Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds
Abstract
1. Introduction
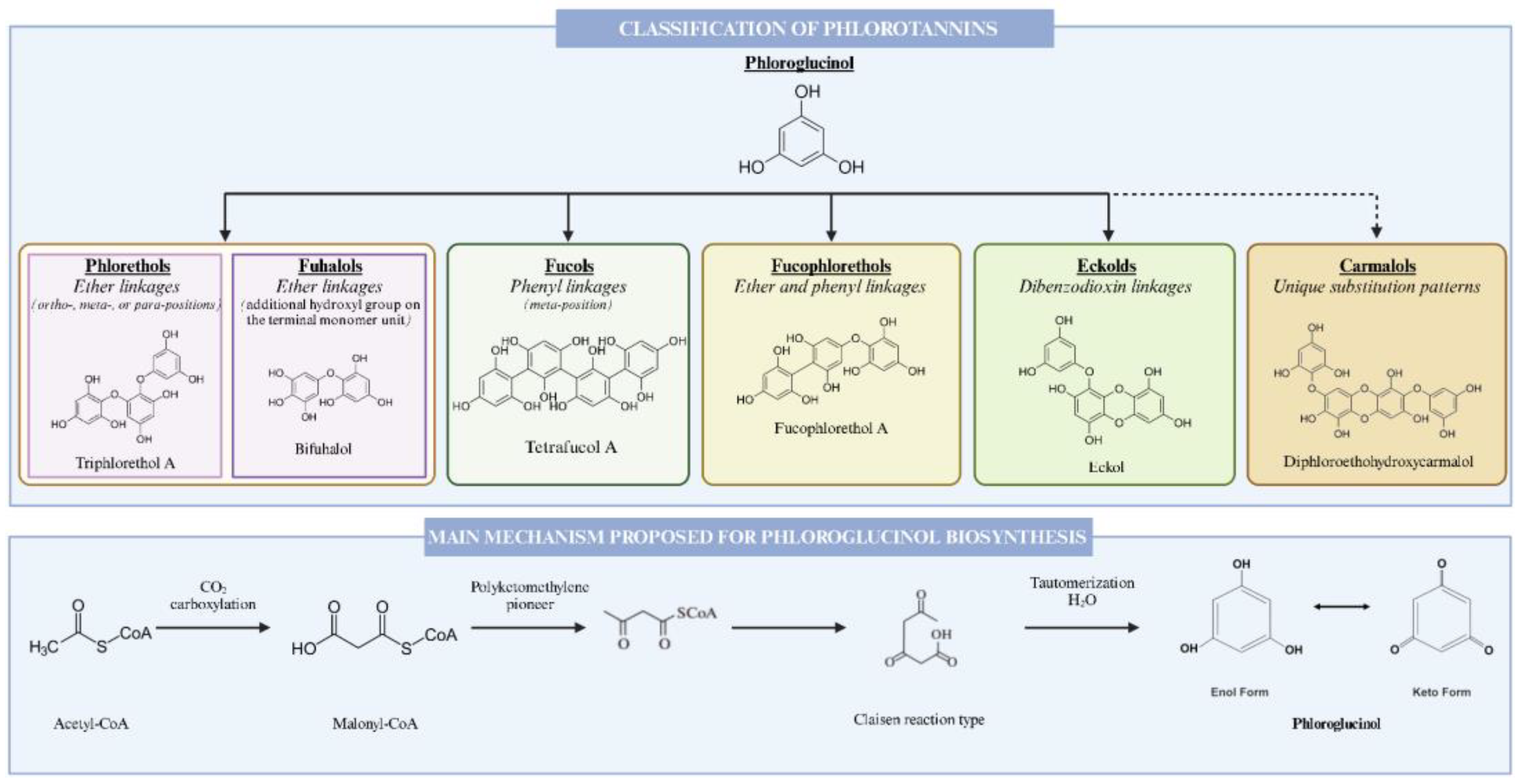
2. Phlorotannins
2.1. Biosynthesis & Structural Classes of Phlorotannins
2.2. Biological Properties of Phlorotannins with Relevance in Disease Treatment
2.2.1. Antioxidant Activity of Phlorotannins
2.2.2. Antimicrobial Properties of Phlorotannins
2.2.3. Anti-Inflammatory Properties of Phlorotannins
2.2.4. Antitumor and Cytotoxic Activity of Phlorotannins
2.2.5. UV-Absorbing Activity
| Properties | Active Compound | Species | Assay | Results (μg/mL) | Ref. |
|---|---|---|---|---|---|
| Antioxidant | 974-A and 974-B | Ecklonia kurome (B) | DPPH | IC50 = 2.4/2.6 | [26] |
| Antioxidant | Dieckol | Ecklonia cava (B) | HOO | IC50 = 3.5 | [52] |
| Antioxidant | Phlorotannin extract | Fucus vesiculosus (B) | DPPH | IC50 = 3.8–4.7 | [50] |
| Ascophylum nodosum (B) | IC50 = 6.3–7.7 | ||||
| Antioxidant | Eckstolonol, dieckol, and phlorofucofuroeckol A | Ecklonia stolonifera (B) | DPPH | IC50 = 2.1/1.5/1.1 | [51] |
| Antioxidant | Eckol, phlorofucofuroeckol A, dieckol and 8,8′-bieckol | Eisenia bicyclis, Ecklonia cava, and Ecklonia kurome (B) | DPPH | IC50 = 6.2, 2.9/3.1/3.5 | [49] |
| IC50 = 2.5/1.9/1.8/1.5 | |||||
| Anti-inflammatory | Phlorotannin extract | Fucus distichus (B) | PMA-stimulated RAW 264.7 cells | IC50 = 37 | [57] |
| Anti-inflammatory | Phlorotannin extract | Eisenia bicyclis and Ecklonia kurome (B) | Inhibitory activity against hyaluronidase | IC50 = 30/35 | [62] |
| Anti-inflammatory | Dieckol, eckol, phlorofucofuroeckol A, and phlorofucofuroeckol B | Ecklonia stolonifera (B) | Inhibited LPS-induced NO and PGE2 | IC50 = 72 and 98 | [56] |
| Phlorofucofuroeckol isomers A and B | Inhibition of NO production | IC50 = 1.7/2.9 | |||
| Anti-inflammatory | Dieckol, eckol, and 7-phloroeckol | Eisenia bicyclis (B) | LPS-induced NO production in RAW 264.7 cells | IC50 = 51.42/52.86/26.87 | [70] |
| Anti-inflammatory | Phlorotannin-purified extracts | A. nodosum and Alaria esculenta (B) | CaCo-2 cell line | IC50 = 33/7 | [71] |
| Antitumor | Phloroglucinol derivate | Ecklonia cava (B) | MCF-7 cell line | IC50 = 2.4 | [63] |
| Dioxinodehydroeckol | IC50 = 24 | ||||
| Antitumor | Dieckol | Ecklonia cava (B) | A2780 and SKOV3 cells | IC50 = 84.3/99.6 | [72] |
| Antitumor | Phlorotannin extracts | Laminaria japonica (B) | BEL-7402 and murine leukemic cells | IC50 = 200/120 | [73] |
| Antitumor | Phlorethols | Costaria costata (B) | Inhibitor of the α-NaGalase of cancer cells | IC50 = 15.2/5.7 | [74] |
| Antitumor | Phlorotannin-purified fractions | Fucus vesiculosus (B) | MKN-28, Caco-2, and HT-29 cell lines | IC50 = 56.3/97.4/118.8 | [75] |
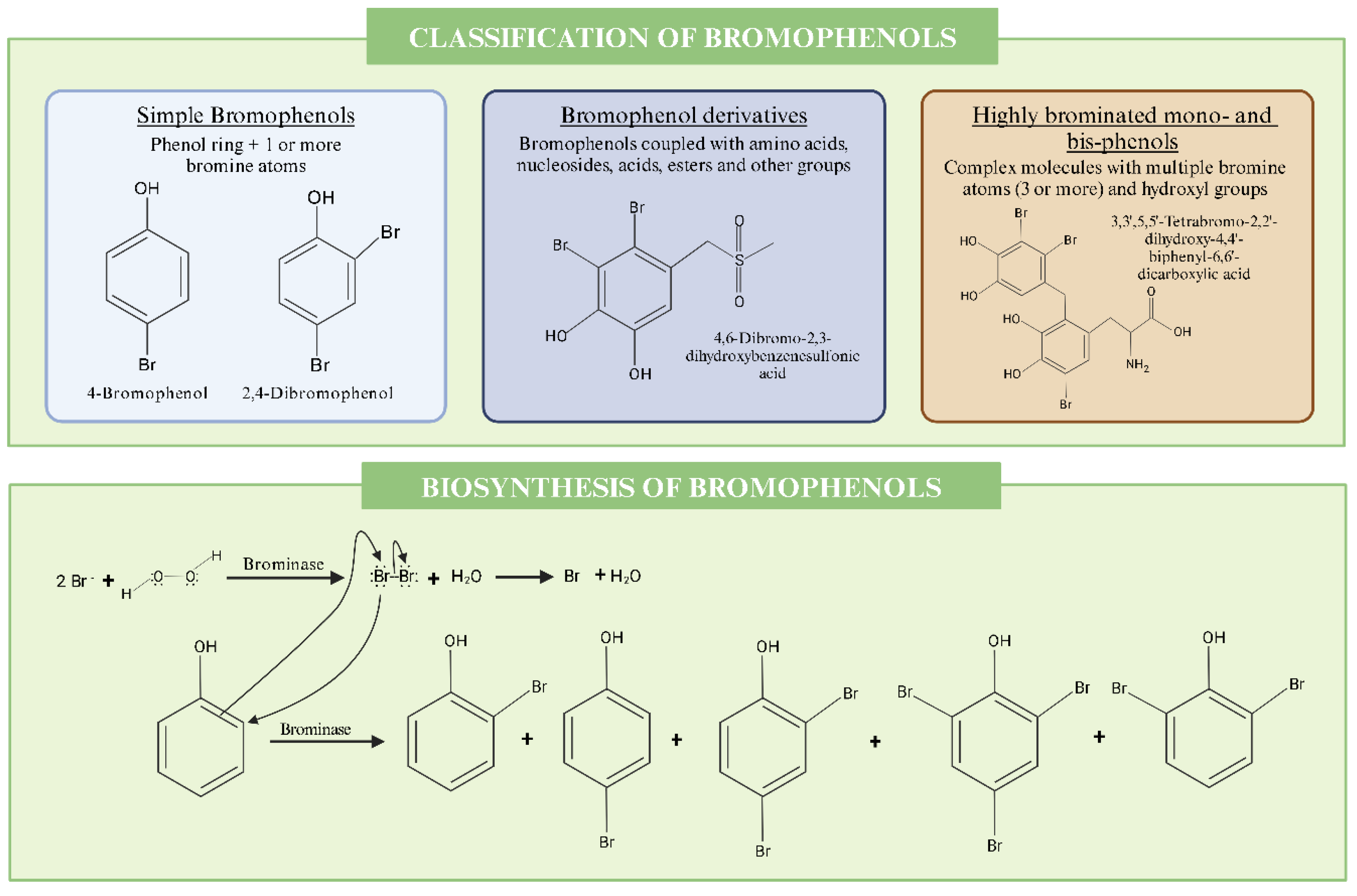
3. Bromophenols
3.1. Biosynthesis & Structural Characterization of Bromophenols
3.2. Biological Properties of Bromophenols with Relevance in Disease Treatment
3.2.1. Antioxidant Activity of Bromophenols
3.2.2. Antiviral Activity of Bromophenols
3.2.3. Other Activities (Anti-Inflammatory Activity, Antibacterial, Antidiabetic, Anti-Obesity, and Enzyme Inhibition) of Bromophenols
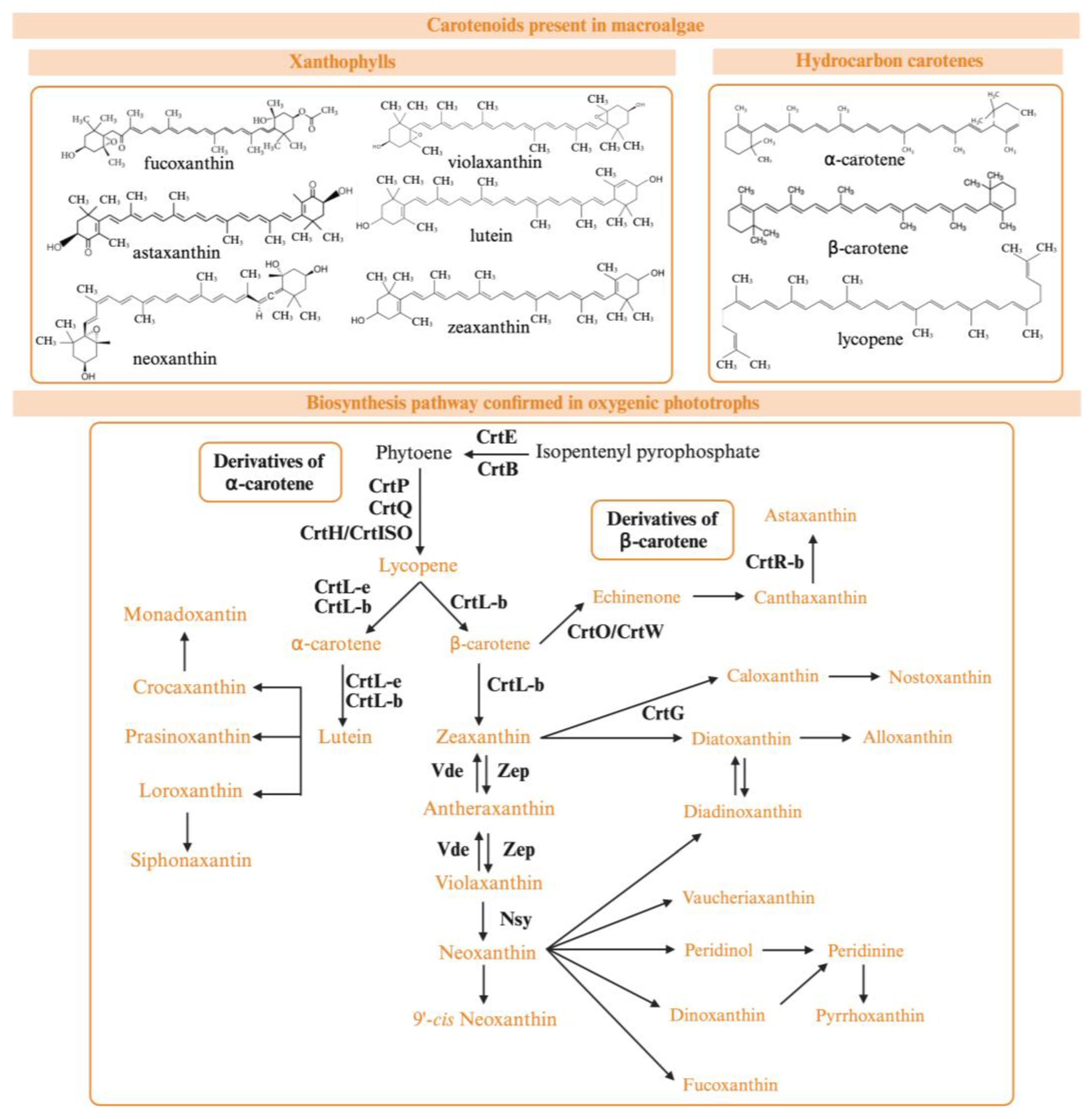
4. Primary Terpenoids: Chlorophylls, Phycobilins, and Carotenoids
4.1. Biosynthesis and Structural Characterization of Primary Terpenoids
4.2. Biological Properties of Natural Pigments with Relevance in Disease Treatment
4.2.1. Antioxidant Activity of Natural Pigments
4.2.2. Neuroprotective Activity of Natural Pigments
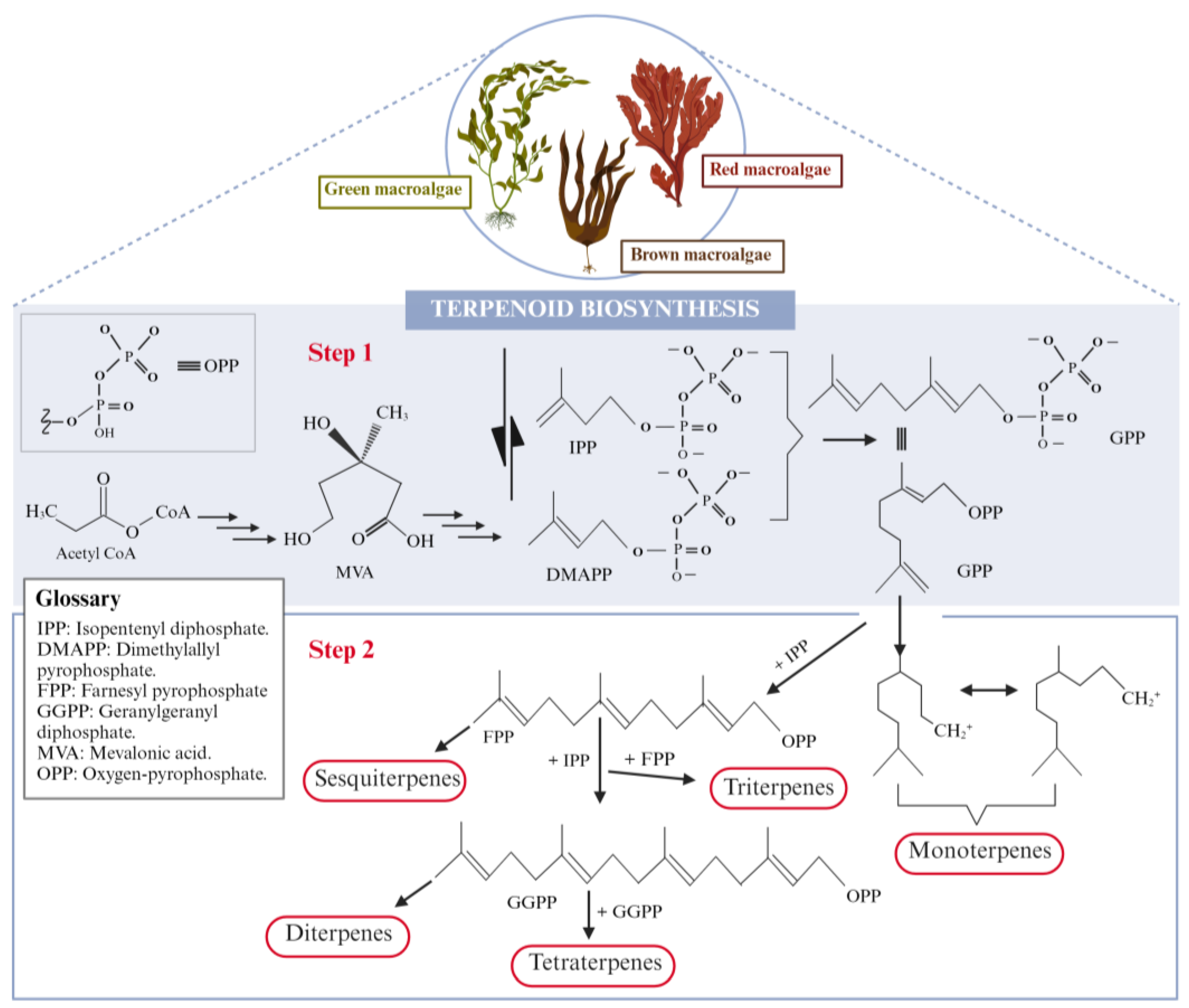
5. Secondary Terpenoids
5.1. Biosynthesis and Structural Characterization of Secondary Terpenoids
5.2. Biological Properties of Terpenoids with Relevance in Disease Treatment
5.2.1. Antioxidant Activity of Terpenoids
5.2.2. Antimicrobial Activity of Terpenoids
5.2.3. Anti-Inflammatory Properties of Terpenoids
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How Many Species of Algae Are There? A Reprise. Four Kingdoms, 14 Phyla, 63 Classes and Still Growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Soler-vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquacultre 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Adarshan, S.; Sree, V.S.S.; Muthuramalingam, P.; Nambiar, K.S.; Sevanan, M.; Satish, L.; Venkidasamy, B.; Jeelani, P.G.; Shin, H. Understanding Macroalgae: A Comprehensive Exploration of Nutraceutical, Pharmaceutical, and Omics Dimensions. Plants 2024, 13, 113. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Cassani, L.; Garcia-Oliveira, P.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A.; Lucini, L. Algal Nutraceuticals: A Perspective on Metabolic Diversity, Current Food Applications, and Prospects in the Field of Metabolomics. Food Chem. 2023, 409, 135295. [Google Scholar] [CrossRef]
- Rico, M.; González, A.G.; Santana-Casiano, M.; González-Dávila, M.; Pérez-Almeida, N.; de Tangil, M.S. Production of Primary and Secondary Metabolites Using Algae. In Prospects and Challenges in Algal Biotechnology; Springer: Singapore, 2017; pp. 311–326. [Google Scholar]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- O’ Brien, R.O.; Hayes, M.; Sheldrake, G.; Tiwari, B.; Walsh, P. Macroalgal Proteins: A Review. Foods 2022, 11, 571. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like Amino Acids (Maas): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates From Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–274. [Google Scholar]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.-J.; Chen, C.-W.; Dong, C.-D. Algal Polysaccharides: Current Status and Future Prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Kolackova, M.; Janova, A.; Dobesova, M.; Zvalova, M.; Chaloupsky, P.; Krystofova, O.; Adam, V.; Huska, D. Role of Secondary Metabolites in Distressed Microalgae. Environ. Res. 2023, 224, 115392. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; ud din Wani, H.M.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A Review of Extraction Methods, Structural Characteristics, Bioactivities, Bioavailability, and Future Trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Flodin, C.; Whitfield, F.B. Biosynthesis of Bromophenols in Marine Algae. Water Sci. Technol. 1999, 40, 53–58. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Preparative Separation of Three Terpenoids from Edible Brown Algae Sargassum fusiforme by High-Speed Countercurrent Chromatography Combined with Preparative High-Performance Liquid Chromatography. Algal Res. 2021, 59, 102449. [Google Scholar] [CrossRef]
- Silva, A.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Morais, S.L.; Echave, J.; Carpena, M.; Xiao, J.; Barroso, M.F.; Simal-Gandara, J.; et al. Recent Advances in Biological Properties of Brown Algae-Derived Compounds for Nutraceutical Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 1283–1311. [Google Scholar] [CrossRef]
- Merhan, O. The Biochemistry and Antioxidant Properties of Carotenoids. In Carotenoids; Cvetkovic, D., Nikolic, G.S., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Healy, L.E.; Zhu, X.; Pojić, M.; Sullivan, C.; Tiwari, U.; Curtin, J.; Tiwari, B.K. Biomolecules from Macroalgae—Nutritional Profile and Bioactives for Novel Food Product Development. Biomolecules 2023, 13, 386. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef] [PubMed]
- Máximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenço, A. Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe. Mar. Drugs 2018, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.-C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and Structural Determination of Two Novel Phlorotannins from the Brown Alga Ecklonia kurome Okamura, and Their Radical Scavenging Activities. Mar. Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Kim, W.R.; Kim, C.Y.; Um, B.H. Determination of Major Phlorotannins in Eisenia bicyclis Using Hydrophilic Interaction Chromatography: Seasonal Variation and Extraction Characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-Performance Liquid Chromatographic Analysis of Phlorotannins from the Brown Alga Fucus vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Boi, V.N.; Trang, N.T.; Cuong, D.X.; Ha, H.T. Antioxidant Phlorotannin from Brown Algae Sargassum dupplicatum: Enzyme-Assissted Extraction and Purification. World 2020, 4, 62–68. [Google Scholar] [CrossRef]
- Kim, S.-K.; Himaya, S.W.A. Chapter 8—Medicinal Effects of Phlorotannins from Marine Brown Algae. In Marine Medicinal Foods; Advances in Food and Nutrition, Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 97–109. [Google Scholar]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.-J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/Function Analysis of a Type III Polyketide Synthase in the Brown Alga Ectocarpus siliculosus Reveals a Biochemical Pathway in Phlorotannin Monomer Biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS Profiling of Low Molecular Weight Phlorotannin Polymers in Ascophyllum Nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; van Staden, J. Perspectives on the Potentials of Phlorotannins in Enhancing Phytoremediation Performance. J. Plant Growth Regul. 2023, 43, 2972–2992. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Call the Eckols: Present and Future Potential Cancer Therapies. Mar. Drugs 2022, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.-S.; Quarta, A.; Ragusa, A.; Jena, M. Algal Phlorotannins as Novel Antibacterial Agents with Reference to the Antioxidant Modulation: Current Advances and Future Directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-Proliferative Activity and Chemical Characterization by Comprehensive Two-Dimensional Liquid Chromatography Coupled to Mass Spectrometry of Phlorotannins from the Brown Macroalga Sargassum muticum Collected on North-Atlantic Coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Lemesheva, V.; Islamova, R.; Stepchenkova, E.; Shenfeld, A.; Birkemeyer, C.; Tarakhovskaya, E. Antibacterial, Antifungal and Algicidal Activity of Phlorotannins, as Principal Biologically Active Components of Ten Species of Brown Algae. Plants 2023, 12, 821. [Google Scholar] [CrossRef]
- Moon, C.; Kim, S.-H.; Kim, J.-C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective Effect of Phlorotannin Components Phloroglucinol and Eckol on Radiation-Induced Intestinal Injury in Mice. Phyther. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as Bioactive Agents from Brown Algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Toth, G.B.; Pavia, H. Removal of Dissolved Brown Algal Phlorotannins Using Insoluble Polyvinylpolypyrrolidone (PVPP). J. Chem. Ecol. 2001, 27, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia Cava (Phaeophyceae): Biological Activities and Potential Health Benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant Activities of Phlorotannins Isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.Q.; Sharma-Shivappa, R.; van Zanten, J. Antioxidant Activity of Phlorotannins from Brown Algae. Int. J. Agric. Biol. Eng. 2017, 10, 184–191. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and Identification of Phlorotannins from Ecklonia Stolonifera with Antioxidant and Anti-Inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.M.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical Components and Its Antioxidant Properties In Vitro: An Edible Marine Brown Alga, Ecklonia Cava. Bioorganic Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Druzhinina, A.; Kaplitsin, P.; Ovchinnikov, D.; Parshina, A.; Kuznetsova, M. Relationship between Radical Scavenging Activity and Polymolecular Properties of Brown Algae Polyphenols. Chem. Pap. 2019, 73, 2377–2385. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal Activity of Phlorotannins from the Brown Alga Ecklonia Kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.Y.; Kim, Y.M.; Park, S.J.; Rho, M.C.; Kim, S.J.; Lee, W.S. Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown Alga Ecklonia Cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Il Kim, J.; Kim, H.R. Anti-Inflammatory Activities of an Ethanol Extract of Ecklonia stolonifera in Lipopolysaccharide-Stimulated RAW 264.7 Murine Macrophage Cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Burgos, P.M.H.; Hernandez-suarez, D.F.; Harris, D. From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar]
- Ferreira, C.A.M.; Félix, R.; Félix, C.; Januário, A.P.; Alves, N.; Novais, S.C.; Dias, J.R.; Lemos, M.F.L. A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing. Biomolecules 2021, 11, 461. [Google Scholar] [CrossRef]
- Wei, R.; Lee, M.S.; Lee, B.; Oh, C.W.; Choi, C.G.; Kim, H.R. Isolation and Identification of Anti-Inflammatory Compounds from Ethyl Acetate Fraction of Ecklonia stolonifera and Their Anti-Inflammatory Action. J. Appl. Phycol. 2016, 28, 3535–3545. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory Activity of Brown Algal Phlorotannins against Hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of Apoptosis by Phloroglucinol Derivative from Ecklonia Cava in MCF-7 Human Breast Cancer Cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Cytotoxic Activities of Phlorethol and Fucophlorethol Derivatives Isolated from Laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Chen, Y.; Liu, H.; Yuan, G.; Fan, Y.; Chen, K. Optimization of the Microwave-Assisted Extraction of Phlorotannins from Saccharina japonica Aresch and Evaluation of the Inhibitory Effects of Phlorotannin-Containing Extracts on HepG2 Cancer Cells. Chin. J. Oceanol. Limnol. 2013, 31, 1045–1054. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, I.K.; Kim, B.J.; Jeong, I.Y.; Shin, T.; Park, J.W.; et al. Eckol Protects V79-4 Lung Fibroblast Cells against γ-Ray Radiation-Induced Apoptosis via the Scavenging of Reactive Oxygen Species and Inhibiting of the c-Jun NH2-Terminal Kinase Pathway. Eur. J. Pharmacol. 2008, 591, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of Phlorotannins Isolated from Ecklonia cava on Melanogenesis and Their Protective Effect against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Cha, S.H.; Heo, S.J.; Lee, S.H.; Kang, S.M.; Jeon, Y.J. Protective Effect of Ecklonia cava on UVB-Induced Oxidative Stress: In Vitro and in Vivo Zebrafish Model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory Effect of Diphlorethohydroxycarmalol on Melanogenesis and Its Protective Effect against UV-B Radiation-Induced Cell Damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-Inflammatory Activity of Edible Brown Alga Eisenia bicyclis and Its Constituents Fucosterol and Phlorotannins in LPS-Stimulated RAW264.7 Macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-Proliferative and Potential Anti-Diabetic Effects of Phenolic-Rich Extracts from Edible Marine Algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, Isolated from the Edible Brown Algae Ecklonia cava, Induces Apoptosis of Ovarian Cancer Cells and Inhibits Tumor Xenograft Growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef]
- Yang, H.; Zeng, M.; Dong, S.; Liu, Z.; Li, R. Anti-Proliferative Activity of Phlorotannin Extracts from Brown Algae Laminaria japonica Aresch. Chin. J. Oceanol. Limnol. 2010, 28, 122–130. [Google Scholar] [CrossRef]
- Bakunina, I.; Imbs, T.; Likhatskaya, G.; Grigorchuk, V.; Zueva, A.; Malyarenko, O.; Ermakova, S. Effect of Phlorotannins from Brown Algae Costaria costata on α-N-Acetylgalactosaminidase Produced by Duodenal Adenocarcinoma and Melanoma Cells. Mar. Drugs 2023, 21, 33. [Google Scholar] [CrossRef]
- Catarino, M.D.; Fernandes, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor Activity of Fucus Vesiculosus-Derived Phlorotannins through Activation of Apoptotic Signals in Gastric and Colorectal Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, H.; Tao, F.M. Molecular Structures and Properties of the Complete Series of Bromophenols: Density Functional Theory Calculations. J. Phys. Chem. A 2005, 109, 5186–5192. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.S.; Dellinger, B. Mechanisms of Dioxin Formation from the High-Temperature Oxidation of 2-Bromophenol. Environ. Sci. Technol. 2005, 39, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, M.; Wang, S.; Li, S.; Cao, P.; Yang, Y.; Lü, Y.; Shi, J.; Xu, N.; Fan, X.; et al. Bromophenols Coupled with Derivatives of Amino Acids and Nucleosides from the Red Alga Rhodomela confervoides. J. Nat. Prod. 2005, 68, 691–694. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J.; Fan, X.; He, L. Bromophenols Coupled with Nucleoside Bases and Brominated Tetrahydroisoquinolines from the Red Alga Rhodomela confervoides. J. Nat. Prod. 2007, 70, 337–341. [Google Scholar] [CrossRef]
- Boztaş, M.; Çetinkaya, Y.; Topal, M.; Gülçin, I.; Menzek, A.; Şahin, E.; Tanc, M.; Supuran, C.T. Synthesis and Carbonic Anhydrase Isoenzymes I, II, IX, and XII Inhibitory Effects of Dimethoxybromophenol Derivatives Incorporating Cyclopropane Moieties. J. Med. Chem. 2015, 58, 640–650. [Google Scholar] [CrossRef]
- Xu, X.; Yin, L.; Wang, Y.; Wang, S.; Song, F. A New Bromobenzyl Methyl Sulphoxide from Marine Red Alga Symphyocladia latiuscula. Nat. Prod. Res. 2013, 27, 723–726. [Google Scholar] [CrossRef]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and Aldose Reductase Inhibitory Effects of Bromophenols from the Red Alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef]
- Agarwal, V.; El Gamal, A.A.; Yamanaka, K.; Poth, D.; Kersten, R.D.; Schorn, M.; Allen, E.E.; Moore, B.S. Biosynthesis of Polybrominated Aromatic Organic Compounds by Marine Bacteria. Nat. Chem. Biol. 2014, 10, 640–647. [Google Scholar] [CrossRef]
- Lin, K.; Zhou, S.; Chen, X.; Ding, J.; Kong, X.; Gan, J. Formation of Hydroxylated Polybrominated Diphenyl Ethers from Laccase-Catalyzed Oxidation of Bromophenols. Chemosphere 2015, 138, 806–813. [Google Scholar] [CrossRef]
- Javan, A.J.; Javan, M.J.; Tehrani, Z.A. Theoretical Investigation on Antioxidant Activity of Bromophenols from the Marine Red Alga Rhodomela confervoides: H-Atom vs. Electron Transfer Mechanism. J. Agric. Food Chem. 2013, 61, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.B.; Lee, J.H.; Chung, S.C.; Shin, J.; Shin, H.J.; Kim, H.K.; Lee, H.S. Antimicrobial Activities of the Bromophenols from the Red Alga Odonthalia corymbifera and Some Synthetic Derivatives. Bioorganic Med. Chem. Lett. 2008, 18, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Hassenklöver, T.; Predehl, S.; Pilli, J.; Ledwolorz, J.; Assmann, M.; Bickmeyer, U. Bromophenols, Both Present in Marine Organisms and in Industrial Flame Retardants, Disturb Cellular Ca2+ Signaling in Neuroendocrine Cells (PC12). Aquat. Toxicol. 2006, 76, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Dong, S.; Hansen, P.E.; Stagos, D.; Lin, X.; Liu, M. Progress of Bromophenols in Marine Algae from 2011 to 2020: Structure, Bioactivities, and Applications. Mar. Drugs 2020, 18, 411. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New Nitrogen-Containing Bromophenols from the Marine Red Alga Rhodomela confervoides and Their Radical Scavenging Activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In Vitro Antiviral Activity of Red Alga, Polysiphonia morrowii Extract and Its Bromophenols against Fish Pathogenic Infectious Hematopoietic Necrosis Virus and Infectious Pancreatic Necrosis Virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Mikami, D.; Kurihara, H.; Kim, S.M.; Takahashi, K. Red Algal Bromophenols as Glucose 6-Phosphate Dehydrogenase Inhibitors. Mar. Drugs 2013, 11, 4050–4057. [Google Scholar] [CrossRef]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Diabetes and Herbal Medicines. Iran. J. Pharmacol. Ther. 2008, 7, 97–106. [Google Scholar]
- Shi, D.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L. Inhibition of Bromophenols against PTP1B and Anti-Hyperglycemic Effect of Rhodomela Confervoides Extract in Diabetic Rats. Chin. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J. Inhibitory Potencies of Bromophenol from Algae Red Algae against A-Glucosidase Activity. Fish. Sci. 1999, 65, 300–303. [Google Scholar] [CrossRef]
- Lijun, H.; Nianjun, X.; Jiangong, S.; Xiaojun, Y.; Chengkui, Z. Isolation and Pharmacological Activities of Bromophenols from Rhodomela confervoides. Chin. J. Oceanol. Limnol. 2005, 23, 226–229. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl Bromophenols with Diverse Dimerization Patterns from the Brown Alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.N.; Lan, L.M.; Tsai, M.Y.; Chen, Y.W.; Hung, H.Y. Comprehensive Exploration of Bromophenol Derivatives: Promising Antibacterial Agents against SA and MRSA. ACS Omega 2024, 9, 40897–40906. [Google Scholar] [CrossRef]
- Carte, B.K.; Troupe, N.; Chan, J.A.; Westley, J.W.; Faulkner, D.J. Rawsonol, an Inhibitor of HMG-CoA Reductase from the Tropical Green Alga Avrainvillea rawsoni. Phytochemistry 1989, 28, 2917–2919. [Google Scholar] [CrossRef]
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols A and B, New Anti-Inflammatory Bromophenols from the Caribbean Marine Red Alga Vidalia obtusaloba. Experientia 1991, 47, 851–853. [Google Scholar] [CrossRef]
- Choi, Y.K.; Ye, B.R.; Kim, E.A.; Kim, J.; Kim, M.S.; Lee, W.W.; Ahn, G.N.; Kang, N.; Jung, W.K.; Heo, S.J. Bis (3-Bromo-4,5-Dihydroxybenzyl) Ether, a Novel Bromophenol from the Marine Red Alga Polysiphonia morrowii That Suppresses LPS-Induced Inflammatory Response by Inhibiting ROS-Mediated ERK Signaling Pathway in RAW 264.7 Macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef]
- Zhang, Y.; Glukhov, E.; Yu, H.; Gerwick, L.; Dorrestein, P.C.; Gerwick, W.H. Monomeric and Dimeric Bromophenols from the Red Alga Ceramium sp. with Antioxidant and Anti-Inflammatory Activities. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-Alzheimer’s Disease Activity of Bromophenols from a Red Alga, Symphyocladia latiuscula (Harvey) Yamada. ACS Omega 2019, 4, 12259–12270. [Google Scholar] [CrossRef]
- Baghel, R.S.; Choudhary, B.; Pandey, S.; Pathak, P.K.; Patel, M.K.; Mishra, A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods 2023, 12, 3642. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A.; Noor, N. Valorization of Natural Colors as Health-Promoting Bioactive Compounds: Phytochemical Profile, Extraction Techniques, and Pharmacological Perspectives. Food Chem. 2021, 362, 130141. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Afraz, M.T.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.A. Recent Progress in Natural Seaweed Pigments: Green Extraction, Health-Promoting Activities, Techno-Functional Properties and Role in Intelligent Food Packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Pereira, L.; Patel, N.B. Pioneering Role of Marine Macroalgae in Cosmeceuticals. Phycology 2022, 2, 172–203. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In Vitro Bioavailability of Chlorophyll Pigments from Edible Seaweeds. J. Funct. Foods 2018, 41, 25–33. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In Vitro Digestion of Chlorophyll Pigments from Edible Seaweeds. J. Funct. Foods 2018, 40, 400–407. [Google Scholar] [CrossRef]
- Haryatfrehni, R.; Dewi, S.C.; Meilianda, A.; Rahmawati, S.; Sari, I.Z.R. Preliminary Study the Potency of Macroalgae in Yogyakarta: Extraction and Analysis of Algal Pigments from Common Gunungkidul Seaweeds. Procedia Chem. 2015, 14, 373–380. [Google Scholar] [CrossRef]
- Praveena, B.; Murthy, S.D. Role of Photosynthetic Pigments in Protection against Oxidative Damege. Int. J. Plant Anim. Environ. Sci. 2013, 4, 167–171. [Google Scholar]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ Pigments and Phenolic Compounds with Antimicrobial Potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Gálová, E.; Šalgovičová, I.; Demko, V.; Mikulová, K.; Ševčovičová, A.; Slováková, L.; Kyselá, V.; Hudák, J. A Short Overview of Chlorophyll Biosynthesis in Algae. Biologia 2008, 63, 947–951. [Google Scholar] [CrossRef]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and Biological Functions. In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 269–284. ISBN 9781119158042. [Google Scholar]
- Larkum, A.W.D.; Kühl, M. Chlorophyll d: The Puzzle Resolved. Trends Plant Sci. 2005, 10, 355–357. [Google Scholar] [CrossRef]
- Li, Y.; Scales, N.; Blankenship, R.E.; Willows, R.D.; Chen, M. Extinction Coefficient for Red-Shifted Chlorophylls: Chlorophyll d and Chlorophyll F. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Montoya, E.J.O.; Dorion, S.; Atehortua-Garcés, L.; Rivoal, J. Phycobilin Heterologous Production from the Rhodophyta Porphyridium Cruentum. J. Biotechnol. 2021, 341, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466. [Google Scholar] [CrossRef] [PubMed]
- Honey, O.; Nihad, S.A.I.; Rahman, M.A.; Rahman, M.M.; Islam, M.; Chowdhury, M.Z.R. Exploring the Antioxidant and Antimicrobial Potential of Three Common Seaweeds of Saint Martin’s Island of Bangladesh. Heliyon 2024, 10, e26096. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. Cooking Effects on Chlorophyll Profile of the Main Edible Seaweeds. Food Chem. 2018, 266, 368–374. [Google Scholar] [CrossRef]
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro-Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective Potential of Phycobiliproteins Extracted from Porphyridium Cruentum. Metabolites 2023, 13, 366. [Google Scholar] [CrossRef]
- Rajauria, G. In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, Y.H.; Nam, T.J. Identification and Antioxidant Activity of Synthetic Peptides from Phycobiliproteins of Pyropia yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef]
- Sanger, G.; Wonggo, D.; Montolalu, L.A.D.Y.; Dotulong, V. Pigments Constituents, Phenolic Content and Antioxidant Activity of Brown Seaweed Sargassum sp. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 012057. [Google Scholar] [CrossRef]
- Hong, D.D.; Thom, L.T.; Ha, N.C.; Thu, N.T.H.; Hien, H.T.M.; Tam, L.T.; Dat, N.M.; Duc, T.M.; Van Tru, N.; Hang, N.T.M.; et al. Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines 2023, 11, 2310. [Google Scholar] [CrossRef]
- Pangestuti, R.; Vo, T.S.; Ngo, D.H.; Kim, S.K. Fucoxanthin Ameliorates Inflammation and Oxidative Reponses in Microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits β-Amyloid Assembly and Attenuates β-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhang, X.; Wu, Q.; Li, W.; Wang, C.X.; Xie, G.B.; Zhou, X.M.; Shi, J.X.; Zhou, M.L. Astaxanthin Offers Neuroprotection and Reduces Neuroinflammation in Experimental Subarachnoid Hemorrhage. J. Surg. Res. 2014, 192, 206–213. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive Compounds in Seaweeds: An Overview of Their Biological Properties and Safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Jia, Q.; Chen, X.; Köllner, T.G.; Bhattacharya, D.; Wong, G.K.S.; Gershenzon, J.; Chen, F. Terpene Biosynthesis in Red Algae Is Catalyzed by Microbial Type but Not Typical Plant Terpene Synthases. Plant Physiol. 2019, 179, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Trindade, P.; Gomes, D.; Guedes de Pinho, P.; Mouga, T.; Andrade, P.B. Codium tomentosum and Plocamium cartilagineum: Chemistry and Antioxidant Potential. Food Chem. 2010, 119, 1359–1368. [Google Scholar] [CrossRef]
- Yassaa, N.; Peeken, I.; Zllner, E.; Bluhm, K.; Arnold, S.; Spracklen, D.; Williams, J. Evidence for Marine Production of Monoterpenes. Environ. Chem. 2008, 5, 391–401. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Mar. Drugs 2022, 20, 789. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578x20903555. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine Algae: A Treasure Trove of Bioactive Anti-Inflammatory Compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A Review of Terpenes from Marine-Derived Fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Miziorko, H.M. Enzymes of the Mevalonate Pathway of Isoprenoid Biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- Habtemariam, S. Antidiabetic Potential of Monoterpenes: A Case of Small Molecules Punching above Their Weight. Int. J. Mol. Sci. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Introduction to Plant Secondary Metabolites—From Biosynthesis to Chemistry and Antidiabetic Action. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Elsevier: Amsterdam, The Netherlands, 2019; Volume 571, pp. 109–132. ISBN 9780081029220. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W. The Current and Potential Therapeutic Uses of Parthenolide. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 61–91. ISBN 9780444640567. [Google Scholar]
- Shapumba, C.W.; Knott, M.; Kapewangolo, P. Antioxidant Activity of a Halogenated Monoterpene Isolated from a Namibian Marine Algal Plocamium Species. J. Food Sci. Technol. 2017, 54, 3370–3373. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with Free-Radical-Scavenging Properties from Marine Macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Shaaban, M.; Abou-El-wafa, G.S.E.; Golz, C.; Laatsch, H. New Haloterpenes from the Marine Red Alga Laurencia papillosa: Structure Elucidation and Biological Activity. Mar. Drugs 2021, 19, 35. [Google Scholar] [CrossRef]
- Mickymaray, S.; Alturaiki, W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules 2018, 23, 3032. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Lee, H.-G.; Nagahawatta, D.P.; Yang, H.-W.; Kang, M.-C.; Jeon, Y.-J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-ΚB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-Inflammatory Effect of Apo-9′-Fucoxanthinone via Inhibition of MAPKs and NF-KB Signaling Pathway in LPS-Stimulated RAW 264.7 Macrophages and Zebrafish Model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef]
- Sumayya, S.S.; Lubaina, A.S.; Murugan, K. Screening of in Vitro Antiviral Activity of Purified Terpenoid Extracts of Selected Sea Weeds against Chikungunya Virus. Int. J. Pharm. Sci. Drug Res. 2020, 12, 320–324. [Google Scholar] [CrossRef]
- De Inés, C.; Argandoña, V.H.; Rovirosa, J.; San-Martín, A.; Díaz-Marrero, A.R.; Cueto, M.; González-Coloma, A. Cytotoxic Activity of Halogenated Monoterpenes from Plocamium cartilagineum. Z. Naturforschung C 2004, 59, 339–344. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Ardiana, N.; Padmi, H.; Ilhami, B.T.K.; Martyasari, N.W.R.; Sunarwidhi, A.L.; Nikmatullah, A.; Widyastuti, S.; Sunarpi, H.; Frediansyah, A. The Antiproliferative and Apoptosis-Inducing Effects of the Red Macroalgae Gelidium latifolium Extract against Melanoma Cells Eka. Molecules 2021, 26, 6568. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological Activities of Two Macroalgae from Adriatic Coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.Y.; Xie, T.; Bai, R. Natural Terpenoids with Anti-Inflammatory Activities: Potential Leads for Anti-Inflammatory Drug Discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A Master Regulator of Cellular Responses in Inflammation, Injury Resolution, and Tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, D.; Huang, J.; Lu, A.; Wang, R.; Jin, Y.; Zhang, R.; Chang, C.; Xu, L.; Xu, L.; et al. Therapeutic Effects of (5R)-5-Hydroxytriptolide on Fibroblast-like Synoviocytes in Rheumatoid Arthritis via LncRNA WAKMAR2/MiR-4478/E2F1/P53 Axis. Front. Immunol. 2021, 12, 605616. [Google Scholar] [CrossRef]
- Khursheed, M.; Ghelani, H.; Jan, R.K.; Adrian, T. Anti-Inflammatory Effects of Bioactive Compounds from Seaweeds, Bryozoans, Jellyfish, Shellfish and Peanut Worms. Mar. Drugs 2023, 21, 524. [Google Scholar] [CrossRef]

| Properties | Active compound | Species | Concentration | Assay | Results | Ref. |
|---|---|---|---|---|---|---|
| Antioxidant | C1 | Vertebrata lanosa (R) | nd | DPPH | IC50 = 7.43 µM | [85] |
| Antioxidant | Nitrogen-containing bromophenols | Rhodomela confervoides (R) | nd | DPPH | IC50 = 5.22 µM | [90] |
| nd | ABTS (TEAC) | 2.87 µM | ||||
| Anti-obesity | Red algae bromophenol extract | Rhodomelaceae (R) | 100 μg/mL | G6PD inhibition in vitro | IC50 = 0.85 µM | [92] |
| Antidiabetic | C2 | Rhodomela confervoides (R) | nd | DPPH | IC50 = 8.28 µM | [52] |
| Anti-cancer | C3 | Rhodomela confervoides (R) | 140 μg/mL | In vitro cytotoxicity against BEL7402 | 3.18 µg/mL | [96] |
| Anti-cancer | C4 | Leathesia nana (B) | 0.1% | In vitro cytotoxicity against BEL7402 | 0.0019 µg/mL | [97] |
| Anti-inflammatory | Vidalols A and B | Vidalia obtusaloba (R) | 1.235 g/kg fw and 54 mg/kg fw | Inhibition of PLA2 in vitro | 50 µg in agar plate | [100] |
| Anti-inflammatory | BBDE | Polysiphonia morrowii (R) | 2 μM | In vitro suppression of LPS induced ROS generation on RAW 264.7 cells | NS | [101] |
| Antioxidant, anti-inflammatory | Lanosol isopropyl ether, Bromourceolatols A-G | Ceramium sp. (R) | 25 μg/mL | 32 µM | [102] | |
| Anti-Alzheimer’s, antidiabetic | C4 | Symphyocladia latiuscula (R) | nd | Inhibition of amyloid plaque aggregation | IC50 = 20 µM | [103] |
| Antimicrobial, antifungal | C5 | Odonthalia corymbifera (R) | nd | Against S. aureus | IC50 = 1.56 µg/mL | [86] |
| nd | Against C. albicanis | |||||
| Antiviral | C6 | Polysiphonia morrowii (R) | 80% | Against INHV | EC50 = 19.0 µg/mL | [91] |
| Against IPNV | EC50 = 8.0 µg/mL | |||||
| Anti-stroke, cardiovascular inflammation | C7 | Leathesia nana (B) | nd | In vitro inhibition of PTP1B | IC50 = 0.84 µmol/L | [94] |
| Inhibition of cholesterol biosynthesis | Rawsonol | Avrainvillea rawsoni (B) | 0.01% dw | In vitro inhibition of IMPDH | IC50 = 7.4 µM | [99] |
| Properties | Active Compound | Species | Concentration | Assay | Results | Ref. |
|---|---|---|---|---|---|---|
| Antioxidant | Lipophilic extract | Hemipristis elongata, Laminaria digitata, and Saccharina latissima (B) | nd | DPPH | EC50 = 98.3 mg/L | [122] |
| nd | Metal ions | EC50 = 228.6–532.4 mg/L | ||||
| nd | FRAP | 8.3–26.3 mg Trolox eq/g dw | ||||
| Antioxidant | Phycobiliproteins | Pyropia yezoensis (R) | 10 µg/mL | ROS | Viable cells increased by 23–32% | [123] |
| Antioxidant | Chlorophyll, fucoxanthin, carotenoid, phycocyanin, phycoerythrin | Sargassum sp. (B) | Chl 0.11 mg/g, Fu 0.04 mg/g, Ca 19.5 mg/g | DPPH | IC50 = 2.584–2.966 mg/mL | [124] |
| Sargassum olygocystum (B) | FRAP | 9.09–14.45 µM Fe2+/mg extract | ||||
| Antioxidant | Fucoxanthin | Sargassum olygocystum (B) | 2.9 mg/g dw | DPPH | IC50 = 3.42 mg/mL | [125] |
| Neuroprotective | Fucoxanthin | NS | nd | PGE2 production | MEI = 2034.2 pg/mL | [126] |
| Neuroprotective | Fucoxanthin | Sargassum olygocystum (B) | 2.9 mg/g dw | MTT assay | Viable cells increased by 91.23–97.69% | [125] |
| Neuroprotective | Astaxanthin | Acetabularia acetabulum (G) | nd | MTT assay | 10,000 µM | [127] |
| Properties | Active Compound | Species | Concentration | Assay | Results | Ref. |
|---|---|---|---|---|---|---|
| Antioxidant | Halogenated monoterpene (1) | Plocamium spp. (R) | nd | DPPH | IC50 = 50 μM; AsA: IC50 = 20 μM | [149] |
| Antioxidant | Limonene and linalool | Codium tomentosum (G) | 28.1 and 3.1% | IC25 = 66 μg/mL | [138] | |
| Menthone (monoterpene) | Plocamium cartilagineum (R) | 4.3% | IC25 = 54 μg/mL | |||
| Antioxidant | Sesquiterpenes | Ulva fasciata (G) | nd | DPPH, ABTS+ | DPPH (89.8%), ABTS+ (82.6%) | [150] |
| Antibacterial | Haloterpenes (aplysiolic acid) | Laurencia papillosa (R) | nd | ADT | S. aureus (10.5 mm) | [151] |
| Antifungal | Diterpenes and sesquiterpenes | Laurencia paniculata (R), Ulva prolifera (G) | 0.18–13.91% | MIC and MFC | C. albicans (MIC = 125 µg/mL; MFC = 125 µg/mL) | [152] |
| Anti-inflammatory | Fucosterol (diterpenoid) | Padina boryana (B) | 125 µg/mL | NO Inhibition | Suppressed the expression of iNOS, COX-2, and PGE2 | [153] |
| Anti-inflammatory | Apo-9-fucoxanthinone (carotenoid) | NS | 25–100 μg/mL | In vivo zebrafish model | Downregulated iNOS, COX-2, TNF-α, and IL-1β | [154] |
| Cytotoxicity | Terpenoid (1) | Gracillaria dura (R) | nd | MTT assay | CC50 (Vero cells = 6; Ribavirin = 2.5 mg/mL); | [155] |
| Cytotoxicity | Halogenated monoterpenes | Plocamium cartilagineum (R) | nd | MTT assay | MIC (SW480 cells = 131; Lindane > 344) | [156] |
| Cytotoxicity | Brassicolene (diterpenoid) | Gelidium latifolium (R) | 107.06 mg GAE/g | MTT assay | CC50 (B16-F10 cells = 84.29 µg/mL) | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpena, M.; Pereira, C.S.G.P.; Silva, A.; Barciela, P.; Jorge, A.O.S.; Perez-Vazquez, A.; Pereira, A.G.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Prieto, M.A. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Mar. Drugs 2024, 22, 478. https://doi.org/10.3390/md22100478
Carpena M, Pereira CSGP, Silva A, Barciela P, Jorge AOS, Perez-Vazquez A, Pereira AG, Barreira JCM, Oliveira MBPP, Prieto MA. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Marine Drugs. 2024; 22(10):478. https://doi.org/10.3390/md22100478
Chicago/Turabian StyleCarpena, Maria, Cláudia S. G. P. Pereira, Aurora Silva, Paula Barciela, A. Olivia S. Jorge, Ana Perez-Vazquez, Antia G. Pereira, João C. M. Barreira, M. Beatriz P. P. Oliveira, and Miguel A. Prieto. 2024. "Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds" Marine Drugs 22, no. 10: 478. https://doi.org/10.3390/md22100478
APA StyleCarpena, M., Pereira, C. S. G. P., Silva, A., Barciela, P., Jorge, A. O. S., Perez-Vazquez, A., Pereira, A. G., Barreira, J. C. M., Oliveira, M. B. P. P., & Prieto, M. A. (2024). Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Marine Drugs, 22(10), 478. https://doi.org/10.3390/md22100478












