Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells
Abstract
1. Introduction
2. Results
2.1. Revision of the Molecular Structure of Curdepsidone A
2.2. Curdepsidone A Inhibited HeLa Cell Viability and Proliferation
2.3. Curdepsidone A Induced G0/G1 Phase Arrest in HeLa Cells
2.4. Curdepsidone A Induced Apoptosis of HeLa Cells
2.5. Curdepsidone A Induced Apoptosis via the Mitochondria Pathway
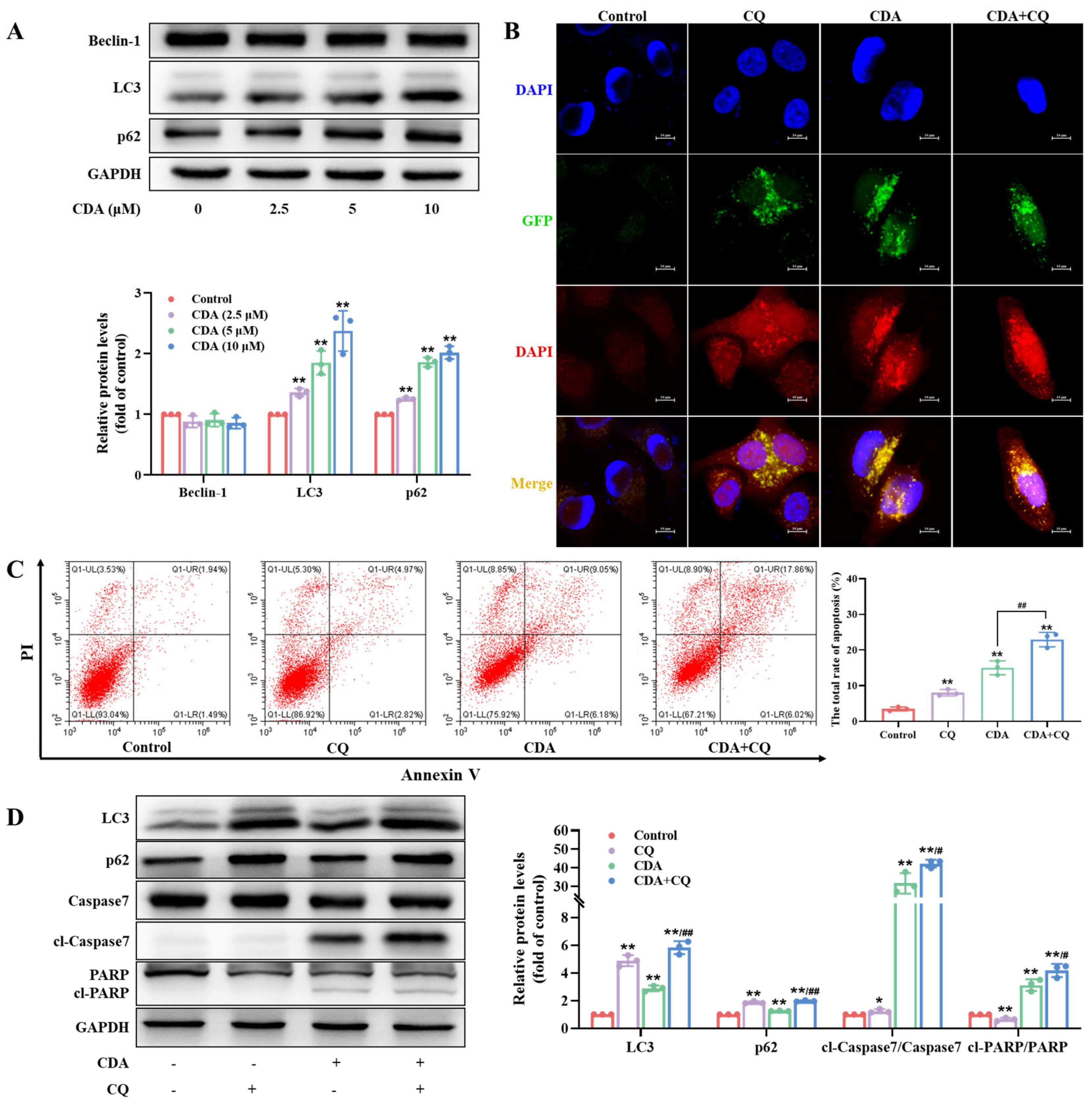
2.6. Curdepsidone A Inhibited Protective Autophagy in HeLa Cells
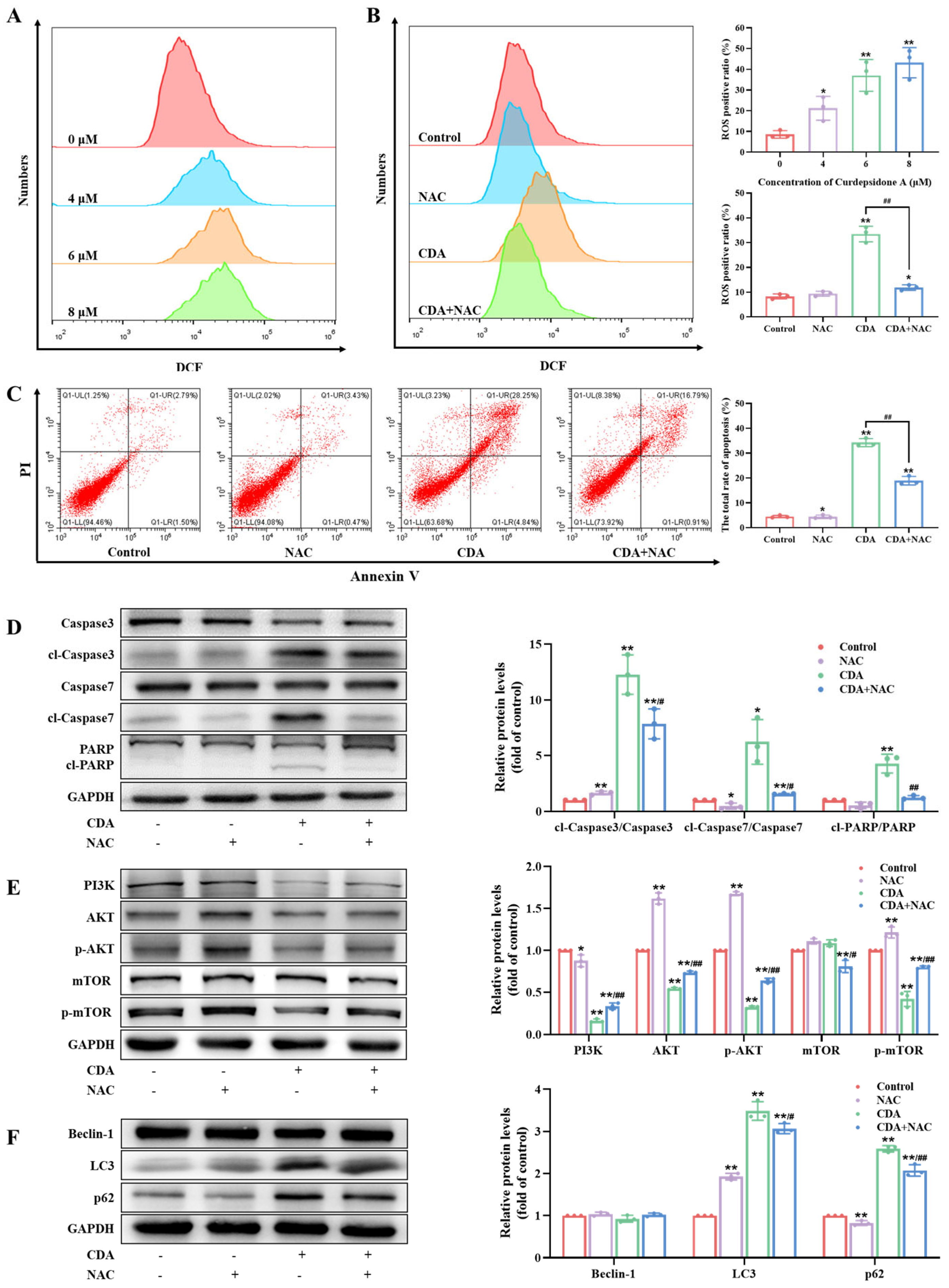
2.7. Curdepsidone A Suppressed the PI3K/AKT Pathway in HeLa Cells
2.8. Curdepsidone A Increased ROS Generation in HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Proliferation Assay
4.5. Cell Cycle Analysis
4.6. Cell Apoptosis Analysis
4.7. MMP Evaluation
4.8. Examination of GFP-RFP-LC3B Punctuation
4.9. Measurement of Intracellular ROS Generation
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA-Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef]
- Zivarpour, P.; Nikkhah, E.; Maleki Dana, P.; Asemi, Z.; Hallajzadeh, J. Molecular and biological functions of gingerol as a natural effective therapeutic drug for cervical cancer. J. Ovarian Res. 2021, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.D.; Yao, H.L.; Shen, L.Q.; Yang, Y.; Lu, L.; Xiao, J.S.; Wang, X.Y.; He, Z.L.; Jiang, L.H. α-Cyperone inhibits the proliferation of human cervical cancer HeLa cells via ROS-mediated PI3K/Akt/mTOR signaling pathway. Eur. J. Pharmacol. 2020, 883, 173355. [Google Scholar] [CrossRef]
- Phuah, N.H.; Azmi, M.N.; Awang, K.; Nagoor, N.H. Suppression of microRNA-629 enhances sensitivity of cervical cancer cells to 1‘S-1’-acetoxychavicol acetate via regulating RSU1. Onco Targets Ther. 2017, 10, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Y.; Xu, S.J.; Xin, X.J.; An, F.L. Perpyrrospirone A, an unprece dente d hirsutellone peroxide from the marine-derived Penicillium citrinum. Chin. Chem. Lett. 2023, 34, 107562. [Google Scholar] [CrossRef]
- Yang, J.; Gong, L.Z.; Guo, M.M.; Jiang, Y.; Ding, Y.; Wang, Z.J.; Xin, X.J.; An, F.L. Bioactive Indole Diketopiperazine Alkaloids from the Marine Endophytic Fungus Aspergillus sp. YJ191021. Mar. Drugs 2021, 19, 157. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef]
- Hu, Z.X.; Ye, Y.; Zhang, Y.H. Large-scale culture as a complementary and practical method for discovering natural products with novel skeletons. Nat. Prod. Rep. 2021, 38, 1775–1793. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Hemu, X.; Tan, N.H.; Wang, Z. OSMAC Strategy: A promising way to explore microbial cyclic peptides. Eur. J. Med. Chem. 2024, 268, 116175. [Google Scholar] [CrossRef]
- Khayat, M.T.; Ghazawi, K.F.; Samman, W.A.; Alhaddad, A.A.; Mohamed, G.A.; Ibrahim, S.R.M. Recent advances on natural depsidones: Sources, biosynthesis, structure-activity relationship, and bioactivities. PeerJ 2023, 11, 15394. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Madrid, A.; Russo, A. Physodic acid sensitizes LNCaP prostate cancer cells to TRAIL-induced apoptosis. Toxicol. In Vitro 2022, 84, 105432. [Google Scholar] [CrossRef] [PubMed]
- Anh, C.V.; Kwon, J.-H.; Kang, J.S.; Lee, H.-S.; Heo, C.-S.; Shin, H.J. Antibacterial and Cytotoxic Phenolic Polyketides from Two Marine-Derived Fungal Strains of Aspergillus unguis. Pharmaceuticals 2022, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- He, R.-J.; Wang, Y.-F.; Yang, B.-Y.; Liu, Z.-B.; Li, D.-P.; Zou, B.-Q.; Huang, Y.-L. Structural Characterization and Assessment of Anti-Inflammatory Activities of Polyphenols and Depsidone Derivatives from Melastoma malabathricum subsp. normale. Molecules 2022, 27, 1521. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, C.; Barbeau, X.; Azelmat, J.; Vaillancourt, K.; Grenier, D.; Lague, P.; Voyer, N. Lobaric acid and pseudodepsidones inhibit NF-κB signaling pathway by activation of PPAR-γ. Biorg. Med. Chem. 2018, 26, 5845–5851. [Google Scholar] [CrossRef] [PubMed]
- Thi Hoang Anh, N.; Mai Anh, N.; Thi Thu Huyen, V.; Thi Dao, P.; Thi Mai Huong, D.; Van Cuong, P.; Thanh Xuan, D.; Huu Tai, B.; Thi Hong Minh, L.; Van Kiem, P. Antimicrobial Activity of Depsidones and Macrocyclic Peptides Isolated from Marine Sponge-Derived Fungus Aspergillus nidulans M256. Chem. Biodivers. 2023, 20, e202301660. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Bunbamrung, N.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Diphenyl ethers and depsidones from the endophytic fungus Aspergillus unguis BCC54176. Tetrahedron 2022, 105, 132612. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Annam, S.S.P. Antimycobacterial activity of acetone extract and isolated metabolites from folklore medicinal lichen Usnea laevis Nyl. against drug-sensitive and multidrug-resistant tuberculosis strains. J. Ethnopharmacol. 2022, 282, 114641. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Majchrzak-Celinska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wojcik, E.; Zarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component-Physodic Acid through the Blood-Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Likitnukul, S.; Tepaarmorndech, S.; Kaewamatawong, T.; Yangchum, A.; Duangtha, C.; Jongjang, P.; Mangmool, S.; Pinthong, D.; Isaka, M. Pyridylnidulin exerts anti-diabetic properties and improves non-alcoholic fatty liver disease in diet-induced obesity mice. Front. Mol. Biosci. 2023, 10, 1208215. [Google Scholar] [CrossRef]
- An, F.L.; Liu, W.H.; Wei, X.C.; Pan, Z.H.; Lu, Y.H. Curdepsidone A, a Depsidone from the Marine-Derived Endophytic Fungus Curvularia sp. IFB-Z10. Nat. Prod. Commun. 2018, 13, 865–866. [Google Scholar] [CrossRef]
- Ding, Y.; An, F.L.; Zhu, X.J.; Yu, H.Y.; Hao, L.L.; Lu, Y.H. Curdepsidones B-G, Six Depsidones with Anti-Inflammatory Activities from the Marine-Derived Fungus Curvularia sp. IFB-Z10. Mar. Drugs 2019, 17, 266. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Sandberg, E.N.; Goel, N.; Bishayee, A. Oncogenic and Tumor Suppressive Components of the Cell Cycle in Breast Cancer Progression and Prognosis. Pharmaceutics 2021, 13, 569. [Google Scholar] [CrossRef]
- Singh, P.; Lim, B. Targeting Apoptosis in Cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef]
- Braicu, C.; Zanoaga, O.; Zimta, A.A.; Tigu, A.B.; Kilpatrick, K.L.; Bishayee, A.; Nabavi, S.M.; Berindan-Neagoe, I. Natural compounds modulate the crosstalk between apoptosis- and autophagy-regulated signaling pathways: Controlling the uncontrolled expansion of tumor cells. Semin. Cancer Biol. 2022, 80, 218–236. [Google Scholar] [CrossRef]
- Cao, W.Y.; Li, J.H.; Yang, K.P.; Cao, D.L. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Satoh, M.; Takemura, Y.; Hamada, H.; Sekido, Y.; Kubota, S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell Int. 2013, 13, 19. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Feng, L.; Fan, J.T.; Wang, J.; Tan, N.H.; Wang, Z. Rubioncolin C, a natural naphthohydroquinone dimer isolated from Rubia yunnanensis, inhibits the proliferation and metastasis by inducing ROS-mediated apoptotic and autophagic cell death in triple-negative breast cancer cells. J. Ethnopharmacol. 2021, 277, 114184. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Gu, L.M.; Li, Y.; Chen, S.M.; You, J.S.; Fan, L.Q.; Wang, Y.D.; Zhao, L.M. Chitooligosaccharides display anti-tumor effects against human cervical cancer cells via the apoptotic and autophagic pathways. Carbohydr. Polym. 2019, 224, 115171. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhong, J.; Bi, Y.; Liu, Y.; Liu, Y.; Guo, J.; Pan, L.; Tan, Y.; Yu, X. Gambogenic acid induces Noxa-mediated apoptosis in colorectal cancer through ROS-dependent activation of IRE1α/JNK. Phytomedicine 2020, 78, 153306. [Google Scholar] [CrossRef]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Degl’Innocenti, D. Marine Migrastatics: A Comprehensive 2022 Update. Mar. Drugs 2022, 20, 273. [Google Scholar] [CrossRef]
- van Andel, L.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H. Review of Chromatographic Bioanalytical Assays for the Quantitative Determination of Marine-Derived Drugs for Cancer Treatment. Mar. Drugs 2018, 16, 246. [Google Scholar] [CrossRef]
- Choi, H.Y.; Ahn, J.H.; Kwon, H.; Yim, J.H.; Lee, D.; Choi, J.H. Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling. Mar. Drugs 2022, 20, 275. [Google Scholar] [CrossRef]
- Janta, S.; Pranweerapaiboon, K.; Vivithanaporn, P.; Plubrukarn, A.; Chairoungdua, A.; Prasertsuksri, P.; Apisawetakan, S.; Chaithirayanon, K. Holothurin A Inhibits RUNX1-Enhanced EMT in Metastasis Prostate Cancer via the Akt/JNK and P38 MAPK Signaling Pathway. Mar. Drugs 2023, 21, 345. [Google Scholar] [CrossRef]
- Koo, M.H.; Shin, M.J.; Kim, M.J.; Lee, S.; So, J.E.; Kim, J.H.; Lee, J.H.; Suh, S.S.; Youn, U.J. Bioactive Secondary Metabolites Isolated from the Antarctic Lichen Himantormia lugubris. Chem. Biodivers. 2022, 19, 7. [Google Scholar]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef]
- Abbastabar, M.; Kheyrollah, M.; Azizian, K.; Bagherlou, N.; Tehrani, S.S.; Maniati, M.; Karimian, A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair 2018, 69, 63–72. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef]
- Patil, A.A.; Bhor, S.A.; Rhee, W.J. Cell death in culture: Molecular mechanisms, detections, and inhibition strategies. J. Ind. Eng. Chem. 2020, 91, 37–53. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Fan, D.M.; Ru, B.B.; Cheng, K.W.; Hu, S.T.; Zhang, J.W.; Li, E.T.S.; Wang, M.F. 6-C-(E-phenylethenyl)naringenin induces cell growth inhibition and cytoprotective autophagy in colon cancer cells. Eur. J. Cancer 2016, 68, 38–50. [Google Scholar] [CrossRef]
- Tang, T.; Xia, Q.J.; Xi, M.R. Dihydroartemisinin and its anticancer activity against endometrial carcinoma and cervical cancer: Involvement of apoptosis, autophagy and transferrin receptor. Singap. Med. J. 2021, 62, 96–103. [Google Scholar] [CrossRef]
- Meng, D.; Li, Z.; Wang, G.; Ling, L.; Wu, Y.; Zhang, C. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed. Pharmacother. 2018, 108, 1617–1627. [Google Scholar] [CrossRef]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Shi, X.; Xu, S. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J. Hazard. Mater. 2020, 398, 122905. [Google Scholar] [CrossRef]





| Cell Line | IC50 of Curdepsidone A (μM) |
|---|---|
| HeLa | 6.28 ± 0.38 |
| BEL-7402 | 17.37 ± 1.84 |
| HepG2 | 8.17 ± 0.42 |
| K562 | 16.76 ± 0.18 |
| SW1116 | 8.85 ± 1.50 |
| MCF-7 | 15.37± 2.10 |
| MGC803 | 12.09 ± 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Li, Z.; Xin, X.; An, F. Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells. Mar. Drugs 2024, 22, 227. https://doi.org/10.3390/md22050227
Xu S, Li Z, Xin X, An F. Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells. Marine Drugs. 2024; 22(5):227. https://doi.org/10.3390/md22050227
Chicago/Turabian StyleXu, Sunjie, Zhimin Li, Xiujuan Xin, and Faliang An. 2024. "Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells" Marine Drugs 22, no. 5: 227. https://doi.org/10.3390/md22050227
APA StyleXu, S., Li, Z., Xin, X., & An, F. (2024). Curdepsidone A Induces Intrinsic Apoptosis and Inhibits Protective Autophagy via the ROS/PI3K/AKT Signaling Pathway in HeLa Cells. Marine Drugs, 22(5), 227. https://doi.org/10.3390/md22050227






