Shotgun-Based Mass Spectrometry Analysis of Phospholipid and Triacylglycerol Molecular Species and Eicosanoids in Salmon Muscle Tissue on Feeding Microbial Oil
Abstract
:1. Introduction
2. Results
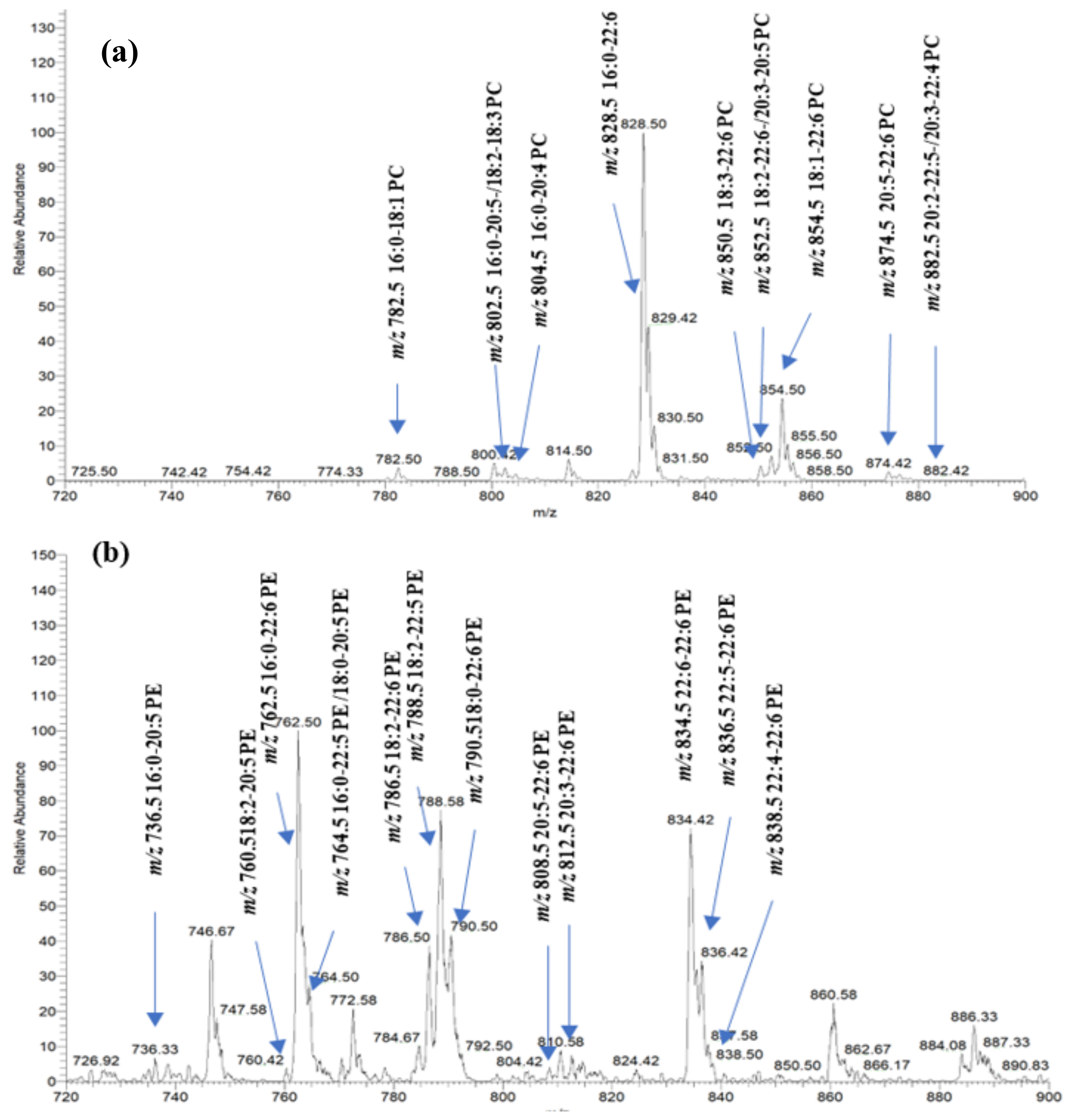
2.1. Identification of PhLs in Salmon Muscle Tissue Using MS Analysis
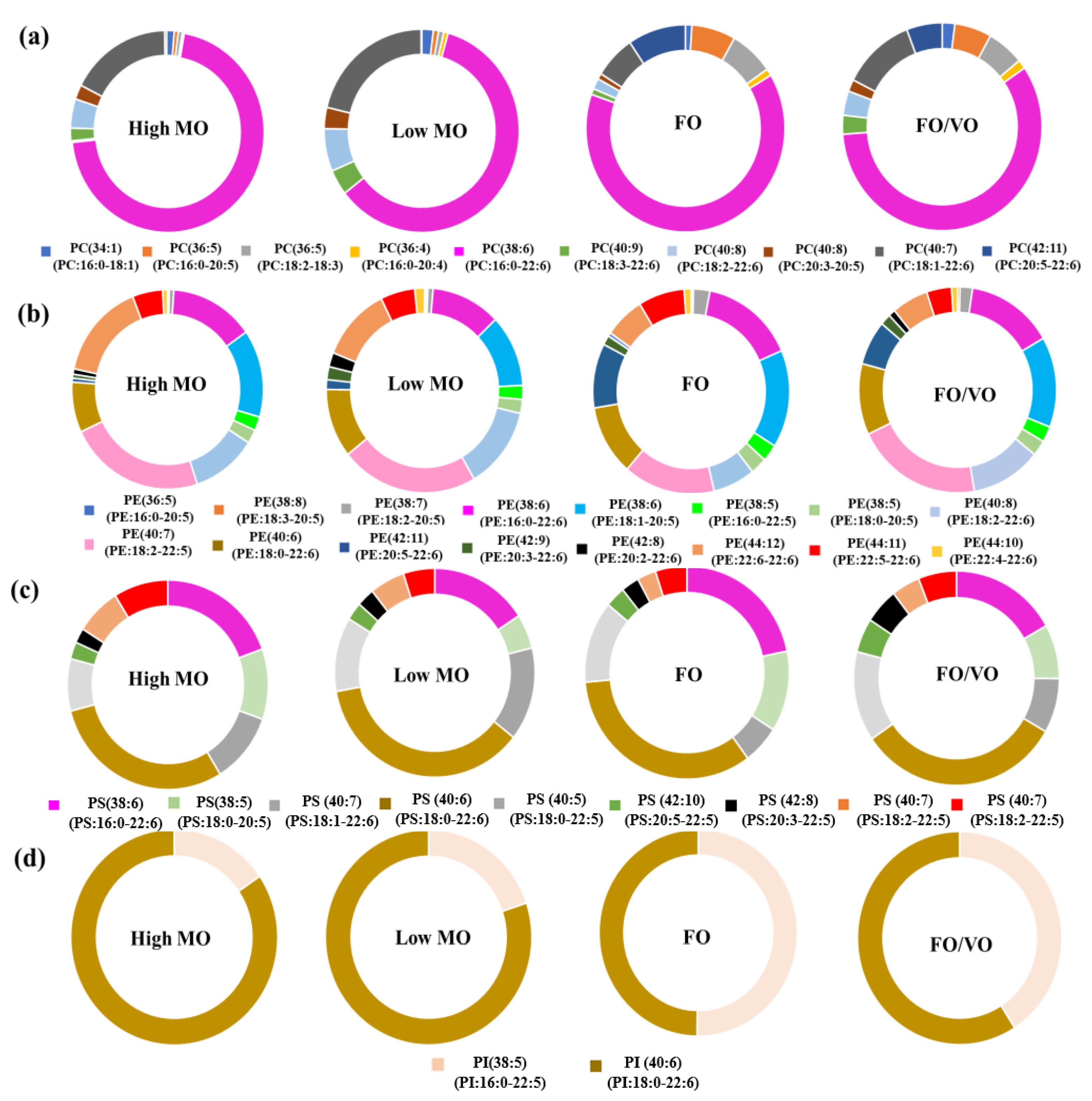
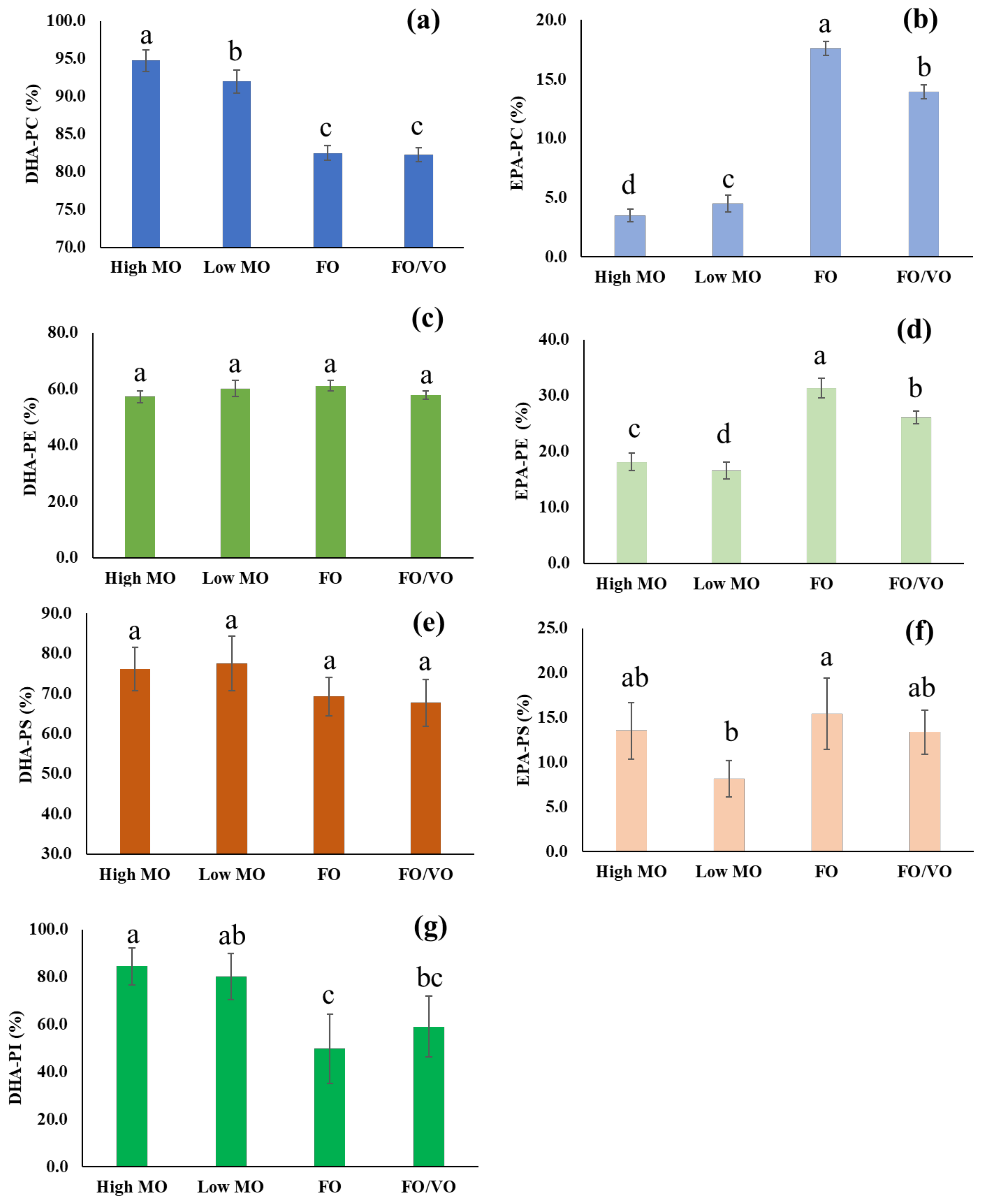
2.2. Changes in the Level of PC, PE, PS, and PI Species in Salmon Muscle Tissue after Being Fed Four Different Diets
2.3. Changes in the Levels of PUFA-Containing TAG Species in Salmon Muscle Tissue after Being Fed Four Different Diets
2.4. Identification of Eicosanoids and Their Quantitative Changes in the Salmon Muscle Tissue When Fed Four Different Diets
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Fish
4.3. Lipid Extraction
4.4. ESI-MS/MS Analysis for the Identification and Quantification of PhLs
4.5. ESI-MS/MS Analysis for the Identification and Quantification of TAG Species
4.6. ESI-MS/MS Analysis for the Identification and Quantification of PGE2 and PGF3α
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lund, E.K. Health Benefits of Seafood; Is It Just the Fatty Acids? Food Chem. 2013, 140, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W. Omega-3 Fatty Acids and Mental Health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Gong, L.; Guo, R.; Dong, W.; Cheung, H.Y. Shotgun Lipidomics Strategy for Fast Analysis of Phospholipids in Fisheries Waste and Its Potential in Species Differentiation. J. Agric. Food Chem. 2012, 60, 9384–9393. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Butka, E.; Wang, X. Comprehensive Quantification of Triacylglycerols in Soybean Seeds by Electrospray Ionization Mass Spectrometry with Multiple Neutral Loss Scans. Sci. Rep. 2014, 4, 6581. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, M.; Han, X. Shotgun Lipidomics in Substantiating Lipid Peroxidation in Redox Biology: Methods and Applications. Red. Biol. 2017, 12, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Lin, X.; Ji, S.; Zhang, Z.; Bo, T.; Guo, D.; Ye, M. Global Profiling and Novel Structure Discovery Using Multiple Neutral Loss/Precursor Ion Scanning Combined with Substructure Recognition and Statistical Analysis (MNPSS): Characterization of Terpene-Conjugated Curcuminoids in Curcuma Longa as a Case Study. Anal. Chem. 2016, 88, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, J.S.; Cruz, V.H.M.; Santos, P.D.S.; Silva, G.R.; Souza, P.M.; Manin, L.P.; Santos, O.O.; Visentainer, J.V. Instantaneous Characterization of Crude Vegetable Oils via Triacylglycerols Fingerprint by Atmospheric Solids Analysis Probe Tandem Mass Spectrometry with Multiple Neutral Loss Scans. Food Cont. 2022, 134, 108710. [Google Scholar] [CrossRef]
- Yang, W.; Shi, X.; Yao, C.; Huang, Y.; Hou, J.; Han, S.; Feng, Z.; Wei, W.; Wu, W.; Guo, D. A Novel Neutral Loss/Product Ion Scan-Incorporated Integral Approach for the Untargeted Characterization and Comparison of the Carboxyl-Free Ginsenosides from Panax Ginseng, Panax Quinquefolius, and Panax Notoginseng. J. Pharm. Biomed. Anal. 2020, 177, 112813. [Google Scholar] [CrossRef]
- Wei, M.; Parrish, C.C.; Guerra, N.I.; Armenta, R.E.; Colombo, S.M. Extracted Microbial Oil from a Novel Schizochytrium sp. (T18) as a Sustainable Lipid Source in Atlantic Salmon Feed: Impacts on Growth and Tissue Fatty Acids. Aquaculture 2021, 534, 736249. [Google Scholar] [CrossRef]
- Nham, T.L.; Miranda, A.F.; Mouradov, A.; Adhikari, B. Physicochemical Characteristics of Protein Isolated from Thraustochytrid Oilcake. Foods 2020, 9, 779. [Google Scholar] [CrossRef]
- Ryan, L.; Symington, A.M. Algal-Oil Supplements Are a Viable Alternative to Fish-Oil Supplements in Terms of Docosahexaenoic Acid (22:6n-3; DHA). J. Funct. Foods 2015, 19, 852–858. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Abellán, V.; Sepulcre, M.P. The role of prostaglandins in the regulation of fish immunity. Mol. Immunol. 2016, 69, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Backlund, M.G.; Mann, J.R.; DuBois, R.N. Mechanisms for the prevention of gastrointestinal cancer: The role of prostaglandin E2. Oncology 2005, 69, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.N.; Plumb, T.J.; Coffman, T.M. Eicosanoids: Lipid mediators of inflammation in transplantation. Spr. Sem. Immun. 2003, 25, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In Vitro Antithrombotic Properties of Salmon (Salmo salar) Phospholipids in a Novel Food-Grade Extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef]
- Yeo, J.D.; Parrish, C.C. Shotgun lipidomics for the determination of phospholipid and eicosanoid profiles in atlantic salmon (Salmo salar L.) muscle tissue using electrospray ionization (ESI)-MS/MS spectrometric analysis. Int. J. Mol. Sci. 2021, 22, 2272. [Google Scholar] [CrossRef]
- Ahern, K.W.; Serbulea, V.; Wingrove, C.L.; Palas, Z.T.; Leitinger, N.; Harris, T.E. Regioisomer-Independent Quantification of Fatty Acid Oxidation Products by HPLC-ESI-MS/MS Analysis of Sodium Adducts. Sci. Rep. 2019, 9, 11197. [Google Scholar] [CrossRef]
- Carter, C.L.; Jones, J.W.; Farese, A.M.; MacVittie, T.J.; Kane, M.A. Inflation-Fixation Method for Lipidomic Mapping of Lung Biopsies by Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging. Anal. Chem. 2016, 88, 4788–4794. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R.; Tocher, D.R.; Napier, J.A.; Betancor, M.B. Profiling Phospholipids within Atlantic Salmon Salmo Salar with Regards to a Novel Terrestrial Omega-3 Oil Source. Metabolites 2022, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H. Tracking phospholipid profiling of muscle from Ctennopharyngodon idellus during storage by shotgun lipidomics. J. Agric. Food Chem. 2011, 59, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Inhamuns, A.J.; Franco, M.R.B. Composition of total, neutral, and phospholipids in Mapará (Hypophthalmus sp.) from the Brazilian Amazonian area. J. Agric. Food Chem. 2001, 49, 4859–4863. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Harvey, T.N.; Bartosova, Z.; Hassani, S.; Bruheim, P.; Sandve, S.R.; Vik, J.O. Diet and Life Stage-Associated Lipidome Remodeling in Atlantic Salmon. J. Agric. Food Chem. 2021, 69, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Jeromson, S.; Mackenzie, I.; Doherty, M.K.; Whitfield, P.D.; Bell, G.; Dick, J.; Shaw, A.; Rao, F.V.; Ashcroft, S.P.; Philp, A.; et al. Lipid Remodeling and an Altered Membrane-Associated Proteome May Drive the Differential Effects of EPA and DHA Treatment on Skeletal Muscle Glucose Uptake and Protein Accretion. Am. J. Physiol.-End. Metabol. 2018, 314, E605–E619. [Google Scholar] [CrossRef]
- Fard, S.G.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How Does High DHA Fish Oil Affect Health? A Systematic Review of Evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef]
- Khalid, W.; Gill, P.; Arshad, M.S.; Ali, A.; Ranjha, M.M.A.N.; Mukhtar, S.; Afzal, F.; Maqbool, Z. Functional Behavior of DHA and EPA in the Formation of Babies Brain at Different Stages of Age, and Protect from Different Brain-Related Diseases. Int. J. Food Prop. 2022, 25, 1021–1044. [Google Scholar] [CrossRef]
- Sierra, S.; Lara-Villoslada, F.; Comalada, M.; Olivares, M.; Xaus, J. Dietary eicosapentaenoic acid and docosahexaenoic acid equally incorporate as decosahexaenoic acid but differ in inflammatory effects. Nutrition 2008, 24, 245–254. [Google Scholar] [CrossRef]
- Trebble, T.M.; Wootton, S.A.; Miles, E.A.; Mullee, M.; Arden, N.K.; Ballinger, A.B.; Stroud, M.A.; Calder, P.C. Prostaglandin E2 production and T-cell function after fish-oil supplementation: Response to antioxidant co-supplementation. Am. J. Clin. Nutr. 2003, 78, 376–382. [Google Scholar] [CrossRef]
- Robinson, J.G.; Stone, N.J. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 39i–49i. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Hals, P.A.; Wang, X.; Xiao, Y.F. Effects of a purified krill oil phospholipid rich in long-chain omega-3 fatty acids on cardiovascular disease risk factors in non-human primates with naturally occurring diabetes type-2 and dyslipidemia. Lipids Health Dis. 2017, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Saha, S.K. Anti-inflammatory and antithrombotic properties of polar lipid extracts, rich in unsaturated fatty acids, from the Irish marine cyanobacterium Spirulina subsalsa. J. Func. Foods 2022, 94, 105124. [Google Scholar] [CrossRef]
- Ferreira, I.; Rauter, A.P.; Bandarra, N.M. Marine sources of DHA-rich phospholipids with anti-Alzheimer effect. Mar Drugs. 2022, 20, 662. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C.; Xue, X.; Wells, J.S.; Collins, S.A.; Anderson, D.M.; Rise, M.L. Growth Performance, Tissue Composition, and Gene Expression Responses in Atlantic Salmon (Salmo salar) Fed Varying Levels of Different Lipid Sources. Aquaculture 2017, 467, 76–88. [Google Scholar] [CrossRef]
- Hu, C.; Duan, Q.; Han, X. Strategies to improve/eliminate the limitations in shotgun lipidomics. Proteomics 2020, 20, 1900070. [Google Scholar] [CrossRef]
- Hsu, F.F. Mass spectrometry-based shotgun lipidomics—A critical review from the technical point of view. Anal. Bioanal. Chem. 2018, 410, 6387–6409. [Google Scholar] [CrossRef]
- Parrish, C.C. Determination of total lipid, lipid classes, and fatty acids in aquatic samples. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999. [Google Scholar]


| High MO | Low MO | FO | FO/VO | |
|---|---|---|---|---|
| PGE2 (ng/g) | 14.6 ± 3.1 b | 7.1 ± 2.7 b | 47.1 ± 8.7 a | 35.8 ± 10.6 a |
| PGF3α (µg/g) | 28.2 ± 6.6 b | 2.0 ± 0.7 c | 36.6 ± 4.9 a | 21.1 ± 4.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, J.; Colombo, S.M.; Guerra, N.I.; Parrish, C.C. Shotgun-Based Mass Spectrometry Analysis of Phospholipid and Triacylglycerol Molecular Species and Eicosanoids in Salmon Muscle Tissue on Feeding Microbial Oil. Mar. Drugs 2024, 22, 11. https://doi.org/10.3390/md22010011
Yeo J, Colombo SM, Guerra NI, Parrish CC. Shotgun-Based Mass Spectrometry Analysis of Phospholipid and Triacylglycerol Molecular Species and Eicosanoids in Salmon Muscle Tissue on Feeding Microbial Oil. Marine Drugs. 2024; 22(1):11. https://doi.org/10.3390/md22010011
Chicago/Turabian StyleYeo, JuDong, Stefanie M. Colombo, Nigel I. Guerra, and Christopher C. Parrish. 2024. "Shotgun-Based Mass Spectrometry Analysis of Phospholipid and Triacylglycerol Molecular Species and Eicosanoids in Salmon Muscle Tissue on Feeding Microbial Oil" Marine Drugs 22, no. 1: 11. https://doi.org/10.3390/md22010011
APA StyleYeo, J., Colombo, S. M., Guerra, N. I., & Parrish, C. C. (2024). Shotgun-Based Mass Spectrometry Analysis of Phospholipid and Triacylglycerol Molecular Species and Eicosanoids in Salmon Muscle Tissue on Feeding Microbial Oil. Marine Drugs, 22(1), 11. https://doi.org/10.3390/md22010011








