Aspergillusidone G Potentiates the Anti-Inflammatory Effects of Polaprezinc in LPS-Induced BV2 Microglia: A Bioinformatics and Experimental Study
Abstract
1. Introduction
2. Results
2.1. Pol Possessed Acceptable ADMET and Drug-likeness Properties in General
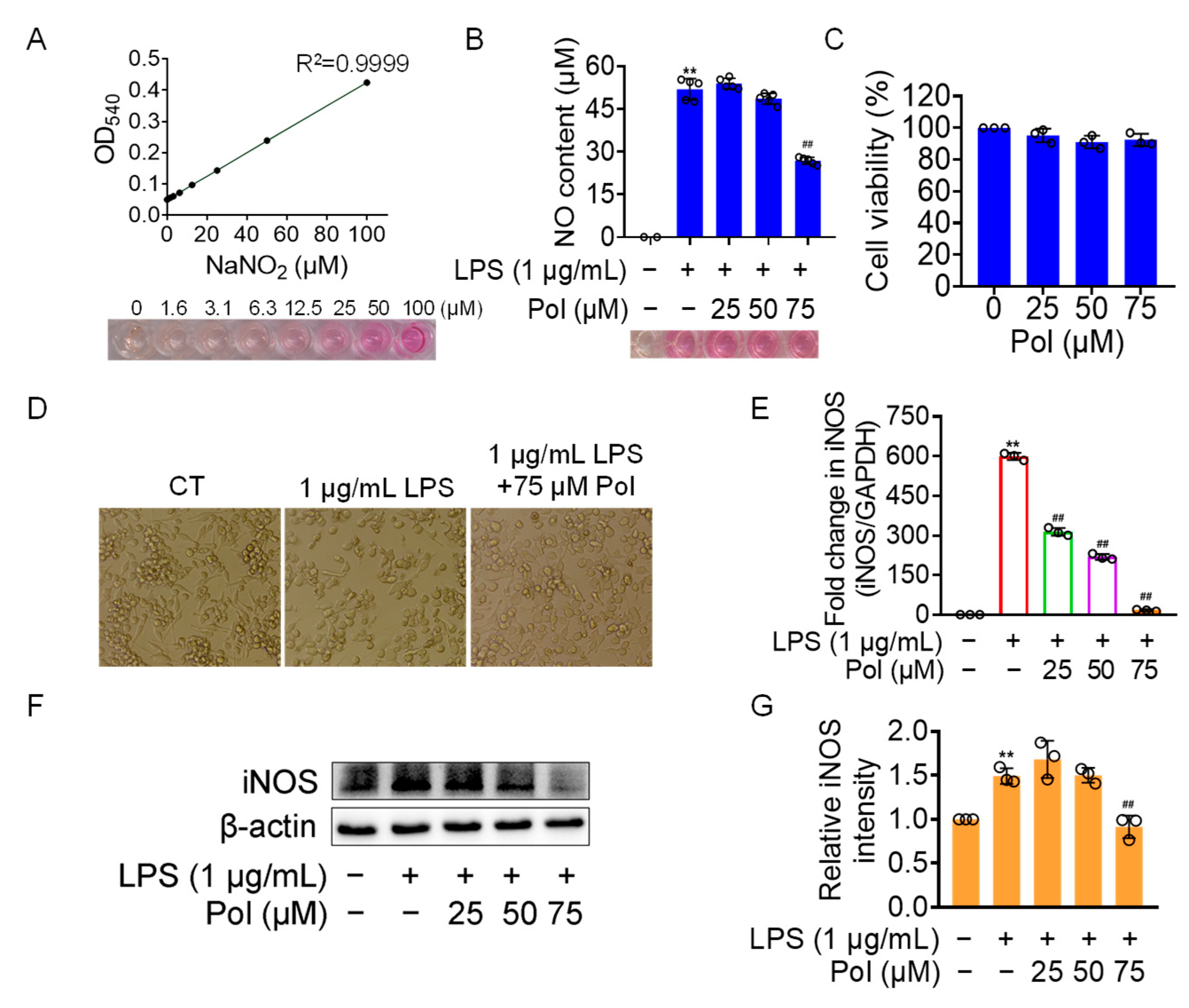
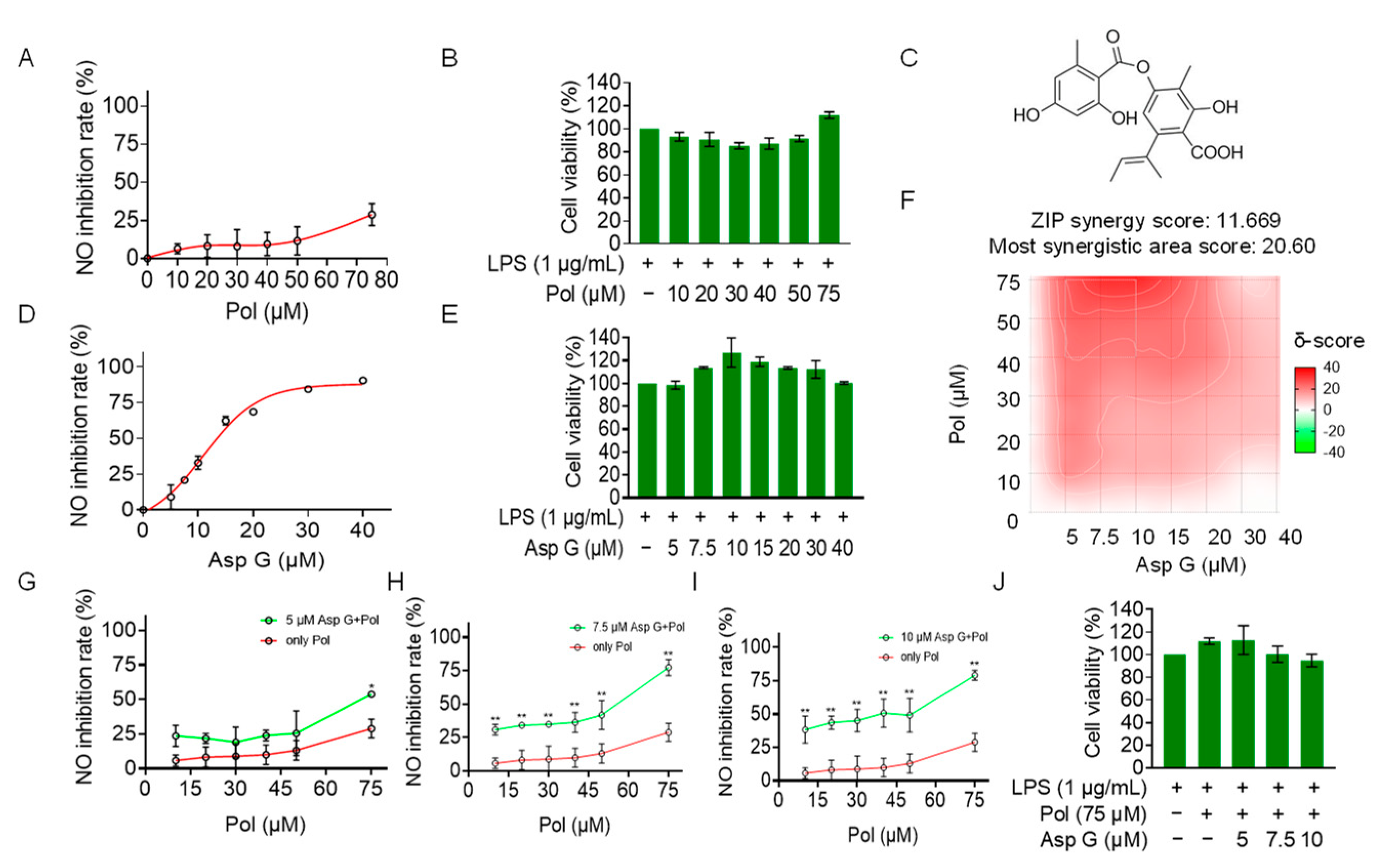
2.2. Pol Alleviated NO Burst in LPS-Induced BV2 Microglia
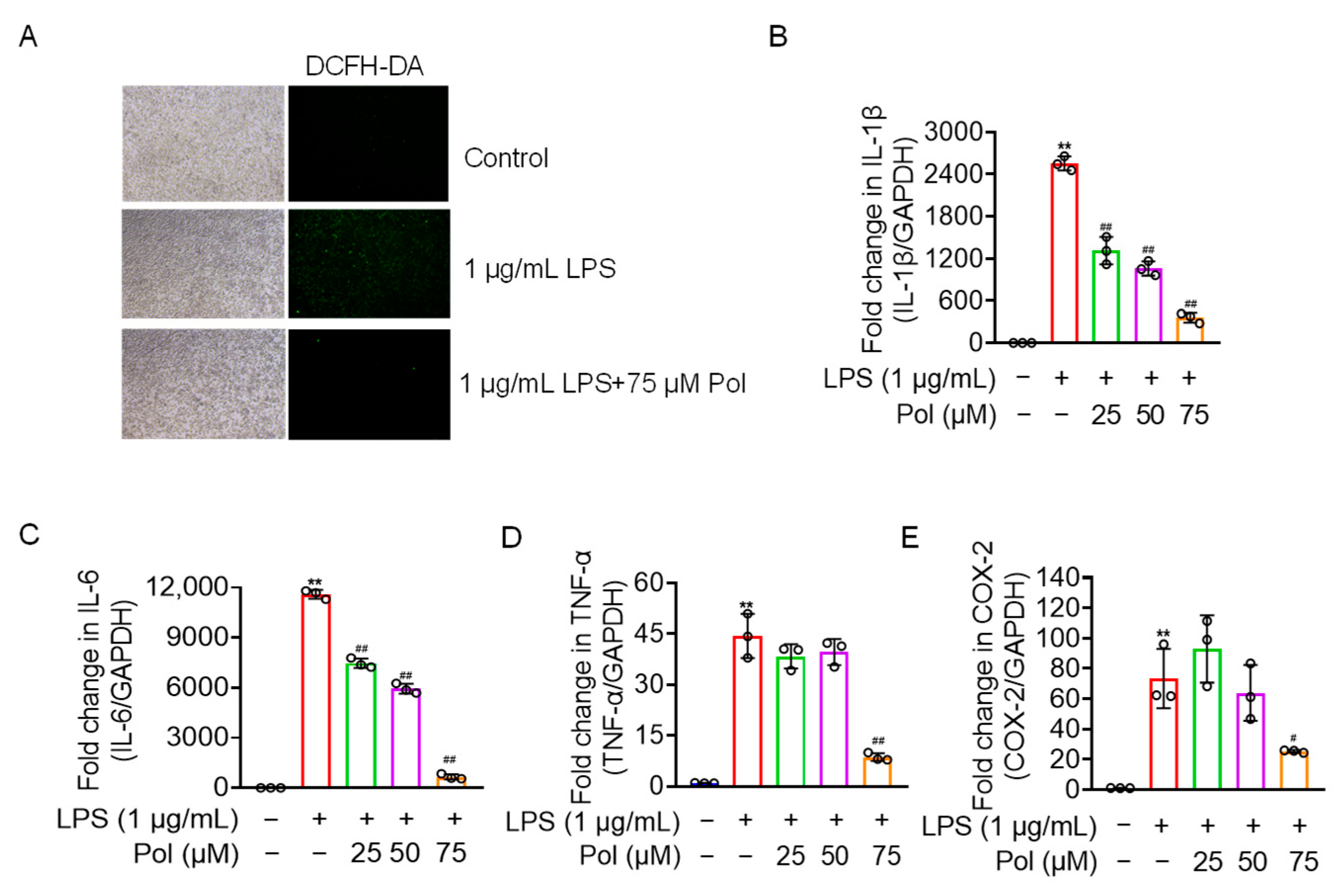
2.3. Pol Relieved the ROS Generation and Expression of Proinflammatory Cytokines and COX-2
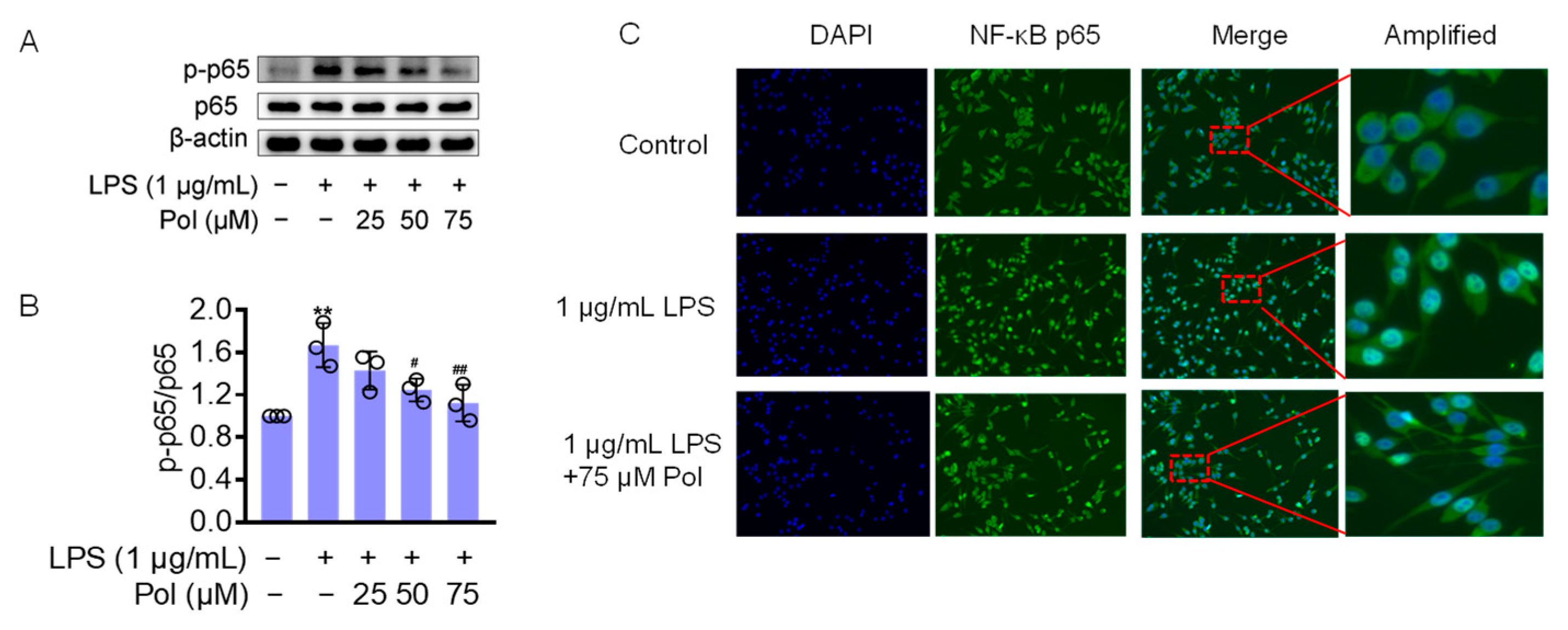
2.4. Pol Attenuated the Activation of NF-κB Pathways in LPS-Treated BV2 Microglia
2.5. Asp G at a Low Dose Synergistically Enhanced the NO Inhibition of Pol in LPS-Induced BV2 Microglia
2.6. Asp G Collaborates with Pol to Further Alleviate Neuroinflammation in LPS-Induced BV2 Microglia
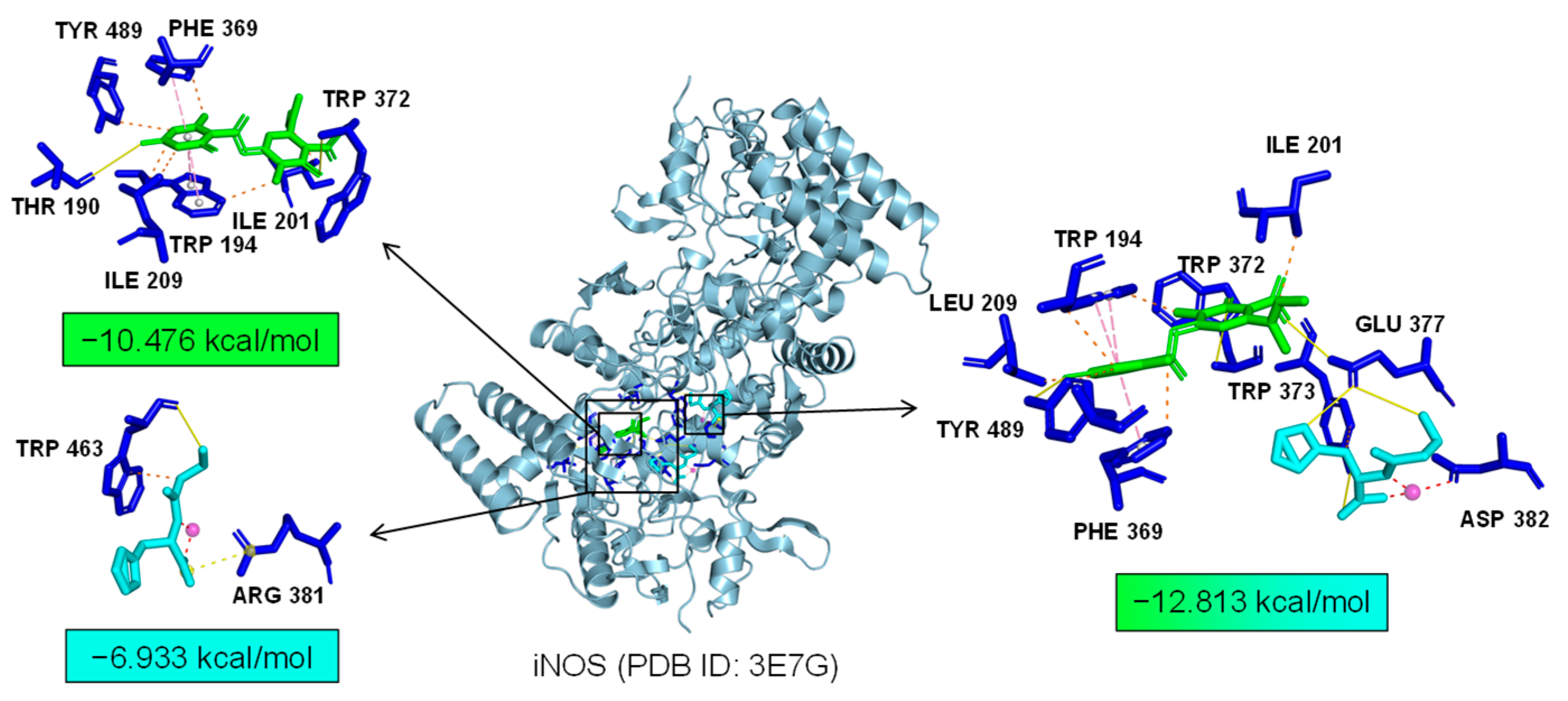
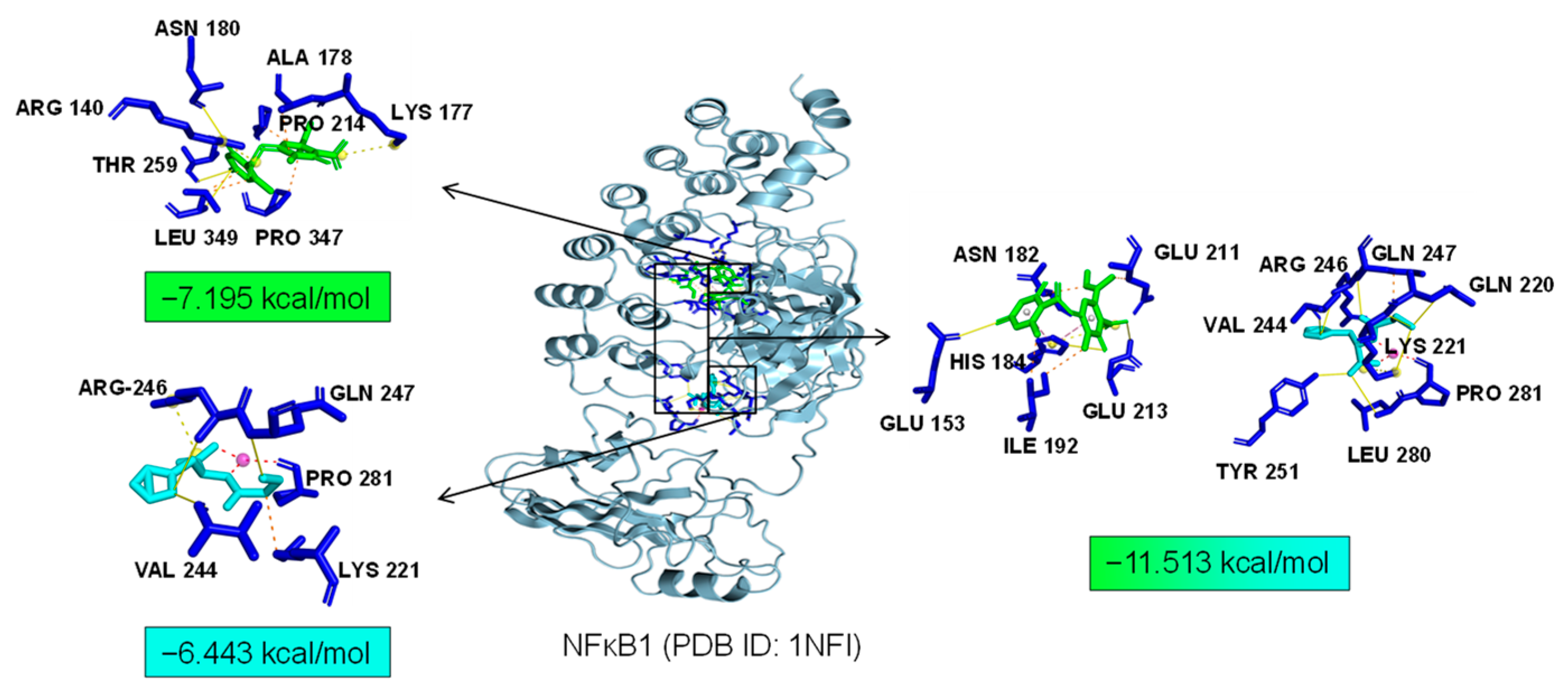
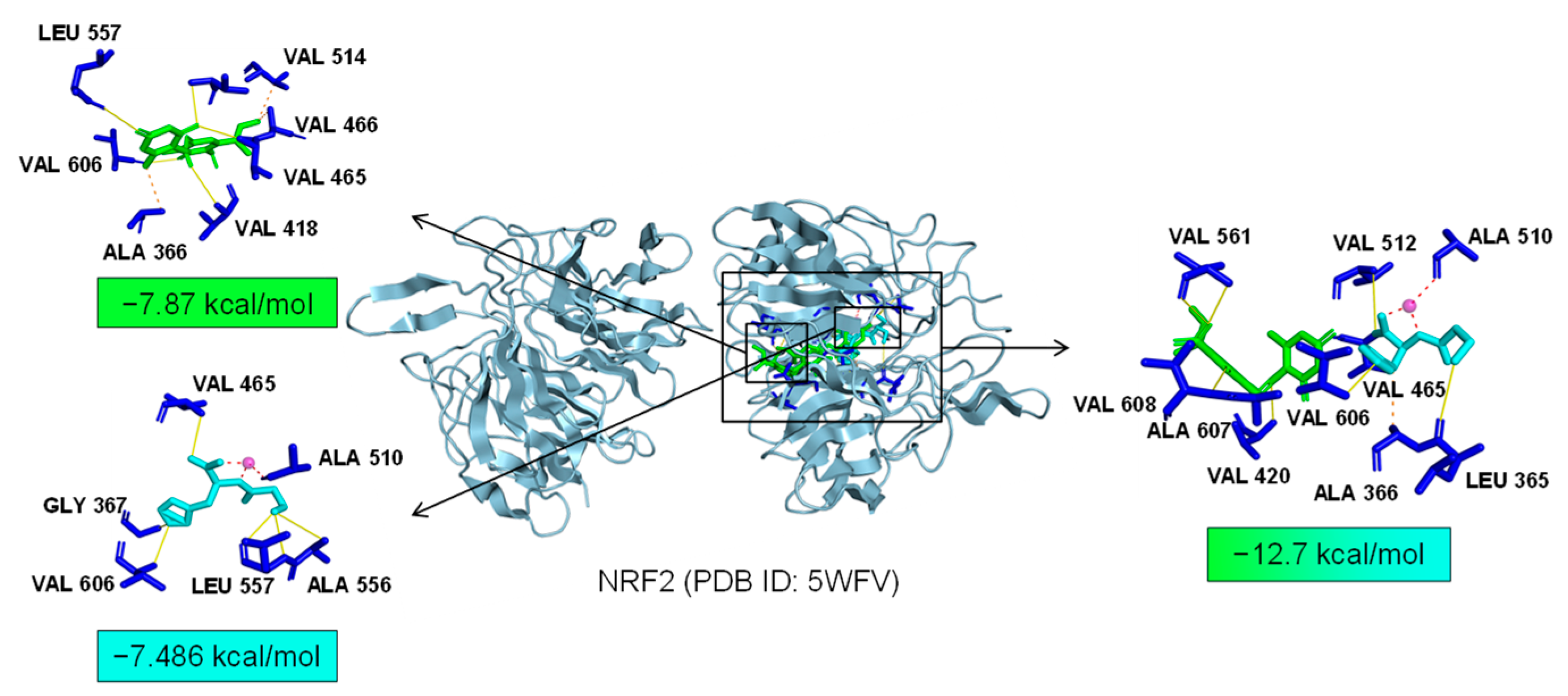
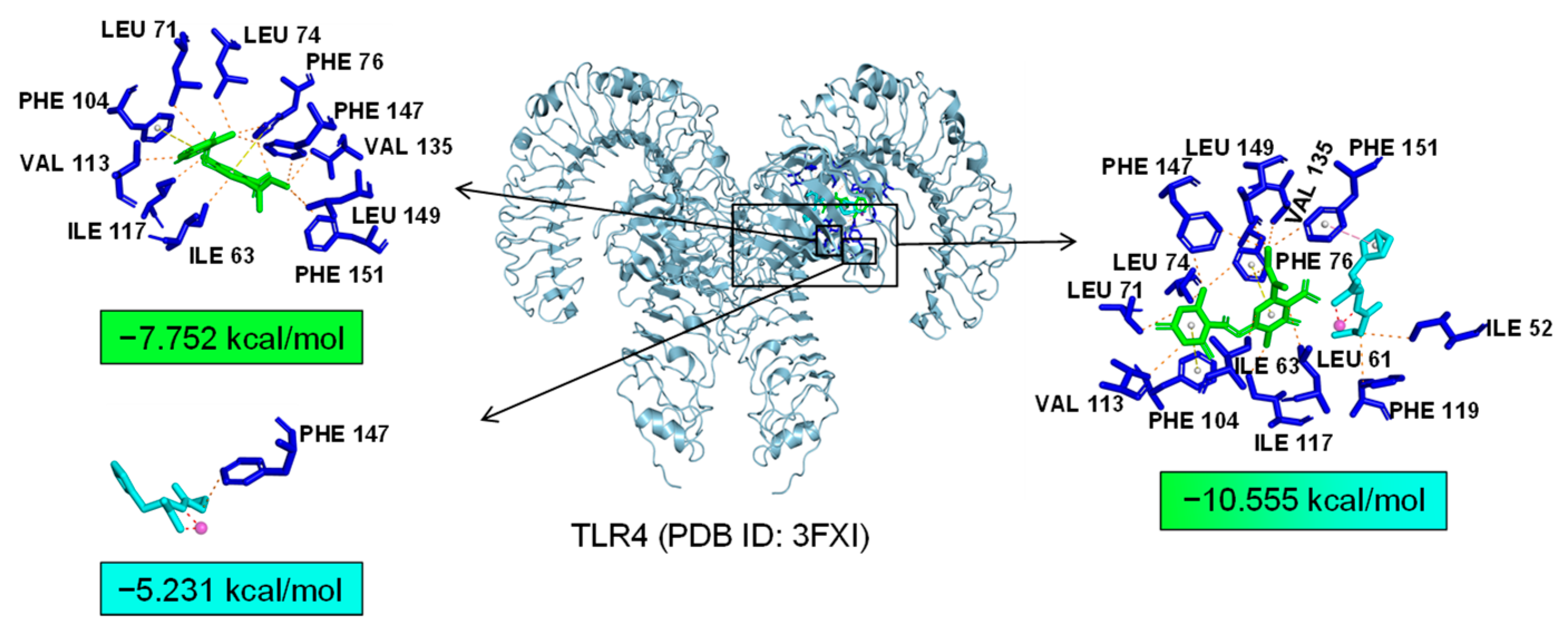
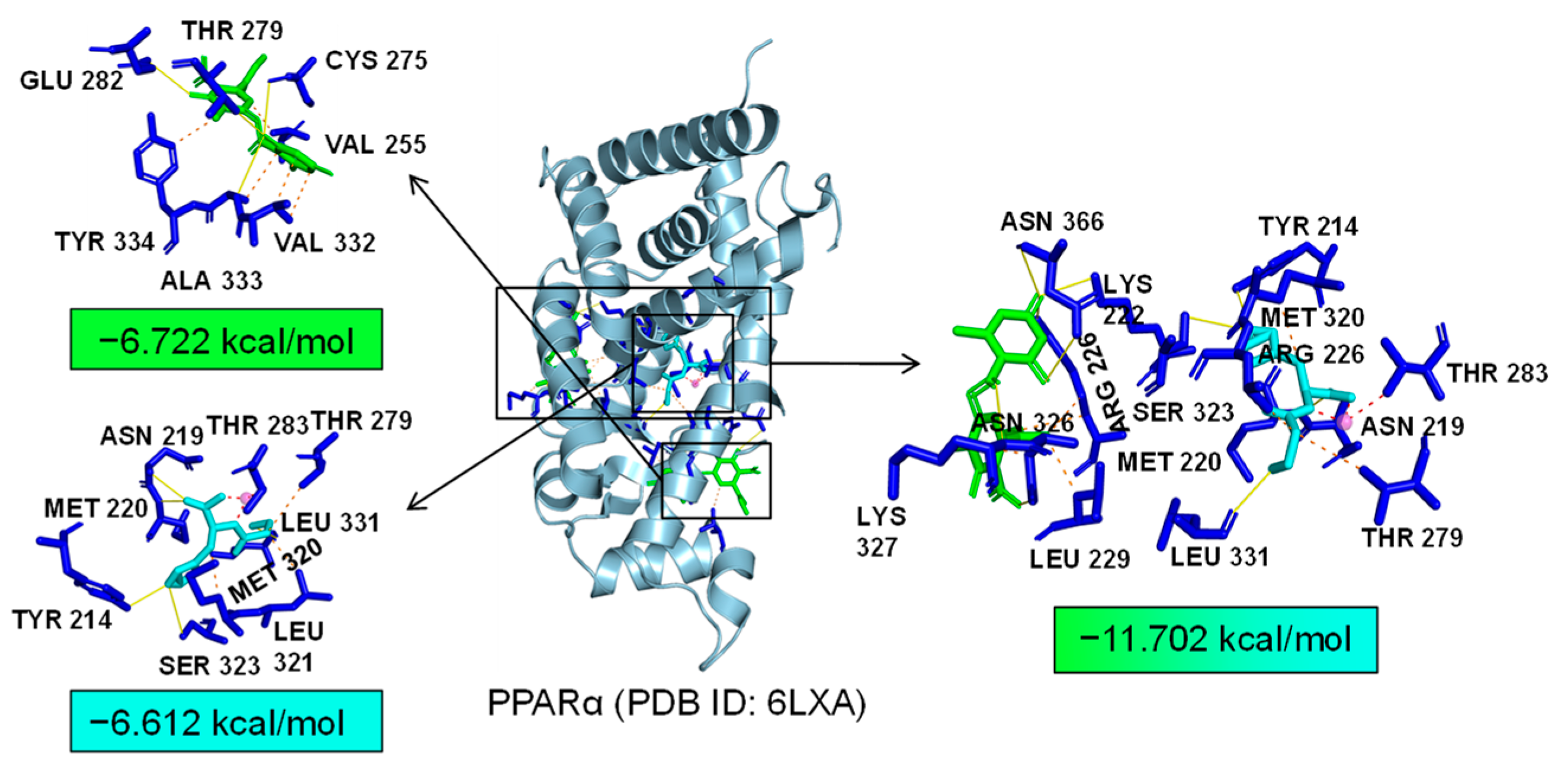
2.7. Bioinformatics Analysis of Potential Synergistic Mechanism
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Condition
4.2. NO Content Measurements
4.3. Cell Viability Detection
4.4. ROS Determination
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Western Blotting
4.7. Immunofluorescence
4.8. Network Pharmacology Prediction
4.9. Molecular Docking
4.10. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hammond, T.R.; Marsh, S.E.; Stevens, B. Immune signaling in neurodegeneration. Immunity 2019, 50, 955–974. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Sosna, J.; Philipp, S.; Albay, R., 3rd; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; LaFerla, F.M.; Glabe, C.G. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chowdhury, S.; Ma, R.; Le, K.X.; Hong, S.; Caldarone, B.J.; Stevens, B.; Lemere, C.A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 2017, 9, eaaf6295. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef]
- Brown, G.C.; Camacho, M.; Williams-Gray, C.H. The endotoxin hypothesis of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Biffo, S.; Grillo, M.; Margolis, F.L. Cellular localization of carnosine-like and anserine-like immunoreactivities in rodent and avian central nervous system. Neuroscience 1990, 35, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Outten, C.E.; O’Halloran, T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292, 2488–2492. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Mizuno, D.; Kawahara, M. The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia. Int. J. Mol. Sci. 2013, 14, 22067–22081. [Google Scholar] [CrossRef] [PubMed]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE 2011, 6, e17971. [Google Scholar] [CrossRef] [PubMed]
- Horning, M.S.; Blakemore, L.J.; Trombley, P.Q. Endogenous mechanisms of neuroprotection: Role of zinc, copper, and carnosine. Brain Res. 2000, 852, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Toyama, S.; Miwa, M.; Itabashi, T.; Sano, H.; Yoneta, T. Residence time of polaprezinc (zinc L-carnosine complex) in the rat stomach and adhesiveness to ulcerous sites. Jpn. J. Pharmacol. 1995, 67, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Adani, G.; Filippini, T.; Michalke, B.; Vinceti, M. Selenium and other trace elements in the etiology of Parkinson’s disease: A systematic review and meta-analysis of case-control studies. Neuroepidemiology 2020, 54, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Liu, M.Y.; Zhong, X.; Wei, M.J. Decreased circulating zinc levels in Parkinson’s disease: A meta-analysis study. Sci. Rep. 2017, 7, 3902. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Alvarez, E.; Soto-Otero, R.; Hermida-Ameijeiras, A.; López-Real, A.M.; Labandeira-García, J.L. Effects of aluminum and zinc on the oxidative stress caused by 6-hydroxydopamine autoxidation: Relevance for the pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta 2002, 1586, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Ajjimaporn, A.; Shavali, S.; Ebadi, M.; Govitrapong, P. Zinc rescues dopaminergic SK-N-SH cell lines from methamphetamine-induced toxicity. Brain Res. Bull. 2008, 77, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ajjimaporn, A.; Swinscoe, J.; Shavali, S.; Govitrapong, P.; Ebadi, M. Metallothionein provides zinc-mediated protective effects against methamphetamine toxicity in SK-N-SH cells. Brain Res. Bull. 2005, 67, 466–475. [Google Scholar] [CrossRef]
- Ajjimaporn, A.; Phansuwan-Pujito, P.; Ebadi, M.; Govitrapong, P. Zinc protects SK-N-SH cells from methamphetamine-induced alpha-synuclein expression. Neurosci. Lett. 2007, 419, 59–63. [Google Scholar] [CrossRef]
- Hristova, V.A.; Beasley, S.A.; Rylett, R.J.; Shaw, G.S. Identification of a novel Zn2+-binding domain in the autosomal recessive juvenile Parkinson-related E3 ligase parkin. J. Biol. Chem. 2009, 284, 14978–14986. [Google Scholar] [CrossRef]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Cassone, M.; Otvos, L., Jr. Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev. Anti-Infect. Ther. 2010, 8, 703–716. [Google Scholar] [CrossRef]
- Di Chio, C.; Previti, S.; De Luca, F.; Bogacz, M.; Zimmer, C.; Wagner, A.; Schirmeister, T.; Zappalà, M.; Ettari, R. Drug combination studies of the dipeptide nitrile CD24 with curcumin: A new strategy to synergistically inhibit rhodesain of trypanosoma brucei rhodesiense. Int. J. Mol. Sci. 2022, 23, 14470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xie, H.; Ji, Z.; He, R.; Xu, M.; He, Y.; Huang, J.; Pan, S.; Hu, Y. Cdk5/p25 specific inhibitory peptide TFP5 rescues the loss of dopaminergic neurons in a sub-acute MPTP induced PD mouse model. Neurosci. Lett. 2016, 632, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A global review on short peptides: Frontiers and perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Bao, H.Y.; Liu, Y.Y.; Nie, Y.Y.; Yang, J.M.; Hong, P.Z.; Zhang, Y. Depsidone derivatives and a cyclopeptide produced by marine fungus Aspergillus unguis under chemical induction and by its plasma induced mutant. Molecules 2018, 23, 2245. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, J.; Kobayashi, R.; Nagata, Y.; Kasahara, S.; Ono, T.; Sawada, M.; Ohata, K.; Kato-Hayashi, H.; Hayashi, H.; Shimizu, M.; et al. Polaprezinc for prevention of oral mucositis in patients receiving chemotherapy followed by hematopoietic stem cell transplantation: A multi-institutional randomized controlled trial. Int. J. Cancer 2021, 148, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R. Molecular basis of etiological implications in Alzheimer’s disease: Focus on neuroinflammation. Mol. Neurobiol. 2013, 48, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Frakes, A.E.; Ferraiuolo, L.; Haidet-Phillips, A.M.; Schmelzer, L.; Braun, L.; Miranda, C.J.; Ladner, K.J.; Bevan, A.K.; Foust, K.D.; Godbout, J.P.; et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 2014, 81, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Wang, J.; Pendergast, A.M. Multifunctional Abl kinases in health and disease. J. Cell Sci. 2016, 129, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Ren, B.; Thelen, A.; Jump, D.B. Peroxisome proliferator-activated receptor alpha inhibits hepatic S14 gene transcription. Evidence against the peroxisome proliferator-activated receptor alpha as the mediator of polyunsaturated fatty acid regulation of s14 gene transcription. J. Biol. Chem. 1996, 271, 17167–17173. [Google Scholar] [CrossRef]
- Staels, B.; Koenig, W.; Habib, A.; Merval, R.; Lebret, M.; Torra, I.P.; Delerive, P.; Fadel, A.; Chinetti, G.; Fruchart, J.C.; et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 1998, 393, 790–793. [Google Scholar] [CrossRef]
- Nishida, K.; Ohta, Y.; Kobayashi, T.; Ishiguro, I. Involvement of the xanthine-xanthine oxidase system and neutrophils in the development of acute gastric mucosal lesions in rats with water immersion restraint stress. Digestion 1997, 58, 340–351. [Google Scholar] [CrossRef]
- Ko, J.K.; Leung, C.C. Ginger extract and polaprezinc exert gastroprotective actions by anti-oxidant and growth factor modulating effects in rats. J. Gastroenterol. Hepatol. 2010, 25, 1861–1868. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Yagi, N.; Matsuyama, K.; Yoshida, N.; Seto, K.; Yoneta, T. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alpha expression in rats with aspirin-induced gastric mucosal injury. Dig. Dis. Sci. 2001, 46, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, L.; Elsherbini, A.; Crivelli, S.M.; Tripathi, P.; Harper, C.; Quadri, Z.; Spassieva, S.D.; Bieberich, E. The S1P receptor 1 antagonist Ponesimod reduces TLR4-induced neuroinflammation and increases Aβ clearance in 5XFAD mice. EBioMedicine 2023, 94, 104713. [Google Scholar] [CrossRef]
- Matsukura, T.; Tanaka, H. Applicability of zinc complex of L-carnosine for medical use. Biochem. Biokhimiia 2000, 65, 817–823. [Google Scholar] [CrossRef]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2022, 25, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiu, J.; Li, X.L.; Aday, S.; Zhang, J.; Conley, G.; Xu, J.; Joseph, J.; Lan, H.; Langer, R.; et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci. Adv. 2021, 7, eabd6889. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Liu, Y.; Zheng, M.; Shi, B. Exosomes based strategies for brain drug delivery. Biomaterials 2023, 293, 121949. [Google Scholar] [CrossRef]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1695. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yuan, J.; Yang, Y.; Yue, Y.; Hu, Z.; Fadera, S.; Chen, H. Incisionless targeted adeno-associated viral vector delivery to the brain by focused ultrasound-mediated intranasal administration. EBioMedicine 2022, 84, 104277. [Google Scholar] [CrossRef] [PubMed]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. suppresses H2O2-induced oxidative damage and LPS-induced inflammation in HT22 hippocampal neurons and BV2 microglia. Antioxidants 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 2.0: Visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020, 48, W488–W493. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhu, Y.; He, G.; Ni, H.; Liu, C.; Ma, L.; Zhang, L.; Shi, D. Dexmedetomidine attenuates neuroinflammation In LPS-stimulated BV2 microglia cells through upregulation of miR-340. Drug Des. Dev. Ther. 2019, 13, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wu, J.; Liu, R.; Wang, S.; Luo, J.; Yang, Y.; Qin, Y.; Li, T.; Zheng, X.; Song, J.; et al. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: Role of Akt(Ser473)/GSK3β(Ser9)-mediated Nrf2 activation. Redox Biol. 2020, 36, 101644. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, J.W.; Lee, H.J.; Jang, G.M.; Jeong, Y.J.; Cho, J.; Seo, J.; Hoe, H.S. Lomerizine inhibits LPS-mediated neuroinflammation and tau hyperphosphorylation by modulating NLRP3, DYRK1A, and GSK3α/β. Front. Immunol. 2023, 14, 1150940. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Meng, T.; Li, Y.; He, D.; Ran, X.; Chen, G.; Guo, W.; Kan, X.; Fu, S.; et al. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson’s disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of quercetin therapeutic targets for Alzheimer disease and type 2 diabetes mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
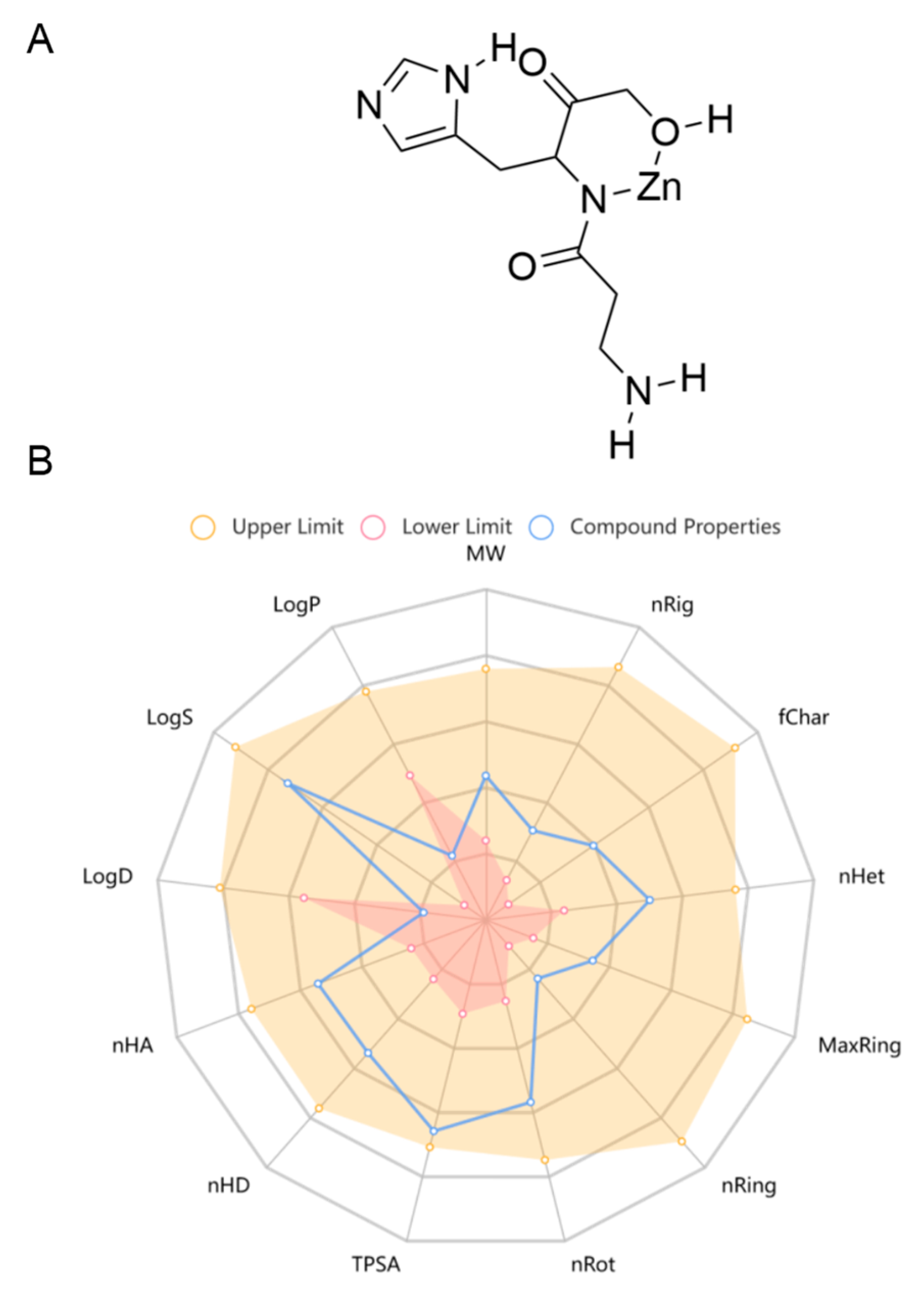



| Property | Value | Potential for Development as a Neurological Drug |
|---|---|---|
| Lipinski rule | Accept (MW: 289.03, logP: −2.875) | Excellent |
| Pfizer rule | Accept (logP: −2.875, TPSA: 123.17) | Excellent |
| Golden Triangle rule | Accept (MW: 289.03, log D: −1.848) | Excellent |
| GSK rule | Accept (MW: 289.03, logP: −2.875) | Excellent |
| Blood–brain barrier penetration | 0.825 | Excellent |
| Human intestinal absorption | 0.506 | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, F.; Zhou, L.; Yang, Z.; Liu, Y.; Zhang, Y. Aspergillusidone G Potentiates the Anti-Inflammatory Effects of Polaprezinc in LPS-Induced BV2 Microglia: A Bioinformatics and Experimental Study. Mar. Drugs 2024, 22, 324. https://doi.org/10.3390/md22070324
Ban F, Zhou L, Yang Z, Liu Y, Zhang Y. Aspergillusidone G Potentiates the Anti-Inflammatory Effects of Polaprezinc in LPS-Induced BV2 Microglia: A Bioinformatics and Experimental Study. Marine Drugs. 2024; 22(7):324. https://doi.org/10.3390/md22070324
Chicago/Turabian StyleBan, Fangfang, Longjian Zhou, Zhiyou Yang, Yayue Liu, and Yi Zhang. 2024. "Aspergillusidone G Potentiates the Anti-Inflammatory Effects of Polaprezinc in LPS-Induced BV2 Microglia: A Bioinformatics and Experimental Study" Marine Drugs 22, no. 7: 324. https://doi.org/10.3390/md22070324
APA StyleBan, F., Zhou, L., Yang, Z., Liu, Y., & Zhang, Y. (2024). Aspergillusidone G Potentiates the Anti-Inflammatory Effects of Polaprezinc in LPS-Induced BV2 Microglia: A Bioinformatics and Experimental Study. Marine Drugs, 22(7), 324. https://doi.org/10.3390/md22070324






