Abstract
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) offer diverse health benefits, such as supporting cardiovascular health, improving cognitive function, promoting joint and musculoskeletal health, and contributing to healthy aging. Despite their advantages, challenges like oxidation susceptibility, low bioavailability, and potential adverse effects at high doses persist. Nanoparticle encapsulation emerges as a promising avenue to address these limitations while preserving stability, enhanced bioavailability, and controlled release. This comprehensive review explores the therapeutic roles of omega-3 fatty acids, critically appraising their shortcomings and delving into modern encapsulation strategies. Furthermore, it explores the potential advantages of metal–organic framework nanoparticles (MOF NPs) compared to other commonly utilized nanoparticles in improving the therapeutic effectiveness of omega-3 fatty acids within drug delivery systems (DDSs). Additionally, it outlines future research directions to fully exploit the therapeutic benefits of these encapsulated omega-3 formulations for cardiovascular disease treatment.
1. Introduction
Cardiovascular diseases (CVDs) remain the world’s leading cause of mortality, claiming a staggering 695,000 lives in the United States alone in 2021 [1], translating to roughly one death every 30 s [2]. According to a 2019 report by the World Health Organization, CVD contributes to 32% of total worldwide fatalities, with 85% of these fatalities attributed to heart attacks or strokes [3]. Major contributors and risk factors for CVD include elevated blood pressure, diabetes, and cholesterol levels, as well as smoking, unhealthy diet, obesity, and physical inactivity [4].
According to a recent report from the American Heart Association, an intake of approximately 3 g of omega-3 fatty acids daily, whether obtained from food or supplements, appears to be the optimal amount for reducing high blood pressure and preventing cardiovascular disease, as indicated by a review of multiple research studies [5]. Omega-3 fatty acids (ω-3 FAs) are categorized as polyunsaturated fatty acids (PUFAs) that include a minimum of a single double bond (C=C) between the carbon atoms in the third and fourth positions from the methyl end of the fatty acid [6,7]. PUFAs are long-chain fatty acids (LC-FAs) found in oily fish like sardines, tuna, and salmon, and other seafood like shellfish, algae, and shrimp, as well as particular plants and nut-based oils [8]. The most common bioactive ω-3 FAs are eicosapentaenoic acid (EPA) (C20:5 ω-3), docosahexaenoic acid (DHA) (C22:6 ω-3), and α-linolenic acid (ALA) (C18:3 ω) [9]. ALA can undergo various elongation and desaturation processes within the body to be converted into EPA and DHA [10]. However, the conversion rates of ALA to EPA and DHA may be relatively low, potentially insufficient to confer health benefits. Therefore, it is essential to ensure adequate intake of EPA and DHA for various aspects of health, including cardiovascular health, brain function, and inflammation regulation. PUFAs are essential nutrients that must be obtained from the diet because the body cannot synthesize them.
PUFAs play numerous physiological roles involving cell signaling and transmission, intercellular contact, membrane fluidity, phospholipid membrane maintenance, reduced inflammation [11,12], and improved fatty acid oxidation [13]. Hence, a deficiency in ω-3 FAs can deteriorate bone health [14,15,16], cardiovascular health [17], and skin and neurological health [13,18] (Figure 1). Along with this, ω-3 FA ingestion is associated with a lowered prevalence of inflammation [19] through the lowering of pro-inflammatory cytokines triggered by oxidative stress in macrophages isolated from female mice [20] and rats [21]. Various studies have also assessed the anti-inhibitory effect of ω-3 FAs on cancers in female mice [22] and cancer cell lines [23]. Over the decades, various preclinical and clinical studies have been conducted using ω-3 FAs to show their efficacy in reducing cardiovascular ailments. This review paper will provide an overview of ω-3 FAs with a focus on their effect on the cardiovascular system, along with shortcomings in the delivery of ω-3 FAs to which nanoparticles could be potentially a viable solution.
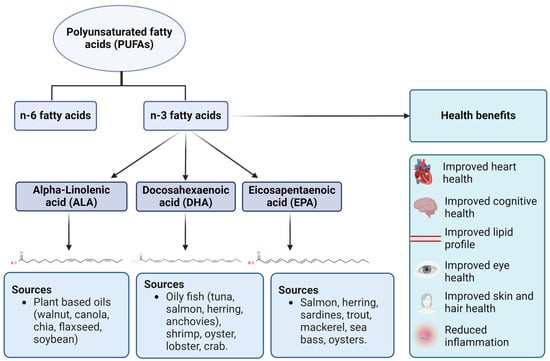
Figure 1.
Types, sources, and health benefits of omega-3 fatty acids. Three types of n-3 fatty acids are ALA (plant-based sources), DHA (animal-based sources), and EPA (animal-based sources). As mentioned earlier, n-3 fatty acids have a wide range of health benefits related to cardiovascular, ocular, cognitive, and dermal health.
1.1. Preclinical Studies
Over the years, various pre-clinical studies conducted on a range of animal experimental units have proved the effectiveness of ω-3 FAs in cardioprotection and decreasing markers of cardiovascular stress, as summarized in Table 1.

Table 1.
Summary of outcomes of pre-clinical animal studies published between 1992 and 2022 on cardiovascular health utilizing diets rich in omega-3 fatty acids (ω-3 FAs). (↓ indicates a positive decrease in outcome). REF = reference or control diet, SF = sheep fat diet, PUFA = polyunsaturated fatty acids, SSO = sunflower seed oil diet, TFO = tuna fish oil diet, EPA = eicosapentaenoic acid, DHA = docosahexaenoic acid, LNA = Linoleic acid.
1.2. Clinical Studies
Several significant meta-analyses of ω-3 FAs and their connection to cardiovascular morbidity and mortality have been published in the last four decades. Some of these studies concluded that fish oil supplementation lowers the risk of cardiovascular events and the mortality rates from CVD, while others did not support this conclusion, as seen in Table 2.

Table 2.
Summary of outcomes of randomized clinical trials (RCTs) published between 1989 and 2021 on cardiovascular health utilizing diets rich in ω-3 FAs. (↓ indicates a positive decrease in outcome, ↓ indicates a negative decrease in outcome, and ↑ indicates a negative increase in outcome).
The differences in research findings might be attributed to the various fatty acids employed in these studies, which ranged fromo-3 carboxylic acid formulation to ethyl esters of EPA, DHA, and ALA. The omega-3 CA employed in the STRENGTH study was EPA as a free fatty carboxylic acid, which has greater absorption in a diet low in fat than EPA and DHA ethyl esters, comparable to the EPA ethyl ester used in the REDUCE-IT study. Consequently, omega-3 CA increases blood levels of EPA and DHA when combined with a diet low in fat, but not with a standard diet. In the STRENGTH study, omega-3 CA was administered independent of eating habits or dietary fat composition, which might culminate in fluctuation in EPA and DHA levels in the blood and perhaps reduce the impact on cardiovascular events. Icosapent ethyl additionally contains approximately 25% higher EPA in each dose than omega-3 CA, resulting in 61% greater EPA levels in blood in REDUCE-IT versus STRENGTH from comparable baseline values [41].
1.3. Epidemiological Studies
Apart from clinical studies, epidemiological studies were also conducted on the effect of ω-3 FAs on the reduction of cardiovascular events. The results of the studies in Table 3 were mixed, with some claiming that ω-3 FAs decrease cardiovascular events and others claiming no such outcome.

Table 3.
Summary of outcomes of cohort studies published between 2000 and 2021 on cardiovascular health utilizing diets rich in ω-3 FAs. (↓ indicates a positive decrease in outcome, ↓ indicates a negative decrease in outcome, and ↑ indicates a negative increase in outcome).
1.4. Case-Control Studies
The number of case-control studies measuring EPA and DHA levels in plasma as a biomarker of ω-3 FA after 2004 declined due to the introduction of the new concept of the omega-3 index (O3I) [50]. The omega-3 index is defined as the proportion of EPA and DHA in a total of 26 distinct fatty acids in the membrane of red blood cells (RBCs), with many studies claiming that an O3I of 8% is required to elicit the cardioprotective effect of ω-3 FAs [51]. There are many reasons why measuring EPA and DHA in erythrocyte membranes is a more accurate method of assessing ω-3 FA intake in the diet. RBC fatty acid content has relatively little biological variability in comparison to plasma. Erythrocyte lipids are nearly entirely composed of phospholipids, and RBC fatty acid content represents tissue fatty acid composition [52]. Table 4 shows the results of a few of the earliest case-control studies.

Table 4.
Summary of outcomes of case-control studies published between 1990 and 2010 on cardiovascular health utilizing diets rich in ω-3 FAs. (↓ indicates a positive decrease in outcome.)
2. Nanoparticles and Administration of Omega-3
2.1. Obstacles in the Effective Administration of Omega-3 PUFAs
There have been concerns regarding the optimal source and route of administration of ω-3 FAs for human consumption. Even though the simplest method of ω-3 FA intake is through eating fish, decreasing fish stocks worldwide and biomagnification of toxic trace elements due to water pollution are proving to be valid concerns [58]. An alternate method is consuming ω-3 FA supplements. The issue with supplements is that they may cause undesired side effects, such as stomach upsets, stale breath, nausea, etc. (Figure 2). Moreover, the release of ω-3 FAs in supplements occurs rapidly, simultaneously delivering the entire amount [59].
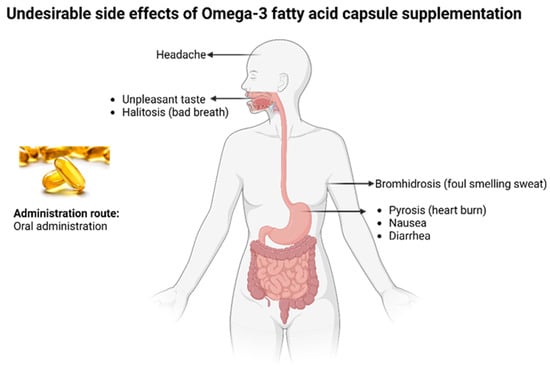
Figure 2.
Common undesirable side effects of omega-3 fish oil capsule supplements.
The bioavailability of long-chain ω-3 PUFA is another complex issue that needs to be tackled. Bioavailability is a relative term that refers to both the rate of absorption and the amount of the substance ingested [60]. Bioavailability encompasses both absorption speed and quantity absorbed. It is influenced by gastrointestinal absorption and transport rates to the portal system. In a broader sense, it considers the amount reaching systemic circulation or the intended destination. Understanding this aids in pharmacokinetics and dietary planning. NPs can significantly improve the pharmacokinetics of drugs by increasing their solubility [61], stability [62], permeability [63], half-life, and residence time [64]. The major cause of the limited bioavailability of long-chain n-3 PUFAs is low solubility in the aqueous gastrointestinal fluids of the GI tract, alongside their vulnerability to chemical breakdown during transit through the stomach [65]. Long-chain omega-3 PUFAs are mostly present in fish as triacylglycerides (TAGs), phospholipids (PLs), and free fatty acids (FFAs) [66]. ω-3 PUFAs, when ingested as pure oil, cannot be completely absorbed by the cells in our intestines, resulting in reduced bioavailability [67]. Fish oils are transformed into oil-in-water emulsions within the mouth and stomach, while emulsified oils are colloidal before consumption. Lipases in the stomach and pancreas subsequently attach to the surface of the lipid drop and begin the process of lipid digestion, which converts TAGs into FFAs and monoacylglycerols (MAGs). The digestive products, FFAs and MAGs, subsequently combine with bile and PLs to produce blended micelles that transport the lipids across the mucus membrane to the epithelial cell surfaces, where they are absorbed. Investigations have demonstrated that the bioavailability of ω-3 PUFAs is enhanced when consummated in an emulsified way rather than in a pure form [65]. Widely ingested edible oils and products have less ω-3 PUFAs compared to the suggested daily guideline; also, the minimal quantity of PUFAs taken is poorly absorbed. The transformation rate of plant-sourced ω-3 FAs, namely, ALA into EPA and DHA, the primary ω-3 PUFAs accountable for the reported benefits, is only 3–6%, with DHA having an inadequate transformation rate maxing out at 1% [68].
Apart from these complications, lipid oxidation is an additional problem that needs to be addressed. Among the different types of ω-3 FAs, EPA and DHA are extremely vulnerable to the oxidation of lipids due to the presence of numerous double bonds [69]. The oxidative degradation of lipids in fish oil, along with other PUFA-rich and fortified foods is a severe issue that frequently results in a decrease in shelf-life, customer acceptance, performance, nutrient content, and quality [70]. The oxidation of these ω-3 FAs results in aldehydes that are toxic to proteins and nucleic acids in the human body, namely 4-hydroxy-2-nonenal and malondialdehyde [71]. The toxicity stems from their capacity to crosslink proteins and attach covalently to nucleic acids.
Lipid oxidation in fish oils is heightened by exposure to light, oxygen, and heat. Lipid oxidation creates three major issues: (i) it produces disagreeable unpleasant flavors, (ii) it decreases the nutritional content of lipid-containing food items, and (iii) free radicals generated during oxidation may contribute to the occurrence of atherosclerosis in the system [72]. Nevertheless, the low oxidative resistance of ω-3 PUFAs makes these oil-enriched foods require potent antioxidant defense to avert oxidative degradation and undesirable flavor formation [73]. Some solutions to stabilize ω-3 PUFAs include providing antioxidants for oxidative stability, mixing and blending with other oils, hydrogenation, and interesterification [68]. However, another innovative solution might be ω-3 FA encapsulation using nanoparticles as part of nanotechnology.
2.2. Nanoencapsulation of Omega-3 PUFAs
Nanoparticles (NPs) are the focal point of nanomedicine, which is a branch of medicine that relies on smart drug delivery technology that can boost the biological action, pharmacological index, and physiological half-life of the drug loaded inside the body [74]. Nanoparticles are increasingly becoming popular as drug delivery systems, especially to treat CVDs [75] and metabolic disorders [76] due to their ability to have a comparatively big surface area that can attach to, adsorb, and transport molecules, including drugs, probes, and protein molecules [77]. Furthermore, for the administration of medication, engineered nanoparticles, as well as the drug itself, can be synthesized at nanoscale and operate as a carrier for themselves [78]. There exists an array of nanoparticles, each having distinct features, advantages, disadvantages, and applications (Table 5).

Table 5.
Characteristics, uses, advantages, and disadvantages of different classes of nanoparticles.
In the past few years, multiple studies have been performed that utilized encapsulated ω-3 FAs within NPs as a drug delivery method. An in vitro study by Deshpande et al. tested a nanotechnology-focused method for administering ω-3 FAs to the walls of vascular vessels alongside other drugs to avoid occlusive vasculopathy after vascular injury [107]. The researchers created an ω-3-FA-rich oil-in-water nanoemulsion composition using flax seed oil naturally rich in ALA, that was administered to cultured vascular cell lines. Combining the administration of 17-βE and CER-laden nanoemulsions had a stronger anti-proliferative impact on vascular smooth muscle cells as compared to endothelial cells [108]. In 2016, the same researchers set out to examine the effectiveness of an ω-3 FA containing 17-βE nanodelivery setup in treating induced atherosclerosis. The study found that a 3-week 17-βE therapy administered in an ω-3-PUFA-encapsulated nanoemulsion setup improved acute vascular damage with just 30% arterial stenosis [109].
Another study conducted by a separate group in 2019 focused on utilizing atorvastatin nano lipid carriers preloaded with ω-3 PUFAs to lower hyperlipidemia. When compared with the commercial formulation, orally administered ω-3-FA-based Atorvastatin reloaded nano lipid carriers resulted in a substantial decrease in low-density lipoprotein and triglyceride levels in the blood [110]. Through various studies, nanoencapsulation has been shown to increase the bioavailability of ω-3 PUFAs in the body. Through their studies, Wakil et al., 2010 and Sanguansri et al., 2013 proved clearly that microencapsulation can improve the availability of FAs [111,112].
Nanofibers like zein have also been used to encapsulate ω-3 PUFAs recently, such as in one study by Busolo et al., where 85 wt% DHA-supplemented fish oil was encapsulated in zein through electrospraying assisted by pressurized gas technology (EAPG). The average particle size and encapsulation efficiency were 3.7 ± 1.8 μm and 84%, respectively. The fortified reconstituted milk with zein/DHA-enriched fish oil microcapsules showed no signs of oxidation even after 45 days in an oxidation test [113].
Whey microgels loaded with ω-3 PUFAs were also tested in a study in 2022 where 85 wt% DHA-enriched algal oil was loaded into whey protein microgels by ball milling. The end product had an average particle size of 250 nm, an average diameter of 380 nm, and a polydispersity index of 0.26, indicating the zeta potential. These protein microgels loaded with omega-3 PUFAs addressed several obstacles in the development and storage of omega-3 PUFA oils, such as long-term oxidative resistance and better sensory and textural qualities [114].
A summary of studies utilizing unique nanoparticles for the encapsulation of ω-3 FAs, their mechanism of production, physiochemical properties, and observed effect is listed below in Table 6.

Table 6.
Summary of studies encapsulating omega-3 fatty acids using different types of nanoparticles between 2013 and 2022 prepared by a plethora of different techniques and having varying physiochemical properties.
The most common mechanism for nanoencapsulation of ω-3 FAs in the literature involves physical methods, as illustrated below in Figure 3.
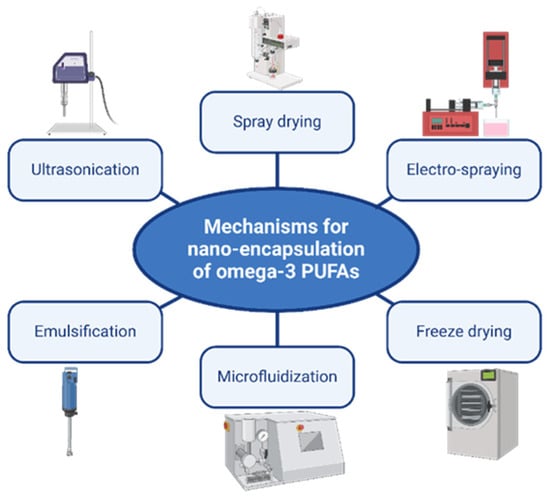
Figure 3.
Common physical mechanisms for nanoencapsulation of omega-3 PUFAs.
However, lipid-based NPs have their drawbacks as well. Due to their precise crystalline form, they display limited drug loading capacity and the likelihood of drug ejection owing to crystallization during storage [115] along with an initial burst discharge of the drug instead of slow controlled drug release [116]. Other disadvantages during oral administration of lipid-based NPs are the formation of gel of hydrophobic lipid dispersion, restricted loading quantity for hydrophilic formulations, and polymorphic transformation [117]. Liposomes are another type of lipid-based NPs that are employed for drug delivery; however, they tend to have a reduced solubility window [91], problems with drug incorporation and encapsulation, high manufacturing costs, and trouble preserving drug integrity and bioactivity during conjugation [75]. Microgels, on the other hand, are complicated and time-consuming to mass produce on a large scale, as the yield and stability of individual microgels is highly variable using the currently available technology [118]. Nanofibers, although super effective in encapsulating omega-3 PUFAs, have their shortcomings too. Their disadvantages include quick disintegration, low mechanical durability, and full dissolution. Therefore, such fibers must be cross-linked to limit their solubility [119].
An alternative to avoid this uncontrolled release would be to use metal–organic framework (MOF) NPs, a novel group of composite nanomaterials, consisting of a combination of inorganic and crystalline organic components [120]. A few of the properties of MOF NPs are their extensive surface area (hollowed-out interior structure) [121], a high degree of porosity, configurable pore dimensions, heat resistance, chemical stability [122], and post-synthesis alterations; these features elevate MOF NPs over lipid-based NPs when it comes to versatility, adaptability, and customizability [123,124]. MOF NPs, such as Material Institute Lavoisier 89 nanoparticles (nanoMIL-89), due to their vast list of perks (Figure 4), can mitigate non-specific drug administration to unsuitable sites, pre-activation of therapeutic agents before reaching the targeted tissue, early immune system approval, and, in certain instances, potentially enhance the pharmacokinetics of drugs at the level of permeability, intake, and dispersion of a drug in the tissue layers [125] (Table 7).
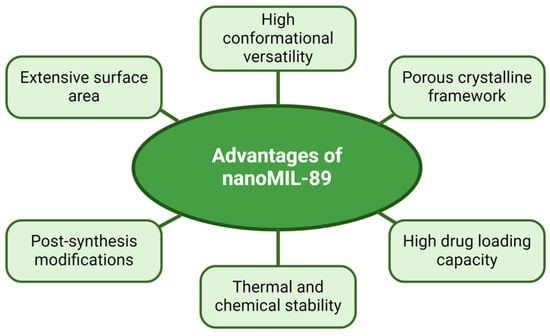
Figure 4.
Advantageous features of a type of MOF; Material Institute Lavoisier 89 nanoparticles (nanoMIL-89). Note: these advantages were based on the study by Al-Ansari et al. [124] that addressed the internalization of MOF NPs in human vascular cells.

Table 7.
Physiochemical properties and related biomedical advantages of MOF NPs.
Keeping the hydrophobic nature of omega-3 FAs in mind, the most crucial design consideration in MOF NPs is to modify the interior of the NPs from hydrophilic to hydrophobic. There are two main approaches to solving the issue of interior hydrophilicity. The first is grafting hydrophobic polymers into the interior wall of the NP, which involves linking hydrophobic polymer chains to the interior of MOF pores to alter the interior for improved encapsulation of omega-3 FAs [131]. The second approach incorporates hydrophobic functional groups within the interior, wherein the MOF linkers are chemically modified to contain hydrophobic groups such as isobutyl, alkyl, and isopropyl functional groups [132].
The possible administration routes of MOF NPs loaded with omega-3 FAs are specific to the system of choice, which, in this review paper, is the cardiovascular system. There are three possible administration routes to focus on for drug delivery to the cardiovascular system: oral, intravenous, and inhalation routes. However, since inhalation relates more to pulmonary ailments [133] and not directly to cardiovascular disease, the former two routes are preferable. Muchow et al., in their study, developed lipid-based omega-3 FA NPs that proved to be patient-friendly and were administered orally [134]. Another consideration for the oral administration of NPs is coating the NP with polymer substances to protect it from the harsh and acidic gastric environment, for instance, coating NPs with poly lactic-co-glycolic acid (PLGA) [135] and chitosan [136]. Omega-3 FA NPs have also successfully been delivered intravenously in some studies [110], resulting in increased hyperlipidemic action.
Based on the toxicology of metal-organic framework MIL-89 nanoparticles on embryonic zebrafish development, Al-Ansari et al. said, “The investigation demonstrates that nanoMIL-89 has no developmental harm on zebrafish embryos at low doses (1–10 μM). High concentrations of nanoMIL-89 (>30 μM) significantly impacted hatching time and heart development. The study proves the safety of nanoMIL89 in biological, environmental, and medicinal applications without cytotoxicity beyond a certain concentration of 30μM” [124].
Other NPs similar to nanoMIL-89, such as MIL88A and MIL101, are also used for the biomedical application of drug delivery. Since both MIL88A and MIL101 share similar features such as small size, high biocompatibility, etc. [137], to nanoMIL-89, they have also been extensively studied for carrying anti-cancer drugs such as curcumin [138] and doxorubicin [139].
SEM and TEM analysis is commonly used to characterize the shape and size of nanoparticles after production, as in the case of nanoMIL-89 below (Figure 5).
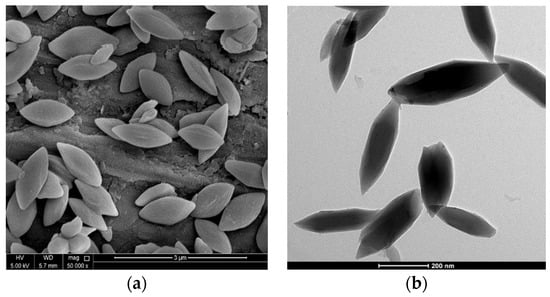
Figure 5.
NanoMIL-89 (iron oxide nanoparticles), a subcategory of MOF nanoparticles. (a) A scanning electron microscope captured an image of nanoMIL-89 (50,000×). (b) A transmission electron microscope captured an image of nanoMIL-89 (5000×).
Paclitaxel (PTX) in microneedle arrays (PTX-MNAs) has higher anticancer efficacy than free PTX (Taxol) in both in vitro and in vivo experiments [140]. Chen and Feng’s research shows that uncoated gold nanoparticles (GNPs) have the potential for skin applications such as penetration, medication loading/release, and combination with physical procedures to treat skin ailments [141]. Gao et al. show that copper sulfide nanoparticles (CuS-TRPV1) can operate as a photothermal switch to minimize atherosclerosis, assisting in cardiac imaging and lowering plaque development in mice, with no long-term damage [142]. Spivak et al. utilized gold NPs loaded with levosimendan (Simdax®) in an in vivo study with Wistar rats and concluded that conjugated AuNPs-Simdax® had a favorable impact on cardiac contractile capacity. Additionally, IV administration of 30 nm AuNPs resulted in accumulation in the endothelial cells of infarcted arteries and capillaries. Necrobiosis and fibrosis were greatly reduced following all treatments. Conjugate (Simdax + AuNPs) injections resulted in a considerably larger hydrothorax reduction compared to Simdax injections alone (p < 0.01), along with improved cardiac contractile ability. Interestingly, AuNP administration yielded results similar to that of the conjugate [143]. In 2020, Li et al. successfully demonstrated that a gold-nanorod-based NP that catalyzes continuous NO production safeguards against cardiovascular damage in vitro [144]. Some studies employ a hybrid NP that combines liposomes coated with metal NPs, as in the case of Bejarano et al., who developed a gold NP-based nanosystem and employed it to optimize the distribution of angiotensin-1–9, which is a cardioprotectant peptide, to the myocardium, helping both hypertension and myocardial remodeling [145]. Hussein et al. were successful in producing ZnO and gum NPs loaded with 400 mg of DHA solution using a one-step solid-state process [146]. A total of 250 mg of DHA were loaded into ZnO NPs using ultrasonication and homogenization methods [147]. Later, the same researcher also synthesized DHA-loaded AgNPs through nanoprecipitation for antidiabetic drug testing [148]. In a study by El-Daly et al., both DHA-Ag NPs and DHA-ZnO NPs were tested side by side to study the expression of the glucose transport cascade [149,150]. Lastly, another study implied that an administration of 400 µg/kg/day gold NPs helps improve myocardial damage induced by isoproterenol in male albino rats [151].
Even though MOF NPs have an array of advantages in their applications, they also come with some drawbacks, such as some of the techniques used to synthesize NPs. The solvothermal and microemulsion techniques are highly expensive. The microemulsion technique uses certain surfactants that are classified as pollutants in the environment. The mechanochemical technique requires a large amount of energy and yields amorphous, not crystalline, NPs, which further cannot be used in X-ray crystal structure analysis. Synthetic techniques for the synthesis of MOFs with effective carbon dioxide absorption capability, such as amine scrubbing, include drawbacks such as high energy usage [152]. Rare-earth-based MOF NPs, namely uranium-, cerium-, lanthanum-, and yttrium-based NPs, are highly expensive, difficult to access, and radioactive, hence dangerous [153]. To conclude, while MOF NPs provide appealing advantages in a variety of applications, their possible downsides highlight the need for additional research and careful evaluation of their usage to maximize their benefits while mitigating related impediments.
3. Conclusions
In conclusion, encapsulating omega-3 fatty acids with nanoparticles offers a potential strategy for improving the vital nutrients’ durability, bioavailability, and targeted administration. Investigators have effectively mitigated oxidation, undesirable taste, and restricted GI absorption linked to free omega-3 fatty acids using various encapsulating strategies, from nanoemulsions to solid lipid and polymeric nanoparticles. Nanoparticle containment has various benefits, including environmental protection, precise release dynamics, and the opportunity to integrate other bioactive substances for synergistic effects. Without a doubt, nanoparticles also have drawbacks that need to be addressed to improve acceptability. Furthermore, the nanoscale dimension of these delivery methods facilitates effective cellular absorption and transit beyond biological barriers, resulting in better therapeutic effects.
Nonetheless, additional investigation is needed to optimize the formulation characteristics of nanoparticles, such as structure, dimension, surface characteristics, and encapsulation efficacy, to maximize the bioavailability and effectiveness of omega-3s. Furthermore, long-term stability trials and in vivo tests are required to evaluate the safety, pharmaceutical kinetics, and medicinal potential of nanoparticle-based omega-3 nanoparticles in a variety of clinical contexts. In summary, applying nanoparticles to encapsulate omega-3 fatty acids shows considerable potential for resolving present problems in omega-3 supplementation, while opening up new options for personalized nutrition and preventative healthcare.
Author Contributions
R.G.: Investigation, Methodology, Writing—Original Draft Preparation; M.A.-B.: Investigation, Writing—Review and Editing; M.A.: Writing—Review and Editing; N.A.M.: Writing—Review and Editing; H.A.-S.: Conceptualization, Resources, Supervision, Writing—Review and Editing, Funding Acquisition; M.M.R.: Conceptualization, Project Administration, Resources, Supervision, Writing—Original Draft, Writing—Review and Editing, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Qatar University [QU Internal grant QUCG-CAS-23, QNRF UREP30-078-3-023].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. Multiple Cause of Death Data on CDC WONDER. 2023. Available online: https://wonder.cdc.gov/mcd.html (accessed on 2 January 2024).
- World Health Organization. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 2 January 2024).
- Centers for Disease Control and Prevention. Heart Disease and Stroke. 2020. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed on 2 January 2024).
- American Heart Association. Consuming about 3 Grams of Omega-3 Fatty Acids a Day May Lower Blood Pressure. Available online: https://www.heart.org/en/news/2022/06/01/consuming-about-3-grams-of-omega-3-fatty-acids-a-day-may-lower-blood-pressure (accessed on 2 January 2024).
- von Schacky, C.; Harris, W. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc. Res. 2007, 73, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J. Essential Fatty Acids, Micronutrient Information Center 179. 2019. Available online: https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids (accessed on 2 January 2024).
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic Effects of Krill Oil are Essentially Similar to Those of Fish Oil but at Lower Doses of EPA and DHA, in Healthy Volunteers. Lipids 2010, 46, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.-K.K.; Gudbrandsen, O.A.; Drotningsvik, A.; Ruyter, B.; Berge, G.M.; Vogt, G.; Nilsson, A. Different Dietary Ratios of Camelina Oil to Sandeel Oil Influence the Capacity to Synthesise and Deposit EPA and DHA in Zucker Fa/Fa Rats. Nutrients 2023, 15, 2344. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Rahman, M.M.; Bhattacharya, A.; Barnes, J.L.; Chandrasekar, B.; Fernandes, G. Docosahexaenoic Acid-Enriched Fish Oil Attenuates Kidney Disease and Prolongs Median and Maximal Life Span of Autoimmune Lupus-Prone Mice. J. Immunol. 2010, 184, 5280–5286. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Bhattacharya, A.; Rahman, M.; Zaman, K.; Banu, J. Effects of n-3 fatty acids on autoimmunity and osteoporosis. Front. Biosci. 2008, 13, 4015–4020. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, H. Dietary polyunsaturated fatty acids, brain function, and mental health. Microb. Ecol. Health Dis. 2017, 28 (Suppl. 1), 1281916. [Google Scholar] [CrossRef]
- Rennie, K.L.; Hughes, J.; Lang, R.; Jebb, S.A. Nutritional management of rheumatoid arthritis: A review of the evidence. J. Hum. Nutr. Diet. 2003, 16, 97–109. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Rahman, M.M.; Banu, J.; Lawrence, R.; McGuff, H.S.; Garrett, I.R.; Fischbach, M.; Fernandes, G. Inhibition of Osteoporosis in Autoimmune Disease Prone MRL/Mpj-FaslprMice by N-3 Fatty Acids. J. Am. Coll. Nutr. 2005, 24, 200–209. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Ouhtit, A.; Halade, G.V.; Rahman, M.M. Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content. Nutrients 2019, 11, 2701. [Google Scholar] [CrossRef] [PubMed]
- Holm, T. Omega-3 fatty acids improve blood pressure control and preserve renal function in hypertensive heart transplant recipients. Eur. Heart J. 2001, 22, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P. Omega-3 Fatty Acids in Psychiatry: A Review. Ann. Clin. Psychiatry 2000, 12, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Ghayas, S.; Jan, H.; Kalaycioglu, G.D.; Moghimi, S.M. Omega-3 fatty acid nanocarriers: Characterization and potential applications. Curr. Opin. Colloid Interface Sci. 2023, 67, 101728. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sun, D.; Rahman, M.; Fernandes, G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J. Nutr. Biochem. 2007, 18, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kesavalu, L.; Bakthavatchalu, V.; Rahman, M.M.; Su, J.; Raghu, B.; Dawson, D.; Fernandes, G.; Ebersole, J.L. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol. Immunol. 2007, 22, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Crovella, S.; Ouhtit, A.; Rahman, S.M.; Rahman, M.M. Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line. Nutrients 2023, 15, 1658. [Google Scholar] [CrossRef]
- McLennan, P.L.; Bridle, T.M.; Abeywardena, M.Y.; Charnock, J.S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am. Heart J. 1992, 123, 1555–1561. [Google Scholar] [CrossRef]
- Madingou, N.; Gilbert, K.; Tomaro, L.; Touchette, C.P.H.; Trudeau, F.; Fortin, S.; Rousseau, G. Comparison of the effects of EPA and DHA alone or in combination in a murine model of myocardial infarction. Prostaglandins Leukot. Essent. Fat. Acids/Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 11–16. [Google Scholar] [CrossRef]
- Baum, J.R.; Dolmatova, E.; Tan, A.; Duffy, H.S. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front. Physiol. 2012, 3, 272. [Google Scholar] [CrossRef]
- Kalish, B.T.; Matte, A.; Andolfo, I.; Iolascon, A.; Weinberg, O.; Ghigo, A.; Cimino, J.; Siciliano, A.; Hirsch, E.; Federti, E.; et al. Dietary ω-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematologica 2015, 100, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Angelotti, A.; Snoke, D.B.; Ormiston, K.; Cole, R.M.; Borkowski, K.; Newman, J.W.; Orchard, T.S.; Belury, M.A. Potential Cardioprotective Effects and Lipid Mediator Differences in Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplemented Mice Given Chemotherapy. Metabolites 2022, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Gilbert, J.F.; Holliday, R.M.; Elwood, P.C.; Fehily, A.M.; Rogers, S.; Sweetnam, P.M.; Deadman, N.M. Effects of changes in fat, fish, and fiber intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 334, 757–761. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Huet, B.; Traber, M. GISSI-Prevenzione trial. Lancet 1999, 354, 1554. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomized open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Einvik, G.; Ole Klemsdal, T.; Sandvik, L.; Hjerkinn, E.M. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 588–592. [Google Scholar] [CrossRef]
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; Del Castillo, U.; Sack, R.; et al. OMEGA, a Randomized, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy After Myocardial Infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n–3 Fatty Acids and Cardiovascular Events after Myocardial Infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomized placebo-controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Stevens, W.; Haynes, R.; Aung, T.; Chen, F.; Buck, G.; Collins, R.; Armitage, J.; ASCEND Study Collaborative Group. ASCEND: A Study of Cardiovascular Events in Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am. Heart J. 2018, 198, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA 2020, 324, 2268. [Google Scholar] [CrossRef] [PubMed]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.; Bistrian, B.; Driscoll, D. Omega-3 Fatty Acids Effect on Major Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA 2021, 325, 1333. [Google Scholar] [CrossRef]
- Gillum, R.F.; Mussolino, M.; Madans, J.H. The relation between fish consumption, death from all causes, and incidence of coronary heart disease. J. Clin. Epidemiol. 2000, 53, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, Y.; Tsugane, S. Intake of Fish and n3 Fatty Acids and Risk of Coronary Heart Disease Among Japanese. Circulation 2006, 113, 195–202. [Google Scholar] [CrossRef]
- Kühn, T.; Teucher, B.; Kaaks, R.; Boeing, H.; Weikert, C.; Buijsse, B. Fish consumption and the risk of myocardial infarction and stroke in the German arm of the European Prospective Investigation into Cancer and Nutrition (EPIC-Germany). Br. J. Nutr. 2013, 110, 1118–1125. [Google Scholar] [CrossRef]
- Nahab, F.; Pearson, K.; Frankel, M.R.; Ard, J.; Safford, M.M.; Kleindorfer, D.; Howard, V.J.; Judd, S. Dietary fried fish intake increases the risk of CVD: The reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Public Health Nutr. 2016, 19, 3327–3336. [Google Scholar] [CrossRef]
- Bonaccio, M.; Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Fish intake is associated with lower cardiovascular risk in a Mediterranean population: Prospective results from the Moli-sani study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Hengeveld, L.M.; Praagman, J.; Beulens, J.W.J.; Brouwer, I.A.; van der Schouw, Y.T.; Sluijs, I. Fish consumption and risk of stroke, coronary heart disease, and cardiovascular mortality in a Dutch population with low fish intake. Eur. J. Clin. Nutr. 2018, 72, 942–950. [Google Scholar] [CrossRef]
- Ward, R.E.; Cho, K.; Nguyen, X.M.T.; Vassy, J.L.; Ho, Y.L.; Quaden, R.M.; Gagnon, D.R.; Wilson, P.W.F.; Gaziano, J.M.; Djoussé, L. Omega-3 supplement use, fish intake, and risk of non-fatal coronary artery disease and ischemic stroke in the Million Veteran Program. Clin. Nutr. 2020, 39, 574–579. [Google Scholar] [CrossRef]
- Pertiwi, K.; Küpers, L.K.; de Goede, J.; Zock, P.L.; Kromhout, D.; Geleijnse, J.M. Dietary and Circulating Long-Chain Omega-3 Polyunsaturated Fatty Acids and Mortality Risk After Myocardial Infarction: A Long-Term Follow-Up of the Alpha Omega Cohort. J. Am. Heart Assoc. 2021, 10, e022617. [Google Scholar] [CrossRef]
- Harris, W.S.; von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Harris, W. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol. Res. 2007, 55, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Thomas, R.M. Biological variability of blood omega-3 biomarkers. Clin. Biochem. 2010, 43, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Gramenzi, A.; Gentile, A.; Fasoli, M.; Negri, E.; Parazzini, F.; La Vecchia, C. Association between certain foods and risk of acute myocardial infarction in women. BMJ 1990, 300, 771–773. [Google Scholar] [CrossRef]
- Siscovick, D.S. Dietary Intake and Cell Membrane Levels of Long-Chain n-3 Polyunsaturated Fatty Acids and the Risk of Primary Cardiac Arrest. JAMA J. Am. Med. Assoc. 1995, 274, 1363. [Google Scholar] [CrossRef]
- Hallgren, C.G.; Göran Hallmans Jansson, J.O.; Marklund, S.L.; Huhtasaari, F.; Schütz, A.; Strömberg, U.; Vessby, B.; Skerfving, S. Markers of high fish intake are associated with a decreased risk of a first myocardial infarction. Br. J. Nutr. 2001, 86, 397–404. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Zampelas, A.; Chrysohoou, C.; Griffin, B.A.; Stefanadis, C.; Toutouzas, P. Fish consumption and the risk of developing acute coronary syndromes: The CARDIO2000 study. Int. J. Cardiol. 2005, 102, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Amani, R.; Noorizadeh, M.; Rahmanian, S.; Afzali, N.; Haghighizadeh, M.H. Nutritional related cardiovascular risk factors in patients with coronary artery disease in IRAN: A case-control study. Nutr. J. 2010, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Calerón, M.; Napier, J.A. New alternative sources of omega-3 fish oil. Adv. Food Nutr. Res. 2023, 1, 343–398. [Google Scholar]
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Br. J. Nutr. 2012, 107 (Suppl. 2), S23–S52. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Lamson, N.G.; Berger, A.; Fein, K.C.; Whitehead, K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 2019, 4, 84–96. [Google Scholar] [CrossRef]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling evolution of protein corona: A prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021, 81, 101081. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef] [PubMed]
- Homroy, S.; Chopra, R.N.; Singh, P.K.; Dhiman, A.; Rama, S.; Talwar, B. Role of encapsulation on the bioavailability of omega-3 fatty acids. Compr. Rev. Food Sci. Food Saf. 2023, 23, e13272. [Google Scholar] [CrossRef] [PubMed]
- Dacaranhe, C.D.; Terao, J. Effect of Phosphatidic Acid and Phosphatidylserine on Lipid Oxidation in Beef Homogenate During Storage and in Emulsified Sardine Oil. J. Food Sci. 2001, 66, 422–427. [Google Scholar] [CrossRef]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Nair, V.; Cooper, C.S.; Vietti, D.E.; Turner, G.A. The chemistry of lipid peroxidation metabolites: Crosslinking reactions of malondialdehyde. Lipids 1986, 21, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Meyer, A.S.; Nissen, J.A.; Holstborg, J.; Hølmer, G. Oxidation in fish-oil-enriched mayonnaise. Eur. Food Res. Technol. 1999, 210, 13–30. [Google Scholar] [CrossRef]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Thomsen, M.; Skibsted, L.H.; Hølmer, G.; Adler-Nissen, J.; Meyer, A.S. Oxidation in fish oil-enriched mayonnaise: 4. Effect of tocopherol concentration on oxidative deterioration. Eur. Food Res. Technol. 2001, 212, 308–318. [Google Scholar] [CrossRef]
- Omer, A.M.; Ziora, Z.M.; Tamer, T.M.; Khalifa, R.E.; Hassan, M.A.; Mohy-Eldin, M.S.; Blaskovich, M.A.T. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saleh, H.A.; Kameno, Y.; Marei, I.; de Nucci, G.; Ahmetaj-Shala, B.; Shala, F.; Kirkby, N.S.; Jennings, L.; Davies, R.P.; et al. Metal-organic framework (MOF) nanomedicine preparations of sildenafil designed for the future treatment of pulmonary arterial hypertension. bioRxiv 2019. [Google Scholar] [CrossRef]
- Mohamed, H.; Mohamed, N.; Macasa, S.; Basha, H.; Adan, A.; Marei, I.; Ding, H.; Triggle, C.; Crovella, S.; Abou-Saleh, H. Managing diabetes with nanomedicine: nanoMIL-89 as a promising drug delivery system for metformin. Res. Sq. 2024; preprint. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Marei, I.; Crovella, S.; Abou-Saleh, H. Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1404. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Preethi, R.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Green Nanomaterials and Nanotechnology for the Food Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–256. [Google Scholar] [CrossRef]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Klinkova, A.; Thérien-Aubin, H. Chapter 6—Polymer nanoparticles. In Nanochemistry: Chemistry of Nanoparticle Formation and Interactions; Klinkova, A., Thérien-Aubin, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 167–215. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780443214479000023 (accessed on 18 March 2024).
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R.R. Polymeric Micelles and their Properties. J. Nanomed. Nanotechnol. 2017, 8, 433. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. BioMed Res. Int. 2021, 2021, e8844030. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef]
- Cavalli, R.; Caputo, O.; Gasco, M.R. Solid lipospheres of doxorubicin and idarubicin. Int. J. Pharm. 1993, 89, R9–R12. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Duong, V.A. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Jha, S.; KSharma, P.; Malviya, R. Liposomal Drug Delivery System for Cancer Therapy: Advancement and Patents. Recent Pat. Drug Deliv. Formul. 2016, 10, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.R.; Osmani, R.A.; Ghodake, P.P.; Shaikh, S.M.; Chavan, S.R. Nanoemulsion: A Review on Novel Profusion in Advanced Drug Delivery. Indian J. Pharm. Biol. Res. 2014, 2, 122. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Ma, R.; Liang, S.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterization and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.F.; El-Monaem, E.M.A.; El-Aqapa, H.G.; Elashery, S.E.A.; Eltaweil, A.S.; El Kady, M.; Khalifa, S.A.M.; Hawash, H.B.; El-Seedi, H.R. Iron oxide nanoparticles and their pharmaceutical applications. Appl. Surf. Sci. Adv. 2022, 11, 100284. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Sahoo, S.K. Applications of Magnetic Nanocomposites in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 47–63. [Google Scholar] [CrossRef]
- Leong, K.H.; Chin, Y.H.; Sim, L.C.; Tan, B.; Dai, C.; Saravanan, P. Physical Properties of Quantum Dots; Elsevier: Amsterdam, The Netherlands, 2022; pp. 687–709. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum Dots—Characterization, Preparation and Usage in Biological Systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Chapter 23—Mesoporous silica nanoparticles: A promising multifunctional drug delivery system. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–621. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323461443000234 (accessed on 4 February 2024).
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Trombino, S.; Calviello, G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: Potential application in cardiovascular and neoplastic diseases. Int. J. Nanomed. 2019, 14, 2809–2828. [Google Scholar] [CrossRef]
- Deshpande, D.; Janero, D.R.; Amiji, M. Engineering of an ω-3 polyunsaturated fatty acid-containing nanoemulsion system for combination C6-ceramide and 17β-estradiol delivery and bioactivity in human vascular endothelial and smooth muscle cells. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 885–894. [Google Scholar] [CrossRef]
- Deshpande, D.; Kethireddy, S.; Janero, D.R.; Amiji, M.M. Therapeutic Efficacy of an ω-3-Fatty Acid-Containing 17-β Estradiol Nano-Delivery System against Experimental Atherosclerosis. Zhu X, editor. PLoS ONE 2016, 11, e0147337. [Google Scholar] [CrossRef]
- Sreedhar, R.; Kumar, V.S.; Bhaskaran Pillai, A.K.; Mangalathillam, S. Omega-3 Fatty Acid Based Nanolipid Formulation of Atorvastatin for Treating Hyperlipidemia. Adv. Pharm. Bull. 2019, 9, 271–280. [Google Scholar] [CrossRef]
- Wakil, A.; Mackenzie, G.; Diego-Taboada, A.; Bell, J.G.; Atkin, S.L. Enhanced Bioavailability of Eicosapentaenoic Acid from Fish Oil After Encapsulation Within Plant Spore Exines as Microcapsules. Lipids 2010, 45, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Sanguansri, L.; Shen, Z.; Weerakkody, R.; Barnes, M.; Lockett, T.; Augustin, M.A. Omega-3 fatty acids in ileal effluent after consuming different foods containing microencapsulated fish oil powder—An ileostomy study. Food Funct. 2013, 4, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Busolo, M.A.; Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innov. Food Sci. Emerg. Technol. 2019, 51, 12–19. [Google Scholar] [CrossRef]
- Wang, G.S.; Chen, H.; Feng, G.; Yuan, Y.; Wan, Z.; Guo, J.; Wang, J.; Yang, X. Polyphenol-Enriched Protein Oleogels as Potential Delivery Systems of Omega-3 Fatty Acids. J. Agric. Food Chem. 2022, 71, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Park, J.W.; Yoon, I.S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharm. 2015, 495, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Ramasamy, T.; Truong, D.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. Preparation and Characterization of Fenofibrate-Loaded Nanostructured Lipid Carriers for Oral Bioavailability Enhancement. AAPS PharmSciTech 2014, 15, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, D.; Li, Q.; Cao, X.; Dong, H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact. Mater. 2022, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; BIHawash, H.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications, and limitations. Heliyon 2023, 9, e16228. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Hirschle, P.; Preiß, T.; Auras, F.; Pick, A.; Völkner, J.; Valdepérez, D.; Witte, G.; Parak, W.J.; Rädler, J.O.; Wuttke, S. Exploration of MOF nanoparticle sizes using various physical characterization methods—Is what you measure what you get? CrystEngComm 2016, 18, 4359–4368. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The Applications of Metal−Organic Frameworks in Electrochemical Sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef]
- Al-Ansari, D.E.; Mohamed, N.A.; Marei, I.; Zekri, A.; Kameno, Y.; Davies, R.P.; Lickiss, P.D.; Rahman, M.M.; Abou-Saleh, H. Internalization of Metal-Organic Framework Nanoparticles in Human Vascular Cells: Implications for Cardiovascular Disease Therapy. Nanomaterials. 2020, 10, 1028. [Google Scholar] [CrossRef]
- Mohamed, N.A. Metal-Organic Frameworks, Their Properties and Future Promises in the Medical Field; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; Available online: https://qspace.qu.edu.qa/handle/10576/48948 (accessed on 12 April 2024).
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Rehman, A.; Mehmood, A.; Ali, A.; Zahra, S.M.; Roobab, U.; Singh, A.; Ibrahim, S.A.; Siddiqui, S.A. Biocompatible Nanomaterials in Food Science, Technology, and Nutrient Drug Delivery: Recent Developments and Applications. Front. Nutr. 2022, 8, 778155. [Google Scholar] [CrossRef]
- Morshed, M.; Chowdhury, E.H. Gene Delivery and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–351. [Google Scholar] [CrossRef]
- Farzan, M.; Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. The processes behind drug loading and release in porous drug delivery systems. Eur. J. Pharm. Biopharm. 2023, 189, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Varma, A.J. Thermal stability of cellulose and their nanoparticles: Effect of incremental increases in carboxyl and aldehyde groups. Carbohydr. Polym. 2014, 114, 339–343. [Google Scholar] [CrossRef]
- Sun, D.; Kim, D.P. Hydrophobic MOFs@Metal Nanoparticles@COFs for Interfacially Confined Photocatalysis with High Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 20589–20595. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Sun, D.T.; Asgari, M.; Oveisi, E.; Trukhina, O.; Bulut, S.; Jamali, A.; Queen, W.L. A new post-synthetic polymerization strategy makes metal-organic frameworks more stable. Chem. Sci. 2019, 10, 4542–4549. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef] [PubMed]
- Muchow, M.; Schmitz, E.I.; Despatova, N.; Maincent, P.; Müller, R.H. Omega-3 fatty acids-loaded lipid nanoparticles for patient-convenient oral bioavailability enhancement. Pharm.-Int. J. Pharm. Sci. 2009, 64, 499–504. [Google Scholar]
- Guo, X.; Zuo, X.; Zhou, Z.; Gu, Y.; Zheng, H.; Wang, X.; Wang, G.; Xu, C.; Wang, F. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 4333. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2009, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Hosseini, M.; Haghgoo, S.; Changizi, V.; Akbari Javar, H.; Khoobi, M.; Riahi Alam, N. Multifunctional MIL-Cur@FC as a theranostic agent for magnetic resonance imaging and targeting drug delivery: In vitro and in vivo study. J. Drug Target. 2020, 28, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Karimi Alavijeh, R.; Akhbari, K. Cancer therapy by nano MIL-n series of metal-organic frameworks. Coord. Chem. Rev. 2024, 503, 215643. [Google Scholar] [CrossRef]
- Xiong, F.; Chen, Y.; Chen, J.; Yang, B.; Zhang, Y.; Gao, H.; Hua, Z.; Gu, N. Rubik-like magnetic nanoassemblies as an efficient drug multifunctional carrier for cancer theranostics. J. Control. Release 2013, 172, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Gao, W.; Sun, Y.; Cai, M.; Zhao, Y.; Cao, W.; Liu, Z.; Cui, G.; Tang, B. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat. Commun. 2018, 9, 231. [Google Scholar] [CrossRef]
- Ya Spivak, M.; Bubnov, R.V.; Yemets, I.M.; Lazarenko, L.M.; Tymoshok, N.O.; Ul’berg, Z.R. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: A theranostic potential for PPP cardiology. EPMA J. 2013, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, J.; Meng, D.; Cai, R.; Gao, X.; Ji, Y.; Wang, L.; Chen, C.; Wu, X. Gold Nanorod-Based Nanoplatform Catalyzes Constant NO Generation and Protects from Cardiovascular Injury. ACS Nano 2020, 14, 12854–12865. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, J.; Rojas, A.; Ramírez-Sagredo, A.; Riveros, A.L.; Morales-Zavala, F.; Flores, Y.; Riquelme, J.A.; Guzman, F.; Araya, E.; Chiong, M.; et al. Light-induced release of the cardioprotective peptide angiotensin-(1–9) from thermosensitive liposomes with gold nanoclusters. J. Control. Release 2020, 328, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; Attia, M.F.; El Bana, M.; El-Daly, S.M.; Mohamed, N.; El-Khayat, Z.; El-Naggar, M.E. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019, 140, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; El-Naggar, M.E.; Badawy, E.; El-Laithy, N.; El-Waseef, M.; Hassan, H.; Abdel-Latif, Y. Homocysteine and Asymmetrical Dimethylarginine in Diabetic Rats Treated with Docosahexaenoic Acid–Loaded Zinc Oxide Nanoparticles. Appl. Biochem. Biotechnol. 2020, 191, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; Rasheed, W.; Ramzy, T.; Nabeeh, M.; Harvy, M.; El-Toukhy, S.; Ali, O.; Raafat, J.; El-Naggar, M. Synthesis of docosahexaenoic acid–loaded silver nanoparticles for improving endothelial dysfunctions in experimental diabetes. Hum. Exp. Toxicol. 2019, 38, 962–973. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, S.M.; Medhat, D.; El-Bana, M.; Abdel-Latif, Y.; El-Naggar, M.E.; Omara, E.A.; Morsy, S.M.; Hussein, J. Stimulatory effect of docosahexaenoic acid alone or loaded in zinc oxide or silver nanoparticles on the expression of glucose transport pathway. Prostaglandins Other Lipid Mediat. 2021, 155, 106566. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Hachem, M.; Ahmmed, M.K. Docosahexaenoic acid-loaded nanoparticles: A state-of-the-art of preparation methods, characterization, functionality, and therapeutic applications. Heliyon 2024, 10, e30946. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Abdelrahman, S.A.; Salama, A.E. Efficacy of gold nanoparticles against isoproterenol-induced acute myocardial infarction in adult male albino rats. Ultrastruct. Pathol. 2017, 41, 168–185. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Shahini, M.H.; Mohammadloo, H.E.; Ramezanzadeh, M.; Ramezanzadeh, B. Recent innovations in synthesis/characterization of advanced nanoporous metal-organic frameworks (MOFs); current/future trends with a focus on the smart anti-corrosion features. Mater. Chem. Phys. 2022, 276, 125420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).










