Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability
Abstract
1. Introduction
2. Marine Animal Co-Products
2.1. Fish
2.2. Crustaceans
2.3. Mollusks
3. Marine Animal Co-Products as a Source of Healthy Lipids
3.1. Fish
3.2. Crustaceans
3.3. Mollusks
4. The Value of Marine Animal Co-Product Lipids for Human Health
5. The Value of Marine Animal Co-Product Lipids for Various Industries
6. Sustainability and Environmental Impact
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F. Maximising the Value of Marine by-Products; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Vieira, H.; Leal, M.C.; Calado, R. Fifty Shades of Blue: How Blue Biotechnology is Shaping the Bioeconomy. Trends Biotechnol. 2020, 38, 940–943. [Google Scholar] [CrossRef]
- Hamilton, H.; Newton, R.; Auchterlonie, N.; Müller, D. Systems approach to quantify the global omega-3 fatty acid cycle. Nat. Food 2020, 1, 59–62. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Seafood lipids and cardiovascular health. Nutrire 2016, 41, 7. [Google Scholar] [CrossRef]
- Dyerberg, J. Fats from marine animals in human nutrition and prevention of cardiovascular diseases. In Atherosclerosis and Cardiovascular Diseases, Proceedings of the Sixth International Meeting on Atherosclerosis and Cardiovascular Diseases, Bologna, Italy, 27–29 October 1986; Lenzi, S., Descovich, G.C., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 261–267. [Google Scholar]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n−3 Fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499S–1504S. [Google Scholar] [CrossRef]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Damaiyanti, D.W.; Tsai, Z.Y.; Masbuchin, A.N.; Huang, C.Y.; Liu, P.Y. Interplay between fish oil, obesity and cardiometabolic diabetes. J. Formos. Med. Assoc. 2023, 122, 528–539. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Nasopoulou, C.; Tsoupras, A.; Zabetakis, I. The Anti-inflammatory Properties of Food Polar Lipids. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Mendivil, C.O. Dietary Fish, Fish Nutrients, and Immune Function: A Review. Front. Nutr. 2020, 7, 617652. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Karadimou, A.; Mulvihill, J.J.E.; Grabrucker, A.M.; Zabetakis, I. The Role of Dietary Lipids in Cognitive Health: Implications for Neurodegenerative Disease. Biomedicines 2022, 10, 3250. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Dash, R.; Sohag, A.A.M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I.S.; Uddin, M.J.; Hannan, M.A. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar. Drugs 2021, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Assisi, A.; Banzi, R.; Buonocore, C.; Capasso, F.; Di Muzio, V.; Michelacci, F.; Renzo, D.; Tafuri, G.; Trotta, F.; Vitocolonna, M.; et al. Fish oil and mental health: The role of n-3 long-chain polyunsaturated fatty acids in cognitive development and neurological disorders. Int. Clin. Psychopharmacol. 2006, 21, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Thirukumaran, R.; Anu Priya, V.K.; Krishnamoorthy, S.; Ramakrishnan, P.; Moses, J.A.; Anandharamakrishnan, C. Resource recovery from fish waste: Prospects and the usage of intensified extraction technologies. Chemosphere 2022, 299, 134361. [Google Scholar] [CrossRef]
- FMI. Marine By-Products Market. Available online: https://www.futuremarketinsights.com/reports/marine-by-products-market (accessed on 10 January 2024).
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- Krueck, N.C.; Ahmadia, G.N.; Possingham, H.P.; Riginos, C.; Treml, E.A.; Mumby, P.J. Marine Reserve Targets to Sustain and Rebuild Unregulated Fisheries. PLoS Biol. 2017, 15, e2000537. [Google Scholar] [CrossRef]
- Issifu, I.; Alava, J.J.; Lam, V.W.Y.; Sumaila, U.R. Impact of Ocean Warming, Overfishing and Mercury on European Fisheries: A Risk Assessment and Policy Solution Framework. Front. Mar. Sci. 2022, 8, 770805. [Google Scholar] [CrossRef]
- zu Ermgassen, P.S.E.; Thurstan, R.H.; Corrales, J.; Alleway, H.; Carranza, A.; Dankers, N.; DeAngelis, B.; Hancock, B.; Kent, F.; McLeod, I.; et al. The benefits of bivalve reef restoration: A global synthesis of underrepresented species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2050–2065. [Google Scholar] [CrossRef]
- Kyzar, T.; Safak, I.; Cebrian, J.; Clark, M.W.; Dix, N.; Dietz, K.; Gittman, R.K.; Jaeger, J.; Radabaugh, K.R.; Roddenberry, A.; et al. Challenges and opportunities for sustaining coastal wetlands and oyster reefs in the southeastern United States. J. Environ. Manag. 2021, 296, 113178. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization: Rome, Italy, 2022; Volume 3. [Google Scholar]
- OECD/FAO. OECD-FAO Agricultural Outlook; OECD Publishing: Paris, France, 2023. [Google Scholar]
- Hicks, D.T. Seafood Safety and Quality: The Consumer’s Role. Foods 2016, 5, 71. [Google Scholar] [CrossRef]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Venugopal, V. Valorization of Seafood Processing Discards: Bioconversion and Bio-Refinery Approaches. Front. Sustain. Food Syst. 2021, 5, 611835. [Google Scholar] [CrossRef]
- Cooney, R.; de Sousa, D.B.; Fernández-Ríos, A.; Mellett, S.; Rowan, N.; Morse, A.P.; Hayes, M.; Laso, J.; Regueiro, L.; Wan, A.H.L.; et al. A circular economy framework for seafood waste valorisation to meet challenges and opportunities for intensive production and sustainability. J. Clean. Prod. 2023, 392, 136283. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste Biomass Valorization 2020, 11, 3223–3246. [Google Scholar] [CrossRef]
- Saravanan, A.; Yuvaraj, D.; Senthil Kumar, P.; Karishma, S.; Rangasamy, G. Fish processing discards: A plausible resource for valorization to renewable fuels production, optimization, byproducts and challenges. Fuel 2023, 335, 127081. [Google Scholar] [CrossRef]
- Samat, A.F.; Muhamad, N.A.S.; Abd Rasib, N.A.; Mohd Hassan, S.S.; Ahmad Sohaimi, K.S.; Iberahim, N.I. The Potential of Biodiesel Production derived from Fish Waste. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012017. [Google Scholar] [CrossRef]
- The Marine Ingredients Organisation. The Global Growth of By-Products. Available online: https://www.iffo.com/global-growth-products (accessed on 10 January 2024).
- Zhang, J.; Akyol, Ç.; Meers, E. Nutrient recovery and recycling from fishery waste and by-products. J. Environ. Manag. 2023, 348, 119266. [Google Scholar] [CrossRef] [PubMed]
- Pounds, A.; Kaminski, A.M.; Budhathoki, M.; Gudbrandsen, O.; Kok, B.; Horn, S.; Malcorps, W.; Mamun, A.A.; McGoohan, A.; Newton, R.; et al. More Than Fish-Framing Aquatic Animals within Sustainable Food Systems. Foods 2022, 11, 1413. [Google Scholar] [CrossRef]
- Regueiro, L.; Newton, R.; Soula, M.; Méndez, D.; Kok, B.; Little, D.C.; Pastres, R.; Johansen, J.; Ferreira, M. Opportunities and limitations for the introduction of circular economy principles in EU aquaculture based on the regulatory framework. J. Ind. Ecol. 2022, 26, 2033–2044. [Google Scholar] [CrossRef]
- Woodgate, S.L.; Wilkinson, R.G. The role of rendering in relation to the bovine spongiform encephalopathy epidemic, the development of EU animal by-product legislation and the reintroduction of rendered products into animal feeds. Ann. Appl. Biol. 2021, 178, 430–441. [Google Scholar] [CrossRef]
- Kumar, V.; Muzaddadi, A.; Mann, S.; Balakrishnan, R.; Bembem, K.; Kalnar, Y. Utilization of Fish Processing Waste: A Waste to Wealth Approach. In Emerging Post-Harvest Engineering and Techological Interventions for Enhancing Farmer’s Incom; ICAR-CIPHET: Ludhiana, India, 2022; pp. 127–131. [Google Scholar]
- European Commission. Oceans and Fisheries–Consumption. Available online: https://oceans-and-fisheries.ec.europa.eu/facts-and-figures/facts-and-figures-common-fisheries-policy/consumption_en (accessed on 10 January 2024).
- Siddiqui, S.A.; Schulte, H.; Pleissner, D.; Schönfelder, S.; Kvangarsnes, K.; Dauksas, E.; Rustad, T.; Cropotova, J.; Heinz, V.; Smetana, S. Transformation of Seafood Side-Streams and Residuals into Valuable Products. Foods 2023, 12, 422. [Google Scholar] [CrossRef]
- Toppe, J.; Olsen, R.L.; Peñarubia, O.R.; James, D.G. Production and Utilization of Fish Silage: A Manual on How to Turn Fish Waste into Profit and a Valuable Feed Ingredient or Fertilizer; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Boronat, Ò.; Sintes, P.; Celis, F.; Díez, M.; Ortiz, J.; Aguiló-Aguayo, I.; Martin-Gómez, H. Development of added-value culinary ingredients from fish waste: Fish bones and fish scales. Int. J. Gastron. Food Sci. 2023, 31, 100657. [Google Scholar] [CrossRef]
- Pedrosa, E.; Costa, C.; Lobato, L.; Mendes, S.; Oliveira, B. Study of the Edible Portion of Some Fishes. Rev. Nutr. 2014, 20, 20–24. [Google Scholar]
- Pinheiro, A.C.D.A.S.; Martí-Quijal, F.J.; Barba, F.J.; Tappi, S.; Rocculi, P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach. Foods 2021, 10, 2030. [Google Scholar] [CrossRef] [PubMed]
- Saima, M.K.; Roohi, I.Z.A. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Cretton, M.; Malanga, G.; Mazzuca Sobczuk, T.; Mazzuca, M. Lipid Fraction from Industrial Crustacean Waste and Its Potential as a Supplement for the Feed Industry: A Case Study in Argentine Patagonia. Waste Biomass Valorization 2021, 12, 2311–2319. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Carotenoids in different body components of Indian shrimps. J. Sci. Food Agric. 2005, 85, 167–172. [Google Scholar] [CrossRef]
- Heu, M.-S.; Kim, J.-S.; Shahidi, F. Components and nutritional quality of shrimp processing by-products. Food Chem. 2003, 82, 235–242. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus vannamei): Compositions and deterioration as affected by iced storage. Food Chem. 2012, 134, 2066–2074. [Google Scholar] [CrossRef]
- Phadtare, I.; Vaidya, H.; Hawboldt, K.; Cheema, S.K. Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes. Mar. Drugs 2021, 19, 259. [Google Scholar] [CrossRef]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B.; Nader, H.B.; Bezerra, R.S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process Biochem. 2012, 47, 570–577. [Google Scholar] [CrossRef]
- Albalat, A.; Nadler, L.E.; Foo, N.; Dick, J.R.; Watts, A.J.R.; Philp, H.; Neil, D.M.; Monroig, O. Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba). Mar. Drugs 2016, 14, 219. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.S.; Hayes, M. Bioprocessing of mussel by-products for value added ingredients. Trends Food Sci. Technol. 2019, 92, 111–121. [Google Scholar] [CrossRef]
- Tokeshi, M.; Ota, N.; Kawai, T. A comparative study of morphometry in shell-bearing molluscs. J. Zool. 2000, 251, 31–38. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Uzcátegui, L.U.M.; Vergara, K.; Martínez Bordes, G. Sustainable alternatives for by-products derived from industrial mussel processing: A critical review. Waste Manag. Res. 2022, 40, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Méndez, L.; Rodríguez, A.; Aubourg, S.P.; Medina, I. Low-Toxicity Solvents for the Extraction of Valuable Lipid Compounds from Octopus (Octopus vulgaris) Waste. Foods 2023, 12, 3631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of Low-Toxicity Solvent Employment for Total Lipid and Tocopherol Compound Extraction from Patagonian Squid By-Products. Foods 2023, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-Y.; Ahn, D.-H.; Wilkinson, G.T.; Chun, B.-S. Extraction of lipids and cholesterol from squid oil with supercritical carbon dioxide. Korean J. Chem. Eng. 2005, 22, 399–405. [Google Scholar] [CrossRef]
- Fitahia, E.M.; Croyal, M.; Raheriniaina, C.E.; Ferchaud-Roucher, V.; Nazih, H. High-Resolution Mass Spectrometry Unravels a Broad Range of Bioactive Lipid Species in Octopus cyanea and Loligo sp. By-products from Southwestern Madagascar. Waste Biomass Valorization 2018, 9, 1787–1793. [Google Scholar] [CrossRef]
- Wang, C.-H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Reclamation of Fishery Processing Waste: A Mini-Review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef]
- Durazzo, A.; Di Lena, G.; Gabrielli, P.; Santini, A.; Lombardi-Boccia, G.; Lucarini, M. Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis. Fishes 2022, 7, 132. [Google Scholar] [CrossRef]
- Durmus, M. Fish oil for human health: Omega-3 fatty acid profiles of marine seafood species. Food Sci. Technol. 2019, 39, 1–12. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and Distribution of Omega-3 Fatty Acids in South Pacific Fish and Shellfish Species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Olgunoglu, İ. Review on Omega-3 (n-3) Fatty Acids in Fish and Seafood. J. Biol. Agric. Healthc. 2017, 7, 37–45. [Google Scholar]
- Irkin, L.C. The Effects of Shellfish Consumption Frequency for Human Health. In Update on Malacology; Sajal, R., Soumalya, M., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 1. [Google Scholar]
- Carboni, S.; Kaur, G.; Pryce, A.; McKee, K.; Desbois, A.P.; Dick, J.R.; Galloway, S.D.R.; Hamilton, D.L. Mussel Consumption as a “Food First” Approach to Improve Omega-3 Status. Nutrients 2019, 11, 1381. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef]
- Dempsey, M.; Rockwell, M.S.; Wentz, L.M. The influence of dietary and supplemental omega-3 fatty acids on the omega-3 index: A scoping review. Front. Nutr. 2023, 10, 1072653. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.; Le, T.T.T.; Pham, M.Q.; Do, T.L.; Vu, M.H.; Nguyen, D.C.; Bach, L.G.; Bui, L.M.; Pham, Q.L. Fatty Acid, Lipid Classes and Phospholipid Molecular Species Composition of the Marine Clam Meretrix lyrata (Sowerby 1851) from Cua Lo Beach, Nghe An Province, Vietnam. Molecules 2019, 24, 895. [Google Scholar] [CrossRef]
- Kizmaz, V. Analysis of lipid classes and the fatty acid composition of fresh and the salted fish, Alburnus tarichi. Cogent Food Agric. 2022, 8, 2126052. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Makhutova, O.N.; Rudchenko, A.E.; Glushchenko, L.A.; Shulepina, S.P.; Kolmakova, A.A.; Gladyshev, M.I. Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes. Biomolecules 2020, 10, 419. [Google Scholar] [CrossRef]
- Biandolino, F.; Prato, E. A preliminary investigation of the lipids and fatty acids composition of Gammarus aequicauda (Crustacea: Amphipoda) and its main food source. J. Mar. Biol. Assoc. UK 2006, 86, 345–348. [Google Scholar] [CrossRef]
- Laudicella, V.A.; Beveridge, C.; Carboni, S.; Franco, S.C.; Doherty, M.K.; Long, N.; Mitchell, E.; Stanley, M.S.; Whitfield, P.D.; Hughes, A.D. Lipidomics analysis of juveniles’ blue mussels (Mytilus edulis L. 1758), a key economic and ecological species. PLoS ONE 2020, 15, e0223031. [Google Scholar]
- Medina, I.; Aubourg, S.P.; Martín, R.P. Composition of phospholipids of white muscle of six tuna species. Lipids 1995, 30, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Domínguez, R.; Wang, M.; Barba, F.J.; Bermúdez, R.; Lorenzo, J.M. Nutritional Profiling and the Value of Processing By-Products from Gilthead Sea Bream (Sparus aurata). Mar. Drugs 2020, 18, 101. [Google Scholar] [CrossRef]
- Kundam, D.; Acham, I.O.; Girgih, A. Bioactive Compounds in Fish and Their Health Benefits. Asian Food Sci. J. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Malcorps, W.; Newton, R.W.; Sprague, M.; Glencross, B.D.; Little, D.C. Nutritional Characterisation of European Aquaculture Processing By-Products to Facilitate Strategic Utilisation. Front. Sustain. Food Syst. 2021, 5, 720595. [Google Scholar] [CrossRef]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9, 190. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; La Barbera, L.; Santulli, A. By-products of farmed European sea bass (Dicentrarchus labrax L.) as a potential source of n-3 PUFA. Biologia 2013, 68, 288–293. [Google Scholar] [CrossRef][Green Version]
- Messina, C.M.; Arena, R.; Manuguerra, S.; La Barbera, L.; Curcuraci, E.; Renda, G.; Santulli, A. Valorization of Side Stream Products from Sea Cage Fattened Bluefin Tuna (Thunnus thynnus): Production and In Vitro Bioactivity Evaluation of Enriched ω-3 Polyunsaturated Fatty Acids. Mar. Drugs 2022, 20, 309. [Google Scholar] [CrossRef] [PubMed]
- Kacem, M.; Sellami, M.; Kammoun, W.; Frikha, F.; Miled, N.; Ben Rebah, F. Seasonal Variations in Proximate and Fatty Acid Composition of Viscera of Sardinella aurita, Sarpa salpa, and Sepia officinalis from Tunisia. J. Aquat. Food Prod. Technol. 2011, 20, 233–246. [Google Scholar] [CrossRef]
- Ovissipour, R.; Abedian Kenari, A.; Motamedzadegan, A.; Nazari, R. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna (Thunnus albacares). Food Bioprocess Technol. 2010, 5, 696–705. [Google Scholar] [CrossRef]
- Aidos, I.; Lourenclo, S.; van der Padt, A.; Luten, J.B.; Boom, R. Stability of Crude Herring Oil Produced from Fresh Byproducts: Influence of Temperature during Storage. J. Food Sci. 2002, 67, 3314–3320. [Google Scholar] [CrossRef]
- Khoddami, A. Quality and fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Euthynnus affinis). Afr. J. Biotechnol. 2012, 11, 1683–1689. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Mohd Ghazali, H. Fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Sardinella lemuru). World Appl. Sci. J. 2009, 7, 127–131. [Google Scholar]
- Shahidi, F.; Naczk, M.; Pegg, R.B.; Synowiecki, J. Chemical composition and nutritional value of processing discards of cod (Gadus morhua). Food Chem. 1991, 42, 145–151. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Carne, A.; Ahmmed, F.; Stewart, I.; Sabrina Tian, H.; Bekhit, A.E.A. Positional distribution of fatty acids and phospholipid composition in King salmon (Oncorhynchus tshawytscha) head, roe and skin using nuclear magnetic resonance spectroscopy. Food Chem. 2021, 363, 130302. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Ahmmed, F.; Stewart, I.; Carne, A.; Tian, H.S.; Bekhit, A.E.A. Omega-3 phospholipids in Pacific blue mackerel (Scomber australasicus) processing by-products. Food Chem. 2021, 353, 129451. [Google Scholar] [CrossRef] [PubMed]
- Guiry, E.J.; Szpak, P.; Richards, M.P. Effects of lipid extraction and ultrafiltration on stable carbon and nitrogen isotopic compositions of fish bone collagen. Rapid Commun. Mass Spectrom. 2016, 30, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Rosidi, W.N.T.M.; Arshad, N.M.; Mohtar, N.F. Characterization of Sardinella fimbriata and Clarias gariepinus bones. Biodivers. J. Biol. Divers. 2021, 22, 1621–1626. [Google Scholar]
- During, A.; Penel, G.; Hardouin, P. Understanding the local actions of lipids in bone physiology. Prog. Lipid Res. 2015, 59, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Mularchuk, P.; Boskey, A. Lipids in bone: Optimal conditions for tissue storage prior to lipid analyses. Calcif. Tissue Int. 1990, 46, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Aitta, E.; Marsol-Vall, A.; Damerau, A.; Yang, B. Enzyme-Assisted Extraction of Fish Oil from Whole Fish and by-Products of Baltic Herring (Clupea harengus membras). Foods 2021, 10, 1811. [Google Scholar] [CrossRef]
- Lee, S.; Koo, M.H.; Han, D.W.; Kim, I.C.; Lee, J.H.; Kim, J.H.; Sultana, R.; Kim, S.Y.; Youn, U.J.; Kim, J.H. Comparison of Fatty Acid Contents and MMP-1 Inhibitory Effects of the Two Antarctic Fish, Notothenia rossii and Champsocephalus gunnari. Molecules 2022, 27, 4554. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Jiang, W.; Yan, X. Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus pelamis) Heads. Molecules 2019, 24, 3189. [Google Scholar] [CrossRef]
- Hu, Z.; Chin, Y.; Liu, J.; Zhou, J.; Li, G.; Hu, L.; Hu, Y. Optimization of fish oil extraction from Lophius litulon liver and fatty acid composition analysis. Fish. Aquat. Sci. 2022, 25, 76–89. [Google Scholar] [CrossRef]
- Gbogouri, G.; Linder, M.; Fanni, J.; Parmentier, M. Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes. Eur. J. Lipid Sci. Technol. 2006, 108, 766–775. [Google Scholar] [CrossRef]
- Abiona, O.O.; Awojide, S.H.; Anifowose, A.J.; Adegunwa, A.O.; Agbaje, W.B.; Tayo, A.S. Quality characteristics of extracted oil from the head and gills of Catfish and Titus fish. Bull. Natl. Res. Cent. 2021, 45, 101. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Zhao, X.; Lu, H.; Zhang, P.; Dong, X.; Wang, Y. Comparison of the Effect of Phospholipid Extracts from Salmon and Silver Carp Heads on High-Fat-Diet-Induced Metabolic Syndrome in C57BL/6J Mice. Mar. Drugs 2023, 21, 409. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Meireles, M.Â.A.; Lopes, B.L.F.; Cabral, F.A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Limam, Z.; Arafa, S.; Sadok, S.; El Abed, A. Lipids and fatty acids composition in the tissues and by-products of two Tunisian shrimp species from the north and south regions. Nutr. Health 2008, 19, 215–220. [Google Scholar] [CrossRef]
- Toyes-Vargas, E.; Robles-Romo, A.; Méndez, L.; Palacios, E.; Civera, R. Changes in fatty acids, sterols, pigments, lipid classes, and heavy metals of cooked or dried meals, compared to fresh marine by-products. Anim. Feed Sci. Technol. 2016, 221, 195–205. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; Santoso, J.; Trilaksani, W.; Nurilmala, M. Extraction and Stability of Carotenoid-Containing Lipids from Hepatopancreas of Pacific White Shrimp (Litopenaeus vannamei). J. Food Process. Preserv. 2015, 39, 10–18. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S. Compositions and yield of lipids extracted from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei) as affected by prior autolysis. Food Chem. 2012, 134, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.S.; Phuong, P.T.D. Bioactive Compounds from By-Products of Shrimp Processing Industry in Vietnam. J. Food Drug Anal. 2012, 20, 194–197. [Google Scholar] [CrossRef]
- O’Leary, C.D.; Matthews, A.D. Lipid class distribution and fatty acid composition of wild and farmed prawn, Penaeus monodon (Fabricius). Aquaculture 1990, 89, 65–81. [Google Scholar] [CrossRef]
- Miniadis-Meimaroglou, S.; Kora, L.; Sinanoglou, V.J. Isolation and identification of phospholipid molecular species in α wild marine shrimp Penaeus kerathurus muscle and cephalothorax. Chem. Phys. Lipids 2008, 152, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Rameshkumar, G.; Prince, A.R. Biochemical composition of shell and flesh of the Indian white shrimp Penaeus indicus (H. milne Edwards 1837). Am.-Eurasian J. Sci. Res. 2009, 4, 191–194. [Google Scholar]
- Tsvetnenko, E.; Kailis, S.; Evans, L.; Longmore, R. Fatty acid composition of lipids from the contents of rock lobster (Panulirus cygnus) cephalothorax. J. Am. Oil Chem. Soc. 1996, 73, 259–261. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant Enrichment of Polyunsaturated Fatty Acids (PUFAs) in the Lipids Extracted by Supercritical CO2 from the Livers of Australian Rock Lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-É. Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: A source of high-valued biomolecules. Bioresour. Technol. 2009, 100, 3332–3342. [Google Scholar] [CrossRef]
- Lv, S.; Xie, S.; Liang, Y.; Xu, L.; Hu, L.; Li, H.; Mo, H. Comprehensive lipidomic analysis of the lipids extracted from freshwater fish bones and crustacean shells. Food Sci. Nutr. 2022, 10, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Muriana, F.J.; Ruiz-Gutierrez, V.; Gallardo-Guerrero, M.L.; Mínguez-Mosquera, M.I. A study of the lipids and carotenoprotein in the prawn, Penaeus japonicus. J. Biochem. 1993, 114, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Song, G.; Wang, H.; Zhang, Y.; Cui, Y.; Xie, H.; Xue, J.; Wang, H. Isolation and lipidomics characterization of fatty acids and phospholipids in shrimp waste through GC/FID and HILIC-QTrap/MS. J. Food Compos. Anal. 2021, 95, 103668. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kijroongrojana, K.; Prodpran, T.; Agustini, T.W. Yield and chemical composition of lipids extracted from solid residues of protein hydrolysis of Pacific white shrimp cephalothorax using ultrasound-assisted extraction. Food Biosci. 2018, 26, 169–176. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted from Cephalothorax of Pacific White Shrimp (Litopenaeus vannamei). Eur. J. Lipid Sci. Technol. 2018, 120, 1700495. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Wang, F.; Zhang, S.; Li, H.; Zhang, Y.; Wang, X.; Liu, K.; Li, X. Separation, identification and cardiovascular activities of phospholipid classes from the head of Penaeus vannamei by lipidomics and zebrafish models. Food Funct. 2021, 12, 2282–2291. [Google Scholar] [CrossRef]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chem. 2007, 103, 1199–1207. [Google Scholar] [CrossRef]
- Guary, J.-C. Lipid class distribution and fatty acid composition of prawn, Penaus japonicus bate. Bull. Jpn. Soc. Sci. Fish. 1974, 40, 1027–1032. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N.M. Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chem. 2012, 134, 308–314. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Siddegowda, G.S.; Sathisha, A.D.; Suresh, P.V. Recovery of carotenoids from ensilaged shrimp waste. Bioresour. Technol. 2007, 98, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, W.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.; Wang, J.; Li, H. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus vannamei Boone): Effect on Yield and Antioxidant Activity. J. Food Process Eng. 2017, 40, e12353. [Google Scholar] [CrossRef]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Carotenoids in crabs from marine and fresh waters of India. LWT-Food Sci. Technol. 2005, 38, 221–225. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.; Martinez-Correa, H.; Paviani, L.; Cabral, F. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag. 2006, 26, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Holanda, H.; Netto, F. Recovery of Components from Shrimp (Xiphopenaeus kroyeri) Processing Waste by Enzymatic Hydrolysis. J. Food Sci. 2006, 71, C298–C303. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.; Mata, S.; Acosta, J.; Cabrera, B.; López-Valdez, L.; Reyes, C.; Barrales-Cureño, H. Obtaining of Astaxanthin from Crab Exosqueletons and Shrimp Head Shells. Biointerface Res. Appl. Chem. 2021, 11, 13516–13523. [Google Scholar]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Ríos-Vázquez, N.J. High-performance liquid chromatography method for the simultaneous quantification of retinol, α-tocopherol, and cholesterol in shrimp waste hydrolysate. J. Chromatogr. A 2006, 1105, 135–139. [Google Scholar] [CrossRef]
- Subra-Paternault, P.; ThongDeng, H.; Grélard, A.; Cansell, M. Extraction of phospholipids from scallop by-product using supercritical CO2/alcohol mixtures. LWT-Food Sci. Technol. 2015, 60, 990–998. [Google Scholar] [CrossRef]
- Savoire, R.; Subra-Paternault, P.; Bardeau, T.; Morvan, E.; Grélard, A.; Cansell, M. Selective extraction of phospholipids from food by-products by supercritical carbon dioxide and ethanol and formulating ability of extracts. Sep. Purif. Technol. 2020, 238, 116394. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Trigo, M.; Prego, R.; Cobelo-García, A.; Medina, I. Nutritional and Healthy Value of Chemical Constituents Obtained from Patagonian Squid (Doryteuthis gahi) By-Products Captured at Different Seasons. Foods 2021, 10, 2144. [Google Scholar] [CrossRef]
- Rodríguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of Healthy-Lipid Content and Oxidative Stability during Oil Extraction from Squid (Illex argentinus) Viscera by Green Processing. Mar. Drugs 2021, 19, 616. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Trigo, M.; González, M.J.; Lois, S.; Medina, I. Comparative Study of Bioactive Lipid Extraction from Squid (Doryteuthis gahi) by-Products by Green Solvents. Foods 2022, 11, 2188. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Rodríguez, A.; Trigo, M.; Medina, I. Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction. Foods 2023, 12, 2649. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kishimura, H.; Chun, B.S. Isolation and characterization of lecithin from squid (Todarodes pacificus) viscera deoiled by supercritical carbon dioxide extraction. J. Food Sci. 2011, 76, C350–C354. [Google Scholar] [CrossRef]
- Hayashi, K. Composition and distribution of lipids in different tissues of the arrow squid Loligo bleekeri. Fish. Sci. 1996, 62, 84–87. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishimura, H. Amount and Composition of Diacyl Glyceryl Ethers in Various Tissue Lipids of the Deep-sea Squid Berryteuthis magister. J. Oleo Sci. 2002, 51, 523–529. [Google Scholar] [CrossRef][Green Version]
- Saito, H.; Sakai, M.; Wakabayashi, T. Characteristics of the lipid and fatty acid compositions of the Humboldt squid, Dosidicus gigas: The trophic relationship between the squid and its prey. Eur. J. Lipid Sci. Technol. 2014, 116, 360–366. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2022, 119, 593–607. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Gimenez, M.S.; Oliveros, L.B.; Gomez, N.N. Nutritional deficiencies and phospholipid metabolism. Int. J. Mol. Sci. 2011, 12, 2408–2433. [Google Scholar] [CrossRef]
- Jeppesen, P.; Christensen, M.; Høy, C.; Brøbech, P. Essential fatty acid deficiency in patients with severe fat malabsoption. Am. J. Clin. Nutr. 1997, 65, 837–843. [Google Scholar] [CrossRef]
- Kuller, L.H. Dietary fat and chronic diseases: Epidemiologic overview. J. Am. Diet. Assoc. 1997, 97, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.S.; Liu, G.; Arasteh, A.; Yin, X.M.; Yan, S. Ability of high fat diet to induce liver pathology correlates with the level of linoleic acid and Vitamin E in the diet. PLoS ONE 2023, 18, e0286726. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tang, Q.; Xu, Y.; Wu, J.; Mao, X.; Li, F.; Wang, S.; Wang, Y. Biotechnology in Future Food Lipids: Opportunities and Challenges. Annu. Rev. Food Sci. Technol. 2023, 14, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Azam, K.; Basher, M.; Asaduzzaman, M.; Hossain, M.; Ali, M. Biochemical assessment of fourteen selected dried fish. Univ. J. Zool. Rajshahi Univ. 2003, 22, 23–26. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K. Changes in lipids of shrimp (Acetes vulgaris) during salting and fermentation. Eur. J. Lipid Sci. Technol. 2017, 119, 1700253. [Google Scholar] [CrossRef]
- Kommuri, P.K.; Mugada, N.; Kondamudi, R.B. Proximate Analysis and Mineral Composition of Commercially Important Spiny Lobsters from Visakhapatnam Coast, Andhra Pradesh, India. Asian J. Fish. Aquat. Res. 2021, 14, 39–47. [Google Scholar] [CrossRef]
- Rosa, R.; Pereira, J.; Nunes, M. Biochemical composition of cephalopods with different life strategies, with special reference to a giant squid, Architeuthis sp. Mar. Biol. 2005, 146, 739–751. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Takahashi, K.; Inoue, Y. Marine by-product phospholipids as booster of medicinal compounds. Adv. Food Nutr. Res. 2012, 65, 31–46. [Google Scholar]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef]
- Nollet, L.M.; Toldrá, F. Handbook of Seafood and Seafood Products Analysis; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- von Schacky, C. Omega-3 index in 2018/19. Proc. Nutr. Soc. 2020, 79, 381–387. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83 (Suppl. 6), 1467s–1476s. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Janneke Dijck-Brouwer, D.A.; Eaton, S.B.; Crawford, M.A.; Cordain, L.; Muskiet, F.A.J. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br. J. Nutr. 2010, 104, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 2009, 139, 804s–819s. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Lenighan, Y.M.; McNulty, B.A.; Roche, H.M. Dietary fat composition: Replacement of saturated fatty acids with PUFA as a public health strategy, with an emphasis on α-linolenic acid. Proc. Nutr. Soc. 2019, 78, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Serini, S.; Chen, Y.Q.; Su, H.-M.; Lim, K.; Cittadini, A.; Calviello, G. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. BioMed Res. Int. 2015, 2015, 143109. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Bae, J.H.; Lim, H.; Lim, S. The Potential Cardiometabolic Effects of Long-Chain ω-3 Polyunsaturated Fatty Acids: Recent Updates and Controversies. Adv. Nutr. 2023, 14, 612–628. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xiong, Q.; Yin, Y.; Ling, Z.; Chen, S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Front. Cardiovasc. Med. 2022, 8, 802306. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Varzideh, F.; Kansakar, U.; Infante, C.; Lombardi, A.; de Donato, A.; Frullone, S.; Santulli, G. Omega-3 fatty acids coordinate glucose and lipid metabolism in diabetic patients. Lipids Health Dis. 2022, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 3, Cd003177. [Google Scholar]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-H.; Han, S.H.; Kim, S.-H.; Eckel, R.H.; Koh, K.K. Cardiovascular effects of omega-3 fatty acids: Hope or hype? Atherosclerosis 2021, 322, 15–23. [Google Scholar] [CrossRef]
- Toth, P.P.; Chapman, M.J.; Parhofer, K.G.; Nelson, J.R. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 17, 100148. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Mukhametov, A.; Yerbulekova, M.; Aitkhozhayeva, G.; Tuyakova, G.; Dautkanova, D. Effects of ω-3 fatty acids and ratio of ω-3/ω-6 for health promotion and disease prevention. Food Sci. Technol. 2022, 42, e58321. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in Fish: Extraction, Fractionation, Importance in Health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Xu, J.; Wang, Y.-M.; Xue, C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Li, L.; Song, G.; Feng, J.; Li, S.; Wang, Y.; Ma, J.; Wang, H. Development of an intelligent surgical knife rapid evaporative ionization mass spectrometry based method for real-time differentiation of cod from oilfish. J. Food Compos. Anal. 2020, 86, 103355. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef]
- Ferreira, I.; Rauter, A.P.; Bandarra, N.M. Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect. Mar. Drugs 2022, 20, 662. [Google Scholar] [CrossRef]
- Sehl, A.; Couëdelo, L.; Chamekh-Coelho, I.; Vaysse, C.; Cansell, M. In vitro lipolysis and lymphatic absorption of n-3 long-chain PUFA in the rat: Influence of the molecular lipid species as carrier. Br. J. Nutr. 2019, 122, 639–647. [Google Scholar] [CrossRef]
- Cansell, M. Marine phospholipids as dietary carriers of long-chain polyunsaturated fatty acids. Lipid Technol. 2010, 22, 223–226. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.S.; Carne, A.; Bekhit, A.E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Grey, A.; Bolland, M. Clinical Trial Evidence and Use of Fish Oil Supplements. JAMA Intern. Med. 2014, 174, 460–462. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Müller, M.; Rainger, G.E.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, R.J.; Torrejon, C. The omega-3 fatty acid nutritional landscape: Health benefits and sources. J. Nutr. 2012, 142, 587s–591s. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526s–1535s. [Google Scholar] [CrossRef] [PubMed]
- Gogus, U.; Smith, C. n-3 Omega fatty acids: A review of current knowledge. Int. J. Food Sci. Technol. 2010, 45, 417–436. [Google Scholar] [CrossRef]
- Nigam, D.; Yadav, R.; Tiwari, U. Omega-3 Fatty Acids and Its Role in Human Health. In Functional Food and Human Health; Rani, V., Yadav, U.C.S., Eds.; Springer: Singapore, 2018; pp. 173–198. [Google Scholar]
- Hama, S.; Ogino, C.; Kondo, A. Enzymatic synthesis and modification of structured phospholipids: Recent advances in enzyme preparation and biocatalytic processes. Appl. Microbiol. Biotechnol. 2015, 99, 7879–7891. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Vítová, M.; Palyzová, A.; Řezanka, T. Plasmalogens–Ubiquitous molecules occurring widely, from anaerobic bacteria to humans. Prog. Lipid Res. 2021, 83, 101111. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Biological Functions of Plasmalogens. In Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1299, pp. 171–193. [Google Scholar]
- Goodenowe, D.B.; Haroon, J.; Kling, M.A.; Zielinski, M.; Mahdavi, K.; Habelhah, B.; Shtilkind, L.; Jordan, S. Targeted Plasmalogen Supplementation: Effects on Blood Plasmalogens, Oxidative Stress Biomarkers, Cognition, and Mobility in Cognitively Impaired Persons. Front. Cell Dev. Biol. 2022, 10, 864842. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Miyazawa, T.; Higuchi, O.; Kinoshita, M.; Miyazawa, T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules 2023, 28, 6328. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joy, M. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: An overview. Food Res. Int. 2020, 137, 109637. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.; Chakraborty, K.; Raola, V.K. New sterols with anti-inflammatory potentials against cyclooxygenase-2 and 5-lipoxygenase from Paphia malabarica. Nat. Prod. Res. 2017, 31, 1286–1298. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Mekinić, I.G. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Elbandy, M. Anti-Inflammatory Effects of Marine Bioactive Compounds and Their Potential as Functional Food Ingredients in the Prevention and Treatment of Neuroinflammatory Disorders. Molecules 2022, 28, 2. [Google Scholar] [CrossRef]
- Tilami, S.K.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Samia Ben, M.-G.; Dhouha, S.-N. Vitamin E: Natural Antioxidant in the Mediterranean Diet. In Vitamin E in Health and Disease; Pınar, E., Júlia Scherer, S., Eds.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–15. [Google Scholar]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Gamna, F.; Spriano, S. Vitamin E: A Review of Its Application and Methods of Detection When Combined with Implant Biomaterials. Materials 2021, 14, 3691. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, P.; Zhu, Y.; He, J.; Zhang, M.; Liu, K.; Lin, H.; Zhai, H.; Li, X.; Ma, Y. Comparative bioactivity profile of phospholipids from three marine byproducts based on the zebrafish model. J. Food Biochem. 2022, 46, e14229. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Zhao, H.; Li, J.; You, H.; Jiang, L.; Hu, J. Activation of Macrophages in vitro by Phospholipids from Brain of Katsuwonus pelamis (Skipjack Tuna). J. Oleo Sci. 2018, 67, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, G.; Song, C.; Gu, S.; Brown, R.E.; Zhang, J.; Zhang, P.; Gagnon, J.; Locke, S.; Stefanova, R.; et al. An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ(25–35). Mar. Drugs 2017, 15, 83. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, P.; López-Caballero, M.E.; Baccan, G.C.; Gómez-Estaca, J. Bioactive and technological functionality of a lipid extract from shrimp (L. vannamei) cephalothorax. LWT 2018, 89, 704–711. [Google Scholar] [CrossRef]
- López-Saiz, C.; Coronado-Aceves, E.; Tavera-Hernández, R.; Espitia-Pinzón, C.; Jiménez-Estrada, M.; Morán-Corrales, P.; Hernández-Zazueta, M. Antibacterial and antimycobacterial activity of white shrimp (Litopenaeus vannamei) exoskeleton and cephalothorax by-products extracts: Fatty acids profile of the active hexanic shrimp cephalothorax extract. Biotecnia 2022, 24, 45–52. [Google Scholar] [CrossRef]
- Fitahia, E.M.; Raheriniaina, C.E.; Bazin, M.A.; Huvelin, J.-M.; Logé, C.; Ranaivoson, E.; Nazih, H. Anti-proliferative and Pro-apoptotic Effect of Dichloromethane Extract of Octopus vulgaris By-Products on Human Breast Cancer Cell Lines. Waste Biomass Valorization 2015, 6, 237–242. [Google Scholar] [CrossRef]
- Toda, Y.; Sato, T.; Hosomi, R.; Harumatsu, S.; Fukuda, S.; Matsuda, Y.; Yoshida, M.; Fukunaga, K. Lipid Components Prepared from an Oyster-extract By-product Decreases Triacylglycerol Contents by Suppressing Acetyl-CoA Carboxylase Activity and Lowering the Stearoyl-CoA Desaturase Index in Rat Livers. Trace Nutr. Res. 2015, 32, 20–26. [Google Scholar]
- Tran, N.K.; Kwon, J.E.; Kang, S.C.; Shim, S.M.; Park, T.S. Crassaostrea gigas oyster shell extract inhibits lipogenesis via suppression of serine palmitoyltransferase. Nat. Prod. Commun. 2015, 10, 349–352. [Google Scholar] [CrossRef]
- Hegde, M.V.; Zanwar, A.A.; Adekar, S.P. Nutrition, Life, Disease, and Death. In Omega-3 Fatty Acids: Keys to Nutritional Health; Hegde, M.V., Zanwar, A.A., Adekar, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–10. [Google Scholar]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Mohebi-Nejad, A.; Bikdeli, B. Omega-3 supplements and cardiovascular diseases. Tanaffos 2014, 13, 6–14. [Google Scholar] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. 2014, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Domínguez, R.; Varzakas, T.; Munekata, P.E.S.; Movilla Fierro, E.; Lorenzo, J.M. Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food. Mar. Drugs 2021, 19, 233. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Nielsen, N.S.; Jacobsen, C. Oxidative stability of fish oil-enriched mayonnaise-based salads. Eur. J. Lipid Sci. Technol. 2010, 112, 476–487. [Google Scholar] [CrossRef]
- Let, M.B.; Jacobsen, C.; Meyer, A.S. Lipid Oxidation in Milk, Yoghurt, and Salad Dressing Enriched with Neat Fish Oil or Pre-Emulsified Fish Oil. J. Agric. Food Chem. 2007, 55, 7802–7809. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocoll. 2016, 61, 155–162. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Srigley, C.; Rader, J.I.T. Content and Composition of Fatty Acids in Marine Oil Omega-3 Supplements. J. Agric. Food Chem. 2014, 62, 7268–7278. [Google Scholar] [CrossRef]
- Erkan, O.N.; Tunçelli, İ.C.; Özden, Ö. Content and economic evaluation of omega-3 fatty acid nutritional supplements. J. Food Nutr. Res. 2023, 62, 14–25. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Bimbo, A.P. Raw material sources for the long-chain omega-3 market: Trends and sustainability. Part 2. In Proceedings of the 99th AOCS Annual Meeting & Expo, Seattle, WA, USA, 19 May 2008. [Google Scholar]
- Rasti, B.; Erfanian, A.; Selamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef]
- Yahoo!Finance. Global Lipid Nutrition Market Report to 2031–By Product, Source, Form, Application, Distribution and Region. Available online: https://finance.yahoo.com/news/global-lipid-nutrition-market-report-102800783.html (accessed on 10 January 2024).
- Laganà, P.; Avventuroso, E.; Romano, G.; Gioffré, M.E.; Patanè, P.; Parisi, S.; Moscato, U.; Delia, S.; Laganà, P.; Avventuroso, E. The Codex Alimentarius and the European legislation on food additives. In Chemistry and Hygiene of Food Additives; Springer: Cham, Switzerland, 2017; pp. 23–32. [Google Scholar]
- Bandarra, N.M.; Campos, R.M.; Batista, I.; Nunes, M.L.; Empis, J.M. Antioxidant synergy of α-tocopherol and phospholipids. J. Am. Oil Chem. Soc. 1999, 76, 905–913. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; León, M.M.; Zamora, R. Antioxidative Activity of Amino Phospholipids and Phospholipid/Amino Acid Mixtures in Edible Oils As Determined by the Rancimat Method. J. Agric. Food Chem. 2006, 54, 5461–5467. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Vaysse, C.; Michalski, M.C. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef]
- Haq, M.; Suraiya, S.; Ahmed, S.; Chun, B.-S. Phospholipids from marine source: Extractions and forthcoming industrial applications. J. Funct. Foods 2021, 80, 104448. [Google Scholar] [CrossRef]
- Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Taneja, N.; Hadia, J.J.; Khopade, A.J. Liposomes as Targeted Drug-Delivery Systems. In Targeted Drug Delivery; Wiley-VCH: Weinheim, Germany, 2022; pp. 69–125. [Google Scholar]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Gulzar, S.; Raju, N.; Nagarajarao, R.C.; Benjakul, S. Oil and pigments from shrimp processing by-products: Extraction, composition, bioactivities and its application—A review. Trends Food Sci. Technol. 2020, 100, 307–319. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammed, A.; Edinur, H.A.; Ghosh, S.; et al. Seafood Discards: A Potent Source of Enzymes and Biomacromolecules with Nutritional and Nutraceutical Significance. Front. Nutr. 2022, 9, 879929. [Google Scholar] [CrossRef]
- Zaffarin, A.S.M.; Ng, S.F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Tocopherols and Tocotrienols as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 61s–94s. [Google Scholar] [CrossRef] [PubMed]
- Espinales, C.; Romero-Peña, M.; Calderón, G.; Vergara, K.; Cáceres, P.J.; Castillo, P. Collagen, protein hydrolysates and chitin from by-products of fish and shellfish: An overview. Heliyon 2023, 9, e14937. [Google Scholar] [CrossRef] [PubMed]
- Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.H.; Kim, S.K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Morganti, A. Transforming nanostructured chitin from crustacean waste into beneficial health products: A must for our society. Nanotechnol. Sci. Appl. 2011, 4, 123–129. [Google Scholar] [CrossRef]
- Massironi, A.; Morelli, A.; Puppi, D.; Chiellini, F. Renewable Polysaccharides Micro/Nanostructures for Food and Cosmetic Applications. Molecules 2020, 25, 4886. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.d.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, K.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Martínez-Macias, M.d.R.; Correa-Murrieta, M.A.; Sanches-Silva, A. Hydroxyapatite recovery from fish byproducts for biomedical applications. Sustain. Chem. Pharm. 2022, 28, 100726. [Google Scholar] [CrossRef]
- Anil, S.; Sweety, V.K.; Joseph, B. Marine-Derived Hydroxyapatite for Tissue Engineering Strategies. In Handbook of the Extracellular Matrix: Biologically-Derived Materials; Maia, F.R.A., Oliveira, J.M., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–26. [Google Scholar]
- Bohnes, F.A.; Hauschild, M.Z.; Schlundt, J.; Laurent, A. Life cycle assessments of aquaculture systems: A critical review of reported findings with recommendations for policy and system development. Rev. Aquac. 2019, 11, 1061–1079. [Google Scholar] [CrossRef]
- Colombo, S.; Beheshti, M.; Parrish, C. Fats and Oils in Aquafeed Formulations. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1–28. [Google Scholar]
- Santigosa, E.; Olsen, R.E.; Madaro, A.; Søfteland, L.; Carr, I. The impact of varying EPA:DHA ratio on Atlantic salmon health and welfare. Aquaculture 2023, 576, 739868. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Nichols, P.D.; Carter, C.G. Sustainable alternatives to dietary fish oil in tropical fish aquaculture. Rev. Aquac. 2019, 11, 1195–1218. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Zou, Y.; Heyndrickx, M.; Debode, J.; Raes, K.; de Pascale, D.; Behan, P.; Giltrap, M.; O’Connor, C.; Solstad, R.G.; Lian, K.; et al. Valorisation of crustacean and bivalve processing side streams for industrial fast time-to-market products: A review from the European Union regulation perspective. Front. Mar. Sci. 2023, 10, 1068151. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of astaxanthin-dimethyldisuccinate (Carophyll® Stay-Pink 10%-CWS) for salmonids, crustaceans and other fish. EFSA Panel Addit. Prod. Subst. Used Anim. Feed 2019, 17, e05920. [Google Scholar]
- Dmytrów, I.; Szymczak, M.; Szkolnicka, K.; Kamiński, P. Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment. Foods 2021, 10, 895. [Google Scholar] [CrossRef]
- Racioppo, A.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Fish Loss/Waste and Low-Value Fish Challenges: State of Art, Advances, and Perspectives. Foods 2021, 10, 2725. [Google Scholar] [CrossRef] [PubMed]
- Guillen, J.; Holmes, S.J.; Carvalho, N.; Casey, J.; Dörner, H.; Gibin, M.; Mannini, A.; Vasilakopoulos, P.; Zanzi, A. A Review of the European Union Landing Obligation Focusing on Its Implications for Fisheries and the Environment. Sustainability 2018, 10, 900. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 10 January 2024).
- Kaanane, A.; Mkadem, H. Valorization Technologies of Marine By-Products. In Innovation in the Food Sector through the Valorization of Food and Agro-Food By-Products; de Barros, A.N., Gouvinhas, I., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–26. [Google Scholar]
- de la Caba, K.; Guerrero, P.; Trung, T.S.; Cruz-Romero, M.; Kerry, J.P.; Fluhr, J.; Maurer, M.; Kruijssen, F.; Albalat, A.; Bunting, S.; et al. From seafood waste to active seafood packaging: An emerging opportunity of the circular economy. J. Clean. Prod. 2019, 208, 86–98. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; van Bueren, E.T.L.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Rudovica, V.; Rotter, A.; Gaudêncio, S.P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L.K.; Alexandrino, D.A.M.; Anne, O.; Arbidans, L.; Atanassova, M.; et al. Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack Towards the Production of High Added-Value Products. Front. Mar. Sci. 2021, 8, 723333. [Google Scholar] [CrossRef]
- Cadavid-Rodríguez, L.S.; Vargas-Muñoz, M.A.; Plácido, J. Biomethane from fish waste as a source of renewable energy for artisanal fishing communities. Sustain. Energy Technol. Assess. 2019, 34, 110–115. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments--Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Paquincha, D.; Martins, F.; Queirós, R.P.; Saraiva, J.A.; Švarc-Gajić, J.; Nastić, N.; Delerue-Matos, C.; Carvalho, A.P. Liquid by-products from fish canning industry as sustainable sources of ω3 lipids. J. Environ. Manag. 2018, 219, 9–17. [Google Scholar] [CrossRef]
- Stoll, J.S.; Bailey, M.; Jonell, M. Alternative pathways to sustainable seafood. Conserv. Lett. 2020, 13, e12683. [Google Scholar] [CrossRef]
- Anderson, C.M.; Himes-Cornell, A.; Pita, C.; Arton, A.; Favret, M.; Averill, D.; Stohs, S.; Longo, C.S. Social and Economic Outcomes of Fisheries Certification: Characterizing Pathways of Change in Canned Fish Markets. Front. Mar. Sci. 2021, 8, 791085. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Granito, R.N.; Renno, A.C.M.; Yamamura, H.; de Almeida, M.C.; Menin Ruiz, P.L.; Ribeiro, D.A. Hydroxyapatite from Fish for Bone Tissue Engineering: A Promising Approach. Int. J. Mol. Cell. Med. 2018, 7, 80–90. [Google Scholar] [PubMed]
- Xing, L.; Wang, Z.; Hao, Y.; Zhang, W. Marine Products As a Promising Resource of Bioactive Peptides: Update of Extraction Strategies and Their Physiological Regulatory Effects. J. Agric. Food Chem. 2022, 70, 3081–3095. [Google Scholar] [CrossRef] [PubMed]
- Adeoti, I.A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Mayookha, V.P.; Bhushan, M. Chapter Ten–Lipid Extraction from Fish Processing Residues for Sustainable Biofuel Production. In Sustainable Fish Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 293–319. [Google Scholar]
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Hülsey, M.J. Shell biorefinery: A comprehensive introduction. Green Energy Environ. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Fiori, L.; Volpe, M.; Lucian, M.; Anesi, A.; Manfrini, M.; Guella, G. From Fish Waste to Omega-3 Concentrates in a Biorefinery Concept. Waste Biomass Valorization 2017, 8, 2609–2620. [Google Scholar] [CrossRef]
- European Commission. Circular Economy Action Plan–The EU’s New Circular Action Plan Paves the Way for a Cleaner and More Competitive Europe. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 10 January 2024).
- European Commission. Bioeconomy Strategy. Available online: https://knowledge4policy.ec.europa.eu/bioeconomy/bioeconomy-strategy_en (accessed on 10 January 2024).
- European Commission. EU Biorefinery Outlook to 2030. Available online: https://research-and-innovation.ec.europa.eu/news/all-research-and-innovation-news/eu-biorefinery-outlook-2030-2021-06-02_en (accessed on 10 January 2024).
- Gilman, E.; Roda, A.P.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P.A.H. Benchmarking global fisheries discards. Sci. Rep. 2020, 10, 14017. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Mahendrakar, N.S. Stability of carotenoids recovered from shrimp waste and their use as colorant in fish sausage. J. Food Sci. Technol. 2010, 47, 77–83. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.-Y.; Liu, Z.-Y.; Yin, F.-W.; Liu, Z.-Q.; Li, D.-Y.; Shahidi, F. Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chem. 2019, 272, 109–116. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.A.S.B.; Minozzo, M.G.; Licodiedoff, S.; Waszczynskyj, N. Physicochemical and sensory characterization of refined and deodorized tuna (Thunnus albacares) by-product oil obtained by enzymatic hydrolysis. Food Chem. 2016, 207, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Uemura, M.; Hosokawa, M. Effective Prevention of Oxidative Deterioration of Fish Oil: Focus on Flavor Deterioration. Annu. Rev. Food Sci. Technol. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Song, G.; Li, L.; Wang, H.; Zhang, M.; Yu, X.; Wang, J.; Shen, Q. Electric Soldering Iron Ionization Mass Spectrometry Based Lipidomics for in Situ Monitoring Fish Oil Oxidation Characteristics during Storage. J. Agric. Food Chem. 2020, 68, 2240–2248. [Google Scholar] [CrossRef]
- Wen, Y.-Q.; Xue, C.-H.; Zhang, H.-W.; Xu, L.-L.; Wang, X.-H.; Bi, S.-J.; Xue, Q.-Q.; Xue, Y.; Li, Z.-J.; Velasco, J.; et al. Concomitant oxidation of fatty acids other than DHA and EPA plays a role in the characteristic off-odor of fish oil. Food Chem. 2023, 404, 134724. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Martin-Martinez, F.J. Biorefineries: Achievements and challenges for a bio-based economy. Front. Chem. 2022, 10, 973417. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Biorefineries as a driver for sustainability: Key aspects, actual development and future prospects. J. Clean. Prod. 2023, 418, 137925. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. Directive 2009/32/EC of the European Parliament and of the Council. Off. J. Eur. Union 2009, 141, 3–11. [Google Scholar]
- de Jesus, S.S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-assisted supercritical fluid extraction: An integral approach to extract bioactive compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Marsol-Vall, A.; Aitta, E.; Guo, Z.; Yang, B. Green technologies for production of oils rich in n-3 polyunsaturated fatty acids from aquatic sources. Crit. Rev. Food Sci. Nutr. 2022, 62, 2942–2962. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Cho, Y.J.; Chun, B.-S. Quality Evaluation of Oil Recovered from By-products of Bigeye Tuna Using Supercritical Carbon Dioxide Extraction. Turk. J. Fish. Aquat. Sci. 2017, 14, 663–672. [Google Scholar]
- Sprick, B.; Linghu, Z.; Amamcharla, J.K.; Metzger, L.E.; Smith, J.S. Selective extraction of phospholipids from whey protein phospholipid concentrate using supercritical carbon dioxide and ethanol as a co-solvent. J. Dairy Sci. 2019, 102, 10855–10866. [Google Scholar] [CrossRef] [PubMed]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO: Rome, Italy, 1995. [Google Scholar]
- Yu, Y.; Hu, L.; Tian, D.; Yu, Y.; Lu, L.; Zhang, J.; Huang, X.; Yan, M.; Chen, L.; Wu, Z.; et al. Toxicities of polystyrene microplastics (MPs) and hexabromocyclododecane (HBCD), alone or in combination, to the hepatopancreas of the whiteleg shrimp, Litopenaeus vannamei. Environ. Pollut. 2023, 329, 121646. [Google Scholar] [CrossRef] [PubMed]
- Ariano, A.; Scivicco, M.; D’Ambola, M.; Velotto, S.; Andreini, R.; Bertini, S.; Zaccaroni, A.; Severino, L. Heavy Metals in the Muscle and Hepatopancreas of Red Swamp Crayfish (Procambarus clarkii) in Campania (Italy). Animals 2021, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Bodin, N.; Abarnou, A.; Le Guellec, A.M.; Loizeau, V.; Philippon, X. Organochlorinated contaminants in decapod crustaceans from the coasts of Brittany and Normandy (France). Chemosphere 2007, 67, S36–S47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.-J.; Mamun, M.; Atique, U.; An, K.-G. Fish Tissue Contamination with Organic Pollutants and Heavy Metals: Link between Land Use and Ecological Health. Water 2023, 15, 1845. [Google Scholar] [CrossRef]
- Rahman, S.A.; Abdullah, N.A.; Chowdhury, A.J.K.; Yunus, K. Fish Scales as a Bioindicator of Potential Marine Pollutants and Carcinogens in Asian Sea Bass and Red Tilapia within the Coastal Waters of Pahang, Malaysia. J. Coast. Res. 2018, 82, 120–125. [Google Scholar]
- Mendoza, L.C.; Nolos, R.C.; Villaflores, O.B.; Apostol, E.M.D.; Senoro, D.B. Detection of Heavy Metals, Their Distribution in Tilapia spp. and Health Risks Assessment. Toxics 2023, 11, 286. [Google Scholar] [CrossRef]
- Cordeli, A.N.; Oprea, L.; Crețu, M.; Dediu, L.; Coadă, M.T.; Mînzală, D.-N. Bioaccumulation of Metals in Some Fish Species from the Romanian Danube River: A Review. Fishes 2023, 8, 387. [Google Scholar] [CrossRef]
- Staniskiene, B.; Matusevicius, P.; Budreckiene, R.; Skibniewska, K.A. Distribution of Heavy Metals in Tissues of Freshwater Fish in Lithuania. Pol. J. Environ. Stud. 2006, 15, 585–591. [Google Scholar]
- Chen, L.; Cai, X.; Cao, M.; Liu, H.; Liang, Y.; Hu, L.; Yin, Y.; Li, Y.; Shi, J. Long-term investigation of heavy metal variations in mollusks along the Chinese Bohai Sea. Ecotoxicol. Environ. Saf. 2022, 236, 113443. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mou, H.; Lin, X.; Zhu, H.; Li, B.; Wang, J.; Junaid, M.; Wang, J. Microplastics in Mollusks: Research Progress, Current Contamination Status, Analysis Approaches, and Future Perspectives. Front. Mar. Sci. 2021, 8, 759919. [Google Scholar] [CrossRef]
- Wang, W.-X.; Lu, G. Heavy Metals in Bivalve Mollusks. In Chemical Contaminants and Residues in Food; Elsevier: Duxford, UK, 2017; pp. 553–594. [Google Scholar]
- Barchiesi, F.; Branciari, R.; Latini, M.; Roila, R.; Lediani, G.; Filippini, G.; Scortichini, G.; Piersanti, A.; Rocchegiani, E.; Ranucci, D. Heavy Metals Contamination in Shellfish: Benefit-Risk Evaluation in Central Italy. Foods 2020, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Colgan, T.J.; Thompson, R.C.; Carolan, J.C. Exposure to microplastics reduces attachment strength and alters the haemolymph proteome of blue mussels (Mytilus edulis). Environ. Pollut. 2019, 246, 423–434. [Google Scholar] [CrossRef]
- Liu, M.; Fan, S.; Rong, Z.; Qiu, H.; Yan, S.; Ni, H.; Dong, Z. Exposure to polychlorinated biphenyls (PCBs) affects the histology and antioxidant capability of the clam Cyclina sinensis. Front. Mar. Sci. 2023, 10, 1076870. [Google Scholar] [CrossRef]
- Pizzurro, F.; Nerone, E.; Ancora, M.; Di Domenico, M.; Mincarelli, L.F.; Cammà, C.; Salini, R.; Di Renzo, L.; Di Giacinto, F.; Corbau, C.; et al. Exposure of Mytilus galloprovincialis to Microplastics: Accumulation, Depuration and Evaluation of the Expression Levels of a Selection of Molecular Biomarkers. Animals 2024, 14, 4. [Google Scholar] [CrossRef]
- Mafra, L.L.; de Souza, D.A.; Menezes, M.; Schramm, M.A.; Hoff, R. Marine biotoxins: Latest advances and challenges toward seafood safety, using Brazil as a case study. Curr. Opin. Food Sci. 2023, 53, 101078. [Google Scholar] [CrossRef]
- Brett, M.M. Food poisoning associated with biotoxins in fish and shellfish. Curr. Opin. Infect. Dis. 2003, 16, 461–465. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E. Bivalve Molluscs as Vectors of Marine Biotoxins Involved in Seafood Poisoning. In Molluscs: From Chemo-Ecological Study to Biotechnological Application; Cimino, G., Gavagnin, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 53–82. [Google Scholar]
- Gerssen, A.; Klijnstra, M.D. The Determination of Marine Biotoxins in Seafood. In Analysis of Food Toxins and Toxicants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 319–362. [Google Scholar]
- Otero, P.; Silva, M. Emerging Marine Biotoxins in European Waters: Potential Risks and Analytical Challenges. Mar. Drugs 2022, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.J.; Horsberg, T.E. Kinetic properties of saxitoxin in Atlantic salmon (Salmo salar) and Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Wang, W.-X.; Lam, P.K.S.; Yu, P.K.N. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquat. Toxicol. 2006, 80, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Mazzillo, F.F.; Pomeroy, C.; Kuo, J.; Ramondi, P.T.; Prado, R.; Silver, M.W. Angler exposure to domoic acid via consumption of contaminated fishes. Aquat. Biol. 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Nakamura, M.; Oshima, Y.; Yasumoto, T. Occurrence of saxitoxin in puffer fish. Toxicon 1984, 22, 381–385. [Google Scholar] [CrossRef]
- Kershaw, J.L.; Jensen, S.-K.; McConnell, B.; Fraser, S.; Cummings, C.; Lacaze, J.-P.; Hermann, G.; Bresnan, E.; Dean, K.J.; Turner, A.D.; et al. Toxins from harmful algae in fish from Scottish coastal waters. Harmful Algae 2021, 105, 102068. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine Biotoxins: Occurrence, Toxicity, Regulatory Limits and Reference Methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef]
- Karp, W.A.; Breen, M.; Borges, L.; Fitzpatrick, M.; Kennelly, S.J.; Kolding, J.; Nielsen, K.N.; Viðarsson, J.R.; Cocas, L.; Leadbitter, D. Strategies Used Throughout the World to Manage Fisheries Discards–Lessons for Implementation of the EU Landing Obligation. In The European Landing Obligation: Reducing Discards in Complex, Multi-Species and Multi-Jurisdictional Fisheries; Uhlmann, S.S., Ulrich, C., Kennelly, S.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–26. [Google Scholar]
- European Parliament, Council of the European Union. Regulation (EC) No 999/2001 of the European Parliament and of the Council. Off. J. Eur. Union 2001, 147, 1–40. [Google Scholar]
- European Parliament, Council of the European Union. Commission Regulation (EU) No 142/2011. Off. J. Eur. Union 2011, 54, 1–254. [Google Scholar]
- Meijer, N.; Van Raamsdonk, L.W.D.; Gerrits, E.W.J.; Appel, M.J. The use of animal by-products in a circular bioeconomy: Time for a TSE road map 3? Heliyon 2023, 9, e14021. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. A New Circular Economy Action Plan; For a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020.
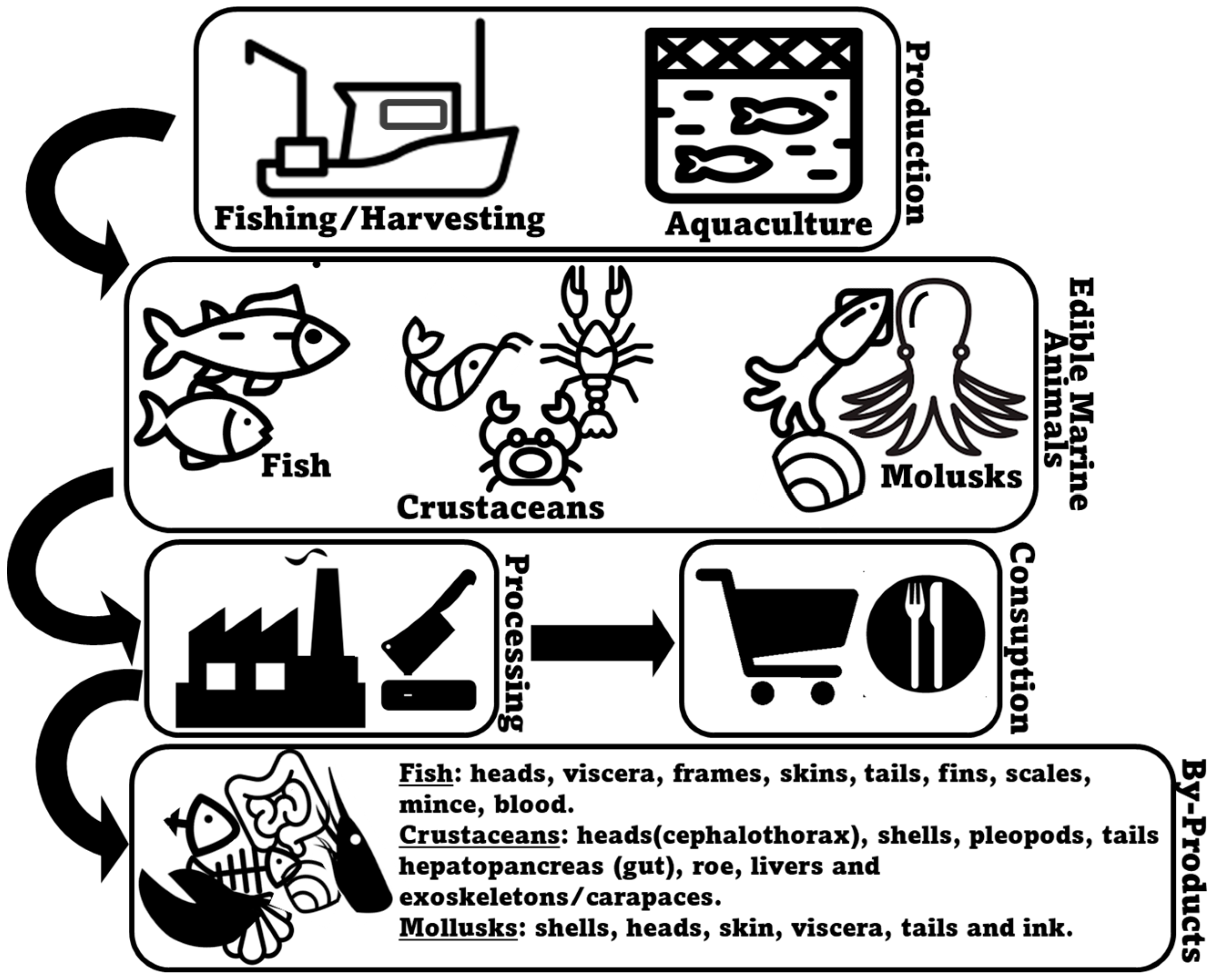
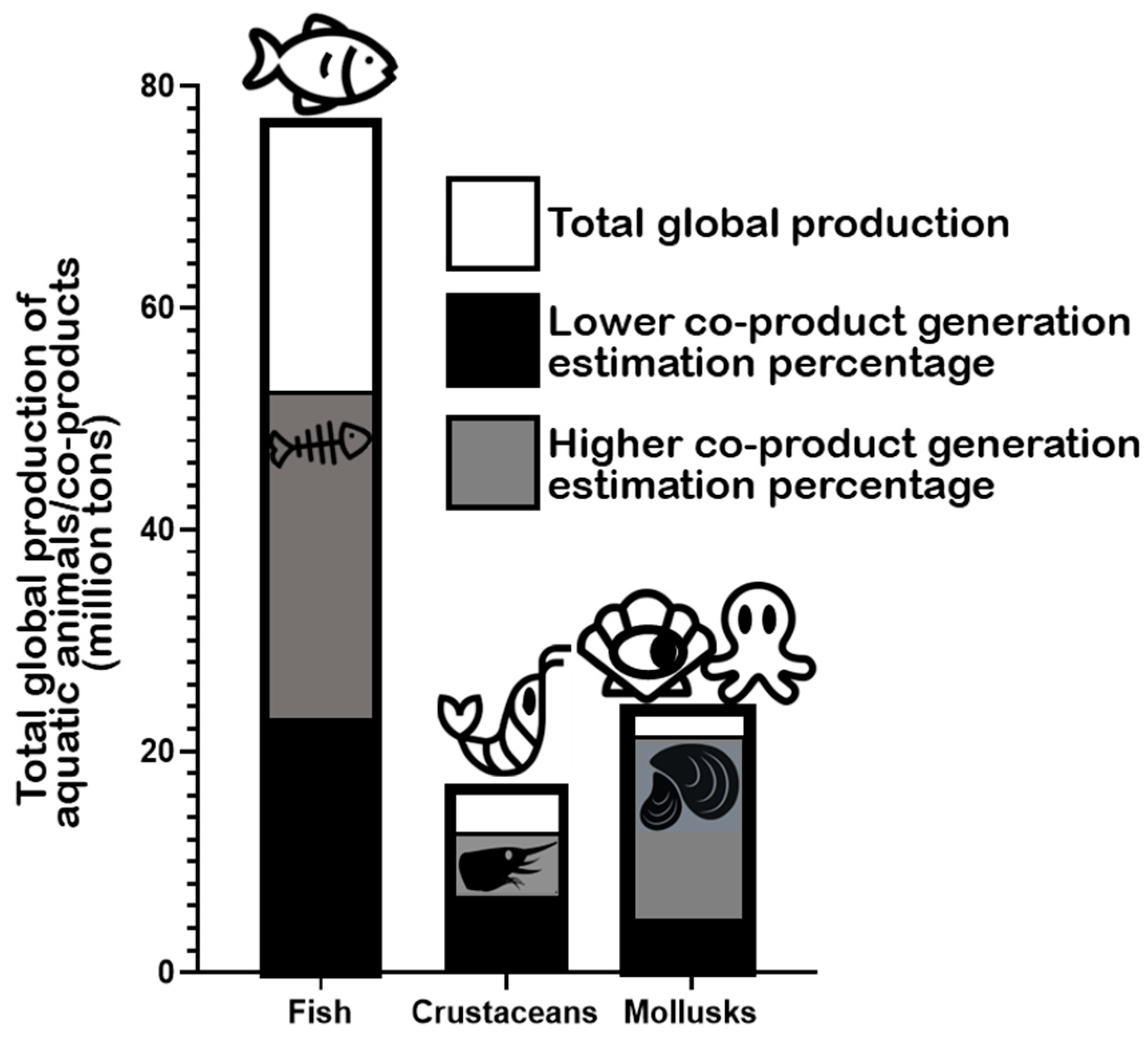
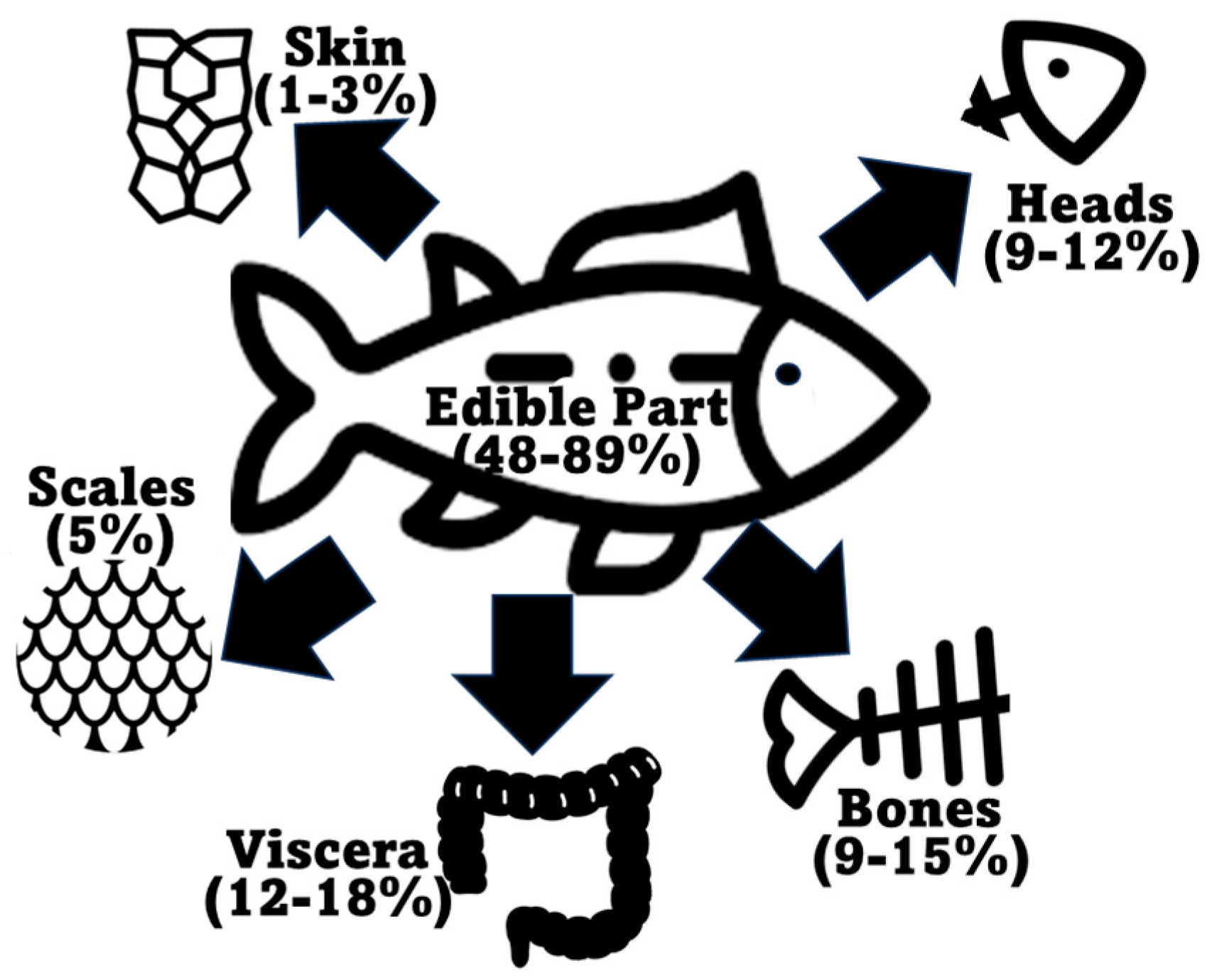

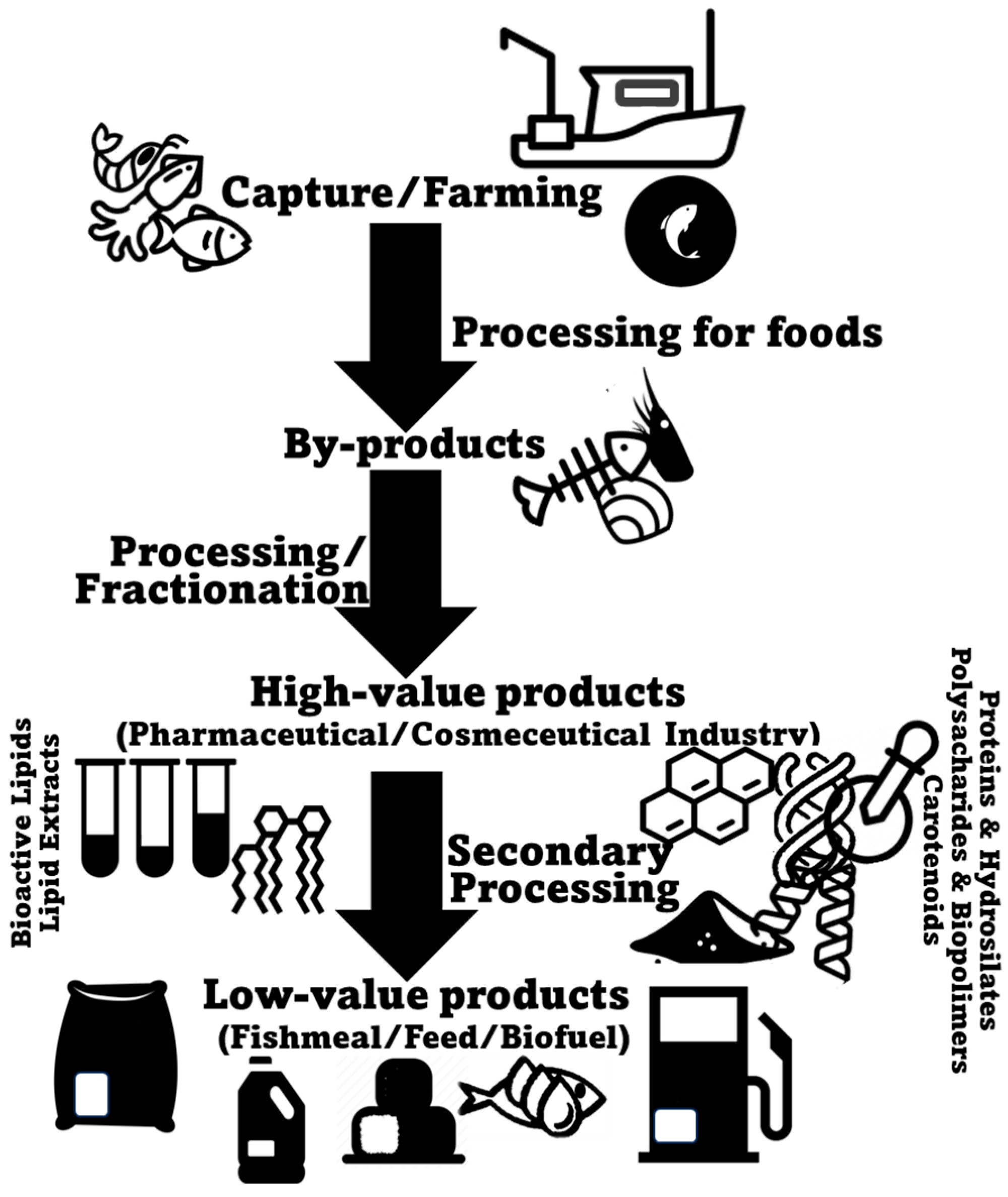
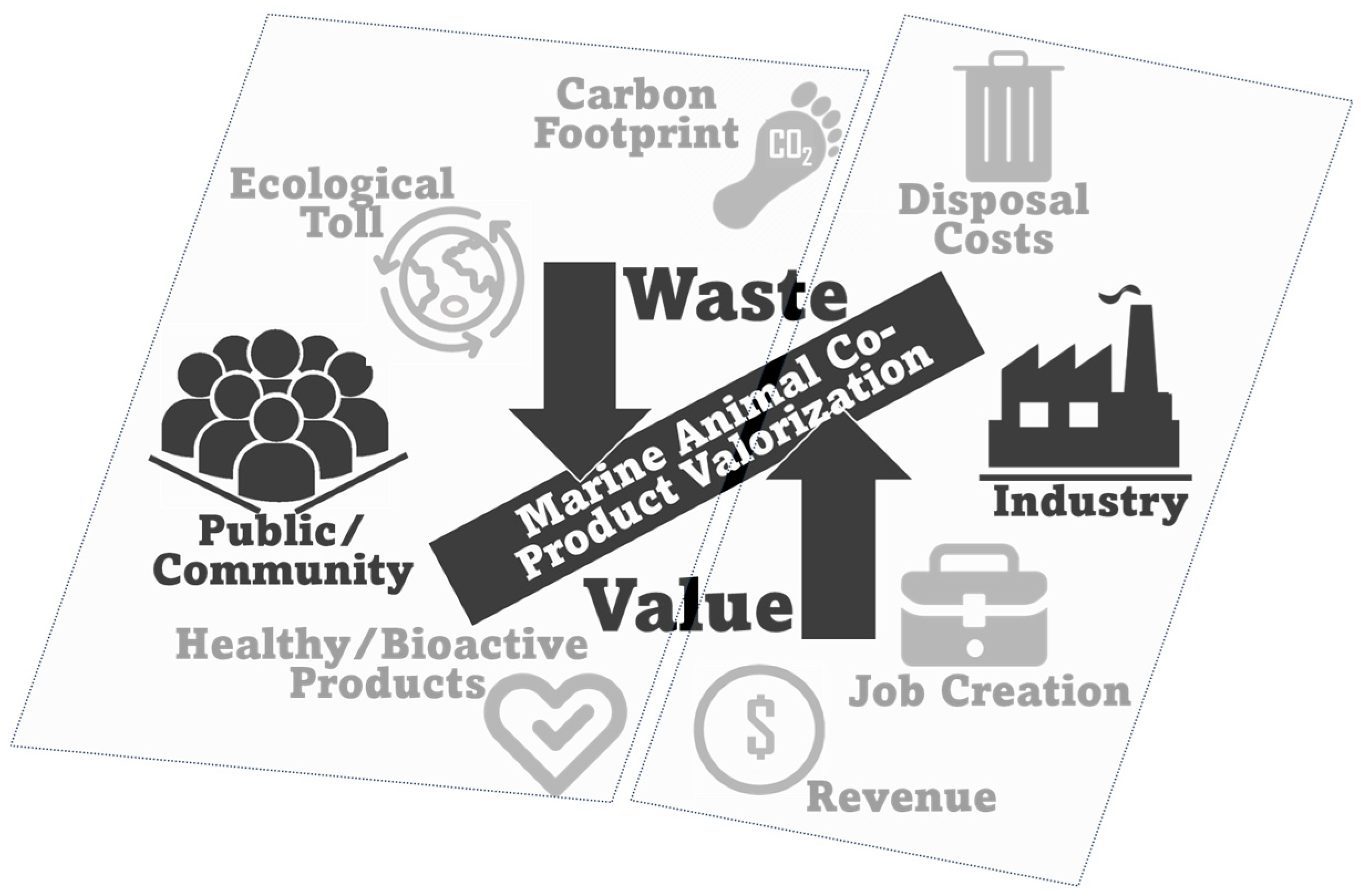
| Species | Co-Product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
|---|---|---|---|---|---|---|---|---|
| Champsocephalus gunnari | Brain | Solvent (hexane) | 0% | 0% | ------ | 0% | 0% | [110] |
| Champsocephalus gunnari | Liver | Solvent (hexane) | 2.29% | 1.34% | 0 | 1.34% | 0% | [110] |
| Champsocephalus gunnari | Stomach | Solvent (hexane) | 35.6% | 32.7% | 0.02 | 15.9% | 14.9% | [110] |
| Champsocephalus gunnari | Skin | Solvent (hexane) | 27.8% | 25.9% | 0 | 15.6% | 8.0% | [110] |
| Clupea harengus | Co-product mix (heads, fins, tails, and viscera) | Bligh and Dyer | 35.5% | 26.4% | 0.34 | 5.6% | 9.2% | [109] |
| Clupea harengus | Minced co-product (heads, frames, skin, and viscera) | Bligh and Dyer | 21.9% | ------ | ------ | 6.4% | 9.4% | [99] |
| Dicentrarchus labrax | Heads | Folch | 28.0% | 12.4% | 1.21 | 3.0% | 5.1% | [93] |
| Dicentrarchus labrax | Frames | Folch | 29.4% | 13.1% | 1.19 | 3.2% | 5.2% | [93] |
| Dicentrarchus labrax | Skin | Folch | 33.8% | 16.6% | 1.00 | 4.0% | 7.5% | [93] |
| Dicentrarchus labrax | Trimmings | Folch | 27.3% | 11.0% | 1.43 | 2.6% | 4.3% | [93] |
| Dicentrarchus labrax | Viscera | Folch | 27.9% | 10.7% | 1.55 | 2.4% | 4.0% | [93] |
| Euthynnus affinis | Heads | Bligh and Dyer | 28.8% | 17.2% | 0.67 | 1.5% | 15.7% | [100] |
| Euthynnus affinis | Intestine | Bligh and Dyer | 27.4% | 17.0% | 0.61 | 2.7% | 14.3% | [100] |
| Euthynnus affinis | Liver | Bligh and Dyer | 24.0% | 15.9% | 0.51 | 1.7% | 14.2% | [100] |
| Gadus morhua | Offal (heads, viscera, and skeletal frames) | Bligh and Dyer | 32.1% | ------ | ------ | 8.9% | 13.3% | [102] |
| Gadus morhua | Liver | Bligh and Dyer | 24.7% | ------ | ------ | 7.7% | 11.4% | [102] |
| Katsuwonus pelamis | Heads | Soxhlet | 12.7% | 9.6% | 0.32 | 1.3% | 6.3% | [111] |
| Lophius litulon | Liver | Soxhlet | 46.6% | ------ | ------ | 1.2% | 8.1% | [112] |
| Notothenia rossii | Brain | Solvent (hexane) | 32.9% | 32.2% | 0.02 | 9.8% | 22.0% | [110] |
| Notothenia rossii | Liver | Solvent (hexane) | 26.0% | 21.8% | 0.13 | 8.4% | 11.5% | [110] |
| Nototheniarossii | Stomach | Solvent (hexane) | 41.6% | 30.8% | 0.32 | 11.3% | 18.6% | [110] |
| Notothenia rossii | Skin | Solvent (hexane) | 35.0% | 31.0% | 0.09 | 16.2% | 10.7% | [110] |
| Salmo salar | Heads | Bligh and Dyer | 35.4% | 27.7% | 0.28 | 8.4% | 12.1% | [113] |
| Salmo salar | Heads | Folch | 31.9% | 16.3% | 0.93 | 3.2% | 4.8% | [93] |
| Salmo salar | Frames | Folch | 31.9% | 15.9% | 0.98 | 3.0% | 4.6% | [93] |
| Salmo salar | Skin | Folch | 31.9% | 15.4% | 1.05 | 2.8% | 4.0% | [93] |
| Salmo salar | Trimmings | Folch | 32.0% | 15.9% | 0.98 | 3.0% | 4.0% | [93] |
| Salmo salar | Viscera | Folch | 25.0% | 10.4% | 1.37 | 1.6% | 2.3% | [93] |
| Sardinella lemuru | Heads | Bligh and Dyer | 26.4% | 17.8% | 0.54 | 1.8% | 16.0% | [101] |
| Sardinella lemuru | Intestine | Bligh and Dyer | 24.9% | 13.6% | 0.83 | 1.7% | 11.9% | [101] |
| Sardinella lemuru | Liver | Bligh and Dyer | 22.7% | 15.7% | 0.44 | 2.8 | 13.0% | [101] |
| Sardinella aurita | Viscera | Bligh and Dyer | 30.5% | 26.1% | 0.15 | 7.4% | 13.6% | [97] |
| Sarpa salpa | Viscera | Bligh and Dyer | 34.8% | 20.4% | 0.71 | 4.1% | 6.0% | [97] |
| Scomber australasicus | Head | EtOH:hexane | 39.9% | 36.6% | 0.09 | 9.1% | 21.9% | [104] |
| Scomber australasicus | Skin | EtOH:hexane | 38.1% | 34.8% | 0.09 | 9.6% | 19.5% | [104] |
| Scomber australasicus | Roe | EtOH:hexane | 47.0% | 44.4% | 0.06 | 11.3% | 27.5% | [104] |
| Scomber australasicus | Male gonads | EtOH:hexane | 44.7% | 42.5% | 0.05 | 12.1% | 24.7% | [104] |
| Scomber scombrus | Heads | Soxhlet | 25.4% | ------ | ------ | 3.6% | 9.3% | [114] |
| Scomber scombrus | Gills | Soxhlet | 12.3% | ------ | ------ | 1.0% | 1.7% | [114] |
| Scophthalmus maximus | Heads | Folch | 36.8% | 22.5% | 0.61 | 4.4% | 11.6% | [93] |
| Scophthalmus maximus | Frames | Folch | 36.5% | 21.7% | 0.64 | 5.2% | 7.9% | [93] |
| Scophthalmus maximus | Skin | Folch | 37.4% | 22.6% | 0.62 | 5.2% | 8.9% | [93] |
| Scophthalmus maximus | Trimmings | Folch | 37.5% | 22.8% | 0.61 | 4.9% | 9.8% | [93] |
| Scophthalmus maximus | Viscera | Folch | 33.3% | 17.7% | 0.86 | 2.7% | 7.6% | [93] |
| Sparus aurata | Fishbone | Bligh and Dyer | 33.8% | 13.6% | 1.48 | 2.8% | 4.6% | [91] |
| Sparus aurata | Frames | Folch | 28.5% | 12.3% | 1.27 | 2.2% | 4.8% | [93] |
| Sparus aurata | Gills | Bligh and Dyer | 31.2% | 11.9% | 1.62 | 1.9% | 4.1% | [91] |
| Sparus aurata | Guts | Bligh and Dyer | 33.1% | 12.1% | 1.75 | 1.8% | 3.5% | [91] |
| Sparus aurata | Heads | Bligh and Dyer | 33.8% | 14.0% | 1.41 | 2.8% | 5.0% | [91] |
| Sparus aurata | Heads | Folch | 28.4% | 12.7% | 1.20 | 2.2% | 5.2% | [93] |
| Sparus aurata | Liver | Bligh and Dyer | 32.2% | 13.6% | 1.38 | 1.9% | 4.9% | [91] |
| Sparus aurata | Skin | Bligh and Dyer | 33.2% | 12.9% | 1.57 | 2.0% | 4.0% | [91] |
| Sparus aurata | Skin | Folch | 29.9% | 13.2% | 1.21 | 2.3% | 5.5% | [93] |
| Sparus aurata | Trimmings | Folch | 29.6% | 13% | 1.23 | 2.2% | 5.4% | [93] |
| Sparus aurata | Viscera | Folch | 28.8% | 12.9% | 1.20 | 1.7% | 5.9% | [93] |
| Thunnus thynnus | Minced side streams | Folch | 33.2% | 29.9% | 0.06 | 9.9% | 13.6% | [96] |
| Species | Co-Product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chionoecetes opilio | Co-product mix (cephalothorax, digestive system, and physiological liquid) | Bligh and Dyer | 24.4% | 21.1% | 0.10 | 9.9% | 8.9% | [128] |
| Commercial crab (no specified species) | Shells | Folch | 35.9% | 23.2% | 0.52 | 12.5% | 9.9% | [129] |
| Commercial shrimp (no specified species) | Shells | Folch | 40.9% | 12.3% | 2.2 | 6.3% | 4.1% | [129] |
| Jasus edwardsii | Hepatopancreas | Soxhlet | 7.8% | 3.1% | 1.52 | 0.9% | 0.9% | [127] |
| Lithodes santolla | Exoskeleton | Bligh and Dyer | 40.0% | 40.0% | 0 | 20.5% | 14.4% | [55] |
| Metapenaeus monoceros | Minced co-product (heads, tails, shells) | Folch | 34.5% | ------ | ------ | 8.9% | 6.9% | [118] |
| Nephrops norvegicus | Heads | Folch | 36.2% | 27.6% | 0.26 | 15.5% | 8.4% | [61] |
| Pandalus borealis | Co-product mix (heads, tails, shells) | Bligh and Dyer | 43.9% | 24.2% | 0.57 | 8.9% | 10.7% | [57] |
| Pandalus borealis | Processing co-product | Soxhlet | 41.1% | 37.1% | 0.11 | 21.1% | 13.9% | [59] |
| Panulirus cygnus | Cephalothorax | Folch | 38.2% | 13.5% | 0.64 | 5.6% | 4.2% | [126] |
| Penaeus japonicus | Hepatopancreas | Folch | 37.2% | 20.0% | 0.82 | 8.4% | 6.1% | [130] |
| Penaeus kerathurus | Cephalothorax | Bligh and Dyer | 44.5% | 28.7% | 0.55 | 14.5% | 13.4% | [124] |
| Penaeus kerathurus | Minced co-product (heads, tails, shells) | Folch | 38.8% | ------ | ------ | 12.2% | 16.1% | [118] |
| Penaeus monodon | Heads | Bligh and Dyer | 44.8% | 29.8% | 0.50 | 15.4% | 13.3% | [123] |
| Penaeus paulensis | Minced co-product (heads, tails, shells) | Bligh and Dyer | 34.6% | 26.0% | 0.30 | 11.7% | 12.2% | [116] |
| Penaeus vannamei | Cephalothorax | Bligh and Dyer | 43.0% | 12.2% | ------ | 5.0% | 7.2% | [131] |
| Penaeus vannamei | Cephalothorax | Folch | 42.5% | 10.5% | ------ | 4.1% | 6.4% | [131] |
| Penaeus vannamei | Cephalothorax | Bligh and Dyer | 39.3% | 14.7% | 1.67 | 4.6% | 8.3% | [58] |
| Penaeus vannamei | Cephalothorax | “Typical solvent extraction” | 48.5% | 24.5% | 0.95 | 9.6% | 13.3% | [132] |
| Penaeus vannamei | Cephalothorax | “Solvent extraction” | 37.5% | 18.1% | 1.08 | 9.2% | 8.1% | [133] |
| Penaeus vannamei | Hepatopancreas | Bligh and Dyer | 37.4% | 10.6% | 2.53 | 2.2% | 6.2% | [58] |
| Penaeus vannamei | Hepatopancreas | Bligh and Dyer | 38.1% | 16.2% | 1.35 | 3.3% | 10.4% | [121] |
| Pleoticus muelleri | Shells | Bligh and Dyer | 52.0% | 50.3% | 0.03 | 21.5% | 22.3% | [55] |
| Pleoticus muelleri | Shells + heads | Bligh and Dyer | 43.9% | 42.3% | 0.04 | 14.9% | 22.0% | [55] |
| Trachypena curvirostris | Co-product mix (heads, tails, shells) | Bligh and Dyer | 48.3% | 26.1% | 0.44 | 10.7% | 10.9% | [57] |
| Hard-Shelled Mollusks | Co-Product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
| Pecten maximus | Pooled mantle, gill, liver, digestive gland, kidney | Supercritical extraction | 42.1% | 40.7% | 0.03 | 20.0% | 12.3% | [149] |
| Pinna rugosa | Viscera | Folch | 41.8% | 37.5% | ------ | 17.0% | 20.0% | [119] |
| Cephalopods | Co-product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
| Commercial squid (no specified species) | Squid viscera oil | Supercritical extraction | 44.7% | ------ | ------ | 15.1% | 24.9% | [69] |
| Doryteuthis gahi | Pooled viscera, heads, skin | Bligh and Dyer | 52.6% | 48.6% | 0.08 | 17.2% | 30.8% | [150] |
| Dosidicus gigas | Viscera | Folch | 36.6% | 34.0% | ------ | 15.5% | 17.8% | [119] |
| Illex argentinus | Viscera | Wet pressing | ------ | ------ | 0.75 | 9.3% | 16.4% | [151] |
| Octopus vulgaris | Pooled co-products | Bligh and Dyer | 49.3% | 36.8% | 0.34 | 12.9% | 22.2% | [67] |
| Sepia officinalis | Viscera | Bligh and Dyer | 44.0% | 26.0% | 0.69 | 11.6% | 6.3% | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, J.P.; Domingues, M.R.; Calado, R. Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability. Mar. Drugs 2024, 22, 73. https://doi.org/10.3390/md22020073
Monteiro JP, Domingues MR, Calado R. Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability. Marine Drugs. 2024; 22(2):73. https://doi.org/10.3390/md22020073
Chicago/Turabian StyleMonteiro, João Pedro, M. Rosário Domingues, and Ricardo Calado. 2024. "Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability" Marine Drugs 22, no. 2: 73. https://doi.org/10.3390/md22020073
APA StyleMonteiro, J. P., Domingues, M. R., & Calado, R. (2024). Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability. Marine Drugs, 22(2), 73. https://doi.org/10.3390/md22020073