Chitotriose Enhanced Antitumor Activity of Doxorubicin through Egr1 Upregulation in MDA-MB-231 Cells
Abstract
:1. Introduction
2. Results
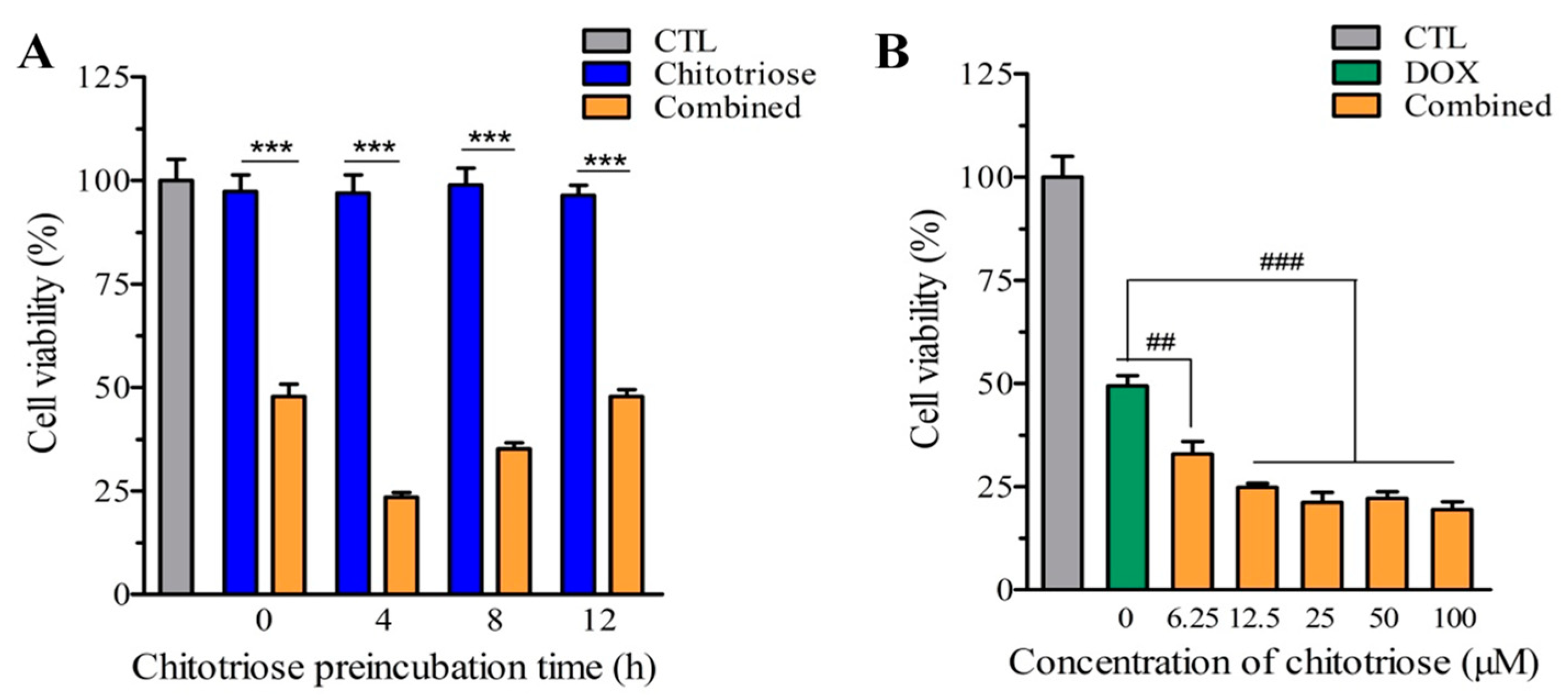
2.1. Chitotriose Impaired the Growth of MDA-MB-231 Cells via Momentary Preincubation
2.2. Chitotriose Initiated Morphological Changes of MDA-MB-231 Cells
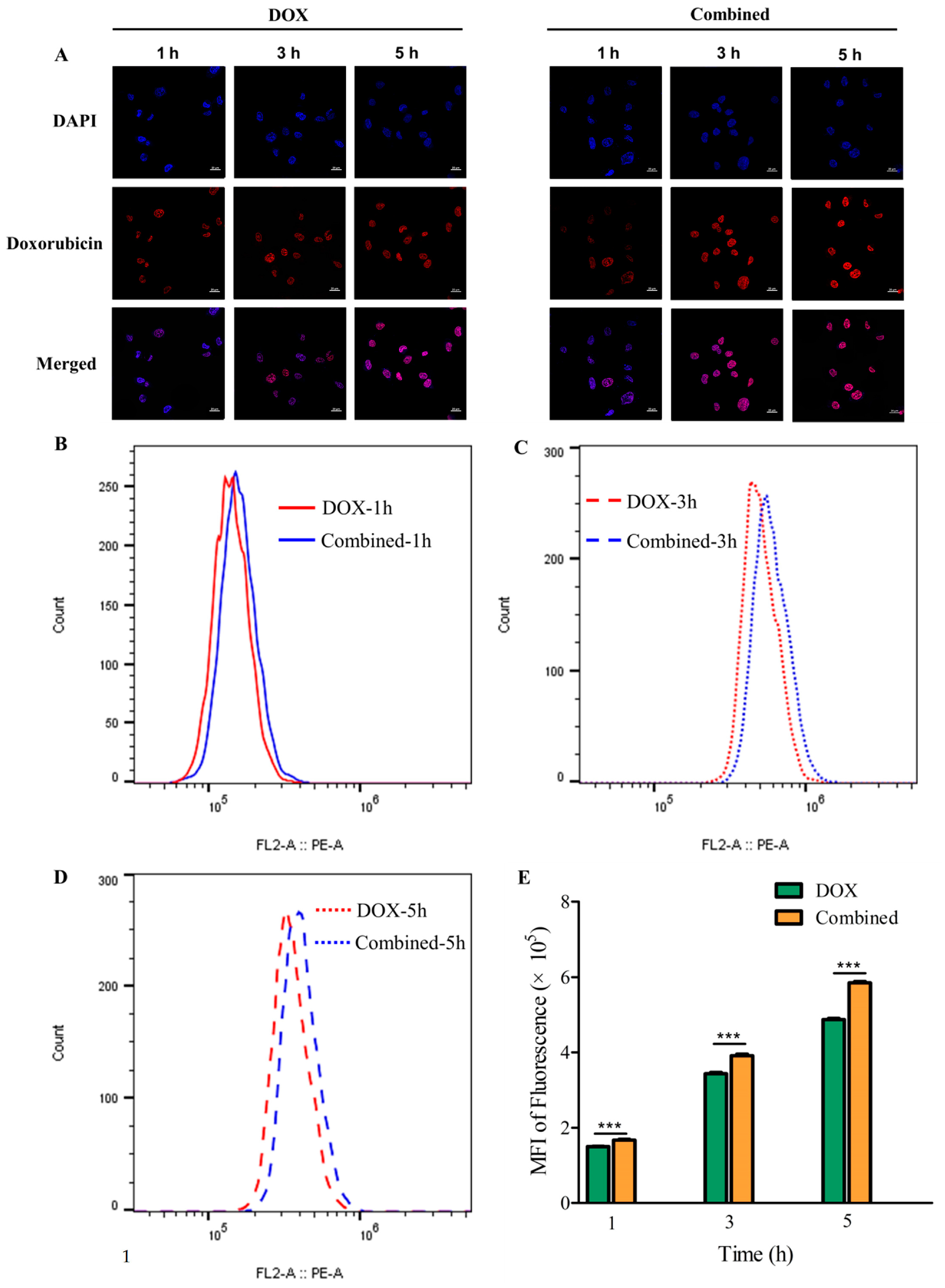
2.3. Chitotriose Enhanced Cell Apoptosis through Promoting Cellular Uptake of Doxorubicin
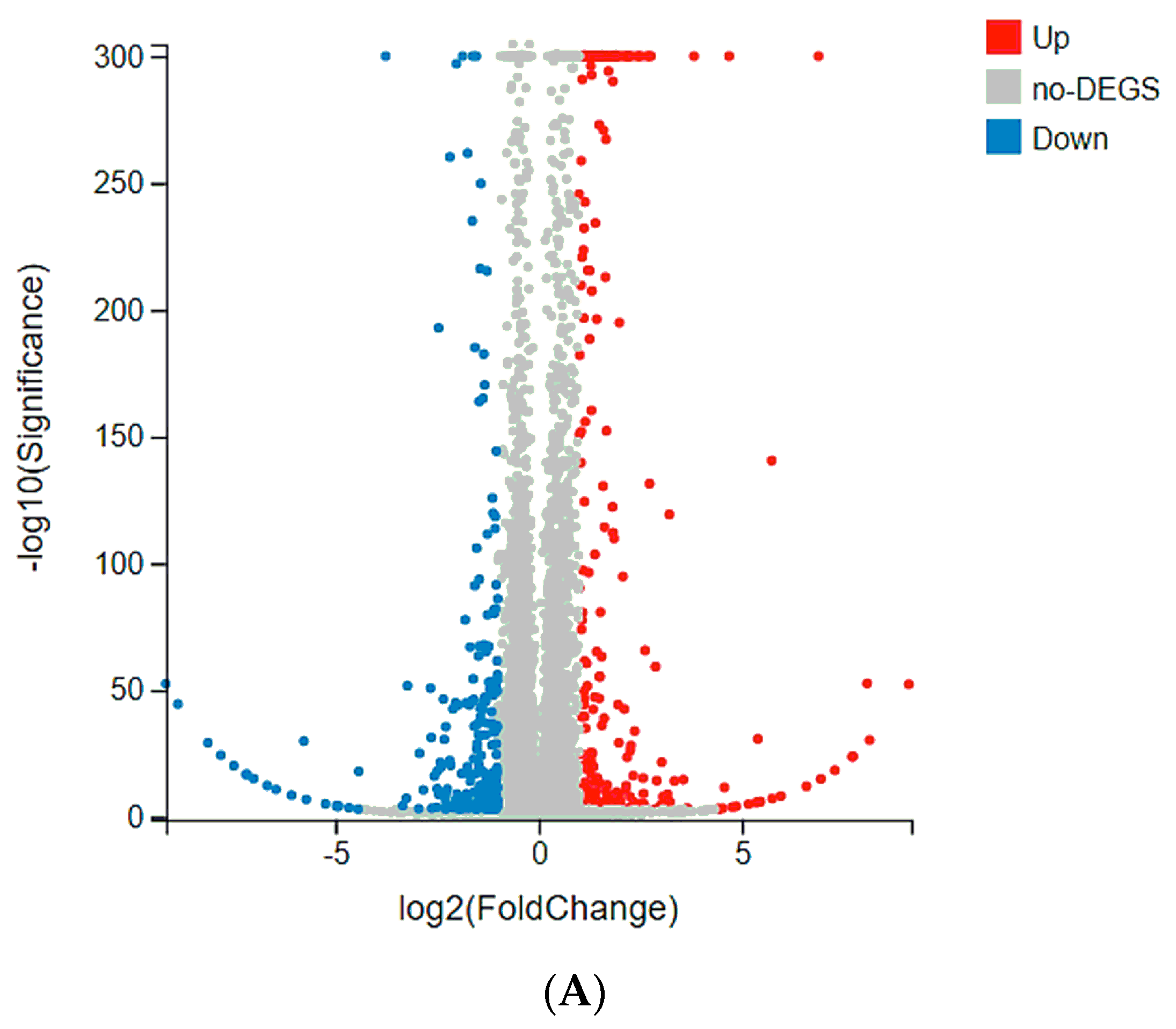
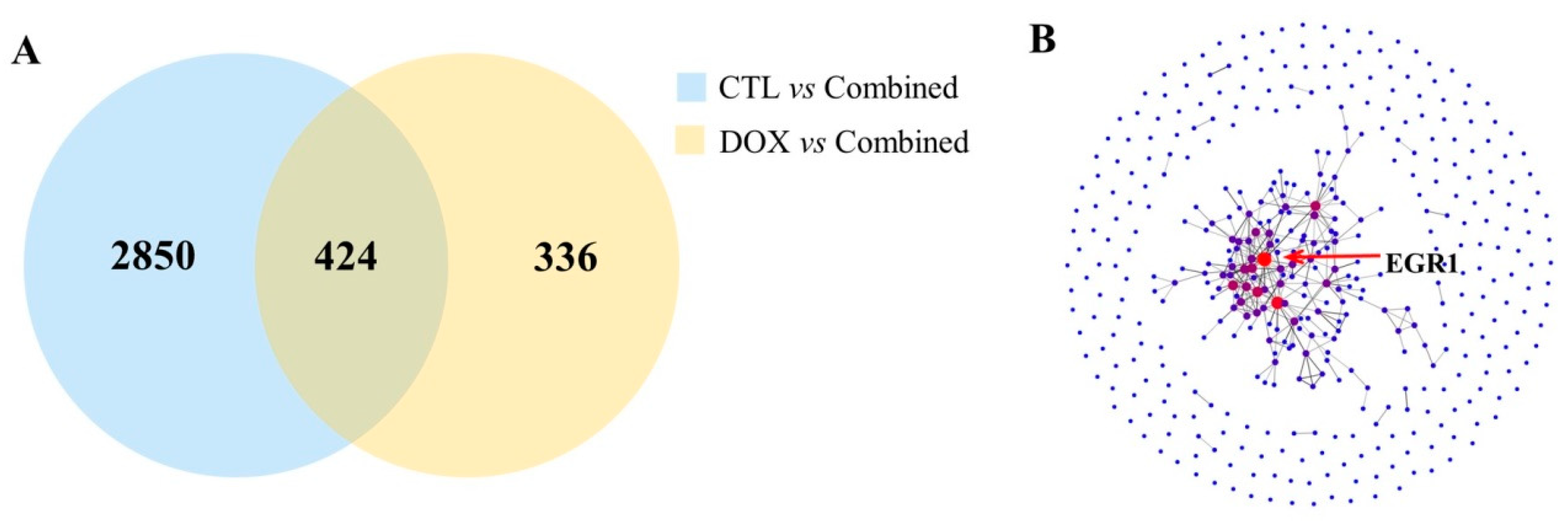
2.4. Tmem61 and Fgl2 Were Identified as Key Genes Correlated with MET
2.5. Upregulation of Egr1 Was Critical for Synergistic Effect of Composite Treatment
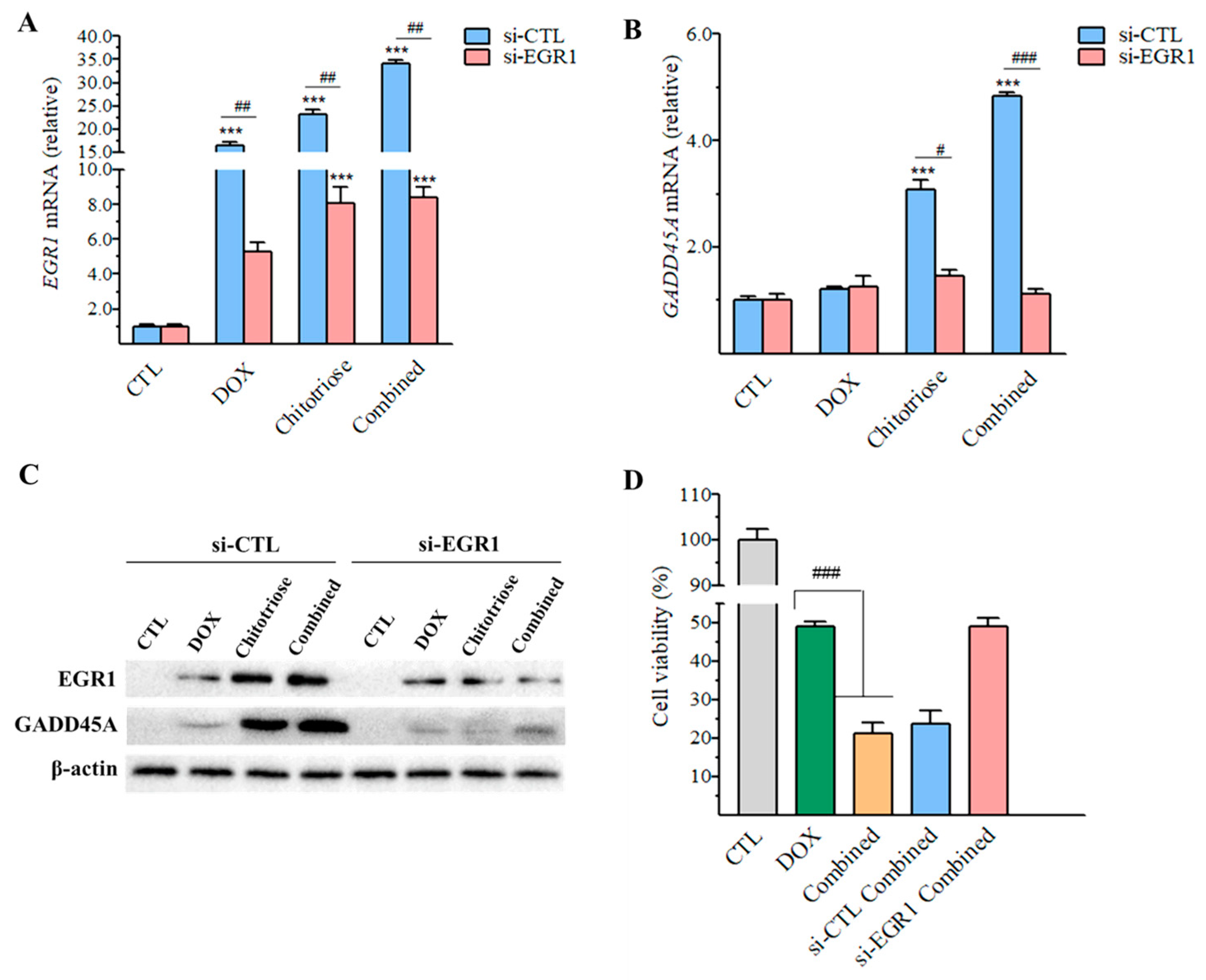
2.6. Downregulation of Egr1 with Small Interfering RNA (siRNA) Transfection Leading to Decrease in GADD45A
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture
4.3. Grouping and Treatment Experiment Design
4.4. Cell Viability Assay
4.5. Field Emission Scanning Electron Microscopy (FESEM)
4.6. Confocal Laser Scanning Microscopy (CLSM)
4.7. Flow Cytometry Analysis of Cellular Uptake
4.8. RNA Isolation, Library Construction and Sequencing
4.9. RT-qPCR Analysis
4.10. siRNA Transfection
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.F.S.; Kluskens, L.D.; Rodrigues, L.R. Triple Negative Breast Cancer: Nanosolutions for a Big Challenge. Adv. Sci. 2015, 2, 1500053. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Ma, D.; Suo, C.; Shi, J.X.; Xue, M.Z.; Hu, X.; Xiao, Y.; Yu, K.D.; Liu, Y.R.; Yu, Y.; et al. Genomic and transcriptomic landscape of triple-negative breast cancers: Subtypes and treatment strategies. Cancer Cell 2019, 35, 428–440. [Google Scholar] [CrossRef]
- Carey, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. Invasive Breast Cancer Version 1.2016 Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 324–354. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F.; et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, 155–166. [Google Scholar] [CrossRef]
- Greenlee, H.; Hershman, D.L.; Jacobson, J.S. Use of antioxidant supplements during breast cancer treatment: A comprehensive review. Breast Cancer Res. Treat. 2009, 115, 437–452. [Google Scholar] [CrossRef]
- Norman, H.A.; Butrum, R.R.; Feldman, E.; Heber, D.; Nixon, D.; Picciano, M.F.; Rivlin, R.; Simopoulos, A.; Wargovich, M.J.; Weisburger, E.K.; et al. The role of dietary supplements during cancer therapy. J. Nutr. 2003, 133, 3794s–3799s. [Google Scholar] [CrossRef]
- Zirpoli, G.R.; Brennan, P.M.; Hong, C.C.; McCann, S.E.; Ciupak, G.; Davis, W.; Unger, J.M.; Budd, G.T.; Hershman, D.L.; Moore, H.C.F.; et al. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast Cancer Res. Treat. 2013, 137, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.S.; O’Prey, J.; Cardaci, S.; Barthet, V.J.A.; Sakamaki, J.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.F.; Yu, H.L.; Zhang, Y.X. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control Release 2005, 102, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, X.L.; Jiang, Y.H.; Hu, X.K.; Mou, H.J.; Li, M.; Guan, H.S. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 2007, 21, 646–654. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.C.; Yuan, S.J.; Yang, X.; Zou, P.; Shao, Y.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Wang, J. Growth-inhibition of S180 residual-tumor by combination of cyclophosphamide and chitosan oligosaccharides in vivo. Life Sci. 2018, 202, 21–27. [Google Scholar] [CrossRef]
- Zou, P.; Yuan, S.J.; Yang, X.; Zhai, X.C.; Wang, J. Chitosan oligosaccharides with degree of polymerization 2-6 induces apoptosis in human colon carcinoma HCT116 cells. Chem. Biol. Interact. 2018, 279, 129–135. [Google Scholar] [CrossRef]
- Mattaveewong, T.; Wongkrasant, P.; Chanchai, S.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-kappa B and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef]
- Petit, V.; Thiery, J.P. Focal adhesions: Structure and dynamics. Biol. Cell 2000, 92, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Chokradjaroen, C.; Theeramunkong, S.; Yui, H.; Saito, N.; Rujiravanit, R. Cytotoxicity against cancer cells of chitosan oligosaccharides prepared from chitosan powder degraded by electrical discharge plasma. Carbohydr. Polym. 2018, 201, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Chen, X.J.; Tian, J.H.; Li, L.H.; Zhao, M.Y.; Jiao, Y.P.; Zhou, C.R. Effect of chitooligosaccharides on cyclin D1, bcl-xl and bcl-2 mRNA expression in A549 cells using quantitative PCR. Chin. Sci. Bull. 2011, 56, 1629–1632. [Google Scholar] [CrossRef]
- Zou, P.; Yuan, S.J.; Yang, X.; Guo, Y.Z.; Li, L.N.; Xu, C.W.; Zhai, X.C.; Wang, J. Structural characterization and antitumor effects of chitosan oligosaccharides against orthotopic liver tumor via NF-κB signaling pathway. J. Funct. Foods 2019, 57, 157–165. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y.I. Effects of the Molecular Weight and the Degree of Deacetylation of Chitosan Oligosaccharides on Antitumor Activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef]
- Kim, E.K.; Je, J.Y.; Lee, S.J.; Kim, Y.S.; Hwang, J.W.; Sung, S.H.; Moon, S.H.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J.; et al. Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. Bioorg. Med. Chem. Lett. 2012, 22, 6136–6138. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Schmit, K.; Michiels, C. TMEM Proteins in Cancer: A Review. Front. Pharmacol. 2018, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Sun, Y.; Han, M.F.; Zhang, L.; Zhu, C.L.; Zhang, W.J.; Guo, H.; Li, J.W.; Yan, W.M.; Gong, F.L.; et al. Role of fibrinogen-like protein 2 prothrombinase/fibroleukin in experimental and human allograft rejection. J. Immunol. 2005, 174, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Rabizadeh, E.; Cherny, I.; Lederfein, D.; Sherman, S.; Binkovsky, N.; Rosenblat, Y.; Inbal, A. The cell-membrane prothrombinase, fibrinogen-like protein 2, promotes angiogenesis and tumor development. Thromb. Res. 2015, 136, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.Z.; Li, Q.L.; Chen, W.F.; Xu, M.D.; Zhang, Y.Q.; Zhong, Y.S.; Ma, L.L.; Hu, J.W.; Cai, M.Y.; He, M.J.; et al. Overexpression of fibrinogen-like protein 2 induces epithelial-to-mesenchymal transition and promotes tumor progression in colorectal carcinoma. Med. Oncol. 2014, 31, 181. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.S.; Shon, Y.H. Suppression of Metastasis of Human Breast Cancer Cells by Chitosan Oligosaccharides. J. Microbiol. Biotechnol. 2009, 19, 629–633. [Google Scholar] [PubMed]
- Thiel, G.; Cibelli, G. Regulation of life and death by the zinc finger transcription factor Egr-1. J. Cell Physiol. 2002, 193, 287–292. [Google Scholar] [CrossRef]
- Redmond, K.L.; Crawford, N.T.; Farmer, H.; D’Costa, Z.C.; O’Brien, G.J.; Buckley, N.E.; Kennedy, R.D.; Johnston, P.G.; Harkin, D.P.; Mullan, P.B. T-box 2 represses NDRG1 through an EGR1-dependent mechanism to drive the proliferation of breast cancer cells. Oncogene 2010, 29, 3252–3262. [Google Scholar] [CrossRef]
- Thyss, R.; Virolle, V.; Imbert, V.; Peyron, J.F.; Aberdam, D.; Virolle, T. NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 2005, 24, 128–137. [Google Scholar] [CrossRef]
- Huang, R.P.; Fan, Y.; deBelle, I.; Niemeyer, C.; Gottardis, M.M.; Mercola, D.; Adamson, E.D. Decreased EGR-1 expression in human, mouse and rat mammary cells and tissues correlates with tumor formation. Int. J. Cancer 1997, 72, 102–109. [Google Scholar] [CrossRef]
- Kang, H.S.; Ock, J.; Lee, H.J.; Lee, Y.J.; Kwon, B.M.; Hong, S.H. Early growth response protein 1 upregulation and nuclear translocation by 2 ‘-benzoyloxycinnamaldehyde induces prostate cancer cell death. Cancer Lett. 2013, 329, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, H.F.; Yu, H.Q.; Chen, Y.; Xu, H.Y.; Zhao, G. The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 2021, 11, 642547. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.N.; Bo, L. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.W.; Zhang, X.G. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]



| KEGG Term | Gene ID | log2 (Chitotriose/CTL) | Q (Chitotriose/CTL) |
|---|---|---|---|
| ko05200: Pathways in cancer | BGI_novel_G000649 (unknown) | −8.87 | 3.24 × 10−45 |
| Frat1 | −2.39 | 2.31 × 10−20 | |
| Gadd45a | 1.66 | 6.16 × 10−268 | |
| Rasgrp1 | 1.58 | 1.23 × 10−4 | |
| Bmp4 | −1.53 | 0 | |
| ko05165: Human papillomavirus infection | BGI_novel_G000649 (unknown) | −8.87 | 3.24 × 10−45 |
| Tmem61 | 3.66 | 5.99 × 10−4 | |
| Fgl2 | −3.34 | 4.24 × 10−5 | |
| BGI_novel_G000366 (unknown) | 2.88 | 6.41 × 10−60 | |
| BGI_novel_G000947 (unknown) | 2.34 | 1.34 × 10−6 | |
| ko04151: PI3K-Akt signaling pathway | Tmem61 | 3.66 | 5.99 × 10−4 |
| Fgl2 | −3.34 | 4.24 × 10−5 | |
| Myc | 2.10 | 0 | |
| Efna4 | −1.80 | 2.03 × 10−78 | |
| Efna1 | −1.42 | 6.58 × 10−33 | |
| ko04510: Focal adhesion | Tmem61 | 3.66 | 5.99 × 10−4 |
| Fgl2 | −3.34 | 4.24 × 10−5 | |
| BGI_novel_G000984 (unknown) | 2.16 | 6.04 × 10−13 | |
| Colq | −1.95 | 1.66 × 10−4 | |
| BGI_novel_G000897 (unknown) | 1.68 | 4.08 × 10−4 | |
| Ko05224: Breast cancer | Myc | 2.10 | 0 |
| Gadd45a | 1.66 | 6.16 × 10−268 | |
| Fos | 1.51 | 5.78 × 10−14 | |
| Jun | 1.33 | 0 | |
| Cdkn1a | 1.19 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ji, K.; Liu, P.; Geng, Y.; Gong, J.; Zhang, C.; Ding, Z.; Xu, Z.; Shi, J. Chitotriose Enhanced Antitumor Activity of Doxorubicin through Egr1 Upregulation in MDA-MB-231 Cells. Mar. Drugs 2024, 22, 26. https://doi.org/10.3390/md22010026
Li H, Ji K, Liu P, Geng Y, Gong J, Zhang C, Ding Z, Xu Z, Shi J. Chitotriose Enhanced Antitumor Activity of Doxorubicin through Egr1 Upregulation in MDA-MB-231 Cells. Marine Drugs. 2024; 22(1):26. https://doi.org/10.3390/md22010026
Chicago/Turabian StyleLi, Heng, Ke Ji, Peng Liu, Yan Geng, Jinsong Gong, Chao Zhang, Zhenzhong Ding, Zhenghong Xu, and Jinsong Shi. 2024. "Chitotriose Enhanced Antitumor Activity of Doxorubicin through Egr1 Upregulation in MDA-MB-231 Cells" Marine Drugs 22, no. 1: 26. https://doi.org/10.3390/md22010026
APA StyleLi, H., Ji, K., Liu, P., Geng, Y., Gong, J., Zhang, C., Ding, Z., Xu, Z., & Shi, J. (2024). Chitotriose Enhanced Antitumor Activity of Doxorubicin through Egr1 Upregulation in MDA-MB-231 Cells. Marine Drugs, 22(1), 26. https://doi.org/10.3390/md22010026








