Protective Effects of an Oligo-Fucoidan-Based Formula against Osteoarthritis Development via iNOS and COX-2 Suppression following Monosodium Iodoacetate Injection
Abstract
1. Introduction
2. Results
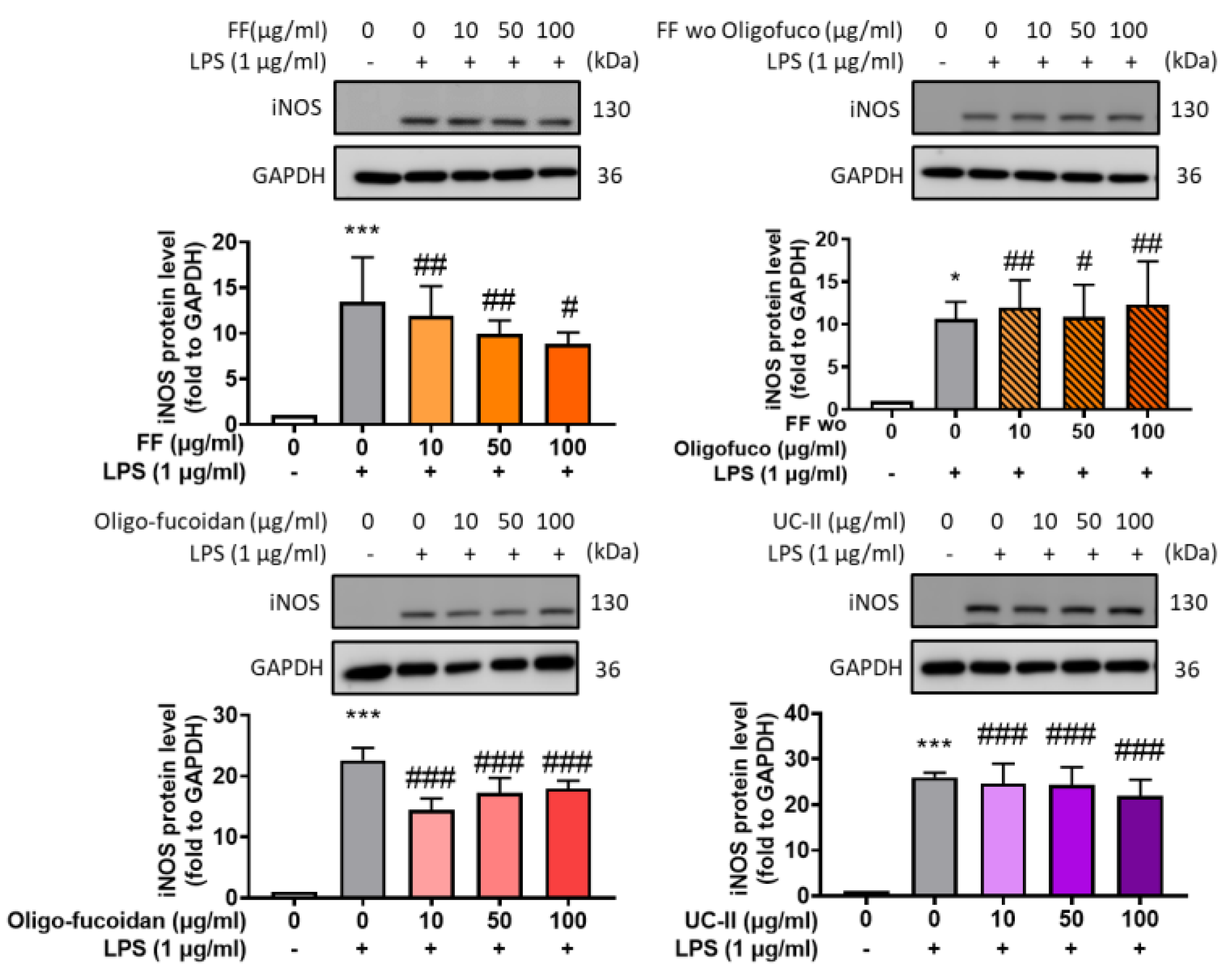
2.1. Formula Selection thorugh Examination of iNOS Expression (In Vitro Study)
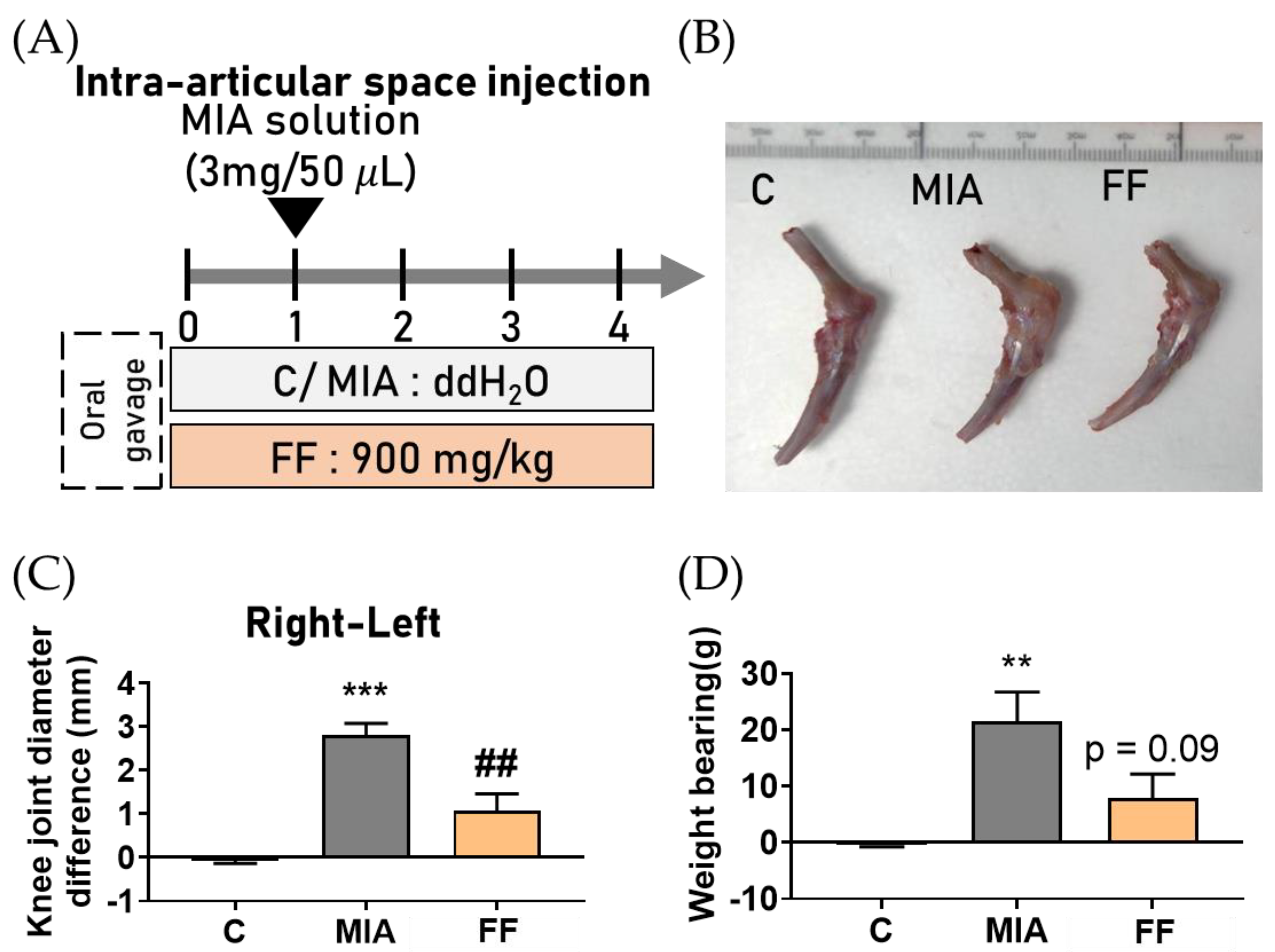
2.2. Protective Effect of the Oligo-Fucoidan-Based Formula (FF) on Joint Swelling Induced by MIA-Induced Osteoarthritis
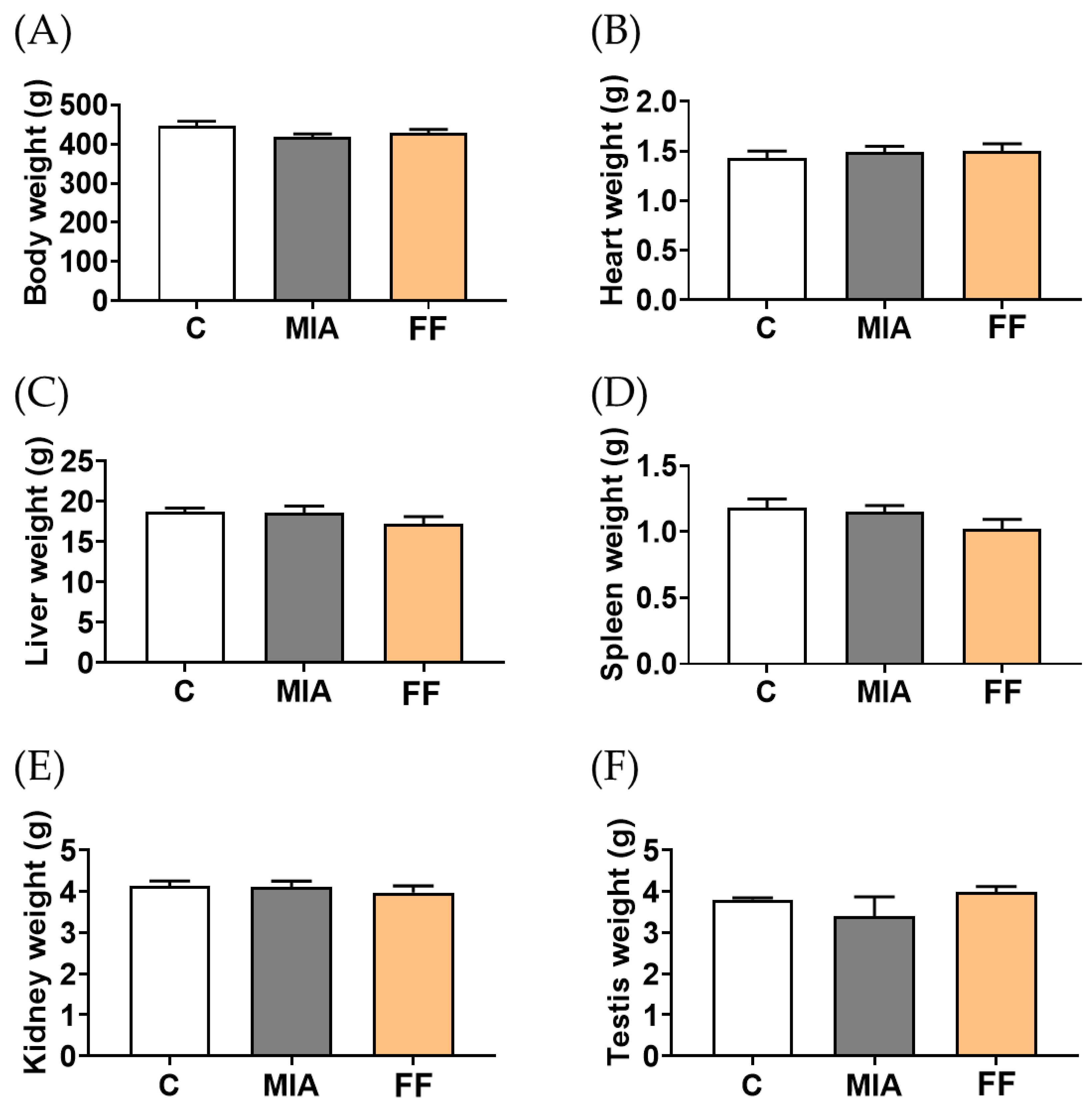
2.3. Oligo-Fucoidan-Based Formula Toxicity Evaluation
2.4. Cytokine Secretion and Malondialdehyde (MDA) Concentration
2.5. Histological Assessment
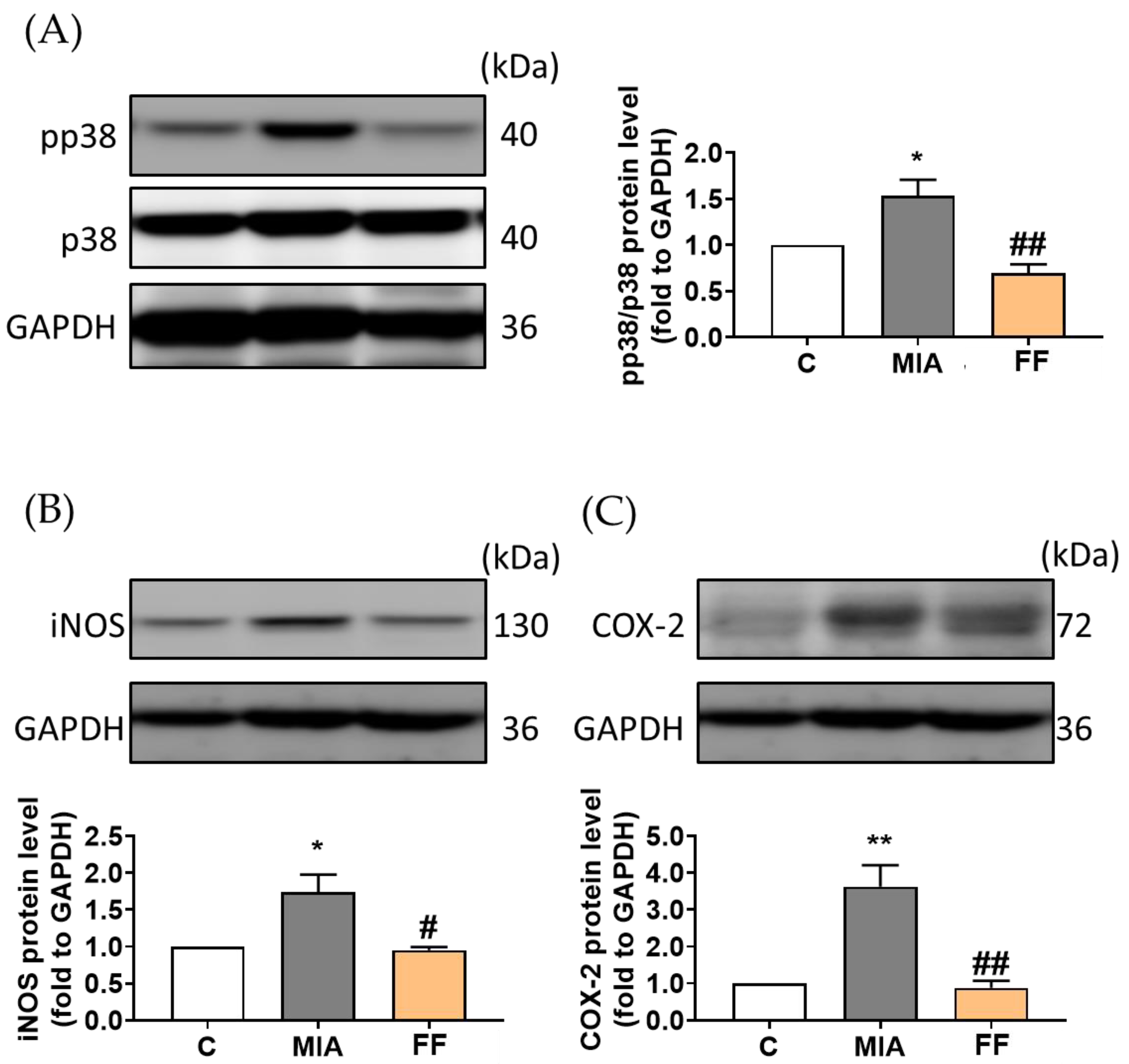
2.6. Modulation of Related Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Animal Osteoarthritis Model and Evaluation
4.4. Bilateral Weight-Bearing Test
4.5. Measurement of Knee Joint Width
4.6. IL-6 Level Measurement
4.7. Thiobarbituric Acid-Reactive Substances (TBARS) Assay
4.8. Histological Examination
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonge, D.P.; Pearson, M.J.; Jones, S.W. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthr. Cartil. 2014, 22, 609–621. [Google Scholar] [CrossRef]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology 2017, 56, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R.J. Chondrocytes expressing intracellular collagen type ii enter the cell cycle and co-express collagen type i in monolayer culture. J. Orthop. Res. 2014, 32, 1503–1511. [Google Scholar] [CrossRef]
- Cicuttini, F.M.; Wluka, A.E. Osteoarthritis: Is oa a mechanical or systemic disease? Nat. Rev. Rheumatol. 2014, 10, 515–516. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.A.; Kim, M.J.; Park, H.J.; Park, B.W.; Seo, S.B.; Roh, S.S. Protective effects of Phellinus linteus mycelium on the development of osteoarthritis after monosodium iodoacetate injection. Evid. Based Complement. Alternat. Med. 2020, 2020, 7240858. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yunus, M.H.M.; Ugusman, A.; Yazid, M.D. Natural compounds affecting inflammatory pathways of osteoarthritis. Antioxidants 2022, 11, 1722. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D. Effects of nonsteroidal anti-inflammatory drugs on chondrocyte metabolism in vitro and in vivo. Am. J. Med. 1987, 83, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of nf-κb, mapk and akt activation in lipopolysaccharide-induced bv2 microglia cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.T.; Chen, H.Y.; Lin, P.H.; Hsia, S.M. Preventive effects of fucoidan and fucoxanthin on hyperuricemic rats induced by potassium oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Barletta, E.; Degl’Innocenti, D. Marine migrastatics: A comprehensive 2022 update. Mar. Drugs 2022, 20, 273. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type ii collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord. 2013, 14, 228. [Google Scholar] [CrossRef]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining organ weight toxicity with bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep. 2020, 10, 6625. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Cherian, D.A.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci. 2019, 11, S297–S300. [Google Scholar] [CrossRef]
- Blake, D.R.; Merry, P.; Unsworth, J.; Kidd, B.L.; Outhwaite, J.M.; Ballard, R.; Morris, C.J.; Gray, L.; Lunec, J. Hypoxic-reperfusion injury in the inflamed human joint. Lancet 1989, 1, 289–293. [Google Scholar] [CrossRef]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and sod2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, 11722. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Deby-Dupont, G.P.; Reginster, J.Y.; Ayache, N.; Pujol, J.P.; Henrotin, Y.E. Regulation by reactive oxygen species of interleukin-1beta, nitric oxide and prostaglandin e(2) production by human chondrocytes. Osteoarthr. Cartil. 2002, 10, 547–555. [Google Scholar] [CrossRef]
- Zhang, W.; Robertson, W.B.; Zhao, J.; Chen, W.; Xu, J. Emerging trend in the pharmacotherapy of osteoarthritis. Front. Endocrinol. 2019, 10, 431. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bally, M.; Dendukuri, N.; Rich, B.; Nadeau, L.; Helin-Salmivaara, A.; Garbe, E.; Brophy, J.M. Risk of acute myocardial infarction with nsaids in real world use: Bayesian meta-analysis of individual patient data. BMJ 2017, 357, j1909. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.R.; Nüesch, E.; Reichenbach, S.; Jüni, P.; Rutjes, A.W. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database Syst. Rev. 2012, 11, Cd007323. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.S.; Chen, J.W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018, 17, 1493–1498. [Google Scholar] [CrossRef]
- Su, Y.; Shen, L.; Xue, J.; Zou, J.; Wan, D.; Shi, Z. Therapeutic evaluation of galangin on cartilage protection and analgesic activity in a rat model of osteoarthritis. Electron. J. Biotechnol. 2021, 53, 8–13. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. Ros/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.S.; Chien, H.W.; Chiou, H.L.; Yang, S.F.; Hsieh, Y.H. Melatonin inhibits naio(3)-induced arpe-19 cell apoptosis via suppression of hif-1α/bnip3-lc3b/mitophagy signaling. Cell Biosci. 2022, 12, 133. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Wang, Q.; Liang, H.; Jiang, X. Fucoidan protects arpe-19 cells from oxidative stress via normalization of reactive oxygen species generation through the ca2+-dependent erk signaling pathway. Mol. Med. Rep. 2015, 11, 3746–3752. [Google Scholar] [CrossRef]
- Meng, X.; Sun, L.; Meng, X.; Bi, Q. The protective effect of ergolide in osteoarthritis: In vitro and in vivo studies. Int. Immunopharmacol. 2024, 127, 111355. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Parimalanandhini, D.; Mahalakshmi, K.; Beulaja, M.; Arumugam, M.; Janarthanan, S.; Palanisamy, S.; You, S.; Prabhu, N.M. Studies on isolation, characterization of fucoidan from brown algae turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 160, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured type ii collagen (uc-ii) in joint health and disease: A review on the current knowledge of companion animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Lindley, J.; Konemann, M.; Minniear, J.; Carroll, B.A.; Hendrick, C.; Goad, J.T.; Rohde, K.; Doss, R.; et al. Comparative therapeutic efficacy and safety of type-ii collagen (uc-ii), glucosamine and chondroitin in arthritic dogs: Pain evaluation by ground force plate. J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. [Google Scholar] [CrossRef]
- Gonzalez-Alvarez, M.E.; Sanchez-Romero, E.A.; Turroni, S.; Fernandez-Carnero, J.; Villafañe, J.H. Correlation between the altered gut microbiome and lifestyle interventions in chronic widespread pain patients: A systematic review. Medicina 2023, 59, 256. [Google Scholar] [CrossRef]
- Kraus, V.B.; Reed, A.; Soderblom, E.J.; Moseley, M.A.; Hsueh, M.F.; Attur, M.G.; Samuels, J.; Abramson, S.B.; Li, Y.J. Serum proteomic panel validated for prediction of knee osteoarthritis progression. Osteoarthr. Cartil. Open 2024, 6, 100425. [Google Scholar] [CrossRef]
- Liao, C.H.; Lai, I.C.; Kuo, H.C.; Chuang, S.E.; Lee, H.L.; Whang-Peng, J.; Yao, C.J.; Lai, G.M. Epigenetic modification and differentiation induction of malignant glioma cells by oligo-fucoidan. Mar. Drugs 2019, 17, 525. [Google Scholar] [CrossRef]
- Chen, L.M.; Yang, P.P.; Al Haq, A.T.; Hwang, P.A.; Lai, Y.C.; Weng, Y.S.; Chen, M.A.; Hsu, H.L. Oligo-fucoidan supplementation enhances the effect of olaparib on preventing metastasis and recurrence of triple-negative breast cancer in mice. J. Biomed. Sci. 2022, 29, 70. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin prevents inflammation in lps-induced raw264.7 macrophages by inhibiting foxo3a/autophagy signaling pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS ONE 2018, 13, e0196625. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, T.; Sousa-Valente, J.; Malcangio, M. The monoiodoacetate model of osteoarthritis pain in the mouse. J. Vis. Exp. 2016, 16, 53746. [Google Scholar]
- Chen, Y.; Yu, Q.; Xu, C.-B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by imagej software. Int. J. Clin. Exp. Med. 2017, 10, 14927–14935. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-F.; Huang, K.-C.; Wang, K.-L.; Huang, Y.-J.; Chen, H.-Y.; Ali, M.; Shieh, T.-M.; Hsia, S.-M. Protective Effects of an Oligo-Fucoidan-Based Formula against Osteoarthritis Development via iNOS and COX-2 Suppression following Monosodium Iodoacetate Injection. Mar. Drugs 2024, 22, 211. https://doi.org/10.3390/md22050211
Chiang Y-F, Huang K-C, Wang K-L, Huang Y-J, Chen H-Y, Ali M, Shieh T-M, Hsia S-M. Protective Effects of an Oligo-Fucoidan-Based Formula against Osteoarthritis Development via iNOS and COX-2 Suppression following Monosodium Iodoacetate Injection. Marine Drugs. 2024; 22(5):211. https://doi.org/10.3390/md22050211
Chicago/Turabian StyleChiang, Yi-Fen, Ko-Chieh Huang, Kai-Lee Wang, Yun-Ju Huang, Hsin-Yuan Chen, Mohamed Ali, Tzong-Ming Shieh, and Shih-Min Hsia. 2024. "Protective Effects of an Oligo-Fucoidan-Based Formula against Osteoarthritis Development via iNOS and COX-2 Suppression following Monosodium Iodoacetate Injection" Marine Drugs 22, no. 5: 211. https://doi.org/10.3390/md22050211
APA StyleChiang, Y.-F., Huang, K.-C., Wang, K.-L., Huang, Y.-J., Chen, H.-Y., Ali, M., Shieh, T.-M., & Hsia, S.-M. (2024). Protective Effects of an Oligo-Fucoidan-Based Formula against Osteoarthritis Development via iNOS and COX-2 Suppression following Monosodium Iodoacetate Injection. Marine Drugs, 22(5), 211. https://doi.org/10.3390/md22050211










