The Composition of Triterpene Glycosides in the Sea Cucumber Psolus peronii: Anticancer Activity of the Glycosides against Three Human Breast Cancer Cell Lines and Quantitative Structure–Activity Relationships (QSAR)
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of the Glycosides
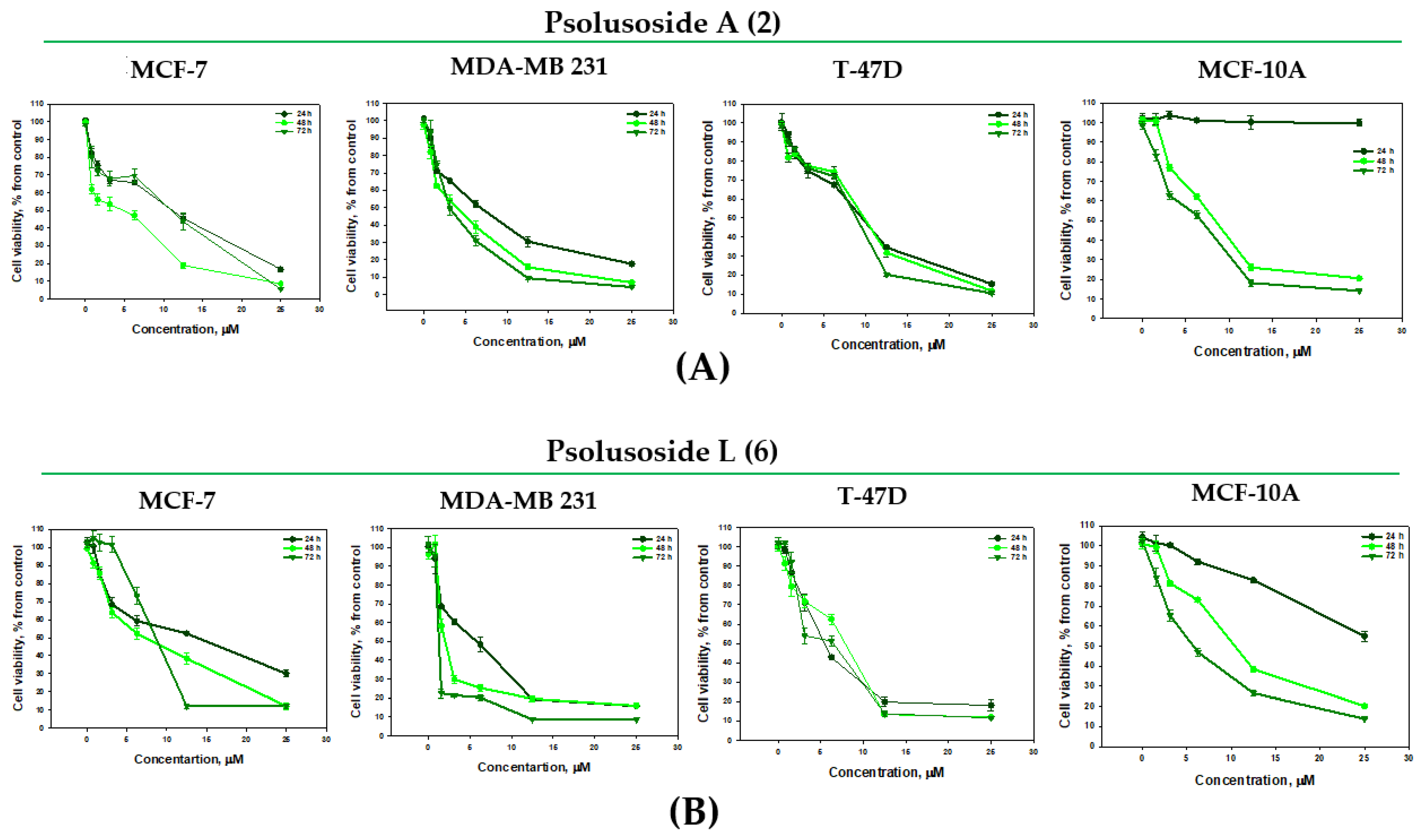
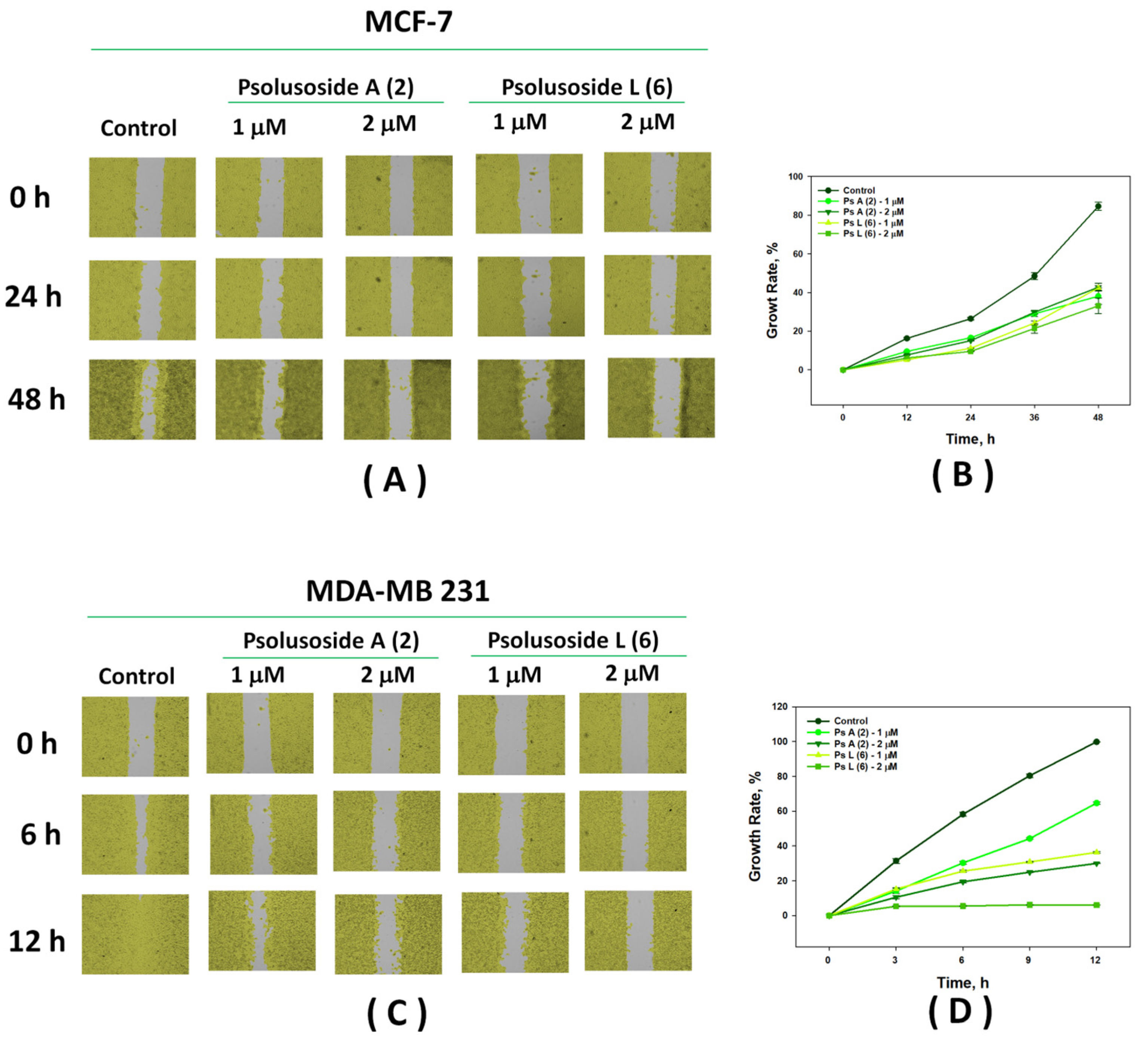
2.2. Bioactivity of the Glycosides
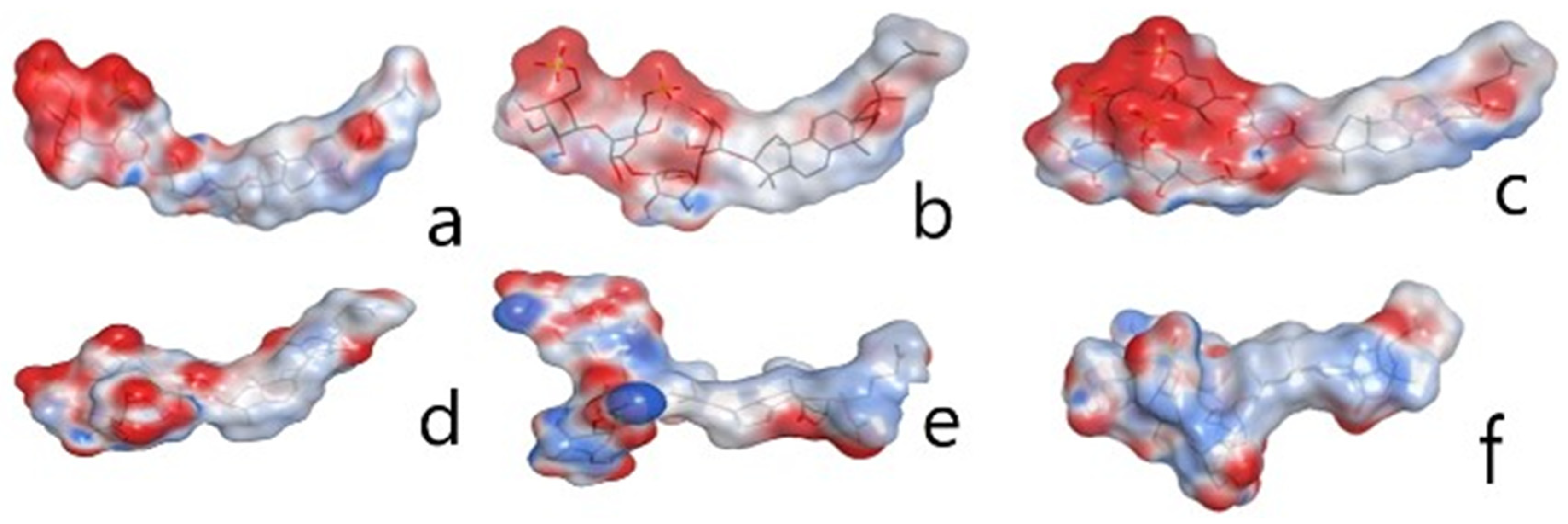
2.3. Quantitative Structure–Activity Relationships
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animals and Cells
3.3. Extraction and Isolation
Peronioside A (1)
3.4. Hemolytic Activity
3.5. Cytotoxic Activity (MTT Assay)
3.6. Colonogenic Assay
3.7. Wound Healing Assay
3.8. Building a QSAR Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A. Progress in the studies of triterpene glycosides from sea cucumbers (Holothuroidea, Echinodermata) between 2017 and 2021. Nat. Prod. Commun. 2021, 16, 10. [Google Scholar] [CrossRef]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Elucidation of molecular diversity and body distribution of saponins in sea cucumber Holothuria forskali (Echinodermata) by mass spectrometry. Comp. Biochem. Physiol. 2009, 152B, 124–134. [Google Scholar] [CrossRef]
- Van Dyck, S.; Flammang, P.; Meriaux, C.; Bonnel, D.; Salzet, M.; Fourmier, I.; Wisztorski, M. Localization of secondary metabolites in marine invertebrates: Contribution of MALDI MSI for the study of saponins in Cuvierian tubules of H. forskali. PLoS ONE 2010, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Quialitative and quantitative saponin contents in five sea cucumbers from Indian Ocean. Mar. Drugs 2010, 8, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Popov, R.S.; Ivanchina, N.V.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Dolmatov, I.Y.; Stonik, V.A.; Dmitrenok, P.S. Metabolite profiling of triterpene glycosides of the Far Eastern sea cucumber Eupentacta fraudatrix and their distribution in various body components using LC-ESI QTOF-MS. Mar. Drugs 2017, 15, 302. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Franco, C.M.M. Distribution of saponins in the sea cucumber Holothuria lessoni; the body wall versus the viscera, and their biological activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Popov, R.S.; Ivanchina, N.V.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Malyarenko, T.V.; Stonik, V.A.; Dmitrenok, P.S. A mass spectrometry database for sea cucumber triterpene glycosides. Metabolites 2023, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Careaga, V.P.; Maier, M.S. Cytotoxic triterpene glycosides from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 515–528. [Google Scholar]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Sea cucumber triterpene glycosides as anticancer agents. In Studies in Natural Product Chemistry; Rahman, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2016; Volume 49, pp. 55–105. [Google Scholar]
- Dyshlovoy, S.A.; Menchinskaya, E.S.; Venz, S.; Rast, S.; Amann, K.; Hauschild, J.; Otte, K.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Intern. J. Cancer 2016, 138, 2450–2465. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Madanchi, R.; Hauschild, J.; Otte, K.; Alsdorf, W.H.; Schumacher, U.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Honecker, F.; et al. The marine triterpene glycoside frondoside A induces p53-independent apoptosis and inhibits autophage in urothelial carcinoma cells. BMC Cancer 2017, 17, 93. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Rast, S.; Hauschild, J.; Otte, K.; Alsdorf, W.H.; Madanchi, R.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Dierlamn, J.; et al. Frondoside A induces AIF-associated caspase independent apoptosis in Burkitt’s lymphoma cells. Leuk. Limphoma 2017, 58, 2905–2915. [Google Scholar] [CrossRef]
- Yun, S.-H.; Sim, E.-H.; Han, S.-H.; Han, J.-Y.; Kim, S.-H.; Silchenko, A.S.; Stonik, V.A.; Park, J.-I. Holotoxin A1 induces apoptosis by activating acid sphingomyelinase and neutral sphingomyelinase in K562 and human primary leukemia cells. Mar. Drugs 2018, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Sim, E.-H.; Han, S.-H.; Kim, T.-R.; Ju, M.-H.; Han, J.-Y.; Jeong, J.-S.; Kim, S.-H.; Silchenko, A.S.; Stonik, V.A.; et al. In vitro and in vivo anti-leukemic effects of cladoloside C2 are mediated by activation of Fas/ceramide syntase 6/P38 kinase/C-Jun NH2-terminal kinase/caspase-8. Oncotarget 2018, 9, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.S.; Dyshlovoy, S.A.; Venz, S.; Jacobsen, C.; Hauschild, J.; Rohlfing, T.; Silchenko, A.S.; Avilov, S.A.; Balabanov, S.; Bokemeyer, C.; et al. Anticancer activity of the marine triterpene glycoside cucumarioside A2-2 in human prostate cancer cells. Mar. Drugs 2024, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.H.; Pislyagin, E.A.; Lin, L.Y.; Menchinskaya, E.S.; Chernikov, O.V.; Kozhemyako, V.B.; Gorpenchenko, T.Y.; Manzhulo, I.V.; Chaikina, E.L.; Agafonova, I.G.; et al. Holothurian triterpene glycoside cucumarioside A2-2 induces macrophages activation and polarization in cancer immunotherapy. Cancer Cell Int. 2023, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Zelepuga, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Structure-activity relationships of holothuroid’s triterpene glycosides and some in silico insights obtained by molecular dynamics study on the mechanisms of their membranolytic action. Mar. Drugs 2021, 19, 604. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Popov, R.S.; Chingizova, E.A.; Menchinskaya, E.S.; Zelepuga, E.A.; Panina, E.G.; Stepanov, V.G.; Kalinin, V.I.; et al. Sulfated triterpene glycosides from the Far Eastern sea cucumber Cucumaria djakonovi: Djakonoviosides C1, D1, E1, and F1; cytotoxicity against human breast cancer cell lines; quantitative structure–activity relationships. Mar. Drugs 2023, 21, 602. [Google Scholar] [CrossRef] [PubMed]
- Mitu, S.A.; Bose, U.; Suwansa-ard, S.; Turner, L.H.; Zhao, M.; Elizur, A.; Ogbourne, S.M.; Shaw, P.N.; Cummins, S.F. Evidence for a saponin biosynthesis pathway in the body wall of the commercially significant sea cucumber Holothuria scabra. Mar. Drugs 2017, 15, 349. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Xun, X.; Wang, J.; Bao, L.; Thimmappa, R.; Ding, J.; Jiang, J.; Zhang, L.; Li, T.; et al. Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discov. 2018, 4, 29. [Google Scholar] [CrossRef]
- Liu, H.; Kong, X.; Chen, J.; Zhang, H. De novo sequencing and transcriptome analysis of Stichopus horrens to reveal genes related to biosynthesis of triterpenoids. Aquaculture 2018, 491, 358–367. [Google Scholar] [CrossRef]
- Claereboudt, E.J.S.; Caulier, G.; Decroo, C.; Colson, E.; Gerbaux, P.; Claereboudt, M.R.; Schaller, H.; Flammang, P.; Deleu, M.; Eeckhaut, I. Triterpenoids in echinoderms: Fundamental differences in diversity and biosynthetic pathways. Mar. Drugs 2019, 17, 352. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Sun, L. Triterpenoid saponin biosynthesis genes and their expression patterns during the development of sea cucumber Apostichopus japonicus. J. Ocean. Limnol. 2021, 39, 2295–2308. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Separation procedures for complicated mixtures of sea cucumber triterpene glycosides with isolation of individual glycosides, their comparison with HPLC/MS metabolomic approach, and biosynthetic interpretation of the obtained structural data. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2022; Volume 72, pp. 103–146. [Google Scholar] [CrossRef]
- Chen, X.; Shao, X.; Li, W.; Zhang, X.; Yu, B. Total synthesis of echinoside A, a representative triterpene glycoside of sea cucumbers. Angew. Chem. 2017, 56, 7648–7653. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, X.; Zhu, K.; Dang, Y.; Yu, B. Synthesis of sea cucumber saponins with antitumor activities. J. Org. Chem. 2020, 85, 12080–12096. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S. Psolusosides C1, C2 and D1, novel triterpene hexaosides from the sea cucumber Psolus fabricii (Psolidae, Dendrochirotida). Nat. Prod. Commun. 2018, 13, 1623–1628. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Popov, R.S.; Chingizova, E.A.; Ermakova, S.P.; Malyarenko, O.S. Structures and bioactivities of six new triterpene glycosides, psolusosides E, F, G, H, H1 and I and the corrected structure of psolusoside B from the sea cucumber Psolus fabricii. Mar. Drugs 2019, 17, 358. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Popov, R.S.; Chingizova, E.A.; Kasakin, M.F. Psolusosides C3 and D2–D5, five novel triterpene hexaosides from the sea cucumber Psolus fabricii (Psolidae, Dendrochirotida): Chemical structures and bioactivities. Nat. Prod. Commun. 2019, 14, 7. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Popov, R.S.; Chingizova, E.A. Structures and bioactivities of psolusosides B1, B2, J, K, L, M, N, O, P, and Q from the sea cucumber Psolus fabricii. The first finding of tetrasulfated marine low molecular weight metabolites. Mar. Drugs 2019, 17, 631. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Avilov, S.A.; Kalinina, E.Y.; Korolkova, O.G.; Kalinovsky, A.I.; Stonik, V.A.; Riguera, R.; Jimenez, C. Structure of eximisoside A, a novel triterpene glycoside from the Far-Eastern sea cucumber Psolus eximius. J. Nat. Prod. 1997, 60, 817–819. [Google Scholar] [CrossRef]
- Murray, A.P.; Muniain, C.; Seldes, A.M.; Maier, M. Patagonicoside A: A novel antifungal disulfated triterpene glycoside from the sea cucumber Psolus patagonicus. Tetrahedron 2001, 57, 9563–9568. [Google Scholar] [CrossRef]
- Careaga, V.P.; Muniain, C.; Maier, M.S. Patagonicosides B and C, two antifungal sulfated triterpene glycosides from the sea cucumber Psolus patagonicus. Chem. Biodivers. 2011, 8, 467–475. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Dmitrenok, P.S.; Chingizova, E.A.; Kalinin, V.I. Unusual structures and cytotoxicities of chitonoidosides A, A1, B, C, D, and E, six triterpene glycosides from the Far Eastern sea cucumber Psolus chitonoides. Mar. Drugs 2021, 19, 449. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Chingizova, E.A.; Kalinin, V.I.; Dmitrenok, P.S. Triterpene glycosides from the Far Eastern sea cucumber Psolus chitonoides: Chemical structures and cytotoxicities of chitonoidosides E1, F., G, and H. Mar. Drugs 2021, 19, 696. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Chingizova, E.A.; Dmitrenok, P.S.; Kalinovsky, A.I.; Rasin, A.B.; Kalinin, V.I. Structures and Biologic activity of chitonoidosides I, J, K, K1 and L—triterpene di-, tri- and tetrasulfated hexaosides from the sea cucumber Psolus chitonoides. Mar. Drugs 2022, 20, 369. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, O.A.; Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A.; Mil’grom, Y.M.; Rashkes, Y.V. New glycosides from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1993, 29, 200–205. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Stepanov, V.R.; Stonik, V.A. Psolusoside A—a new triterpene glycoside from the holothurian Psolus fabricii. Chem. Nat. Compd. 1983, 19, 753–754. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), version 2020.09; Chemical Computing Group ULC: Montreal, QC, Canada, 2020.
- Wildman, S.A.; Crippen, G.M. Prediction of physiochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Sauer, W.H.B.; Schwarz, M.K. Molecular shape diversity of combinatorial libraries: A prerequisite for broad bioactivity. J. Chem. Inf. Comput. Sci. 2003, 43, 987–1003. [Google Scholar] [CrossRef]
- Labute, P. LowModeMD–implicit low mode velocity filtering applied to conformational search of macrocycles and protein loops. J. Chem. Inf. Model. 2010, 50, 792–800. [Google Scholar] [CrossRef]
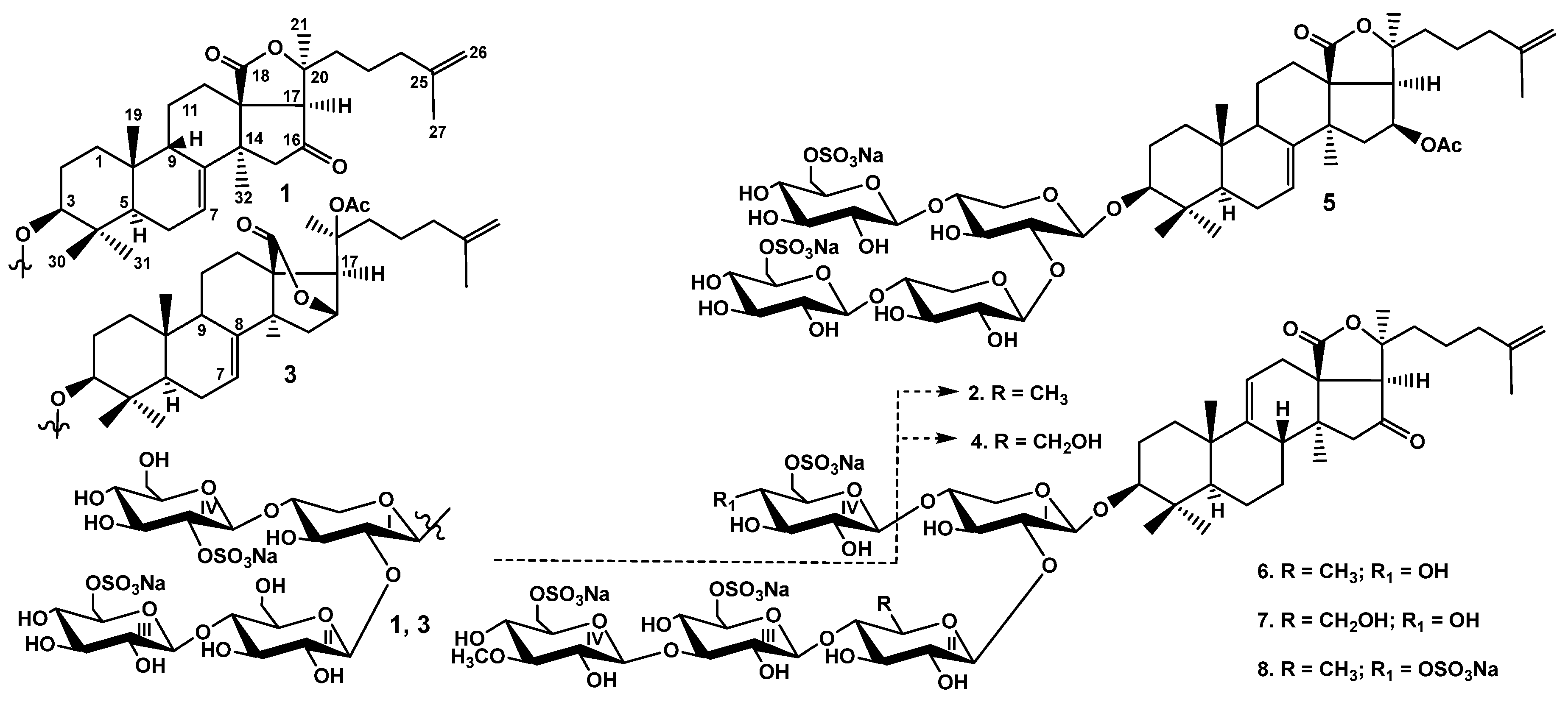



| Position | δC mult. a | δH mult. (J in Hz) b | HMBC | ROESY |
|---|---|---|---|---|
| 1 | 35.7 CH2 | 1.36 m | H-19 | |
| 1.32 m | ||||
| 2 | 26.8 CH2 | 2.01 m | ||
| 1.86 m | H-19, H-30 | |||
| 3 | 89.3 CH | 3.15 dd (3.9; 11.7) | C: 31, C:1 Xyl1 | H-1, H-5, H-31, H1-Xyl1 |
| 4 | 39.3 C | |||
| 5 | 48.2 CH | 0.90 brd (11.5) | H-1, H-3, H-31 | |
| 6 | 23.1 CH2 | 1.88 m | H-31 | |
| 7 | 121.8 CH | 5.92 m | ||
| 8 | 143.9 C | |||
| 9 | 47.0 CH | 3.52 brd (14.1) | H-6, H-12, H-19 | |
| 10 | 35.5 C | |||
| 11 | 22.3 CH2 | 1.83 m | H-1 | |
| 12 | 29.5 CH2 | 2.22 m | C: 18 | H-17, H-21, H-32 |
| 1.57 m | H-32 | |||
| 13 | 56.8 C | |||
| 14 | 45.6 C | |||
| 15 | 51.9 CH2 | 2.66 d (15.4) | C: 13, 16, 17, 32 | H-7, H-32 |
| 2.31 d (15.4) | C: 14, 16, 32 | |||
| 16 | 214.2 C | |||
| 17 | 63.4 CH | 2.90 s | C: 12, 13, 16, 18, 20, 21 | H-12, H-21, H-22, H-32 |
| 18 | 179.3 C | |||
| 19 | 23.9 CH3 | 1.08 s | C: 1, 5, 9, 10 | H-2, H-6, H-9 |
| 20 | 83.8 C | |||
| 21 | 26.1 CH3 | 1.48 s | C: 17, 20, 22 | H-12, H-17 |
| 22 | 38.2 CH2 | 1.69 m | H-17, H-24 | |
| 1.54 m | ||||
| 23 | 22.1 CH2 | 1.71 m | ||
| 1.42 m | ||||
| 24 | 37.8 CH2 | 1.88 m | C: 23, 25, 26, 27 | H-22 |
| 25 | 145.5 C | |||
| 26 | 110.4 CH2 | 4.69 brs | ||
| 4.68 brs | ||||
| 27 | 22.1 CH3 | 1.62 s | C: 24, 25, 26 | |
| 30 | 17.2 CH3 | 1.03 s | C: 3, 4, 5, 31 | H-2, H-31 |
| 31 | 28.5 CH3 | 1.14 s | C: 3, 4, 5, 30 | H-3, H-5, H-6, H-30, H-1 Xyl1 |
| 32 | 31.8 CH3 | 1.17 s | C: 8, 13, 14, 15 | H-7, H-12, H-15, H-17 |
| Atom | δC mult. a, b, c | δH mult. (J in Hz) d | HMBC | ROESY |
|---|---|---|---|---|
| Xyl1 (1→C-3) | ||||
| 1 | 104.8 CH | 4.59 d (7.7) | C: 3 | H-3; H-3, 5 Xyl1 |
| 2 | 81.1 CH | 4.05 t (8.8) | C: 1 Glc2; 1,3 Xyl1 | H-1 Glc2 |
| 3 | 75.2 CH | 4.23 t (8.8) | C: 2, 4 Xyl1 | H-1, 5 Xyl1 |
| 4 | 78.7 CH | 4.12 m | C: 1 Glc4 | H-1 Glc4 |
| 5 | 63.8 CH2 | 4.49 m | ||
| 3.78 dd (9.9; 12.7) | H-1, 3 Xyl1 | |||
| Glc2 (1→2Xyl1) | ||||
| 1 | 104.2 CH | 5.14 d (8.1) | C: 2 Xyl1 | H-2 Xyl1; H-3, 5 Glc2 |
| 2 | 75.3 CH | 3.86 t (8.1) | C: 1, 3 Glc2 | |
| 3 | 75.3 CH | 3.99 t (8.1) | C: 2, 4 Glc2 | H-1, 5 Glc2 |
| 4 | 82.2 CH | 3.90 t (8.1) | C: 3 Glc2; 1 Glc3 | H-1 Glc3; H-6 Glc2 |
| 5 | 75.9 CH | 3.72 m | H-1, 3 Glc2 | |
| 6 | 61.5 CH2 | 4.33 d (10.2) | ||
| 4.28 m | ||||
| Glc3 (1→4Glc2) | ||||
| 1 | 104.6 CH | 4.84 d (6.8) | C: 4 Glc2 | H-4 Glc2; H-3, 5 Glc3 |
| 2 | 74.2 CH | 3.81 t (9.7) | C: 1, 3 Glc3 | H-4 Glc3 |
| 3 | 76.8 CH | 4.10 t (9.7) | C: 2, 4 Glc3 | H-1 Glc3 |
| 4 | 70.8 CH | 3.94 t (9.7) | C: 3, 5, 6 Glc3 | H-6 Glc3 |
| 5 | 75.6 CH | 4.04 m | H-1 Glc3 | |
| 6 | 67.4 CH2 | 5.03 brd (12.6) | ||
| 4.68 m | ||||
| Glc4 (1→4Xyl1) | ||||
| 1 | 101.1 CH | 4.94 d (7.0) | C: 4 Xyl1 | H-4 Xyl1; H-3, 5 Glc4 |
| 2 | 80.6 CH | 4.77 t (8.3) | C: 1, 3 Glc4 | H-4 Glc4 |
| 3 | 76.9 CH | 4.31 t (8.3) | C: 2, 4 Glc4 | H-1, 5 Glc4 |
| 4 | 70.7 CH | 3.92 t (8.3) | C: 5, 6 Glc4 | H-2 Glc4 |
| 5 | 77.5 CH | 3.88 m | H-1 Glc4 | |
| 6 | 61.8 CH2 | 4.35 brd (11.8) | ||
| 4.03 dd (6.3; 11.8) | C: 5 Glc4 |
| Glycosides | IC50, µM, Erythrocytes | Cytotoxicity, IC50 µM | |||
|---|---|---|---|---|---|
| MCF-10A | MCF-7 | T-47D | MDA-MD-231 | ||
| peronioside A (1) | 30.80 ± 1.01 | >50.0 | >50.0 | >50.0 | >50.0 |
| psolusoside A (2) | 1.05 ± 0.12 | >50.0 | 11.17 ± 0.40 | 9.52 ± 0.13 | 6.09 ± 0.87 |
| psolusoside B (3) | 74.57 ± 1.21 | >50.0 | >50.0 | >50.0 | >50.0 |
| DS-psolusoside B | 25.78 ± 2.55 | 46.22 ± 0.98 | 33.88 ± 1.64 | 31.20± 0.41 | 18.74 ± 1.65 |
| psolusoside G (4) | 2.70 ± 0.09 | >50.0 | 11.43 ± 0.36 | 16.04 ± 1.17 | 10.99 ± 0.36 |
| psolusoside I (5) | 8.49 ± 0.49 | >50.0 | >50.0 | 19.56 ± 0.61 | 33.12 ± 0.85 |
| psolusoside L (6) | 1.83 ± 0.14 | 33.08 ± 0.29 | 13.74 ± 0.31 | 5.45 ± 0.04 | 6.39 ± 0.30 |
| psolusoside N (7) | 17.41 ± 0.41 | >50.0 | >50.0 | >50.0 | 39.30 ± 2.89 |
| psolusoside P (8) | 7.77 ± 0.71 | >50.0 | >50.0 | 37.09 ± 0.83 | 33.29 ± 1.31 |
| cisplatin | - | 82.63 ± 4.62 | 70.39 ± 2.15 | 63.57 ± 1.94 | 66.23 ± 1.07 |
| Glycosides | Time, h | Selectivity Index | ||
|---|---|---|---|---|
| MCF-7 | T-47D | MDA-MB-231 | ||
| psolusoside A (2) | 24 | 4.47 | 5.25 | 8.21 |
| 48 | 1.84 | 0.86 | 2.18 | |
| 72 | 0.59 | 0.76 | 2.09 | |
| psolusoside L (6) | 24 | 2.41 | 6.07 | 5.18 |
| 48 | 1.33 | 1.25 | 5.83 | |
| 72 | 0.66 | 0.91 | 4.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Popov, R.S.; Chingizova, E.A.; Menchinskaya, E.S.; Zelepuga, E.A.; Tabakmakher, K.M.; Stepanov, V.G.; Kalinin, V.I. The Composition of Triterpene Glycosides in the Sea Cucumber Psolus peronii: Anticancer Activity of the Glycosides against Three Human Breast Cancer Cell Lines and Quantitative Structure–Activity Relationships (QSAR). Mar. Drugs 2024, 22, 292. https://doi.org/10.3390/md22070292
Silchenko AS, Kalinovsky AI, Avilov SA, Popov RS, Chingizova EA, Menchinskaya ES, Zelepuga EA, Tabakmakher KM, Stepanov VG, Kalinin VI. The Composition of Triterpene Glycosides in the Sea Cucumber Psolus peronii: Anticancer Activity of the Glycosides against Three Human Breast Cancer Cell Lines and Quantitative Structure–Activity Relationships (QSAR). Marine Drugs. 2024; 22(7):292. https://doi.org/10.3390/md22070292
Chicago/Turabian StyleSilchenko, Alexandra Sergeevna, Anatoly Ivanovich Kalinovsky, Sergey Anatolievich Avilov, Roman Sergeevich Popov, Ekaterina Alexandrovna Chingizova, Ekaterina Sergeevna Menchinskaya, Elena Alexandrovna Zelepuga, Kseniya Mikhailovna Tabakmakher, Vadim Georgievich Stepanov, and Vladimir Ivanovich Kalinin. 2024. "The Composition of Triterpene Glycosides in the Sea Cucumber Psolus peronii: Anticancer Activity of the Glycosides against Three Human Breast Cancer Cell Lines and Quantitative Structure–Activity Relationships (QSAR)" Marine Drugs 22, no. 7: 292. https://doi.org/10.3390/md22070292
APA StyleSilchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Popov, R. S., Chingizova, E. A., Menchinskaya, E. S., Zelepuga, E. A., Tabakmakher, K. M., Stepanov, V. G., & Kalinin, V. I. (2024). The Composition of Triterpene Glycosides in the Sea Cucumber Psolus peronii: Anticancer Activity of the Glycosides against Three Human Breast Cancer Cell Lines and Quantitative Structure–Activity Relationships (QSAR). Marine Drugs, 22(7), 292. https://doi.org/10.3390/md22070292








