Abstract
The skin is the largest organ of the human body, composed of a diverse range of cell types, non-cellular components, and an extracellular matrix. With aging, molecules that are part of the extracellular matrix undergo qualitative and quantitative changes and the effects, such as a loss of skin firmness or wrinkles, can be visible. The changes caused by the aging process do not only affect the surface of the skin, but also extend to skin appendages such as hair follicles. In the present study, the ability of marine-derived saccharides, L-fucose and chondroitin sulphate disaccharide, to support skin and hair health and minimize the effects of intrinsic and extrinsic aging was investigated. The potential of the tested samples to prevent adverse changes in the skin and hair through stimulation of natural processes, cellular proliferation, and production of extracellular matrix components collagen, elastin, or glycosaminoglycans was investigated. The tested compounds, L-fucose and chondroitin sulphate disaccharide, supported skin and hair health, especially in terms of anti-aging effects. The obtained results indicate that both ingredients support and promote the proliferation of dermal fibroblasts and dermal papilla cells, provide cells with a supply of sulphated disaccharide GAG building blocks, increase ECM molecule production (collagen and elastin) by HDFa, and support the growth phase of the hair cycle (anagen).
1. Introduction
The skin is the largest organ in the body, consisting of many cellular layers and a diverse array of cells. As the human body ages, the quantity and quality of key skin building molecules, such as collagen, elastin, and proteoglycans, decreases; the composition of the extracellular matrix is altered; and the skin becomes thinner and less resistant to environmental conditions [1]. Numerous changes taking place at the cellular and molecular level also become visible on the skin surface as wrinkles or skin laxity. The aging process occurs as a result of external and internal factors, for example, genetic, hormonal, chronological, and environmental factors, sun exposure, dietary habits, and lifestyle. Intrinsic aging can be characterized by three main features: atrophy of the dermis due to loss of collagen, degeneration in the elastic fiber network, and loss of hydration. Extrinsic aging is often referred to as photoaging, as it is primarily caused by ultraviolet (UV) exposure. The distinguishing feature of photoaging is an accumulation of elastotic material in the upper and middle dermis (solar elastosis) [2]. The progressive impact of aging is particularly visible in the skin. The proportion of activated fibroblasts in the dermis changes and the density of the extracellular matrix decreases. The integrity of the skin is disturbed, skin becomes thinner, and wound healing and regenerative abilities are compromised [3,4]. The changes caused by the aging process do not only affect the surface of the skin, but also extend to skin appendages, such as hair follicles [5]. Progressive hair loss and reduced regeneration capacity of the hair follicle was observed in humans and rodents [6,7,8]. Regeneration of hair follicles is orchestrated by epithelial–mesenchymal interactions between stem/progenitor cells and fibroblasts comprising the dermal papilla [9,10]. Dermal papilla cells are responsible for controlling proliferation and differentiation of epithelial progenitors through releasing a cascade of signaling molecules [11]. Age-related hair loss (sometimes referred to as senescent alopecia) is associated with a miniaturization of hair follicles and a reduction in dermal papilla cell numbers [12].
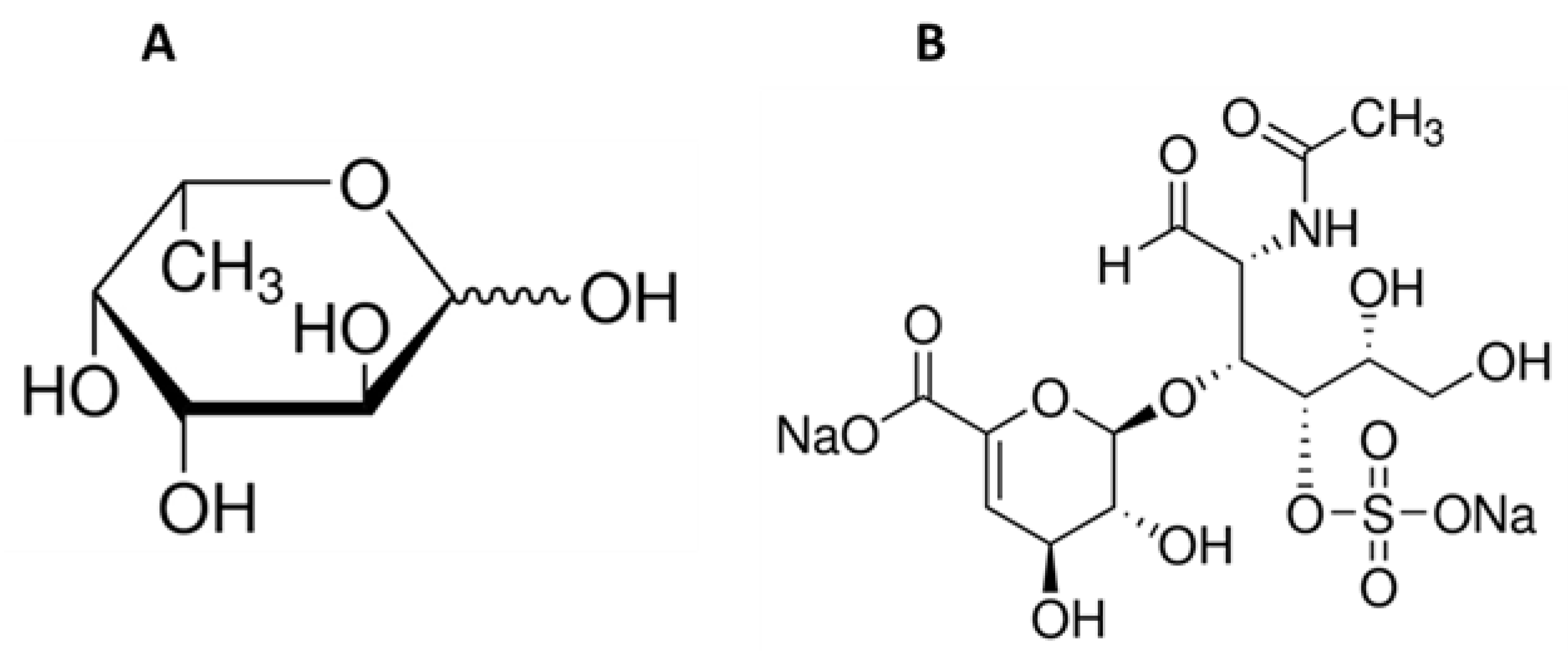
Water, a source of life on Earth and essential for all living organisms, also represents the origin of biologically active chemicals which are incredibly valuable for the pharmaceutical industry. Marine organisms are a source of many ingredients which have various useful roles in maintaining health. They can be applied in the fight against reactive oxygen species (ROS) [13,14,15,16,17] and in the treatment or prevention of infectious diseases [18,19,20,21], cancer diseases [22,23,24,25,26], diabetes [27,28], and obesity [29,30,31], as well as cardiovascular illnesses caused by hypertension [32,33]. Marine-derived compounds are used to produce drugs, pharmaceuticals, and supplements, as well as cosmetics. Various bioactive extracts from marine plants and animals are used in cosmetics acting as photo-aging inhibitors [34,35,36], skin whitening agents [37,38,39], or antiacne agents [40]. Some of these valuable ingredients are not found outside the marine environment. An example of such a group of compounds might be fucose-containing sulfated polysaccharides, with the best-known representative fucoidan. Fucoidans can be characterized by variability in chemical structure, dependent on the source, season, or plant maturity, but L-fucose (Figure 1A) is always the main component and may exceed 90% of the total fucoidan sugar composition [41]. Marine sulphated polysaccharide naturally occur in brown algae (Phaeophyta) and seawater invertebrates such as echinoderms (sea urchins and cucumbers) (class Echinoidea and Holothuroidea). The sea cucumber is also a source of another compound that occurs exclusively in the marine environment—fucosylated chondroitin sulfate [42]. This compound belongs to the glycosaminoglycan group and has the same backbone as mammalian chondroitin sulfate (D-glucuronic acid and N-acetyl-D-galactosamine; Figure 1B) but is distinguished by the presence of sulfated L-fucose [43]. This unusual sugar, characterized by the lack of a hydroxyl group on the 6-position carbon and the L-configuration, occurs in nature in tiny amounts and can be found in humans, bacteria, plants, and fungi [44,45]. L-fucose is a major component of human milk oligosaccharides and can also be found in mucin glycoproteins and glycolipids [44,46,47].
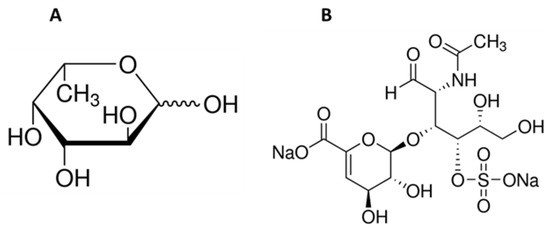
Figure 1.
Chemical structure of L-fucose (A) and chondroitin disaccharide ∆di-4S sodium salt (B).
Fucoidans extracted from many marine sources, as well as glycosaminoglycans, are known for their bioactivities, including their effects on the skin and hair. Fucoidans obtained from various marine macroalgae promote the proliferation of dermal fibroblasts and dermal papilla cells, stimulate hair growth in vitro and in vivo, and have anti-aging properties [48,49,50,51,52,53,54,55]. Glycosaminoglycans, including chondroitin sulphate, have been proven to be skin anti-ageing ingredients/agents/compounds, wound healing promoters, and antiapoptotic agents [56,57,58,59].
In the present study, the effect of single compounds, the basic units of fucoidan and chondroitin sulphate, L-fucose and chondroitin disaccharide ∆di-4S sodium salt, on skin- and hair-derived cells was investigated. We aimed to understand the bioactivity of tested samples on the cellular level and to assess if they can be considered a potential treatment for skin and hair ageing. The potential ability of L-fucose and chondroitin disaccharide to prevent adverse changes in the skin and hair caused by ageing processes was analyzed. Commonly known hair growth markers, such as cell proliferation and the production of alkaline phosphatase and glycosaminoglycans, were tested. In order to look deeper into the hair-related bioactivity of the tested compounds, an analysis of hair-cycle-related genes by qRT-PCR was performed. The influence of the tested samples on skin health was investigated by performing a proliferation assay, an assessment of the extracellular matrix composition, and quantification of type I collagen and elastin. To the knowledge of the authors, the data obtained in the present study are the first of its kind. The bioactivities of L-fucose and chondroitin disaccharide ∆di-4S sodium salt, the building units of larger molecules with proven biological activities, have not been investigated previously in the skin and hair cellular models used in our study.
2. Results
2.1. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Dermal Fibroblast Proliferation Rate
The proliferation rate of fibroblasts in the dermis slows down through aging and a decrease in the total number of dermal cells is observed. To determine whether the tested samples have the ability to affect the proliferation rate of human dermal fibroblasts, PrestoBlue™ Cell Viability Reagent was used.
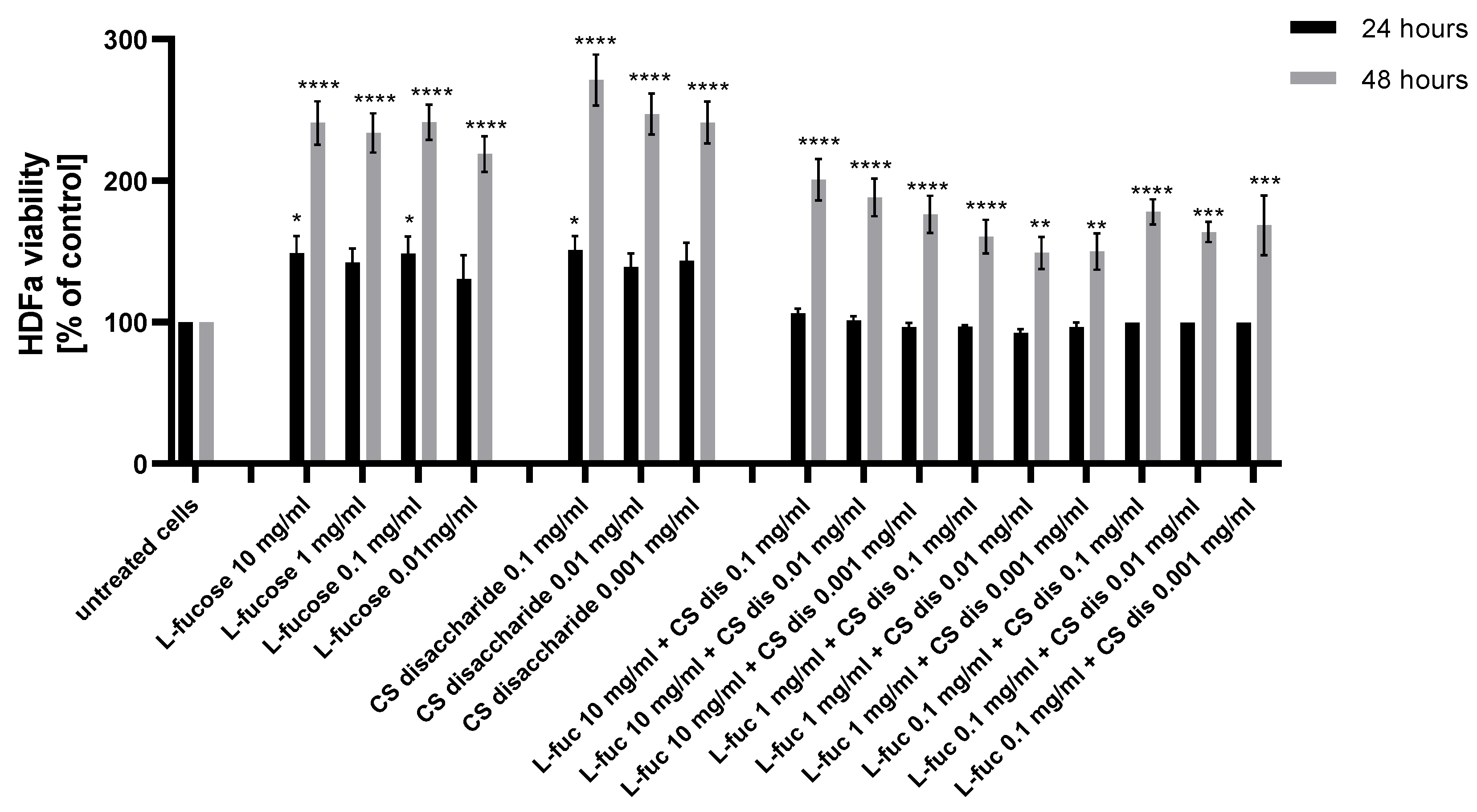
As can be seen from Figure 2, human dermal fibroblast exposure to L-fucose and chondroitin sulphate disaccharide resulted in a statistically significant increase in the cellular proliferation rate.
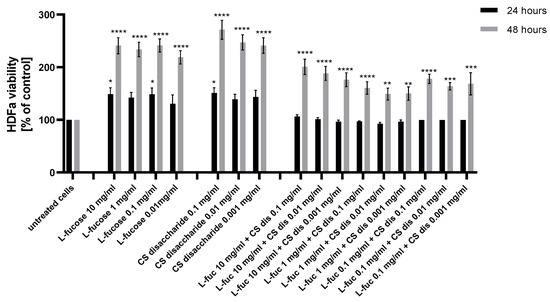
Figure 2.
Effect of L-fucose and chondroitin disaccharide Δdi-4S sodium salt on cell proliferation of human dermal fibroblasts. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
The effect was observed for all tested samples after 48 h of incubation. None of the tested samples were cytotoxic against used cell lines, with the lowest value of 92.5% obtained after 24 h treatment with a combination of 1 mg/mL L-fucose and 0.01 mg/mL chondroitin sulphate disaccharide. Two samples with concentrations of L-fucose of 10 mg/mL and 0.1 mg/mL and of chondroitin sulphate disaccharide of 0.1 mg/mL and 0.001 mg/mL resulted in a statistically significant increase in HDFa proliferation after 24 h incubation. Chondroitin sulphate disaccharide sodium salt has the strongest proliferative effect on human dermal fibroblasts. The effect of separate treatment of dermal fibroblasts with tested L-fucose and chondroitin sulphate disaccharide in terms of the obtained proliferation values was significantly better in comparison to the cell’s exposure to treatment with the samples combined.
2.2. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Dermal Papilla Cell Proliferation Rate
To examine whether the tested L-fucose and chondroitin sulphate disaccharide have the ability to affect the growth phase of the hair cycle (anagen), the proliferation rate of human dermal fibroblasts was investigated by using PrestoBlue™ Cell Viability Reagent.
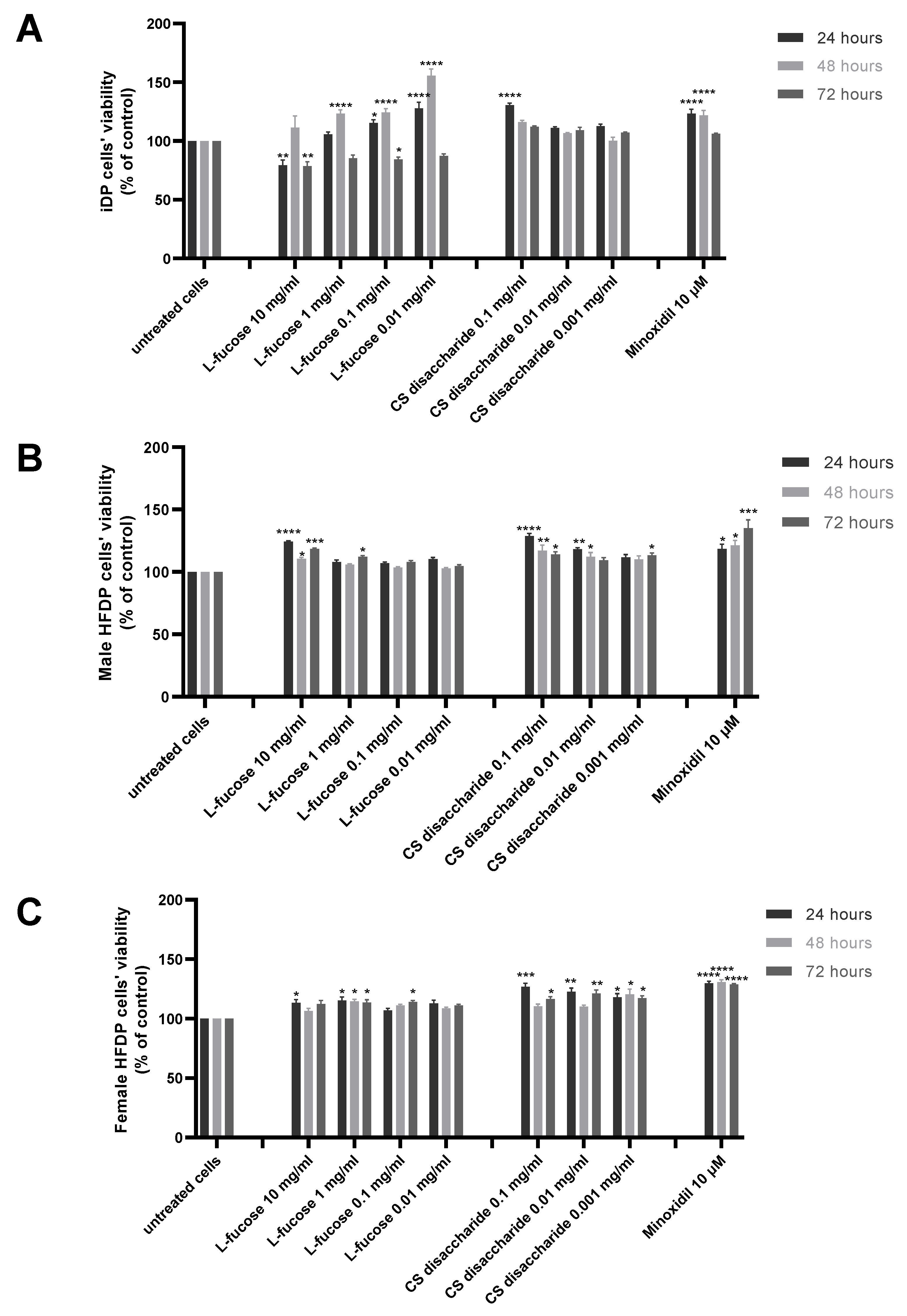
The results of experiments performed on three types of dermal papilla cells are presented in Figure 3. Two tested concentrations of L-fucose, 10 mg/mL and 0.1 mg/mL, caused a statistically significant decrease in the proliferation of immortalized dermal papilla cells (Figure 2A); however, the obtained results do not demonstrate a cytotoxic effect of the tested sample. In the case of primary cell lines (Figure 2B,C), the tested L-fucose and chondroitin disaccharide Δdi-4S sodium salt significantly stimulated the proliferation of male- and female-derived dermal papilla cells. A statistically significant increase in hair cell proliferation in comparison to the untreated cells was also observed for 10 µM minoxidil.
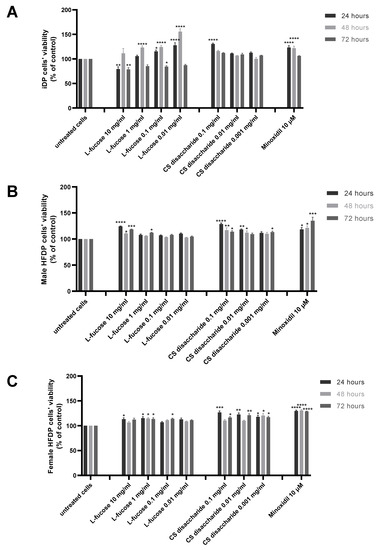
Figure 3.
Effect of L-fucose and chondroitin disaccharide Δdi-4S sodium salt on cell proliferation of dermal papilla cells: immortalized (A), primary male (B), and primary female (C). Cells were treated with different concentrations of the tested samples for 24, 48, and 72 h, and proliferation was assessed using PrestoBlue® Cell Viability Reagent. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
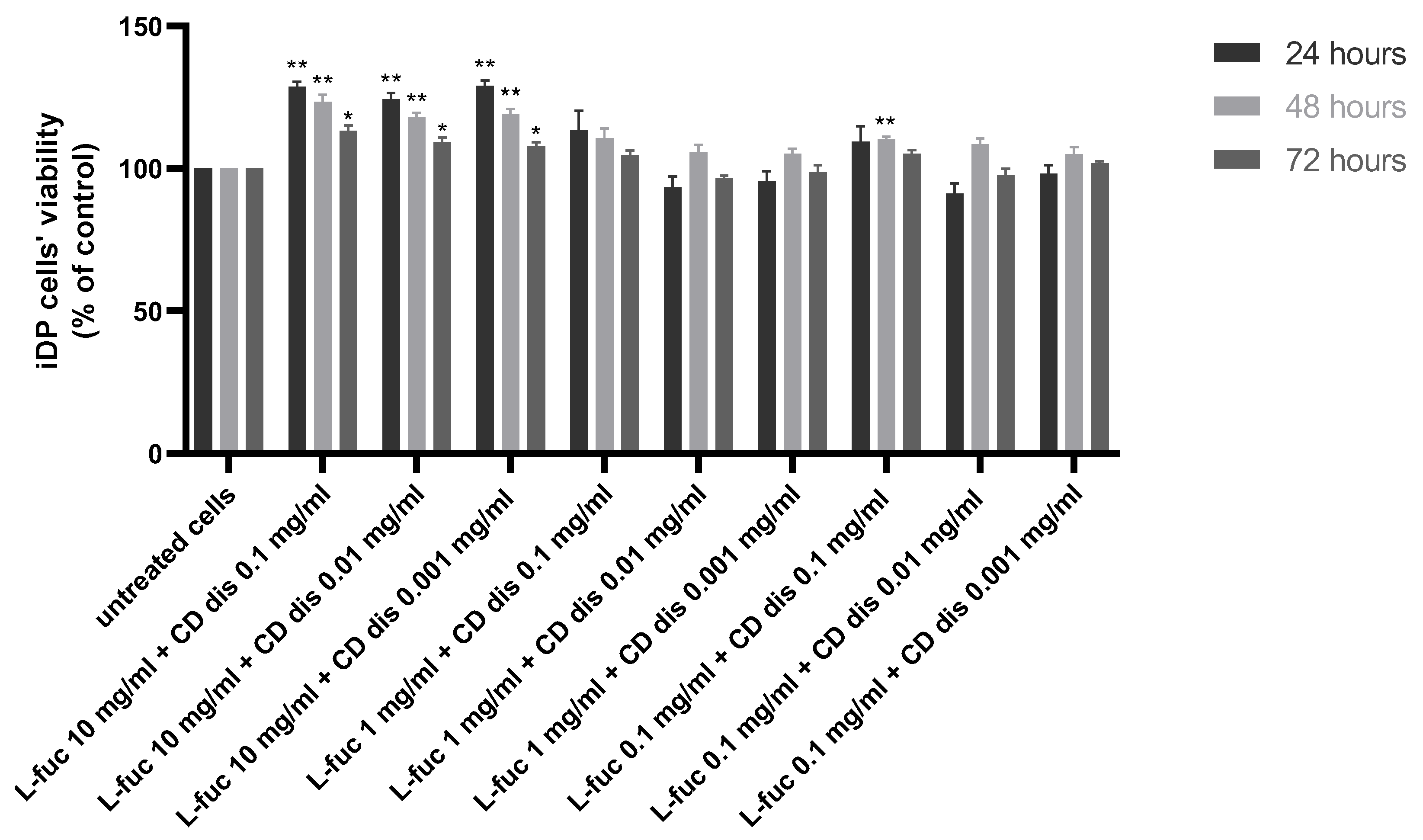
The combination of 10 mg/mL L-fucose with the three different concentrations of chondroitin sulphate disaccharide caused a statistically significant increase in immortalized dermal papilla cell proliferation at all tested timepoints (Figure 4).
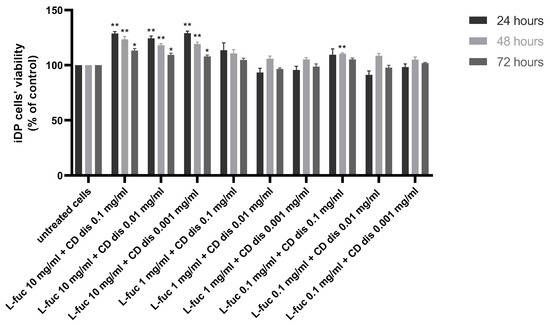
Figure 4.
Effect of the simultaneous treatment of immortalized dermal papilla cells with L-fucose and chondroitin disaccharide Δdi-4S sodium salt. Cells were treated with different concentrations of tested samples for 24, 48, and 72 h, and proliferation was assessed using PrestoBlue® Cell Viability Reagent. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01.
2.3. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Alkaline Phosphatase Levels in Dermal Papilla Cells
To investigate whether the tested samples influence the anagen phase of hair growth, the level of alkaline phosphatase production by immortalized and primary dermal papilla cells was assessed.
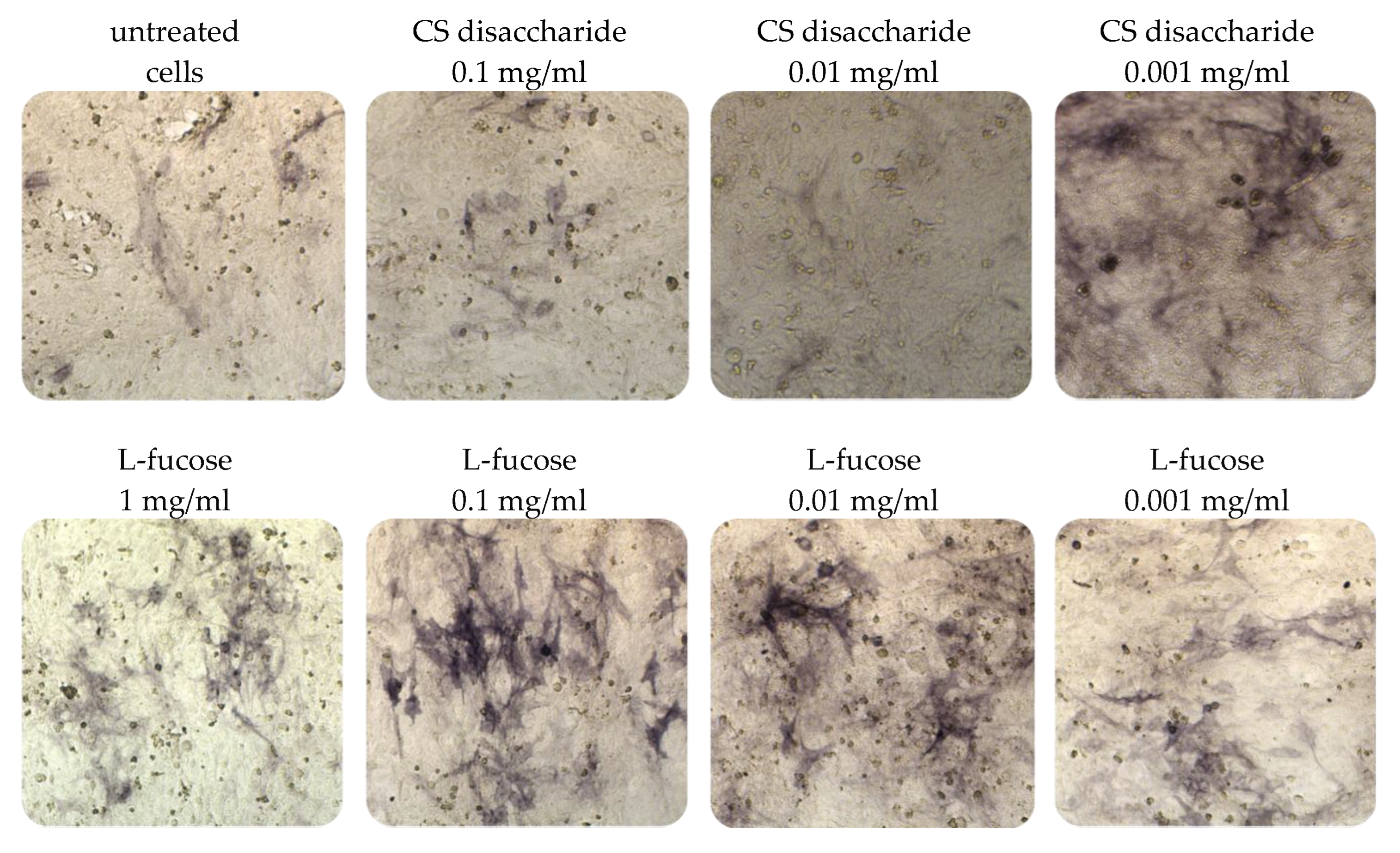
Immortalized dermal papilla cells cultured for 120 h in media containing L-fucose or chondroitin sulphate disaccharide sodium salt displayed alkaline phosphatase activities (Figure 5). The results of the histological analysis showed an increase in the enzyme activity (bluish violet color). The highest intensity of staining in comparison to untreated cells was observed when dermal papilla cells were cultured with two concentrations of L-fucose, 0.1 mg/mL and 0.01 mg/mL. Fewer violet cells were noted in the chondroitin sulphate disaccharide-treated group, especially at a concentration of 0.001 mg/mL.
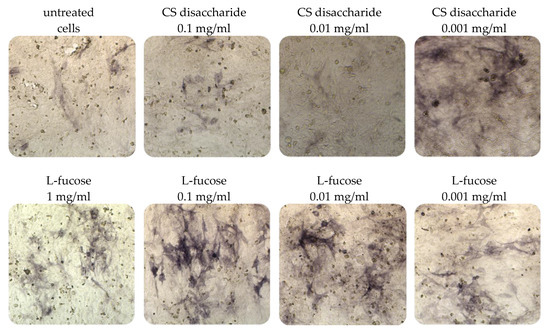
Figure 5.
Effect of L-fucose and chondroitin disaccharide ∆di-4S sodium salt on alkaline phosphatase production by immortalized dermal papilla cells. Cells were treated with different concentrations of the tested samples for 120 h and stained with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) to visualize alkaline phosphatase activity (bluish violet color).
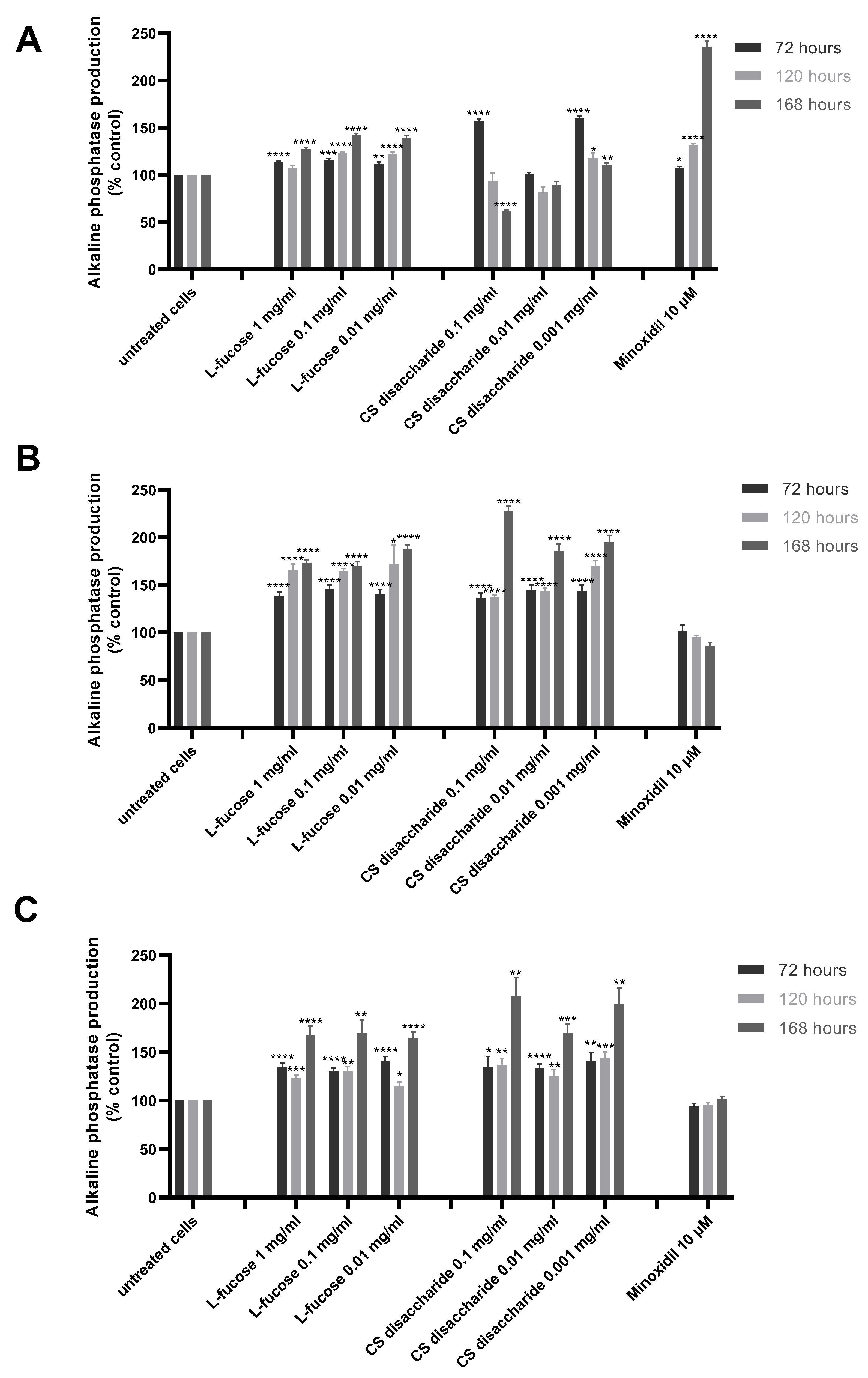
The results from the quantitative analysis of enzyme production indicated that tested samples significantly increase alkaline phosphatase expression, this was especially visible in the case of L-fucose used at the three sampled concentrations and chondroitin sulphate disaccharide at the lowest concentration, 0.001 mg/mL (Figure 6A).
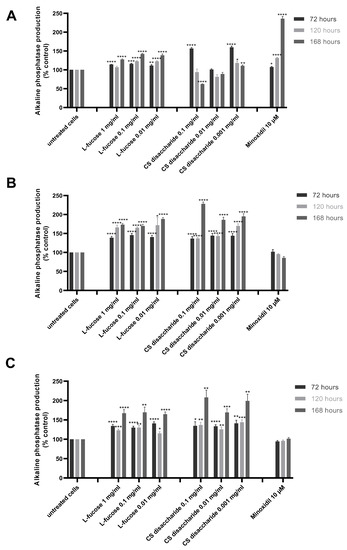
Figure 6.
Effect of L-fucose and chondroitin disaccharide Δdi-4S sodium salt on alkaline phosphatase production by dermal papilla cells: immortalized (A), primary male (B), and primary female (C). Cells were treated with different concentrations of tested samples for 72, 120, and 168 h. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
An increased production of the enzyme also resulted in iDP cell exposure to 10 µM minoxidil. However, the sample did not stimulate alkaline phosphatase production by primary cells. Similar conclusions were drawn by other authors. Lee et al. [60] analyzed the effect of valproic acid and minoxidil on primary human dermal papilla cells. The authors have concluded that valproic acid and not minoxidil increased the alkaline phosphatase production level by the tested cells. The observed stimulation of enzyme synthesis was caused by the up-regulation of the Wnt/β-catenin pathway. The relation between different Wnt isoforms and an increased production of alkaline phosphatase has been reported previously [61,62]. In a study by Lee et al., minoxidil did not affect the Wnt/β-catenin signaling pathway [60]. The positive effects of minoxidil action on the hair cycle are related to the ERK and protein kinase B (Akt) pathways [63]. Kwack et al. [64] compared the biological activity of five immortalized human scalp dermal papilla cell lines to the primary dermal papilla cells (male origin). It was found that alkaline phosphatase was expressed in KNU201 immortalized cells as well as in the early passage of male dermal papilla cells (p2). Primary cells at passage 6 did not produce alkaline phosphatase. The immortalized cell line KNU201 as well as the cell line used in our study was established using a simian virus 40T (SV40T) and the human telomerase reverse transcriptase (hTERT) gene.
Female- and male-derived dermal papilla cell lines produced a significantly higher concentration of the tested enzyme when treated with L-fucose and chondroitin sulphate disaccharide (Figure 6B,C). These results were observed for all tested concentrations of samples after 72, 120, and 168 h of incubation. An increased enzyme activity was observed with increasing incubation time.
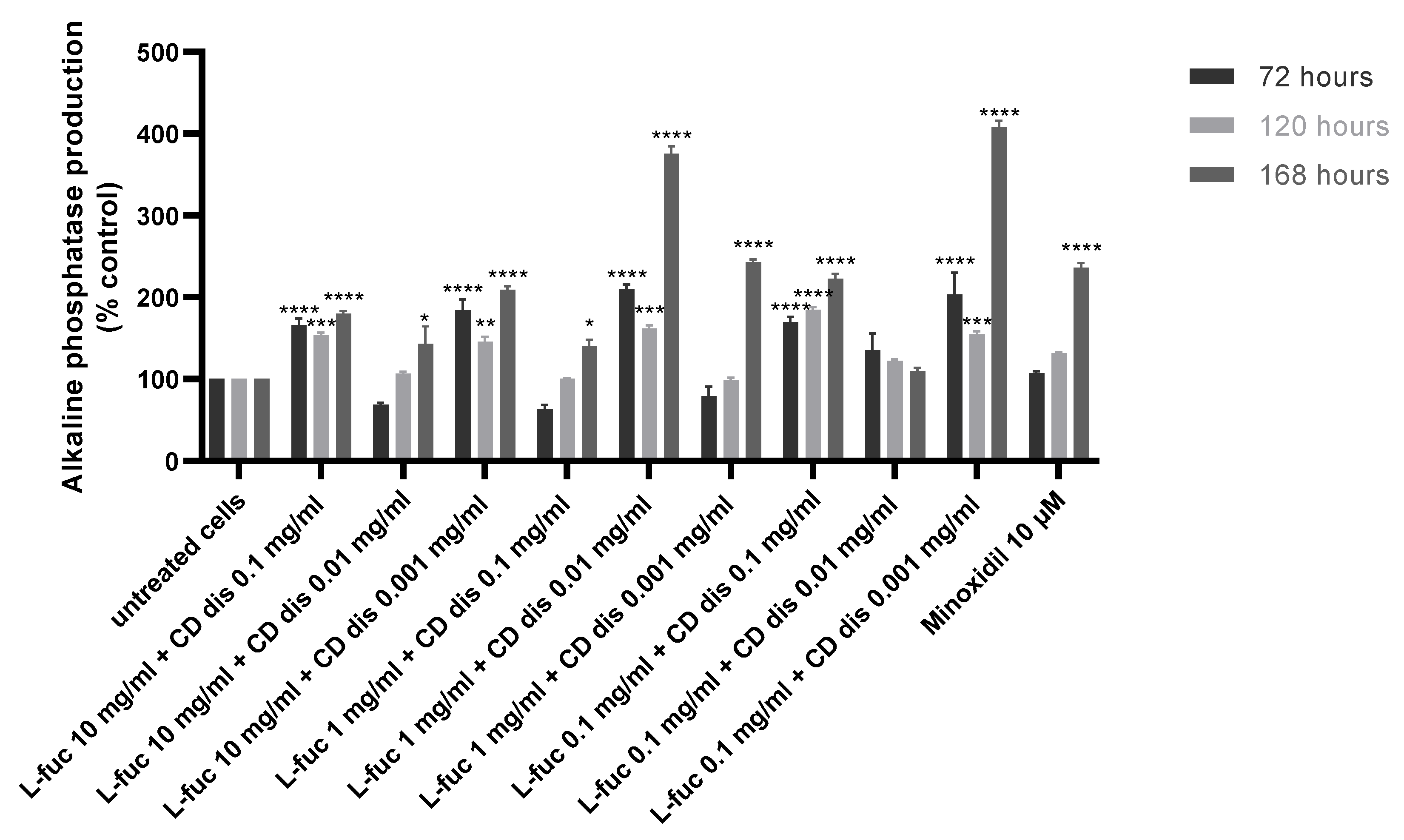
The production of alkaline phosphatase by immortalized dermal papilla was stimulated when tested samples were used in combination, especially after 168 h of treatment (Figure 7).
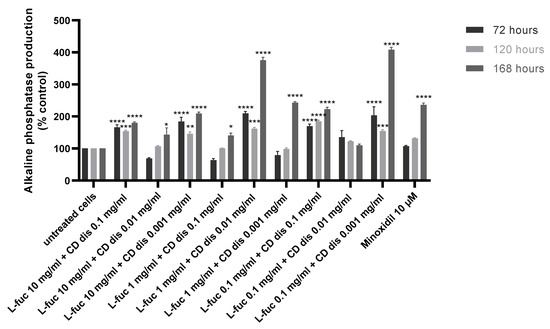
Figure 7.
Effect of simultaneous treatment with L-fucose and chondroitin disaccharide Δdi-4S sodium salt on alkaline phosphatase production by immortalized dermal papilla cells. Cells were treated with different concentrations of tested samples for 72, 120, and 168 h. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
2.4. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Glycosaminoglycan (GAG) Production in Dermal Papilla Cells
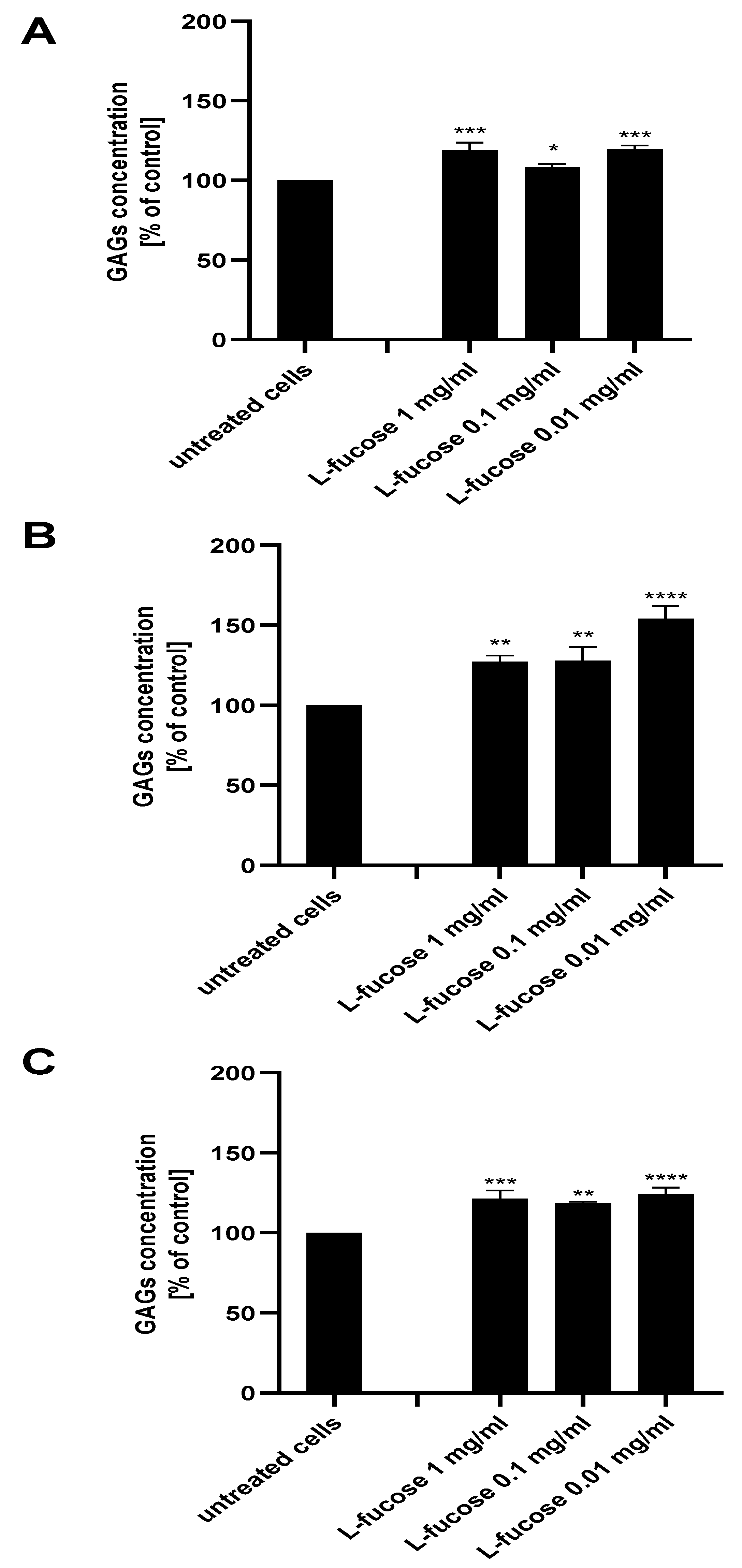
The extracellular matrix of the hair follicle dermal papilla is rich in glycosaminoglycans, with the expression varying during the hair growth cycle and being maximal in the growth phase (anagen). The ability of the tested samples to support the anagen phase of hair growth was tested with an assessment of glycosaminoglycan production by the three dermal papilla cell lines.
As can be seen in Figure 8, treatment of all three types of dermal papilla cell lines resulted in a statistically significant increase in glycosaminoglycan production by the cells. The highest expression of sulphated GAGs was obtained for male HFDPC, the levels of detected molecules were 127%, 128%, and 154% for concentrations of 1 mg/mL, 0.1 mg/mL, and 0.01 mg/mL, respectively.
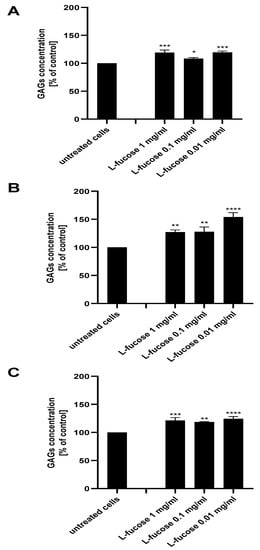
Figure 8.
Effect of L-fucose on glycosaminoglycan production by dermal papilla cells: immortalized (A), primary male (B), and primary female (C). Cells were treated with different concentrations of the tested sample for 96 h. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
2.5. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Collagen Synthesis in Fibroblasts
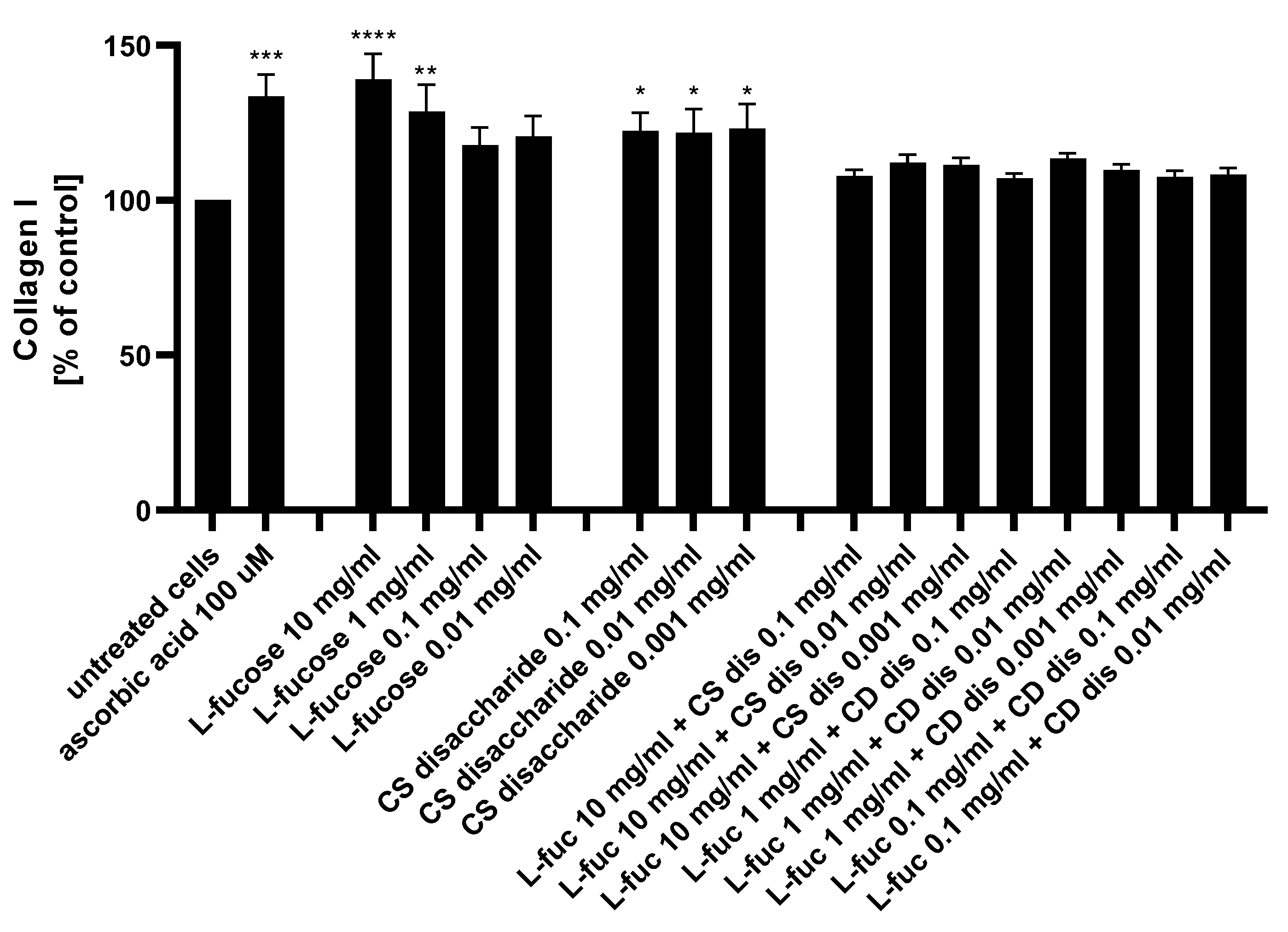
The potential of the tested samples to stimulate collagen I production by dermal fibroblasts was assessed using the ELISA technique. The results are presented in Figure 9.
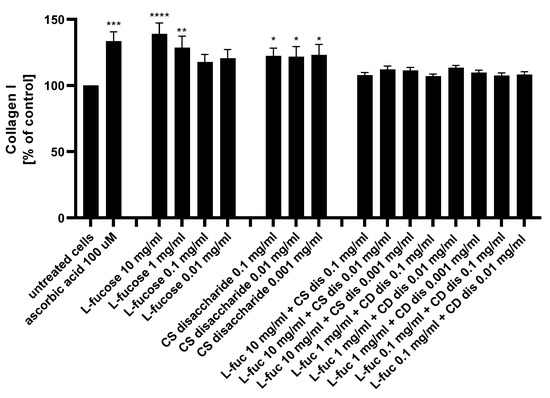
Figure 9.
Collagen I production measured by ELISA in HDFa after treatment with L-fucose and chondroitin disaccharide Δdi-4S sodium salt. HDFa was treated with different concentrations of the tested samples for 48 h. Results are presented as a percentage of the untreated cells ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
L-fucose caused a statistically significant increase in collagen production when used at the two highest concentrations, 10 mg/mL and 1 mg/mL. Human dermal fibroblasts treated with three concentrations of chondroitin sulphate disaccharide Δdi-4S exhibited a statistically significant increase in collagen production levels. There was a lack of significant changes in the protein expression observed when cells were exposed to the combination of both tested samples. Collagen production levels were reduced below those achieved by either L-fucose and chondroitin sulphate disaccharide alone. When L-fucose was used alone at a concentration of 10 mg/mL, the collagen production level was significantly higher when compared to the protein expression obtained after simultaneous treatment with the same concentration of L-fucose and 0.1 mg/mL (p < 0.01), 0.01 mg/mL (p < 0.05), and 0.001 mg/mL (p < 0.05) chondroitin sulphate disaccharide.
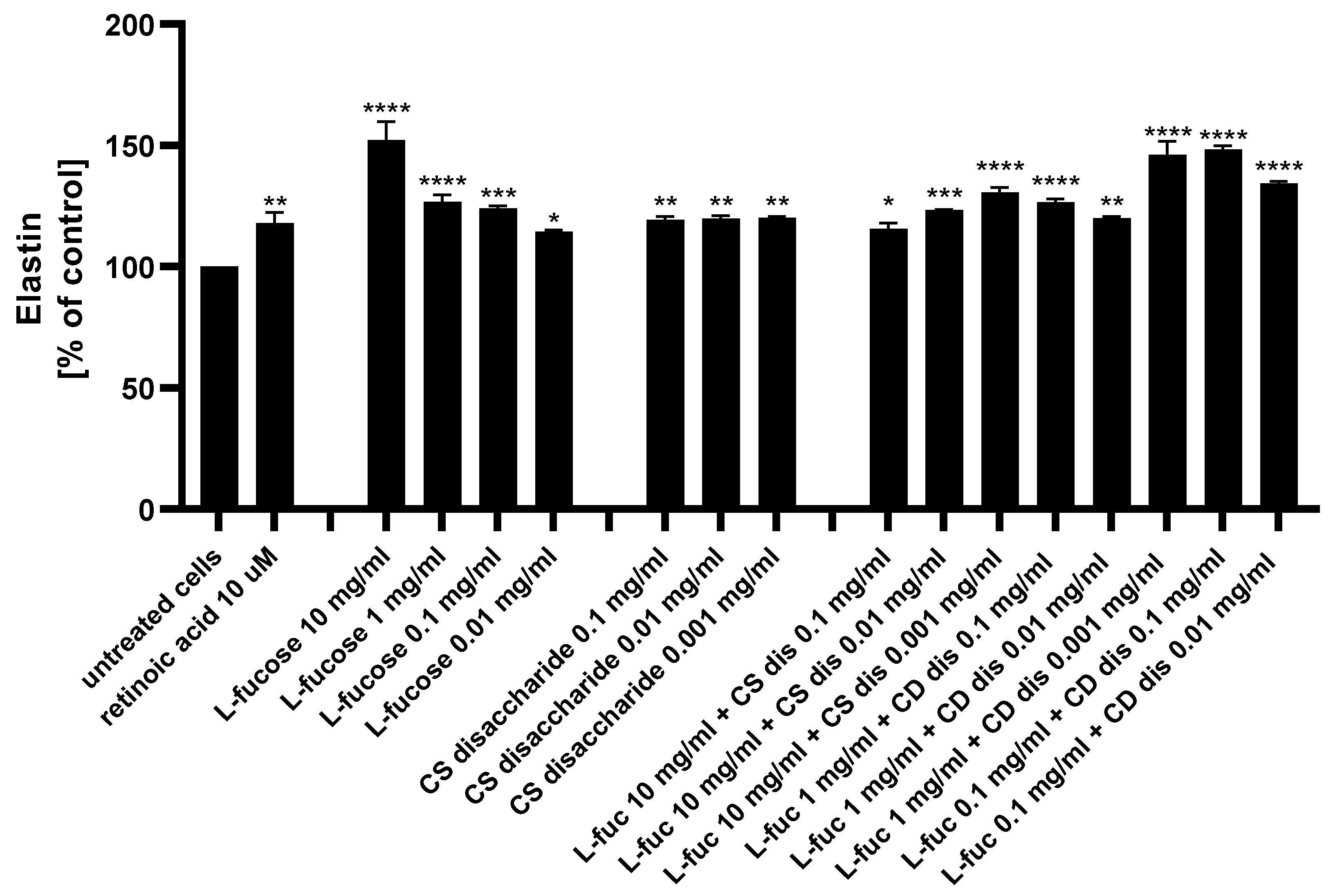
2.6. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Elastin Synthesis in Fibroblasts
The ability of L-fucose and chondroitin sulphate disaccharide Δdi-4S to stimulate fibroblasts to produce one of the major components of the extracellular matrix, elastin, was analyzed using ELISA.
As presented in Figure 10, both tested samples, used alone or in combination, exhibited a statistically significant increase in elastin production by human dermal fibroblasts. Of the 15 treatments evaluated, 4 caused a significantly higher increase in the protein expression when compared to the positive control: 10 µM retinoic acid (p < 0.01)–10 mg/mL L-fucose; 1 mg/mL L-fucose + 0.001 mg/mL CS dis; 0.1 mg/mL L-fucose + 0.1 mg/mL CS dis; and 0.1 mg/mL L-fucose + 0.001 mg/mL CS dis. When L-fucose was used alone at a concentration of 10 mg/mL, the elastin expression level was significantly higher compared to the protein production obtained after simultaneous treatment with the same concentration of L-fucose and 0.1 mg/mL (p < 0.0001), 0.01 mg/mL (p < 0.001), and 0.001 mg/mL (p < 0.01) chondroitin sulphate disaccharide. A combination of 0.1 mg/mL L-fucose and 0.1 mg/mL chondroitin sulphate disaccharide resulted in a significantly higher elastin production than that achieved by either L-fucose or chondroitin sulphate disaccharide alone.
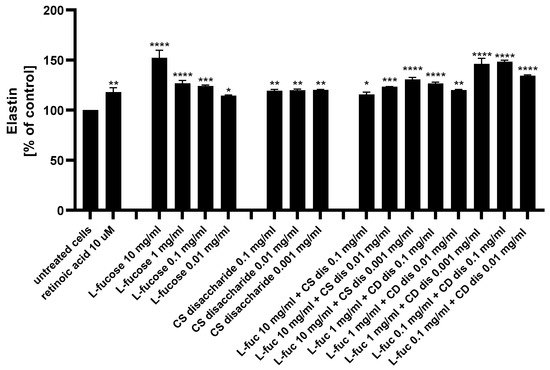
Figure 10.
Elastin production measured by ELISA in HDFa after treatment with L-fucose and chondroitin disaccharide Δdi-4S sodium salt. HDFa was treated with different concentrations of the tested samples for 24 h. Results are presented as a percentage of the untreated cells ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
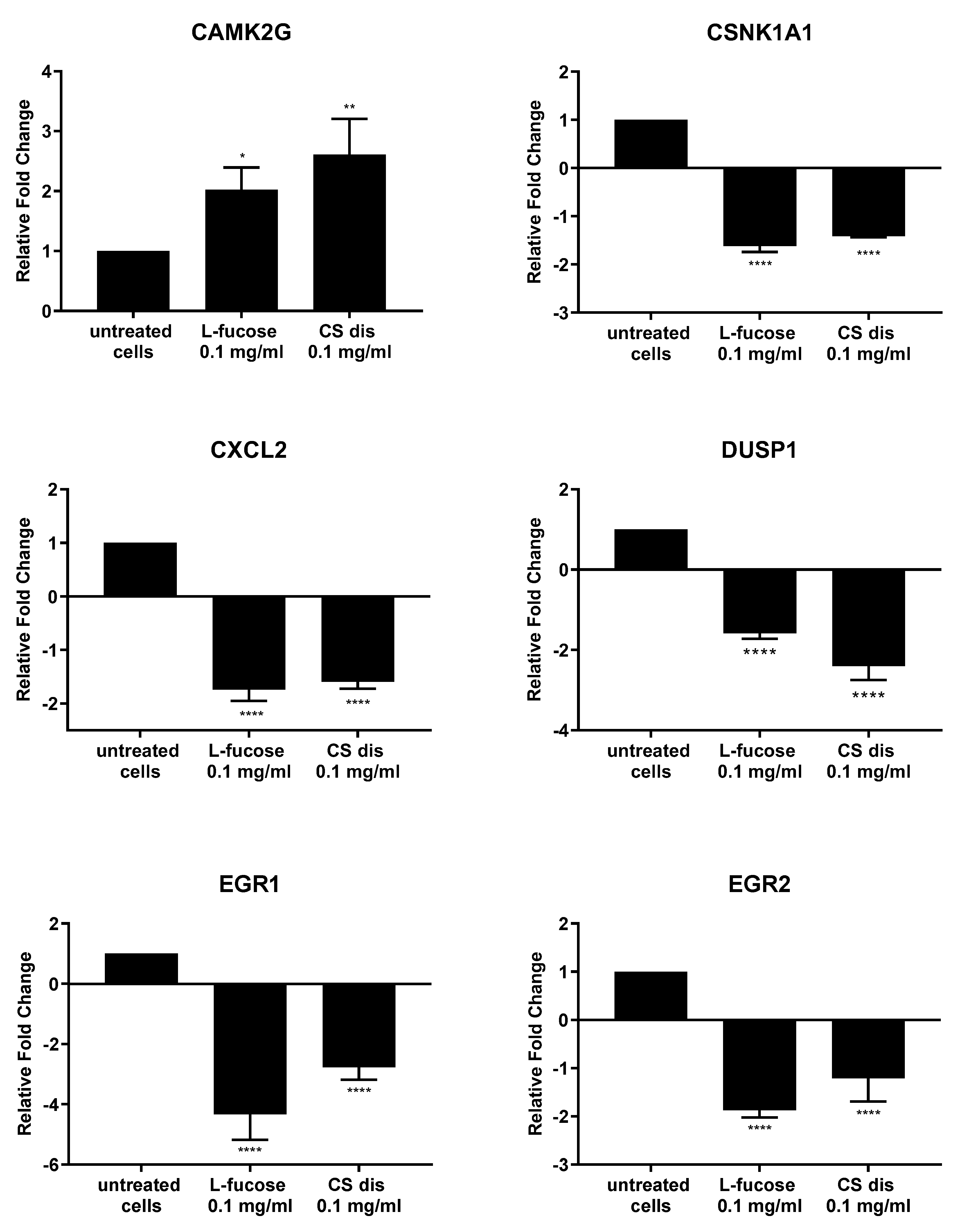
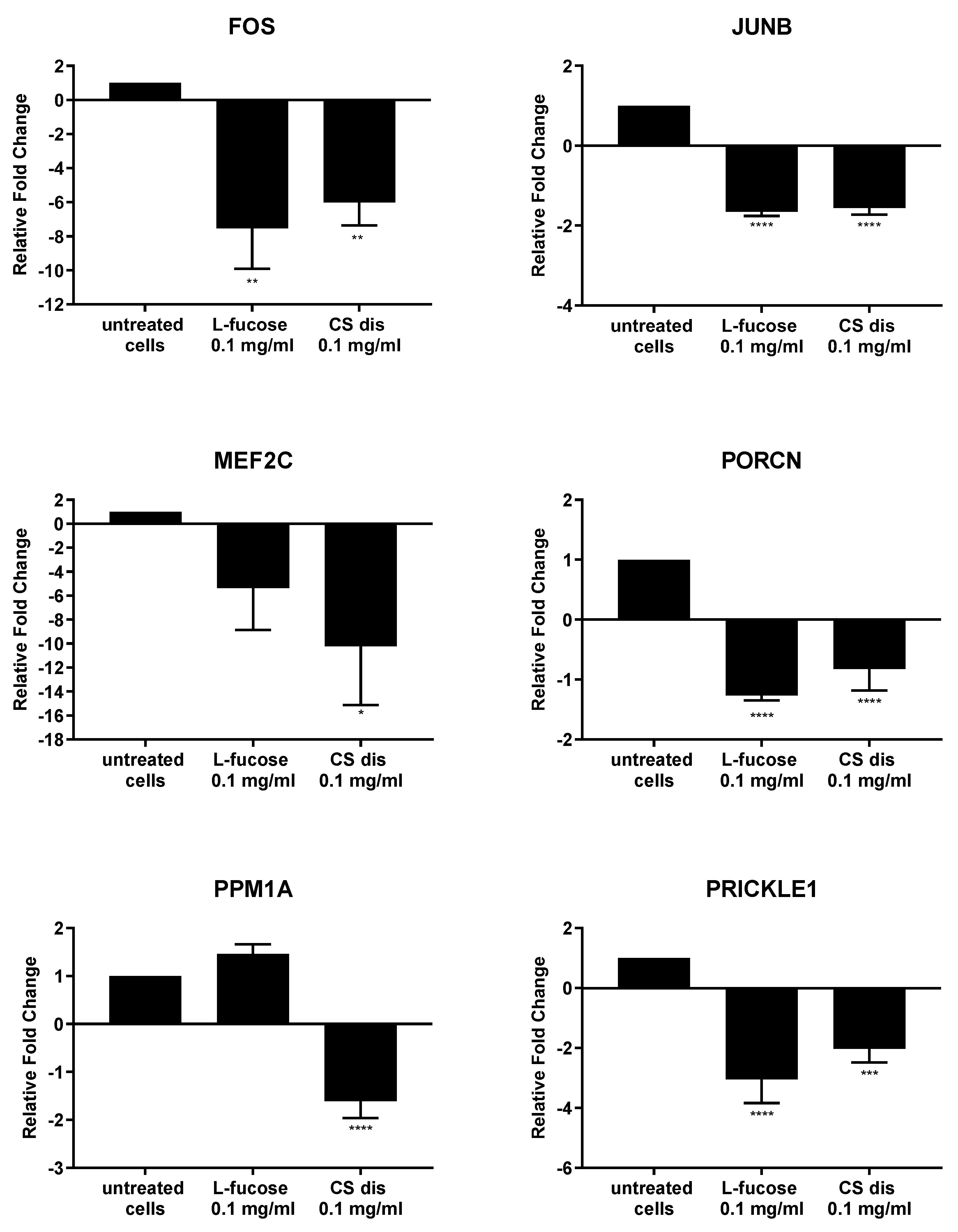
2.7. Effect of L-fucose and Chondroitin Disaccharide ∆di-4S Sodium Salt on Gene Expression
As can be seen from Figure 11, both tested samples, L-fucose and chondroitin sulphate disaccharide, caused similar changes in the genetic level. The only exception is the PPM1A gene, the expression of which was significantly down-regulated by CS disaccharide and up-regulated (lack of statistical significance) by L-fucose. In general, treatment of male dermal papilla cells with L-fucose caused an up-regulation of the expression of two genes (CAMK2G and PPM1A) and down-regulation of ten (CSNK1A1; CXCL2; DUSP1; EGR1; EGR2; FOS; JUNB, MEF2C; PORCN; and PRICKLE1). Chondroitin sulphate disaccharide at a concentration of 0.1 mg/mL up-regulated the expression of one gene of interest, CAMK2G, and down-regulated the expression of all other eleven genes.
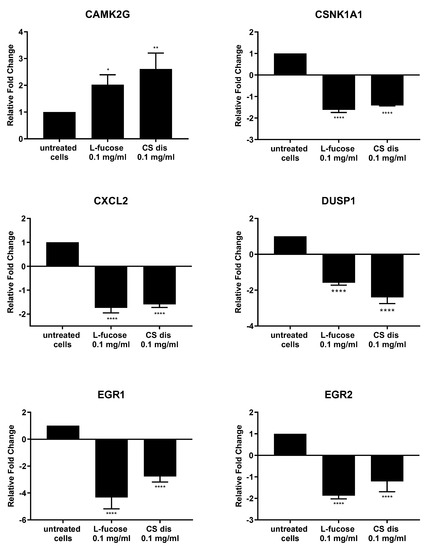
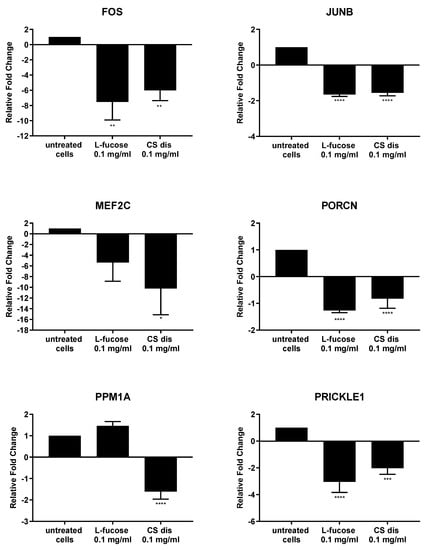
Figure 11.
l-fucose’s and chondroitin disaccharide Δdi-4S sodium salt’s effect on gene expression in male human dermal papilla cells. The data are presented as the means ± SEM. Statistical significance in comparison to the negative control (untreated cells) was assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
3. Discussion
In the present study, the effect of L-fucose and chondroitin disaccharide Δdi-4S sodium salt on skin and hair cells was investigated. Both compounds are building blocks of larger molecules with proven skin and hair health promoting properties, fucoidan and glycosaminoglycan chondroitin sulphate. Prior work has documented the potential of both tested compounds to support skin and hair health, however, to the best of authors knowledge, the assessment of bioactivity of the samples, used separately and in combination, in four human cell lines, both immortalized and primary, was not considered previously in the literature.
Proliferative properties of tested L-fucose and chondroitin sulphate disaccharide were analyzed in this study. The obtained results indicate that tested compounds positively affect proliferation rate of all four types of tested cell lines. This is highly valued in the context of approach for skin rejuvenation, anti-aging and hair growth. Dermal fibroblast proliferation and skin turnover are associated with and characteristic of both intrinsic and extrinsic skin aging pathways.
Cellular proliferation is a very important parameter of skin and hair regeneration and is evaluated for every molecule that potentially play a role in hair and skin biology. Proliferation is the most often tested marker of activity of dermal papilla cells, as it determines their growth and mitotic index [65]. Dermal fibroblasts proliferation is not only a parameter considered for the antiaging agents, but also plays a role in wound healing and skin regeneration [66]. Neri et al. [58] investigated hair growth promoting effect of GAGs extracted from ascidian tunics.
After separation and purification steps, researchers obtained three fractions differing in the ratio of ∆di-6S and ∆di-4S disaccharide components. It was found that none of the tested fraction showed toxicity on human follicle dermal papilla cells and fractions F3 used at the concentrations 50 and 100 μg/mL significantly increased cell proliferation. Proliferative effect was also observed for a combination of fractions F2 and F3 after 120 hours of incubation. The results obtained in our study prove proliferative effect of chondroitin sulphate disaccharide Δdi-4S sodium salt as well. As proteoglycans and building them glycosaminoglycans are present in dermal papilla cells in large quantities, especially in the phase of hair growth, anagen, the mechanism of action of the tested compound might be based on the delivery of building units for these larger molecules [67,68]. Glycosaminoglycans and proteoglycans are important components of not only hair follicles, but skin extracellular matrix as well. The most abundant skin proteoglycans- versican, decorin, and biglycan, belong to the class of chondroitin sulphate or dermatan sulphate proteoglycans [69]. The observed proliferative properties of tested disaccharides on human dermal fibroblasts might be connected to the fact that tested compound is a building unit for skin proteoglycans, molecules involve in the regulation of cell adhesion, migration and proliferation [70]. Another tested compound, L-fucose also showed proliferative activity in dermal papilla cells and dermal fibroblasts. Similar results were obtained by Péterszegi et al. [71]. L-fucose used at concentrations 1 and 10 µg/mL statistically significant increased proliferation of human skin fibroblasts.
Dermal papilla cells produce a huge variety of growth factors, cytokines and other molecules acted as a marker of hair growth. One of these secreted molecules is alkaline phosphatase [65]. This zinc-metallo enzyme (EC 3.1.3.1) is widely distributed in high metabolic rate cells [72]. Hair follicles as a highly proliferating and regenerative tissue also express substantial amount of alkaline phosphatase. The activity of the enzyme changed during the hair growth cycle—with a maximal level obtained during anagen phase of hair growth [73]. On that basis it can be assumed that the enzyme act as a marker of the hair growth phase. Results of present in vitro study on dermal papilla cells revealed that tested L-fucose and chondroitin sulphate Δdi-4S disaccharide promote expression of alkaline phosphatase and through that activity support the anagen phase of hair cycle. The increase in the enzyme level after treatment with potential hair growth promoters was observed and documented previously in the literature. Treatment of human dermal papilla cells with rice bran mineral extract resulted in increase in enzyme production. The hair growth promoting activity of tested sample was connected with the activation of β-catenin/Wnt signaling pathway - one of the most important for growth and development of hair follicles pathway [74]. The Wnt signaling pathway is an evolutionarily conserved pathway regulating cell migration, cell polarity, neural patterning and organogenesis during embryonic development [75]. Within the group of Wnt pathways we can distinguish canonical (Wnt-β-catenin) and noncanonical signalling (the Wnt/Ca2+, the Wnt/planar cell polarity (PCP) and Wnt/Ror2) [76,77] and all are involved in hair follicle development [63,78,79,80,81].
Male follicle dermal papilla cells treatment with L-fucose and chondroitin sulphate disaccharide resulted in increased expression of CAMK2G (Figure 11), gene involves in Wnt/Ca2+ pathway. Calmodulin-dependent kinase II (CamKII), product of the gene expression, is stimulated by calcium released from the endoplasmic reticulum and activates transcription factor NFAT in noncanonical Wnt pathway [82]. PRICKLE1, another gene from the Wnt noncanonical signalling pathway, was found to be significantly downregulated by the tested compounds. The gene encodes prickle planar cell polarity protein 1, a nuclear receptor involve in the Wnt/PCP pathway. Several studies indicate that PRICKLE1 may function as a negative regulator of canonical Wnt/β-catenin pathway [83,84,85,86]. Moreover, the significant upregulation of CSNK1A1 was observed when primary cells were treated with tested samples. Protein encoded by the gene, casein kinase 1α (CK1α), is a component of the β-catenin degradation complex and acts as a critical regulator of the Wnt signaling pathway [87,88,89]. Casein kinase 1α in responsible for β-catenin phosphorylation, which initiates cascade of signals leading to the β-catenin destabilization, ubiquitination and subsequently proteasomal degradation [90]. The obtained RT-PCR results may indicate that the activity of L-fucose and chondroitin sulphate disaccharide is mediated through Wnt signalling pathways.
Downregulation of two genes, FOS and JUNB, which protein products are the members of activator protein 1 (AP-1) family of transcription factors was observed in the present study. AP1 proteins are responsible for gene expression regulation and are involved in a variety of cellular processes, like proliferation, differentiation or apoptosis [91,92,93,94]. One of the signalling pathway AP-1 proteins are involved in is TGF-β-Smad. It was observed that knockdown of two subunits of AP-1, JUN and FOS or using inhibitors, T-5224 and SR11302, resulted in downregulation of TGF-β1 [95]. TGF- β acts as a negative regulator of hair growth, reduces the anagen phase and promotes early enter the catagen [96,97]. The synthesis of transforming growth factor-beta2 in dermal papilla cells as a result of dihydrotestosterone activity is an initial step of a cascade of molecular events leading to the premature entry into catagen [97]. The results obtained in the study may suggest that hair growth properties of tested samples are connected with the inhibition of TGF-β pathway, involved in the anagen reduction and catagen progression.
In the present study, the effect of L-fucose on glycosaminoglycans production by dermal papilla cells was investigated. Results showed increase in GAGs level after treatment with tested sample. Positive effect of L-fucose activity was observed in all three types of dermal papilla cells, both immortalized and primary male- and female-derived. L-fucose is an element of many glycoproteins as a terminal or core monosaccharide of glycan chain, can be found in proteoglycans like keratan sulphate or chondroitin sulphate. Due to the terminal location of the L-fucose, it might act as a recognition site for other molecules or acceptor molecule for the attachment of further saccharides [98]. It was proven that the dermal part of hair follicle, consisting of dermal papilla and connective tissue sheath, is rich in extracellular matrix compounds, such as glycosaminoglycans (GAGs) [99,100,101,102].
The role of these molecules and associated proteoglycans is stabilizing, participating in fibrillogenesis and epithelial-stromal interactions during hair follicle morphogenesis, interacting with secreted growth factors and modulating the signal molecules exchange between the epithelium and the dermal papilla [68,103]. It was reported by Fernández-Martos et al. [104] that a defined GAG matrix promoted and supported the sustained growth of human hair follicles ex vivo. Hair follicular units, obtained from human scalp samples, were grown in supplemented Williams E medium containing 1.5% HC007 containing GAGs (high and low molecular weight hyaluronic acid chondroitin sulfate, dermatan sulfate, keratan sulfate and heparan sulfate, purity ≥ 95%). It was found that the treatment supported cell proliferation and hair follicle growth—after 8 days of ex vivo culture strong cell proliferation phenotype was maintained, after 16 days a significant increase in the number of cells was observed. Moreover, researchers noted increase in hair shaft length in about 50% of hair follicles grown in 1.5% HC007.
A decrease in skin hydration, loss of compounds that are components of extracellular matrix, such as hyaluronic acid, collagen or elastin, increased activity of matrix-degrading metalloproteinases are associated with the aging process and are visible on the skin in the form of wrinkles or loss of firmness [2,105]. The dermis consists mainly of collagen fibers, which make up 70% of its volume. The most abundant collagen in the skin is type I collagen. It is a fibrous structural protein with significant tensile strength giving the skin its strength and toughness [106]. Collagen is produced by dermal cells, fibroblasts. This type of cells is responsible for production the other fibrillar skin protein, elastin. This component of extracellular matrix provides skin with its stretch and resilience, exemplified by the skins ability to return to their original conformation [107]. Compounds that have an ability to stimulate fibroblasts to produce extracellular matrix molecules - collagen and elastin, and thus compensate for the aging resulting changes, are considered to be an anti-aging agents. In the present study, increased synthesis of collagen and elastin was observed as a result of dermal fibroblasts treatment with L-fucose and chondroitin sulphate disaccharide (Figure 9 and Figure 10). Robert et al. [108] investigated the effect of L-fucose on elastin biosynthesis on human skin fibroblasts in explant cultures. L-fucose used at concentration 10 µg/mL causes increase in elastin production of about 40%. It was also found that 10 µg/mL L-fucose inhibited collagen biosynthesis. The obtained results are in agreement with our finding. All four tested concentrations of L-fucose used in our study caused statistically significance increase in elastin level, however in the case of collagen the significant increase was observed only for the highest two concentrations, 1% w/v and 0.1% w/v (corresponding to 10 mg/mL and 1 mg/mL). Kitazawa et al. [109] analysed effect of low molecular weight chondroitin sulphate, high molecular weight chondroitin sulphate and chondroitin sulphate disaccharides on ECM molecules production by normal human dermal fibroblasts. The cells were treated with tested samples and the gene expression levels of extracellular matrix-related proteins after 12 h was evaluated. Disaccharide CS significantly increased gene expression levels of COL1A1 and DCN, ELN and SMAD2 when compared with the normal group. The effect of high-molecular-weight CS on gene expression levels was not observed. Significant increase in collagen and elastin protein expression level measured by ELISA was noted as well.
The obtained results reveal that both tested compounds, L-fucose and chondroitin sulphate disaccharide, support skin and hair health, especially in terms of anti-aging effects. Treatment with tested samples provides the approaches to addressing both intrinsic and extrinsic aging of skin and hair. Tested compounds support and promote proliferation of dermal fibroblast and dermal papilla cells, provide cells with a supply of sulphated disaccharide GAG building blocks, increase ECM molecules production, collagen and elastin, by HDFa, support growth phase of hair cycle, anagen.
4. Materials and Methods
4.1. Materials
L-fucose (F2252; ≥99%) and chondroitin disaccharide Δdi-4S sodium salt (C4045) were purchased from Sigma-Aldrich Co. Ltd. (Saint Louis, MO, USA).
4.2. Cells Culture
The human dermal fibroblasts cell line, HDFa, was purchased from Gibco® Cell Culture. Cells were cultured in complete Dulbecco’s modified Eagle’s medium (DMEM; GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) containing 5% fetal bovine serum (FBS; GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) and 2 mM L-glutamine (GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C in a 5% CO2 humidified atmosphere.
The immortalized human dermal papilla cells, iDPC, were purchased from Kyungpook National University (South Korea). Cells were cultured in complete Dulbecco’s modified Eagle’s medium (DMEM; GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) with GlutaMAX™, 10% FBS, and penicillin/streptomycin (100 unit/mL and 100 μg/mL, respectively; GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA).
4.3. Proliferation Assay of Dermal Papilla Cells
Dermal papilla cells were seeded at a density of 10,000 cells per well in 96-well plates in 100 μL of complete DMEM. Cells were allowed to adhere for 24 h. Treatment media were prepared to contain an appropriate concentration of L-fucose and chondroitin disaccharide Δdi-4S sodium salt in complete cell culture media. Treatments were performed in triplicate wells. Cells were cultured for a further 24, 48, and 72 h. No media changes were performed between initial treatment and cell assays. Only the medium was used to treat controls cells. After exposure for the desired period of time, 11 µL of PrestoBlue reagent was added to each well of the 96-well plate. Plates were incubated in the dark for 2 h at 37 °C. The fluorescence was read using a 560 nm excitation/590 nm emission filter set (10 nm bandwidth). Fluorescence data in wells containing cells were corrected for background fluorescence using cell-free media control replicates.
4.4. Proliferation Assay of Human Dermal Fibroblasts
Human dermal fibroblasts (HDFa) were seeded at a density of 5000 cells per well in 96-well plates in 100 μL complete medium. Cells were allowed to adhere overnight. Treatment media were prepared to contain an appropriate concentration of L-fucose and chondroitin disaccharide Δdi-4S sodium salt in complete cell culture media. Treatments were performed in triplicate wells. Cells were cultured for a further 24, 48, and 72 h. Only the medium was used to treat controls cells. After exposure for the desired period of time, 11 µL PrestoBlue reagent was added to each well of the 96-well plate. Plates were incubated in the dark for 2 h at 37 °C. The fluorescence was read using a 560 nm excitation/590 nm emission filter set (10 nm bandwidth).
4.5. Measurement of Alkaline Phosphatase Level
The SensoLyte® Alkaline Phosphatase Assay Kit (AnaSpec) was used for the quantitative evaluation of enzyme production by dermal papilla cells. Cells were seeded in transparent 12-well plates at a density of 30,000 cells per well in 2 mL of complete media. Cells were allowed to adhere overnight. The treatment medium was prepared to contain an appropriate concentration of L-fucose and chondroitin disaccharide Δdi-4S sodium salt in complete DMEM. Cells were assessed for alkaline phosphatase expression at 72, 120, and 168 h. Cells were gently washed twice with 1X assay buffer. Lysis buffer (1X assay buffer with TritonX-100) was added to each well. Cells were transferred into Eppendorf tubes and subjected to three freeze–thaw cycles to ensure cells lysis. Cell suspensions were centrifuged at 2500× g for 10 min at 4 °C. The supernatants were used in an AP assay according to the manufacturer’s protocol.
4.6. Histological Analysis of Alkaline Phosphatase
Dermal papilla cells were seeded at a density of 10,000 cells in each 8 Cell Culture Chamber FlexiPERM® (Sarstedt AG & Co., Nümbrecht, Germany) on a microscope slide. Cells were allowed to grow for 24 h and were incubated with appropriate concentrations of L-fucose and chondroitin disaccharide Δdi-4S sodium salt for 120 h. A BCIP/NBT tablet (SigmaFastTM BCIP-NBT; Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in 10 mL distilled water to prepare the substrate solution. Cells were washed with Wash Buffer (Tween20 in PBS) without disrupting the cell monolayer. After liquid aspiration, cells were covered to a depth of 2–3 mm with ice-cold 100% methanol and were allowed to fix for 15 min at −20 °C. Cells were then washed with Wash Buffer. The BCIP/NBT substrate solution was added to cover the cellular monolayer. The slides were incubated at room temperature in the dark for 10 min. The substrate solution was aspirated and the cell monolayer was washed with Wash Buffer. Cells were analyzed under a light microscope.
4.7. Measurement of GAGs Level
A Blyscan™ Glycosaminoglycan assay, a quantitative dye-binding method, was used for the analysis of sulphated glycosaminoglycans. Dermal papilla cells were seeded at a density of 200,000 cells in T25 flasks containing 4 mL of complete media. Cells were allowed to adhere overnight. Treatment media were prepared to contain an appropriate concentration of stimulants, L-fucose, and chondroitin disaccharide Δdi-4S sodium salt. Media were removed from all T25 flasks and retained for the sGAG assay. Cells were washed with PBS, drained, and 2 mL of Papain Extraction Reagent was added to each flask. Cells were incubated with Papain Extraction reagent at 65 °C with occasional mixing. After 3 h, the digested extracts were removed from the flasks and centrifuged at 10,000 rpm for 10 min. An amount of 50 μL of each supernatant was retained for the sGAG assay according to the manufacturer’s protocol. The sGAG concentration was obtained from the standard curve.
4.8. Measurement of Collagen Synthesis
Human dermal fibroblasts were seeded in 6-well plates at a density of 200,000 cells/well. Treatment media were prepared to contain an appropriate concentration of L-fucose and chondroitin disaccharide Δdi-4S sodium salt in DMEM with 1% of FBS. Cells were incubated with the tested compounds for 120 h. The synthesis of type I collagen by human dermal fibroblasts was determined using a commercially available enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
4.9. Measurement of Elastin Synthesis
Human dermal fibroblasts were seeded in 6-well plates at a density of 200,000 cells/well. Treatment media were prepared to contain an appropriate concentration of L-fucose and chondroitin disaccharide Δdi-4S sodium salt. Cells were incubated with the tested compounds for 120 h. The synthesis of elastin by human dermal fibroblasts was determined using a commercially available enzyme-linked immunosorbent assay (BT-Labs, Birmingham, UK) according to the manufacturer’s protocol.
4.10. RNA Extraction and Quantitative RT-PCR
Total RNA was extracted from the male dermal papilla cells using a High Pure RNA Isolation kit (Roche) according to the manufacturer’s protocol. A total of 500 ng of DNA-free RNA was subjected to reverse transcription using a qScript cDNA Synthesis Kit (Quanta bio, Beverly, MA, USA). The relative cDNA levels were measured using FastStart Essential DNA Green Master (Roche) in a LightCycler® 96 System (Roche). Two housekeeping genes, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and ACTB (actin beta), were used as reference genes in the calculations of relative expressions. The relative mRNA expression level was calculated using the 2−ΔΔCT analysis method. All measurements were repeated in three biological and three technical replicates. The primers used for the quantitative PCR are shown in Table 1.

Table 1.
Sequences of primer pairs used in RT-PCR.
4.11. Statistical Analysis
All results were expressed as the means ± SEM from three independent experiments. GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) and Dunnets’s multiple comparison tests were used to analyze significant differences (p < 0.05) between the mean values of the individual group.
Author Contributions
Conceptualization, H.M.; data curation, A.A.; formal analysis, A.A.; funding acquisition, H.M.; investigation, A.A.; methodology, A.A.; project administration, H.M.; resources, H.M.; supervision, H.M.; validation, H.M. and A.A.; visualization, A.A.; writing, A.A.; writing—review and editing, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Enterprise Ireland, grant number IP/2014/0444.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sparavigna, A. Role of the Extracellular Matrix in Skin Aging and Dedicated Treatment-State of the Art. Plast. Aesthet. Res. 2020, 2020, 14. [Google Scholar] [CrossRef]
- Uitto, J. The Role of Elastin and Collagen in Cutaneous Aging: Intrinsic Aging versus Photoexposure. J. Drugs Dermatol. 2008, 7, s12–s16. [Google Scholar] [PubMed]
- Mahmoudi, S.; Mancini, E.; Xu, L.; Moore, A.; Jahanbani, F.; Hebestreit, K.; Srinivasan, R.; Li, X.; Devarajan, K.; Prélot, L.; et al. Heterogeneity in Old Fibroblasts Is Linked to Variability in Reprogramming and Wound Healing. Nature 2019, 574, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Desprez, P.Y.; Campisi, J.; Velarde, M.C. Cell Autonomous and Non-Autonomous Effects of Senescent Cells in the Skin. J. Investig. Dermatol. 2015, 135, 1722–1726. [Google Scholar] [CrossRef]
- Shin, W.; Rosin, N.L.; Sparks, H.; Sinha, S.; Rahmani, W.; Sharma, N.; Workentine, M.; Abbasi, S.; Labit, E.; Stratton, J.A.; et al. Dysfunction of Hair Follicle Mesenchymal Progenitors Contributes to Age-Associated Hair Loss. Dev. Cell 2020, 53, 185–198.e7. [Google Scholar] [CrossRef]
- Matsumura, H.; Mohri, Y.; Thanh Binh, N.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Stem Cells: Hair Follicle Aging Is Driven by Transepidermal Elimination of Stem Cells via COL17A1 Proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef]
- Chen, C.C.; Murray, P.J.; Jiang, T.X.; Plikus, M.V.; Chang, Y.T.; Lee, O.K.; Widelitz, R.B.; Chuong, C.M. Regenerative Hair Waves in Aging Mice and Extra-Follicular Modulators Follistatin, Dkk1, and Sfrp4. J. Investig. Dermatol. 2014, 134, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Keyes, B.E.; Segal, J.P.; Heller, E.; Lien, W.-H.; Chang, C.-Y.; Guo, X.; Oristian, D.S.; Zheng, D.; Fuchs, E. Nfatc1 Orchestrates Aging in Hair Follicle Stem Cells. Proc. Natl. Acad. Sci. USA 2013, 110, E4950–E4959. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A Two-Step Mechanism for Stem Cell Activation during Hair Regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Deschene, E.R.; Zito, G.; Gonzalez, D.G.; Saotome, I.; Haberman, A.M.; Greco, V. Live Imaging of Stem Cell and Progeny Behaviour in Physiological Hair-Follicle Regeneration. Nature 2012, 487, 496–499. [Google Scholar] [CrossRef]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular Dissection of Mesenchymal–Epithelial Interactions in the Hair Follicle. PLoS Biol. 2005, 3, e331. [Google Scholar] [CrossRef]
- Elliott, K.; Stephenson, T.J.; Messenger, A.G. Differences in Hair Follicle Dermal Papilla Volume Are Due to Extracellular Matrix Volume and Cell Number: Implications for the Control of Hair Follicle Size and Androgen Responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef]
- Šližyte, R.; Mozuraityte, R.; Martínez-Alvarez, O.; Falch, E.; Fouchereau-Peron, M.; Rustad, T. Functional, Bioactive and Antioxidative Properties of Hydrolysates Obtained from Cod (Gadus Morhua) Backbones. Process. Biochem. 2009, 44, 668–677. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.K. Purification and Characterization of Antioxidative Peptide Derived from Muscle of Conger Eel (Conger Myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant Properties of a New Antioxidative Peptide from Algae Protein Waste Hydrolysate in Different Oxidation Systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in Vitro Antioxidative Effects of Giant Squid Muscle Peptides on Free Radical-Mediated Oxidative Systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.G.; Lee, J.K.; Park, H.G.; Jeon, J.K.; Kim, S.K. Antioxidant Peptides Isolated from the Marine Rotifer, Brachionus Rotundiformis. Process. Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a Novel Antimicrobial Peptide from Jellyfish Aurelia Aurita with Structural Features of Defensins and Channel-Blocking Toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef]
- Desjardine, K.; Pereira, A.; Wright, H.; Matainaho, T.; Kelly, M.; Andersen, R.J. Tauramamide, a Lipopeptide Antibiotic Produced in Culture by Brevibacillus Laterosporus Isolated from a Marine Habitat: Structure Elucidation and Synthesis. J. Nat. Prod. 2007, 70, 1850–1853 . [Google Scholar] [CrossRef]
- Wang, W.; Kim, H.; Nam, S.J.; Rho, B.J.; Kang, H. Antibacterial Butenolides from the Korean Tunicate Pseudodistoma Antinboja. J. Nat. Prod. 2012, 75, 2049–2054 . [Google Scholar] [CrossRef]
- Vidal-Dupiol, J.; Ladrière, O.; Destoumieux-Garzón, D.; Sautière, P.E.; Meistertzheim, A.L.; Tambutté, E.; Tambutté, S.; Duval, D.; Fouré, L.; Adjeroud, M.; et al. Innate Immune Responses of a Scleractinian Coral to Vibriosis. J. Biol. Chem. 2011, 286, 22688–22698. [Google Scholar] [CrossRef]
- Alemán, A.; Pérez-Santín, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gómez-Guillén, M.C.; Montero, P. Squid Gelatin Hydrolysates with Antihypertensive, Anticancer and Antioxidant Activity. Food Res. Int. 2011, 44, 1044–1051. [Google Scholar] [CrossRef]
- Ramos, A.; Castro-Carvalho, B.; Prata-Sena, M.; Dethoup, T.; Buttachon, S.; Kijjoa, A.; Rocha, E. Crude Extracts of Marine-Derived and Soil Fungi of the Genus Neosartorya Exhibit Selective Anticancer Activity by Inducing Cell Death in Colon, Breast and Skin Cancer Cell Lines. Pharmacogn. Res. 2016, 8, 8–15. [Google Scholar] [CrossRef]
- Choo, G.S.; Lee, H.N.; Shin, S.A.; Kim, H.J.; Jung, J.Y. Anticancer Effect of Fucoidan on DU-145 Prostate Cancer Cells through Inhibition of PI3K/Akt and MAPK Pathway Expression. Mar. Drugs 2016, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.J.; Kwon, T.H.; Park, G.H.; Song, H.M.; Lee, S.J.; Park, N.H.; Jeong, J.B. In Vitro Anticancer Activity of Phlorofucofuroeckol a via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells. Mar. Drugs 2016, 14, 69. [Google Scholar] [CrossRef]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer Activity of Sea Cucumber Triterpene Glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeon, Y.J. Anti-Diabetic Effects of Brown Algae Derived Phlorotannins, Marine Polyphenols through Diverse Mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Zhang, D.; Fujii, I.; Lin, C.; Ito, K.; Guan, H.; Zhao, J.; Shinohara, M.; Matsukura, M. The Stimulatory Activities of Polysaccharide Compounds Derived from Algae Extracts on Insulin Secretion in Vitro. Biol. Pharm. Bull. 2008, 31, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In Vitro and in Vivo Evidence for a Satiating Effect of Fish Protein Hydrolysate Obtained from Blue Whiting (Micromesistius Poutassou) Muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Nobile, V.; Duclos, E.; Michelotti, A.; Bizzaro, G.; Negro, M.; Soisson, F. Supplementation with a Fish Protein Hydrolysate (Micromesistius Poutassou): Effects on Body Weight, Body Composition, and CCK/GLP-1 Secretion. Food Nutr. Res. 2016, 60, 29857. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a Sulfated Polysaccharide, Inhibits Adipogenesis through the Mitogen-Activated Protein Kinase Pathway in 3T3-L1 Preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef]
- Xie, C.L.; Kim, J.S.; Ha, J.M.; Choung, S.Y.; Choi, Y.J. Angiotensin I-Converting Enzyme Inhibitor Derived from Cross-Linked Oyster Protein. BioMed Res. Int. 2014, 2014, 379234. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Ryu, B.; Kim, S.-K. Active Peptides from Skate (Okamejei Kenojei) Skin Gelatin Diminish Angiotensin-I Converting Enzyme Activity and Intracellular Free Radical-Mediated Oxidation. Food Chem. 2014, 143, 246–255. [Google Scholar] [CrossRef]
- Ryu, B.M.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-Photoaging Activity and Inhibition of Matrix Metalloproteinase (MMP) by Marine Red Alga, Corallina Pilulifera Methanol Extract. Radiat. Phys. Chem. 2009, 78, 98–105 . [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-Induced Skin Carcinogenesis in SKH-1 Mice by Brown Algae Polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of Phlorotannins Isolated from Ecklonia Cava on Melanogenesis and Their Protective Effect against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Kang, H.Y.; Yoon, T.J.; Lee, G.J. Whitening Effects of Marine Pseudomonas Extract. Ann. Dermatol. 2011, 23, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-Pigmentary Activity of Fucoxanthin and Its Influence on Skin MRNA Expression of Melanogenic Molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase Inhibitors Isolated from the Edible Brown Alga Ecklonia Stolonifera. Arch. Pharm. Res. 2004, 27. [Google Scholar] [CrossRef]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.I.; Terada, R.; Nozaki, H. The Novel Anti-Propionibacterium Acnes Compound, Sargafuran, Found in the Marine Brown Alga Sargassum Macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural Characterization of Fucoidan from Laminaria Hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure-Function Relationship. ACS Appl. Bio. Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.G.; Vicente, C.P.; Zancan, P.; Mourão, P.A.S. Different Antithrombotic Mechanisms among Glycosaminoglycans Revealed with a New Fucosylated Chondroitin Sulfate from an Echinoderm. Blood Coagul. Fibrinolysis 2000, 11, 563–573 . [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and Biological Function in Mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds Inhibit Proliferation of Melanoma Cells and Induce Apoptosis by Activation of Caspase-3 in Vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Mller-Werner, B.; Jelinek, J.; Stahl, B. Variation of Human Milk Oligosaccharides in Relation to Milk Groups and Lactational Periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.H.; Lynch, B.S.; Baldwin, N.; Dakoulas, E.W.; Roy, S.; Moore, C.; Thorsrud, B.A.; Röhrig, C.H. Safety Evaluation of the Human-Identical Milk Monosaccharide, l-Fucose. Regul. Toxicol. Pharmacol. 2015, 72, 39–48. [Google Scholar] [CrossRef]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, C.Y.; Yang, C.C.; Lee, T.M.; Chang, J.S. Hair Growth-Promoting Effects of Sargassum Glaucescens Oligosaccharides Extracts. J. Taiwan Inst. Chem. Eng. 2022, 134, 104307. [Google Scholar] [CrossRef]
- Shin, K.; Kim, T.-S.; Kyung, J.; Kim, D.; Park, D.; Choi, E.-K.; Lee, S.-P.; Yang, W.-S.; Kang, M.-H.; Kim, Y.-B. Effectiveness of the Combinational Treatment of Laminaria Japonica and Cistanche Tubulosa Extracts in Hair Growth. Lab. Anim. Res. 2015, 31, 24–32 . [Google Scholar] [CrossRef]
- Shin, H.; Cho, A.R.; Kim, D.Y.; Munkhbayer, S.; Choi, S.J.; Jang, S.; Kim, S.H.; Shin, H.C.; Kwon, O. Enhancement of Human Hair Growth Using Ecklonia Cava Polyphenols. Ann. Dermatol. 2016, 28, 15–21. [Google Scholar] [CrossRef]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan Modulates the Effect of Transforming Growth Factor (TGF)-Beta1 on Fibroblast Proliferation and Wound Repopulation in in Vitro Models of Dermal Wound Repair. Biol. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Lee, W.W.; Jeon, Y.J. Fucoidan Isolated from Hizikia Fusiforme Suppresses Ultraviolet B-Induced Photodamage by down-Regulating the Expressions of Matrix Metalloproteinases and pro-Inflammatory Cytokines via Inhibiting NF-ΚB, AP-1, and MAPK Signaling Pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Song, Y.S.; Li, H.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Choi, H.R.; Park, K.C. Fucoidan Promotes the Reconstruction of Skin Equivalents. Korean J. Physiol. Pharmacol. 2014, 18, 327–331. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan Inhibits UVB-Induced MMP-1 Expression in Human Skin Fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef]
- Min, D.; Park, S.; Kim, H.; Lee, S.H.; Ahn, Y.; Jung, W.; Kim, H.J.; Cho, Y.W. Potential Anti-Ageing Effect of Chondroitin Sulphate through Skin Regeneration. Int. J. Cosmet. Sci. 2020, 42, 520–527. [Google Scholar] [CrossRef]
- D’Agostino, A.; La Gatta, A.; Stellavato, A.; Cimini, D.; Corsuto, L.; Cammarota, M.; D’Agostino, M.; Schiraldi, C. Potential of Biofermentative Unsulfated Chondroitin and Hyaluronic Acid in Dermal Repair. Int. J. Mol. Sci. 2022, 23, 1686. [Google Scholar] [CrossRef]
- Neri, T.A.N.; Palmos, G.N.; Park, S.Y.; Jung, T.S.; Choi, B.D. Hair Growth-Promoting Activities of Glycosaminoglycans Extracted from the Tunics of Ascidian (Halocynthia Roretzi). Polymers 2022, 14, 1096. [Google Scholar] [CrossRef]
- Kim, B.J.; No, Y.A.; Lee, Y.; Kim, M.N.; Hong, C.K.; Yoo, K.H.; Kim, Y.M.; Hwang, J.H.; Kong, S.Y. Use of Cream Containing Mucus Secreted by Snails Has an Anti-Aging Effect on Skin. Korean J. Dermatol. 2015, 53, 430–436. [Google Scholar]
- Lee, S.H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.S.; Min, D.S.; Kim, H.Y.; Choi, K.Y. Valproic Acid Induces Hair Regeneration in Murine Model and Activates Alkaline Phosphatase Activity in Human Dermal Papilla Cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Han, Q.; Zhao, Y.; Liu, H.; et al. Treatment of MSCs with Wnt1a-Conditioned Medium Activates DP Cells and Promotes Hair Follicle Regrowth. Sci. Rep. 2014, 4, 5432. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Seo, C.H.; Kwack, M.H.; Oh, J.M.; Lee, H.W.; Gopal, A.; Sung, Y.K.; Jeong, S.Y.; Lee, S.W.; et al. Macrophage-Derived Extracellular Vesicle Promotes Hair Growth. Cells 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Yang, J.M.; Won, G.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Establishment and Characterization of Five Immortalized Human Scalp Dermal Papilla Cell Lines. Biochem. Biophys. Res. Commun. 2018, 496, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla Cells as in Vitro Screening Model for Hair Growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef]
- Westgate, G.E.; Messenger, A.G.; Watson, L.P.; Gibson, W.T. Distribution of Proteoglycans during the Hair Growth Cycle in Human Skin. J. Investig. Dermatol. 1991, 96, 191–195. [Google Scholar] [CrossRef]
- Malgouries, S.; Thibaut, S.; Bernard, B.A. Proteoglycan Expression Patterns in Human Hair Follicle. Br. J. Dermatol. 2008, 158, 234–342. [Google Scholar] [CrossRef]
- Carrino, D.A.; Sorrell, J.M.; Caplan, A.I. Age-Related Changes in the Proteoglycans of Human Skin. Arch. Biochem. Biophys. 2000, 373, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N.; Kinsella, M.G.; Qwarnström, E.E. The Role of Proteoglycans in Cell Adhesion, Migration and Proliferation. Curr. Opin. Cell Biol. 1992, 4, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Péterszegi, G.; Isnard, N.; Robert, A.M.; Robert, L. Studies on Skin Aging. Preparation and Properties of Fucose-Rich Oligo- and Polysaccharides. Effect on Fibroblast Proliferation and Survival. Biomed. Pharmacother. 2003, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, A.C.; Rappolee, D.A.; Millan, J.L.; Manes, T.; Ziomek, C.A.; Theodosiou, N.G.; Werb, Z.; Pedersen, R.A.; Schultz, G.A. Two Alkaline Phosphatase Genes Are Expressed during Early Development in the Mouse Embryo. Development 1990, 110, 555–564. [Google Scholar] [CrossRef]
- Iida, M.; Ihara, S.; Matsuzaki, T. Hair Cycle-Dependent Changes of Alkaline Phosphatase Activity in the Mesenchyme and Epithelium in Mouse Vibrissal Follicles. Dev. Growth Differ. 2007, 49, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kwon, S.J.; Jang, H.J.; Seo, Y.K. Rice Bran Mineral Extract Increases the Expression of Anagen-Related Molecules in Human Dermal Papilla through Wnt/Catenin Pathway. Food Nutr. Res. 2017, 61, 1412792. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Huelsken, J.; Behrens, J. The Wnt Signalling Pathway. J. Cell Sci. 2002, 115, 3977–3978. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, X.; Guo, H.; Deng, F.; Yang, J.; Li, Y. Wnt5a Suppresses β-Catenin Signaling during Hair Follicle Regeneration. Int. J. Med. Sci. 2016, 13, 603–610. [Google Scholar] [CrossRef]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt Signaling Maintains the Hair-Inducing Activity of the Dermal Papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a Novel Activator of Wnt/β-Catenin Signalling, Promotes Hair Regrowth and Hair Follicle Neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Morroniside Regulates Hair Growth and Cycle Transition via Activation of the Wnt/β-Catenin Signaling Pathway. Sci. Rep. 2018, 8, 13785. [Google Scholar] [CrossRef]
- Chen, J.; Chuong, C.M. Patterning Skin by Planar Cell Polarity: The Multi-Talented Hair Designer. Exp. Dermatol. 2012, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.; Kim, G.S.; Cheng, A.G. Making Sense of Wnt Signaling—Linking Hair Cell Regeneration to Development. Front. Cell Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef]
- Carreira-Barbosa, F.; Concha, M.L.; Takeuchi, M.; Ueno, N.; Wilson, S.W.; Tada, M. Prickle 1 Regulates Cell Movements during Gastrulation and Neuronal Migration in Zebrafish. Development 2003, 130, 4037–4046. [Google Scholar] [CrossRef]
- Veeman, M.T.; Slusarski, D.C.; Kaykas, A.; Louie, S.H.; Moon, R.T. Zebrafish Prickle, a Modulator of Noncanonical Wnt/Fz Signaling, Regulates Gastrulation Movements. Curr. Biol. 2003, 13, 680–685. [Google Scholar] [CrossRef]
- Chan, D.W.; Chan, C.; Yam, J.W.P.; Ching, Y.; Ng, I.O.L. Prickle-1 Negatively Regulates Wnt/β-Catenin Pathway by Promoting Dishevelled Ubiquitination/Degradation in Liver Cancer. Gastroenterology 2006, 131, 1218–1227. [Google Scholar] [CrossRef]
- Dyberg, C.; Papachristou, P.; Haug, B.H.; Lagercrantz, H.; Kogner, P.; Ringstedt, T.; Wickström, M.; Johnsen, J.I. Planar Cell Polarity Gene Expression Correlates with Tumor Cell Viability and Prognostic Outcome in Neuroblastoma. BMC Cancer 2016, 16, 259. [Google Scholar] [CrossRef]
- Schittek, B.; Sinnberg, T. Biological Functions of Casein Kinase 1 Isoforms and Putative Roles in Tumorigenesis. Mol. Cancer 2014, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.K.; Virshup, D.M. Casein Kinase 1: Complexity in the Family. Int. J. Biochem. Cell Biol. 2011, 43, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Pribluda, A.; Goldstein, R.E.; Morgenstern, Y.; Brachya, G.; Cojocaru, G.; Snir-Alkalay, I.; Burstain, I.; Haffner-Krausz, R.; Jung, S.; et al. CKIα Ablation Highlights a Critical Role for P53 in Invasiveness Control. Nature 2011, 470, 409–413. [Google Scholar] [CrossRef]
- Amit, S.; Hatzubai, A.; Birman, Y.; Andersen, J.S.; Ben-Shushan, E.; Mann, M.; Ben-Neriah, Y.; Alkalay, I. Axin-Mediated CKI Phosphorylation of β-Catenin at Ser 45: A Molecular Switch for the Wnt Pathway. Genes Dev. 2002, 16, 1066–1076. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a Regulator of Cell Life and Death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: A Double-Edged Sword in Tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Durchdewald, M.; Angel, P.; Hess, J. The Transcription Factor Fos: A Janus-Type Regulator in Health and Disease. Histol. Histopathol. 2009, 24, 1451–1461. [Google Scholar] [CrossRef]
- Shaulian, E. AP-1-The Jun Proteins: Oncogenes or Tumor Suppressors in Disguise? Cell Signal. 2010, 22, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wan, X.; Zou, Y.; Chen, Z.; Zhong, A. Transforming Growth Factor Beta (TGF-β) Is Activated by the CtBP2-P300-AP1 Transcriptional Complex in Chronic Renal Failure. Int. J. Biol. Sci. 2020, 16, 204–215. [Google Scholar] [CrossRef]
- Tsuji, Y.; Denda, S.; Soma, T.; Raftery, L.; Momoi, T.; Hibino, T. A Potential Suppressor of TGF-β Delays Catagen Progression in Hair Follicles. J. Investig. Dermatol. Symp. Proc. 2003, 8, 65–68. [Google Scholar] [CrossRef]
- Hibino, T.; Nishiyama, T. Role of TGF-Beta2 in the Human Hair Cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Glycans and Glycosaminoglycans in Neurobiology: Key Regulators of Neuronal Cell Function and Fate. Biochem. J. 2018, 475, 2511–2545. [Google Scholar] [CrossRef]
- Bernard, B.A. Hair Cycle Dynamics: The Case of the Human Hair Follicle. J. Soc. Biol. 2003, 197, 57–61. [Google Scholar] [CrossRef]
- Couchman, J.R. Rat Hair Follicle Dermal Papillae Have an Extracellular Matrix Containing Basement Membrane Components. J. Investig. Dermatol. 1986, 87, 762–767. [Google Scholar] [CrossRef]
- Chiu, H.C.; Chang, C.H.; Chen, J.S.; Jee, S.H. Human Hair Follicle Dermal Papilla Cell, Dermal Sheath Cell and Interstitial Dermal Fibroblast Characteristics. J. Formos. Med. Assoc. 1996, 95, 667–674. [Google Scholar] [PubMed]
- Braun-Falco, O. The Histochemistry of the Hair Follicle. In The Biology of Hair Growth; Elsevier: Amsterdam, The Netherlands, 1958; pp. 65–90. [Google Scholar]
- Du Cros, D.L.; LeBaron, R.G.; Couchman, J.R. Association of Versican with Dermal Matrices and Its Potential Role in Hair Follicle Development and Cycling. J. Investig. Dermatol. 1995, 105, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martos, S.; Calvo-Sánchez, M.; García-Alonso, K.; Castro, B.; Hashtroody, B.; Espada, J. Sustained Human Hair Follicle Growth Ex Vivo in a Glycosaminoglycan Hydrogel Matrix. Int. J. Mol. Sci. 2019, 20, 1741. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue Elasticity and the Ageing Elastic Fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef]
- Robert, L.; Fodil-Bourahla, I.; Bizbiz, L.; Robert, A.M. Effects of L-Fucose and Fucose-Rich Oligo- and Polysaccharides (FROP-s) on Collagen Biosynthesis by Human Skin Fibroblasts. Modulation of the Effect of Retinol, Ascorbate and α-Tocopherol. Biomed. Pharmacother. 2004, 58, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Kano, S.; Hashimoto, F.; Sugibayashi, K.; Tokudome, Y. Chondroitin Sulfate Disaccharide Enhances Extracellular Matrix-Related Gene and Protein Expression in Normal Human Dermal Fibroblasts in Vitro. J. Jpn. Cosmet. Sci. Soc. 2015, 39, 1–6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).