Phytochemical Composition, Anti-Inflammatory Property, and Anti-Atopic Effect of Chaetomorpha linum Extract
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of C. linum Extract
2.1.1. UPLC-MS/MS Analysis
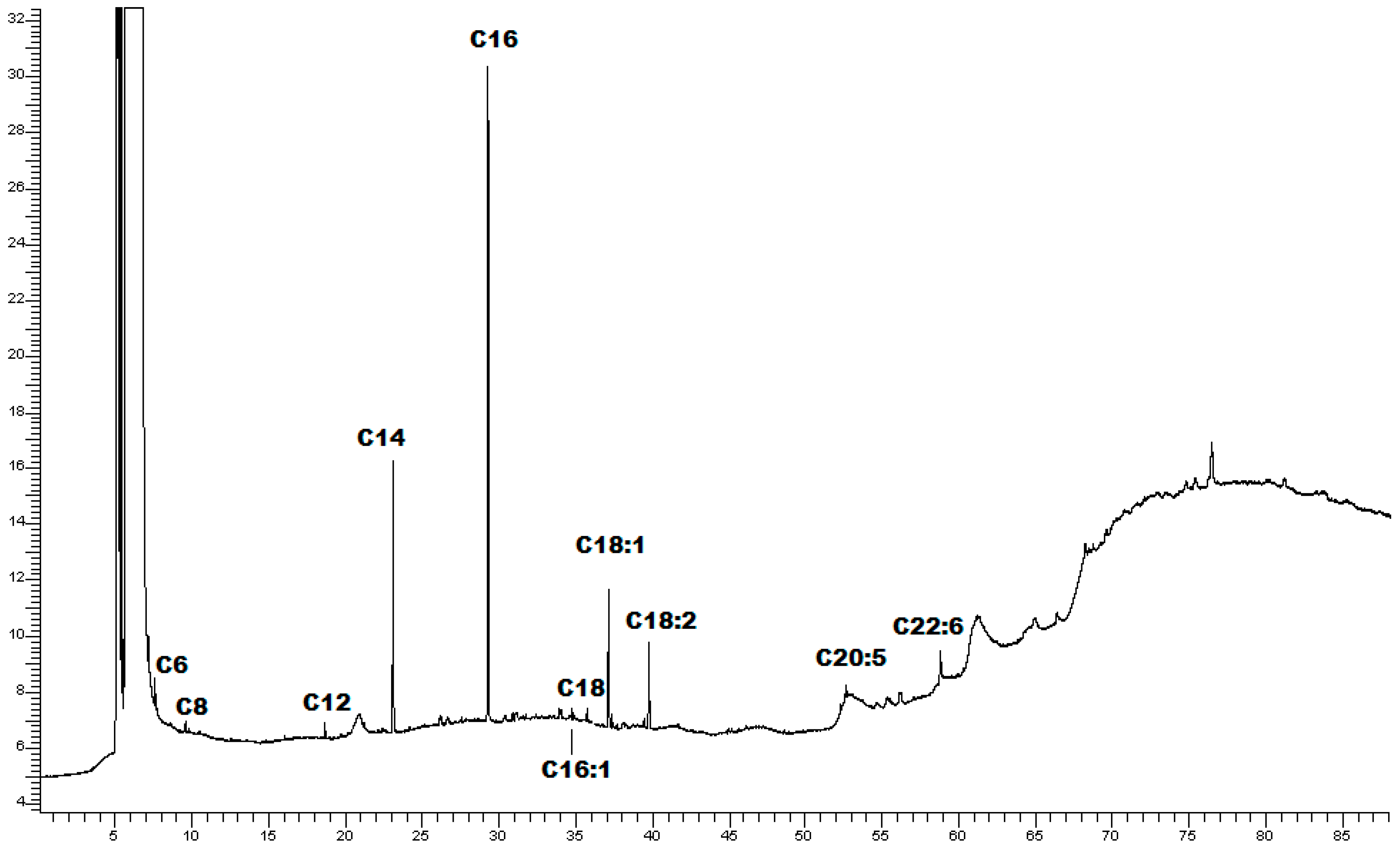
2.1.2. GC-FID Analysis
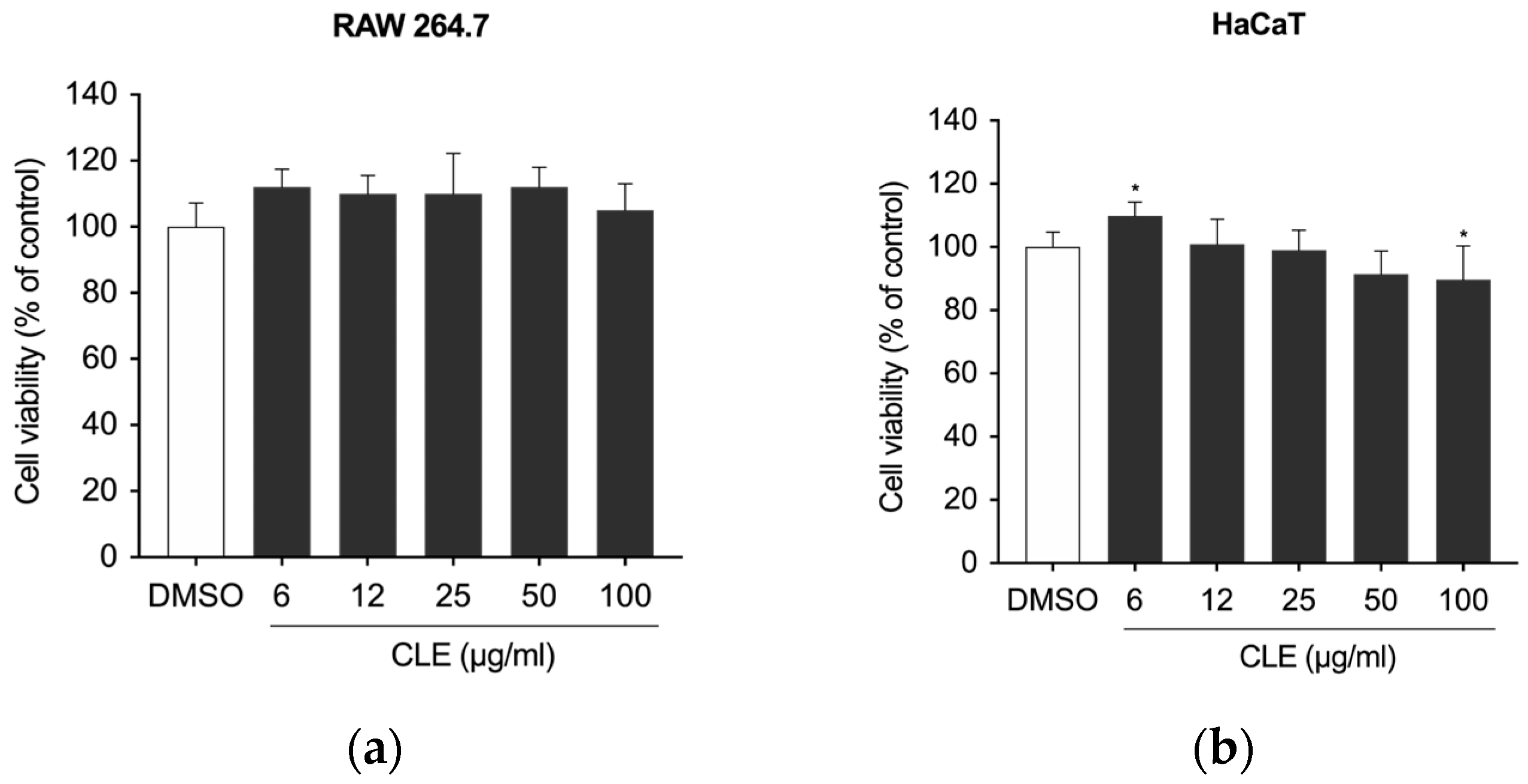
2.2. The Effect of CLE on Cell Viability of RAW 264.7 and HaCaT Cells
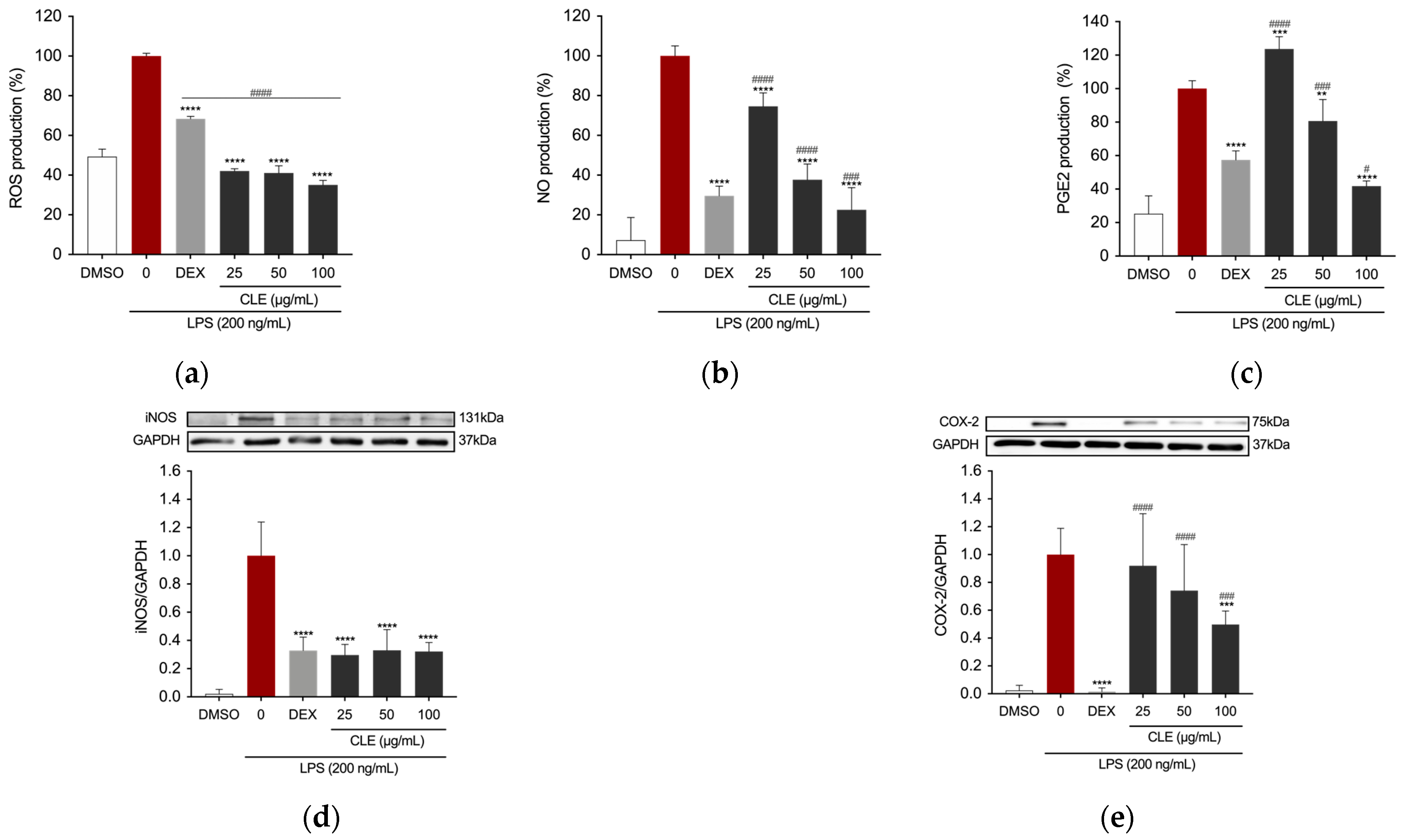
2.3. The Effects of CLE on Inflammatory Mediators in LPS-Stimulated RAW 264.7 Cells
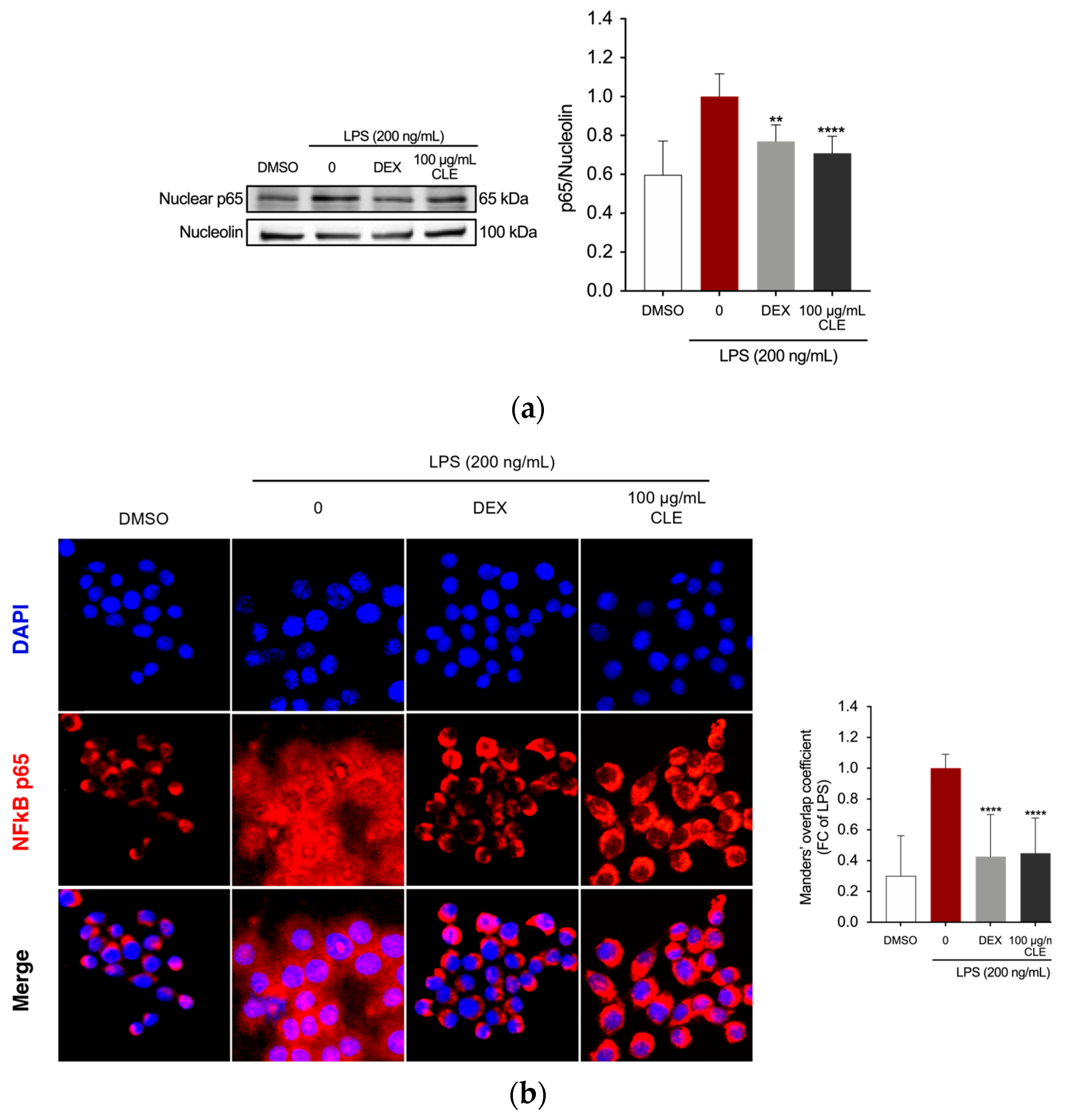
2.4. The Effect of CLE on NF-κB Activation in LPS-Stimulated RAW 264.7 Cells
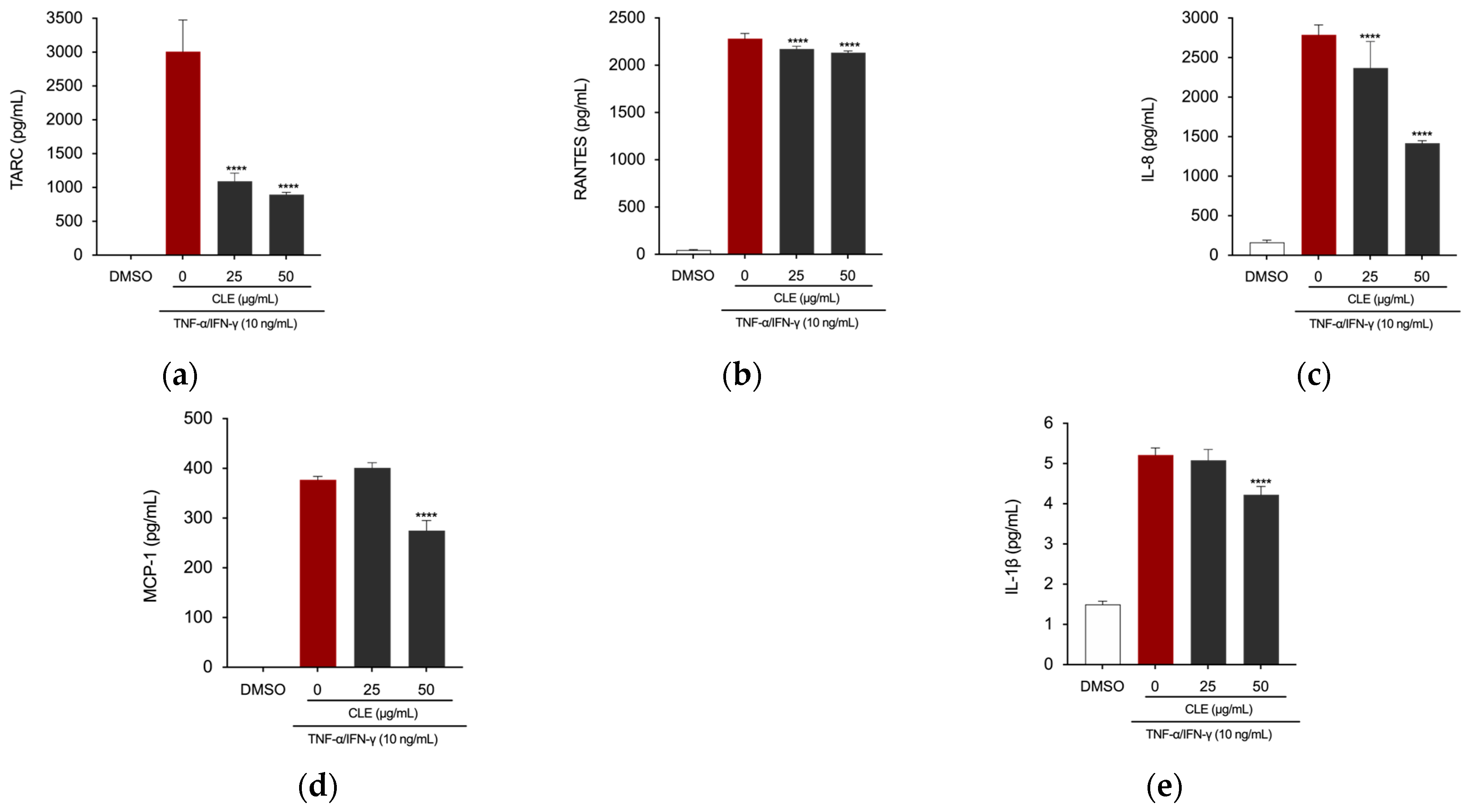
2.5. CLE Reduced Inflammation in TNF-α/IFN-γ Insulted HaCaT Cells
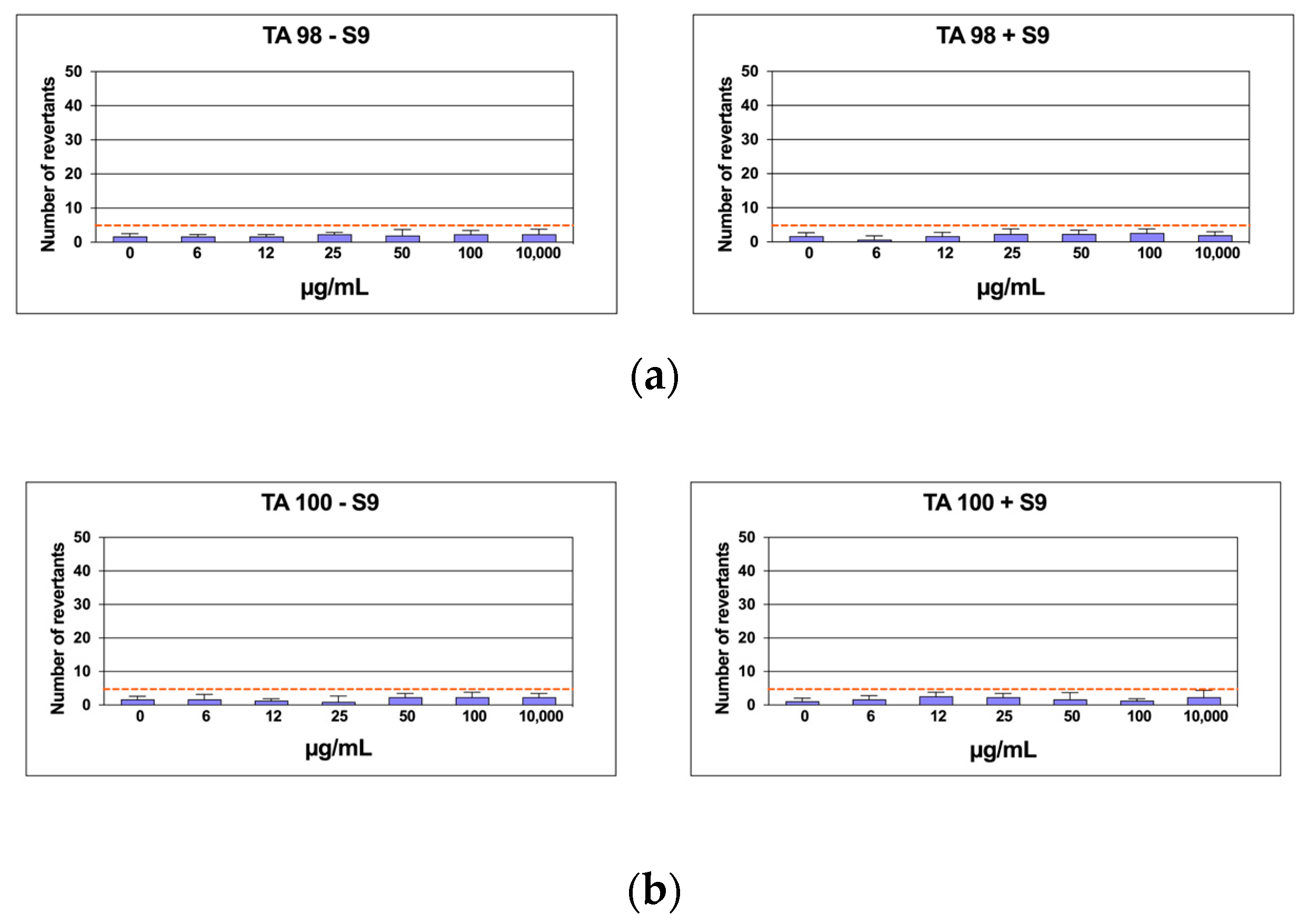
2.6. Mutagenicity Assay: Ames Test
2.7. In Silico Results
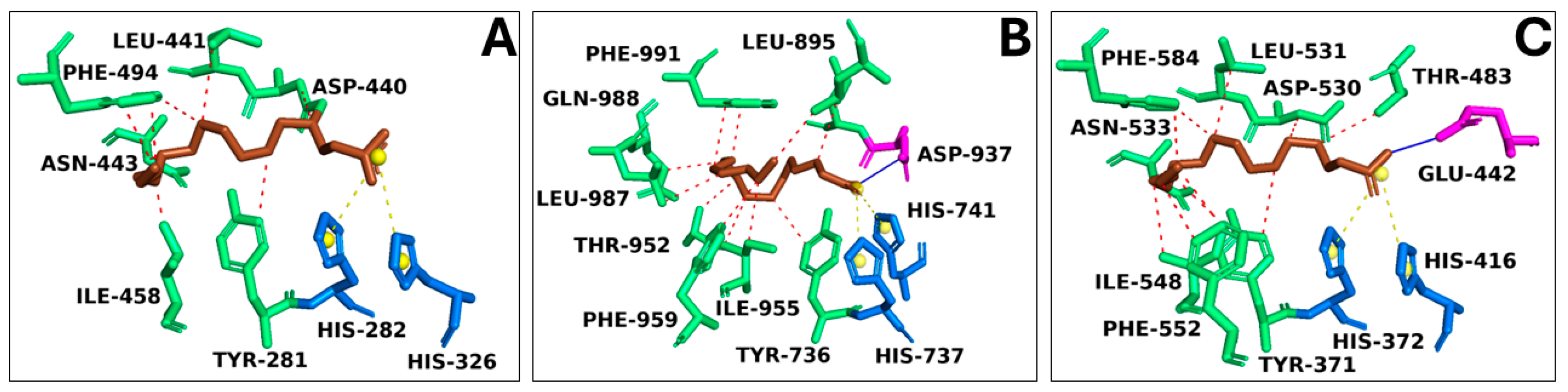
Target/Compound Virtual Screening
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. The Collection of Chaetomorpha linum and the Preparation of Algae Extract (CLE)
4.3. NMR Spectroscopy
4.4. UPLC-MS/MS
4.5. GC-FID
4.6. In Vitro Anti-Inflammatory Activity
4.6.1. Cell Cultures
4.6.2. RAW 264.7 and HaCaT Cells Viability
4.6.3. Cell Stimulation
4.6.4. The Quantification of Intracellular ROS Generation
4.6.5. The Determination of NO Production
4.6.6. Immunofluorescence Study
4.6.7. Protein Extraction
4.6.8. Western Blotting
4.6.9. Enzyme-Linked Immunosorbent (ELISA) Assay
4.7. Mutagenicity Assay: Ames Test
4.8. Statistical Analysis
4.9. In Silico Studies
Structural Optimization and Resources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deepika, C.; Ravishankar, G.A.; Rao, A.R. Potential Products from Macroalgae: An Overview. In Sustainable Global Resources of Seaweeds Volume 1; Springer International Publishing: Berlin, Germany, 2022; pp. 17–44. [Google Scholar]
- Jaworowska, A.; Murtaza, A. Seaweed Derived Lipids Are a Potential Anti-Inflammatory Agent: A Review. Int. J. Environ. Res. Public. Health 2023, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Degtyaryov, E.; Radzyukevich, Y.; Buryak, V. Therapeutic Potential of Plant Oxylipins. Int. J. Mol. Sci. 2022, 23, 14627. [Google Scholar] [CrossRef] [PubMed]
- Market Analysis and Insights: Global Biomedical Materials Market. Available online: https://www.marketgrowthreports.com/global-biomedical-materials-market-21051012 (accessed on 28 March 2024).
- Ripol, A.; Cardoso, C.; Afonso, C.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N.M. Composition, Anti-Inflammatory Activity, and Bioaccessibility of Green Seaweeds from Fish Pond Aquaculture. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty Acid Profiles of the Main Lipid Classes of Green Seaweeds from Fish Pond Aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef]
- Amaro, H.M.; Pagels, F.; Tavares, T.G.; Costa, I.; Sousa-Pinto, I.; Guedes, A.C. Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology 2022, 1, 469–482. [Google Scholar] [CrossRef]
- Lenzi, M. Artificial Top Layer Sediment Resuspension To Counteract Chaetomorpha Linum (Muller) Kutz Blooms In A Eutrophic Lagoon. Three Years Full-Scale Experience. J. Aquac. Mar. Biol. 2017, 5, 00114. [Google Scholar] [CrossRef]
- Sorce, C.; Persiano Leporatti, M.; Lenzi, M. Growth and Physiological Features of Chaetomorpha Linum (Müller) Kütz. in High Density Mats. Mar. Pollut. Bull. 2018, 129, 772–781. [Google Scholar] [CrossRef]
- Bastianoni, S.; Coppola, F.; Tiezzi, E.; Colacevich, A.; Borghini, F.; Focardi, S. Biofuel Potential Production from the Orbetello Lagoon Macroalgae: A Comparison with Sunflower Feedstock. Biomass Bioenergy 2008, 32, 619–628. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Licursi, D.; Mussi, L.; Balestri, E.; Lardicci, C. Levulinic Acid Production from the Green Macroalgae Chaetomorpha Linum and Valonia Aegagropila Harvested in the Orbetello Lagoon. Chem. Eng. Trans. 2019, 74, 103–108. [Google Scholar] [CrossRef]
- Renzi, M.; Giovani, A.; Focardi, S.E. Biofuel Production from the Orbetello Lagoon Macrophytes: Efficiency of Lipid Extraction Using Accelerate Solvent Extraction Technique. J. Environ. Prot. 2013, 4, 1224–1229. [Google Scholar] [CrossRef][Green Version]
- Piovani, D.; Nikolopoulos, G.K.; Bonovas, S. Non-Communicable Diseases: The Invisible Epidemic. J. Clin. Med. 2022, 11, 5939. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of Allergic Diseases and Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Cadau, S.; Gault, M.; Berthelemy, N.; Hsu, C.-Y.; Danoux, L.; Pelletier, N.; Goudounèche, D.; Pons, C.; Leprince, C.; André-Frei, V.; et al. An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care Ingredients. Int. J. Mol. Sci. 2022, 23, 12880. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Kuypers, D.R.J.; Sarwal, M. Calcineurin Inhibitor Nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef]
- Bechstein, W.O. Neurotoxicity of Calcineurin Inhibitors: Impact and Clinical Management. Transpl. Int. 2000, 13, 313–326. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the Efficacy and Side Effects of Antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef]
- Buchman, A.L. Side Effects of Corticosteroid Therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Koh, P.O.; Kang, C.; Kim, E. Rosa Davurica Pall. Improves DNCB-Induced Atopic Dermatitis in Mice and Regulated TNF-Alpa/IFN-Gamma-Induced Skin Inflammatory Responses in HaCaT Cells. Phytomedicine 2021, 91, 153708. [Google Scholar] [CrossRef]
- Nur Husna, S.M.; Tan, H.-T.T.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 2022, 9, 874114. [Google Scholar] [CrossRef]
- Dierick, B.J.H.; van der Molen, T.; Flokstra-de Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.H.; van Boven, J.F.M. Burden and Socioeconomics of Asthma, Allergic Rhinitis, Atopic Dermatitis and Food Allergy. Expert. Rev. Pharmacoecon. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, F.; Zhang, L. Advances and Highlights in Allergic Rhinitis. Allergy 2021, 76, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Kang, S.-C.; Jin, B.-R.; An, H.-J. Euphorbia Hirta Leaf Ethanol Extract Suppresses TNF-α/IFN-γ-Induced Inflammatory Response via Down-Regulating JNK or STAT1/3 Pathways in Human Keratinocytes. Life 2022, 12, 589. [Google Scholar] [CrossRef]
- Maiello, N.; Comberiati, P.; Giannetti, A.; Ricci, G.; Carello, R.; Galli, E. New Directions in Understanding Atopic March Starting from Atopic Dermatitis. Children 2022, 9, 450. [Google Scholar] [CrossRef]
- Yang, L.; Fu, J.; Zhou, Y. Research Progress in Atopic March. Front. Immunol. 2020, 11, 559823. [Google Scholar] [CrossRef]
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The New Medicine of Beauty. Mo. Med. 2011, 108, 60. [Google Scholar]
- Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.H.; Kim, S.K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef]
- Lee, J.; Hyun, C.G. Natural Products for Cosmetic Applications. Molecules 2023, 28, 534. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, D.S.; Shawky, E.; Ibrahim, R.S.; Mohyeldin, M.M. Comprehensive Metabolomics Unveil the Discriminatory Metabolites of Some Mediterranean Sea Marine Algae in Relation to Their Cytotoxic Activities. Sci. Rep. 2022, 12, 8094. [Google Scholar] [CrossRef] [PubMed]
- Sutour, S.; XU, T.; Casabianca, H.; Paoli, M.; de Rocca-Serra, D.; Garrido, M.; Pasqualini, V.; Aiello, A.; Castola, V.; Bighelli, A. Chemical Composition of Extracts from Chaetomorpha Linum (Miller) Kütz. A Potential Use in the Cosmetic Industry. Int. J. Phytocosmetics Nat. Ingred. 2015, 2, 5. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional Profiling of the LPS Induced NF-ΚB Response in Macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef]
- Trezza, A.; Geminiani, M.; Cutrera, G.; Dreassi, E.; Frusciante, L.; Lamponi, S.; Spiga, O.; Santucci, A. A Drug Discovery Approach to a Reveal Novel Antioxidant Natural Source: The Case of Chestnut Burr Biomass. Int. J. Mol. Sci. 2024, 25, 2517. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Acquaviva, M.I.; Angilé, F.; Cavallo, R.A.; Cecere, E.; Del Coco, L.; Fanizzi, F.P.; Gerardi, C.; Narracci, M.; Petrocelli, A. Screening of Chaetomorpha Linum Lipidic Extract as a New Potential Source of Bioactive Compounds. Mar. Drugs 2019, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Cecere, E.; Licciano, M.; Petrocelli, A.; Sicuro, B.; Giangrande, A. Integrated Multitrophic Aquaculture By-Products with Added Value: The Polychaete Sabella Spallanzanii and the Seaweed Chaetomorpha Linum as Potential Dietary Ingredients. Mar. Drugs 2019, 17, 677. [Google Scholar] [CrossRef]
- Biandolino, F.; Prato, E. A Preliminary Investigation of the Lipids and Fatty Acids Composition of Gammarus Aequicauda (Crustacea: Amphipoda) and Its Main Food Source. J. Mar. Biol. Assoc. United Kingd. 2006, 86, 345–348. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae. Mar. Drugs 2016, 14, 23. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Nitrate and Phosphate Regimes Induced Lipidomic and Biochemical Changes in the Intertidal Macroalga Ulva Lactuca (Ulvophyceae, Chlorophyta). Plant Cell Physiol. 2014, 55, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty Acids of Marine Algae from the Pacific Coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- George, A.; Chinnappan, S.; Chintamaneni, M.; Kotak, C.V.; Choudhary, Y.; Kueper, T.; Radhakrishnan, A.K. Anti-Inflammatory Effects of Polygonum Minus (Huds) Extract (LineminusTM) in in-Vitro Enzyme Assays and Carrageenan Induced Paw Edema. BMC Complement. Altern. Med. 2014, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Terme, N.; Chénais, B.; Fournière, M.; Bourgougnon, N.; Bedoux, G. Algal Derived Functional Lipids and Their Role in Promoting Health. In Recent Advances in Micro and Macroalgal Processing; Wiley: Hoboken, NJ, USA, 2021; pp. 370–417. [Google Scholar]
- Kim, H.; Shin, H.Y.; Jeong, E.J.; Lee, H.D.; Hwang, K.C.; Yu, K.W.; Lee, S.; Lee, S. Antioxidant and Anti-Inflammatory Activities of Sargassum Macrocarpum Extracts. Antioxidants 2022, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia Turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Leitner, P.D.; Jakschitz, T.; Gstir, R.; Stuppner, S.; Perkams, S.; Kruus, M.; Trockenbacher, A.; Griesbeck, C.; Bonn, G.K.; Huber, L.A.; et al. Anti-Inflammatory Extract from Soil Algae Chromochloris Zofingiensis Targeting TNFR/NF-ΚB Signaling at Different Levels. Cells 2022, 11, 1407. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Daletos, G.; Proksch, P.; Andrade, P.B.; Valentão, P. Anti-Inflammatory Potential of Monogalactosyl Diacylglycerols and a Monoacylglycerol from the Edible Brown Seaweed Fucus Spiralis Linnaeus. Mar. Drugs 2014, 12, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.I.; Meyer, B.J.; Winberg, P.C.; Ranson, M.; Skropeta, D. Selecting Australian Marine Macroalgae Based on the Fatty Acid Composition and Anti-Inflammatory Activity. J. Appl. Phycol. 2015, 27, 2111–2121. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Rey, F.; Costa, E.; Moreira, A.S.P.; Abreu, M.H.; Domingues, P.; Lillebø, A.I.; Calado, R.; Rosário Domingues, M. Insights of Species-Specific Polar Lipidome Signatures of Seaweeds Fostering Their Valorization in the Blue Bioeconomy. Algal Res. 2021, 55, 102242. [Google Scholar] [CrossRef]
- Arguelles-Peña, K.; Olguín-Rojas, A.; Acosta-Osorio, A.A.; Carrera, C.; Barbero, G.F.; Ángel García-Alvarado, M.; Del Carmen Rodríguez-Jimenes, G.; Olguín-Rojas, K.; Acosta-Osorio, J.A.; Carrera, A.A.; et al. An Evaluation of the Equilibrium Properties in Hexane and Ethanol Extractive Systems for Moringa Oleifera Seeds and Fatty Acid Profiles of the Extracts. Separations 2021, 8, 217. [Google Scholar] [CrossRef]
- Grima, E.M.; Medina, A.R.; Giménez, A.G.; Sánchez Pérez, J.A.; Camacho, F.G.; García Sánchez, J.L. Comparison between Extraction of Lipids and Fatty Acids from Microalgal Biomass. J. Am. Oil Chem. Soc. 1994, 71, 955–959. [Google Scholar] [CrossRef]
- Food and Drug Administration Substances Generally Recognized as Safe (Final Rule) RIA; 2016. Available online: https://www.fda.gov/about-fda/economic-impact-analyses-fda-regulations/summary-substances-generally-recognized-safe-final-rule (accessed on 28 March 2024).
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of Astaxanthin from Microalga Haematococcus Pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Seibert, K.; Zhang, Y.; Leahy, K.; Hauser, S.; Masferrer, J.; Perkins, W.; Lee, L.; Isakson, P. Pharmacological and Biochemical Demonstration of the Role of Cyclooxygenase 2 in Inflammation and Pain. Proc. Natl. Acad. Sci. USA 1994, 91, 12013–12017. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Perna, F.; Masini, E. INOS/COX-2 Pathway Interaction: A Good Molecular Target for Cancer Treatment. Curr. Enzym. Inhib. 2005, 1, 97–105. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, Y.H.; Koh, D.; Lim, Y.; Shin, S.Y. Chrysin Inhibits NF-ΚB-Dependent CCL5 Transcription by Targeting IκB Kinase in the Atopic Dermatitis-Like Inflammatory Microenvironment. Int. J. Mol. Sci. 2020, 21, 7348. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Hwang, B.S.; Hwang, Y.; Jeong, Y.T.; Jeong, D.W.; Oh, Y.T. Anti-Inflammatory and Antiatopic Effects of Rorippa Cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells. Molecules 2023, 28, 5463. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Lakshmanan, V.; Palakodeti, D.; Ginhoux, F.; Raghavan, S. Epithelial-Macrophage Crosstalk Initiates Sterile Inflammation in Embryonic Skin. Front. Immunol. 2021, 12, 718005. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Ponce, A.; Tiruneh, M.W.; Lee, J.; Guerrero-Juarez, C.F.; Kuhn, J.; David, J.A.; Dammeyer, K.; Mc Kell, R.; Kwong, J.; Rabbani, P.S.; et al. Keratinocyte-Macrophage Crosstalk by the Nrf2/Ccl2/EGF Signaling Axis Orchestrates Tissue Repair. Cell Rep. 2020, 33, 108417. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Al Tamimi, M.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An Update on Clinical Importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Tresini, M. Oxidative Stress and Gene Regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Kolb, N.; Werner, E.R.; Pfeilschifter, J. Coordinated Induction of Inducible Nitric Oxide Synthase and GTP-Cyclohydrolase I Is Dependent on Inflammatory Cytokines and Interferon-γ in HaCaT Keratinocytes: Implications for the Model of Cutaneous Wound Repair. J. Investig. Dermatol. 1998, 111, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Takuathung, M.N.; Potikanond, S.; Sookkhee, S.; Mungkornasawakul, P.; Jearanaikulvanich, T.; Chinda, K.; Wikan, N.; Nimlamool, W. Anti-Psoriatic and Anti-Inflammatory Effects of Kaempferia Parviflora in Keratinocytes and Macrophage Cells. Biomed. Pharmacother. 2021, 143, 112229. [Google Scholar] [CrossRef] [PubMed]
- Viard-Leveugle, I.; Gaide, O.; Jankovic, D.; Feldmeyer, L.; Kerl, K.; Pickard, C.; Roques, S.; Friedmann, P.S.; Contassot, E.; French, L.E. TNF-α and IFN-γ Are Potential Inducers of Fas-Mediated Keratinocyte Apoptosis through Activation of Inducible Nitric Oxide Synthase in Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2013, 133, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Shiu, P.H.T.; Li, J.; Zheng, C.; Rangsinth, P.; Li, R.; Cheung, Q.T.L.; Lau, A.H.Y.; Chan, J.C.K.; Kwan, Y.W.; Cheung, T.M.Y.; et al. Amauroderma Rugosum Extract Suppresses Inflammatory Responses in Tumor Necrosis Factor Alpha/Interferon Gamma-Induced HaCaT Keratinocytes. Molecules 2022, 27, 6533. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Pisani, L.P.; Baker, E.J.; Marat, A.D.; Valenzuela, C.A.; Miles, E.A.; Calder, P.C. Anti-Inflammatory Effects of Oleic Acid and the Anthocyanin Keracyanin Alone and in Combination: Effects on Monocyte and Macrophage Responses and the NF-ΚB Pathway. Food Funct. 2021, 12, 7909–7922. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Jeon, H.; Kim, I.-H.; Jeong, H.S.; Lee, J. Anti-Inflammatory Effects of Stearidonic Acid Mediated by Suppression of NF-ΚB and MAP-Kinase Pathways in Macrophages. Lipids 2017, 52, 781–787. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Santos, F.; Monteiro, J.P.; Duarte, D.; Melo, T.; Lopes, D.; da Costa, E.; Domingues, M.R. Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria Bifurcata and Invasive Sargassum Muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity. Antioxidants 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Reddy, R.; Jha, B. Quantification of Selected Endogenous Hydroxy-Oxylipins from Tropical Marine Macroalgae. Mar. Biotechnol. 2014, 16, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Morisseau, C.; Inceoglu, B.; Schmelzer, K.; Tsai, H.-J.; Jinks, S.L.; Hegedus, C.M.; Hammock, B.D. Naturally Occurring Monoepoxides of Eicosapentaenoic Acid and Docosahexaenoic Acid Are Bioactive Antihyperalgesic Lipids. J. Lipid Res. 2010, 51, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-Inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic Acid Esters of Hydroxy Linoleic Acids Are Anti-Inflammatory Lipids Found in Plants and Mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef] [PubMed]
- Ngu, E.-L.; Tan, C.-Y.; Lai, N.J.-Y.; Wong, K.-H.; Lim, S.-H.; Ming, L.C.; Tan, K.-O.; Phang, S.-M.; Yow, Y.-Y. Spirulina Platensis Suppressed INOS and Proinflammatory Cytokines in Lipopolysaccharide-Induced BV2 Microglia. Metabolites 2022, 12, 1147. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.J.-Y.; Ngu, E.-L.; Pang, J.-R.; Wong, K.-H.; Ardianto, C.; Ming, L.C.; Lim, S.-H.; Walvekar, S.G.; Anwar, A.; Yow, Y.-Y. Carrageenophyte Kappaphycus Malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-ΚB and ERK Signaling Pathways. Mar. Drugs 2022, 20, 534. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 409585. [Google Scholar] [CrossRef]
- O’Brien, J.J.; O’Callaghan, J.P.; Miller, D.B.; Chalgeri, S.; Wennogle, L.P.; Davis, R.E.; Snyder, G.L.; Hendrick, J.P. Inhibition of Calcium-Calmodulin-Dependent Phosphodiesterase (PDE1) Suppresses Inflammatory Responses. Mol. Cell. Neurosci. 2020, 102, 103449. [Google Scholar] [CrossRef]
- Dubois, M.; Picq, M.; Némoz, G.; Lagarde, M.; Prigent, A.-F. Inhibition of the Different Phosphodiesterase Isoforms of Rat Heart Cytosol by Free Fatty Acids. J. Cardiovasc. Pharmacol. 1993, 21, 522–529. [Google Scholar] [CrossRef]
- Scapin, G.; Patel, S.B.; Chung, C.; Varnerin, J.P.; Edmondson, S.D.; Mastracchio, A.; Parmee, E.R.; Singh, S.B.; Becker, J.W.; Van der Ploeg, L.H.T.; et al. Crystal Structure of Human Phosphodiesterase 3B: Atomic Basis for Substrate and Inhibitor Specificity. Biochemistry 2004, 43, 6091–6100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, M.-S.; Chen, Y.; Geng, J.; Robinson, H.; Houslay, M.D.; Cai, J.; Ke, H. Structures of the Four Subfamilies of Phosphodiesterase-4 Provide Insight into the Selectivity of Their Inhibitors. Biochem. J. 2007, 408, 193–201. [Google Scholar] [CrossRef] [PubMed]
- O’ Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of Protein from Four Different Seaweeds Using Three Different Physical Pre-Treatment Strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef] [PubMed]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.-E.; Turgeon, S.L. Characterization of Antibacterial Activity from Protein Hydrolysates of the Macroalga Saccharina Longicruris and Identification of Peptides Implied in Bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive Properties of Peptides Obtained by Enzymatic Hydrolysis from Protein Byproducts of Porphyra Columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- SAITO, M. Antihypertensive Effect of Nori-Peptides Derived from Red Alga Porphyra Yezoensis in Hypertensive Patients. Am. J. Hypertens. 2002, 15, A210. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra Spp). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and Identification of a Novel ACE Inhibitory Peptide from Marine Alga Gracilariopsis Lemaneiformis Protein Hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Li, P.; Ying, J.; Chang, Q.; Zhu, W.; Yang, G.; Xu, T.; Yi, H.; Pan, R.; Zhang, E.; Zeng, X.; et al. Effects of Phycoerythrin from Gracilaria Lemaneiformis in Proliferation and Apoptosis of SW480 Cells. Oncol. Rep. 2016, 36, 3536–3544. [Google Scholar] [CrossRef]
- Hung, L.D.; Hirayama, M.; Ly, B.M.; Hori, K. Biological Activity, CDNA Cloning and Primary Structure of Lectin KSA-2 from the Cultivated Red Alga Kappaphycus Striatum (Schmitz) Doty Ex Silva. Phytochem. Lett. 2015, 14, 99–105. [Google Scholar] [CrossRef]
- Nam, T.-J. A Glycoprotein from Porphyra Yezoensis Produces Anti-Inflammatory Effects in Liposaccharide-Stimulated Macrophages via the TLR4 Signaling Pathway. Int. J. Mol. Med. 2011, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, I.N.L.; Quinderé, A.L.G.; Rodrigues, J.A.G.; de Sousa Oliveira Vanderlei, E.; Ribeiro, N.A.; da Conceição Rivanor, R.L.; Ribeiro, K.A.; Coura, C.O.; Pereira, K.M.A.; Chaves, H.V.; et al. Dual Effects of a Lectin from the Green Seaweed Caulerpa Cupressoides Var. Lycopodium on Inflammatory Mediators in Classical Models of Inflammation. Inflamm. Res. 2015, 64, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Anyanji, V.U.; Mustapha, N.M.; Lim, S.-L.; Mohamed, S. Seaweed (Eucheuma Cottonii) Reduced Inflammation, Mucin Synthesis, Eosinophil Infiltration and MMP-9 Expressions in Asthma-Induced Rats Compared to Loratadine. J. Funct. Foods 2015, 19, 710–722. [Google Scholar] [CrossRef]
- LEE, H.-A.; KIM, I.-H.; NAM, T.-J. Bioactive Peptide from Pyropia Yezoensis and Its Anti-Inflammatory Activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.X.; de Brito, T.V.; Fontenelle, T.P.C.; Damasceno, R.O.S.; de Souza, M.H.L.P.; de Souza Lopes, J.L.; Beltramini, L.M.; dos Reis Barbosa, A.L.; Freitas, A.L.P. Lectin from Red Algae Amansia Multifida Lamouroux: Extraction, Characterization and Anti-Inflammatory Activity. Int. J. Biol. Macromol. 2021, 170, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Vernarelli, J.A.; Lambert, J.D. Flavonoid Intake Is Inversely Associated with Obesity and C-Reactive Protein, a Marker for Inflammation, in US Adults. Nutr. Diabetes 2017, 7, e276. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Li, K.; Hu, W.; Yang, Y.; Wen, H.; Li, W.; Wang, B. Anti-Inflammation of Hydrogenated Isoflavones in LPS-Stimulated RAW264.7 Cells via Inhibition of NF-ΚB and MAPK Signaling Pathways. Mol. Immunol. 2023, 153, 126–134. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Bin Emran, T.; Dhama, K. Antioxidant Effects of Seaweeds and Their Active Compounds on Animal Health and Production—A Review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Yeo, I.J.; Park, J.H.; Jang, J.S.; Lee, D.Y.; Park, J.E.; Choi, Y.E.; Joo, J.H.; Song, J.K.; Jeon, H.O.; Hong, J.T. Inhibitory Effect of Carnosol on UVB-Induced Inflammation via Inhibition of STAT3. Arch. Pharm. Res. 2019, 42, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio-protocol 2021, 11, e3877. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef]
- Geminiani, M.; Gambassi, S.; Millucci, L.; Lupetti, P.; Collodel, G.; Mazzi, L.; Frediani, B.; Braconi, D.; Marzocchi, B.; Laschi, M.; et al. Cytoskeleton Aberrations in Alkaptonuric Chondrocytes. J. Cell Physiol. 2017, 232, 1728–1738. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2000, 455, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The Harmonizome: A Collection of Processed Datasets Gathered to Serve and Mine Knowledge about Genes and Proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.J.; O’Donovan, C.; Magrane, M.; Alpi, E.; Antunes, R.; Bely, B.; Bingley, M.; Bonilla, C.; Britto, R.; et al. UniProt: The Universal Protein Knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Janson, G.; Paiardini, A. PyMod 3: A Complete Suite for Structural Bioinformatics in PyMOL. Bioinformatics 2021, 37, 1471–1472. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Koebel, M.R.; Schmadeke, G.; Posner, R.G.; Sirimulla, S. AutoDock VinaXB: Implementation of XBSF, New Empirical Halogen Bond Scoring Function, into AutoDock Vina. J. Cheminform. 2016, 8, 27. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 Update: Improved Access to Chemical Data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Trezza, A.; Spiga, O.; Sgaragli, G.; Bova, S. Ca v 1.2 Channel Current Block by the PKA Inhibitor H-89 in Rat Tail Artery Myocytes via a PKA-Independent Mechanism: Electrophysiological, Functional, and Molecular Docking Studies. Biochem. Pharmacol. 2017, 140, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Spiga, O.; Trezza, A.; Frosini, M.; Floriddia, E.; Teodori, E.; Dei, S.; Saponara, S. In Vitro and in Silico Analysis of the Vascular Effects of Asymmetrical N,N-Bis(Alkanol)Amine Aryl Esters, Novel Multidrug Resistance-Reverting Agents. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1033–1043. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
| Chemical Class | Name | Retention Time (min) | Formula | Calculated MW | m/z | Reference Ion | Mass Error (ppm) |
|---|---|---|---|---|---|---|---|
| Lipids | Palmitin | 41.679 | C19H38O4 | 330.2777 | 331.2849 | [M+H]+ | 1.99 |
| Oleic acid | 50.271 | C18H34O2 | 282.2555 | 281.2482 | [M−H]− | −1.54 | |
| Stearidonic acid | 24.921 | C18H28O2 | 276.2094 | 277.2167 | [M+H]+ | 1.81 | |
| Hydroxymyristic acid | 40.396 | C14H28O3 | 244.2033 | 243.196 | [M−H]− | −2.14 | |
| Hydroxylauric acid | 35.009 | C12H24O3 | 216.172 | 215.1647 | [M−H]− | −2.61 | |
| Dihydroxypalmitic acid | 37.957 | C16H34O4 | 290.2461 | 291.2534 | [M+H]+ | 1.38 | |
| 9(10)-EpODE | 38.428 | C18H30O3 | 294.2204 | 295.2276 | [M+H]+ | 2.9 | |
| Hydroxylinoleic acid | 40.866 | C18H32O3 | 296.236 | 297.2432 | [M+H]+ | 2.8 | |
| Lauramide | 35.481 | C12H25NO | 199.1942 | 200.2014 | [M+H]+ | 2.71 | |
| 8-Pentadecenal | 41.627 | C15H28O | 224.2136 | 223.2063 | [M−H]− | −2.03 | |
| Amino acids | Valine | 2.043 | C5H11NO2 | 117.0793 | 118.0866 | [M+H]+ | 2.5 |
| Norleucine | 1.877 | C6H13NO2 | 131.0948 | 132.1021 | [M+H]+ | 1.18 | |
| Thymine | 8.042 | C5H6N2O2 | 126.0433 | 127.0505 | [M+H]+ | 2.52 | |
| Terpenoids | Carnosic acid | 31.801 | C20H28O4 | 332.1984 | 333.2056 | [M+H]+ | −1.23 |
| Rosmanol | 28.817 | C20H26O5 | 346.1783 | 347.1856 | [M+H]+ | 0.89 | |
| Methyl dehydroabietate | 40.729 | C21H30O2 | 314.2241 | 315.2313 | [M+H]+ | −1.7 | |
| Kaempferol | 12.302 | C15H10O6 | 286.0479 | 285.0407 | [M−H]− | 0.66 | |
| Flavonoids | Apigenin | 32.899 | C15H10O5 | 270.0532 | 269.0459 | [M−H]− | 1.42 |
| 3′-O-Methylequol | 23.484 | C16H16O4 | 272.1052 | 271.0979 | [M−H]− | 1.06 |
| Fatty Acid | RT (min) | Area % * |
|---|---|---|
| Caproic acid C6:0 | 7.21 | 1.66 ± 0.19 |
| Caprylic acid C8:0 | 9.53 | 0.33 ± 0.06 |
| Lauric acid C12:0 | 18.68 | 1.06 ± 0.08 |
| Tridecanoic acid C13:0 | 20.16 | 1.38 ± 0.09 |
| Myristic C14:0 | 23.09 | 20.46 ± 2.12 |
| Palmitic acid C16:0 | 29.27 | 52.18 ± 1.87 |
| Palmitoleic acid C16:1 | 31.09 | 1.14 ± 0.15 |
| Stearic acid C18:0 | 35.51 | 1.00 ± 0.07 |
| Oleic acid C18:1 | 37.12 | 11.70 ± 1.24 |
| Linoleic acid C18:2 | 39.74 | 7.35 ± 1.42 |
| Eicosapentanoic acid C20:5 | 52.62 | 0.24 ± 0.05 |
| Docosahexaenoic acid C22:6 | 58.81 | 2.65 ± 0.18 |
| Collection Date | Voucher Number | Type | Species Name | Location | GPS Point |
|---|---|---|---|---|---|
| 16 May 2021 | CL01 | Green | Chaetomorpha linum | Orbetello | 42°26′15.1″ N 11°11′38.7″ E |
| 18 May 2021 | CL02 | Green | Chaetomorpha linum | Orbetello | 42°26′15.1″ N 11°11′38.7″ E |
| 25 May 2021 | CL03 | Green | Chaetomorpha linum | Orbetello | 42°26′15.1″ N 11°11′38.7″ E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frusciante, L.; Geminiani, M.; Trezza, A.; Olmastroni, T.; Mastroeni, P.; Salvini, L.; Lamponi, S.; Bernini, A.; Grasso, D.; Dreassi, E.; et al. Phytochemical Composition, Anti-Inflammatory Property, and Anti-Atopic Effect of Chaetomorpha linum Extract. Mar. Drugs 2024, 22, 226. https://doi.org/10.3390/md22050226
Frusciante L, Geminiani M, Trezza A, Olmastroni T, Mastroeni P, Salvini L, Lamponi S, Bernini A, Grasso D, Dreassi E, et al. Phytochemical Composition, Anti-Inflammatory Property, and Anti-Atopic Effect of Chaetomorpha linum Extract. Marine Drugs. 2024; 22(5):226. https://doi.org/10.3390/md22050226
Chicago/Turabian StyleFrusciante, Luisa, Michela Geminiani, Alfonso Trezza, Tommaso Olmastroni, Pierfrancesco Mastroeni, Laura Salvini, Stefania Lamponi, Andrea Bernini, Daniela Grasso, Elena Dreassi, and et al. 2024. "Phytochemical Composition, Anti-Inflammatory Property, and Anti-Atopic Effect of Chaetomorpha linum Extract" Marine Drugs 22, no. 5: 226. https://doi.org/10.3390/md22050226
APA StyleFrusciante, L., Geminiani, M., Trezza, A., Olmastroni, T., Mastroeni, P., Salvini, L., Lamponi, S., Bernini, A., Grasso, D., Dreassi, E., Spiga, O., & Santucci, A. (2024). Phytochemical Composition, Anti-Inflammatory Property, and Anti-Atopic Effect of Chaetomorpha linum Extract. Marine Drugs, 22(5), 226. https://doi.org/10.3390/md22050226












