Effects of Shrimp Shell-Derived Chitosan on Growth, Immunity, Intestinal Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System
Abstract
1. Introduction
2. Results
2.1. Growth Performance
2.2. Immunological Response
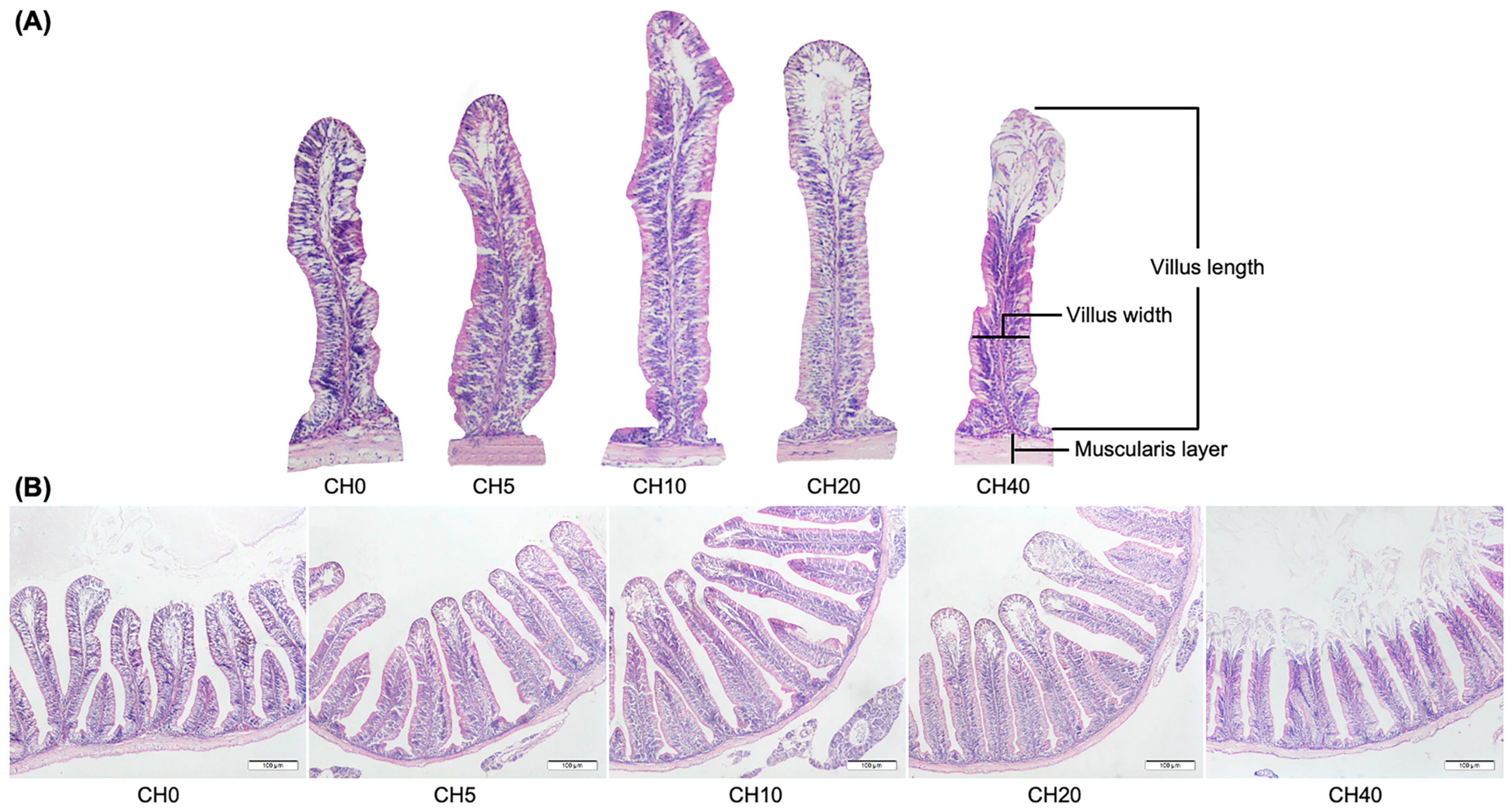
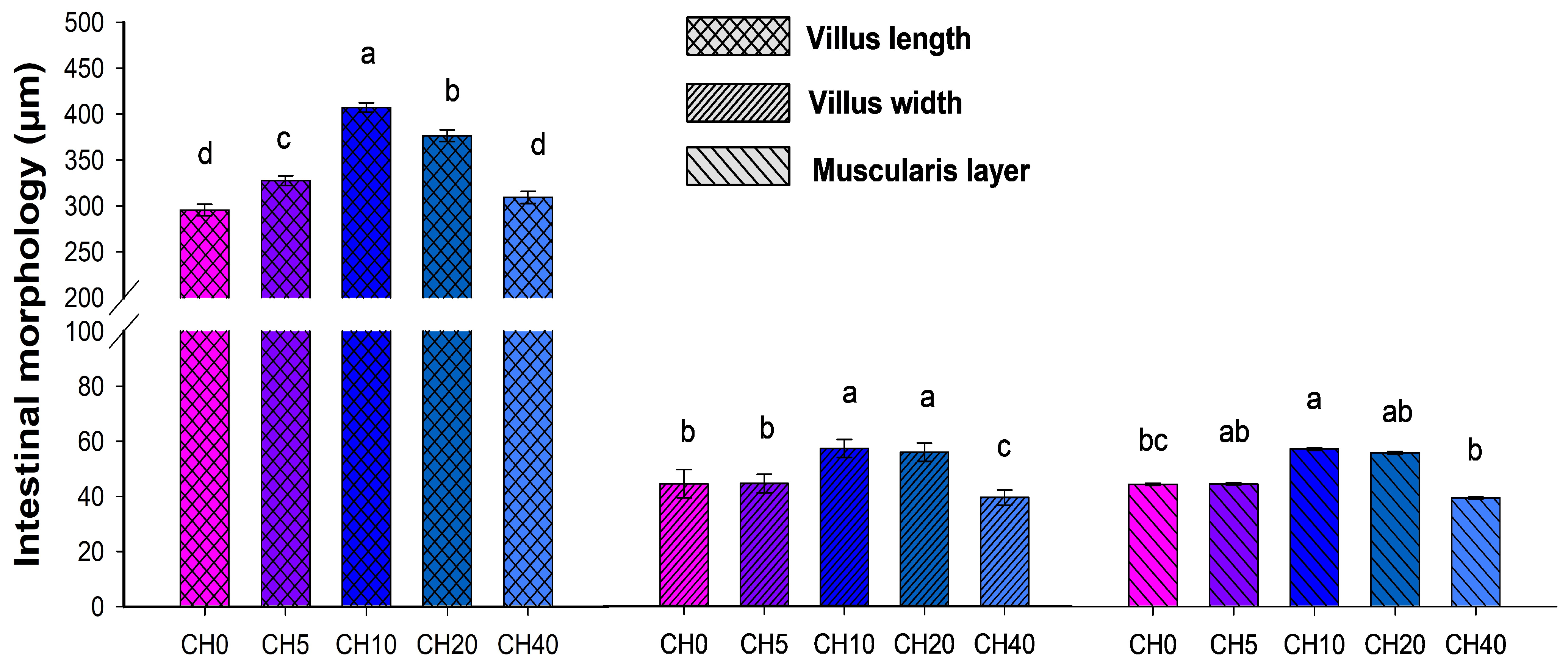
2.3. Histological Analysis
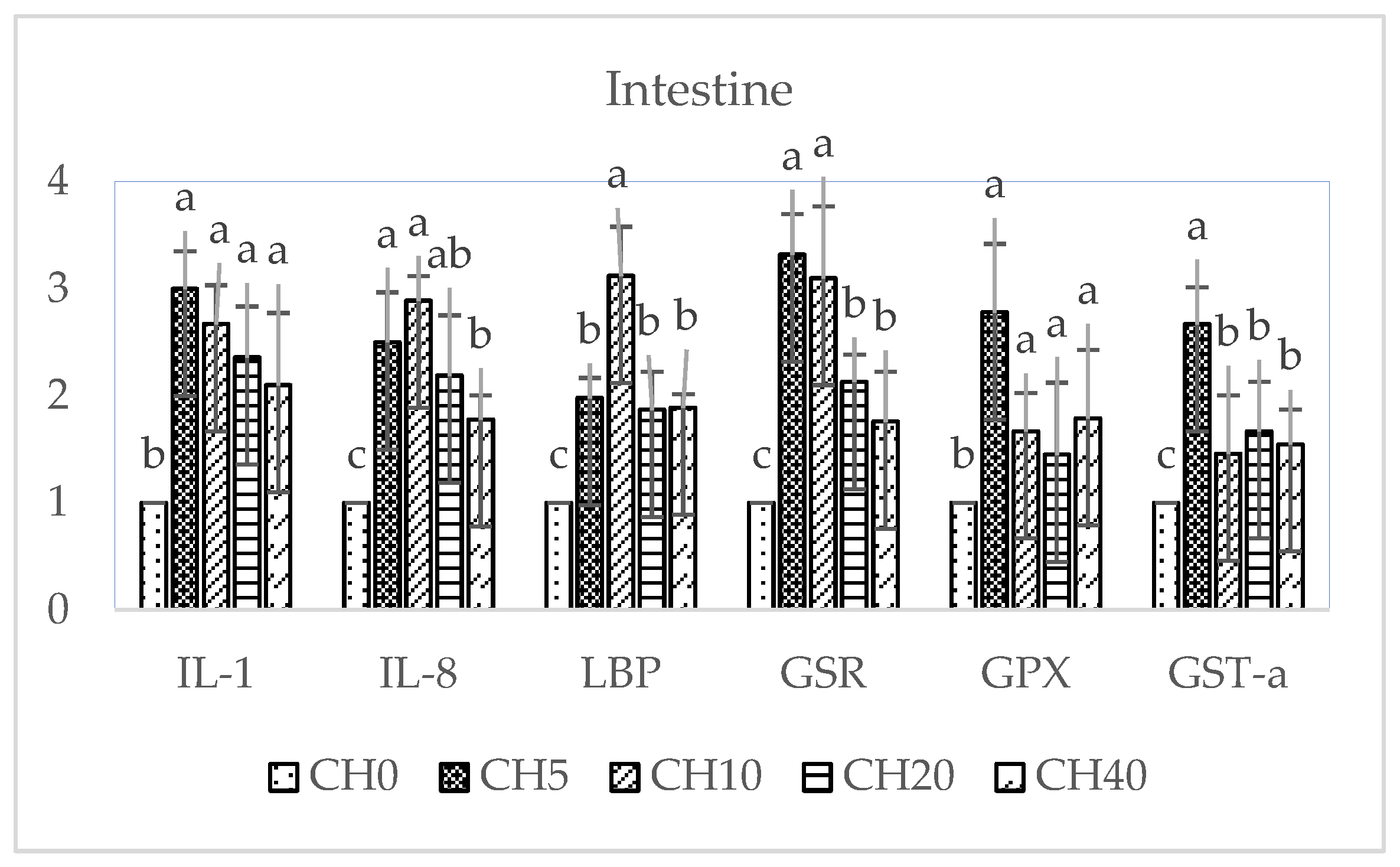
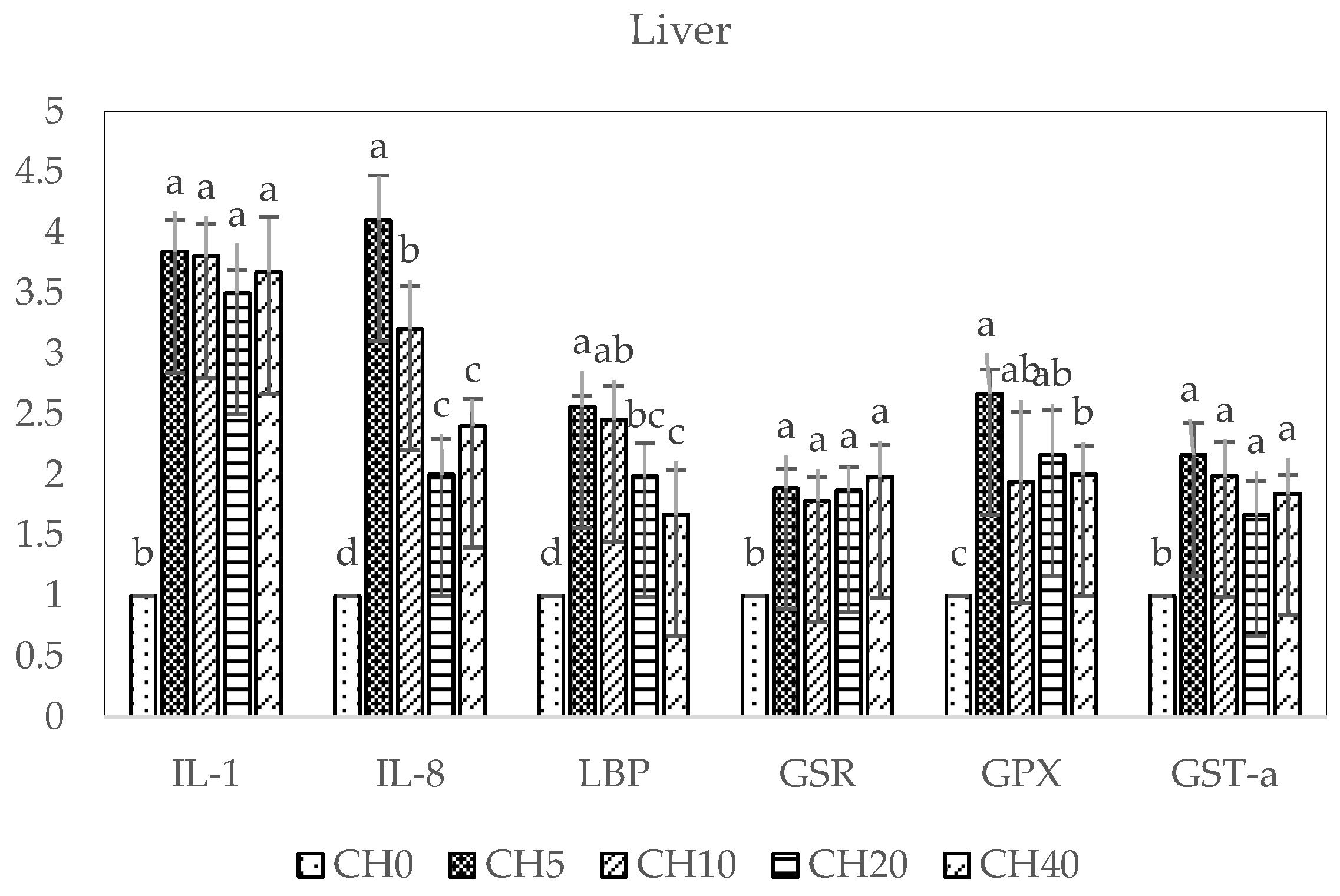
2.4. Immune and Antioxidant-Related Gene Expressions
3. Discussion
4. Materials and Methods
4.1. Nile Tilapia Husbandry
4.2. Diet Preparation and Experimental Design
4.2.1. Preparation of Chitosan (CH)
4.2.2. Experimental Design
4.3. Biofloc Water Preparation
4.4. Growth Performance
4.5. Immunological Analysis
4.5.1. Sample Collection
4.5.2. Immunological Parameter Analysis
4.6. Histopathology Analysis
4.7. Quantitative Real-Time PCR (qPCR)
4.7.1. Tissue Sampling, Total RNA Isolation, and cDNA Synthesis
4.7.2. Quantitative Real-Time PCR
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sampathkumar, K.; Yu, H.; Loo, S.C.J. Valorisation of Industrial Food Waste into Sustainable Aquaculture Feeds. Future Foods 2023, 7, 100240. [Google Scholar] [CrossRef]
- Gule, T.T.; Geremew, A. Dietary Strategies for Better Utilization of Aquafeeds in Tilapia Farming. Aquac. Nutr. 2022, 2022, 9463307. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.F.A.; Hassan, H.U.; Yones, A.-M.; Abdel-Tawwab, Y.A.; Metwalli, A.A.A.-T. Assessing the Effect of Different Feeding Frequencies Combined with Stocking Density, Initial Weight, and Dietary Protein Ratio on the Growth Performance of Tilapia, Catfish and Carp. Sci. Afr. 2021, 12, e00806. [Google Scholar] [CrossRef]
- Syed, R.; Masood, Z.; Hassan, H.U.; Khan, W.; Mushtaq, S.; Ali, A.; Gul, Y.; Jafari, H.; Habib, A.; Shah, M.I.A. Growth Performance, Haematological Assessment and Chemical Composition of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Fed Different Levels of Aloe Vera Extract as Feed Additives in a Closed Aquaculture System. Saudi J. Biol. Sci. 2022, 29, 296–303. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.-F.M.; Fitzsimmons, K. From Africa to the World—The Journey of Nile Tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Delphino, M.; Joshi, R.; Alvarez, A.T. Economic Appraisal of Using Genetics to Control Streptococcus agalactiae in Nile Tilapia under Cage and Pond Farming System in Malaysia. Sci. Rep. 2022, 12, 8754. [Google Scholar] [CrossRef] [PubMed]
- Suyamud, B.; Chen, Y.; Quyen, D.T.T.; Dong, Z.; Zhao, C.; Hu, J. Antimicrobial Resistance in Aquaculture: Occurrence and Strategies in Southeast Asia. Sci. Total Environ. 2024, 907, 167942. [Google Scholar] [CrossRef]
- Henriksson, P.J.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking Factors Influencing Antimicrobial Use in Global Aquaculture and Their Implication for Management: A Review from a Systems Perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef]
- Guimarães, M.C.; Cerezo, I.M.; Fernandez-Alarcon, M.F.; Natori, M.M.; Sato, L.Y.; Kato, C.A.; Moriñigo, M.A.; Tapia-Paniagua, S.; Dias, D.d.C.; Ishikawa, C.M. Oral Administration of Probiotics (Bacillus subtilis and Lactobacillus plantarum) in Nile Tilapia (Oreochromis niloticus) Vaccinated and Challenged with Streptococcus agalactiae. Fishes 2022, 7, 211. [Google Scholar] [CrossRef]
- Nurmalasari; Liu, C.-H.; Maftuch, I.M.; Hu, S.-Y. Dietary Supplementation with Prebiotic Chitooligosaccharides Enhances the Growth Performance, Innate Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus). Fishes 2022, 7, 313. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.-X.; Li, W.; Xu, R.; Qiao, F.; Du, Z.-Y.; Zhang, M.-L. Effects of Dietary Mannan Oligosaccharides (MOS) Supplementation on Metabolism, Inflammatory Response and Gut Microbiota of Juvenile Nile Tilapia (Oreochromis niloticus) Fed with High Carbohydrate Diet. Fish Shellfish Immunol. 2022, 130, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, F.; Iri, A.; Rezaei Shadegan, M.; Bigdeli, M.; Heydarzadeh Barzegar, D.; Zamani, V.; Barkhordar, M.; Houshmand, P.; Zare Mehrabadi, E.; Hedayati, A. Toxicity Effect of Silver Nitrate on Some Hematological Indices of Nil Tilapia (Oreochromis niloticus) Fed with Different Levels of Prebiotic Oyster Mushroom (Pleurotus ostreatus). J. Aquat. Physiol. Biotechnol. 2022, 9, 141–160. [Google Scholar]
- Outama, P.; Le Xuan, C.; Wannavijit, S.; Lumsangkul, C.; Linh, N.V.; Montha, N.; Tongsiri, S.; Chitmanat, C.; Van Doan, H. Modulation of Growth, Immune Response, and Immune-Antioxidant Related Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared under Biofloc System Using Mango Peel Powder. Fish Shellfish Immunol. 2022, 131, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- del Valle, J.C.; Bonadero, M.C.; Gimenez, A.V.F. Saccharomyces Cerevisiae as Probiotic, Prebiotic, Synbiotic, Postbiotics and Parabiotics in Aquaculture: An Overview. Aquaculture 2023, 569, 739342. [Google Scholar] [CrossRef]
- Khanjani, M.H.; da Silva, L.O.B.; Fóes, G.K.; Vieira, F.d.N.; Poli, M.A.; Santos, M.; Emerenciano, M.G.C. Synbiotics and Aquamimicry as Alternative Microbial-Based Approaches in Intensive Shrimp Farming and Biofloc: Novel Disruptive Techniques or Complementary Management Tools? A Scientific-Based Overview. Aquaculture 2023, 567, 739273. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and Chitosan Derived from Crustacean Waste Valorization Streams Can Support Food Systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Wani, A.K.; Akhtar, N.; Mir, T.u.G.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; El Messaoudi, N.; et al. Eco-Friendly and Safe Alternatives for the Valorization of Shrimp Farming Waste. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ravanipour, M.; Bagherzadeh, R.; Mahvi, A.H. Fish and Shrimp Waste Management at Household and Market in Bushehr, Iran. J. Mater. Cycles Waste Manag. 2021, 23, 1394–1403. [Google Scholar] [CrossRef]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive Utilization of Shrimp Waste Based on Biotechnological Methods: A Review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Mansyur, N.; Hanudin, E.; Purwanto, B.; Utami, S. The Nutritional Value of Shrimp Waste and Its Response to Growth and N Uptake Efficiency by Corn. IOP Conf. Ser. Earth Environ. Sci. 2021, 748, 012013. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Zhang, D.; Wei, S.; Sun, Q.; Xia, Q.; Shi, W.; Ji, H.; Liu, S. Comparison of the Proximate Composition and Nutritional Profile of Byproducts and Edible Parts of Five Species of Shrimp. Foods 2021, 10, 2603. [Google Scholar] [CrossRef] [PubMed]
- Susetyaningsih, R.; Suntoro, S.; Gunawan, T.; Budiastuti, M.T.S. Impact of Shrimp Pond Waste on Water Quality (Case Study of Trisik Lagoon in Yogyakarta). AIP Conf. Proc. 2020, 2296, 020050. [Google Scholar]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste Production in Aquaculture: Sources, Components and Managements in Different Culture Systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- El Amri, H.; Boukharta, M.; Zakham, F.; Ennaji, M.M. Emergence and Reemergence of Viral Zoonotic Diseases: Concepts and Factors of Emerging and Reemerging Globalization of Health Threats. In Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 619–634. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Hosney, A.; Ullah, S.; Barčauskaitė, K. A Review of the Chemical Extraction of Chitosan from Shrimp Wastes and Prediction of Factors Affecting Chitosan Yield by Using an Artificial Neural Network. Mar. Drugs 2022, 20, 675. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, W.; Qu, C.; Gu, J.; Yin, H.; Jia, Z.; Song, L.; Du, Y. Chitosan Oligosaccharides Inhibit Epithelial Cell Migration through Blockade of N-Acetylglucosaminyltransferase V and Branched GlcNAc Structure. Carbohydr. Polym. 2017, 170, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yuan, S.; Yang, X.; Zhai, X.; Wang, J. Chitosan Oligosaccharides with Degree of Polymerization 2–6 Induces Apoptosis in Human Colon Carcinoma HCT116 Cells. Chem.-Biol. Interact. 2018, 279, 129–135. [Google Scholar] [CrossRef]
- Jitprasertwong, P.; Khamphio, M.; Petsrichuang, P.; Eijsink, V.G.H.; Poolsri, W.; Muanprasat, C.; Rangnoi, K.; Yamabhai, M. Anti-Inflammatory Activity of Soluble Chitooligosaccharides (CHOS) on VitD3-Induced Human THP-1 Monocytes. PLoS ONE 2021, 16, e0246381. [Google Scholar] [CrossRef]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by Chitosan Oligosaccharide in Intestinal Epithelial Cells: Mechanism of Action and Potential Applications in Intestinal Disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Chang, P.; Li, X.; Gao, Z.; Sun, Y. The Inhibitory Effect of Chitosan Oligosaccharides on β-Site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE1) in HEK293 APPswe Cells. Neurosci. Lett. 2018, 665, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan Oligosaccharide: Biological Activities and Potential Therapeutic Applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Bezrodnykh, E.A.; Antonov, Y.A.; Berezin, B.B.; Kulikov, S.N.; Tikhonov, V.E. Molecular Features of the Interaction and Antimicrobial Activity of Chitosan in a Solution Containing Sodium Dodecyl Sulfate. Carbohydr. Polym. 2021, 270, 118352. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Ebrahimi, B.; Rajaei, A.; Movahednejad, M.H.; Rastegari, H.; Taghavi, E.; Aghbashlo, M.; Gupta, V.K.; Lam, S.S. Producing Submicron Chitosan-Stabilized Oil Pickering Emulsion Powder by an Electrostatic Collector-Equipped Spray Dryer. Carbohydr. Polym. 2022, 294, 119791. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Li, C.; Hou, T.; Wen, C.; Kong, S.; Ma, D.; Sun, C.; Li, S. Effects of Chitosan–Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 419. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; del Pilar González, M.; Murado, M.A. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; World Aquaculture Society: Sorrento, LA, USA, 2009; ISBN 1-888807-16-4. [Google Scholar]
- McCusker, S.; Warberg, M.B.; Davies, S.J.; Valente, C.d.S.; Johnson, M.P.; Cooney, R.; Wan, A.H. Biofloc Technology as Part of a Sustainable Aquaculture System: A Review on the Status and Innovations for Its Expansion. Aquac. Fish Fish. 2023, 33, 331–352. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Boardman, G.D.; Craig, S.R.; Flick, G.J., Jr.; McLean, E. Use of Microbial Flocs Generated from Tilapia Effluent as a Nutritional Supplement for Shrimp, Litopenaeus vannamei, in Recirculating Aquaculture Systems. J. World Aquac. Soc. 2008, 39, 72–82. [Google Scholar] [CrossRef]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc Technology in Aquaculture: Beneficial Effects and Future Challenges. Aquaculture 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mohammadi, A.; Emerenciano, M.G.C. Microorganisms in Biofloc Aquaculture System. Aquac. Rep. 2022, 26, 101300. [Google Scholar] [CrossRef]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The Prospects of Biofloc Technology (BFT) for Sustainable Aquaculture Development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, Digestive Activity, Welfare, and Partial Cost-Effectiveness of Genetically Improved Farmed Tilapia (Oreochromis niloticus) Cultured in a Recirculating Aquaculture System and an Indoor Biofloc System. Aquaculture 2014, 422, 1–7. [Google Scholar] [CrossRef]
- Xu, W.-J.; Morris, T.C.; Samocha, T.M. Effects of C/N Ratio on Biofloc Development, Water Quality, and Performance of Litopenaeus vannamei Juveniles in a Biofloc-Based, High-Density, Zero-Exchange, Outdoor Tank System. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Lee, J.-H.; Choi, J.-H.; Choi, Y.J.; Jo, A.-H.; Choi, C.Y.; Kang, J.-C.; Kim, J.-H. The Application and Future of Biofloc Technology (BFT) in Aquaculture Industry: A Review. J. Environ. Manag. 2023, 342, 118237. [Google Scholar] [CrossRef]
- Chutia, A.; Xavier, K.M.; Shamna, N.; Rani, A.B. Application of Bioflocculating Agent in Inoculum Enhances Quality of Biofloc and Influences Growth, Feed Utilization and Stress Responses of GIFT Tilapia Reared in-situ. Aquaculture 2022, 553, 738050. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, Y.; Liu, W.; Xu, L.; Yang, Y.; Zhou, Z. Effects of Chito-Oligosaccharides Supplementation on Growth Performance, Intestinal Cytokine Expression, Autochthonous Gut Bacteria and Disease Resistance in Hybrid Tilapia Oreochromis niloticus♀× Oreochromis aureus♂. Fish Shellfish Immunol. 2014, 40, 267–274. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Yuan, X.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Chitooligosaccharide Supplementation in Low-Fish Meal Diets for Pacific White Shrimp (Litopenaeus vannamei): Effects on Growth, Innate Immunity, Gut Histology, and Immune-Related Genes Expression. Fish Shellfish Immunol. 2018, 80, 405–415. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; Salem, M.E.-S. Effects of Dietary Chitosan Supplementation on Farmed Fish; a Review. Rev. Aquac. 2020, 12, 438–452. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Yu, Y.-P. Dietary Supplementation of Chitin and Chitosan Depresses Growth in Tilapia, Oreochromis niloticus × O. aureus. Aquaculture 1999, 179, 439–446. [Google Scholar] [CrossRef]
- Romana-Eguia, M.R.R.; Parado-Estepa, F.D.; Salayo, N.D.; Lebata-Ramos, M.J.H. Resource Enhancement and Sustainable Aquaculture Practices in Southeast Asia: Challenges in Responsible Production of Aquatic Species: Proceedings of the International Workshop on Resource Enhancement and Sustainable Aquaculture Practices in Southeast Asia 2014 (RESA); Aquaculture Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2015; ISBN 971-9931-04-3. [Google Scholar]
- Kamali Najafabad, M.; Imanpoor, M.R.; Taghizadeh, V.; Alishahi, A. Effect of Dietary Chitosan on Growth Performance, Hematological Parameters, Intestinal Histology and Stress Resistance of Caspian Kutum (Rutilus frisii Kutum Kamenskii, 1901) Fingerlings. Fish Physiol. Biochem. 2016, 42, 1063–1071. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Effects of Chitosan Nanoparticles on Survival, Growth and Meat Quality of Tilapia, Oreochromis nilotica. Nanotoxicology 2011, 5, 425–431. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Chen, H.; Zhou, Q.; Zhang, Y.; Huang, X.; Huang, Z.; Li, T.; Zhou, C.; Ma, Z. Effects of Dietary Chitosan on the Growth, Health Status and Disease Resistance of Golden Pompano (Trachinotus ovatus). Carbohydr. Polym. 2023, 300, 120237. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, T.; Wu, Q.; Zhou, C.; Ma, Z.; Lin, H. Effects of Dietary Astragalus Polysaccharides on Growth, Health and Resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef]
- Abd El-Naby, F.S.; Naiel, M.A.; Al-Sagheer, A.A.; Negm, S.S. Dietary Chitosan Nanoparticles Enhance the Growth, Production Performance, and Immunity in Oreochromis niloticus. Aquaculture 2019, 501, 82–89. [Google Scholar] [CrossRef]
- Denji, K.A.; Mansour, M.R.; Akrami, R.; Ghobadi, S.; Jafarpour, S.; Mirbeygi, S. Effect of Dietary Prebiotic Mannan Oligosaccharide (MOS) on Growth Performance, Intestinal Microflora, Body Composition, Haematological and Blood Serum Biochemical Parameters of Rainbow Trout (Oncorhynchus mykiss) Juveniles. J. Fish. Aquat. Sci. 2015, 10, 255. [Google Scholar]
- Wang, Y.; Zhao, K.; Li, L.; Song, X.; He, Y.; Ding, N.; Li, L.; Wang, S.; Liu, Z. A Review of the Immune Activity of Chitooligosaccharides. Food Sci. Technol. 2023, 43, e97822. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Al-Sagheer, A.A.; Negm, S.S.; Naiel, M.A. Dietary Combination of Chitosan Nanoparticle and Thymol Affects Feed Utilization, Digestive Enzymes, Antioxidant Status, and Intestinal Morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Eaton, P.; Nascimento, H.; Gião, M.S.; Ramos, Ó.S.; Belo, L.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X. Antioxidant Activity of Chitooligosaccharides upon Two Biological Systems: Erythrocytes and Bacteriophages. Carbohydr. Polym. 2010, 79, 1101–1106. [Google Scholar] [CrossRef]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant Activities of Chitosans and Its Derivatives in in Vitro and in Vivo Studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Bøgwald, J. SS-Glucans as Conductors of Immune Symphonies. Fish Shellfish Immunol. 2008, 25, 384–396. [Google Scholar] [CrossRef]
- Alves, A.P.d.C.; Paulino, R.R.; Pereira, R.T.; da Costa, D.V.; e Rosa, P.V. Nile Tilapia Fed Insect Meal: Growth and Innate Immune Response in Different Times under Lipopolysaccharide Challenge. Aquac. Res. 2021, 52, 529–540. [Google Scholar] [CrossRef]
- Conforto, E.; Vílchez-Gómez, L.; Parrinello, D.; Parisi, M.G.; Esteban, M.Á.; Cammarata, M.; Guardiola, F.A. Role of Mucosal Immune Response and Histopathological Study in European Eel (Anguilla anguilla L.) Intraperitoneal Challenged by Vibrio anguillarum or Tenacibaculum soleae. Fish Shellfish Immunol. 2021, 114, 330–339. [Google Scholar] [CrossRef]
- Marshall, W.S.; Bellamy, D. The 50 Year Evolution of in Vitro Systems to Reveal Salt Transport Functions of Teleost Fish Gills. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 275–280. [Google Scholar] [CrossRef]
- McNeilly, T.N.; Naylor, S.W.; Mahajan, A.; Mitchell, M.C.; McAteer, S.; Deane, D.; Smith, D.G.; Low, J.C.; Gally, D.L.; Huntley, J.F. Escherichia Coli O157: H7 Colonization in Cattle Following Systemic and Mucosal Immunization with Purified H7 Flagellin. Infect. Immun. 2008, 76, 2594–2602. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate Immunity of Fish (Overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Galagarza, O.A.; Smith, S.A.; Drahos, D.J.; Eifert, J.D.; Williams, R.C.; Kuhn, D.D. Modulation of Innate Immunity in Nile Tilapia (Oreochromis niloticus) by Dietary Supplementation of Bacillus subtilis Endospores. Fish Shellfish Immunol. 2018, 83, 171–179. [Google Scholar] [CrossRef]
- Park, Y.-K.; Kim, M.-H.; Park, S.-C.; Cheong, H.-S.; Jang, M.-K.; Nah, J.-W.; Hahm, K.-S. Investigation of the Antifungal Activity and Mechanism of Action of LMWS-Chitosan. J. Microbiol. Biotechnol. 2008, 18, 1729–1734. [Google Scholar]
- Chang, Q.; Liang, M.; Wang, J.; Sun, J. Influence of Chitosan on the Growth and Non-Specific Immunity of Japanese Sea Bass (Lateolabrax japonicus). Mar. Fish. Res. 2006, 27, 17–22. [Google Scholar]
- Gopalakannan, A.; Arul, V. Immunomodulatory Effects of Dietary Intake of Chitin, Chitosan and Levamisole on the Immune System of Cyprinus carpio and Control of Aeromonas hydrophila Infection in Ponds. Aquaculture 2006, 255, 179–187. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Arizcun, M.; Meseguer, J.; Esteban, M.A. Comparative Skin Mucus and Serum Humoral Defence Mechanisms in the Teleost Gilthead Seabream (Sparus aurata). Fish Shellfish Immunol. 2014, 36, 545–551. [Google Scholar] [CrossRef]
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.; Jaturasitha, S.; Esteban, M.Á.; Ringø, E.; Van Doan, H. The Effects Gotu Kola (Centella asiatica) Powder on Growth Performance, Skin Mucus, and Serum Immunity of Nile Tilapia (Oreochromis niloticus) Fingerlings. Aquac. Rep. 2020, 16, 100239. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.; Moate, R.; Davies, S.; Spring, P.; Sweetman, J.; Bradley, G. Dietary Mannan Oligosaccharide Supplementation Modulates Intestinal Microbial Ecology and Improves Gut Morphology of Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef]
- Geda, F.; Rekecki, A.; Decostere, A.; Bossier, P.; Wuyts, B.; Kalmar, I.; Janssens, G. Changes in Intestinal Morphology and Amino Acid Catabolism in Common Carp at Mildly Elevated Temperature as Affected by Dietary Mannanoligosaccharides. Anim. Feed Sci. Technol. 2012, 178, 95–102. [Google Scholar] [CrossRef]
- Daniels, C.L.; Merrifield, D.L.; Boothroyd, D.P.; Davies, S.J.; Factor, J.R.; Arnold, K.E. Effect of Dietary Bacillus spp. and Mannan Oligosaccharides (MOS) on European Lobster (Homarus gammarus L.) Larvae Growth Performance, Gut Morphology and Gut Microbiota. Aquaculture 2010, 304, 49–57. [Google Scholar] [CrossRef]
- Chen, G.; Yin, B.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Supplementing Chitosan Oligosaccharide Positively Affects Hybrid Grouper (Epinephelus fuscoguttatus♀× E. lanceolatus♂) Fed Dietary Fish Meal Replacement with Cottonseed Protein Concentrate: Effects on Growth, Gut Microbiota, Antioxidant Function and Immune Response. Front. Mar. Sci. 2021, 8, 707627. [Google Scholar]
- Hahor, W.; Thongprajukaew, K.; Suanyuk, N. Effects of Dietary Supplementation of Oligosaccharides on Growth Performance, Gut Health and Immune Response of Hybrid Catfish (Pangasianodon gigas × Pangasianodon hypophthalmus). Aquaculture 2019, 507, 97–107. [Google Scholar] [CrossRef]
- Zhou, Q.-C.; Buentello, J.A.; Gatlin III, D.M. Effects of Dietary Prebiotics on Growth Performance, Immune Response and Intestinal Morphology of Red Drum (Sciaenops ocellatus). Aquaculture 2010, 309, 253–257. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Li, H.; Xu, W.; He, J.; Li, X.; Jiang, G. Effects of Dietary Supplementation of Fructooligosaccharide on Growth Performance, Body Composition, Intestinal Enzymes Activities and Histology of Blunt Snout Bream (Megalobrama amblycephala) Fingerlings. Aquac. Nutr. 2013, 19, 886–894. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, W.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.; Tang, L.; Zhang, Y.; Zhou, X.; Feng, L. Gossypol Reduced the Intestinal Amino Acid Absorption Capacity of Young Grass Carp (Ctenopharyngodon idella). Aquaculture 2018, 492, 46–58. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsunari, H.; Sugita, T.; Furuita, H.; Masumoto, T.; Iwashita, Y.; Amano, S.; Suzuki, N. Optimization of the Supplemental Essential Amino Acids to a Fish Meal-Free Diet Based on Fermented Soybean Meal for Rainbow Trout Oncorhynchus mykiss. Fish. Sci. 2012, 78, 359–366. [Google Scholar] [CrossRef]
- Morales, A.; Gómez, T.; Villalobos, Y.D.; Bernal, H.; Htoo, J.K.; González-Vega, J.C.; Espinoza, S.; Yáñez, J.; Cervantes, M. Dietary Protein-Bound or Free Amino Acids Differently Affect Intestinal Morphology, Gene Expression of Amino Acid Transporters, and Serum Amino Acids of Pigs Exposed to Heat Stress. J. Anim. Sci. 2020, 98, skaa056. [Google Scholar] [CrossRef]
- Sakai, M.; Hikima, J.; Kono, T. Fish Cytokines: Current Research and Applications. Fish. Sci. 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The Chemokine Superfamily Revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Fu, G.H.; Liu, F.; Xia, J.H.; Yue, G.H. The LBP Gene and Its Association with Resistance to Aeromonas hydrophila in Tilapia. Int. J. Mol. Sci. 2014, 15, 22028–22041. [Google Scholar] [CrossRef]
- Fath El-Bab, A.F.; Majrashi, K.A.; Sheikh, H.M.; Shafi, M.E.; El-Ratel, I.T.; Neamat-Allah, A.N.; El-Raghi, A.A.; Elazem, A.Y.A.; Abd-Elghany, M.F.; Abdelnour, S.A. Dietary Supplementation of Nile Tilapia (Oreochromis niloticus) with β-Glucan and/or Bacillus coagulans: Synergistic Impacts on Performance, Immune Responses, Redox Status and Expression of Some Related Genes. Front. Vet. Sci. 2022, 9, 1011715. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological Significance of Phospholipid Hydroperoxide Glutathione Peroxidase (PHGPx, GPx4) in Mammalian Cells. Free Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Ahlf, W.; Heise, S. Sediment Toxicity Assessment: Rationale for Effect Classes (5 Pp). J. Soils Sediments 2005, 5, 16–20. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The Basics of Bio-Flocs Technology: The Added Value for Aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The Biofloc Technology (BFT) in Indoor Tanks: Water Quality, Biofloc Composition, and Growth and Welfare of Nile Tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Mirzakhani, N.; Ebrahimi, E.; Jalali, S.A.H.; Ekasari, J. Growth Performance, Intestinal Morphology and Nonspecific Immunity Response of Nile Tilapia (Oreochromis niloticus) Fry Cultured in Biofloc Systems with Different Carbon Sources and Input C: N Ratios. Aquaculture 2019, 512, 734235. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent Progress towards the Application of Biofloc Technology for Tilapia Farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Linh, N.V.; Van Nguyen, D.; Khongdee, N.; Wannavijit, S.; Outama, P.; Le Xuan, C.; Mahatheeranont, S.; Sookwong, P.; Le, T.D.; Hoseinifar, S.H. Influence of Black Rice (Oryza sativa L.) Bran Derived Anthocyanin-Extract on Growth Rate, Immunological Response, and Immune-Antioxidant Gene Expression in Nile Tilapia (Oreochromis niloticus) Cultivated in a Biofloc System. Fish Shellfish Immunol. 2022, 128, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Suthongsa, S.; Pichyangkura, R.; Kalandakanond-Thongsong, S.; Thongsong, B. Effects of Dietary Levels of Chito-Oligosaccharide on Ileal Digestibility of Nutrients, Small Intestinal Morphology and Crypt Cell Proliferation in Weaned Pigs. Livest. Sci. 2017, 198, 37–44. [Google Scholar] [CrossRef]
- Keereelang, J.; Mangumphan, K.; Chitmanat, C.; Tongsiri, S.; Linh, N.V.; Van Doan, H. Dietary Effect of Lactobacillus plantarum (TISTR 912) on Digestive Enzyme Activity, Growth Performance, Immune Response, and Disease Resistance of Black Sharkminnow (Labeo chrysophekadion) against Aeromonas hydrophila Infection. Aquac. Rep. 2022, 27, 101409. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| CH0 | CH5 | CH10 | CH20 | CH40 | p-Value | |
|---|---|---|---|---|---|---|
| IW (g) | 13.48 ± 0.03 | 13.66 ± 0.05 | 13.55 ± 0.03 | 13.56 ± 0.08 | 13.47 ± 0.05 | 0.499 |
| FW (g) | ||||||
| 4 weeks | 29.45 ± 1.33 | 28.97 ± 0.98 | 31.72 ± 0.28 | 29.06 ± 0.38 | 28.87 ± 1.27 | 0.470 |
| 8 weeks | 50.44 ± 0.67 b | 50.23 ± 0.62 b | 50.69 ± 0.54 a | 50.60 ± 1.20 b | 50.29 ± 1.95 b | 0.049 |
| WG (g) | ||||||
| 4 weeks | 15.97 ± 1.26 | 15.30 ± 0.93 | 18.17 ± 0.44 | 15.49 ± 0.45 | 15.40 ± 1.20 | 0.228 |
| 8 weeks | 36.96 ± 1.30 b | 36.56 ± 0.04 b | 40.14 ± 0.83 a | 37.03 ± 0.85 b | 36.81 ± 0.47 b | 0.050 |
| SGR (%/day) | ||||||
| 4 weeks | 2.60 ± 0.13 ab | 2.50 ± 0.10 b | 2.83 ± 0.07 a | 2.54 ± 0.06 ab | 2.54 ± 0.13 ab | 0.032 |
| 8 weeks | 2.20 ± 0.04 b | 2.17 ± 0.00 b | 2.29 ± 0.03 a | 2.19 ± 0.04 b | 2.19 ± 0.01 b | 0.047 |
| FCR | ||||||
| 4 weeks | 0.76 ± 0.03 a | 0.76 ± 0.01 a | 0.75 ± 0.03 a | 0.75 ± 0.02 a | 0.75 ± 0.02 a | 0.479 |
| 8 weeks | 1.02 ± 0.01 a | 1.07 ± 0.04 a | 1.08 ± 0.04 a | 1.02 ± 0.03 a | 1.07 ± 0.08 a | 0.855 |
| SR (%) | ||||||
| 4 weeks | 96.67 ± 1.67 a | 95.00 ± 2.89 a | 98.33 ± 3.33 a | 98.33 ± 1.67 a | 98.33 ± 1.67 a | 0.046 |
| 8 weeks | 96.67 ± 1.67 a | 95.00 ± 2.89 a | 98.33 ± 3.33 a | 98.33 ± 1.67 a | 98.33 ± 1.67 a | 0.046 |
| CH0 | CH5 | CH10 | CH20 | CH40 | p-Value | ||
|---|---|---|---|---|---|---|---|
| 4 weeks | SMLA | 0.217 a ± 0.01 | 0.205 b ± 0.01 | 0.211 ab ± 0.01 | 0.195 b ± 0.01 | 0.201 b ± 0.01 | 0.048 |
| SMPA | 0.313 a ± 0.02 | 0.287 b ± 0.01 | 0.304 ab ± 0.01 | 0.261 b ± 0.02 | 247 b ± 0.03 | 0.050 | |
| 8 weeks | SMLA | 0.249 a ± 0.01 | 0.215 b ± 0.01 | 0.220 ab ± 0.01 | 0.201 b ± 0.01 | 0.215 b ± 0.01 | 0.001 |
| SMPA | 0.213 a ± 0.04 | 0.193 b ± 0.06 | 0.204 ab ± 0.06 | 0.181 b ± 0.02 | 147 b ± 0.02 | 0.036 | |
| CH0 | CH5 | CH10 | CH20 | CH40 | p-Value | ||
|---|---|---|---|---|---|---|---|
| 4 weeks | SL | 0.297 a ± 0.01 | 0.275 bc ± 0.01 | 0.289 ab ± 0.01 | 0.258 c ± 0.01 | 0.286 b ± 0.01 | 0.038 |
| SP | 0.443 b ± 0.02 | 0.449 b ± 0.02 | 0.501 a ± 0.01 | 0.388 c ± 0.01 | 0.392 c ± 0.01 | 0.015 | |
| 8 weeks | SL | 0.248 a ± 0.01 | 0.225 bc ± 0.01 | 0.240 ab ± 0.01 | 0.212 c ± 0.01 | 0.231 b ± 0.01 | 0.049 |
| SP | 0.477 b ± 0.05 | 0.438 abc ± 0.04 | 0.522 a ± 0.03 | 0.377 c ± 0.02 | 0.392 bc ± 0.03 | 0.041 | |
| CH0 | CH5 | CH10 | CH20 | CH40 | |
|---|---|---|---|---|---|
| Fish meal | 200 | 200 | 200 | 200 | 200 |
| Corn meal | 150 | 150 | 150 | 150 | 150 |
| Soybean meal | 390 | 390 | 390 | 390 | 390 |
| Wheat flour | 70 | 70 | 70 | 70 | 70 |
| Rice bran | 150 | 150 | 150 | 150 | 150 |
| Soybean oil | 2 | 2 | 2 | 2 | 2 |
| Chitosan solution (mL) | 0 | 5 | 10 | 20 | 40 |
| Binder | 20 | 20 | 20 | 20 | 20 |
| Premix 1 | 10 | 10 | 10 | 10 | 10 |
| Vitamin C 98% | 8 | 8 | 8 | 8 | 8 |
| Total (g) | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate composition of the experimental diets (%) | |||||
| Crude protein | 32.80 | 32.00 | 32.60 | 32.40 | 32.50 |
| Crude lipid | 2.85 | 2.75 | 2.63 | 2.78 | 2.88 |
| Fiber | 3.68 | 3.74 | 3.44 | 3.72 | 3.55 |
| Ash | 7.59 | 7.86 | 7.75 | 7.35 | 7.91 |
| Dry matter | 99.16 | 98.40 | 98.35 | 97.77 | 97.54 |
| Gross Energy (cal/g) | 4273.00 | 4261.50 | 4253.90 | 4262.00 | 4245.00 |
| Genes | Primer Sequence (5′-3′) | Tm (°C) | Product Size (bp) | Reference |
|---|---|---|---|---|
| 18S-rRNA | GTGCATGGCCGTTCTTAGTT CTCAATCTCGTGTGGCTGAA | 60 | 150 | XR_003216134 |
| IL-1 | GTCTGTCAAGGATAAGCGCTG ACTCTGGAGCTGGATGTTGA | 59 | 200 | XM_019365844 |
| IL-8 | CTGTGAAGGCATGGGTGTG GATCACTTTCTTCACCCAGGG | 59 | 196 | NM_001279704 |
| LBP | ACCAGAAACTGCGAGAAGGA GATTGGTGGTCGGAGGTTTG | 59 | 200 | XM_013271147 |
| GST-α | ACTGCACACTCATGGGAACA TTAAAAGCCAGCGGATTGAC | 60 | 190 | NM_001279635 |
| GPX | GGTGGATGTGAATGGAAAGG CTTGTAAGGTTCCCCGTCAG | 60 | 190 | NM_001279711 |
| GSR | CTGCACCAAAGAACTGCAAA CCAGAGAAGGCAGTCCACTC | 60 | 172 | XM_005467348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linh, N.V.; Lubis, A.R.; Dinh-Hung, N.; Wannavijit, S.; Montha, N.; Fontana, C.M.; Lengkidworraphiphat, P.; Srinual, O.; Jung, W.-K.; Paolucci, M.; et al. Effects of Shrimp Shell-Derived Chitosan on Growth, Immunity, Intestinal Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System. Mar. Drugs 2024, 22, 150. https://doi.org/10.3390/md22040150
Linh NV, Lubis AR, Dinh-Hung N, Wannavijit S, Montha N, Fontana CM, Lengkidworraphiphat P, Srinual O, Jung W-K, Paolucci M, et al. Effects of Shrimp Shell-Derived Chitosan on Growth, Immunity, Intestinal Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System. Marine Drugs. 2024; 22(4):150. https://doi.org/10.3390/md22040150
Chicago/Turabian StyleLinh, Nguyen Vu, Anisa Rilla Lubis, Nguyen Dinh-Hung, Supreya Wannavijit, Napatsorn Montha, Camilla Maria Fontana, Phattawin Lengkidworraphiphat, Orranee Srinual, Won-Kyo Jung, Marina Paolucci, and et al. 2024. "Effects of Shrimp Shell-Derived Chitosan on Growth, Immunity, Intestinal Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System" Marine Drugs 22, no. 4: 150. https://doi.org/10.3390/md22040150
APA StyleLinh, N. V., Lubis, A. R., Dinh-Hung, N., Wannavijit, S., Montha, N., Fontana, C. M., Lengkidworraphiphat, P., Srinual, O., Jung, W.-K., Paolucci, M., & Doan, H. V. (2024). Effects of Shrimp Shell-Derived Chitosan on Growth, Immunity, Intestinal Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System. Marine Drugs, 22(4), 150. https://doi.org/10.3390/md22040150











