Hot-Melt Extrusion Drug Delivery System-Formulated Haematococcus pluvialis Extracts Regulate Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages
Abstract
1. Introduction
2. Results
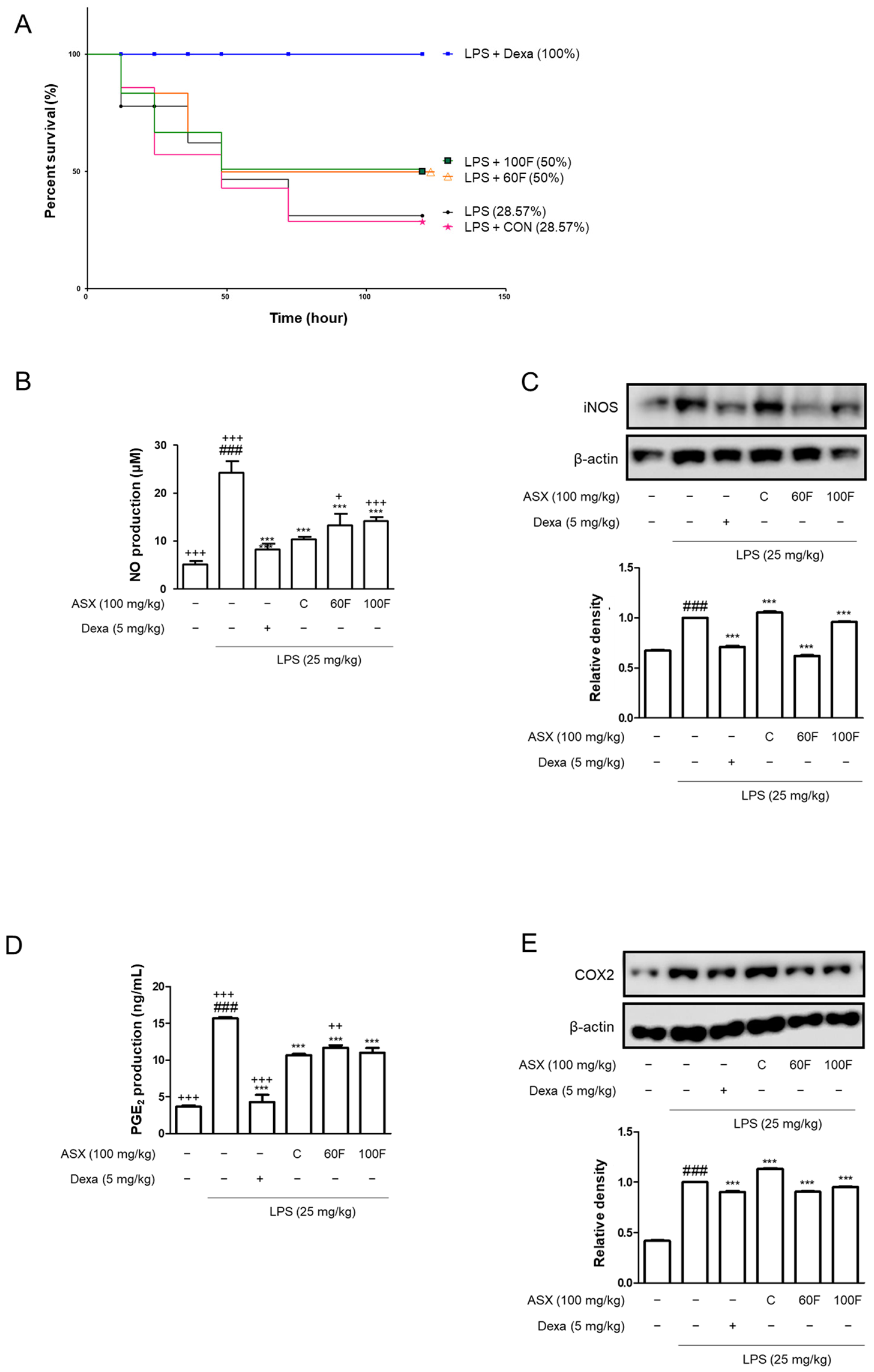
2.1. ASX-60F and ASX-100F Treatments Reduced the Mortality and Pro-Inflammatory Biomarker Expression in LPS-Stimulated Mice
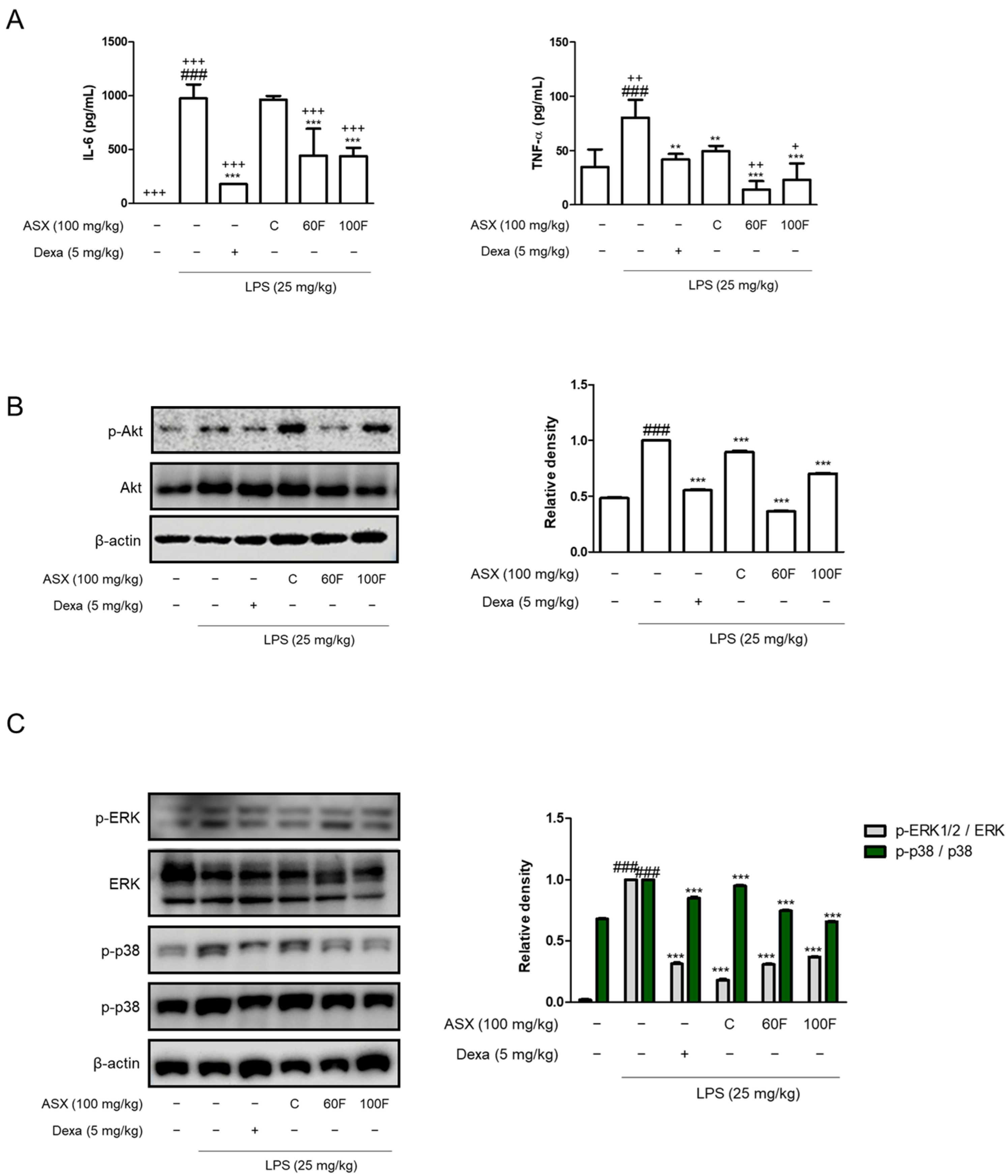
2.2. ASX-60F and ASX-100F Downregulate Inflammatory Responses via ERK/p-38 MAPK Signaling in LPS-Stimulated Mice
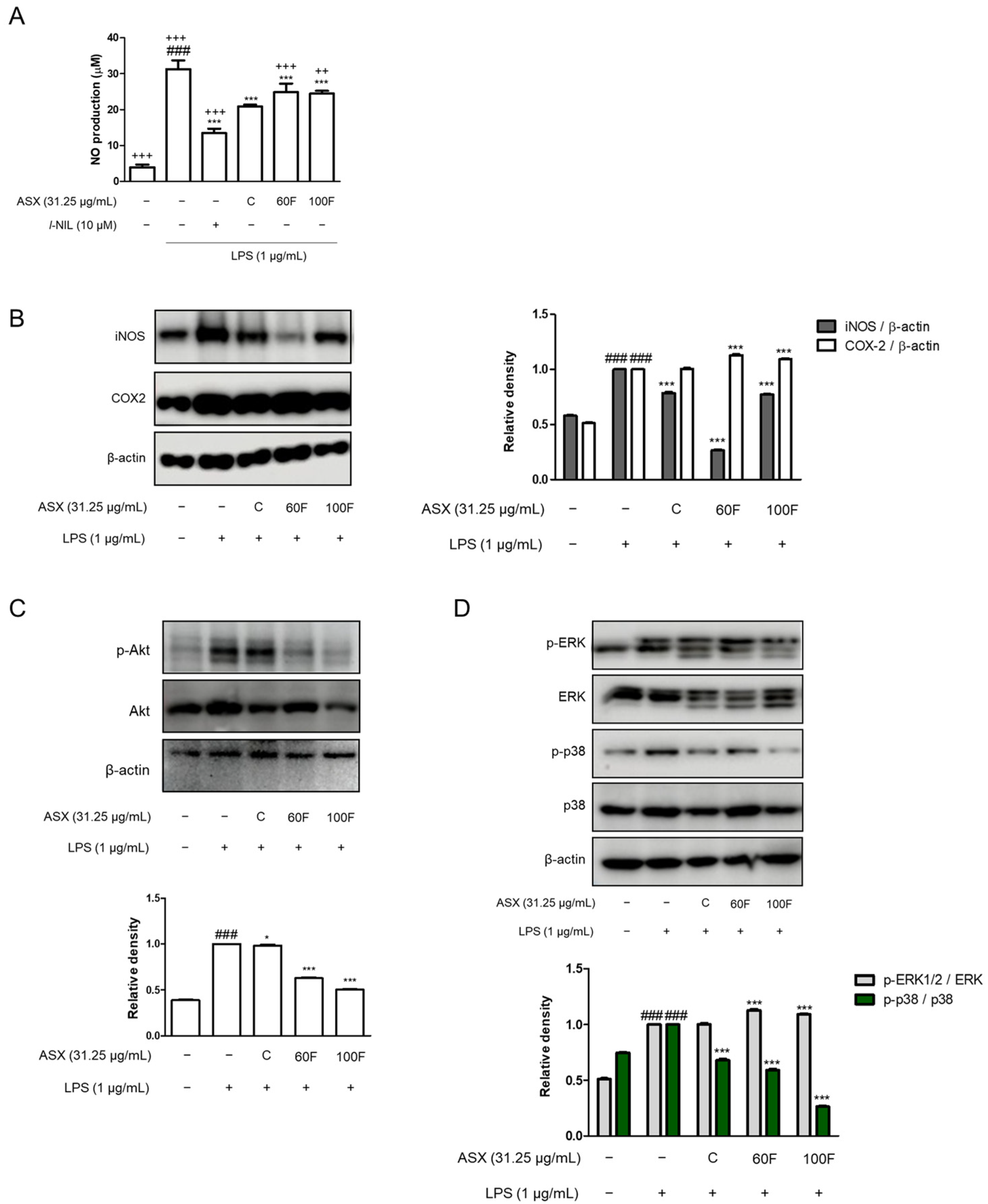
2.3. ASX-60F and ASX-100F Inhibited ERK/p-38 MAPK Signaling in LPS-Induced Inflammatory Responses in Macrophages
2.4. ASX-60F and ASX-100F Inhibited Excessive ROS Production in LPS-Stimulated RAW 264.7 Macrophages
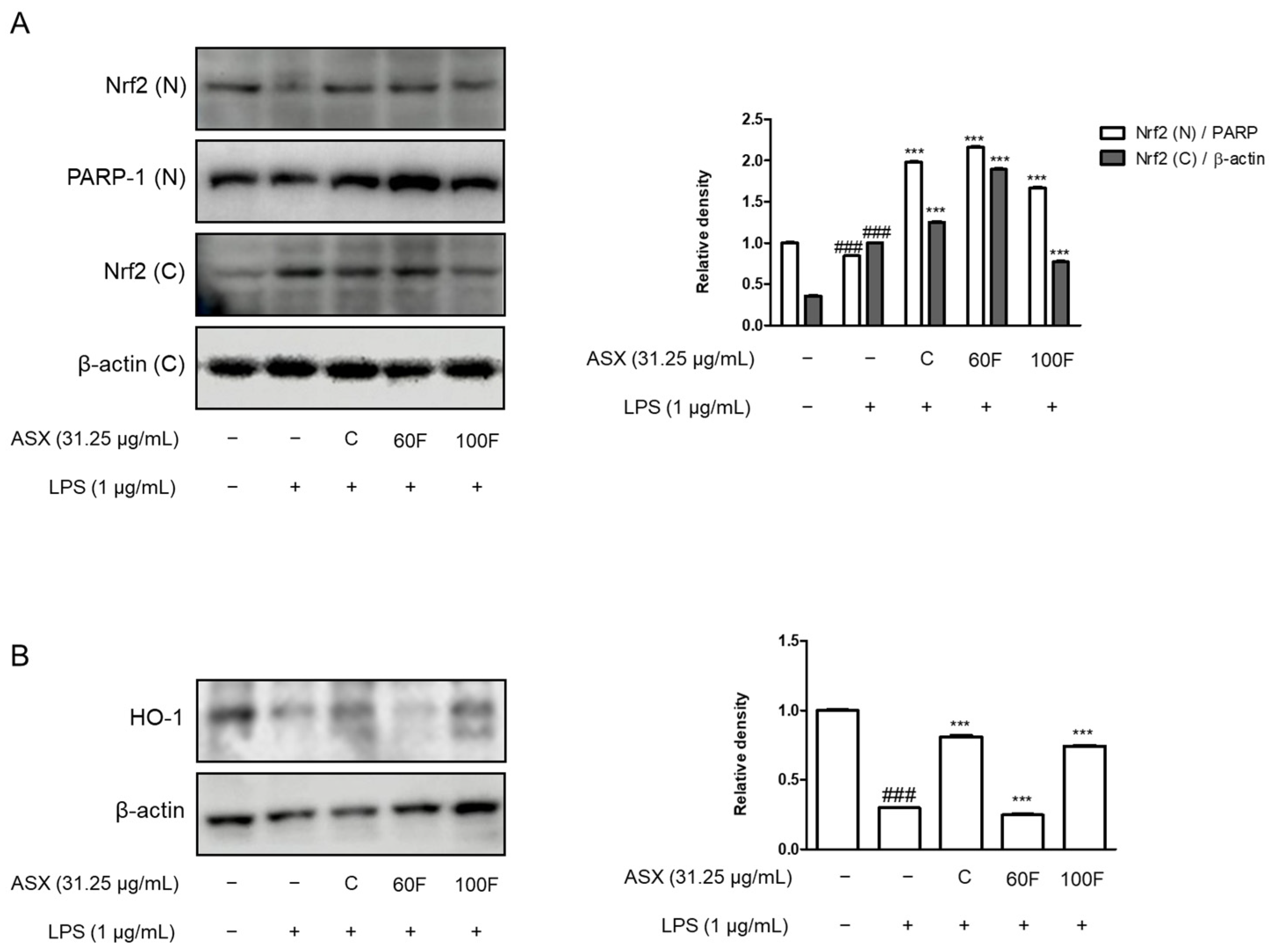
2.5. ASX-60F and ASX-100F Enhanced Nrf2 and HO-1 Protein Expression in LPS-Stimulated RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
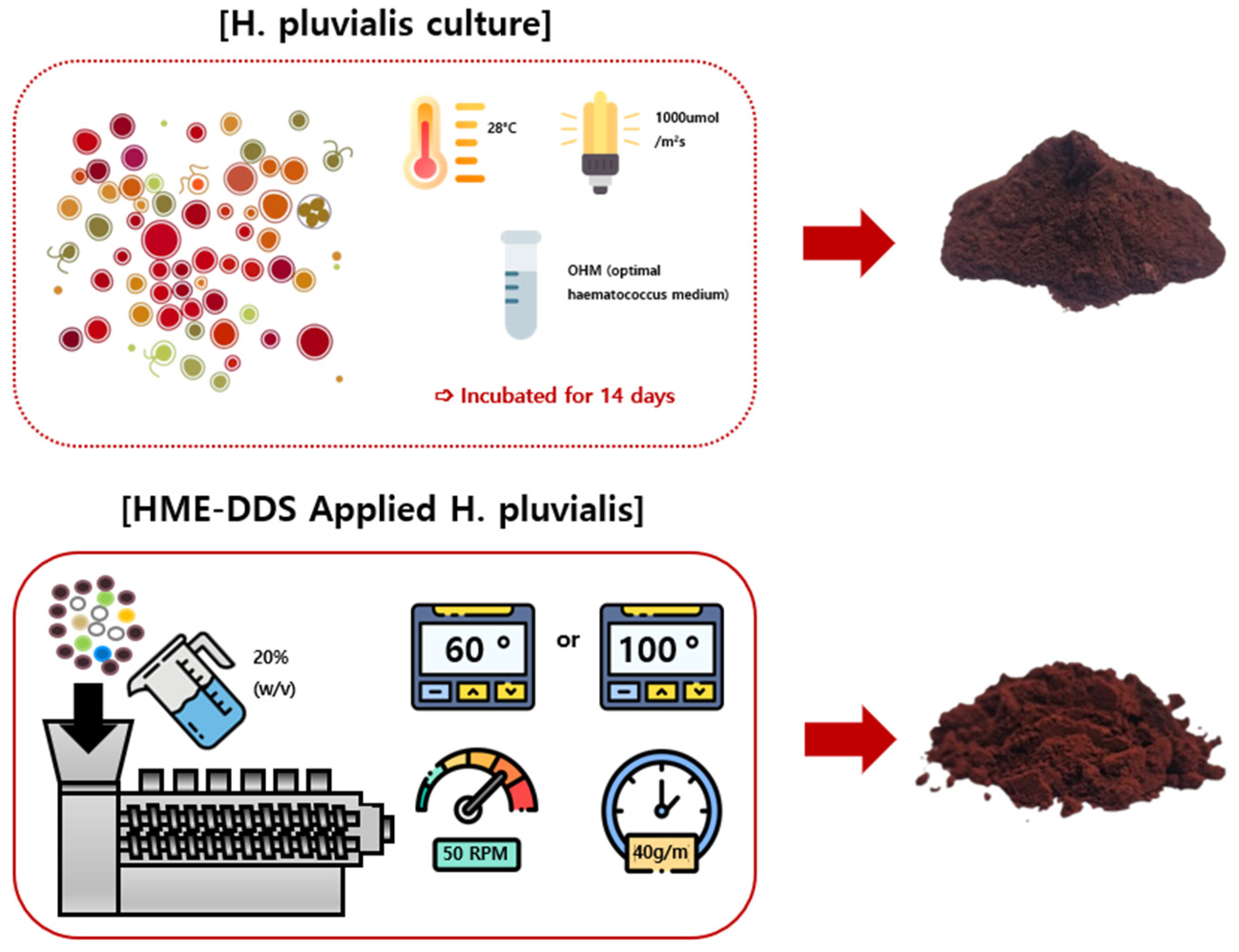
4.2. Preparation of C, 60F, and 100F (HME-DDS-Applied H. pluvialis) Extracts
4.3. Cell Culture and Sample Treatment
4.4. Experimental Animals and Sample Treatment
4.5. NO Production Assay
4.6. PGE2 Assay
4.7. Cytokine Assays (TNF-α and IL-6 Production)
4.8. Nuclear Extraction
4.9. Western Blot Analysis
4.10. Intracellular ROS Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Milton, R.; Gillespie, D.; Dyer, C.; Taiyari, K.; Carvalho, M.J.; Thomson, K.; Sands, K.; Portal, E.A.R.; Hood, K.; Ferreira, A.; et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: An international multisite prospective observational study. Lancet Glob. Health 2022, 10, e661–e672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-El-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U. Inflammation. Nature 2008, 454, 427. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.F.; Barreto, F.; da Silva, E.G.; Andrades, M.E.; Guimaraes, E.L.; Behr, G.A.; Moreira, J.C.; Bernard, E.A. Regulation of LPS stimulated ROS production in peritoneal macrophages from alloxan-induced diabetic rats: Involvement of high glucose and PPARgamma. Life Sci. 2007, 81, 153–159. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef]
- Fiorelli, S.; Porro, B.; Cosentino, N.; Di Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability:an In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gonczi, M.; Czirjak, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pinter, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxidative Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef]
- van Heijst, N.; Whiting, P.; Dutcher, J.R. Solubilization of Hydrophobic Astaxanthin in Water by Physical Association with Phytoglycogen Nanoparticles. Biomacromolecules 2024, 25, 4110–4117. [Google Scholar] [CrossRef]
- Li, Y.; Hu, K.; Huang, C.; Hu, Y.; Ji, H.; Liu, S.; Gao, J. Improvement of solubility, stability and antioxidant activity of carotenoids using deep eutectic solvent-based microemulsions. Colloids Surf. B Biointerfaces 2022, 217, 112591. [Google Scholar] [CrossRef]
- Patil, H.; Vemula, S.K.; Narala, S.; Lakkala, P.; Munnangi, S.R.; Narala, N.; Jara, M.O.; Williams, R.O., 3rd; Terefe, H.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation-Where Are We Now? AAPS PharmSciTech 2024, 25, 37. [Google Scholar] [CrossRef]
- Zheng, Y.; Pokorski, J.K. Hot melt extrusion: An emerging manufacturing method for slow and sustained protein delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1712. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.H.; Kim, H. Anti-Oxidant and Anti-Inflammatory Effects of Astaxanthin on Gastrointestinal Diseases. Int. J. Mol. Sci. 2022, 23, 15471. [Google Scholar] [CrossRef]
- Go, E.J.; Ryu, B.R.; Ryu, S.J.; Kim, H.B.; Lee, H.T.; Kwon, J.W.; Baek, J.S.; Lim, J.D. An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. Int. J. Mol. Sci. 2021, 22, 12377. [Google Scholar] [CrossRef]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef]
- Gil, T.Y.; Jin, B.R.; Cha, Y.Y.; An, H.J. Magnoliae flos Downregulated Lipopolysaccharide-Induced Inflammatory Responses via NF-kappaB/ERK-JNK MAPK/STAT3 Pathways. Mediat. Inflamm. 2022, 2022, 6281892. [Google Scholar] [CrossRef]
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock—Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 2020, 104, 573–585. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Zhao, J.; Meng, F.; Yao, C.; Bao, E.; Sun, N.; Chen, X.; Cheng, W.; Hua, H.; et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation. Theranostics 2022, 12, 976–998. [Google Scholar] [CrossRef]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Y.; Xie, X.; Yao, D.; Ding, C.; Chen, M. Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-kappaB. Am. J. Transl. Res. 2019, 11, 1884–1894. [Google Scholar]
- Xie, W.J.; Hou, G.; Wang, L.; Wang, S.S.; Xiong, X.X. Astaxanthin suppresses lipopolysaccharide-induced myocardial injury by regulating MAPK and PI3K/AKT/mTOR/GSK3beta signaling. Mol. Med. Rep. 2020, 22, 3338–3346. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Y.; Wu, Q.; Sun, X.; Zhang, W.Z.; Xu, X.L.; Chen, W. An Overview of Drug Delivery Nanosystems for Sepsis-Related Liver Injury Treatment. Int. J. Nanomed. 2023, 18, 765–779. [Google Scholar] [CrossRef]
- Partheniadis, I.; Karantzalis, A.E.; Shah, R.R.; Al-Zoubi, N.; Nikolakakis, I. Influence of compression at elevated temperature on the compactibility of thermo-mechanically processed polymers. Chem. Eng. Res. Des. 2020, 156, 64–75. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.; Jing, X.; Wang, J.; Wang, H.; Chen, F.; Wang, W.; Shao, Y.; Cui, X. Astaxanthin suppresses oxidative stress and calcification in vertebral cartilage endplate via activating Nrf-2/HO-1 signaling pathway. Int. Immunopharmacol. 2023, 119, 110159. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Li, B.; Nasser, M.I.; Masood, M.; Adlat, S.; Huang, Y.; Yang, B.; Luo, C.; Jiang, N. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 126, 110074. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.M.; Park, S.W.; Kim, H.J.; Lee, J.H.; Lee, D.U.; Chang, K.C. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem. Toxicol. 2013, 55, 386–395. [Google Scholar] [CrossRef]
- Rostami, S.; Alyasin, A.; Saedi, M.; Nekoonam, S.; Khodarahmian, M.; Moeini, A.; Amidi, F. Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: A randomized, triple-blind placebo-controlled clinical trial. Front. Endocrinol. 2023, 14, 1144323. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Donoso, A.; Gonzalez-Duran, J.; Munoz, A.A.; Gonzalez, P.A.; Agurto-Munoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin Protects Dendritic Cells from Lipopolysaccharide-Induced Immune Dysfunction. Mar. Drugs 2021, 19, 346. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, M.; Xiao, Z.; Zhang, J.; Li, X.; Wang, A. Protective effect of astaxanthin against multiple organ injury in a rat model of sepsis. J. Surg. Res. 2015, 195, 559–567. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Park, S.K.; Park, J.H.; Jeong, H.R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.S. Donepezil Regulates LPS and Abeta-Stimulated Neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-kappaB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Wu, M.F.; Huang, Y.H.; Chiu, L.Y.; Cherng, S.H.; Sheu, G.T.; Yang, T.Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef] [PubMed]
- Hoa, N.L.H.; Ngoc, N.T.B.; Dung, N.T.T.; Dung, N.H.; Hoa, H.T.M.; Hung, N.H.; Kim, D.G.; Cuong, T.V. Two-stage LEDs illuminated plastic bag photobioreactor for production of astaxanthin from Haematococcus pluvialis and manipulated egg yolk pigment by astaxanthin. Tạp Chí Khoa Học Và Công Nghệ 2023, 6, 8. [Google Scholar] [CrossRef]

| Composition | Percentage (%) |
|---|---|
| Astaxanthin | 70 |
| HPCD | 20 |
| Lecithin | 2.5 |
| Ascorbyl Palmitate | 5 |
| Vit. C. | 1.5 |
| Vit. E. | 1 |
| Total | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, T.-Y.; Sim, H.-Y.; Lee, H.-Y.; Ryu, S.; Baek, J.-S.; Kim, D.G.; Sim, J.; An, H.-J. Hot-Melt Extrusion Drug Delivery System-Formulated Haematococcus pluvialis Extracts Regulate Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Mar. Drugs 2024, 22, 512. https://doi.org/10.3390/md22110512
Gil T-Y, Sim H-Y, Lee H-Y, Ryu S, Baek J-S, Kim DG, Sim J, An H-J. Hot-Melt Extrusion Drug Delivery System-Formulated Haematococcus pluvialis Extracts Regulate Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Marine Drugs. 2024; 22(11):512. https://doi.org/10.3390/md22110512
Chicago/Turabian StyleGil, Tae-Young, Ha-Yeon Sim, Ha-Yeon Lee, Suji Ryu, Jong-Suep Baek, Dae Geun Kim, Jaehoon Sim, and Hyo-Jin An. 2024. "Hot-Melt Extrusion Drug Delivery System-Formulated Haematococcus pluvialis Extracts Regulate Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages" Marine Drugs 22, no. 11: 512. https://doi.org/10.3390/md22110512
APA StyleGil, T.-Y., Sim, H.-Y., Lee, H.-Y., Ryu, S., Baek, J.-S., Kim, D. G., Sim, J., & An, H.-J. (2024). Hot-Melt Extrusion Drug Delivery System-Formulated Haematococcus pluvialis Extracts Regulate Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Marine Drugs, 22(11), 512. https://doi.org/10.3390/md22110512









