun, H.; Wang, Y.; Yang, Z.; Zhang, G.; Jiang, S.; Yang, W. Polysaccharide from alfalfa activates RAW264.7 macrophages through MAPK and NF-kB signaling pathways. Int. J. Biol. Macromol. 2019, 126, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, J.-F.; Zha, X.-Q.; Cui, S.-H.; Cao, L.; Luo, J.-P. Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydr. Polym. 2015, 134, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.N.; Hyun, S.B.; Ahn, K.J.; Hyun, C.-G. Immunomodulatory effects of Abelmoschus esculentus water extract through MAPK and NF-κB signaling in RAW264.7 cells. Biotechnol. Notes 2022, 3, 38–44. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kwon, M.-S.; Choi, J.-W.; Shin, T.; No, H.K.; Choi, J.-S.; Byun, D.-S.; Kim, J.-I.; Kim, H.-R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW264.7 murine macrophage cells. J. Agricul. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef]
- Lim, J.; Rod-in, W.; Monmai, C.; Jang, A.Y.; Choi, J.; Park, W.-J. In vitro immune-enhancement and anti-inflammatory effects of fatty acids extracted from the Halocynthia aurantium gonad on RAW264.7 macrophages. Nutrients 2022, 14, 4510. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Cai, R.-L.; Gao, Y.; Qi, Y. Lipid-soluble extracts from Salvia miltiorrhiza inhibit production of LPS-induced inflammatory mediators via NF-κB modulation in RAW264.7 cells and perform anti-inflammatory effects in vivo. Phytother. Res. 2012, 26, 1195–1204. [Google Scholar] [CrossRef]
- Son, H.J.; Eo, H.J.; Park, G.H.; Jeong, J.B. Heracleum moellendorffii root extracts exert immunostimulatory activity through TLR2/4-dependent MAPK activation in mouse macrophages, RAW264.7 cells. Food Sci. Nutr. 2021, 9, 514–521. [Google Scholar] [CrossRef]
- Wang, T.; Wu, F.; Jin, Z.; Zhai, Z.; Wang, Y.; Tu, B.; Yan, W.; Tang, T. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem. Toxicol. 2014, 64, 177–183. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-kB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cejas, J.R.; Almansa, E.; Villamandos, J.E.; Badía, P.; Bolaños, A.; Lorenzo, A. Lipid and fatty acid composition of ovaries from wild fish and ovaries and eggs from captive fish of white sea bream (Diplodus sargus). Aquaculture 2003, 216, 299–313. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices, of raw roe of Blue Fin Tuna (Thunnus thynnus L.) and their salted product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Huynh, M.D.; Kitts, D.D.; Hu, C.; Trites, A.W. Comparison of fatty acid profiles of spawning and non-spawning Pacific herring, Clupea harengus pallasi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 504–511. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef]
- Holub, D.J.; Holub, B.J. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell. Biochem. 2004, 263, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Torrejon, C.; Jung, U.J.; Deckelbaum, R.J. n-3 Fatty acids and cardiovascular disease: Actions and molecular mechanisms. Prostaglandins Leukot Essent Fat. Acids 2007, 77, 319–326. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, G.S.; Monmai, C.; Rod-in, W.; Jang, A.Y.; Park, W.J. Immunomodulatory activities of Ammodytes personatus egg lipid in RAW264.7 cells. Molecules 2021, 26, 6027. [Google Scholar] [CrossRef]
- Choi, G.S.; Lim, J.H.; Rod-In, W.; Jung, S.K.; Park, W.J. Anti-inflammatory properties of neutral lipids, glycolipids, and phospholipids isolated from Ammodytes personatus eggs in LPS-stimulated RAW264.7 cells. Fish Shellfish Immunol. 2022, 131, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.M.; Tokranov, A.M. Specific features of distribution, some features of biology, and the dynamics of catches of smooth lumpsucker Aptocyclus ventricosus (Cyclopteridae) in waters of the Pacific Ocean off the Kuril Islands and Kamchatka. J. Ichthyol. 2008, 48, 81–95. [Google Scholar] [CrossRef]
- Solomatov, S.F.; Orlov, A.M. Smooth lumpsucker Aptocyclus ventricosus in the northwestern Sea of Japan: Distribution and some life history traits. Fish. Aquat. Life 2018, 26, 5–20. [Google Scholar] [CrossRef]
- Okazaki, T.; Stevenson, D.E.; Kai, Y.; Ueda, Y.; Hamatsu, T.; Yamashita, Y. Genetic population structure and demographic history of a pelagic lumpsucker, Aptocyclus ventricosus. Environ. Biol. Fish 2020, 103, 283–289. [Google Scholar] [CrossRef]
- Kim, I.-S.; Park, H.-J.; Jeong, B.-Y.; Moon, S.-K. Food components characteristics of the muscles and roes of Smooth Lumpsucker Aptocyclus ventricosus and Korai bikunin Liparis ingens from the East sea, Korea. Korean J. Fish. Aquat. Sci. 2020, 53, 809–815. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health Bbenefits throughout life. Advances in Nutrition 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Kim, H.; Park, J.J.; Kim, J.W.; Lee, J.S. Ultrastructure of Integument of the Smooth Lumpsucker, Aptocyclus ventricosus (Pallas, 1769)(Teleostei: Cyclopteridae). Korean J. Ichthyol. 2016, 28, 147–155. [Google Scholar]
- Kobayashi, K. Larvae of the smooth lumpsucker, Aptocyclus ventricosus (Pallas), with discussion on revision of the taxonomy of the species. Bull. Fac. Fish. Hokkaido Univ. 1962, 13, 153–164. [Google Scholar]
- Rincón-Cervera, M.Á.; Suárez-Medina, M.D.; Guil-Guerrero, J.L. Fatty acid composition of selected roes from some marine species. Eur. J. Lipid Sci. Technol. 2009, 111, 920–925. [Google Scholar] [CrossRef]
- Han, L.; Yu, J.; Chen, Y.; Cheng, D.; Wang, X.; Wang, C. Immunomodulatory activity of docosahexenoic acid on RAW264.7 cells activation through GPR120-mediated signaling pathway. J. Agric. Food Chem. 2018, 66, 926–934. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Uday Kumar, P.; Nimgulkar, C.; Dinesh Kumar, B. Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Res. Int. 2014, 62, 1054–1061. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Chu, T.-W.; Danata, R.H.; Risjani, Y.; Shih, H.-T.; Hu, S.-Y. Hepcidin-expressing fish eggs as a novel food supplement to modulate immunity against pathogenic infection in Zebrafish (Danio rerio). Sustainability 2020, 12, 4057. [Google Scholar] [CrossRef]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.H.; Brown, G.D.; Gordon, S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2004, 23, 901–944. [Google Scholar] [CrossRef] [PubMed]
- Boscá, L.; Zeini, M.; Través, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Rod-in, W.; Monmai, C.; Shin, I.-S.; You, S.; Park, W.J. Neutral lipids, glycolipids, and phospholipids, isolated from Sandfish (Arctoscopus japonicus) eggs, exhibit anti-inflammatory activity in LPS-stimulated RAW264.7 cells through NF-κB and MAPKs pathways. Mar. Drugs 2020, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.J.; Park, Y.; Kwon, H.Y.; Park, G.H. Immune-enhancing effects of Hibiscus syriacus roots in RAW264.7 macrcophages. Food Agric. Immunol. 2022, 33, 617–626. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Yang, L.; Jiang, J.-G.; Zheng, C.-Y.; Zhu, W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara Engl. Food Funct. 2017, 8, 796–807. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, L.; Yu, B.; Chen, K.; Liu, B.; Liu, J.; Qin, G.; Liu, C.; Liu, H.; Chen, K. Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation. Carbohydr. Polym. 2016, 149, 112–120. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lin, H.Y.; Wang, C.C.; Zhang, M.; Lin, Y.Y.; Huang, F.Y.; Lin, Y.Z.; Tan, G.H. Exopolysaccharide from Paecilomyces lilacinus modulates macrophage activities through the TLR4/NF-κB/MAPK pathway. Mol. Med. Rep. 2019, 20, 4943–4952. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, A.; Harorli, T.; Limtanyakul, J.; Hiller, K.-A.; Bosl, C.; Bolay, C.; Reichl, F.-X.; Schmalz, G.; Schweikl, H. Inhibition of cytokine and surface antigen expression in LPS-stimulated murine macrophages by triethylene glycol dimethacrylate. Biomaterials 2009, 30, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Bruch, D.; Kittur, D.S. Ginger extract inhibits LPS induced macrophage activation and function. BMC Complement. Altern. Med. 2008, 8, 1. [Google Scholar] [CrossRef]
- Kumar, A.; Sawhney, G.; Kumar Nagar, R.; Chauhan, N.; Gupta, N.; Kaul, A.; Ahmed, Z.; Sangwan, P.L.; Satheesh Kumar, P.; Yadav, G. Evaluation of the immunomodulatory and anti-inflammatory activity of Bakuchiol using RAW 264.7 macrophage cell lines and in animal models stimulated by lipopolysaccharide (LPS). Int. Immunopharmacol. 2021, 91, 107264. [Google Scholar] [CrossRef]
- Yun, Y.; Han, S.; Park, E.; Yim, D.; Lee, S.; Lee, C.-K.; Cho, K.; Kim, K. Immunomodulatory activity of betulinic acid by pro-ducing pro-inflammatory cytokines and activation of macrophages. Arch. Pharm. Res. 2003, 26, 1087–1095. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of Fucoidan: A review. Polym. 2020, 12, 2338. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Park, W.J.; Kothapalli, K.S.D.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef]
- Garces, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.G.; Rod-in, W.; Jung, J.J.; Jung, S.K.; Lee, S.-m.; Park, W.J. Lipids Extracted from Aptocyclus ventricosus Eggs Possess Immunoregulatory Effects on RAW264.7 Cells by Activating the MAPK and NF-κB Signaling Pathways. Mar. Drugs 2024, 22, 368. https://doi.org/10.3390/md22080368
Lee SG, Rod-in W, Jung JJ, Jung SK, Lee S-m, Park WJ. Lipids Extracted from Aptocyclus ventricosus Eggs Possess Immunoregulatory Effects on RAW264.7 Cells by Activating the MAPK and NF-κB Signaling Pathways. Marine Drugs. 2024; 22(8):368. https://doi.org/10.3390/md22080368
Chicago/Turabian StyleLee, Seul Gi, Weerawan Rod-in, Jun Jae Jung, Seok Kyu Jung, Sang-min Lee, and Woo Jung Park. 2024. "Lipids Extracted from Aptocyclus ventricosus Eggs Possess Immunoregulatory Effects on RAW264.7 Cells by Activating the MAPK and NF-κB Signaling Pathways" Marine Drugs 22, no. 8: 368. https://doi.org/10.3390/md22080368
APA StyleLee, S. G., Rod-in, W., Jung, J. J., Jung, S. K., Lee, S.-m., & Park, W. J. (2024). Lipids Extracted from Aptocyclus ventricosus Eggs Possess Immunoregulatory Effects on RAW264.7 Cells by Activating the MAPK and NF-κB Signaling Pathways. Marine Drugs, 22(8), 368. https://doi.org/10.3390/md22080368






