Exopolysaccharide Production from Marine-Derived Brevundimonas huaxiensis Obtained from Estremadura Spur Pockmarks Sediments Revealing Potential for Circular Economy
Abstract
1. Introduction
2. Results and Discussion
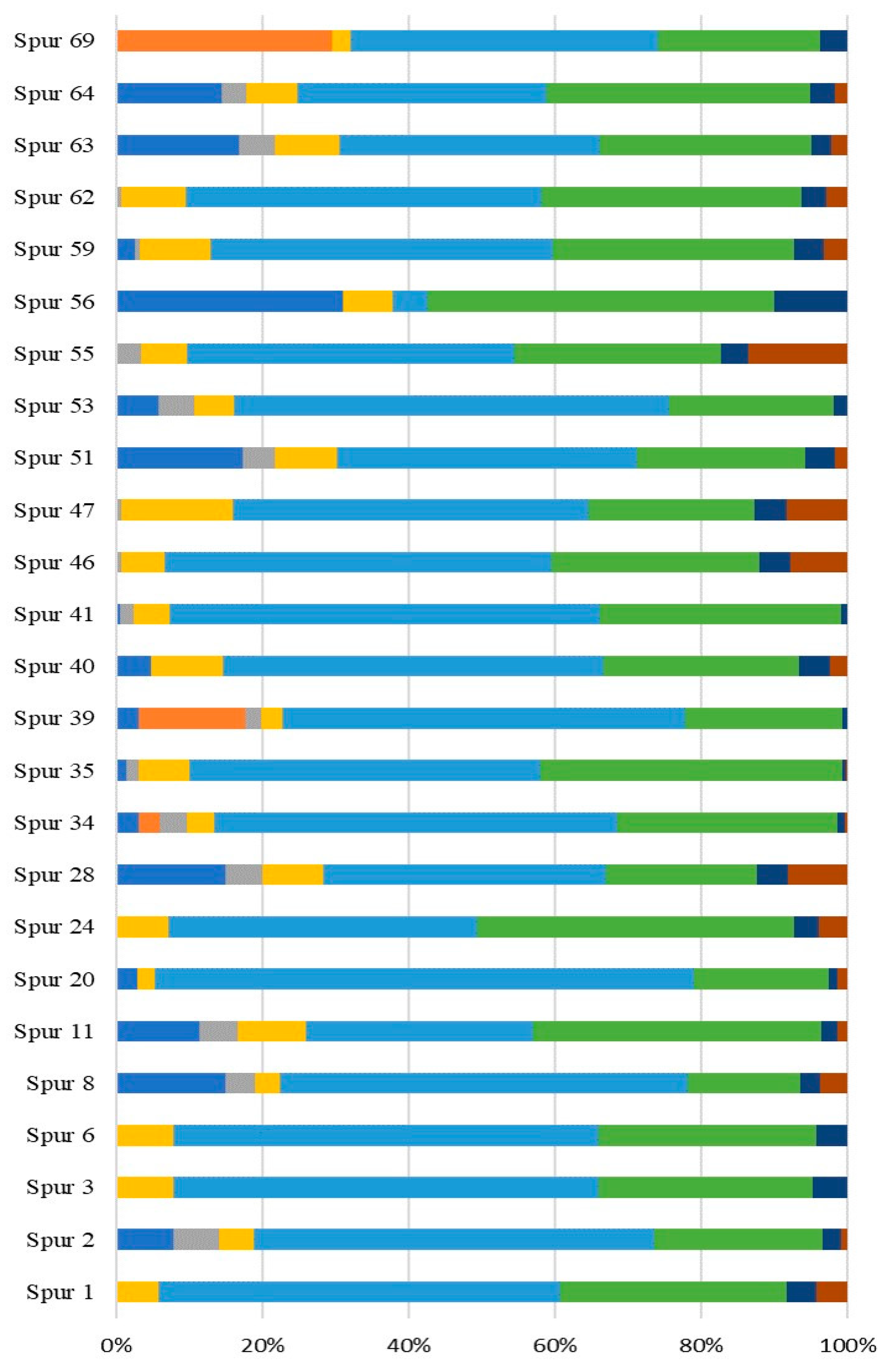
2.1. Characterization of EPS Producing Bacterial Strain
2.2. Taxonomic Identification of the Higher Yield Marine-Derived Bacteria EPS Producers

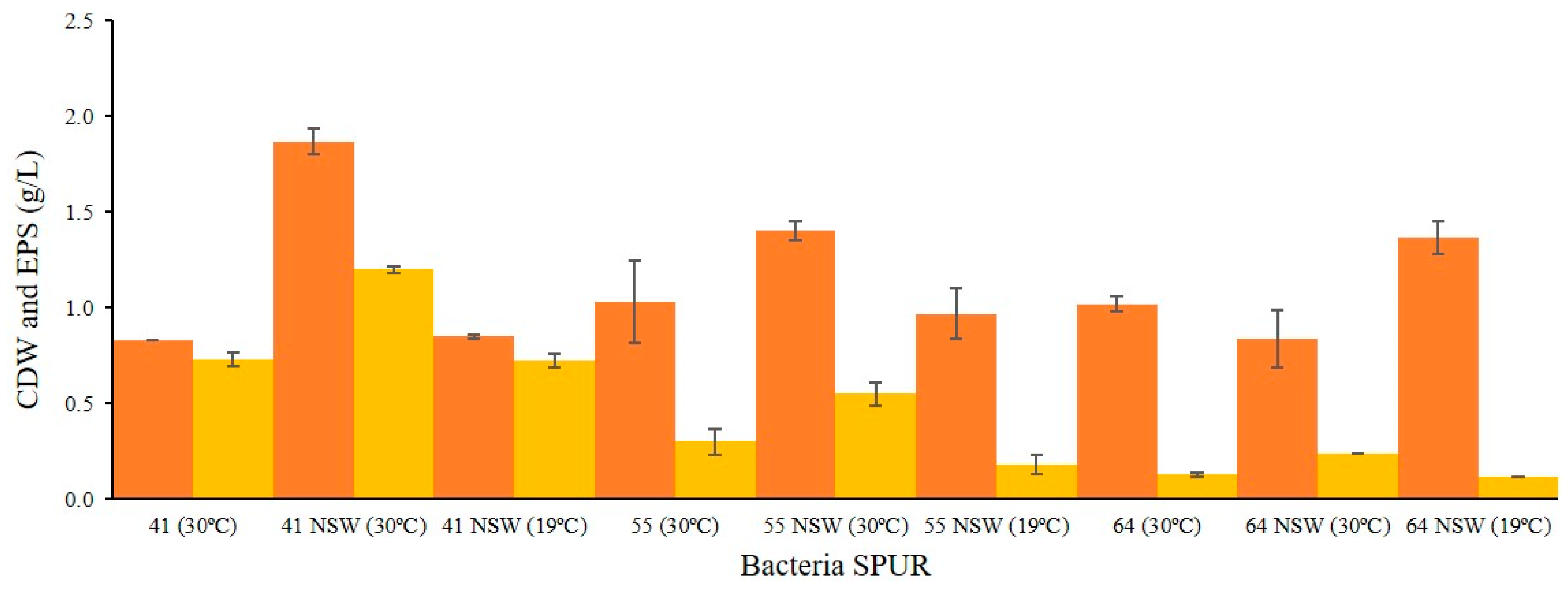
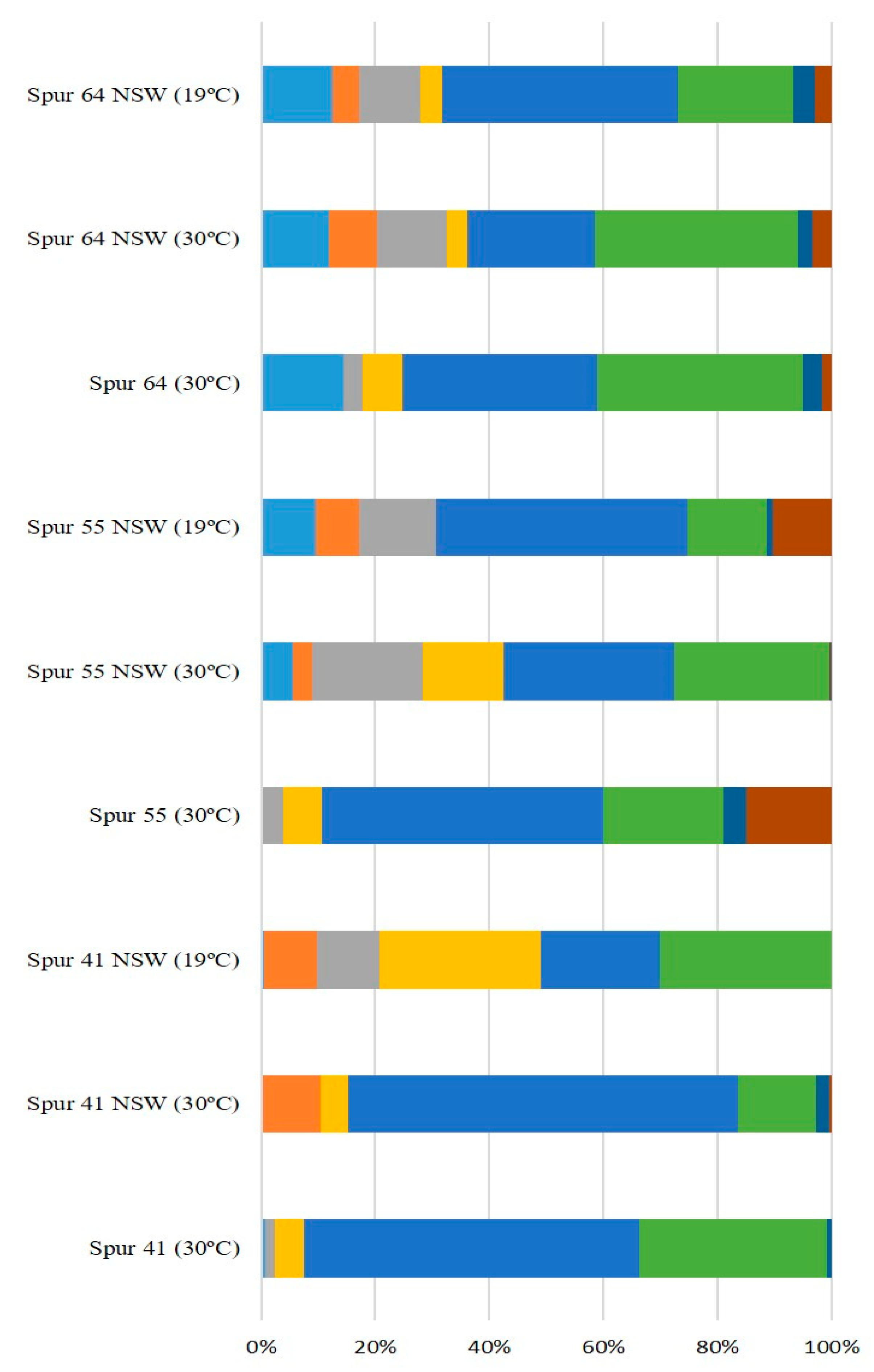
2.3. Effect of NSW and Temperature on Bacterial Growth and EPS Production
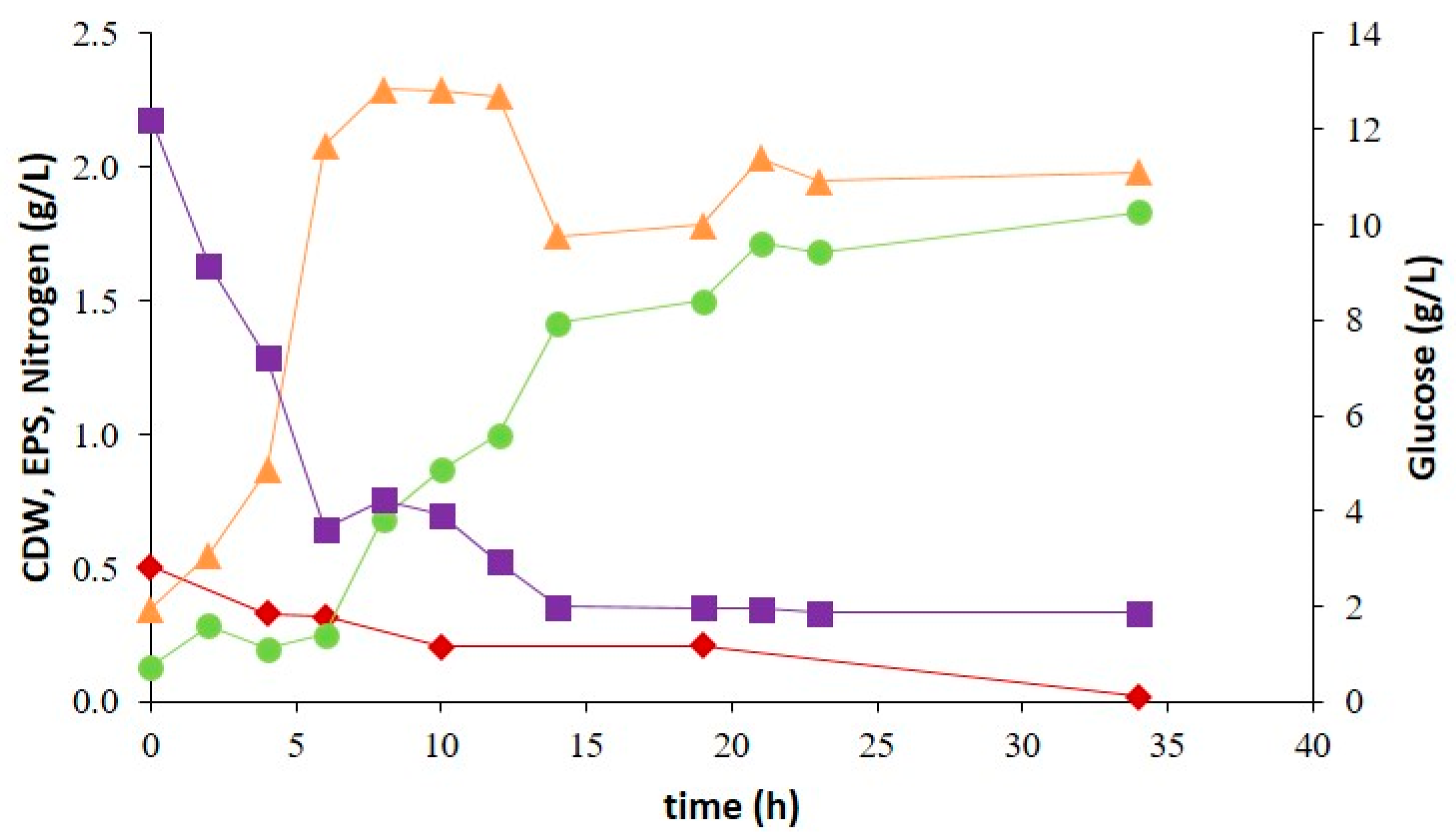
2.4. Bioreactor Cultivation of Strain SPUR-41
3. Materials and Methods
3.1. Isolation and Screening of Marine-Derived Bacteria to Produce EPS
3.2. DNA Extraction, 16S rRNA Gene Amplification, Sequencing, and Taxonomic Identification of Bacteria
3.3. Taxonomic Classification and Phylogenetic Analysis
3.4. Bioreactor Cultivation
3.5. Analytical Techniques
3.6. Biopolymer Characterization
3.7. Molecular Weight Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahu, P. A comprehensive review of saline effluent disposal and treatment: Conventional practices, emerging technologies, and future potential. Water Reuse 2021, 11, 33–65. [Google Scholar] [CrossRef]
- Antranikian, G.; Streit, W.R. Microorganisms harbor keys to a circular bioeconomy making them useful tools in fighting plastic pollution and rising CO2 levels. Extremophiles 2022, 26, 10. [Google Scholar] [CrossRef]
- Dufourcq, R.; Chalkiadakis, E.; Fauchon, M.; Deslandes, E.; Kerjean, V.; Chanteau, S.; Petit, E.; Guezennec, J.; Dupont-Rouzeyrol, M. Isolation and partial characterization of bacteria (Pseudoalteromonas sp.) with potential antibacterial activity from a marine costal environment from New Caledonia. Lett. Appl. Microbiol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef]
- Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. Exopolysaccharides from Marine Microbes: Source, Structure and Application. Mar. Drugs. 2022, 20, 512. [Google Scholar] [CrossRef]
- Torres, C.A.V.; Ferreira, A.R.V.; Freitas, F.; Reis, M.A.M.; Coelhoso, I.; Sousa, I.; Alves, V.D. Rheological studies of the fucose-rich exopolysaccharide FucoPol. Int. J. Biol. Macromol. 2015, 79, 611–617. [Google Scholar] [CrossRef]
- Vijayendra, S.V.N.; Shamala, T.R. Film forming microbial biopolymers for commercial applications—A review Film forming microbial biopolymers for commercial applications—A review. Crit. Rev. Biotechnol. 2014, 34, 338–357. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Torres, C.A.V.; Marques, R.; Antunes, S.; Alves, V.D.; Sousa, I.; Maria, R.; Oliveira, R.; Freitas, F.; Reis, M.A.M. Kinetics of production and characterization of the fucose-containing exopolysaccharide from Enterobacter A47. J. Biotechnol. 2011, 156, 261–267. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biotechnology of Microbial Exopolysaccharides; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Iyer, A.; Mody, K.; Jha, B. Emulsifying properties of a marine bacterial exopolysaccharide. Enzym. Microb. Technol. 2006, 38, 220–222. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Michela, M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum -BMS strain. Carbohydr. Polym. 2020, 256, 117523. [Google Scholar] [CrossRef]
- Barbosa, V.; Honório, J.; Lopes, P.; Santos, K.; Vieira, E.A.; Iacomini, M.; Silva, C.M.; dos Santos, K.M.O.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT Food Sci. Technol. 2020, 127, 109349. [Google Scholar]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Costaouëc, T.L.; Cérantola, S.; Ropartz, D.; Ratiskol, J.; Sinquin, C.; Colliec-jouault, S.; Boisset, C. Structural data on a bacterial exopolysaccharide produced by a deep-sea Alteromonas macleodii strain. Carbohydr. Polym. 2012, 90, 49–59. [Google Scholar] [CrossRef]
- Lelchat, F.; Cozien, J.; Costaouec, T.L.; Brandilly, C.; Schmitt, S.; Baudoux, A.; Colliec-Jouault, S.; Boisset, C. Exopolysaccharide biosynthesis and biodegradation by a marine hydrothermal Alteromonas sp. strain. Appl. Microbiol. Biotechnol. 2015, 99, 2637–2647. [Google Scholar] [CrossRef]
- Raguenes, G.; Christen, R.; Guezennec, J.; Pignet, P.; Barbier, G. Vibrio diabolicus sp. nov, a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int. J. Syst. Evol. Microbiol. 1997, 47, 989–995. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Lee, H.K.; Chun, J.; Moon, E.Y.; Ko, S.H.; Lee, D.S.; Lee, H.S.; Bae, K.S. Hahella chejuensis gen. nov., sp. nov., an extracellular-polysaccharide-producing marine bacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 661–666. [Google Scholar] [CrossRef]
- Chug, R.; Mathur, S.; Kothari, S.L.; Harish; Gour, V.S. Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration. Biochem. Biophys. Rep. 2021, 26, 100972. [Google Scholar] [CrossRef]
- Solmaz, K.B.; Ozcan, Y.; Mercan, D.N.; Bozkaya, O.; Ide, S. Characterization and Production of Extracellular Polysaccharides (EPS) by Bacillus pseudomycoides U10. Environments 2018, 5, 63. [Google Scholar] [CrossRef]
- Joulak, I.; Finore, I.; Nicolaus, B.; Leone, L.; Moriello, A.S.; Attia, H.; Poli, A.; Azabou, S. Evaluation of the production of exopolysaccharides by newly isolated Halomonas strains from Tunisian hypersaline environments. Int. J. Biol. Macromol. 2019, 138, 658–666. [Google Scholar] [CrossRef]
- Roca, C.; Lehmann, M.; Torres, C.A.V.; Baptista, S.; Gaudêncio, S.P.; Freitas, F.; Reis, M.A.M. Exopolysaccharide production by a marine Pseudoalteromonas sp. strain isolated from Madeira Archipelago ocean sediments. New Biotechnol. 2016, 33, 460–466. [Google Scholar] [CrossRef]
- Abdelnasser, S.M.; Yahya, S.M.M.; Mohamed, W.F.; Asker, M.M.; Shady, H.M.A.; Mahmoud, M.G.; Gadallah, M.A. Antitumor exopolysaccharides derived from novel marine Bacillus: Isolation, characterization aspect and biological activity. Asian Pac. J. Cancer Prev. 2017, 18, 1847–1854. [Google Scholar]
- Hassan, S.W.M.; Ibrahim, H.A.H. Production, Characterization and Valuable Applications of Exopolysaccharides from Marine Bacillus subtilis SH1. Pol. J. Microbiol. 2017, 66, 449–461. [Google Scholar] [CrossRef]
- Singh, K.; Sundharam, S.S.; Krishnamurthi, S.; Roy, A. Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 2019, 127, 240–249. [Google Scholar]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Gouveia, A.R.; Pinheiro, C.; Torres, C.A.V.; Grandfils, C.; Reis, M.A.M. Controlled production of exopolysaccharides from Enterobacter A47 as a function of carbon source with demonstration of their film and emulsifying abilities. Appl. Biochem. Biotechnol. 2014, 172, 641–657. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Dubey, S.K. Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23. Lett. Appl. Microbiol. 2016, 43, 571–577. [Google Scholar] [CrossRef]
- Nichols, C.M.; Bowman, J.P.; Guezennec, J. Effects of incubation Temperature on Growth and Production of Exopolysaccharides by an Antarctic Sea Ice Bacterium Grown in Batch Culture. Appl. Environ. Microbiol. 2005, 71, 3519–3523. [Google Scholar] [CrossRef]
- Carrión, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and Properties of Two Novel Exopolysaccharides Synthesized by a Thermophilic Bacterium Aeribacillus pallidus. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Finore, I.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Isolation, characterization and optimization of EPSs produced by a cold-adapted Marinobacter isolate from Antarctic seawater. Antarct. Sci. 2019, 31, 69–79. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A review of the role of extracellular polymeric substances (EPS) in wastewater treatment s ystems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Wei, L.; Zong, Z. Genome-based taxonomy of Brevundimonas with reporting Brevundimonas huaxiensis sp. nov. Microbiol. Spectrum. 2021, 9, e00111-21. [Google Scholar] [CrossRef]
- Teresa, M.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar]
- Djolo, Y.; Dossounon, D.; Blaghen, M. Exopolysaccharide production by Brevundimonas diminuta isolated from Marchica lagoon ecosystem in Morocco. Int. J. App. Microbiol. Biotechnol. Res. 2019, 7, 79–85. [Google Scholar]
- Mohamed, S.S.; Amer, S.K.; Selim, M.S.; Rifaat, H.M. Characterization and applications of exopolysaccharide produced by marine Bacillus altitudinis MSH2014 from Ras Mohamed, Sinai, Egypt. Egypt. J. Basic Appl. Sci. 2018, 5, 204–209. [Google Scholar] [CrossRef]
- Verhoef, E.; Waard, P.; Ratto, M.; Voragen, A.G.J. Structural elucidation of the EPS of slime producing Brevundimonas vesicularis sp. isolated from a paper machine. Carbohydr. Res. 2002, 337, 1821–1831. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Feng, R.; Chen, J.; Alwathnani, H.A.; Xu, W.; Rensing, C. Characterization of Two Highly Arsenic-Resistant Caulobacteraceae Strains of Brevundimonas nasdae: Discovery of a New Arsenic Resistance Determinant. Int. J. Mol. Sci. 2022, 23, 5619. [Google Scholar] [CrossRef]
- Isfahani, F.M.; Tahmourespour, A.; Hoodaji, M.; Ataabadi, M.; Mohammadi, A. Characterizing the new bacterial isolates of high yielding exopolysaccharides under hypersaline conditions. J. Clean. Prod. 2018, 185, 922–928. [Google Scholar] [CrossRef]
- Halder, U.; Mazumder, K.; Kumar, K.J.; Bandopadhyay, R. Structural insight into a glucomannan-type extracellular polysaccharide produced by a marine Bacillus altitudinis SORB11 from Southern Ocean. Sci. Rep. 2022, 12, 16322. [Google Scholar] [CrossRef]
- Ravi, G.; Sampath, G.; Srinivas, B.; Sarika, K.; Govindarajan, R.K.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities. J. King Saud Univ. Sci. 2021, 33, 101571. [Google Scholar] [CrossRef]
- Bandopadhyay, R. Comparative study between a Southern Ocean psychrophile and a hot water spring thermophilic microbe: Biological potential, exopolysaccharide production and characterization, biological activity of exopolysaccharide-AgNO3 nanoparticle conjugate. J. Microb. Biochem. Technol. 2016, 8, 53. [Google Scholar]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.; Anzelmo, G.; Poli, A.; Nicolaus, B. A Novel EPS-Producing Strain of Bacillus licheniformis Isolated from a Shallow Vent Off Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar] [CrossRef]
- Banerjee, A.; Mohammed Breig, S.J.; Gómez, A.; Sánchez-Arévalo, I.; González-Faune, P.; Sarkar, S.; Bandopadhyay, R.; Vuree, S.; Cornejo, J.; Tapia, J.; et al. Optimization and Characterization of a Novel Exopolysaccharide from Bacillus haynesii CamB6 for Food Applications. Biomolecules 2022, 12, 834. [Google Scholar] [CrossRef]
- Ko, S.; Lee, H.; Park, S.; Lee, H. Optimal Conditions for the Production of Exopolysaccharide by Marine Microoranism Hahella chejebsis. Biotechnol Bioprocess Eng. 2000, 5, 181–185. [Google Scholar] [CrossRef]
- Lee, H.K.; Park, S.; Lee, J.; Lee, H. Effects of Aeration Rates on Production of Extracellular Polysaccharide EPS-R, by Marine Bacterium Hahuella chejuensis. Biotechnol Bioprocess Eng. 2001, 6, 259–362. [Google Scholar] [CrossRef]
- Kojic, M.; Vujcic, M.; Banina, A.; Cocconcelli, P.; Cerning, J.; Topisirovic, L. Analysis of Exopolysaccharide Production by Lactobacillus casei CG11, Isolated from Cheese. Appl. Environ. Microbiol. 1992, 58, 4086–4088. [Google Scholar] [CrossRef]
- Torres, C.A.V.; Antunes, S.; Ricardo, A.R.; Grandfils, C.; Reis, M.A.M. Study of the interactive effect of temperature and ph on exopolysaccharide production by Enterobacter A47 using multivariate statistical analysis. Bioresour. Technol. 2012, 119, 148–156. [Google Scholar] [CrossRef]
- Peng, L.; Qiao, S.; Xu, Z.; Guan, F.; Ding, Z.; Gu, Z.; Zhang, L.; Shi, G. Effects of culture conditions on monosaccharide composition og Ganoderma lucidum exopolysaccharide and on activities of related enzymes. Carbohydr. Polym. 2015, 133, 104–109. [Google Scholar] [CrossRef]
- Grobben, G.J.; Chin-Joe, I.; Kitzen, V.A.; Boels, I.C.; Boer, F.; Sikkema, J.; Smith, M.R.; de Bont, J.A.M. Enhancement of Exopolysaccharide Production by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 with a Simplified Defined Medium. Appl. Environ. Microbiol. 1998, 64, 1333–1337. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Alves, V.D.; Moppert, X.; Guézennec, J.; Freitas, F.; Reis, M.A.M. Characterization and Biotechnological Potential of Extracellular Polysaccharides Synthesized by Alteromonas Strains Isolated from French Polynesia Marine Environments. Mar. Drugs 2021, 19, 522. [Google Scholar] [CrossRef]
- Asker, M.S.; El Sayed, O.H.; Mahmoud, M.G.; Yahya, S.M.; Mohamed, S.S.; Selim, M.S.; El Awady, M.S.; Abdelnasse, S.M.; Elsoud, M.M.A. Production of exopolysaccharides from novel marine bacteria and anticancer activity against hepatocellular carcinoma cells (HepG2). Bull. Natl. Res. Cent. 2018, 42, 30. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Nachiyar, C.; Kumar, G.; Sunkar, S.; Badsha, I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of Pseudomonas fluorescens. Biocatal. Agric. Biotechnol. 2018, 16, 320–325. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Shi, B. A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Pinto-Almeida, A.; Bauermeister, A.; Luppino, L.; Grilo, I.R.; Oliveira, J.; Sousa, J.R.; Petras, D.; Rodrigues, C.F.; Prieto-Davó, A.; Tasdemir, D.; et al. The diversity, metabolomics profiling, and the pharmacological potential of Actinomycetes isolated from the Estremadura Spur Pockmarks (Portugal). Mar. Drugs 2022, 20, 21. [Google Scholar] [CrossRef]
- Pereira, F.; Almeida, J.R.; Paulino, M.; Grilo, I.R.; Macedo, H.; Cunha, I.; Sobral, R.G.; Vasconcelos, V.; Gaudêncio, S.P. Antifouling napyradiomycins from marine-derived Actinomycetes Streptomyces aculeolatus. Mar. Drugs 2020, 18, 63. [Google Scholar] [CrossRef]
- Gontang, E.A.; Fenical, W.; Jensen, P.R. Phylogenetic Diversity of gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 2007, 73, 3272–3282. [Google Scholar] [CrossRef]
- MAFFT. Available online: https://mafft.cbrc.jp/alignment/server/ (accessed on 15 January 2023).

| Strain | Location | Carbon Source | Cultivation Time (h) | CDW (g/L) | μ (h−1) | EPS (g/L) | rEPS (g/L d−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SPUR-41 Batch | Ocean sediments, Estremadura Spur, Portugal | Glucose | 34 | 2.29 | 0.25 | 1.83 | 1.30 | This study |
| Brevundimonas diminuta Batch | Marchica lagoon, Morocco | Glucose | 120 | 0.7 | n.a. | 2 | 0.40 | [37] |
| Pseudomonas sp. MD12-642 Batch | Ocean sediments, Madeira Archipelago, Portugal | Glucose | 15 | n.a | 0.60 | 2.5 | 1.56 | [23] |
| Halomonas elongate Batch | Sehline Kerkennah Salt Lake, Tunisia | Glucose | 120 | n.a | n.a | 0.16 | 0.03 | [22] |
| Halomonas halophila Batch | Sehline Kerkennah Salt Lake, Tunisia | Glucose | 120 | n.a | n.a | 0.11 | 0.04 | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalão, M.; Fernandes, M.; Galdon, L.; Rodrigues, C.F.; Sobral, R.G.; Gaudêncio, S.P.; Torres, C.A.V. Exopolysaccharide Production from Marine-Derived Brevundimonas huaxiensis Obtained from Estremadura Spur Pockmarks Sediments Revealing Potential for Circular Economy. Mar. Drugs 2023, 21, 419. https://doi.org/10.3390/md21070419
Catalão M, Fernandes M, Galdon L, Rodrigues CF, Sobral RG, Gaudêncio SP, Torres CAV. Exopolysaccharide Production from Marine-Derived Brevundimonas huaxiensis Obtained from Estremadura Spur Pockmarks Sediments Revealing Potential for Circular Economy. Marine Drugs. 2023; 21(7):419. https://doi.org/10.3390/md21070419
Chicago/Turabian StyleCatalão, Marta, Mafalda Fernandes, Lorena Galdon, Clara F. Rodrigues, Rita G. Sobral, Susana P. Gaudêncio, and Cristiana A. V. Torres. 2023. "Exopolysaccharide Production from Marine-Derived Brevundimonas huaxiensis Obtained from Estremadura Spur Pockmarks Sediments Revealing Potential for Circular Economy" Marine Drugs 21, no. 7: 419. https://doi.org/10.3390/md21070419
APA StyleCatalão, M., Fernandes, M., Galdon, L., Rodrigues, C. F., Sobral, R. G., Gaudêncio, S. P., & Torres, C. A. V. (2023). Exopolysaccharide Production from Marine-Derived Brevundimonas huaxiensis Obtained from Estremadura Spur Pockmarks Sediments Revealing Potential for Circular Economy. Marine Drugs, 21(7), 419. https://doi.org/10.3390/md21070419









