Improvement of the Antioxidant and Antitumor Activities of Benzimidazole-Chitosan Quaternary Ammonium Salt on Drug Delivery Nanogels
Abstract
1. Introduction
2. Results
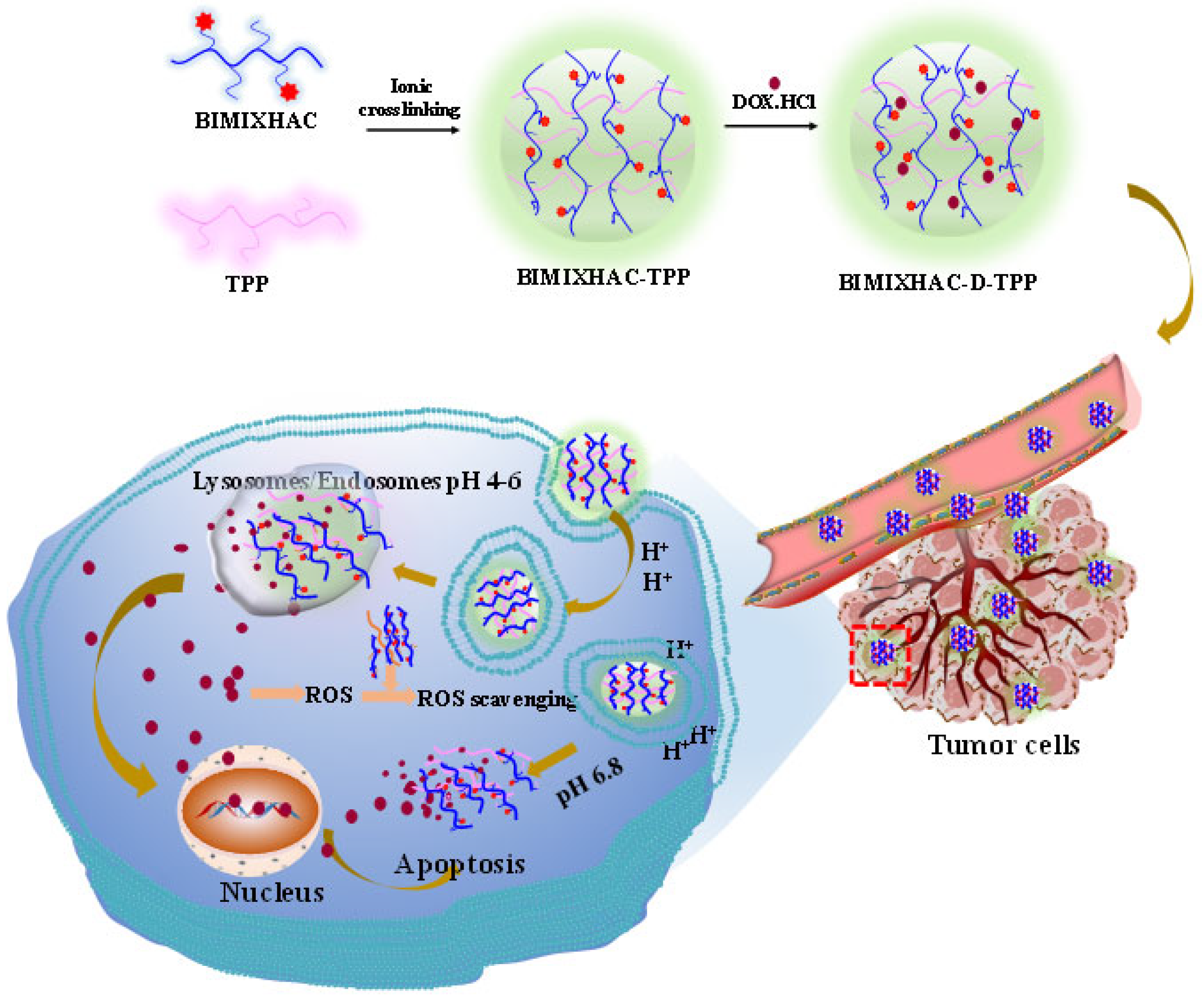
2.1. Chemical Synthesis and Characterization
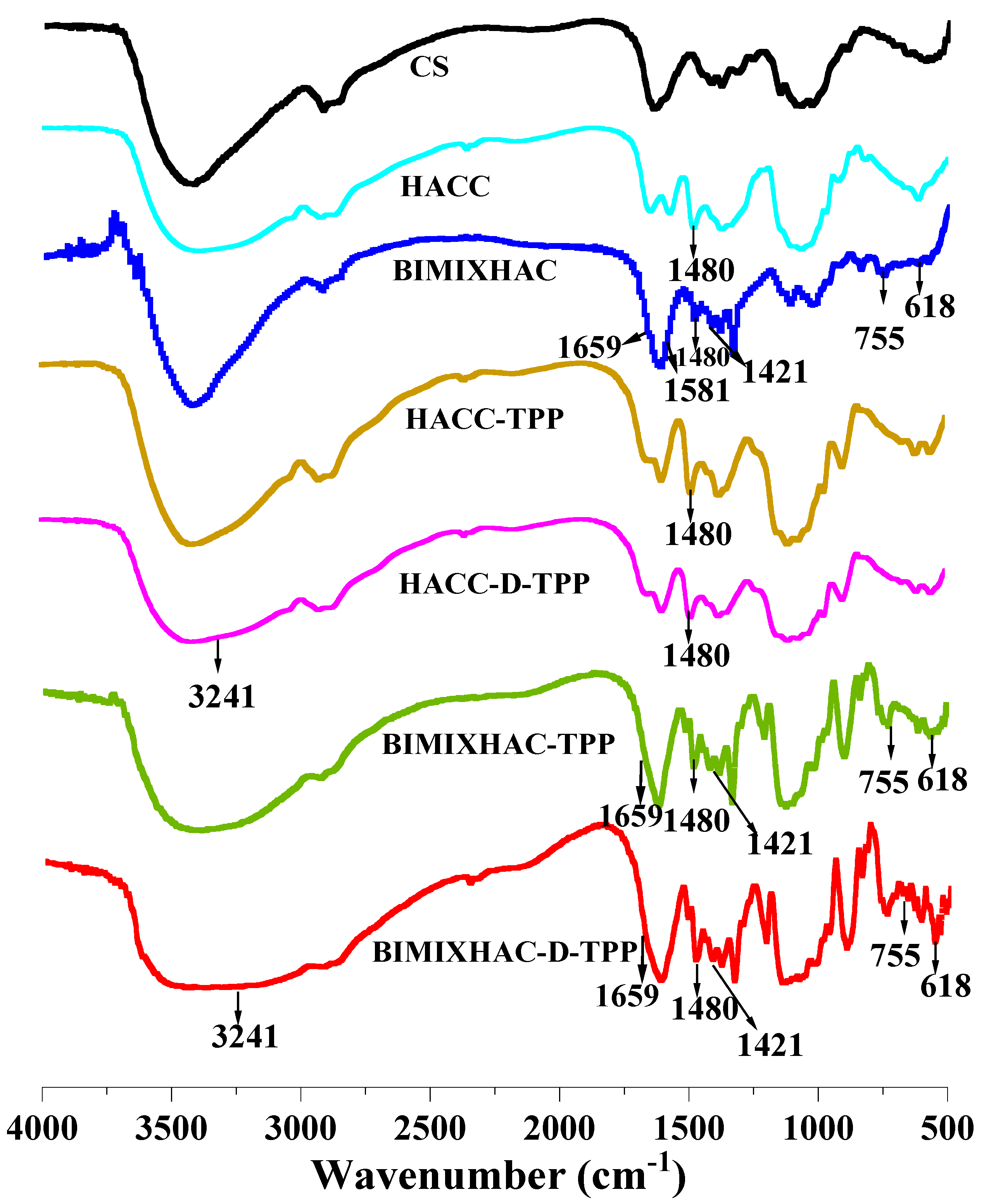
2.1.1. FT-IR Spectra
H NMR Spectroscopy
2.2. Characterization of Nanogels
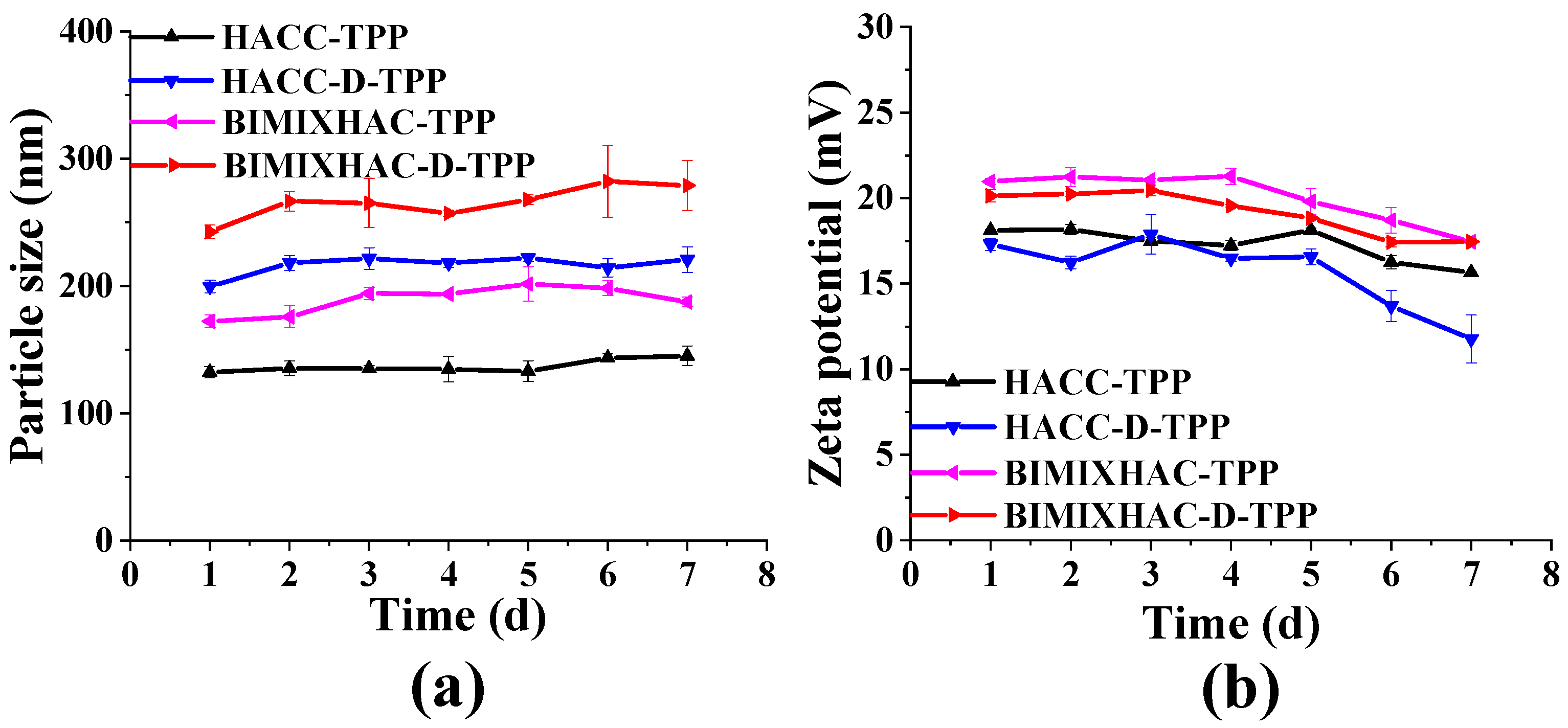
2.2.1. Zeta Potential, Particle Size, Polydispersity Index (PDI), and Stability of Nanogels
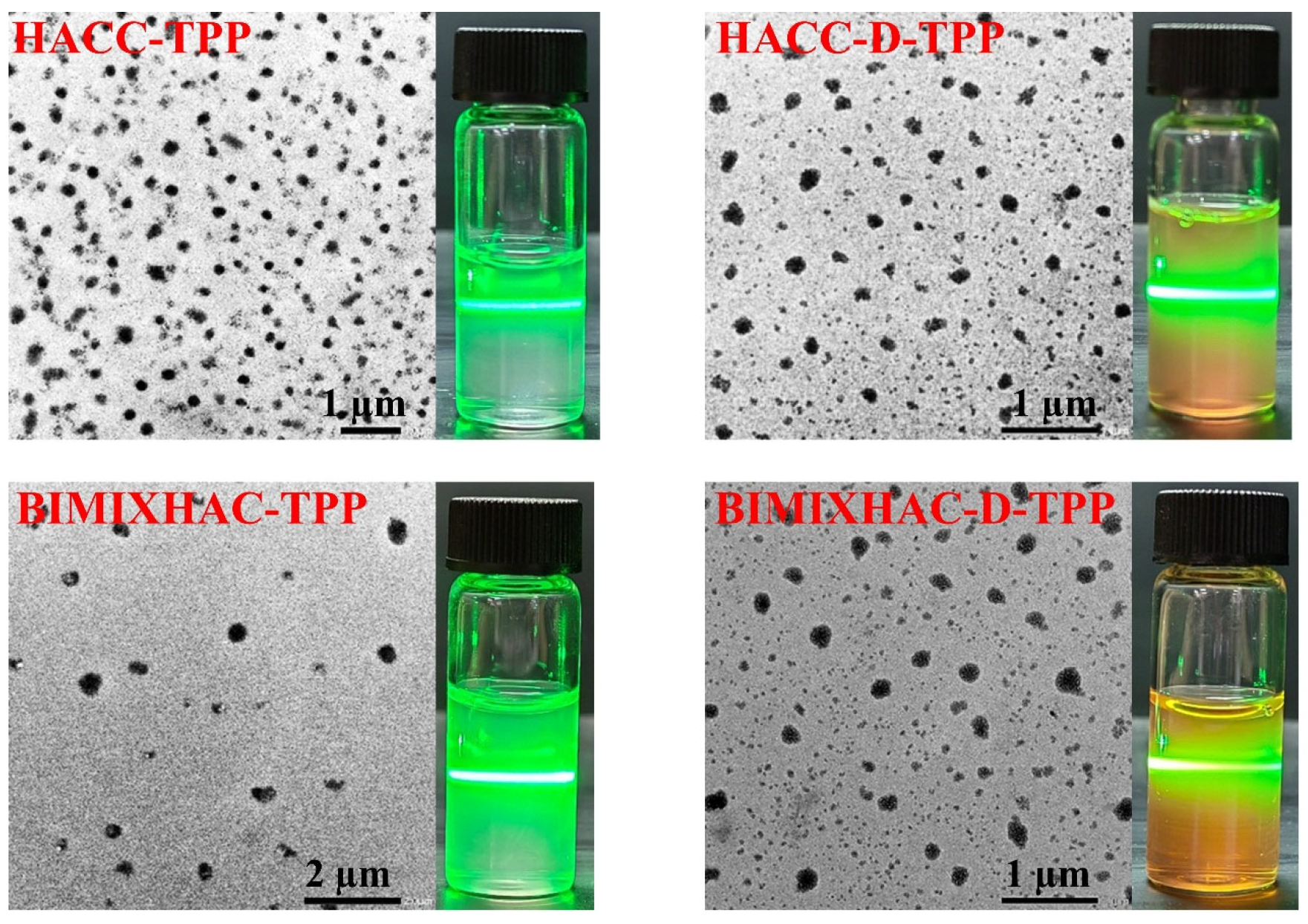
2.2.2. Morphology of Nanogels
2.2.3. Drug Entrapped Efficiency and Loading Efficiency of Nanogels
2.3. In Vitro Drug Release
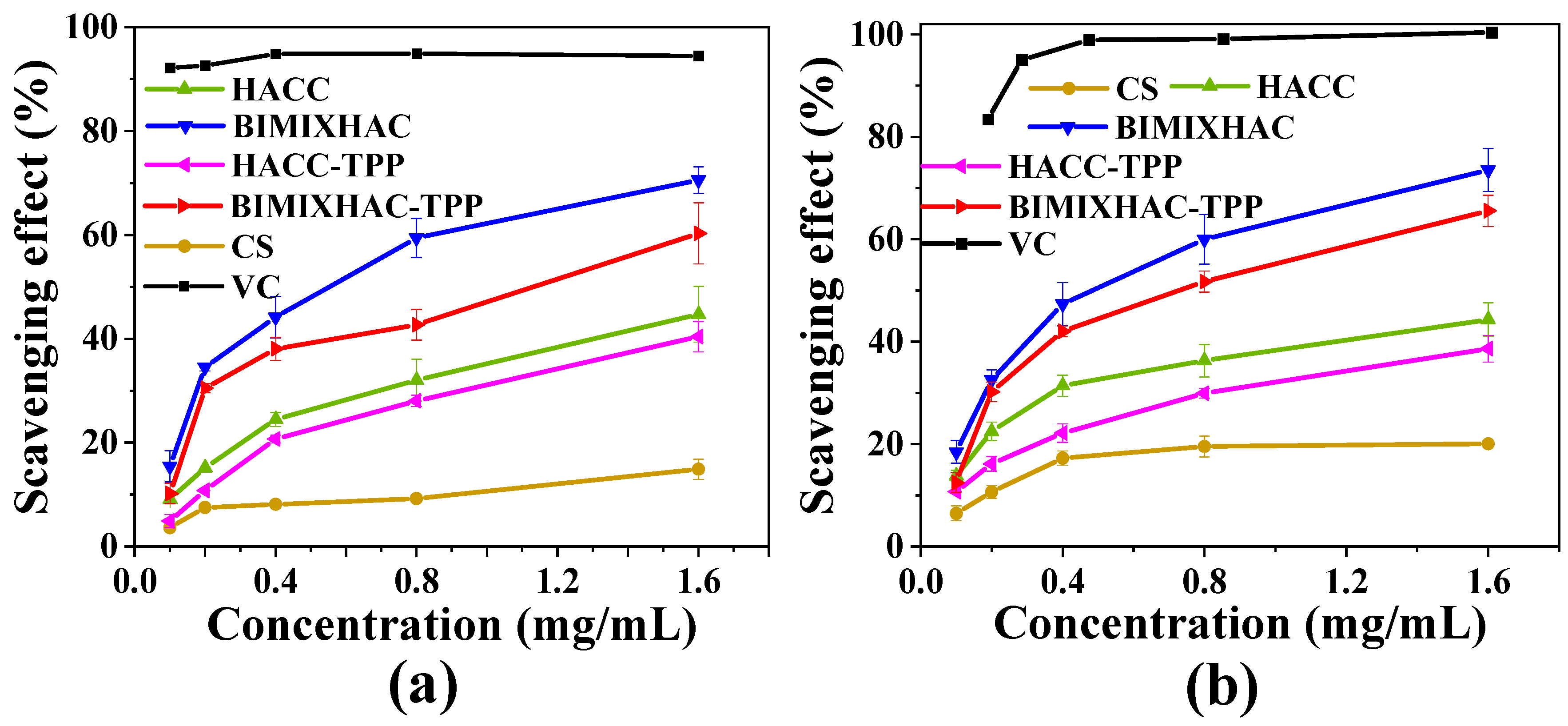
2.4. Antioxidant Activity
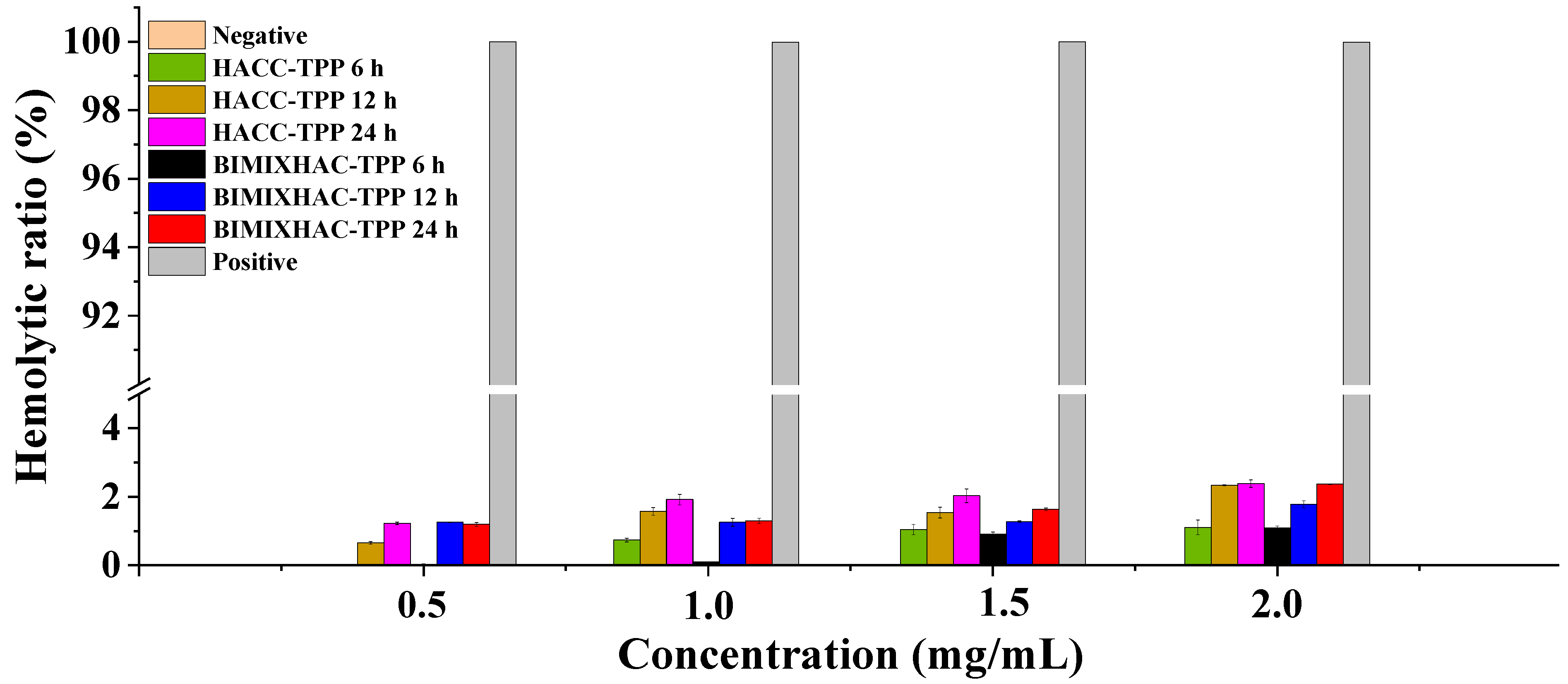
2.5. Hemolysis Analysis
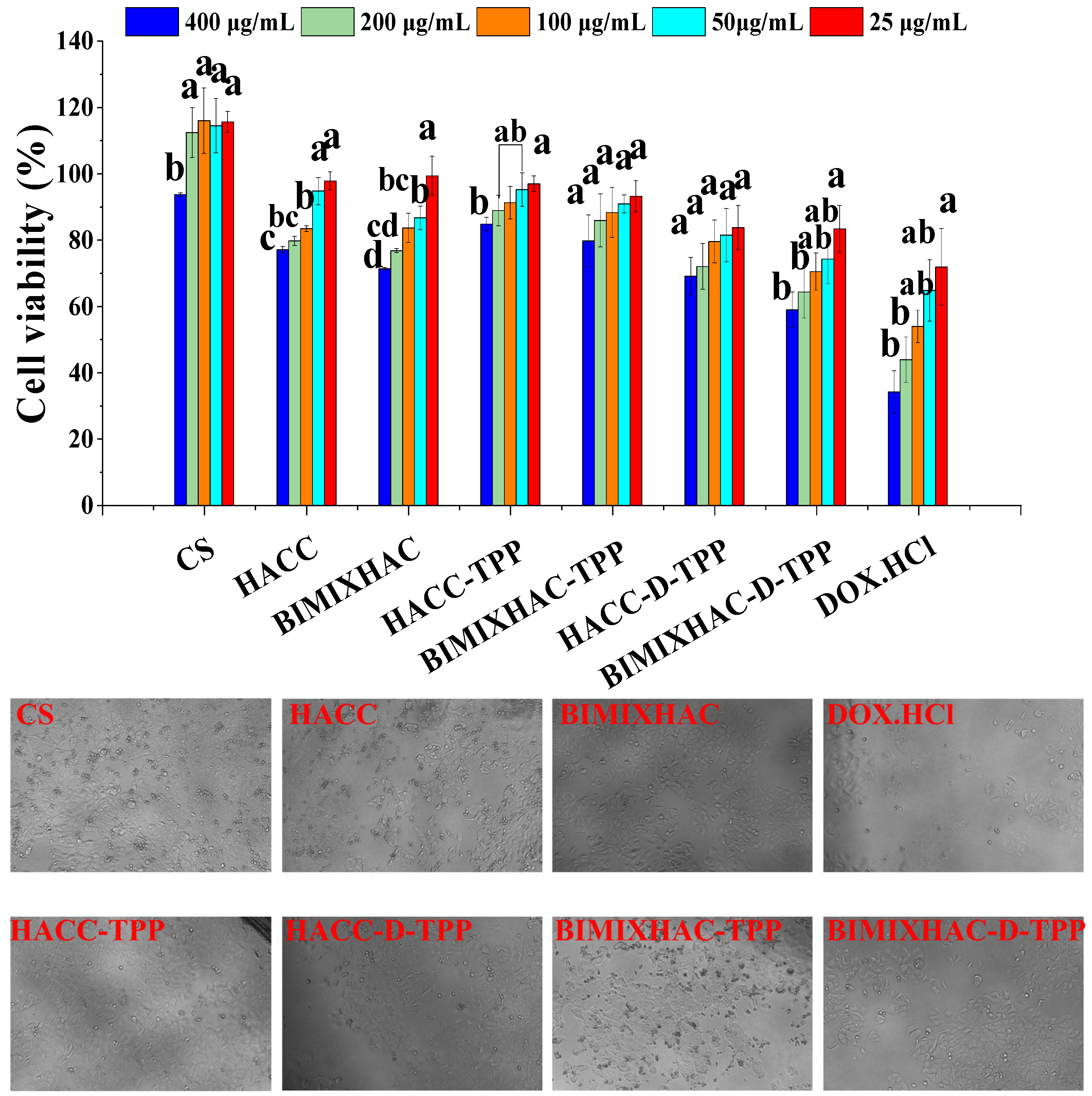
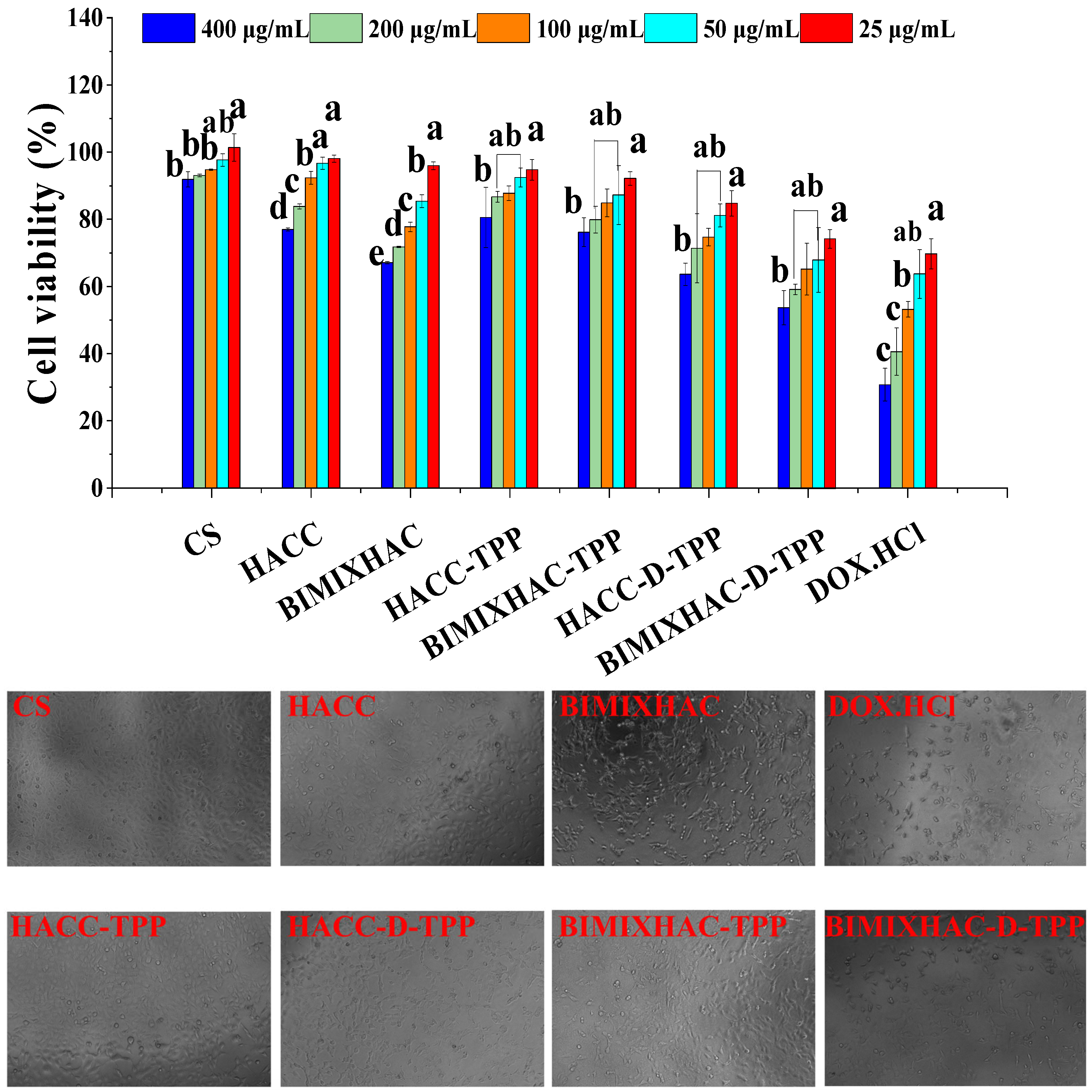
2.6. Cytotoxicity Assay
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hydroxypropyl Trimethyl Ammonium Chloride Chitosan (HACC), and HACC Grafting Benzimidazolic Acid (BIMIXHAC)
3.3. Analytical Methods
3.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.4. Preparation of Nanogels Based on HACC and BIMIXHAC
3.5. Characterization of Nanogels
3.5.1. Zeta Potential, Particle Size, Polydispersity Index (PDI) and Storage Stability of Nanogels
3.5.2. Morphology
3.5.3. Drug Entrapped Efficiency (EE) and Loading Efficiency (LE) of Nanogels
3.6. In Vitro Drug Release
3.7. Antioxidant Assay
3.7.1. DPPH Radical Scavenging Activity
3.7.2. Superoxide Anion Radical Scavenging Activity
3.8. Hemocompatibility Assay
3.9. Cell Cytotoxicity Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.L.; Song, S.S.; Wang, M.; Wang, H.X. Transforming a highly toxic agent DM1 into injectable safe nanomedicines via prodrug self-assembly for the treatment of taxane-resistant cancer. Nanoscale 2023, 15, 10110–10124. [Google Scholar] [CrossRef]
- Oroudjev, E.; Lopus, M.; Wilson, L.; Audette, C.; Provenzano, C.; Erickson, H.; Kovtun, Y.; Chari, R.; Jordan, M.A. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol. Cancer Ther. 2010, 9, 2700–2713. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Li, S.H.; Xu, G.; Man, X.Y.; Yang, T.F.; Zhang, Z.L.; Liang, H.; Yang, F. Developing a Ruthenium(III) Complex to Trigger Gasdermin E-Mediated Pyroptosis and an Immune Response Based on Decitabine and Liposomes: Targeting Inhibition of Gastric Tumor Growth and Metastasis. J. Med. Chem. 2023, 66, 13072–13130. [Google Scholar] [CrossRef]
- Christowitz, C.; Davis, T.; Isaacs, A.; van Niekerk, G.; Hattingh, S.; Engelbrecht, A.M. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 2019, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.K.; Trumpp, A.; Müller-Tidow, C. Therapy resistance mechanisms in hematological malignancies. Int. J. Cancer 2023, 152, 340–347. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.L.; Xie, D.D.; Zhou, M.; Liao, J.X.; Wu, H.L.; Dai, Y.; Huang, J.B.; Zhao, Y. PLGA Nanoparticles Containing VCAM-1 Inhibitor Succinobucol and Chemotherapeutic Doxorubicin as Therapy against Primary Tumors and Their Lung Metastases. Pharmaceutics 2023, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Soflou, R.; Karkhaneh, A. Redox-Sensitive multifunctional hyaluronic acid-based nanomicelles with Fine-controlled anticancer drug release. Int. J. Pharm. 2022, 629, 122402. [Google Scholar] [CrossRef]
- Renu, K.; Abilash, V.G.; Pichiah, P.B.T.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy–An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Singh, M.; Nicol, A.T.; DelPozzo, J.; Wei, J.; Singh, M.; Nguyen, T. Demystifying the relationship between metformin, AMPK, and doxorubicin cardiotoxicity. Front. Cardiovasc. Med. 2022, 9, 839644. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int. J. Mol. Sci. 2015, 17, 14. [Google Scholar] [CrossRef]
- Kassner, N.; Huse, K.; Martin, H.J.; Godtel-Armbrust, U.; Metzger, A.; Meineke, I.; Brockmoller, J.; Klein, K.; Zanger, U.M.; Maser, E.; et al. Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metab. Dispos. 2008, 36, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Lahoti, T.S.; Patel, D.; Thekkemadom, V.; Beckett, R.; Ray, S.D. Doxorubicin-induced in vivo nephrotoxicity involves oxidative stress-mediated multiple pro- and anti-apoptotic signaling pathways. Curr. Neurovasc. Res. 2012, 9, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.B.; Hong, L.; Cai, X.Y.; Xiao, B.; Zhang, P.; Shao, L. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar]
- Li, D.L.; Hill, J.A. Cardiomyocyte autophagy and cancer chemotherapy. J. Mol. Cell. Cardiol. 2014, 71, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, J.D.; Sun, S.M.; Lin, H.; Zhang, H.L.; Zhong, Z.Q.; Chi, J.F.; Guo, H.Y. Protective effect of urotensin II receptor antagonist urantide and exercise training on doxorubicin-induced cardiotoxicity. Sci. Rep. 2023, 13, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Mukai, S.A.; Sasaki, Y.; Akiyoshi, K. Nanogel Tectonics for Tissue Engineering: Protein Delivery Systems with Nanogel Chaperones. Adv. Healthc. Mater. 2018, 7, 23–78. [Google Scholar] [CrossRef]
- Sankaranarayanan, A.; Ramprasad, A.; Ganesh, S.S.; Ganesh, H.; Ramanathan, B.; Shanmugavadivu, A.; Selvamurugan, N. Nanogels for bone tissue engineering-from synthesis to application. Nanoscale 2023, 15, 10206–10222. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef]
- Geyik, G.; Guncum, E.; Isiklan, N. Design and development of pH-responsive alginate-based nanogel carriers for etoposide delivery. Int. J. Biol. Macromol. 2023, 250, 126242. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Wang, S.H.; Wu, F.G. Nanogels: Synthesis, properties, and recent biomedical applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cuenot, S.; Radji, S.; Alem, H.; Demoustier-Champagne, S.; Jonas, A.M. Control of swelling of responsive nanogels by nanoconfinement. Small 2012, 8, 2978–2985. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.Y.; Zhang, A.T.; Cheng, F.F.; Cui, L.; Liu, J.Q.; Xia, Y.Z. Biodegradable polymeric architectures via reversible deactivation radical polymerizations. Polymers 2018, 10, 758. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, E.; Özkan, B.; Güngör, S.; Özsoy, Y. Biopolymer-Based Nanogel Approach in Drug Delivery: Basic Concept and Current Developments. Pharmaceutics 2023, 15, 1644. [Google Scholar] [CrossRef]
- Li, H.P.; Zhou, Z.W.; Zhang, F.R.; Guo, Y.X.; Yang, X.; Jiang, H.L. A networked swellable dextrin nanogels loading Bcl2 siRNA for melanoma tumor therapy. Nano Res. 2018, 11, 4627–4642. [Google Scholar] [CrossRef]
- Goldberg, M.; Langer, R.; Jia, X.Q. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef]
- Myerson, J.W.; McPherson, O.; DeFrates, K.G.; Towslee, J.H.; Marcos-Contreras, O.A.; Shuvaev, V.V.; Braender, B.; Composto, R.J.; Muzykantov, V.R.; Eckmann, D.M. Cross-linker-Modulated Nanogel Flexibility Correlates with Tunable Targeting to a Sterically Impeded Endothelial Marker. ACS Nano 2019, 13, 11409–11421. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, T.G.; Zhuang, Y.P.; He, T.D.; Wu, X.L.; Su, L.; Kang, J.; Chang, J.; Wang, H.J. Sodium Alginate Hydrogel-Mediated Cancer Immunotherapy for Postoperative in Situ Recurrence and Metastasis. ACS Biomater. Sci. Eng. 2021, 7, 5717–5726. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, M.; Wang, H.; Guo, Y.; Cheng, X.L.; Zhao, T.; Wang, H.Q.; Zhang, Y.F.; Ma, Y.; Tao, W.W. Exosome-sheathed ROS-responsive nanogel to improve targeted therapy in perimenopausal depression. J. Nanobiotechnol. 2023, 21, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Jang, M.S.; Sun, X.S.; Liu, C.L.; Lee, J.H.; Li, Y.; Fu, Y. CD44-mediated tumor homing of hyaluronic acid nanogels for hypoxia-activated photodynamic therapy against tumor. Colloid Surf. B 2023, 228, 113395. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.F.; Chen, J.; Xu, R. Recent Progress in Bio-Responsive Drug Delivery Systems for Tumor Therapy. Front. Bioeng. Biotechnol. 2022, 10, 916952. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Heawchaiyaphum, C.; Yoshiyama, H.; Iizasa, H.; Burassakarn, A.; Tumurgan, Z.; Ekalaksananan, T.; Pientong, C. Epstein-Barr Virus Promotes Oral Squamous Cell Carcinoma Stemness through the Warburg Effect. Int. J. Mol. Sci. 2023, 24, 14072. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, W.A.; Wu, Q.; Hitomi, M.; Rahim, N.; Kim, Y.; Sloan, A.E.; Weil, R.J.; Nakano, I.; Sarkaria, J.N.; Stringer, B.W.; et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat. Neurosci. 2013, 16, 1373–1384. [Google Scholar] [CrossRef]
- Raines, L.N.; Huang, S.C. Is glucose the scapegoat for tumor evasion. Cancer Cell. 2021, 39, 907–909. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Bhattacharjee, S. Chemical, physical, and biological stimuli-responsive nanogels for biomedical applications (mechanisms, concepts, and advancements): A review. Int. J. Biol. Macromol. 2023, 226, 535–553. [Google Scholar] [CrossRef]
- Cinay, G.E.; Erkoc, P.; Alipour, M.; Hashimoto, Y.; Sasaki, Y.; Akiyosh, K.; Kizilel, S. Nanogel-Integrated pH-Responsive Composite Hydrogels for Controlled Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 370–380. [Google Scholar] [CrossRef]
- Pragti, B.K.; Kundu, B.K.; Singh, S.; Ranjith, W.A.C.; Sarkar, S.; Sonawane, A.; Mukhopadhyay, S. Chitosan-Biotin-Conjugated pH-Responsive Ru(II) Glucose Nanogel: A Dual Pathway of Targeting Cancer Cells and Self-Drug Delivery. ACS Appl. Mater. Interfaces 2023, 15, 43345–43358. [Google Scholar] [CrossRef]
- Mahmoodzadeh, F.; Ghorbani, M.; Jannat, B. Glutathione and pH-responsive chitosan-based nanogel as an efficient nanoplatform for controlled delivery of doxorubicin. J. Drug Deliv. Sci. Technol. 2019, 54, 101315. [Google Scholar] [CrossRef]
- Li, D.Y.; Xu, W.S.; Liu, H. Fabrication of chitosan functionalized dual stimuli-responsive injectable nanogel to control delivery of doxorubicin. Colloid Polym. Sci. 2023, 301, 879–891. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Gujarathi, P.; Pansare, P.; Tripathi, S. A comprehensive review on alginate-based delivery systems for the delivery of chemotherapeutic agent: Doxorubicin. Carbohydr. Polym. 2021, 259, 117696. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Zhong, S.L.; Xu, L.F.; Wang, J.F.; Zhang, Z.Q.; Gao, Y.; Cui, X.J. Review on design strategies and considerations of polysaccharide-based smart drug delivery systems for cancer therapy. Carbohydr. Polym. 2022, 279, 119013. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.; Alvi, M.A.U.R.; Niazi, M.B.K.; Batool, M.; Bhatti, M.F.; Khan, A.L.; Khan, A.U.; Jamil, T.; Ahmad, N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr. Polym. 2019, 207, 17–25. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, J.J.; Mi, Y.Q.; Miao, Q.; Tan, W.Q.; Guo, Z.Y. Preparation of imidazole acids grafted chitosan with enhanced antioxidant, antibacterial and antitumor activities. Carbohydr. Polym. 2023, 315, 120978. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.Q.; Zhang, J.J.; Zhang, L.L.; Li, Q.; Cheng, Y.Z.; Guo, Z.Y. Synthesis, Characterization, and Evaluation of Nanoparticles Loading Adriamycin Based on 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan Grafting Folic Acid. Polymers 2021, 13, 2229. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Tan, W.Q.; Zhang, J.J.; Mi, Y.Q.; Miao, Q.; Guo, Z.Y. Preparation and Characterization of Chitosan Derivatives Bearing Imidazole Ring with Antioxidant, Antibacterial, and Antifungal Activities. Starch-Stärke 2023, 75, 2200204. [Google Scholar] [CrossRef]
- Pan, Q.Y.; Zhou, C.; Yang, Z.M.; He, Z.Y.; Wang, C.; Liu, Y.H.; Song, S.H.; Gu, H.; Hong, K.Q.; Yu, L.J.; et al. Preparation and characterization of chitosan derivatives modified with quaternary ammonium salt and quaternary phosphate salt and its effect on tropical fruit preservation. Food Chem. 2022, 387, 132878. [Google Scholar] [CrossRef]
- Tang, F.L.; Lv, L.M.; Lu, F.; Rong, B.; Li, Z.Q.; Lu, B.T.; Yu, K.; Liu, J.W.; Dai, F.Y.; Wu, D.Y.; et al. Preparation and characterization of N-chitosan as a wound healing accelerator. Int. J. Biol. Macromol. 2016, 93, 1295–1303. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Huang, K.X.; Li, J.R.; Wang, K.; Zhang, K.; Tang, X.Y. Preparation and characterization of carboxymethyl cellulose containing quaternized chitosan for potential drug carrier. Int. J. Biol. Macromol. 2020, 154, 1392–1399. [Google Scholar] [CrossRef]
- Mendapara, J.V.; Vaghasiya, M.D.; Rajani, D.P.; Ahmad, I.; Patel, H.; Kumari, P. Benzimidazole and piperidine containing novel 1,2,3-triazole hybrids as anti-infective agents: Design, synthesis, in silico and in vitro antimicrobial efficacy. J. Biochem. Mol. Toxicol. 2023, 37, e23526. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, S.; Iqbal, U.; Yousuf, S.; Siddiqi, H.M.; Liaqat, F.; Rehman, H.M.; Akhtar, Z.; El-Shafei, A.; Shahzad, N. Synthesis of new benzimidazole based ruthenium (II) dyes for application in dye-sensitized solar cells with detailed spectroscopic and theoretical evaluation. J. Mol. Struct. 2023, 1289, 135860. [Google Scholar] [CrossRef]
- Wang, S.; Pi, L.; Wen, H.; Yu, H.; Yang, X. Evaluation of novel magnetic targeting microspheres loading adriamycin based on carboxymethyl chitosan. J. Drug Deliv. Sci. Technol. 2020, 55, 101388. [Google Scholar] [CrossRef]
- Xu, X.F.; Li, Y.G.; Wang, F.H.; Lv, L.; Liu, J.Y.; Li, M.N.; Guo, A.J.; Jiang, J.J.; Shen, Y.Y.; Guo, S.R. Synthesis, in vitro and in vivo evaluation of new norcantharidin-conjugated hydroxypropyltrimethyl ammonium chloride chitosan derivatives as polymer therapeutics. Int. J. Pharm. 2013, 453, 610–619. [Google Scholar] [CrossRef]
- Mahalingam, S.; Murugesan, A.; Thiruppathiraja, T.; Lakshmipathi, S.; Makhanya, T.R.; Gengan, R.M. Green synthesis of benzimidazole derivatives by using zinc boron nitride catalyst and their application from DFT (B3LYP) study. Heliyon 2022, 8, e11480. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter. Methods 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Z.; Gao, Y.; Shi, Y.B.; Qiu, S.N.; Lin, G.M.; Ding, X.B.; Wang, W.G.; Feng, Y.H.; Wang, F.; Qiao, J.W. Design of in vitro biomimetic experimental system and simulation analysis for transvascular transport of nano-preparation. Microvas. Res. 2024, 151, 104597. [Google Scholar] [CrossRef]
- Panda, P.K.; Jain, S.K. Doxorubicin bearing peptide anchored PEGylated PLGA nanoparticles for the effective delivery to prostate cancer cells. J. Drug Deliv. Sci. Technol. 2023, 86, 104667. [Google Scholar] [CrossRef]
- Kelishomi, R.B.; Ejtemaeemehr, S.; Tavangar, S.M.; Rahimian, R.; Mobarakeh, J.I.; Dehpour, A.R. Morphine is protective against doxorubicin-induced cardiotoxicity in rat. Toxicology 2008, 243, 96–104. [Google Scholar] [CrossRef]
- Rahimi_Balaei, M.; Momeny, M.; Babaeikelishomi, R.; Mehr, S.E.; Tavangar, S.M.; Dehpour, A.R. The modulatory effect of lithium on doxorubicin-induced cardiotoxicity in rat. Eur. J. Pharmacol. 2010, 641, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, M.; Abdollahi, A.; Abdolahi, F.; Abdolghaffari, A.H.; Dehpour, A.R.; Jazaeri, F. Protective effects of magnesium sulfate against doxorubicin induced cardiotoxicity in rats. Life Sci. 2018, 207, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Li, N.N.; Wang, R.; Sun, Y.Y.; He, X.Y.; Lu, X.Y.; Chu, L.X.; Sun, K.X. Enhanced therapeutic efficacy of doxorubicin against multidrug-resistant breast cancer with reduced cardiotoxicity. Drug Deliv. 2023, 30, 2189118. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Fan, C.; Wang, H.; Jia, C.Y.; Li, X.T.; Yang, J.R.; Zhang, T.; Gao, S.; Min, X.; Huang, J. Type VI secretion system-associated FHA domain protein TagH regulates the hemolytic activity and virulence of Vibrio cholera. Gut Microbes 2022, 14, e2055440. [Google Scholar] [CrossRef]
- Qiu, J.G.; Tomeh, M.A.; Jin, Y.; Zhang, B.; Zhao, X.B. Microfluidic formulation of anticancer peptide loaded ZIF-8 nanoparticles for the treatment of breast cancer. J. Colloid Interf. Sci. 2023, 15, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.Q.; Zhang, J.J.; Tan, W.Q.; Miao, Q.; Li, Q.; Guo, Z.Y. Preparation of Doxorubicin-Loaded Carboxymethyl-β-Cyclodextrin/Chitosan Nanoparticles with Antioxidant, Antitumor Activities and pH-Sensitive Release. Mar. Drugs 2022, 20, 278. [Google Scholar] [CrossRef]
- Ma, B.; Li, Q.; Mi, Y.Q.; Zhang, J.J.; Tan, W.Q.; Guo, Z.Y. pH-responsive nanogels with enhanced antioxidant and antitumor activities on drug delivery and smart drug release. Int. J. Biol. Macromol. 2023, 315, 120978. [Google Scholar] [CrossRef]



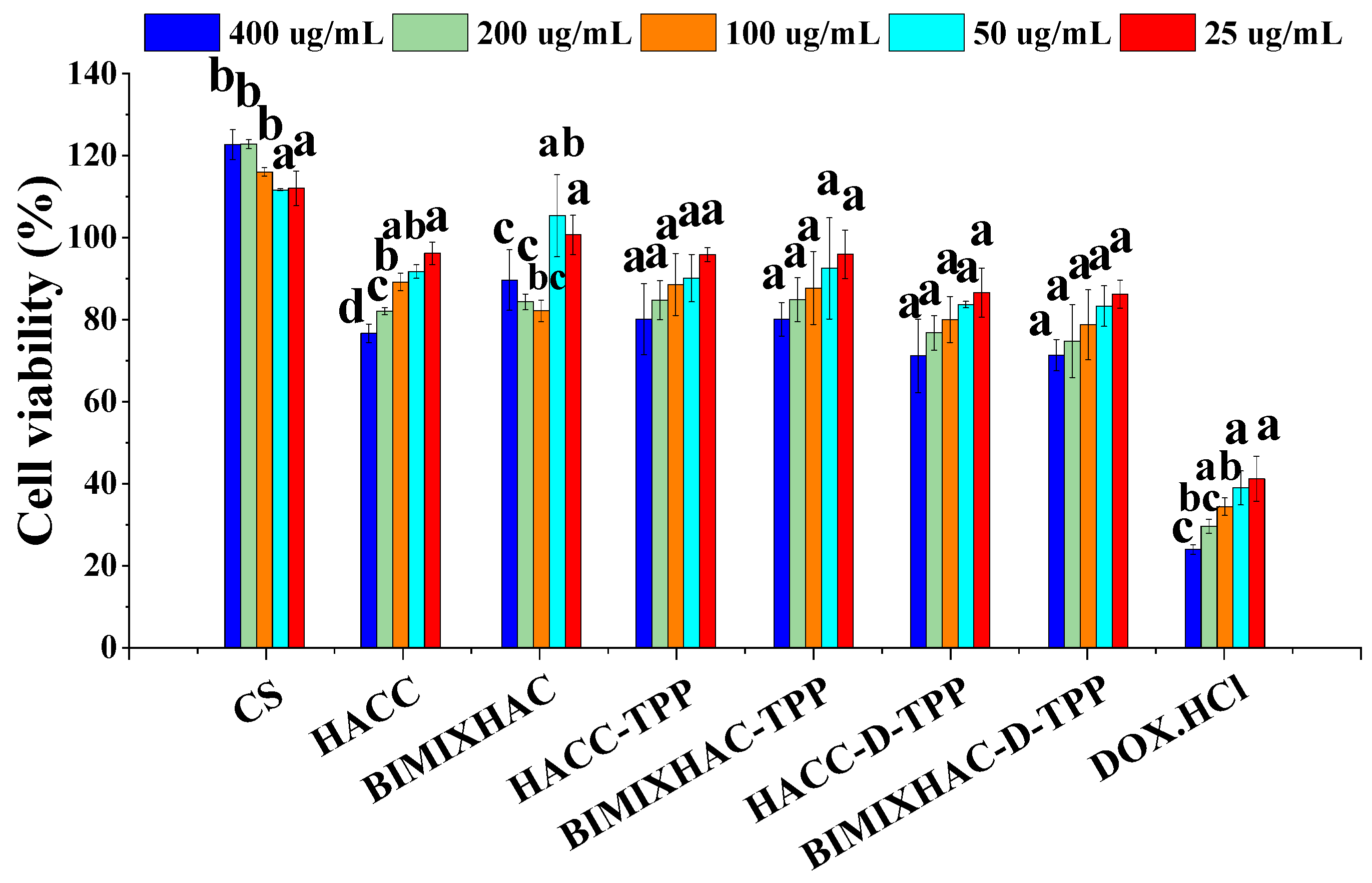
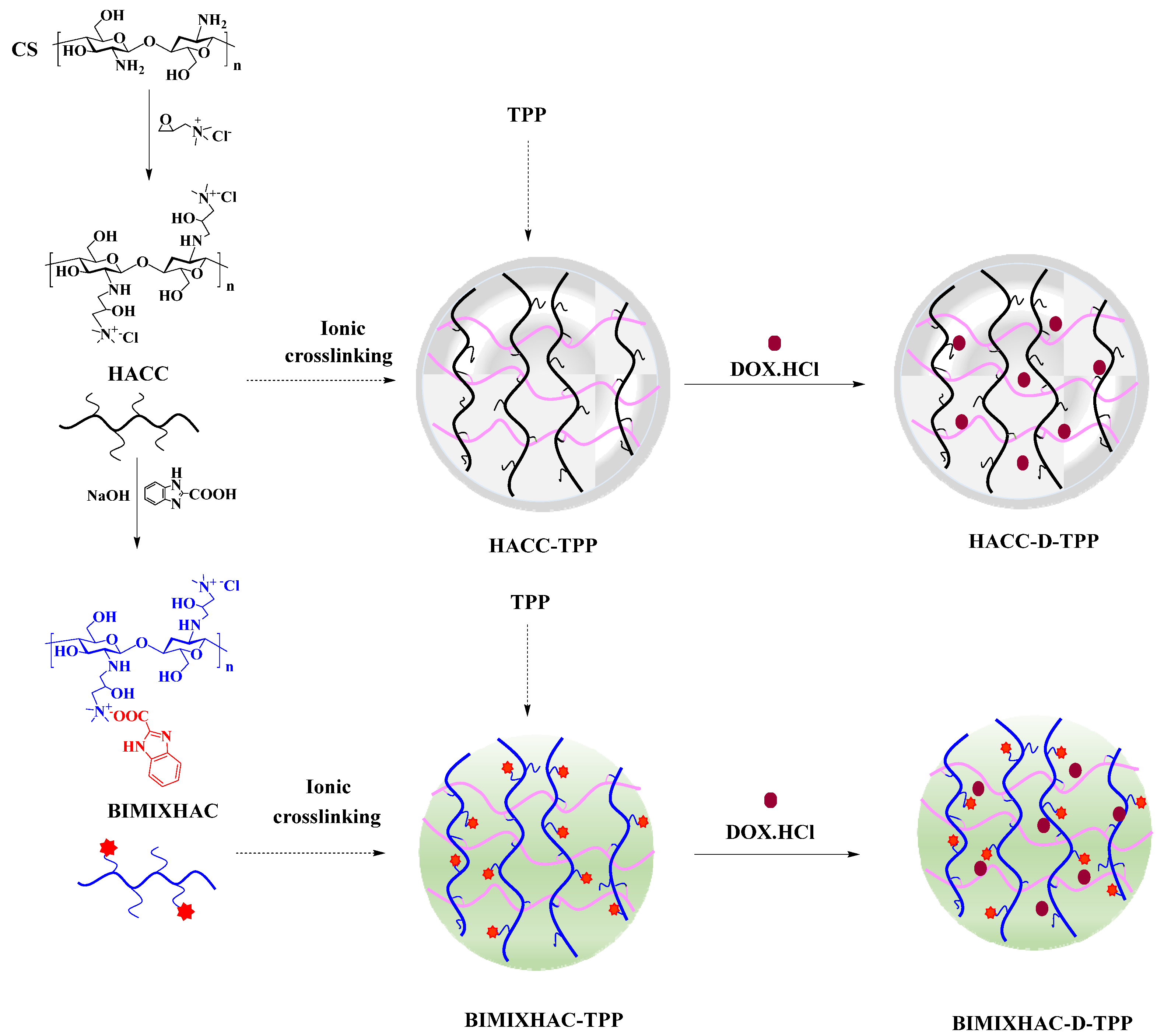
| Sample | Size (nm) | Zeta Potential (mV) | PDI (%) | EE (%) | LE (%) |
|---|---|---|---|---|---|
| HACC-TPP | 132.21 ± 4.51 | 18.12 ± 0.06 | 28.18 ± 1.09 | – | – |
| HACC-D-TPP | 199.6 ± 4.87 | 20.69 ± 0.27 | 28.25 ± 0.91 | 90.02 ± 0.25 | 26.74 ± 0.07 |
| BIMIXHAC-TPP | 172.33 ± 4.95 | 20.97 ± 0.17 | 26.39 ± 0.51 | – | – |
| BIMIXHAC-D-TPP | 242.78 ± 5.31 | 20.12 ± 0.36 | 27.67 ± 0.44 | 93.17 ± 0.27 | 31.17 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Li, Q.; Zhang, J.; Mi, Y.; Tan, W.; Guo, Z. Improvement of the Antioxidant and Antitumor Activities of Benzimidazole-Chitosan Quaternary Ammonium Salt on Drug Delivery Nanogels. Mar. Drugs 2024, 22, 40. https://doi.org/10.3390/md22010040
Ma B, Li Q, Zhang J, Mi Y, Tan W, Guo Z. Improvement of the Antioxidant and Antitumor Activities of Benzimidazole-Chitosan Quaternary Ammonium Salt on Drug Delivery Nanogels. Marine Drugs. 2024; 22(1):40. https://doi.org/10.3390/md22010040
Chicago/Turabian StyleMa, Bing, Qing Li, Jingjing Zhang, Yingqi Mi, Wenqiang Tan, and Zhanyong Guo. 2024. "Improvement of the Antioxidant and Antitumor Activities of Benzimidazole-Chitosan Quaternary Ammonium Salt on Drug Delivery Nanogels" Marine Drugs 22, no. 1: 40. https://doi.org/10.3390/md22010040
APA StyleMa, B., Li, Q., Zhang, J., Mi, Y., Tan, W., & Guo, Z. (2024). Improvement of the Antioxidant and Antitumor Activities of Benzimidazole-Chitosan Quaternary Ammonium Salt on Drug Delivery Nanogels. Marine Drugs, 22(1), 40. https://doi.org/10.3390/md22010040






